A feasibility trial achieved positive outcomes with a sensory-adapted dental environment to enhance oral care for children with ASD.
MeSH TERMS: child development disorders, pervasive; dental care; environment; occupational therapy; sensation disorders; stress, physiological
Abstract
OBJECTIVE. To provide an example of an occupational therapy feasibility study and evaluate the implementation of a randomized controlled pilot and feasibility trial examining the impact of a sensory-adapted dental environment (SADE) to enhance oral care for children with autism spectrum disorder (ASD).
METHOD. Twenty-two children with ASD and 22 typically developing children, ages 6–12 yr, attended a dental clinic in an urban hospital. Participants completed two dental cleanings, 3–4 mo apart, one in a regular environment and one in a SADE. Feasibility outcome measures were recruitment, retention, accrual, dropout, and protocol adherence. Intervention outcome measures were physiological stress, behavioral distress, pain, and cost.
RESULTS. We successfully recruited and retained participants. Parents expressed satisfaction with research study participation. Dentists stated that the intervention could be incorporated in normal practice. Intervention outcome measures favored the SADE condition.
CONCLUSION. Preliminary positive benefit of SADE in children with ASD warrants moving forward with a large-scale clinical trial.
According to Tickle-Degnen (2013) and others (Sturkenboom et al., 2013), feasibility studies are underutilized in occupational therapy. This underuse is problematic because threats to the validity of a subsequent intervention study may not be uncovered until a significant investment in resources has already been made. The two basic intentions of a feasibility study are (1) to assess the potential for successful implementation of a subsequent randomized controlled trial and (2) to uncover and reduce threats to validity. The purpose of this article is to present an example of an occupational therapy pilot and feasibility study funded by the National Institute of Dental and Craniofacial Research (NIDCR).
It is well established that oral care is an important component of pediatric health care. Poor oral health and the diseases that may result from it can lead to difficulties with eating, speech impediments, pain, sleep disturbances, missed days of work or school, and decreased self-esteem, causing a negative effect on people’s health and quality of life (Casamassimo, 1996; Owens, Kerker, Zigler, & Horwitz, 2006; U.S. Department of Health and Human Services [HHS], 2010). However, despite the importance of oral care, disparities exist for children with special health care needs in access to and practice of oral care in the United States, with oral care being the most frequently cited unmet health care need (Child and Adolescent Health Measurement Initiative, 2011; Lewis, 2009; Lewis, Robertson, & Phelps, 2005).
Children with autism spectrum disorder (ASD) often have oral care challenges that are exacerbated by sensory processing difficulties (Stein, Polido, & Cermak, 2012, 2013; Stein, Polido, Mailloux, Coleman, & Cermak, 2011). Such children are highly anxious and exhibit negative behavioral reactions when exposed to standard sensory characteristics of the dental operatory, such as bright fluorescent lights, touch in or around the mouth, and the taste and smell of various oral care products, making it difficult for dentists to provide treatment (Stein et al., 2013). Stein et al. (2013) used cut scores of sensory responsivity to identify children with ASD who were overresponsive to sensory stimuli versus those who were not. In this study, 65% of parents of overresponders with ASD, compared with 39% of parents of children with ASD who were not overresponsive to stimuli, reported moderate to severe behavioral challenges at the dentist. Likewise, significantly more parents of overresponders with ASD (45%) than parents of children with ASD who were not overresponders (12%) reported that their child exhibited increased uncooperative behaviors in the dental office. These findings support the view that sensory overresponsivity is significantly associated with difficulty with dental cleanings and with increased uncooperative behaviors during dental care, key assumptions of the proposed study.
The design of our intervention was based on the hypothesis that children’s arousal and anxiety are, in part, related to the sensory environment to which they are exposed. Therefore, we hypothesized that if the noxious sensory characteristics of the dental environment could be reduced, children would be less anxious and exhibit decreased uncooperative behaviors. Adaptations to the environment were based on sensory integration theory (Parham & Mailloux, 2010), information on multisensory environments (Shapiro, 2011), and a previous study using sensory adaptations to the dental environment with children with developmental disabilities (Shapiro, Melmed, Sgan-Cohen, & Parush, 2009).
This pilot and feasibility study was designed to build the foundation for an intervention study, the sensory-adapted dental environment (SADE) study, to evaluate the utility of adapting the sensory characteristics of the dental environment to reduce anxiety and behavioral distress and therefore enhance oral care for children with ASD. We compared children’s physiological stress (the primary study outcome) in two dental conditions: a regular dental environment (RDE) and a SADE. Establishing feasibility for this intervention protocol was especially important because few published studies have used this specific intervention technique (Bowen et al., 2009). It is also notable that the NIDCR, which funded this study, requires investigators to seek funding for the conduct of a planning study before submitting an application for a full-scale clinical trial.
In a discussion of how to design a feasibility study, Bowen et al. (2009) outlined eight areas of focus ranging from demand to practicality. Following the rubric proposed by Thabane et al. (2010) and adapted for occupational therapy by Tickle-Degnen (2013), we assessed the practical aspects of our proposed intervention study, specifically its scientific basis, processes, resources, and management. This paradigm, and our experience with its implementation, is described and discussed using the rubric presented by Tickle-Degnen. Bowen et al.’s eight focus areas are defined in Table 1 and applied to our SADE feasibility study, along with specific questions to be addressed in the research.
Table 1.
Areas of Focus in Designing a Feasibility Study
| Area of Focus | Definition | Application to the SADE Study |
| Applicability | How do the intended recipients, both targeted participants and those implementing the program, react to the intervention? | How do children with ASD and TD ages 6–12 respond to SADE? How do their parents and dental professionals react to the intervention? |
| Demand | Do people in the target population see a need for the intervention? Are they willing to try it? | Results of prior research have indicated that children with ASD experience great difficulty receiving oral care at the dentist and that sensory sensitivities are associated with this difficulty (Stein, Polido, & Cermak, 2012, 2013; Stein, Polido, Mailloux, Coleman, & Cermak, 2011; Stein, Polido, Najera, & Cermak, 2012). |
| Implementation | What is the extent to which, likelihood that, and manner in which an intervention can be fully implemented as planned and proposed? | The population at our recruitment site were largely Spanish-speaking Latinos. Latinos are underrepresented in medical research; there is not good evidence regarding whether this situation is due to their views on research, a lack of interest in participating, or barriers in recruitment methods. Moreover, the dental clinic reported a 30%–40% no-show rate; therefore, we were concerned about our ability to retain participants once recruited. Questions we addressed included
|
| Practicality | To what extent can an intervention be delivered when resources, time, commitment, or some combination of these are constrained in some way? | What are the constraints of implementing the intervention in a large dental clinic in a children’s hospital? What will we need in terms of person hours for the consent process, verification of autism diagnosis, questionnaire completion, dental cleaning, and video analysis? Will adequate space (private room for dental cleaning) be available? Are there hospital policies or procedures that need to be considered to obtain approval to implement the intervention? |
| Adaptation | Will procedures need to be changed to be appropriate in a new situation? It is important to describe the actual modifications that are made to accommodate the context and requirements of a different format, media, or population. | See description of SADE adaptations in Table 2. |
| Integration | What level of system change will be needed to integrate a new program or process into an existing infrastructure? Will change in the organizational setting or the social or physical environment be necessary? | To what extent will the dental staff be willing to incorporate the SADE process into their daily routine? Can we work with the Cancer Research Informatics Core at the university’s cancer center to develop a database management system? |
| Expansion | What is the potential for success of an already-successful intervention with a different population or in a different setting? | Our program was based on an application of SADE with young children with intellectual and developmental disabilities in a dental clinic in Israel (Shapiro, Melmed, Sgan-Cohen, & Parush, 2009). We wanted to examine its use in the United States with a different population. |
| Limited-efficacy testing | Many feasibility studies are designed to test an intervention in a limited way such as with a convenience sample, with intermediate rather than final outcomes, with shorter follow-up periods, or with limited statistical power. | We used outcome measures, randomized controlled group assignment, and intervention procedures designed for our final study; however, we did use a smaller convenience sample.
|
Note. ASD = autism spectrum disorder; RCT = randomized controlled trial; SADE = sensory-adapted dental environment; TD = typically developing.
Method
Research Design
This pilot and feasibility study used an experimental randomized crossover design. We examined behavioral and physiological distress, pain, and sensory discomfort of children with ASD and typically developing (TD) children during routine dental cleanings in two conditions, an RDE and a SADE, presented in a counterbalanced manner. This study was approved for human participants by the Committee on Clinical Investigations of Children’s Hospital Los Angeles (CCI–11–00250) and by the institutional review board of the University of Southern California Health Sciences (HS–12–00521); consent was obtained from all parents of children participating in the study, and child assent was obtained when appropriate.
Participants
Eligible participants included Spanish- and English-speaking families with TD children or children with ASD between ages 6 and 12 yr. Participants were required to have experienced at least one prior dental cleaning, but not within the previous 4–6 mo.
Children with ASD were required to have a confirmed ASD diagnosis using the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999); this measure was completed as part of study procedures if documentation was not available from a previous assessment conducted by a licensed psychologist. Of our ASD group, 64% received diagnosis confirmation via the ADOS specifically for this study; most of the remaining 8 children had been administered the ADOS within the previous 3 yr.
Children with significant motor impairments such as cerebral palsy or any known genetic, endocrine, or metabolic dysfunctions were excluded from both groups in the study. Children in the TD group with a sibling diagnosed with ASD were excluded, as were those with identified psychological disorders (e.g., attention deficit hyperactivity disorder, bipolar disorder, anxiety disorder).
Recruitment
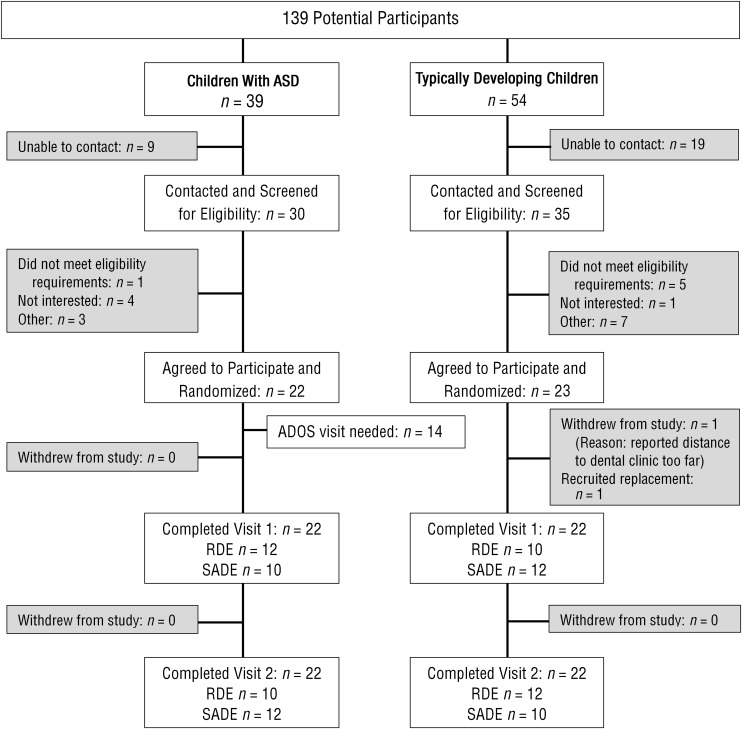
Participants were recruited from the dental clinic of a large urban children’s hospital that provides services to an ethnically diverse (60% Hispanic, 16% African American, 5% Asian) and low-income (90%) population. Records for upcoming scheduled patients were reviewed for initial eligibility (Figure 1), and those who met the criteria were contacted by phone. If parents expressed interest in learning more about the study, we scheduled a visit to further describe the study, answer questions, and obtain consent.
Figure 1.
Study flowchart.
Screening of online medical records based on upcoming appointments, followed by chart review for preliminary eligibility to identify children with ASD and typically developing children ages 6 to 12 yr old, yielded 139 potential participants (not all potential participants were contacted).
Note. ADOS = Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder; RDE = regular dental environment; SADE = sensory-adapted dental environment.
Because this was a pilot and feasibility study, participants in each group (ASD and TD) were of comparable chronological age but were not matched on other features (e.g., gender, ethnicity, mental age).
Procedures
Although we originally planned to obtain consent and survey completion from families at the time of the child’s first study-related dental visit, we realized that this process would take too long, and many children would have difficulty waiting. Therefore, families living within 45 mi of the dental clinic were offered the opportunity to complete the consent form and surveys in their home with a member of the research team. In addition, if the child was home, he or she was shown the electrodes for psychophysiological measurement and encouraged to touch them. We followed a similar process for families who provided consent at the dental clinic immediately before the first dental cleaning.
After completion of the consent process, each child participated in two dental cleanings, 3–4 mo apart. Children were randomized to their first assigned cleaning environment, either the RDE or SADE. Approximately 2 wk before each dental visit, a social story describing the dental cleaning (including application of electrodes) was sent to the parent, who was asked to read the story several times to the child before the dental visit.
Measures
Descriptive Measures.
We obtained demographics (child’s gender, age, ethnicity, and race; parent’s education level) with a questionnaire. ASD diagnosis was confirmed through administration of the ADOS. Competence in communication was measured with parent report on the Expressive Language subtest of the Communication domain of the Vineland Adaptive Behavior Scales, Second Edition (Vineland II; Sparrow, Cicchetti, & Balla, 2005); this subtest also served as a proxy measure for IQ because it is strongly correlated with IQ (Klin et al., 2007). Anxiety was measured by parent report, with general anxiety assessed with the Child and Adolescent Symptom Inventory’s Anxiety Scale (Sukhodolsky et al., 2008) and dental anxiety measured by the Children’s Fear Survey Schedule–Dental Subscale (Cuthbert & Melamed, 1982). Sensory processing difficulties were assessed by parent responses on the Short Sensory Profile (Dunn, 1999).
Outcome Measures.
Physiological stress was assessed throughout the dental cleaning by means of electrodermal activity (EDA), which measures the activation of the sympathetic, fight-or-flight nervous system and increases in times of stress (Dawson, Schell, & Filion, 2007). EDA measures included skin conductance levels and nonspecific skin conductance responses. Overt anxiety and distress behaviors were measured by dentist-report scales (Anxiety and Cooperation Scale [Veerkamp, Gruythuysen, van Amerogen, Hoogstraten, & Weerheijm, 1995] and Frankl Scale [Frankl, Shiere, & Fogels, 1962]) and researcher-completed video coding of the dental cleaning (Children’s Dental Behavior Rating Scale [CDBRS], developed for this study). Additional measurements, such as child report of pain (Faces Pain Scale–Revised; Hicks, von Baeyer, Spafford, van Korlaar, & Goodenough, 2001) and sensory discomfort (Dental Sensory Sensitivity Scale, developed by Leah Stein and Sharon Cermak for this study), were collected. Last, time to complete cleaning, number of hands required to stabilize the child for safety, and need for pharmacological methods were collected as proxies for cost.
Intervention
The SADE intervention is based on two theoretical models or frameworks, multisensory environments (Shapiro, 2011) and sensory integration theory (Ayres, 1972; Parham & Mailloux, 2010), and consists of modification of the sensory environment of the dental office. The specific sensory modifications that make up the SADE intervention are described in Table 2. The intervention used portable equipment costing less than $5,000.
Table 2.
Overview of Sensory-Adapted Dental Environmental Modifications
| Sensory Modality | Modifications |
| Visual | Blackout curtains were placed over windows, overhead fluorescent and dental lamps were turned off, and the dentist wore a headlamp to direct light directly into the child’s mouth and minimize light in the child’s eyes. Slow-moving visual color effects (Snoezelen) shone on the ceiling in the child’s visual field. |
| Auditory | Calming, rhythmic music played throughout the visit (Gibson, 1994). |
| Tactile | Deep pressure was provided by a butterfly-type wrap (adapted from Shapiro, Melmed, Sgan-Cohen, Eli, & Parush, 2007), weighted with a regular pediatric dental X-ray vest. The wings wrapped around the child from shoulders to toes and provided a deep hugging pressure to produce a calming effect. |
Results
In this section, we examine the study participants and the study’s scientific basis, process, resources, and management, as proposed by Thabane et al. (2010) and adapted for occupational therapy by Tickle-Degnen (2013).
Participants
The participants were 22 children with ASD and 22 TD children, ages 6–12 yr. Most of the ASD (82%) and TD (96%) groups were White and self-identified as Hispanic or Latino (68% ASD, 82% TD). In the TD group, 46% of the children were male and 54% were female; in the ASD group, children were predominantly male (82% male, 18% female). Approximately 25% of the parents in both the ASD and the TD groups spoke Spanish as their primary language; 75% spoke English. All children were English speaking. The mean age of the ASD group was 8.3 yr; for the TD group, it was 8.2 yr. Scores on the Vineland II indicated that 91% (n = 20) of participants in the ASD group were in the moderately low (3rd–17th percentile rank) to low (≤ 2nd percentile rank) categories compared with 14% (n = 3) of the TD participants. Likewise, 100% of the ASD participants were reported to have sensory processing differences (n = 1, probable difference; n = 21 definite difference), compared with 36% of the TD participants (n = 7, probable difference; n = 1, definite difference).
Scientific Basis
As noted earlier, this study was based on prior research indicating that children with ASD experience challenges with oral care and that these difficulties are associated with sensory processing difficulties (Stein et al., 2011, 2013). Therefore, we hypothesized that decreasing the noxious sensory characteristics of the dental environment would reduce behavioral and physiological distress during oral care in children with ASD.
Process
Recruitment and Retention.
This feasibility study indicated that we were able to successfully recruit and retain participants for the study. Of the 139 potential participants identified, 65 were contacted to obtain the necessary 45 eligible participants. Of the 45 participants who consented, 44 completed all study-related activities. We consider this 98% completion rate extremely successful given the 30%–40% no-show rate of the dental clinic.
We used several strategies to successfully recruit and retain participants. First, we modified the study protocol to include the opportunity for a home visit before the first study-related dental visit. Of the families, 59% (13 in the TD group and 13 in the ASD group) consented and completed surveys at their home before the first dental visit; 34% (9 TD and 6 ASD) consented and completed surveys at the dental clinic, and 9% (1 TD and 3 ASD) consented at the dental clinic but completed the surveys at their home after the first dental visit.
Second, as part of the SADE study, we had greater scheduling flexibility than the dental clinic’s regular procedures allowed. We called participants a few days before their visit to confirm their appointment, and if the family’s availability had changed, we were able to reschedule the visit. In addition, we sent the participants a social story to read to their child, which served as another reminder. Last, families received free dental cleanings for their children and were given small stipends to serve as compensation for time and transportation expenses for each visit. Using these strategies, we had only 1 participant who did not arrive for a scheduled dental appointment.
Acceptability, Validity, and Process Findings Regarding Measures.
Psychophysiological Measures.
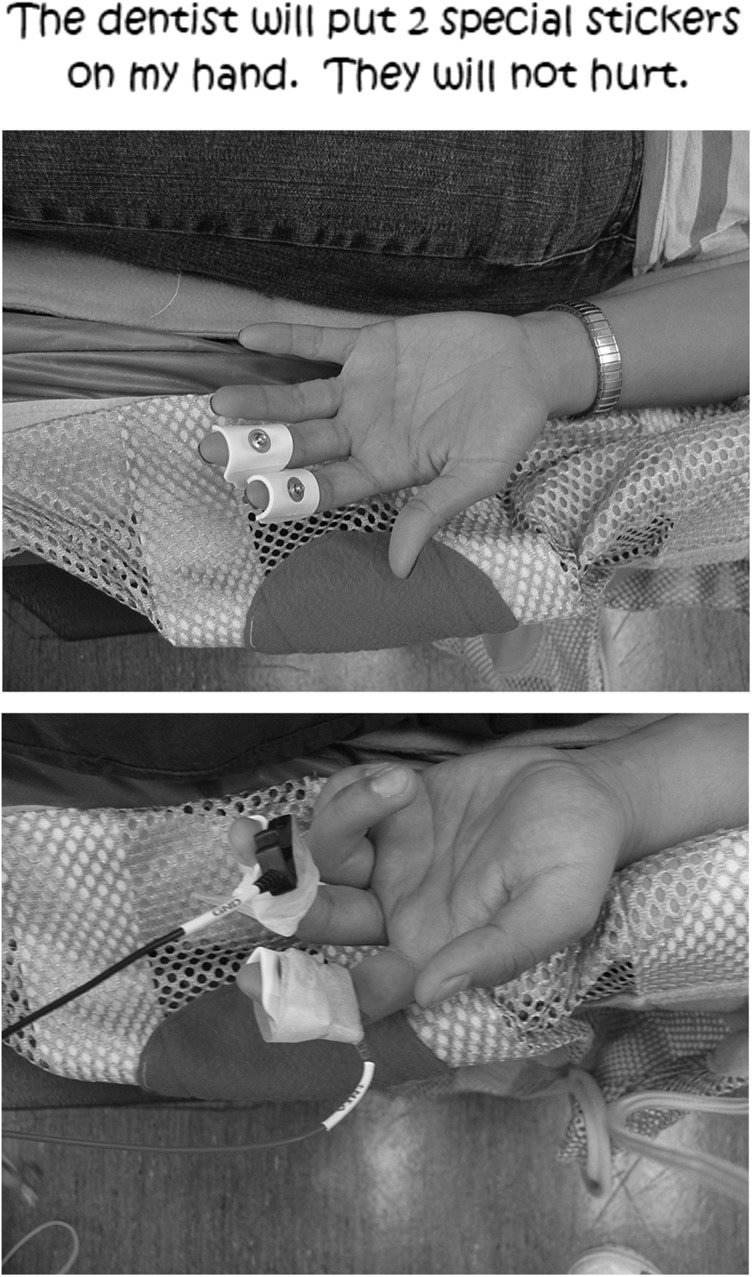
One concern we had before the study was whether children would accept electrodes placed on their fingers. For this reason, we used a social story to prepare the children for the “stickers” (electrodes; see Figure 2). Also, before the dental cleaning, the child was shown the electrodes for psychophysiological measurement and encouraged to touch them and practice putting them on their fingers, their parents' fingers, or both. Moreover, the home visit allowed the research team to develop rapport with the family because the occupational therapist completing the consent process (Leah Stein) was also present at the oral cleaning; this rapport may have helped the child accept the staff and electrodes. As such, all children were able to tolerate the electrodes during dental cleaning, even though our ASD group was lower functioning (per Vineland II scores) and had sensory processing difficulties (per Short Sensory Profile scores). Although this prior familiarity with research staff may have helped children accept the electrode placement, it may also have inadvertently reduced anxiety during the dental procedure.
Figure 2.
Social story page for electrodes.
Another consideration was whether the children would be able to sit sufficiently still to record EDA activity without movement artifacts during the dental cleaning. To accommodate the possibility that we would not be able to obtain artifact-free data, we included a 2-min rest during the dental cleaning. However, rather than being helpful, many children found it difficult to sit still during this period. Conversely, children were able to sit sufficiently still during the cleaning to obtain EDA data. Therefore, we will not include this rest period in the subsequent clinical trial.
Questionnaire Measures.
Although all questionnaires were available in English and Spanish, depending on parent preference, we found it necessary to be available to answer parents’ questions during survey completion.
Video-Coding Measure.
We developed the CDBRS for this study to identify overt behavioral distress during dental care. High interrater reliability was found (κ = .97, p < .001). The measure differentiated between ASD and TD group distress; however, it was less robust in distinguishing between the two dental environments. Also, the process of coding was labor and time intensive; therefore, we plan to reexamine the coding scale.
Intervention Fidelity and Acceptability.
We undertook several approaches to ensure treatment fidelity. First, the dentist treating participants underwent training in sensory processing, autism, and the SADE procedure. Second, we administered a fidelity checklist that monitored adherence to the treatment protocol, confirming that all adaptations were used as planned and all assessments were administered.
At the conclusion of the study, we interviewed the participating dental professionals to examine their perceptions of the feasibility and acceptability of the SADE intervention. Overall, we received excellent feedback from the dental clinic. The dentist stated that the headlamp took getting used to and was not fully comfortable, but indicated liking the butterfly wrap and feeling more relaxed in the SADE condition. Moreover, the dentist reported that the SADE did not affect her ability to perform the oral cleaning.
To examine acceptability to the participants, we asked children how they felt about the SADE adaptations and recorded any components that the child rejected. More than 95% of children accepted all components of SADE; 1 child with ASD stated that he did not like the music and requested that we turn it off, and 1 TD child reported that she did not like having the lights dimmed because she was afraid of the dark. Parents provided positive comments about the intervention as well, but we did not collect these data in a systematic manner—another change that will take place in the subsequent clinical trial.
Resources
Resources in the dental clinic were sufficient for this pilot trial but need to be more fully examined to determine whether personnel dedicated solely to this project are needed for a large-scale clinical trial. Moreover, only two private rooms in the dental clinic can be adapted for our protocol; thus, consideration of the need for an additional site or space is warranted.
Monetary Resources.
Unexpected or unplanned costs occurred in several areas. First, we added a home visit for the consent and questionnaire process, adding cost in the form of mileage expenses, staff time, and compensation to families. However, this visit increased rapport between the investigator and the family, which may have contributed to our excellent rate of recruitment and retention of participants. We plan to include this extra visit in the larger intervention. Second, the home visit worked best with two research staff, one interacting primarily with the parent and the other with the participating child, siblings, or both so the parent could concentrate on completing the questionnaires. In addition, early evenings seemed to be the best time for families. As such, for safety reasons, we recommend sending two members of the research team for this visit, despite the increased cost.
Third, we anticipated that approximately 20% of families of children with ASD would need an ADOS administration to confirm an ASD diagnosis. However, 64% of the participants with ASD required administration of the ADOS, increasing cost. Also, many of the ADOS reports we did obtain were incomplete, were outdated, or did not provide item scores, so although we could confirm an ASD diagnosis, we could not calculate an autism severity score.
Fourth, we were required to have some study materials professionally translated into Spanish so we could include Spanish-speaking families in our study, which was more costly than we anticipated. We also ensured that one of our research team members was bilingual to communicate with Spanish-speaking families. However, these materials are now available for future research.
Last, because of the darkened environment in the SADE, we had to use a more expensive camera with night-vision capabilities instead of the camera originally planned.
Recruitment Resources.
Another unexpected factor was the high prevalence of children with moderate to severe ASD in our sample, based on Vineland II scores, because we expected a broader range of severity. We believe this may reflect the population of children with ASD served in a hospital-based dental clinic, in which children with moderate to severe language and cognitive impairment were overrepresented. Thus, even though we were able to recruit a sufficient number of children, we did not have a representative range of language and IQ abilities. This is important for determining whether the intervention is appropriate for the larger population of children with ASD. Thus, additional sources of recruitment will be needed to identify a wider range of children with ASD.
Management
Acquiring approval from two institutional review boards was more time consuming than expected and took approximately 12 mo. Multiple iterations were required with the full committee review, and after approval from the hospital review board, we were also required to submit a facilitated review to the review board at the university where our offices are located.
A major purpose of the pilot study, from the perspective of the funding agency, was the development of a data management system. Extensive efforts were expended working with the large Cancer Research Informatics Core affiliated with our university and a statistician in developing a data management system. The system itself was designed to minimize data entry errors, for example, by setting possible ranges for each item. However, one challenge we experienced was that after the system had already been developed, we decided to use double entry of data to further reduce examiner error, and the database was not set up for that. Also, we did not adequately standardize data entry across examiners, such as the method of entering the date (e.g., 6-2-99 vs. 06-02-1999) or entering the time of day (e.g., AM or am). These minor differences resulted in multiple items being identified as not matching and required returning to our data management developer. We now expect to be able to build on this database with adjustments as a result of what we have learned.
As part of our preparation for a larger clinical trial, we developed an intervention manual with practical guidelines and a manual of procedures as required by the funding agency. These were reviewed by all members of the team and will serve as the basis for our proposed large-scale trial. Throughout the process of conducting this pilot study, it became clear that the larger clinical trial will require more administrative positions with clearly defined responsibilities.
Discussion
Oral health is important for children’s psychological and physiological health (Casamassimo, 1996; HHS, 2010). However, despite the importance of proper oral care, dental care is the most frequently cited unmet health care need for children with special health care needs, with parents frequently reporting fair or poor condition of teeth in these children (Child and Adolescent Health Measurement Initiative, 2011; Lewis, 2009; Lewis et al., 2005). One group of children with special health care needs that may be at particular risk for poor oral health is children with ASD. Although autism itself is not a direct cause of dental deficit, it is considered an indicator of high caries risk, with caries incidence linked to behaviors and life factors that are prevalent with the disorder (Marshall, Sheller, & Mancl, 2010; Murshid, 2011), including sensory processing difficulties (Stein et al., 2011, 2013).
Incorporation of a pilot and feasibility study was extremely helpful in planning for a large-scale randomized controlled trial. The pilot study demonstrated that a SADE can be implemented within a dental clinic in an urban hospital, and patients, families, dentists, clinic staff, and investigators responded positively to the experience. Before embarking on a large intervention trial, we plan to incorporate the following lessons learned.
First, we now understand the importance of developing rapport with research participants; therefore, we plan to perform a separate consent and baseline assessment visit in the home setting before the first dental visit. However, although these home visits were helpful in increasing research quality, because of costs home visits may not be a practical strategy to enhance clinical care. Second, the inclusion of Spanish-speaking personnel to ensure parental understanding of the consent process and overall study was critical; it was not needed in the dental clinic because the clinic in which we conducted the study had a large Spanish-speaking population, and bilingual dental staff were part of the team. Third, we plan to reexamine the coding system for the videotaped dental visits. The goal will be to more clearly separate the behaviors that interrupted dental treatment (e.g., screaming, biting) from those that indicated mild patient distress (e.g., grimacing), and identify those that best discriminate between the RDE and SADE. Last, we plan to add a quantifiable instrument to examine the dental professionals’, parents’, and children’s perceptions of the intervention. Mixed methods incorporating both quantitative and qualitative study techniques are invaluable in examining treatment efficacy.
It is important for occupational therapy practitioners to consider different ways to improve health care for children with ASD. One way is to work as a member of the dental care team for children with ASD. Adapting the sensory environment to lessen the noxious sensory stimuli encountered during dental care is a valuable contribution. Our feasibility study was successful in pointing out the strengths and weaknesses in our study. Like Tickle-Degnen (2013), we strongly advocate for the need for feasibility studies in occupational therapy before conducting large-scale clinical trials. Our hope is that after a larger-scale clinical trial, this type of innovative treatment will aid in diminishing the current disparity in oral health in this population.
Limitations and Future Research
Because this was a pilot and feasibility study, we were less stringent with the diagnosis confirmation date; therefore, not all participants had been administered the ADOS in the past 12 mo. Approximately 65% of our ASD group received diagnosis confirmation via the ADOS specifically for this study; most of the remaining 8 children had the ADOS administered within the previous 3 yr. In our next study, all recruited children will receive confirmation of an ASD diagnosis with a study-related administration of the ADOS.
Children with ASD are a heterogeneous group; however, in our sample the majority of children with ASD were lower functioning in terms of their level of expressive language. Therefore, our findings may not generalize to children with ASD with more verbal skill. For our next study, we plan to use the same recruitment techniques but also to recruit from additional sources to ensure inclusion of participants with a broader range of autism severity.
Last, because our study was a feasibility and pilot study, it had a small sample size and thus did not allow us to examine potential moderating or mediating variables of the SADE’s efficacy. In a future clinical trial, we plan to include a larger, appropriately powered sample size to enable us to investigate the potential impacts of general anxiety, dental anxiety, sensory overresponsivity, communication level, and severity of ASD.
Implications for Occupational Therapy Practice
The results of this study have the following implications for occupational therapy practice:
Occupational therapy practitioners are uniquely situated to view a variety of health care challenges in a different light. One such challenge is oral care in children with ASD.
Occupational therapy practitioners can help decrease health-related disparities by incorporating their knowledge of sensory processing in stressful health-related situations.
A SADE is feasible to implement and shows the potential to reduce behavioral distress, physiological stress, pain, and sensory discomfort in children with ASD.
It was essential to conduct this feasibility study before conducting a large-scale randomized controlled trial.
Conclusion
This study elucidated the SADE’s strengths and weaknesses, providing us with important knowledge that enabled the research team to make appropriate adjustments to the study protocol and procedures before applying for grant funding for a larger-scale clinical trial. We concur with Tickle-Degnen (2013) in suggesting that occupational science and occupational therapy researchers design and conduct feasibility studies to assess interventions on their scientific basis, processes, resources, and management.
We are excited to continue this research with a larger and more diverse group of children to examine the use of a SADE. This treatment technique has implications not only for children with ASD visiting the dentist but also for people with ASD of any age, as well as for people with other disabilities and TD children with dental anxiety, sensory processing difficulties, or both.
Acknowledgments
This study was funded by the National Institute of Dental and Craniofacial Research (1R34DE022263-01; Sharon Cermak, principal investigator), by a seed grant from the University of Southern California Ostrow School of Dentistry, and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (T32HD064578; Florence Clark, principal investigator). We thank the University of Southern California Mrs. T. H. Chan Division of Occupational Science and Occupational Therapy for its continued support; Michele Shapiro and Anat Baniel from Beit Issie Shapiro, Ra’nana, Israel, for their original research on sensory adaptations in the dental environment and for providing consultation throughout this project; Trudy Mallinson for her assistance with Rasch analysis in developing the CDBRS coding scale; Mae Aghili for her participation and skill in performing the study dental cleanings; Irina Zamora for assistance in confirming diagnosis of children with ASD; Elyse Peterson and Lauren St. Hilaire for their assistance in psychometric testing of the CDBRS, data collection, and interrater reliability assessments of measures; and Daniela Florindez, our bilingual research assistant. Our ClinicalTrials.gov identifier is NCT02077985.
Contributor Information
Sharon A. Cermak, Sharon A. Cermak, EdD, OTR/L, is Professor, Mrs. T. H. Chan Division of Occupational Science and Occupational Therapy, Herman Ostrow School of Dentistry, University of Southern California, Los Angeles; cermak@usc.edu
Leah I. Stein Duker, Leah I. Stein Duker, PhD, OTR/L, is Postdoctoral Fellow, Division of Occupational Science and Occupational Therapy, Herman Ostrow School of Dentistry, University of Southern California, Los Angeles
Marian E. Williams, Marian E. Williams, PhD, is Associate Professor of Clinical Pediatrics, Keck School of Medicine, University of Southern California University Center for Excellence in Developmental Disabilities, Children’s Hospital Los Angeles
Christianne Joy Lane, Christianne Joy Lane, PhD, is Assistant Professor, Division of Biostatistics, Department of Preventive Medicine, University of Southern California, Los Angeles.
Michael E. Dawson, Michael E. Dawson, PhD, is Professor, Department of Psychology, Dana and David Dornsife College of Letters, Arts and Sciences, University of Southern California, Los Angeles
Ann E. Borreson, Ann E. Borreson, MD, is Research Associate, Division of Occupational Science and Occupational Therapy, Herman Ostrow School of Dentistry, University of Southern California, Los Angeles
José C. Polido, José C. Polido, DDS, MS, is Division Head—Dentistry, Children’s Hospital Los Angeles, and Assistant Professor of Clinical Dentistry, Herman Ostrow School of Dentistry, University of Southern California, Los Angeles
References
- Ayres A. J. (1972). Sensory integration and learning disorders. Los Angeles: Western Psychological Services. [Google Scholar]
- Bowen D. J., Kreuter M., Spring B., Cofta-Woerpel L., Linnan L., Weiner D., . . . Fernandez M. (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36, 452–457. http://dx.doi.org/10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassimo P. (1996). Bright futures in practice: Oral health. Arlington, VA: National Center for Education in Maternal and Child Health. [Google Scholar]
- Child and Adolescent Health Measurement Initiative. (2011). National survey of children with special health care needs 2000–10 [Data set]. Retrieved from http://www.childhealthdata.org/browse/survey?s=1
- Cuthbert M. I., & Melamed B. G. (1982). A screening device: Children at risk for dental fears and management problems. ASDC Journal of Dentistry for Children, 49, 432–436. [PubMed] [Google Scholar]
- Dawson M. E., Schell A. M., & Filion D. L. (2007). The electrodermal system. In Cacioppo J. T., Tassinary L. G., & Bernston G. G. (Eds.), Handbook of psychophysiology (3rd ed., pp. 159–181). Cambridge, England: Cambridge University Press. [Google Scholar]
- Dunn W. (1999). Sensory Profile: User manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Frankl S. N., Shiere F. R., & Fogels H. R. (1962). Should the parent remain with the child in the dental operatory? ASDC Journal of Dentistry for Children, 29, 150–163. [Google Scholar]
- Gibson D. (1994). Solitudes: Exploring nature with music Retrieved from http://www.allmusic.com/album/the-classics-2-exploring-nature-with-music-mw0000916892
- Hicks C. L., von Baeyer C. L., Spafford P. A., van Korlaar I., & Goodenough B. (2001). The Faces Pain Scale–Revised: Toward a common metric in pediatric pain measurement. Pain, 93, 173–183. http://dx.doi.org/10.1016/S0304-3959(01)00314-1 [DOI] [PubMed] [Google Scholar]
- Klin A., Saulnier C. A., Sparrow S. S., Cicchetti D. V., Volkmar F. R., & Lord C. (2007). Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. Journal of Autism and Developmental Disorders, 37, 748–759. http://dx.doi.org/10.1007/s10803-006-0229-4 [DOI] [PubMed] [Google Scholar]
- Lewis C. W. (2009). Dental care and children with special health care needs: A population-based perspective. Academic Pediatrics, 9, 420–426. http://dx.doi.org/10.1016/j.acap.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C., Robertson A. S., & Phelps S. (2005). Unmet dental care needs among children with special health care needs: Implications for the medical home. Pediatrics, 116, e426–e431. http://dx.doi.org/10.1542/peds.2005-0390 [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., & Risi S. (1999). Autism Diagnostic Observation Schedule manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Marshall J., Sheller B., & Mancl L. (2010). Caries-risk assessment and caries status of children with autism. Pediatric Dentistry, 32, 69–75. [PubMed] [Google Scholar]
- Murshid E. Z. (2011). Characteristics and dental experiences of autistic children in Saudi Arabia: Cross-sectional study. Journal of Autism and Developmental Disorders, 41, 1629–1634. http://dx.doi.org/10.1007/s10803-011-1188-y [DOI] [PubMed] [Google Scholar]
- Owens P. L., Kerker B. D., Zigler E., & Horwitz S. M. (2006). Vision and oral health needs of individuals with intellectual disability. Mental Retardation and Developmental Disabilities Research Reviews, 12, 28–40. http://dx.doi.org/10.1002/mrdd.20096 [DOI] [PubMed] [Google Scholar]
- Parham D., & Mailloux Z. (2010). Sensory integration. In Case-Smith J. & O’Brien J. (Eds.), Occupational therapy for children (6th ed.). Maryland Heights, MO: Mosby. [Google Scholar]
- Shapiro M. (2011). Beit Issie Shapiro’s approach to multi-sensory environments (Snoezelen). Ra’nana, Israel: Rotem. [Google Scholar]
- Shapiro M., Melmed R. N., Sgan-Cohen H. D., Eli I., & Parush S. (2007). Behavioural and physiological effect of dental environment sensory adaptation on children’s dental anxiety. European Journal of Oral Sciences, 115, 479–483. http://dx.doi.org/10.1111/j.1600-0722.2007.00490.x [DOI] [PubMed] [Google Scholar]
- Shapiro M., Melmed R. N., Sgan-Cohen H. D., & Parush S. (2009). Effect of sensory adaptation on anxiety of children with developmental disabilities: A new approach. Pediatric Dentistry, 31, 222–228. [PubMed] [Google Scholar]
- Sparrow S. S., Cicchetti D. V., & Balla D. A. (2005). Vineland Adaptive Behavior Scales (2nd ed.). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Stein L. I., Polido J. C., & Cermak S. A. (2012). Oral care and sensory concerns in autism. American Journal of Occupational Therapy, 66, e73–e76. http://dx.doi.org/10.5014/ajot.2012.004085 [DOI] [PubMed] [Google Scholar]
- Stein L. I., Polido J. C., & Cermak S. A. (2013). Oral care and sensory over-responsivity in children with autism spectrum disorders. Pediatric Dentistry, 35, 230–235. [PubMed] [Google Scholar]
- Stein L. I., Polido J. C., Mailloux Z., Coleman G. G., & Cermak S. A. (2011). Oral care and sensory sensitivities in children with autism spectrum disorders. Special Care in Dentistry, 31, 102–110. http://dx.doi.org/10.1111/j.1754-4505.2011.00187.x [DOI] [PubMed] [Google Scholar]
- Stein L. I., Polido J. C., Najera S. O., & Cermak S. A. (2012). Oral care experiences and challenges in children with autism spectrum disorders. Pediatric Dentistry, 34, 387–391. [PubMed] [Google Scholar]
- Sturkenboom I. H., Graff M. J., Borm G. F., Veenhuizen Y., Bloem B. R., Munneke M., & Nijhuis-van der Sanden M. W. (2013). The impact of occupational therapy in Parkinson’s disease: A randomized controlled feasibility study. Clinical Rehabilitation, 27, 99–112. http://dx.doi.org/10.1177/0269215512448382 [DOI] [PubMed] [Google Scholar]
- Sukhodolsky D. G., Scahill L., Gadow K. D., Arnold L. E., Aman M. G., McDougle C. J., . . . Vitiello B. (2008). Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology, 36, 117–128. http://dx.doi.org/10.1007/s10802-007-9165-9 [DOI] [PubMed] [Google Scholar]
- Thabane L., Ma J., Chu R., Cheng J., Ismaila A., Rios L. P., . . . Goldsmith C. H. (2010). A tutorial on pilot studies: The what, why and how. BMC Medical Research Methodology, 10, 1–10. http://dx.doi.org/10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle-Degnen L. (2013). Nuts and bolts of conducting feasibility studies. American Journal of Occupational Therapy, 67, 171–176. http://dx.doi.org/10.5014/ajot.2013.006270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2010). Healthy People 2020: Oral health Retrieved from http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=32
- Veerkamp J. S., Gruythuysen R. J., van Amerogen J., Hoogstraten H., & Weerheijm K. L. (1995). Dentist’s ratings of child dental patients’ anxiety. Community Dentistry and Oral Epidemiology, 23, 356–359. http://dx.doi.org/10.1111/j.1600-0528.1995.tb00262.x [DOI] [PubMed] [Google Scholar]