Abstract
As FK506 binding proteins (FK506BPs) are known to play an important role in the regulation of a variety of biological processes related to cell survival, this study was designed to examined the protective effects of FK506 binding protein 12 (FK506BP) on low humidity air flow induced dry eye in a rat model using transduced PEP-1-FK506BP. After the topical application of PEP-1-FK506BP, tear volumes were markedly increased and significant prevention of cornea damage was observed compared with dry eye rats. Further, immunohistochemical analysis demonstrated that PEP-1-FK506BP markedly prevented damage to the cornea, the bulbar conjunctiva, and the palpebral conjunctiva epithelial lining compared with dry eye rats. In addition, caspase-3 and PARP expression levels were found to be decreased. These results demonstrated that topical application of PEP-1-FK506BP significantly ameliorates dry eye injury in an animal model. Thus, we suggest that PEP-1-FK506BP can be developed as a new ophthalmic drop to treat dry eye diseases. [BMB Reports 2015; 48(3): 153-158]
Keywords: Dry eye disease, FK506BP, Tear volume, Protein therapy, Protein transduction
INTRODUCTION
Dry eye is characterized by abnormal changes involving the quality or quantity of tears, and the integrity of ocular surface cells (1, 2). These changes lead to desiccation of the ocular surface, and can also cause ocular injury (3). Tear dynamics are maintained by various factors such as blink frequency, tear production, and evaporation from the ocular surface. The typical clinical manifestations of dry eye include various such symptoms as ocular irritation, blurred vision, infection and ulceration. Many people are affected by dry eye diseases in the USA (4). Several studies have demonstrated that dry eye diseases are induced by various factors including aging, hormonal changes, environmental factors, and inflammation (5-9). In the past few decades, various models have been developed to induce abnormal changes in dry eye experimental animals. However, most models are known to have disadvantages such as difficulty excluding the complex influence of surgical insults or the adverse effects of pharmacologic agents. Thus, studies have suggested that ideal dry eye models should use minimally invasive procedures on the ocular surface (10-12). The ocular surface is constantly exposed to air and low humidity, which cause dry eye damage by evaporation of the tear fluid (13). Several studies have shown that dry eye models using low humidity, which appear to reflect clinical conditions in patients with dry eye disorders, are also an effective approach to testing the possible treatments of dry eye disorders (1-3).
The immunophilin family of proteins, FK506 binding proteins (FK506BPs), is known to play an important role in the regulation of a variety of biological processes such as DNA damage response. Recent studies have indicated that FK506BPs to be emerging as a new protein for the therapeutic treatment of tumors (14, 15). FK506 binding protein 12 (FK506BP) is a smaller member, and contains a peptidyl prolyl cis/trans isomerase (PPIase) domain. FK506BP strongly interacts with immunosuppressive drugs such as FK506 and rapamycin (16, 17). We previously demonstrated that transduced PEP-1-FK506BP inhibits inflammatory response in both Raw 264.7 cells and in an animal model. Further, topical application of PEP-1-FK506BP markedly inhibited cytokine/chemokine expression levels in an atopic dermatitis animal model, thereby demonstrating improvement of atopic dermatitis (18, 19). Recently, we demonstrated that transduction of PEP-1-FK506BP into human corneal epithelial (HCE-2) cells and mice corneal tissue significantly improved Botulinum toxin A-induced dry eye diseases (20).
Protein transduction domains (PTDs) facilitate the delivery of proteins into cells and tissues, including the brain (21). Research has demonstrated the potential of PTD fusion proteins as a powerful tool in the therapeutic application of proteins to treat a variety of diseases (22-33).
The purpose of this study was to determine whether topically applied PEP-1-FK506BP could ameliorate the symptoms of dry eye in a low humidity air flow induced dry eye model. We demonstrated herein that PEP-1-FK506BP markedly inhibited dry eye disease. In the dry eye disease model, PEP-1-FK506BP also protected against the expression of caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) in the corneal epithelium. Therefore, we suggest the topical application of PEP-1-FK506BP as a potential therapeutic agent for dry eye diseases.
RESUTLS AND DICSUSSION
Effect of PEP-1-FK506BP on tear volume
In this study, a low humidity air flow induced rat dry eye model was used, which was developed to test the effects of potential therapeutic drugs for the treatment of damage to the ocular surface induced by environmental factors such as temperature, humidity, and air flow (2, 13). Several studies have demonstrated many people to suffer from the symptoms of dry eye induced by such environmental factors (34, 35).
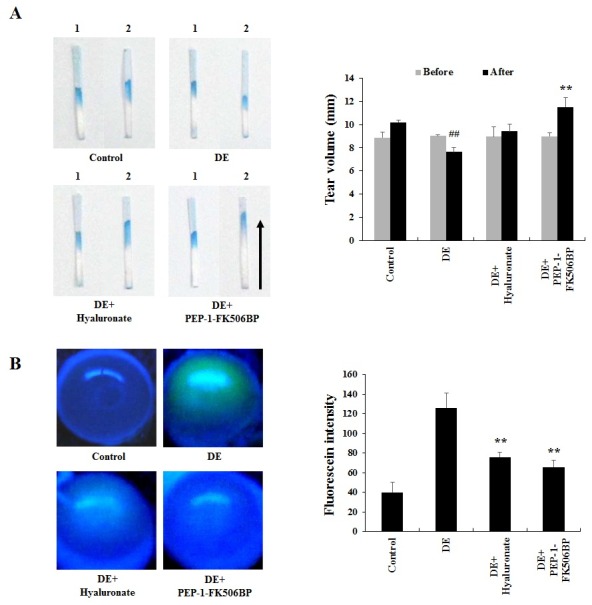
The Schirmer test using phenol red thread is regarded as a routine method to assess tear volume. Under dry eye conditions, tear volumes are markedly decreased (11, 36). To assess the effect of PEP-1-FK506BP on tear volume in dry eye rats, PEP-1-FK506BP and hyaluronate were applied 6 times at 1 hr-intervals, starting from 30 min before exposure to dry air flow. The tear volumes were measured before and after dry air exposure. As shown in Fig. 1A, tear volumes were increased after dry air exposure in the normal control rats. However, in the dry eye rat group, significantly decrease of the tear volumes were observed after exposure to dry air. In contrast, the group treated with PEP-1-FK506BP showed a marked increase in tear volume compared with the dry eye rat group. Further, the PEP-1-FK506BP-treated group also demonstrated a greater increase in tear volume compared with the hyaluronate-treated group, used as a negative control. These results indicated that the decrease in tear volumes was significantly ameliorated by treatment with PEP-1-FK506BP in dry eye conditions.
Fig. 1. Effects of PEP-1-FK506BP on tear volume and corneal damage. Tear volume (A) and fluorescein intensity (B) measured in rat eye after exposure to dry air flow with or without application of PEP-1-FK506BP. The decrease in tear volume and increase in fluorescein intensity was significantly ameliorated in the PEP-1-FK506BP-treated group compared with the dry eye induced (DE) group. ##P < 0.01 compared with the control group. **P < 0.01 compared with the DE group. Lanes are as follows: lane 1, before dry air exposure; lane 2, after dry air exposure.
Corneal fluorescein staining
Corneal damage in animals is known to cause a marked increase in fluorescein staining (3). The fluorescent dye test is one of the most prevalent methods used in preclinical animal tests. Increase of the remnant dye in the eyeball indicates an increase of corneal permeability and damage (1, 37, 38). Therefore, the protective effect of PEP-1-FK506BP on dry eye injury was examined with corneal fluorescein staining. As shown in Fig. 1B, dry eye rats showed significantly increased corneal fluorescein staining compared with normal control rats, while the PEP-1-FK506BP-treated group showed significantly reduced staining compared with dry eye groups, similar to those treated with hyaluronate. These results indicate that PEP-1-FK506BP has potential as a therapeutic agent against dry eye disease.
Effect of PEP-1-FK506BP on corneal and conjunctival epithelium injury
Corneal and conjunctiva epithelial damage characterized by desquamation of the surface epithelial lining is a common histopathological feature of dry eyes (39, 40). Herein, the histopathology changes to the cornea and conjunctiva of the rats was examined. As shown in Figs. 2A and 3A, the damaged corneal epithelial regions of were markedly increased in the dry eye group. However, the group treated with PEP-1-FK506BP demonstrated significant decrease in the damage to the corneal epithelial regions. In addition, the PEP-1-FK506BP-treated group showed significant increase in the total thicknesses of the cornea, corneal epithelia and stroma compared with the dry eye group.
Fig. 2. Histological analysis of the corneal epithelia (A), bulbar conjunctiva epithelia (B), and palpebral conjunctiva epithelia (C). Normal control group (a), dry eye induced (DE) group (b), DE + hyaluronate group (c), and DE + PEP-1-FK506BP group (d). Arrows indicate the thickness of the total cornea (ThT), epithelium (ThE), and stroma (ThS). *P < 0.05 and **P < 0.01 compared with the DE group. Scale bar = 80 μm.
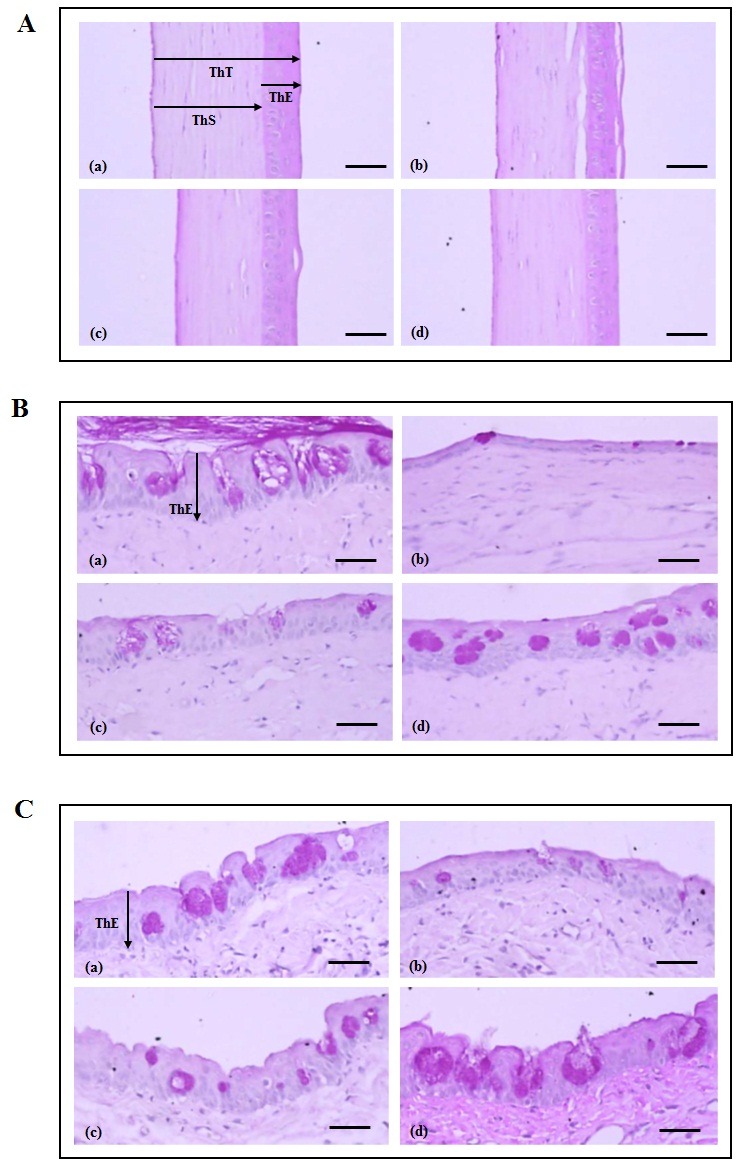
Fig. 3. Histomorphological analysis of the corneal epithelia (A) and bulbar/palpebral conjunctiva epithelia (B). *P < 0.05 and **P < 0.01 compared with the DE group.
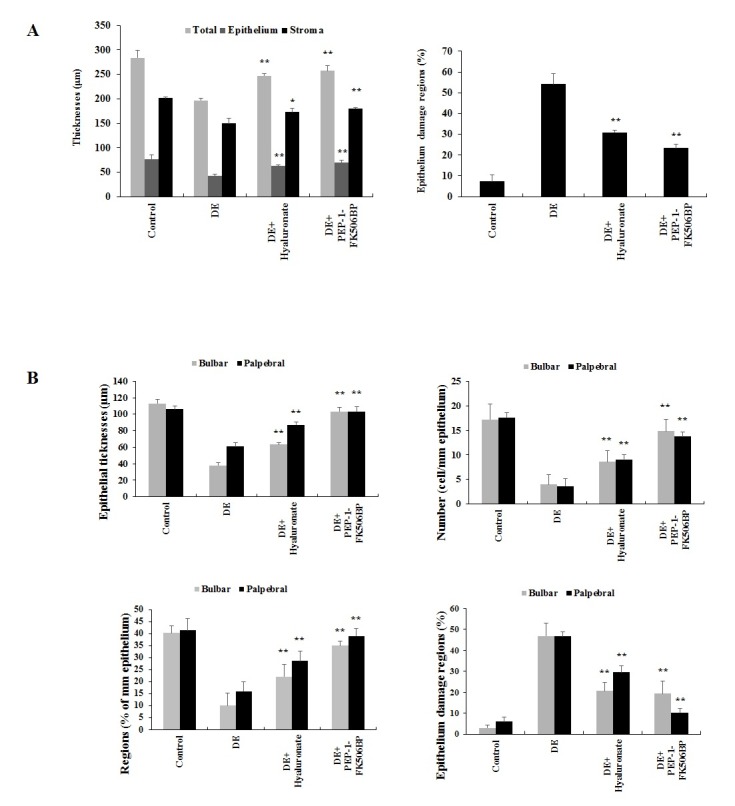
Examination of the bulbar conjunctiva epithelia revealed the dry eye rats to have a significant decrease in conjunctiva epithelial thickness, as well as a decreased number and percentage of mucous-producing cells, with a marked increase in the regions with epithelial damage. The group treated with PEP-1-FK506BP showed significantly increased thickness of the conjunctiva compared with the dry eye rats (Figs. 2B and 3B). The palpebral conjunctiva epithelia revealed similar patterns to the bulbar conjunctiva epithelia (Figs. 2C and 3B). These results indicated that PEP-1-FK506BP markedly inhibited histopathological changes resulting from dry eye conditions, and showed similar or more favorable inhibitory effects on the histopathological changes to the cornea and conjunctiva induced by dry eye than did hyaluronate.
Recently, several studies have demonstrated that trehalose could improve the appearance of ocular surface epithelial diseases via desiccation through suppression of apoptosis. Other studies have also shown that the levels of apoptosis in mice of the dry eye model were significantly higher than in control mice. As the exact mechanisms are not yet known, these studies suggested that further research is needed to understand the exact mechanism for dry eye diseases, which may provide new therapies for their treatment (2, 3).
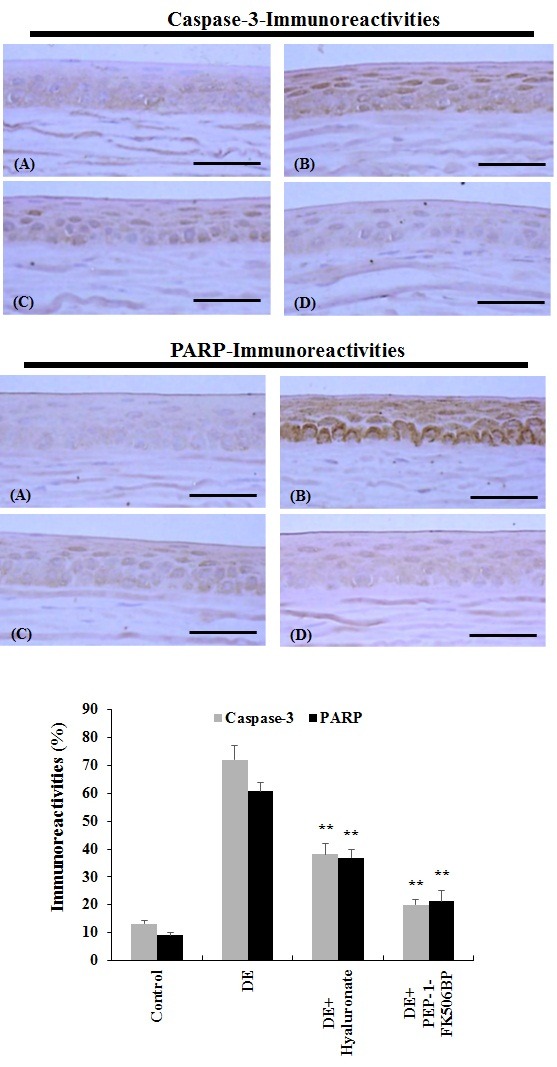
Herein, we investigated the effect of PEP-1-FK506BP on caspase-3 and PARP expression levels in the dry eye rat model using immunohistochemistry. As shown in Fig. 4, the dry eye rats showed a marked increase in the amount of caspase-3 and PARP immunoreactive regions in the corneal epithelia compared with the normal control group. However, the group treated with PEP-1-FK506BP showed a significant decrease in the amount of caspase-3 and PARP immunoreactive regions in the corneal epithelia. Furthermore, the protective effects of PEP-1-FK506BP were greater than those found after treatment with hyaluronate. These results indicate that PEP-1-FK506BP has an anti-apoptotic effect on the cornea epithelium, and may be a good potential therapeutic agent against dry eye diseases.
Fig. 4. Anti-apoptotic effects of PEP-1-FK506BP in the corneal epithelium. The expression levels of caspase-3 and PARP were significantly increased in the corneal epithelium of dry eye rats. Normal control group (A), dry eye induced (DE) group (B), DE + hyaluronate group (C), and DE + PEP-1-FK506BP group (D). **P < 0.01 compared with the DE group. Scale bar = 80 μm.
Kubo et al. (2008) demonstrated that TAT-peroxiredoxin 6 recombinant protein efficiently protects eye lens epithelial cells and opacity, suggesting that this recombinant protein may provide an effective approach to delay cataracts (22). In addition, Liu et al. (2014) showed that Tat-heat shock protein 27 (HSP27) transduced into human lens epithelial cells protected against UV-induced apoptosis. These authors suggested that protein therapy via protein transduction may be a useful tool to address a variety of eye diseases (29).
In summary, we demonstrated that topical application of PEP-1-FK506BP markedly inhibited damage caused by dry eye in a low humidity air flow induced dry eye rat model. Therefore, we suggest that PEP-1-FK506BP may be a potential therapeutic agent, and can be developed in the form of new ophthalmic drops for the treatment of dry eye diseases.
MATERIALS AND METHODS
Purification and preparation of PEP-1-FK506BP proteins
The cell-permeable PEP-1-FK506BP protein was prepared as previously described (18-20). A Bradford assay was used to estimate the protein concentration (41). PEP-1-FK506BP was dissolved in phosphate buffered saline (PBS) before being applied topically to the animals’ eyes (0.01 mg/kg of protein in 5 μl). Hyaluronate (5 μl; Samil Pharm, Co., Korea) was used as a reference.
Experimental animals
Male Sprague-Dawley (SD) rats (6-week-old, 180-200 g) were used after an acclimatization period of 8 days. The animals were housed at 23℃ and a relative humidity of 45%. They were exposed to a 12 h light-dark cycle and had free access to food and water. All experimental procedures involving animals and their care conformed to the Guide for the Care and Use of Laboratory Animals of the National Veterinary Research and Quarantine Service of Korea, and were approved by the Hallym Medical Center Institutional Animal Care and Use Committee.
Low humidity air flow induced dry eye model
The rats were divided into four groups (each n = 8). Group 1 was used as a control, with saline (5 μl) dropped into the eyes of normal rats. In Group 2, saline (5 μl) was dropped into the eyes of dry eye induced rats. In Group 3, 0.1% sodium hyaluronate was dropped into the eyes of dry eye induced rats as a reference. In Group 4, PEP-1-FK506BP (0.01 mg/kg of protein in 5 μl) was dropped into the eyes of dry eye induced rats.
The central region of the corneal epithelium (0.4 mm2) was scraped mechanically with an ophthalmic surgical blade under anesthesia of 25 mg/kg intraperitomeal injection of Zoletile mixture (Zoletile 50; Virbac Lab, France). The rats were then placed in a desiccation room (temperature, 28℃ and humidity, 25-30%) with a constant air flow (2.4 m/sec) for 5 h. PEP-1-FK506BP and hyaluronate were applied to the appropriate groups 6 times at 1 hr-intervals, initiated 30 min before exposure to the dry air flow.
Schirmer test
A Schirmer test was performed to measure tear fluid secretion. A Schirmer tear test strip (1 × 15 mm; cobalt chloride paper, Toyo Roshi Kaisha, Japan) was placed on the temporal side of the lower eyelid margin for 1 min after scraping of the corneal epithelium, before initiation of exposure to the dry eye inducing conditions and at the end of the 5 h of exposure. The length of the moistened area from the edge was measured to an accuracy of 0.5 mm using an electronic digital caliper (Mytutoyo, Japan) (1, 36).
Fluorescein staining
The damaged areas were photographed after the 5 h of exposure to dry eye inducing conditions by applying a 1% fluorescein solution (Sigma-Aldrich, St. Louis, MO, USA). Fluorescein solutions were topically applied to the eye spaces, and then the eyelids were taped closed. 1 h after application of the fluorescein solution, the remaining solution was removed and the eyeballs were extracted and photographed under a blue light tungsten lamp. The stained area was digitized with an optical scanner and quantified using image-analysis software (Image J, NIH, USA) (1, 37, 38).
Histological analysis
The rats were euthanized with an overdose of Zoletile, and the eyeballs and bulbar conjunctivas were removed and fixed in Davidson’s solution (37.5% ethanol, 12.5% acetic acid, and 25% formaldehyde). The palpebral conjunctivas were also separated and fixed in 10% neutral buffered formalin. The eye and palpebral specimens were embedded in paraffin, crosssectioned, and stained with hematoxylin and eosin for observation of the cornea, and with periodic acid Schiff (PAS) for mucous producing goblet cells in both conjunctivas (39, 40). The morphology of the corneal, bulbar, and palpebral conjunctiva and goblet cells were measured using a digital image analyzer (DMI-300, DMI, Korea).
Changes of immunoreactivity to caspase-3 and PARP on the cornea and conjunctive were observed by immunohistochemistrical methods using purified primary antibodies (Cell Signaling Technology Inc, MA, USA) with an avidin-biotin-peroxidase (ABC) and peroxidase substrate kit (Vector Labs, Burlingame, CA, USA) (42, 43).
Statistical analysis
All statistical analysis was carried out in SPSS (6.1.3; USA). Data were analyzed by one way ANOVA, followed by the least-significant differences multi comparison test. Further, statistical differences between experimental groups were analyzed by the Kruskal-Wallis H and Mann-Whitney U test. P values of < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by a Priority Research Centers Program grant (2009-0093812). It was also supported in part by a MidCareer Researcher Program grant (2012R1A2A2A06043084) through the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning in the Republic Korea, by a grant from the Korean Health Technology R&D Project (A120960), Ministry of Health & Welfare, Republic of Korea, and by a grant from Hallym University (HRF-2014-03-009).
References
- 1.Nakamura S, Shibuya M, Nakashima H, Imagawa T, Uehara M, Tsubota K. D-β-hydroxybutyrate protects against corneal epithelial disorders in a rat dry eye model with jogging board. Invest Ophthalmol Vis Sci. (2005);46:2679–2687. doi: 10.1167/iovs.04-1344. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zhang X, Zhang J, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci. (2008);49:1386–1391. doi: 10.1167/iovs.07-0744. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zhang X, Liu M, et al. Trehalose protects against ocular surface disorders in experimental murine dry eye through suppression of apoptosis. Exp Eye Res. (2009);89:311–318. doi: 10.1016/j.exer.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Ade Exp Med Biol. (2002);506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- 5.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. (2001);286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 6.Khaw PT, Shah P, Elkington AR. Injury to the eye. BMJ. (2004);328:36–38. doi: 10.1136/bmj.328.7430.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. (2006);25:900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 8.Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. (2007);14:507–515. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini H, Nejabat M. A potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses. (2007);68:799–801. doi: 10.1016/j.mehy.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Maitchouk DY, Beuerman RW, Ohta T, Stern M, Varnell RJ. Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol. (2000);118:246–252. doi: 10.1001/archopht.118.2.246. [DOI] [PubMed] [Google Scholar]
- 11.Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. (2001);42:96–100. [PubMed] [Google Scholar]
- 12.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. (2002);43:632–638. [PubMed] [Google Scholar]
- 13.Rolando M, Refojo MF. Tear evaporimeter for measuring water evaporation rate from the tear film under controlled conditions in humans. Exp Eye Res. (1983);36:25–33. doi: 10.1016/0014-4835(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 14.Romano S, D’Angelillo A, Pacelli R, et al. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. (2010);17:145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- 15.Robson T, James IJ. The therapeutic and diagnostic potential of FKBPL: a novel anticancer protein. Drug Discov Today. (2012);17:544–548. doi: 10.1016/j.drudis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Kay JE. Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochem J. (1996);314:361–385. doi: 10.1021/bi9522312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivery MT. Immunophilins: switched on protein binding domains? Med Res Rev. (2000);20:452–484. doi: 10.1002/1098-1128(200011)20:6<452::aid-med2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Jeong HJ, Kim DW, et al. Transduced PEP-1-FK506BP inhibits the inflammatory response in the Raw 264.7 cells and mouse models. Immunobiol. (2011);216:771–781. doi: 10.1016/j.imbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Sohn EJ, Kim DW, et al. Transduced PEP-1-FK506BP ameliorates atopic dermatitis in NC/Nga mice. J Invest Dermatol. (2011);131:1477–1485. doi: 10.1038/jid.2011.49. [DOI] [PubMed] [Google Scholar]
- 20.Kim DW, Lee SH, Ku SK, et al. Transduced PEP-1-FK506BP ameliorates corneal injury in Botulinum toxin A-induced dry eye mouse model. BMB Rep. (2013);46:124–129. doi: 10.5483/BMBRep.2013.46.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. (2002);13:52–56. doi: 10.1016/S0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 22.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. (2008);294:C842–C855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- 23.Ahn EH, Kim DW, Kang HW, et al. Transduced PEP-1-ribosomal protein S3 (rpS3) ameliorates 12-O-tetradecanoyl phorbol-13-acetate-induced inflammation in mice. Toxicol. (2010);276:192–197. doi: 10.1016/j.tox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Dietz GP. Cell-penetrating peptide technology to deliver chaperones and associated factors in diseases and basic research. Curr Pharm Biotechol. (2010);11:167–174. doi: 10.2174/138920110790909731. [DOI] [PubMed] [Google Scholar]
- 25.Kwon SW, Sohn EJ, Kim DW, et al. Anti-inflammatory effect of transduced PEP-1-heme oxygenase-1 in Raw 264.7 cells and a mouse edema model. Biochem Biophys Res Commun. (2011);411:354–359. doi: 10.1016/j.bbrc.2011.06.147. [DOI] [PubMed] [Google Scholar]
- 26.Shibagaki N, Okamoto T, Mitsui H, Inozume T, Kanzaki M, Shimada S. Novel immunotherapeutic approaches to skin cancer treatments using protein transduction technology. J Dermatol Sci. (2011);61:153–161. doi: 10.1016/j.jdermsci.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ahn EH, Kim DW, Shin MJ, et al. PEP-1-ribosomal protein S3 protects dopaminergic neurons in an MPTP-induced Parkinson’s disease mouse model. Free Radic Biol Med. (2013);55:36–45. doi: 10.1016/j.freeradbiomed.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 28.An JJ, Eum WS, Kwon HS, et al. Protective effects of skin permeable epidermal and fibroblast growth factor against ultraviolet-induced skin damage and human skin wrinkles. J Cosmet Dermatol. (2013);12:287–295. doi: 10.1111/jocd.12067. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Hou J, Xia ZY, et al. Recombinant PTD-Cu/Zn SOD attenuates hypoxia-reoxygenation injury in cardiomyocytes. Free Radic Res. (2013);47:386–393. doi: 10.3109/10715762.2013.780286. [DOI] [PubMed] [Google Scholar]
- 30.Zhou G, Shan P, Hu X, Zheng X, Zhou S. Neuroprotective effect of TAT PTD-Ngb fusion protein on primary cortical neurons against hypoxia-induced apoptosis. Neurol Sci. (2013);34:1771–1778. doi: 10.1007/s10072-013-1333-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Yu R, Shi Y, et al. Transduced protein transduction domain linked HSP27 protected LECs against UVB radiation-induced damage. Exp Eye Res. (2014);120:36–42. doi: 10.1016/j.exer.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Shin MJ, Kim DW, Lee YP, et al. Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic Biol Med. (2014);67:195–210. doi: 10.1016/j.freeradbiomed.2013.10.815. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Huang L, Wu Q, et al. A recombinant trans-membrane protein hMn-SOD-R9 inhibits the proliferation of cervical cancer cells in vitro. Mol Cell Biochem. (2014);385:79–86. doi: 10.1007/s11010-013-1816-2. [DOI] [PubMed] [Google Scholar]
- 34.Sommer HJ, Johnen J, Schongen P, Stolze HH. Adaptation of the tear film to work in air-conditioned rooms (office-eye syndrome). Ger J Ophthalmol. (1994);3:406–408. [PubMed] [Google Scholar]
- 35.Backman H, Haghighat F. Indoor-air quality and ocular discomfort. J Am Optom Assoc. (1999);70:309–316. [PubMed] [Google Scholar]
- 36.Nakamura S, Okada S, Umeda Y, Saito F. Development of a rabbit model of tear film instability and evaluation of viscosity of artificial tear preparations. Cornea. (2004);23:390–397. doi: 10.1097/00003226-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Koh S, Watanabe H, Hosohata J, et al. Diagnosing dry eye using a blue-free barrier filter. Am J Ophthalmol. (2003);136:513–519. doi: 10.1016/S0002-9394(03)00317-9. [DOI] [PubMed] [Google Scholar]
- 38.Steinfeld A, Lux A, Maier S, Suverkrup R, Diestehorst M. Bioavailability of fluorescein from a new drug delivery system in human eyes. Br J Ophthalmol. (2004);88:48–53. doi: 10.1136/bjo.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura S, Shibuya M, Saito Y, et al. Protective effect of D-beta-hydroxybutyrate on corneal epithelia in dry eye conditions through suppression of apoptosis. Invest Ophthalmol Vis Sci. (2003);44:4682–4688. doi: 10.1167/iovs.03-0198. [DOI] [PubMed] [Google Scholar]
- 40.Higuchi A, Ueno R, Shimmura S, Suematsu M, Dogru M, Tsubota K. Albumin rescues ocular epithelial cells from cell death in dry eye. Curr Eye Res. (2007);32:83–88. doi: 10.1080/02713680601147690. [DOI] [PubMed] [Google Scholar]
- 41.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Anal Biochem. (1976);72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Cole KK, Perez-Polo JR. Poly (ADP-ribose) polymerase inhibition prevents both apoptotic-like delayed neuronal death and necrosis after H2O2 injury. J Neurochem. (2002);82:19–29. doi: 10.1046/j.1471-4159.2002.00935.x. [DOI] [PubMed] [Google Scholar]
- 43.Koo TB, Han MS, Tadashi Y, Seong WJ, Choi JY. Differential expression of the metastasis suppressor KAI1 in decidual cells and trophoblast giant cells at the feto-maternal interface. BMB Rep. (2013);46:507–512. doi: 10.5483/BMBRep.2013.46.10.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


