Abstract
Although the short isoform of ErbB3-binding protein 1 (Ebp1), p42 has been considered to be a potent tumor suppressor in a number of human cancers, whether p42 suppresses tumorigenesis of lung cancer cells has never been clarified. In the current study we investigated the tumor suppressor role of p42 in non-small cell lung cancer cells. Our data suggest that the expression level of p42 is inversely correlated with the cancerous properties of NSCLC cells and that ectopic expression of p42 is sufficient to inhibit cell proliferation, anchorage-independent growth, and invasion as well as tumor growth in vivo. Interestingly, p42 suppresses Akt activation and overexpression of a constitutively active form of Akt restores the tumorigenic activity of A549 cells that is ablated by exogenous p42 expression. Thus, we propose that p42 Ebp1 functions as a potent tumor suppressor of NSCLC through interruption of Akt signaling. [BMB Reports 2015; 48(3): 159-165]
Keywords: AKT, Ebp1, EGFR, Non-small cell lung cancer, Tumor suppressor
INTRODUCTION
To date, two isoforms of Ebp1 (ErbB3 receptor-binding protein; also known as proliferation-associated protein 2G4 or PA2G4) have been characterized: p48 and p42 (1). p48 is 54 amino acids longer than p42 at its N-terminus and promotes cell proliferation and contributes to neuronal survival (2). Previously we proposed that p48 is an oncoprotein that is highly expressed in human gliomas and leads to down-regulation of p53 protein expression, promoting tumorigenesis in vitro and in vivo and resulting in poor clinical outcome (3, 4). Patients with breast cancers that express high levels of Ebp1/PA2G4 have poor clinical outcomes, suggesting that Ebp1 may promote aggressive behavior (5). In addition, Ebp1 expression increased with disease progression in prostate cancer (6). Thus, overexpression of the long form of Ebp1 (p48) has an oncogenic function.
In contrast to the oncogenic potential of p48, the short isoform of Ebp1, p42, has been considered a tumor suppressor because it binds to tumor suppressor retinoblastoma protein (Rb), thus inhibiting E2F-1 mediated transcription (7, 8), and strongly suppresses both androgen receptor (AR)-mediated transcription and tumorigenesis of prostate cancer cells and salivary adenoid carcinoma cell metastasis in mice (9, 10). This is consistent with our observation that p42 Ebp1 suppresses cancerous growth of glioma cells and reduces the size of tumor in glioma mouse models (3). Moreover, p42 is ubiquitinated and degraded in various human cancer cells, accounting for its rare detection by immunoblotting (11). Collectively, these findings suggest that the shorter isoform of Ebp1 acts as a potential tumor suppressor in various human cancers.
Lung cancer is the leading cause of cancer-related death throughout the world. In particular, non-small cell lung cancer (NSCLC), including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, is the predominant type of lung cancer (12, 13). Although mounting evidence suggests that p42 possesses tumor suppressive activity, the role of p42 in lung cancer has not been investigated. In this report, we demonstrated that low p42 expression is associated with high tumorigenicity of NSCLC cells and that restoration of p42 in NSCLC cells functionally impeded their malignant behavior, providing evidence that p42 acts as tumor suppressor that inhibits cell proliferation and tumor growth of NSCLC.
RESULTS
p42 Ebp1 protein expression represses the oncogenicity of lung cancer cells
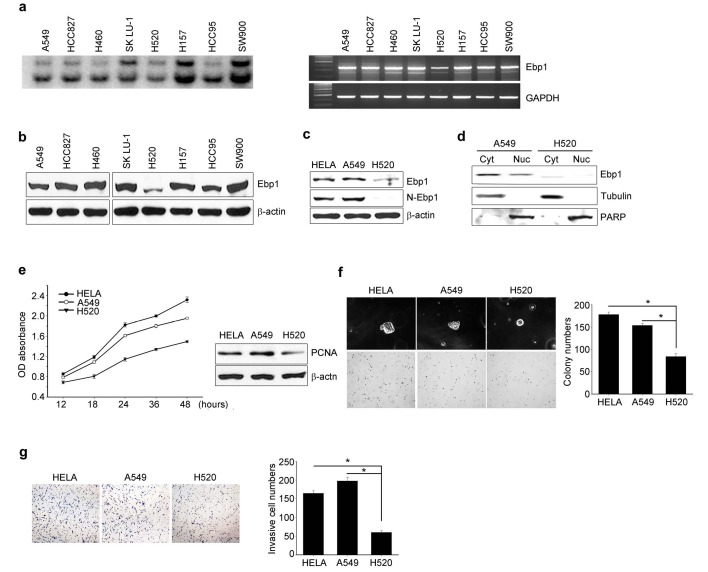
We examined the mRNA and protein expression levels of the two Ebp1 isoforms in several NSCLC cell lines. In all tested cells, northern blotting and RT-PCR analysis demonstrated the existence of two distinct Ebp1 mRNA species (2.2 kb and 1.7 kb; Fig. 1A), consistent with the previous finding that Ebp1 encodes two isoforms: p48 and p42 (1). However, Immunoblotting analysis selectively revealed a 48-kDa band (Fig. 1B), indicating that p48 is the major isoform detected in NSCLC cells. Among the tested NSCLC cells, only H520 cells expressed detectable level of the smaller isoform of Ebp1, although this p42 expression was at a low level (Fig. 1B). To confirm whether the Ebp1 protein expressed in H520 was indeed the p42 isoform, we performed immunoblotting analysis with two different antibodies, anti-N-Ebp1 antibody (specific for p48) and anti-Ebp1 (which detects both p48 and p42) and found that anti-N-Ebp1 antibody did not detect p48 Ebp1 in H520 cells (Fig. 1C). This finding was supported by subcellular fraction analysis. Endogenous Ebp1 in A549 was detected in both cytoplasm and nucleus whereas in H520 cells the majority of Ebp1 was present in the cytoplasm, confirming that the Ebp1 expressed in H520 is the p42 isoform (Fig. 1D).
Fig. 1. p42 Ebp1 protein expression represses the oncogenicity of lung cancer cells. (A) Ebp1 mRNA expression was determined by northern blotting (left) and RT-PCR (right). (B and C) Immunoblot analysis of Ebp1 protein expression with specific antibodies as indicated. β-actin was used as an internal loading control. (D) Subcellular fractions of A549 and H520 cells were subjected to immunoblot analysis with anti-Ebp1 antibody. The purity of each fraction was confirmed by anti-PARP (nucleus) and anti-tubulin (cytosol) antibodies. (E) Determination of viable cell number by colorimetric MTT assay (left); immunoblotting of cell lysate with the indicated antibodies (right). (F) Representative digital microscopic images of colony-forming cells (left). The number of colonies is presented under the bar graphs (right). (G) Invasive cells were fixed and stained, and representative areas were photographed (left). Invasive cells were counted at 100× magnification (right).
To determine the role of p42 in the tumorigenicity of lung cancer cells, we compared cell proliferation, anchorage-independent growth, and in vitro invasion between H520 cells that express detectable level of p42 protein and A549 cells that express only p48 protein. HeLa cells, which are known to express p48 (1), were used as a positive control. The cell proliferation rate of A549 cells was higher than that of H520 cells (Fig. 1E left), correlating with the low expression of proliferating cell nuclear antigen (PCNA) protein in H520 cells (Fig. 1E, right). To determine whether p42 expression is involved in the colonogenicity of lung cancer cells, we tested the ability of A549 and H520 cells to form colonies in soft agar. While A549 cells formed approximately 150 colonies, H520 cells formed fewer than 100 colonies, which were smaller (Fig. 1F). Moreover, in vitro invasion assays with Matrigel demonstrated that the ability of H520 cells to digest and penetrate the Matrigel barrier was attenuated (63.5% and 70% inhibition of invasion compared with HeLa and A549 cells, respectively) (Fig. 1G). Thus, our data suggest that endogenous expression of p42 Ebp1 probably leads to the relatively lower tumorigenicity of H520 cells compared with A549 cells.
p42 inhibited A549 cell growth and motility in vitro
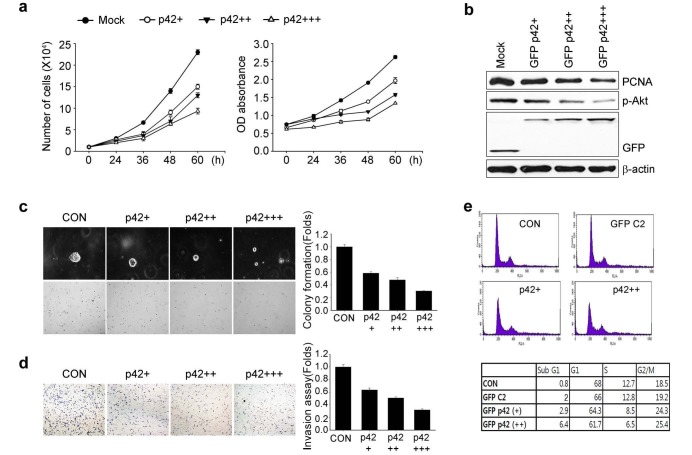
Based on the finding that p42-expressing H520 cells exhibit low oncogenic activity, we hypothesized that p42 might be a tumor suppressor, at least in NSCLC. To test this hypothesis we transfected p42 construct into A549 cells that do not express endogenous p42 protein. Overexpression of p42 in A549 cells elicited notable suppression of cell proliferation and reduced PCNA expression (Figs. 2A and B). Moreover, forced expression of p42 in A549 cells markedly suppressed anchorage-independent growth and in vitro invasion (Figs. 2C and 2D).
Fig. 2. p42 inhibits A549 cell growth and motility in vitro. A549 cells were transfected with vector control or GFP-p42 in a dose-dependent manner (1, 2 and 4 μg). (A) Cell proliferation assay (left) or colorimetric MTT assay (right) of cells transfected with GFP-p42. (B) Immunoblot analysis of cells in (A). (C) Colony formation and (D) Invasion assay. A representative photomicrograph is provided (left). The number of colonies and invasive cells are presented under the bar graphs (right). (E) Cells were labeled with PI and analyzed by flow cytometry.
To explore the tumor suppressive effect of p42, we tested whether p42 expression affects A549 cell cycle kinetics in vitro. Fluorescence activated cell sorting (FACS) analysis demonstrated that increased expression of p42 caused notable accumulation of the sub-G1 population and increased G2 phase population, with a reduction in G1 and S phase populations (Fig. 2E). In contrast, no changes in the number of cells in G1 or S phase were observed in GFP-vector transfected cells compared with non-transfected A549 cells. Presumably, overexpression of p42 prohibits G1/S phase progression and delaying G2 phase progression thereby inhibiting tumor cell growth and enhancing cell death.
Overexpression of p42 increases susceptibility of A549 cells to apoptosis
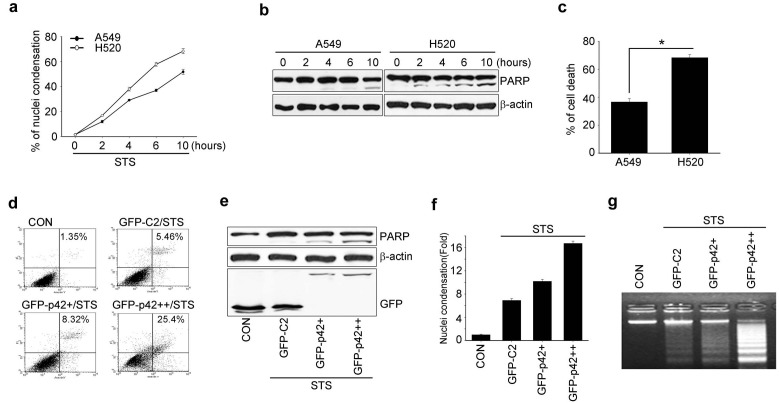
Because higher expression of p42 exerted the accumulation of sub-G1 population (Fig. 2E), we investigated whether p42 expression increased the apoptotic sensitivity of lung cancer cells under genotoxic stress using staurosporine (STS), a general kinase inhibitor. Compared with A549 cells, treatment of H520 cells with STS induced a rapid increase in condensed chromatin in a time-dependent manner (Fig. 3A) and revealed clear cleavage of poly (ADP-ribose) polymerase (PARP), a wellknown hallmark of apoptosis (Fig. 3B). In addition, 60% of H520 cells stained positive in Tunel assay compared with 40% of A549 cells (Fig. 3C). Thus, the relatively high level of p42 expression in H520 cells is associated with enhanced susceptibility to apoptosis.
Fig. 3. Overexpression of p42 increases susceptibility of A549 cells to apoptosis. (A) A549 and H520 cells were treated with STS (100 nM) for the indicated times and stained with DAPI. The number of cells with nuclear condensation was counted. (B) Whole cell lysates from (A) were collected and 30 μg protein was used for immunodetection of target proteins. (C) A549 and H520 cells were treated with STS (100 nM) for 16 hours and apoptotic cell death was determined by Tunel assay. The percentage of positive Tunel-stained cells is presented under the bar graphs. *P < 0.05. (D-G) A549 cells were transfected with vector control or GFP p42 (2 and 4 μg) in a dose-dependent manner, followed by treatment with 100 nM STS. (D) Apoptotic cells were detected by flow cytometry after staining for Annexin V. (E) Immunoblot analysis with the indicated antibodies. (F) The condensed nuclei were counted under a fluorescence microscope after staining with DAPI and the results are presented under the bar graph. (G) Genomic DNA was extracted and 10 μg DNA was analyzed by electrophoresis in a 2% agarose gel.
As p42-expressing H520 cells showed enhanced sensitivity to STS-induced apoptosis, we investigated the effects of p42 overexpression on sensitivity to apoptosis by introducing p42 in A549 cells. FACS analysis with Anexin V staining showed that overexpression of p42 in A549 cells notably enhanced apoptotic cell death following STS treatment in a p42 dose-dependent manner (Fig. 3D). Moreover, PARP proteolysis was substantially enhanced in A549 cells upon increased expression of p42 (Fig. 3E), indicating that p42 overexpression results in enhanced sensitivity to apoptosis induction upon exposure to toxic insults such as STS treatment.
To confirm that p42 increased the vulnerability of A549 cells to certain apoptotic stimuli, we investigated hallmarks of the terminal stage of apoptosis, chromatin condensation and DNA breakdown. Upon STS treatment A549 cells showed distinct formation of discrete apoptotic bodies when p42 was expressed, and isolated nuclei from p42-expressing A549 cells contained highly fragmented DNA (Figs. 3F and G). Thus, our data demonstrate that p42 overexpression in NSCLC cells promotes apoptosis, and give rise to the possibility that forced expression of p42 in some, if not all, NSCLCs might prevent tumor cells from escaping apoptosis.
p42 suppresses in vivo tumorigenicity of A549 cells, inhibiting EGFR signaling
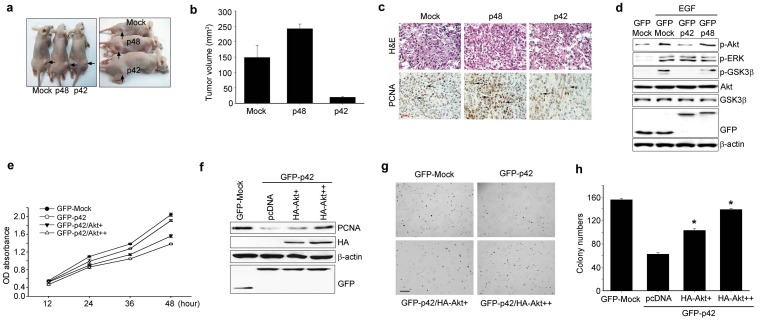
We next examined the anti-tumorigenic potential of p42-transfected A549 cells in nude mice. Tumor growth was first noticed at day 12 after injection in all mice that were injected with mock or GFP-p48-expressing A549 cells. In contrast, no tumors formed in mice injected with GFP-p42-expressing A549 cells within the same time period. Palpable tumors were first observed in mice that were implanted with the GFP- p42-expressing A549 cells at day 20 post-injection. The average tumor volume was measured 28 days post-injection. Representative images of tumors from xenograft mice at day 28 after injection are displayed in Fig. 4A, and show that tumors derived from A549/p42 cells were smaller and paler than those derived from control cells, whereas A549/p48-derived tumors were larger (Fig. 4B). Mice injected with p42-expressing cells displayed decreased tumor volumes (23.71 ± 4.61 mm3, P = 0.009) compared with cells expressing the control vector (141.47 ± 6.82 mm3, P = 0.025) (Fig. 4B), while p48-expressing cells resulted in increased tumor volume (246.24 ± 9.58 mm3, P = 0.01). Proliferating cell nuclear antigen (PCNA) was less frequently expressed in nude mice injected with p42-expressing cells than in control or p48 injected group (Fig. 4C). Taken together, our in vivo data indicate that overexpression of p42 Ebp1 in A549 cells abrogates A549-induced tumor growth whereas p48 over-expression stimulates tumor growth of NSCLC.
Fig. 4. p42 suppresses in vivo tumorigenicity of A549 cells, inhibiting EGFR signaling A549 cells that were stably transfected with GFP C2, GFP p42, or GFP p48 were injected into nude mice. After 28 days the mice were photographed and representative tumor formation is shown (A). (B) Tumor volumes were measured 28 days after tumor inoculation, n = 5. (C) Tumors from nude mice were explanted, fixed in formalin, and embedded in paraffin. Proliferative status was evaluated by H & E staining and with an anti-PCNA antibody. Arrows indicate PCNA-positive cells (bottom). (D) A549 cells were transfected with the indicated constructs and treated with EGF for 10 minutes. Whole cell protein (30 μg) was analyzed by immunoblotting with the indicated antibodies. (E) A549 cells transfected as shown, were subjected to MTT assay. (F) A549 cells, transfected as shown, were subjected to immunoblot analysis. (G and H) Colony forming assay with A549 cells transfected as indicated.
Epidermal growth factor receptor (EGFR) is an attractive target for the development of therapeutic agents (14) because it is known to promote cell growth and function as an oncogene and is expressed in up to 80-90% of NSCLCs (15). To explore the molecular mechanism of the tumor suppressive effect of p42 in NSCLC, we tested whether p42 expression affects Akt, a representative downstream effector of EGFR. Expression of phosphorylated Akt was diminished in p42-expressing cells upon EGF exposure whereas its expression was not changed in cells expressing p48 or control vector (Fig. 4D, first panel). However, the phosphorylation status of extracellular signal regulated kinase (ERK) upon EGF treatment was not altered in p42-expressing cells (Fig. 4D second panel). Thus p42 probably inhibits activation of Akt but not Erk.
To further investigate the inhibitory role of p42 on Akt activity, we transfected p42-expresssing A549 cells with a constitutively active (CA) form of HA-Akt or control vector and monitored whether active Akt restored the cancerous properties of A549 cells that were suppressed by p42 expression. Indeed, transfection of CA-HA-Akt cells highly promoted growth of p42-expressing A549 cells, correlating with enhanced expression of PCNA in these cells (Figs. 4E and F). Consistent with this observation, CA-HA-Akt expression strongly enhanced anchorage-independent growth of p42-expressing A549 cells compared with p42-expressing cells transfected with control vector (Figs. 4G and F). Thus, p42 expression is sufficient to suppress the cancerous properties of NSCLC, which is closely associated with its inhibitory effect on Akt.
DISCUSSION
The identification and characterization of tumor suppressor genes provides vital information for the development of new therapeutic agents to treat cancer. In the present study, we provide the first evidence that p42 Ebp1 is a potent tumor suppressor in human lung cancer cells. Previous studies and data presented here show that both p48 and p42 mRNA are expressed in almost all tissues and cell lines tested at comparable levels, but the protein expression level of p42 is extremely low or undetectable in many tumor cell lines and tumor tissues including NSCLC cell lines, highlighting a possible tumor suppressive function of p42. In this study, among the tested lung cancer cell lines we compared the cancerous properties of two cell lines: H520, which expresses p42, and A549, which lacks p42 expression (Fig. 1). Considering the tumor suppressive activity of p42, H520 cells would be expected to have lower rates of proliferation, soft agar growth, and invasion potential than A549 cells, as was indeed observed in our study (Fig. 1). This is consistent with previous invasion analysis of multilayered raft cultures of lung cancer cells (16). Moreover, forced expression of p42 in A549 cell, which has no p42 expression affected on cancerous properties of A549 cells, inhibiting its proliferation, anchorage-independent growth, and invasion (Figs. 2A-D) as well as in vivo tumor growth in xenograft mice model, supporting our hypothesis that p42 protein suppresses tumor growth (Figs. 4A-C).
Activation of EGFR signaling in NSCLC leads to cell growth and enhanced survival, which contributes to tumor growth, metastasis, and resistance to therapy through the PI3K/Akt pathway (17). We previously showed that the p48 isoform of Ebp1, which possesses oncogenic potential, associates with active Akt and enhances its kinase activity. Intriguingly, results of this study indicate that p42 suppresses Akt activation. Overexpression of CA-Akt in p42-expressing A549 cells restored the cancerous properties of these cells (Figs. 4D-H), implying that p42 elicits its tumor suppressive effect through ablation of Akt signaling.
Although p42 and p48 differ in only the N-terminal 54 amino acids, these two iso-proteins exert contradictory tumor suppressive and oncogenic effects. The molecular mechanism underlying this difference in function remains unknown. Recent crystal structure analysis of Ebp1 indicates that loss of the N-terminal 54 residues, which form one and half a-helices, exposes a hydrophobic domain of p42 to hydrophilic solvent and causes refolding of the protein, resulting in recruitment of new binding partners to stabilize p42 (18). Thus, identifying the distinctive binding partners of p42 and p48 will lead to better understanding of the contrasting not only physiological functions of Ebp1 isoforms in tumorigenesis but also their specificity in various cancer types including NSCLC.
MATERIALS AND METHODS
Cell culture and antibodies
All cell lines were purchased from the American Type Culture Collection, Manassas, VA and maintained in RPMI medium with 10% FBS and 100 units of penicillin-streptomycin in a humidified incubator at 37℃ with 5% CO2. Antibodies were obtained from Cell Signaling Technology (Akt, phospho-Akt, phospho-GSK3, phospho-ERK, and PARP) or Calbiochem (PCNA). Anti-N-p48 antibody specific for the p48 isoform and anti-Ebp1 antibodies were generated by our laboratory. Staurosporine (STS) was purchased from Calbiochem.
Proliferation, invasion, and colony formation assay
Transfected cells were plated (5 × 104 cells/60-mm dish) in complete media, and viable cell numbers were counted at 0, 24, 36, 48, and 60 hours. Invasion assays were performed as described (19) using a Matrigel invasion assay kit (BD Bioscience, Inc), according to the manufacturer’s instructions. For the colony forming assay, 5 × 103 cells were seeded on 0.35% soft agar (supplemented with complete medium) and cultured for 21 days. The cells were fixed and stained with crystal violet stain, and then counted in five random fields.
Northern blotting and RT-PCR
RT-PCR was performed as previously described (20). Total RNA was extracted from cells with TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription reaction was performed using the Superscript IITM kit (Invitrogen). Expression of Ebp1 2 isoforms was verified by GoTaq® Green Master mix (Promega). Primers used were: forward, 5’-ACAGCCTGTGGCTGGGAAGGG-3’; reverse, 5’-CTTCAAAGGGGAGAAGTG-3’.
Flow cytometry
Flow cytometry analysis was performed as previously described, with minor modifications (21). Transfected cells were harvested by trypsinization. 2 × 106 cells were stained with the Annexin V-FITC apoptosis detection kit (BioBud) or fixed in 70% ethanol at -20 ℃ for at least 24 hours. The pellets were resuspended in 50 μl phosphate/citrate buffer (0.2 M Na2HPO4, 0.1 M citrate, pH 7.5) for 30 minutes at RT. The cells were washed with PBS and incubated with propidium iodide solution (20 μg/ml pI, 20 μg/ml RNase A in PBS) for 30 minutes at RT. The samples were analyzed with the FacsCalibur system (Becton Dickinson, USA).
Animal experiments
Animal studies and all associated procedures were approved by the Sungkyunkwan University School of Medicine Institutional Animal Care and Use Committee (IACUC). Briefly, 6- to 8-week-old athymic nude (nu/nu) mice were housed in laminar-flow cabinets under specific-pathogen-free conditions. A549 cells stably expressing GFP-p48, GFP-p42, or GFP-vector control (5 × 106 cells) were injected subcutaneously into the right flank of the nude mice (n = 5 mice per group). Tumor volume was calculated from the major (a) and minor (b) axis of the tumors using the following formula: V = a × b2/2.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1120090) and by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090118).
References
- 1.Liu Z, Ahn JY, Liu X, Ye K. Ebp1 isoforms distinctively regulate cell survival and differentiation. Proc Natl Acad Sci U S A. (2006);103:10917–10922. doi: 10.1073/pnas.0602923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn JY, Liu X, Liu Z, et al. Nuclear Akt associates with PKC-phosphorylated Ebp1, preventing DNA fragmentation by inhibition of caspase-activated DNase. EMBO J. (2006);25:2083–2095. doi: 10.1038/sj.emboj.7601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CK, Nguyen TL, Joo KM, et al. Negative regulation of p53 by the long isoform of ErbB3 binding protein Ebp1 in brain tumors. Cancer Res. (2010);70:9730–9741. doi: 10.1158/0008-5472.CAN-10-1882. [DOI] [PubMed] [Google Scholar]
- 4.Kim CK, Lee SB, Nguyen TL, et al. Long isoform of ErbB3 binding protein, p48, mediates protein kinase B/Aktdependent HDM2 stabilization and nuclear localization. Exp Cell Res. (2012);318:136–143. doi: 10.1016/j.yexcr.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Ou K, Kesuma D, Ganesan K, et al. Quantitative profiling of drug-associated proteomic alterations by combined 2-nitrobenzenesulfenyl chloride (NBS) isotope labeling and 2DE/MS identification. J Proteome Res. (2006);5:2194–2206. doi: 10.1021/pr060115n. [DOI] [PubMed] [Google Scholar]
- 6.Gannon PO, Koumakpayi IH, Le Page C, Karakiewicz PI, Mes-Masson AM, Saad F. Ebp1 expression in benign and malignant prostate. Cancer Cell Int. (2008);8:18-2867–8-18. doi: 10.1186/1475-2867-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia X, Cheng A, Lessor T, Zhang Y, Hamburger AW. Ebp1, an ErbB-3 binding protein, interacts with Rb and affects Rb transcriptional regulation. J Cell Physiol. (2001);187:209–217. doi: 10.1002/jcp.1075. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Woodford N, Xia X, Hamburger AW. Repression of E2F1-mediated transcription by the ErbB3 binding protein Ebp1 involves histone deacetylases. Nucleic Acids Res. (2003);31:2168–2177. doi: 10.1093/nar/gkg318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Chen W, Zhang Y, Hamburger AW, Pan H, Zhang Z. Suppression of salivary adenoid cystic carcinoma growth and metastasis by ErbB3 binding protein Ebp1 gene transfer. Int J Cancer. (2007);120:1909–1913. doi: 10.1002/ijc.22541. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang XW, Jelovac D, et al. The ErbB3- binding protein Ebp1 suppresses androgen receptor-mediated gene transcription and tumorigenesis of prostate cancer cells. Proc Natl Acad Sci U S A. (2005);102:9890–9895. doi: 10.1073/pnas.0503829102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Oh SM, Okada M, et al. Human BRE1 is an E3 ubiquitin ligase for Ebp1 tumor suppressor. Mol Biol Cell. (2009);20:757–768. doi: 10.1091/mbc.E08-09-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. (2000);355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 13.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. (2006);24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 14.Engelman JA, Cantley LC. The role of the ErbB family members in non-small cell lung cancers sensitive to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. (2006);12:4372s–4376s. doi: 10.1158/1078-0432.CCR-06-0795. [DOI] [PubMed] [Google Scholar]
- 15.Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev. (2004);30:1–17. doi: 10.1016/j.ctrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Okoh V, Young GD, Winokur TS, Garver RI., Jr A novel assay for the quantification of invasion from raft cultures of lung carcinomas. Clin Exp Metastasis. (2004);21:1–6. doi: 10.1023/B:CLIN.0000017159.12425.c9. [DOI] [PubMed] [Google Scholar]
- 17.Donev IS, Wang W, Yamada T, et al. Transient PI3K inhibition induces apoptosis and overcomes HGF-mediated resistance to EGFR-TKIs in EGFR mutant lung cancer. Clin Cancer Res. (2011);17:2260–2269. doi: 10.1158/1078-0432.CCR-10-1993. [DOI] [PubMed] [Google Scholar]
- 18.Monie TP, Perrin AJ, Birtley JR, et al. Structural insights into the transcriptional and translational roles of Ebp1. EMBO J. (2007);26:3936–3944. doi: 10.1038/sj.emboj.7601817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn JY, Rong R, Kroll TG, Van Meir EG, Snyder SH, Ye K. PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J Biol Chem. (2004);279:16441–16451. doi: 10.1074/jbc.M312175200. [DOI] [PubMed] [Google Scholar]
- 20.Xie H, Hu J, Pan H, Lou Y, Lv P, Chen Y. Adenovirus vector-mediated FAM176A overexpression induces cell death in human H1299 non-small cell lung cancer cells. BMB Rep. (2014);47:104–109. doi: 10.5483/BMBRep.2014.47.2.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai M, Ni J, Shen S, Huang Q, Wu J, Le Y, Yu L. Aurora-A kinase-inactive mutants disrupt the interaction with Ajuba and cause defects in mitotic spindle formation and G2/M phase arrest in HeLa cells. BMB Rep. (2014);47:631–636. doi: 10.5483/BMBRep.2014.47.11.250. [DOI] [PMC free article] [PubMed] [Google Scholar]