Abstract
Teratomas comprise a spectrum of tumours that have striking imaging appearances and are commonly considered when evaluating a mass in the female pelvis. A subgroup of these tumours located in an extragonadal abdominopelvic location, in contrast, are extremely rare and can affect both sexes. Extragonadal teratomas can occur at all ages, are particularly unusual in adults and can cause confusion in the differential diagnosis, especially in children. Familiarity with the imaging features of the spectrum of teratomas within the abdominal cavity is therefore of great importance, as radiological diagnosis can guide treatment, prevent delays in diagnosis and avoid sequelae. This article summarizes the radiological appearances of these rare extragonadal tumours in adults in relation to their pathology, malignant potential, location and behaviour. Although uncommon, teratomas should be considered in the differential diagnosis of extragonadal abdominal masses, particularly in young adults.
Teratomas are neoplasms with tissue components resembling normal derivatives of the three germ layers. They comprise a spectrum of tumours with striking imaging appearances and are commonly considered when evaluating pelvic masses in females. Extragonadal abdominopelvic teratomas are, in contrast, very rare and are seen in both sexes. They represent only 1–5% of germ cell tumours (GCTs)1 and therefore may not be readily considered by clinicians. Their radiological appearances are, however, characteristic, and familiarity with these features will lead to more accurate radiological diagnoses that not only guide treatment but also prevent avoidable complications from diagnostic delay.
Teratomas can present at any age. They contain tissue elements foreign to the organ or anatomic site of origin.2 Arising from totipotent germ cells, they contain derivatives of one to all three germ cell layers (the ectoderm, endoderm and mesoderm)2–4 and occur most frequently in the ovary or testis. Gonadal teratomas are well described; however, the extragonadal variants are under-represented in the literature. These encompass mature cystic teratomas (MCTs), which are most frequently tridermal lineage, and epidermoid cysts, which are frequently classified pathologically as monodermal teratomas (MTs) (Table 1).
Table 1.
The histopathological classification of teratomas
| Lineage | Tumour |
|---|---|
| Bi-/tridermal | Mature cystic/benign teratoma |
| Bi-/tridermal | Immature teratoma (containing immature neural/neuroectodermal tissue) |
| Bi-/tridermal | Malignant (teratoma plus malignant foci) |
| Monodermal | Epidermoid cyst |
| Monodermal | Struma ovarii |
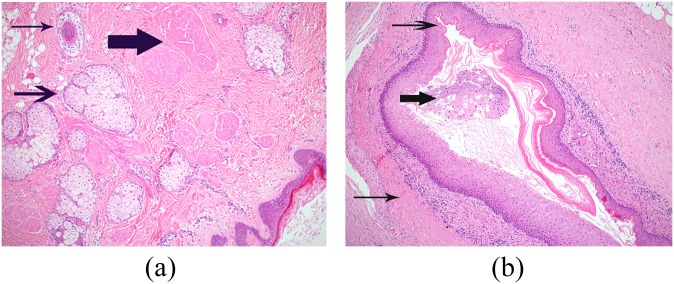
Histologically, both MCT (Figure 1a) and MT (Figure 1b) are lined by squamous epithelium but MTs lack skin appendageal structures. Postulated theories for the extragonadal location of teratomas include origin from displaced germ cells during embryologic migration,1 ectopic ovarian tissue and ovarian dermoids reimplanting in an extragonadal site.3
Figure 1.
(a) Photomicrograph of mature cystic teratoma with a fibrous wall lined by keratinizing stratified squamous epithelium (bottom right of field). The wall contains skin appendageal structures, including sebaceous follicles (arrow), a hair follicle (thin arrow) and arrector pili muscle bundles (thick arrow). (b) Photomicrograph of monodermal teratoma with a fibrous tissue wall of the cyst (thin arrow), lined by mature, keratinizing stratified squamous epithelium (arrow). Keratin flakes and keratinous debris are present within the lumen (thick arrow).
LOCATION
Extragonadal GCTs are a group of tumours that can be subdivided into seminomatous and non-seminomatous GCTs. This latter group is further divided into subtypes including embryonal cell carcinoma, choriocarcinoma, yolk sac tumour (YST), teratomas and mixed GCTs, and the latter can be a combination of any. These tumours typically arise in midline sites, although their frequency at specific sites (Table 2) varies with age. Within the abdomen and pelvis, the most common location is the sacrococcygeal region (Figure 2). These MCTs most frequently diagnosed in infancy, have an incidence of 1 per 30,000–43,000 live births4 and are the most common teratomas of childhood accounting for 40% of those in the first two decades.2 In adults, they occur most commonly in middle-aged females.4
Table 2.
The frequency of teratomas arising at different extragonadal anatomical locations. Adapted from Tapper and Lack2
| Anatomical location | Frequency (%) |
|---|---|
| Sacrococcygeal | 67.0 |
| Head and neck | 9.0 |
| Retroperitoneum | 8.0 |
| Mediastinum | 7.0 |
| Brain/spine | 6.0 |
| Liver | 1.5 |
| Abdominal wall/back | 1.5 |
Figure 2.
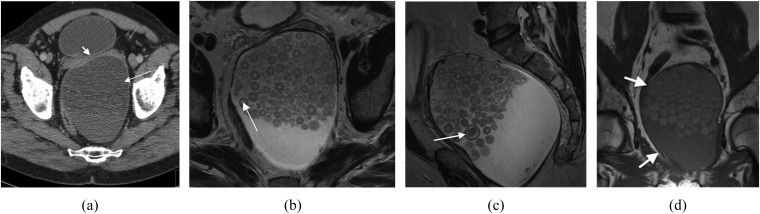
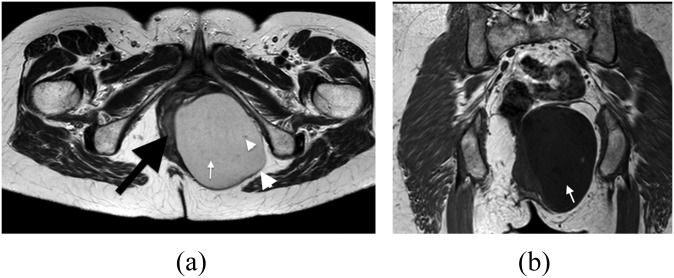
A 64-year-old male with a history of urinary frequency. This was histopathologically confirmed as a mature cystic teratoma unusually containing epithelial nests with a Pagetoid pattern of growth. (a) Axial contrast-enhanced CT shows a cystic pre-sacral mass (arrow) closely applied to the confluence of the seminal vesicles (arrow head). (b) Axial and (c) sagittal plane T2 weighted MR images showing the mass containing multifaceted layering densities (arrows). (d) Coronal T1 weighted MR image showing preserved fascial planes (arrows).
Retroperitoneal MCTs (Figure 3) are also more common in childhood accounting for <5% of all extragonadal GCTs.1 They may cause considerable diagnostic confusion, if thought to represent Wilms' tumours or neuroblastomas.2 These MCTs are rare in adults and are seen more frequently in females.
Figure 3.
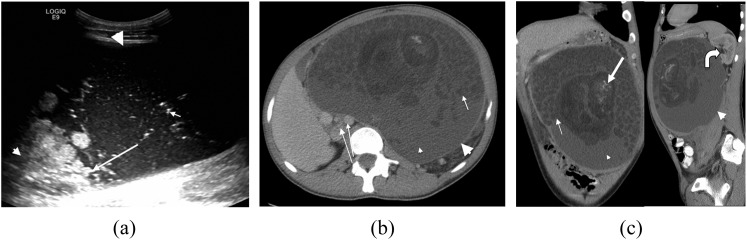
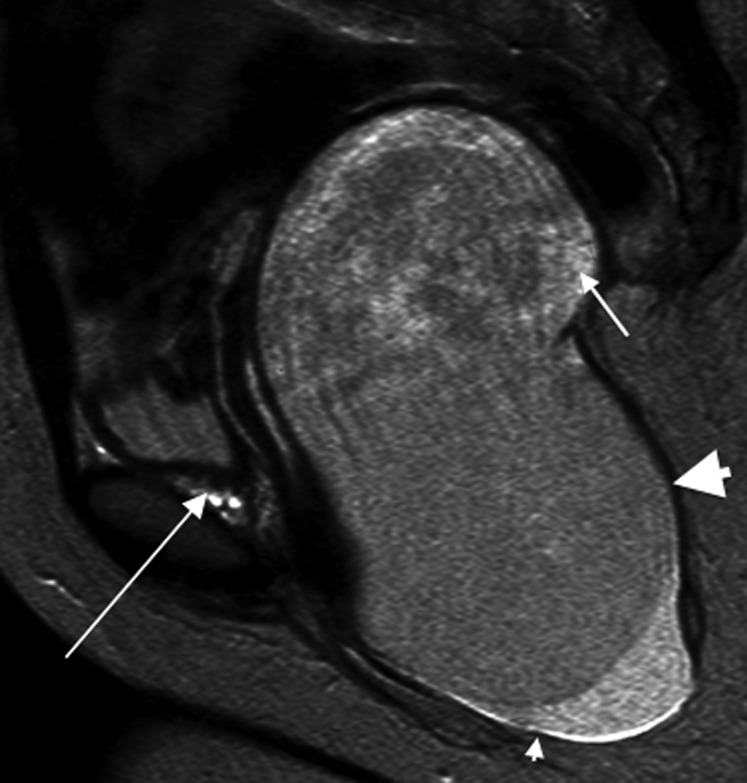
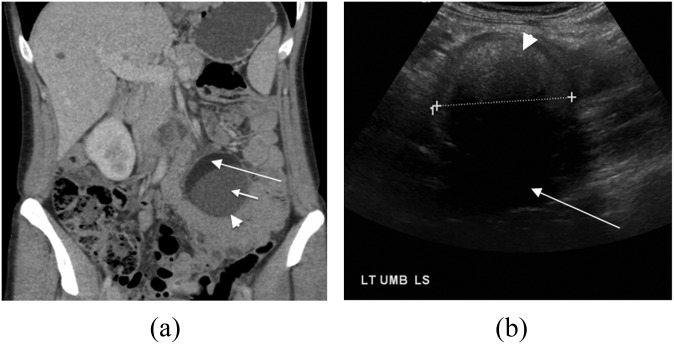
A 21-year-old male with a slowly growing abdominal mass histopathologically confirmed as a mature cystic teratoma (Figure 1a). (a) Ultrasound image showing the predominantly hypoechoic lesion (large arrowhead), containing areas of hyperechogenicity (long arrow), floating densities (short arrow) and acoustic shadowing (small arrowhead). (b) Axial and (c) coronal and sagittal axial contrast-enhanced CT images showing an encapsulated mass arising from the left retroperitoneum (large arrowheads), containing fluid (small arrowheads), multiple hypoattenuating spherules (short arrows) and areas of central calcification (thick arrow). The aorta and inferior vena cava (long thin arrows) are displaced to the right. The left kidney is displaced with prominence of the renal pelvis (curved arrow).
Teratomas arising in the liver and abdominal wall are rare, presenting most often as masses in newborns.2 Reported supradiaphragmatic sites include the mediastinum, brain and neck.
MTs of the abdomen and pelvis are rare, most frequently occurring in middle-aged females.5 They usually occur in the pre-sacral space5 (Figure 4) but can also arise in the ischiorectal space (Figure 5) and perineum (Figure 6). When found in the retroperitoneum, they usually arise from the spleen.
Figure 4.
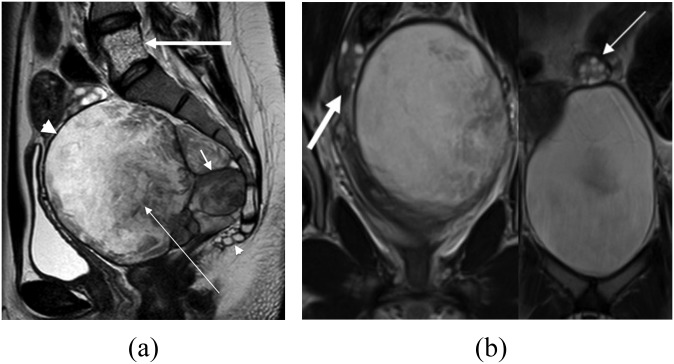
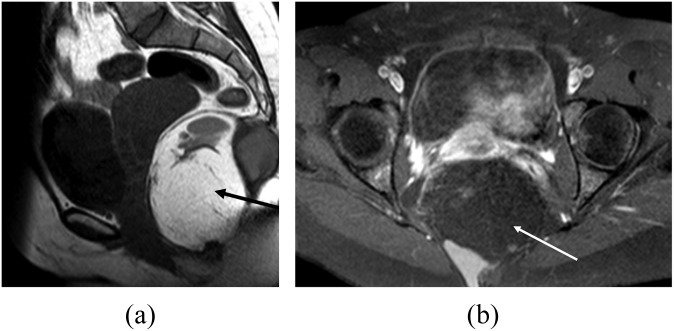
A 23-year-old asymptomatic female underwent investigation for polycystic ovarian syndrome. Histology confirmed a monodermal teratoma. (a) T2 weighted MR image in sagittal plane showing the lesion as a lobulated (short arrow) well-circumscribed (large arrowhead), complex and partly cystic mixed signal mass (thin arrow) anterior to the sacrum and coccyx (small arrowhead). Incidental note is made of a haemangioma within the vertebral body of L5 (thick arrow). (b) Coronal T2 weighted MR image showing the normal right (thick arrow) and left (thin arrow) ovaries.
Figure 5.
A 46-year-old female with a painful mass in her left buttock, which was confirmed histopathologically to be a monodermal teratoma. (a) Axial T2 weighted MR image showing a well-circumscribed ovoid mass arising from the left ischiorectal fossa (large arrowhead), separate from the uterus and ovaries and extending into the left hemipelvis. The rectum is displaced to the right (black arrow). The lesion contains complex myxoid and fluid morphology (white arrow) with small areas of inhomogeneity (small arrowhead). (b) Coronal T1 weighted MR image showing the low signal of the cystic mass (arrow).
Figure 6.
A 28-year-old female with an enlarging monodernal teratoma in the left buttock (histopathology shown in Figure 1b). Sagittal T2 weighted image showing a large smooth mass (large arrowhead) with areas of high signal secondary to fluid (short arrow), arising from the left perineum (small arrowhead) and extending into the left hemipelvis and left pelvic side wall. The lesion displaces the vagina and uterus to the right (not shown) and lies separately from the left ovary (long arrow).
Histologically, teratomas vary from well-differentiated benign lesions containing mature elements (MCTs) to immature tumours containing incompletely differentiated foetal-appearing tissues (often of neural or neuroectodermal differentiation).6 In pre-pubertal children, the immature elements within a teratoma are not associated with malignant behaviour; however, in adults, mediastinal immature teratomas can be aggressive with a poor prognosis.1,7
More rarely, teratomas may contain frankly malignant components of several tissue lineages. Generally, cystic teratomas are more likely to be benign and solid teratomas more likely to be malignant.5 However, benign mature teratomas can contain large solid components known as Rokitansky protruberances (RP). These are thickened areas within the cyst wall from which dermal appendages such as teeth, bone and hair may arise if present. It should be noted that immature teratomas without prominent solid components can occasionally be seen.6 Alpha-fetoprotein tumour markers can be of assistance in distinguishing malignancy but may be only raised in up to 50% of the cases.6
MALIGNANT TRANSFORMATION
Malignant transformation in MCT is rare and reported to be between 1% and 12.5% for sacrococcygeal lesions.4 The most common malignancies arising in teratomas are squamous carcinoma, carcinoid tumour and adenocarcinoma,4 with neuroendocrine carcinoma and extramammary Paget's disease also rarely described.4 Malignant change in MT is considered to be exceptionally rare.
PRESENTATION AND COMPLICATIONS
Extragonadal teratomas (EGTs) may present as incidental findings, as a mass with/without pain or with gastrointestinal or urinary symptoms. Complications such as haemorrhage, infection and rupture may occur, the latter possibly resulting in a chemical peritonitis.
IMAGING
MCTs have a number of characteristic imaging features. A plain film may reveal a soft tissue or fat density mass that can show rim calcification or teeth-like components. Although 74% of benign teratomas contain calcification, this can also occur in 25% of malignant teratomas.3
Ultrasound evaluation can reveal a complex (Figure 3a), anechoic or hyperechoic mass due to fluid, fat, hair and calcification. Hyperechoic mural nodules may be seen projecting from the cyst wall or may demonstrate acoustic shadowing (Figure 7b).8
Figure 7.
A 42-year old female with a palpable abdominal mass, confirmed as a mature cystic teratoma. (a) Coronal axial contrast-enhanced CT showing a lesion within the mesentery of the small bowel (arrowhead), which is of mixed fat, fluid (small arrow) and soft tissue attenuation. Note the layering of fat (long arrow). (b) Ultrasound image showing a lesion of hyper-/iso-echogenicity (arrowhead) with acoustic shadowing (arrow).
CT imaging of MCT often reveals a smooth thin-walled unilocular lesion containing solid RP. These foci arise from the wall and may have palm tree-like appearance. They may also contain fluid, fat and calcification in any combination (Figures 3b,c and 7a). The central cavity may contain hypoattenuating fluid (Figure 7a) possibly with multiple low-attenuation spheres or “marbles” (Figure 2a), thought to consist non-dependent fat density keratin,4 which may form secondary to hypovascularity. The floating spherules in this series are often poorly defined, and although of low attenuation do not demonstrate a consistently negative Hounsfield Unit as would typically be seen in fat, and are often poorly defined. There may be a fat/fluid level (Figure 7a) or heterogeneity due to differing tumour components (Figure 3c).
MRI of MCTs show high T1 weighted signal intensity depending on the proportion of fat within the tumour. They may be variable or hyperintense on T2 weighted imaging (T2WI) (Figure 2b,c). Chemical shift between the fatty and watery content is a characteristic finding on MR (Figure 8a,b).6 Other features include dependent layering, palm tree-like RP or frond-like RP, and fat fluid levels. A thickened wall with an irregular margin may be a sign of malignant transformation.6 Mobile spherules if present are seen more distinctly than on CT (Figure 2b,c). They are non-dependent, layering and faceted in their appearance. The cases presented in this series demonstrate that they are very variable in the extent of their fatty signal. Immature teratomas contain immature neural tissue in variable quantities that are usually seen as solid parts.6
Figure 8.
Characteristic fat suppression of a mature cystic teratoma (MCT). (a) Sagittal T1 weighted MR image showing the high signal fat within this sacrococcygeal MCT (black arrow). (b) Axial fat-saturated T1 weighted MR image showing corresponding loss of signal within the mass on fat suppression (white arrow).
Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) values in benign teratomas may show higher signal intensity on DWI and lower ADC values than do other benign and malignant neoplasms.8 This is thought to be due to keratin and solid RP, making them unreliable in distinguishing benign from malignant teratomas.9
The plain film appearance of MT can be a non-calcified mass. With ultrasonography, they frequently appear as a hypoechogenic mass with slight posterior enhancement. Heterogeneity within the mass, if present, may be explained by keratin.10 On CT, they are thin-walled cystic masses of homogenous fluid attenuation, and in our experience, without enhancement. The ultrasound and CT appearances are not specific enough to differentiate them from other cystic masses.5 On MRI, they tend to follow the signal intensity of fluid being hypointense on T1 weighted imaging (Figure 5b) and hyperintense on T2WI (Figures 4, 5a and 6) but may show hypointense foci on T2WI, possibly secondary to keratin.10
Positron emission tomography CT can help to distinguish malignant teratomas in the pre-treatment work-up. After treatment, some patients may have residual masses needing characterization. Although residual viable tumours show intense fludeoxyglucose uptake when compared with mature teratomas, it is difficult to differentiate residual MCT from necrosis or scar.11
As a general over view, ultrasound is used most often to initially highlight a mass and define its relative location. CT provides greater information about the location, nature, staging and the effect of the lesion on surrounding organs. MR is superior in evaluating the constituents of the mass. It provides fine detail on its relationship to adjacent fascial planes, which is of particular relevance for surgery.
MANAGEMENT
Differentiation between benign and malignant lesions using clinical examination, imaging and tumour markers guides the treatment of EGT. Imaging features suggestive of malignancy include a greater proportion of solid components within the mass, a thickened irregular wall, extension into adjacent tissue planes, local/regional lymphadenopathy and metastases. Histopathological confirmation is the gold standard, and the treatment of mature and immature teratomas is predominantly surgical. The prognosis is excellent for benign teratomas with complete resection,3,6 with no additional therapy being required.
In cases of malignant teratoma, treatment with cisplatin, etoposide and bleomycin is advocated. There is a paucity of data available in the literature regarding event-free survival (EFS) and overall survival (OS) in relation to the resection of teratomas. However, the UK Children's cancer study group found that even in incomplete resection of mature tetatomas, the 5-year EFS was 92% and the OS rate was 99%. Incomplete resection of immature teratoma resulted in a 5-year EFS of 85.9% and an OS of 95.1%.12 Recurrence can be seen in benign or malignant teratomas, the latter mostly YST in children.12
CONCLUSION
Abdominopelvic extragonadal teratomas are very rare in adults but have characteristic radiology. Despite these features, owing to their rarity they can cause diagnostic confusion. Histopathological confirmation is advised before treatment, which is predominantly surgical. Familiarity with their radiological appearances is therefore necessary, and they should be considered when evaluating an abdominopelvic mass, particularly in the young adult.
REFERENCES
- 1.McKenney JK, Heerema-McKenney A, Rouse RV. Extragonadal germ cell tumours: a review with emphasis on pathologic features, clinical prognostic variables and differential diagnostic considerations. Adv Anat Pathol 2007; 14: 69–92. [DOI] [PubMed] [Google Scholar]
- 2.Tapper D, Lack EE. Teratomas in infancy and childhood. A 54-year experience at the Children’s Hospital Medical Center. Ann Surg 1983; 198: 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett CE, Khan A, Pisal N. Parasitic dermoid cyst managed laparoscopically in a 29-year-old woman: a case report. J Med Case Rep 2009; 3: 63. doi: 10.1186/1752-1947-3-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thway K, Polson A, Pope R, Thomas JM, Fisher C. Extramammary Paget disease in a retrorectal dermoid cyst: report of a unique case. Am J Surg Pathol 2008; 32: 635–9. doi: 10.1097/PAS.0b013e318158427b [DOI] [PubMed] [Google Scholar]
- 5.Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH, et al. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics 2004; 24: 1353–65. doi: 10.1148/rg.245045017 [DOI] [PubMed] [Google Scholar]
- 6.Ueno T, Tanaka YO, Nagata M, Tsunoda H, Anno I, Ishikawa S, et al. Spectrum of germ cell tumors: from head to toe. Radiographics 2004; 24: 387–404. [DOI] [PubMed] [Google Scholar]
- 7.Marina N, London WB, Frazier AL, Lauer S, Rescorla F, Cushing B, et al. Prognostic factors in children with extragonadal malignant germ cell tumors: a paediatric intergroup study. J Clin Oncol 2006; 24: 2544–8. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama T, Yoshimitsu K, Irie H, Aibe H, Tajima T, Nishie A, et al. Diffusion-weighted echo-planar MR imaging and ADC mapping in the differential diagnosis of ovarian cystic masses: usefulness of detecting keratinoid substances in mature cystic teratomas. J Magn Reson Imaging 2005; 22: 271–8. [DOI] [PubMed] [Google Scholar]
- 9.Fujii S, Kakite S, Nishihara K, Kanasaki Y, Harada T, Kigawa J, et al. Diagnostic accuracy of diffusion-weighted imaging in differentiating benign from malignant ovarian lesions. J Magn Reson Imaging 2008; 28: 1149–56. doi: 10.1002/jmri.21575 [DOI] [PubMed] [Google Scholar]
- 10.Yang DM, Yoon MH, Kim HS, Oh YH, Ha SY, Oh JH, et al. Presacral epidermoid cyst: imaging findings with histopathologic correlation. Abdom Imaging 2001; 26: 79–82. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara Y, Zasadny KR, Grossman HB, Francis IR, Clarke MF, Wahl RL. Germ cell tumor: differentiation of viable tumor, mature teratoma and necrotic tissue with FDG PET and kinetic modeling. Radiology 1999; 211: 249–56. [DOI] [PubMed] [Google Scholar]
- 12.Mann JR, Gray ES, Thornton C, Raafat F, Robinson K, Collins GS, et al. Mature and immature extracranial teratomas in children: the UK Children’s Cancer Study Group Experience. J Clin Oncol 2008; 26: 3590–7. [DOI] [PubMed] [Google Scholar]