Abstract
Objective. To evaluate the impact of counseling in a simulated medication adherence activity.
Design. Students were randomized into 2 groups: patient medication monograph only (PMMO) and patient medication monograph with counseling (PMMC). Both groups received a fictitious medication and monograph. Additionally, the PMMC group received brief counseling. A multiple-choice, paper-based survey instrument was used to evaluate simulated food-drug interactions, adherence, and perceptions regarding the activity’s value and impact on understanding adherence challenges.
Assessment. Ninety-two students participated (PMMC, n=45; and PMMO, n=47). Overall, a significantly higher incidence of simulated food-drug interactions occurred in the PMMO group (30%) vs the PMMC group (22%) (p=0.02). Doses taken without simulated food-drug interactions were comparable: 46.2% (PMCC) vs 41.9% (PMMO) (p=0.19). The average number of missed doses were 3.2 (PMMC) vs 2.8 (PMMO) (p=0.55). Approximately 70% of the students found the activity to be valuable and 89% believed it helped them better understand adherence challenges.
Conclusion. This activity demonstrated the challenges and important role of counseling in medication adherence.
Keywords: adherence, counseling, pharmacy, simulation, student
INTRODUCTION
Medication adherence can be defined as the degree to which a patient’s disease and drug therapy management correspond to the established treatment plan developed with health care providers.1 Nonadherence includes incorrect duration of therapy, incorrect dosing, and incorrect frequency or timing of medication administration.2 Lack of medication adherence has significant consequences on both patients and the health care system; in fact, it has been associated with an increase in morbidity and mortality, as well as an increase in overall health care costs.3-5 Medication adherence among patients with chronic conditions averages about 50%, so understanding the barriers to adherence and developing strategies to increase adherence rates could have a significant impact on improving disease-related outcomes.1
Pharmacists are in a unique position to improve medication adherence by using beneficial tools such as increased patient education.6 Counseling by pharmacists in both the inpatient and outpatient settings can improve medication adherence and persistence.7,8 Various organizations, such as the National Council on Patient Information and Education, the Pharmacy Quality Alliance, and the American Association of Colleges of Pharmacy have partnered with the National Consumer League to create a campaign that increases patient awareness and encourages provider discussion with patients regarding adherence.9,10
The Center for the Advancement of Pharmacy Education specifically delineates pharmacist-provided education as an objective for students.11 Although the Accreditation Council for Pharmacy Education Standards do not directly outline pharmacist-delivered education in relation to medication adherence, they do include medication adherence within example performance competencies for basic patient assessment.12 In their review of medication adherence education in schools of pharmacy, Rickles and colleagues demonstrated that interventions to promote adherence are not taught comprehensively and that further nationwide curricular modification is necessary.10
Various studies in education have utilized simulated medication scenarios to illustrate the concepts of medication adherence.13-17 In a recent study by Ulbrich and colleagues, 69 second-year pharmacy students were required to participate in a project in which they were given 6 medications and a complex regimen to follow for 6 days. They were given jellybeans in medication vials labeled with fictitious drug names, use, route of administration, and directions. Presurvey and postsurvey responses demonstrated that students missed a considerable number of doses and described a greater level of empathy for patients in regard to adherence to complex regimens.16 In another study of 65 doctor of pharmacy students in their third year, Slain and colleagues assessed adherence to 2 different placebo antiretroviral regimens. Although overall adherence to the regimens was higher among pharmacy students than among actual patients with HIV, less than 50% of the students adhered fully to dietary restrictions.18
At The Ernest Mario School of Pharmacy, the Pharmacy Communications course sequence is comprised of 2 separate 2-credit courses delivered over the fall and spring semesters of the third professional (P3) year of the pharmacy curriculum. Both courses consist of 6 small, class-size sections (approximately 30-35 students) and integrate lectures, group activities, and one-on-one counseling sessions for the development of student interpersonal, professional, and clinical communications skills.
Approximately half of the classes are comprised of the one-on-one counseling sessions. Topics focus on a wide variety of prescription and nonprescription medications. Twelve postdoctoral PharmD fellows from the Rutgers Pharmaceutical Industry Fellowship Program (2 per section) participate in the lecture delivery and counseling activity grading.
First-hand experience with the challenges of taking a medication with a food-drug interaction may demonstrate to students the importance of counseling and how a pharmacist can improve outcomes. The objective of this instructional activity was to evaluate and demonstrate to students through simulation the potential impact of counseling on patients’ medication adherence.
DESIGN
In the spring of 2012, a lecture was created that reviewed the challenges associated with medication adherence, specifically issues faced by patients and how to overcome barriers to full adherence. Accompanying the lecture material was an interactive medication adherence challenge (MAC) activity, illustrating potential adherence issues through personal experience.
At the conclusion of a scheduled counseling practical examination, students were given the option of participating in the activity. A fictitious prescription medication, “dasdegvolinase (DDV) 400mg Geltabs,” represented by JellyBelly jelly beans, was distributed to participants along with a patient medication monograph. The monographs were developed using examples of handouts from community pharmacies. Each vial contained 10 jelly beans and was labeled with the directions “Take 1 tablet by mouth twice a day.”
Students were randomized into 2 groups using an alternating alphabetical selection process. The groups were designated Patient Medication Monograph Only (PMMO) and Patient Medication Monograph with Counseling (PMMC). The PMMO group only received medication and a monograph, whereas the PMMC group received medication, a monograph, and a brief (approximately 30 seconds to 1 minute) individual counseling session reviewing the following instructions: (1) Take each medication twice a day (as close to 12 hours apart as possible) to help maintain consistent levels of the medication. Ask what times would work best for the student to plan a scheduled time. (2) [EMPHASIZE]: Avoid eating foods containing wheat/gluten within 2 hours before or 4 hours after taking DDV (unless labeled gluten free). Examples included: pasta, breads/breaded products, beer, cakes/cookies/muffins, cereal, dressings, gravy, matzo, sauces, soups and soup bases, white flour (3) Food labels with wheat, barley, and rye listed as ingredients indicate wheat/gluten present so they should not be consumed with DDV.
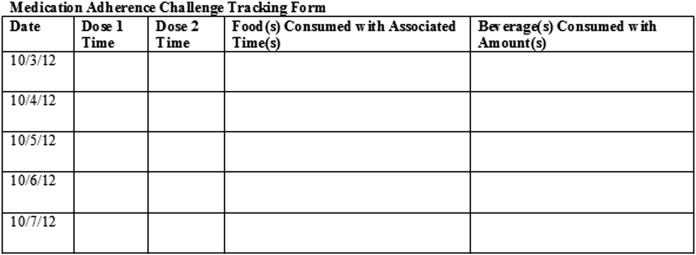
Students were directed not to share information with other students to minimize influencing adherence behavior. Both groups were instructed to track the time each dose was taken and all foods and/or beverages consumed, with associated times, using a MAC tracking form (Figure 1).
Figure 1.
Medication Adherence Challenge Tracking Form
The following week, tracking forms were used to assess adherence. Students were asked to use their MAC tracking form to complete a multiple-choice, paper-based survey instrument evaluating adherence by noting the number of missed doses. Survey drafts were developed and tested during the activity’s initial implementation the prior year. General student feedback was solicited at the beginning of class and collected during class discussions and survey completion. Based on this input, edits were made to simplify and streamline completion of the tracking form and assessment. During the pilot program, students were asked multiple questions rather than one about adherence and compliance to the prescribed regimen (Appendix 1). The question formats were identified by students as being confusing and unclear. Explanations of questions had to be provided throughout the survey completion portion of the class. To address this issue for the following school year, each dose was listed as a separate question and covered both adherence and compliance. In addition, the pilot program revealed that some students completed the survey without participating in the program or participated but provided inaccurate answers. Therefore, questions 1 (MAC participation) and 12 (information accuracy) were added to reduce the potential for inaccuracies or falsified information. Data identified as either falsified or inaccurate were not included in the final analysis. Although both surveys were tested, neither was validated.
Students transcribed answers onto machine-readable answer sheets (eg, Scantron, Apperson etc.) for data collection purposes. Foods that could potentially interact with DDV were also reviewed during this time to assess dietary restriction compliance. The survey evaluated student perceptions regarding the activity’s value and assessed the impact on student understanding of medication adherence challenges. This study was deemed exempt by the Rutgers University Office of Research and Sponsored Programs Institutional Review Board.
Student experiences, adherence perceptions, and other elements gathered from this activity, were incorporated throughout the concurrent lecture portion of the class. The lecture included interactive discussions, feedback, questions, and comments. Survey results were tabulated and shared with students to demonstrate the potential impact of pharmacist counseling on medication adherence.
Minimal finance- and workforce-related resources were required for the development and implementation of the activity. Financial support for this program was provided by the Pharmacy Practice and Administration Department. The supplies needed for the project included jelly beans, vials, labels, bags, and informational sheets. The direct costs for this activity were approximately $186 but did not factor in roughly 6 hours of time spent by a work-study student filling and labeling the medication vials and bags. An additional 10 hours, over 4 meetings, were spent planning and developing the activity materials (eg, handouts, medication tracking form, etc.). For individuals looking to recreate this activity in a large course, an initial financial stipend of $250 plus 10 hours of time would be appropriate based on potential variations in cost.
EVALUATION AND ASSESSMENT
Students completed a paper-based survey instrument documenting the potential for food-drug interactions and missed doses 2 times every day for 5 days following the start of the fictitious therapy (ie, 10 observation points). They were also asked 2 questions about the value of the exercise in terms of developing as a pharmacist and helping students understand the challenges of medication adherence. Students documented regimen adherence and beverage/food consumption compliance over the 5-day study phase via the MAC tracking form, which was also used to complete the survey.
Survey responses were collected from the PMMO and PMMC groups. At each time point, 3 outcomes were possible: (1) No food-drug interaction; (2) Documented food-drug interaction; or (3) A missed dose. Data were later aggregated into a dichotomous outcome defined as: (1) Positive (no food-drug interaction) or (2) Negative (interaction or missed dose).
Chi-square tests were used to assess the differences in rates of outcomes comparing the PMMO group to the PMMC group. Because of the small cell sizes, the Fisher exact test was also used, where appropriate. Each of the 10 observation points was analyzed individually. One series of analyses examined the rates of the 3 possible outcomes at every time point. When assessing the 3 outcomes, separate tests were conducted including and then excluding the missed dose responses. The dichotomized outcomes (positive and negative) were then assessed at each time point. The number of missed doses was compared using the nonparametric Kruskal-Wallis test. Finally, data from each of the 10 data points were pooled (ie, arrayed) into 1 overall observation point and the rates of the 3 outcomes and the dichotomized outcomes were assessed. The 2 questions evaluating the value of the exercise were also analyzed by respondent.
A MAC tracking form was used to maintain consistency in student data tracking and reporting. The tracking form was intended to help students identify the necessary information for documentation and improve the accuracy and consistency of data reporting. Survey question 12 was also included to ascertain and exclude inaccurate data provided by students who self-identified as not honestly or accurately documenting and reporting how they took their medication. Students were encouraged to answer question 12 correctly, as no penalty would occur, regardless of their answer choice. Data sets identified as inaccurate were not included in the final analysis. Similarly, all data associated with students who provided incorrect responses (eg, selection of “E” on multiple-choice survey questions where only “A” and “B” were options) were not included in the data assessment.
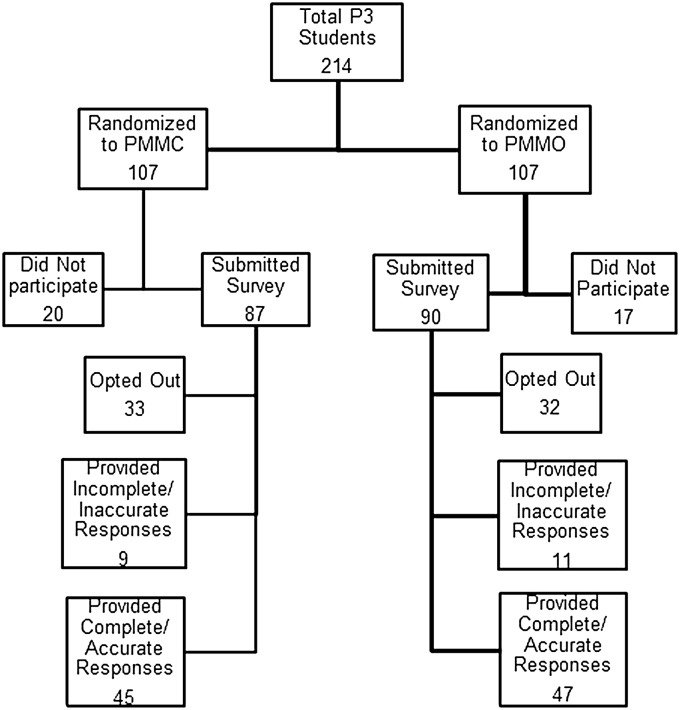
Since the MAC activity and its assessments were voluntary, approximately half (47.7%) of the class did not take part in the exercise and/or its evaluation. Figure 2 highlights student participation in the study. Nearly a third (30.4%) of the students indicated they did not participate and 17.3% did not complete the survey. Of those who participated and completed the survey, 43% did so completely and accurately (PMMC, n=45; and PMMO, n=47). Data was only included from students who indicated that they completed the medication tracking form and survey accurately. Student surveys containing incomplete or inaccurate documentation of adherence tracking were excluded from the data assessment.
Figure 2.
Distribution of Student Participation
The average number of missed doses per respondent among the PMMO group was 2.8 vs 3.2 in the PMMC group. The difference was not significantly different (p=0.55). Overall, 46.2% of the PMMC group’s doses were taken without any simulated food-drug interaction, compared to 41.9% of the PMMO group (p=0.19). When evaluating doses taken that simulated an interaction with food, there was a higher incidence in the PMMO group (30%) vs the PMMC group (22%) (p=0.02). With the exception of the dose to be taken in the evening of the third day, the PMMC group consistently maintained a lower drug interaction incidence with gluten compared to the PMMO group. At the end of the study period, 89.1% of the students felt they were able to better understand the challenges associated with taking medications as prescribed, while 69.6% felt the activity helped them develop as a pharmacist.
DISCUSSION
The medication adherence activity was designed to evaluate and demonstrate the impact of counseling on adherence and foster discussions regarding medication adherence challenges. Faculty members felt that interactive learning (ie, taking medications) provided students with an opportunity to better understand the impact of counseling and the challenges associated with compliance. The study evaluated adherence and drug interaction rates of medications taken by students who were counseled (the PMMC group) vs not counseled (the PMMO group). It was expected that the PMMC group would have fewer simulated drug interactions, and the counseling they received would provide students the ability to see the impact of counseling patients, even with a brief session. Ultimately, the project supported the anticipated outcomes with 89.1% of students feeling that they better understood the medication adherence challenges faced by patients.
Studies have shown that medication adherence demonstrations using simulated medication regimens reinforce taught concepts regarding the challenges of complex dosing, as well as dietary restrictions.16,18 Additionally, the use of an adherence tool, such as an automated medication dispenser, has also been incorporated into medication simulation activities.19 This study was the first in the authors’ knowledge to examine the impact of patient counseling on improving adherence using student participants. Students were able to see first-hand how counseling led to decreased drug interactions.
Similar to feedback from previous studies,16-19 students indicated that this activity was beneficial in helping them understand the challenges with medication adherence. The activity and postdiscussions highlighted key adherence and compliance related issues, such as potential food-drug interactions, difficulties in remembering to take medications, and managing a medication regimen. Overall, 69.6% of students reported the activity helped them develop as a pharmacist. Although significant differences were not detected when comparing the PMMC and PMMO group results for individual doses, the PMMC group more consistently maintained a lower drug interaction incidence with gluten than the PMMO group did.
During study development, some potential obstacles and limitations were anticipated, based on the class structure, information delivery and tracking, and student and fellow involvement. Efforts were made to address and minimize their impact on study implementation and results tracking. Since the Pharmacy Communications course consisted of 6 separate sections, each taught by 2 fellows, consistency in the delivery of messages and counseling by fellows was a concern. To reduce potential variability, fellows were given specific guidance on content and how to counsel students in the PMMC group. Since a comprehensive patient counseling session, consisting of open-ended questions and a thorough review of medication use (dosing, side effects, drug interactions, etc.) was not conducted, the potential impact of such a session on compliance could not be gauged but may demonstrate additional benefits if used in future studies.
Although measures were taken to minimize or avoid factors potentially affecting study implementation and results, some limitations may have still affected the activity, study, and data assessment. A primary limitation was the relatively small sample size of study participants. From the original 214 students, only 92 useable response sets were received for an overall response rate of 43%. Information from students who did not choose to participate was not queried or solicited. Thus, it was not possible to determine if the volunteers were a representative sample of the entire pharmacy class. The small sample sizes (47 in the PMMO group and 45 in the PMMC group) limited the ability to detect significant differences in many of the individual time-point assessments. Further, as noted previously, the questionnaire was not validated. The evaluation period of only 5 days may have been adequate but not representative of the long-term course for most medication regimens. Further studies would also need to evaluate if counseling impacts interactions as more time lapse occurs. In addition, demographic information to assess any correlations between successful behaviors and personal characteristics, such as age, gender, academic performance, etc., was not collected from respondents. Such information, along with an assessment of potential differences between study participants and nonparticipants might be a consideration of future studies. Although the hypothetical case for this evaluation was a food/drug interaction, it was not possible to assess whether the adherence behaviors would be the same for other types of interactions (eg, a potential fatal drug-drug interaction).
Since students self-monitored and reported medication adherence and compliance with the food restrictions, the accuracy of the information could not be verified. Future studies could require students to return unused medications to confirm adherence. Information sharing among students may have also impacted outcomes (eg, PMMC providing guidance to PMMO students who did not receive counseling). Anecdotal student comments gathered during poststudy discussions indicated that some students shared counseling session details with other study participants. This may have resulted in falsely improved compliance for students in the PMMO group who received additional counseling about food/drug interactions. Including multiple medication regimens of varying degrees of dosing schedules, complexities, interactions, etc., may help reduce the incidence of information sharing among students. Students may have also viewed this evaluation as a classroom exercise and may not have behaved, or recorded their behaviors, with the same veracity as an actual medication regimen.
Although educational experiences were the same for both groups, individual knowledge differences may have affected results. The education and medication awareness of pharmacy students may also be higher than awareness among the general public. This could have resulted in elevated adherence and compliance rates across both groups. If the PMMO study group subjects were knowledgeable about handling potential food/drug interactions, their compliance rates may have been falsely elevated compared to a noncounseled general public group. Finally, during poststudy discussions, some students indicated consuming all of their doses at once since the medication was candy. Substituting a flavorless placebo (eg, empty capsule) may reduce the chance of students prematurely consuming all of their doses based on taste.
SUMMARY
This medication adherence challenge activity demonstrated the barriers associated with medication adherence and the value of patient counseling. By including a drug interaction, this activity simulated the difficulty of a more complex medication regimen to students in a Pharmacy Communications course. This strategy will continue to be utilized in teaching the challenges associated with medication adherence and can be incorporated as an activity in other schools of pharmacy.
ACKNOWLEDGMENTS
The authors would like to thank Lisa Degnan, Abhay Patel, and Janice Weinstein for their assistance and contributions to this project.
Appendix 1. Medication Adherence Challenge Assessment Pilot Survey Questions 1 and 2 of 10
Please answer the following questions based on information obtained from your medication tracking form and the wheat/gluten food list provided to you. Use the accompanying Scantron to document your answers. Please leave all identifying information blank on the Scantron.
- 1. For Day 1 of your therapy, which of the following statements most accurately represents how you took your medication?
- A. I took both of my medication doses.
- B. I missed my first dose but took my second dose.
- C. I took my first dose but missed my second dose.
- D. I missed both doses.
- 2. For Day 1 of your therapy, which of the following statements most accurately represents how you took your medication with respect to wheat/gluten containing foods?
- A. For both doses, I did not have wheat/gluten containing foods within 2 hours before or 4 hours after.
- B. For my first dose, I had wheat/gluten containing foods within 2 hours before or 4 hours after but not for my second dose.
- C. For my second dose, I had wheat/gluten containing foods within 2 hours before or 4 hours after but not for my first dose.
- D. For both doses, I had wheat/gluten containing foods within 2 hours before or 4 hours after.
REFERENCES
- 1.Sabate E. World Health Organization. Geneva: World Health Organization; 2003. Adherence to long-term therapies: evidence for action. [Google Scholar]
- 2.Bubalo J, Clark RK, Jiing SS, et al. Medication adherence: pharmacist perspective. J Am Pharm Assoc. 2010;50(3):394–406. doi: 10.1331/JAPhA.2010.08180. [DOI] [PubMed] [Google Scholar]
- 3.Ho PM, Bryson CL, Runsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 4.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 5.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 6.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 7.Taitel M, Jiang J, Rudkin K, Ewing S, Duncan I. The impact of pharmacist face-to-face counseling to improve medication adherence among patients initiating statin therapy. Patient Prefer Adherence. 2012;6:323–329. doi: 10.2147/PPA.S29353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saranagam P, London MS, Snowden SS, et al. Impact of pharmacist discharge medication therapy counseling and disease state education: pharmacist assisting at routine medical discharge (Project PhARMD) Am J Med Qual. 2013;28(4):292–300. doi: 10.1177/1062860612461169. [DOI] [PubMed] [Google Scholar]
- 9.Script Your Future. National Consumers League. http://www.scriptyourfuture.org/our-partners. Accessed January 12, 2014.
- 10.Rickles NM, Garrelts L, Hess K, et al. Teaching medication adherence in US colleges and schools of pharmacy. Am J Pharm Educ. 2012;76(5) doi: 10.5688/ajpe76579. Article 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina MS, Plaza CM, Stowe CD, et al. Center for the Advancement of Pharmacy Education 2013 educational outcomes. Am J Pharm Educ. 2013;77(8) doi: 10.5688/ajpe778162. Article 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Accreditation standards and guidelines for the professional degree program leading to the doctor of pharmacy degree. Accreditation Council for Pharmacy Education. https://www.acpe-accredit.org/pdf/S2007Guidelines2.0_ChangesIdentifiedInRed.pdf. Accessed January 12, 2014.
- 13.Divine HS, Cain JC. Assessing the effect of a polypharmacy medication adherence simulation project in a geriatrics course in a college of pharmacy. J Am Geriatr Soc. 2009;57(8):1487–1491. doi: 10.1111/j.1532-5415.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor DM, Savageau JA, Centerbar DB, Wamback KN, Ingle JS, Lomerson NJ. Lesson in a pill box: teaching about the challenges of medication adherence. Fam Med. 2009;41(2):99–104. [PubMed] [Google Scholar]
- 15.Singla DL, MacKinnon GE, MacKinnon KJ, Younis W, Field B. Interdisciplinary approach to teaching medication adherence to pharmacy and osteopathic medical students. J Am Osteopath Assoc. 2004;104(3):127–132. [PubMed] [Google Scholar]
- 16.Ulbrich T, Hamer D, Lehotsky K. Second-year pharmacy students’ perceptions of adhering to a complex simulated medication regimen. Am J Pharm Educ. 2012;76(1) doi: 10.5688/ajpe76111. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton El, Transue ER, Comes SE, Paauw DS. Placebo HAART regimen as a method for teaching medication adherence issues to students. J Gen Intern Med. 2005;20(6):541–545. doi: 10.1111/j.1525-1497.2005.0096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slain D, Casdorph D, McIntire T. Assessment of an antiretroviral adherence sensitivity training exercise in the doctor of pharmacy curriculum. Am J Pharm Educ. 2002;66(Fall):277–280. [Google Scholar]
- 19.Darbishire PL, Plake KS, Kiersma ME, White JK. An introductory practice experience on improving medication adherence. Am J Pharm Educ. 2012;76(3) doi: 10.5688/ajpe76342. Article 42. [DOI] [PMC free article] [PubMed] [Google Scholar]