Abstract
Objective:
The second tumour (ST) occurrence is a relatively uncommon late complication of radiotherapy but represents one of the most significant issues, especially in childhood oncology. We describe our experience with patients who developed second brain neoplasm following cranial irradiation in childhood.
Methods:
We identified nine patients who received radiotherapy owing to central nervous system tumour in childhood and subsequently developed the second brain tumour. The full clinical and radiological documentation and histopathological reports were reviewed. Risk factors such as age at irradiation, latency period to ST diagnosis, radiotherapy doses and volumes and other therapy methods were evaluated. We correlated the ST location with the three levels of irradiation dose (high, >40 Gy; medium, 25–40 Gy; and low <25 Gy).
Results:
Five meningiomas and four gliomas occurred as the ST after the mean time of 11.7 years after radiotherapy. The average age of children during irradiation was 4.6 years. The shorter latency time to the ST induction was found in children treated with chemotherapy (9 years vs 17.2 years). Seven STs developed in the area of high and moderate dose (>25 Gy), only two low-grade gliomas appeared in the low-dose region.
Conclusion:
Our data suggest that the STs usually develop in the brain tissues that received doses >25 Gy in patients irradiated at a young age.
Advances in knowledge:
The low-dose volume seems not to be so significant for second brain neoplasm induction. Therefore, the modern intensity-modulated radiotherapy technique could be safely applied in paediatric patients.
The second tumour (ST) occurrence is a multifactorial event, depending on the factors associated with therapy and with the clinical characteristics of the patient, such as age or genetic predisposition.1–5 The cranial irradiation, especially the radiation dose and the field size, is known as the significant risk factor for developing second brain neoplasms.1,6–11 However, details of this relationship are still not clear. The mechanisms of the tumour induction are complex.2 The radio-induced tumour is defined by many authors as a new mass, histologically different from the original tumour, occurring after delay in irradiated areas, and not related to phacomatosis.6,9,10 This late complication of radiotherapy is not common but is especially important in young patients with a long life expectancy. Among factors that limit better understanding of ST's aetiology is the lack of long-term follow-up that is often interrupted when irradiated children become adults. In this study, a single institution's experience was presented with nine second brain tumours that appeared following central nervous system (CNS) radiotherapy in childhood.
METHODS AND MATERIALS
In the period between 1980 and 2008, 1404 children underwent CNS irradiation in the Radiotherapy Department Maria Sklodowska-Curie Memorial Cancer Center (MSCMCC) in Warsaw, Poland. Unfortunately, we have no information on the completion of the follow-up for the whole group and because of that this group was not used in any analysis. From 1997 to 2013, among patients mentioned above, we identified 11 second brain tumours. To select these patients, the following criteria were used: more than a 2-year period between the initial irradiation and the ST detection, and histological difference between primary and second neoplasm. Two patients were excluded from the further evaluation owing to the presence of neurofibromatosis Type 2 that could pre-dispose neoplasm development. We limited our study to the brain location of STs, bypassing other locations so that the group was homogenous enough to be able to draw some conclusions. All the available records for each patient were reviewed, especially clinical documentation, radiographic examinations and histological reports.
Children's mean age at primary CNS radiotherapy was 4.6 years (range, 1–12 years). Initial diagnoses were four cases of ependymoma, and one medulloblastoma, meningosarcoma (meningioma meningotheliale after further consultation), high-grade glioma, primitive neuroectodermal tumour and atypical teratoid rhabdoid tumour. All children had surgical resection, and six of them received chemotherapy before irradiation. Patients were irradiated according to the standards of the era in which they were diagnosed. Therefore, most of them underwent the two-dimensional planning with the dose prescribed to the midplane of the cranial fields. Seven children received the cranial or craniospinal irradiation with boost to the tumour bed in doses for the brain in the range 25–40 Gy (mean, 33.6 Gy) and for the tumour bed 45–55 Gy. The other two children were irradiated for the tumour bed only up to the dose of 45 and 54 Gy, respectively.
The latency period was defined as the time from the end of radiation therapy to the ST diagnosis. The dose of delivered radiotherapy was estimated on the basis of our archival documentation: port radiographs and irradiation plans. Doses were divided into three levels: high-dose region (>40 Gy) corresponded to the tumour bed dose, medium from 25 to 40 Gy that was generally associated with elective brain dose and the third region of low dose <25 Gy applied outside tumour bed in patients irradiated for this area only. The correlation between the ST location and the irradiation dose was classified based on diagnostic MRI and radiotherapy documentation. The assessment examples are shown in Figures 1 and 2.
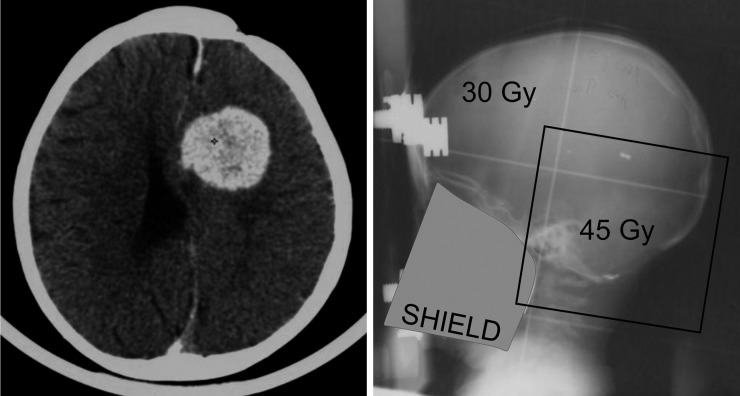
Figure 1.
Second primary meningioma outside the boost field/in the brain field (the medium-dose area).
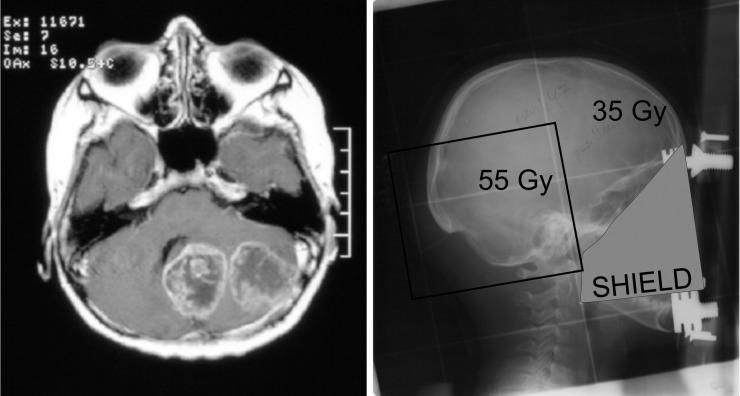
Figure 2.
Second primary glioma in the boost field (the high-dose area).
The overall survival (OS) after the second neoplasm diagnosis was estimated with the Kaplan–Meier product-limit method.12
RESULTS
All patients gained remission after the primary therapy and did not experience recurrence until developing the new tumour. The mean interval between the initial radiotherapy and the ST diagnosis was 11.7 years (range, 2.9–20.0 years). This latency time was longer when irradiation was preceded only by surgery and amounted 17.2 years vs 9.0 years when additional chemotherapy was administered. All STs were diagnosed histopathologically. There were five meningiomas and four gliomas.
Meningioma
Meningiomas were diagnosed at the mean patient age of 18.5 years (range, 10–32 years) with the average time of 13 years (range, 5.2–20.0 years) after the primary CNS irradiation. Only one meningioma appeared during a time period shorter than 10 years. Initially, all children received cranial or craniospinal radiotherapy at the mean age of 5.2 years (range, 1.3–12.0 years). All cases of second meningiomas developed in the medium- or high-dose areas in range from 25 to 55 Gy, but only one anaplastic tumour emerged in full-dose region. The surgical treatment of STs was performed in all patients. Additionally, the patient with atypical meningioma received chemotherapy, and in the other one with anaplastic neoplasm, radiosurgery was applied. All are alive with the average follow-up of 10.4 years after ST diagnosis (range, 2–16 years). In the patient with anaplastic tumour, the subsequent meningioma developed in another location, 9 months after ST diagnosis. Currently, he has reached complete remission after resection. New meningiomas also occured in Patient 1 but they were not resected because of the lack of symptoms and the stable radiological image.
Glioma
For the four patients who were diagnosed with gliomas as the second neoplasms, the mean age was 14.1 years (range, 6.2–25.7 years) with the average interval of 10 years (range, 2.9–17.0 years) after radiotherapy.
High-grade glioma
Two high-grade gliomas developed in the full-dose area (55 Gy) in children irradiated at the craniospinal fields with boost to the tumour bed. In these patients, the average latency period from irradiation was 11 years, and the mean age at radiotherapy was 5.7 years. The surgical treatment, reirradiation and chemotherapy (temozolomid) were administered owing to a second high-grade glioma diagnosis. One patient died after 2.7 years and the other one has currently been in complete remission with a follow-up of 6 months after ST diagnosis.
Low-grade glioma
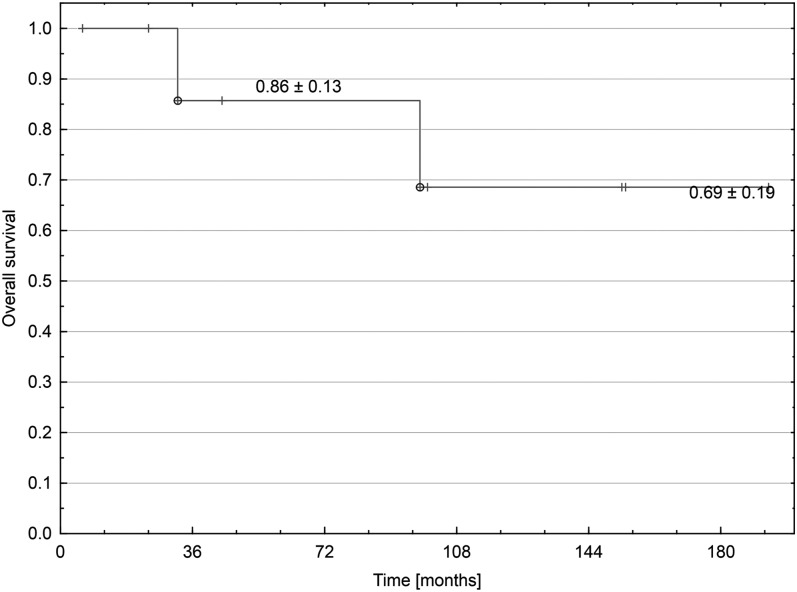
Two cases of low-grade gliomas occurred in the low-dose area (<25 Gy) in patients irradiated to the tumour bed only at an average age of 2.1 years. STs developed after mean time of 9 years after radiotherapy. One patient has currently been in complete remission after the tumour resection, with a follow-up of 3.7 years since ST diagnosis. The other one died because of a third neoplasm (spindle cell synovial sarcoma) that developed in the same location as glioma 7 years after its resection. The patient clinical characteristics and treatment outcome are described in Table 1. Actuarial plot of OS after ST diagnosis is shown in Figure 3.
Table 1.
Patient characteristics
| Patient | Primary tumour |
Second tumour |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at RT (years) | Histology/location | RT: dose (Gy)/target | Other therapy | Age at diagnosis (years) | Histology/location | Time RT to ST (years) | Dose level | Outcome/follow-up from ST (years) | |
| 1 | 1.3 | Medulloblastoma posterior fossa | 35/brain 29/spine 45/tumour bed |
Surgery chth |
13.7 | Meningioma meningotheliale/suprasellar | 12.2 | High | New meningiomas/16.0 |
| 2 | 1.0 | Meningosarcoma hemisphere right |
45/tumour bed |
Surgery chth |
16.2 | 1. Glioma GI/frontal lobe left 2. Spindle cell synovial sarcoma/frontal lobe left |
15.2 | Low | Death/8.2 |
| 3 | 6.3 | Glioma GIV parietal lobe |
40/brain 30/spine 55/tumour bed |
Surgery | 21.0 | Meningioma transitionale/frontal lobe left | 14.5 | High | NED/12.7 |
| 4 | 8.5 | Ependymoma GIII posterior fossa |
35/CNS 55/tumour bed |
Surgery | 25.7 | Glioma GIV/posterior fossa | 17.0 | High | Death/2.7 |
| 5 | 12.0 | Ependymoma GII hemisphere left |
35/brain 55/tumour bed |
Surgery | 32.0 | Meningioma anaplasticum/hemisphere left | 20.0 | High | NED/2.0 |
| 6 | 1.8 | Ependymoma posterior fossa |
30/CNS 45/tumour bed |
Surgery chth |
15.6 | Meningioma GI/parietal lobe right | 13.5 | Medium | NED/8.3 |
| 7 | 4.5 | PNET hemisphere left |
35/CNS 55/tumour bed |
Surgery chth |
10.0 | Meningioma atypicum/frontal lobe left | 5.2 | High | NED/12.8 |
| 8 | 3.2 | Ependymoma GII/III posterior fossa |
54/tumour bed |
Surgery chth |
6.2 | Glioma GII/frontal lobe right | 2.9 | Low | NED/3.7 |
| 9 | 3.0 | ATRT hemisphere right |
25/CNS 55/tumour bed |
Surgery chth |
8.2 | Glioma GIII/IV/hemisphere right | 5.0 | High | NED/0.5 |
ATRT, atypical teratoid rhabdoid tumour; chth, chemotherapy; CNS, central nervous system; G, grade; NED, no evidence of disease; PNET, primitive neuroectodermal tumour; RT, radiotherapy; ST, second tumour.
Figure 3.
Overall survival since the second tumour diagnosis.
DISCUSSION
Primary CNS tumours represent the second most common group of neoplasms in childhood. Radiotherapy is one of the most important treatment methods but is connected with serious late complications. Neglia et al1 in a large study concluded that the exposure to the radiation therapy is the essential factor for a new CNS tumour development in survivors of the childhood cancers. Similarly, Inskip and Curtis5 stated that the risk of subsequent solid cancers was higher among persons whose initial treatment for childhood cancer included radiotherapy. The expected incidence to develop a second neoplasm in children with CNS malignancy is 0.2% as compared with the incidence of 1.4% observed after treatment.5 Therefore, it is vital to develop the criteria for the reduction of the risk of radio-induced cancers by the optimization of the treatment planning process. As stated in Tubiana2 review, the aim of the treatment should be to deliver the minimal effective radiation therapy rather than maximal tolerable dose.
In our country, the incidence of STs in children is poorly documented. It is mainly because of their long latency period extending into adulthood. During this time, a lot of patients have changed their medical centre, and their follow-up information has been lost. Because of that, in our analysis, the evaluation of the cumulative incidence of STs could not be performed. This little group did not let us to conduct a statistical analysis of risk factors. Only the preliminary observations and hypothesis are presented.
The linear relationship between the radiation dose and the ST induction in the low-dose region was demonstrated in a large study about atomic bomb survivors.13 However, these results were not confirmed in clinical studies, which suggested that the risk of carcinogenesis does not decrease at the high-dose regions. As stated in the main reviews, most of the secondary tumours emerge close to the irradiated fields in the high- and medium-dose zones.1,6,9,10,14 Diallo et al11 stated that in a cohort of 115 STs, 12% of them were found in the central area of the irradiated volume and 66% in the beam-bordering region. In the Galloway analysis, the dose to the ST site was usually in the range from 20 to 36 Gy.10 In our study, only two from nine second tumours occurred in the low-dose region (<25 Gy). The three high-grade neoplasms developed in the primary tumour bed boost field and the four others in the dose area >25 Gy.
There is no clear correlation between the radiotherapy dose and the malignancy of the ST; however, a high-dose irradiation was performed in most of the high-grade gliomas and meningiomas of survivors.9 In our experience, all three cases of high-grade tumours developed in the region that received a dose of 55 Gy.
The importance of patient age during radiation therapy is emphasized by all available studies about the STs.1,3,14 Tubiana2 in a large review highlighted that the risk of radio-induced neoplasms decreases with age. It could be explained by the higher number of stem cells in tissues at young age, their high proliferative rate and the promotion by growth hormones.2 In our group, six of the nine STs developed in children irradiated at <5 years of age.
The diagnosis of radiation-induced meningioma is difficult and sometimes controversial because it is not distinguishable from de novo meningioma, which is the common brain tumour in adults.8 The relationship between the irradiation and meningiomas could be explained by the high radiosensitivity of meninges, especially in children. Galloway et al10,15 in their analysis demonstrated that more than half of the second brain tumours were meningiomas and in the other study concluded that radiation-induced meningioma often occurred long after the primary therapy and could be successfully treated, with a 5-year OS rate of 89%. Similar conclusions were formulated by Vinchon et al6 in a longitudinal study about the radiation-induced tumours in children. In our study, meningiomas were also the most common STs, developing after a longer period time than gliomas (13 vs 10 years) in the moderate- and high-dose regions (>25 Gy). In the mean follow-up of 10.4 years, all patients with second meningiomas were alive. In most cases, surgery was sufficient therapy.
Although our ability to make conclusions about radiation-induced neoplasms is limited by a small sample size, we observed that chemotherapy could shorten the time to the ST development. All cases of second malignancies, which occurred in children who had not received chemotherapy, needed over 10 years (mean, 17.2 years) to develop, contrary to the remaining patients (mean, 9 years). The radiation might have synergistic interactions with chemotherapeutic agents in the new neoplasm induction, and patients treated with chemotherapy had a trend towards shorter intervals before developing ST.14 Duffner et al16 conducted a study in the Pediatric Oncology Group in which 198 children younger than 3 years of age with brain neoplasms were treated with prolonged post-operative chemotherapy in order to delay radiotherapy. Five patients developed second malignancies including two solid tumours with a cumulative risk at 8 years of 11.3%. The potential causative factors for this high rate include the prolonged use of alkylating agents and etoposide.16 Paediatric neuro-oncologists have generally concentrated on the long-term toxicity of irradiation, whereas some subsequent malignancies are strongly associated with chemotherapy.5 We should modernize our radiotherapy techniques in the treatment of paediatric patients to minimize serious late effects such as the STs. In some cases, the replacement of X-ray by protons could result in a reduced volume of normal tissue exposure, with a consequent reduction in the incidence of radio-induced neoplasms especially in low-dose region.17 However, the risk of ST occurrence in high-dose area may seem to remain the same independent of the kind of irradiation beam.
Our data suggest that the radiation dose, target volume, chemotherapy addition and young age at the time of radiotherapy should be involved in the development the second primary brain tumour. The low-dose volume seems to be not as significant for second brain neoplasm induction as we previously thought. Therefore, the fear of applying the new intensity-modulated radiotherapy technique is not reasonable. Modern radiotherapy minimizes the irradiated tissue volume, especially in the region of high and moderate doses. More data from different centres are required to exactly define the relationship between initial therapy and the induction of new tumours. The incidence of ST could be estimated only by long-term studies.
REFERENCES
- 1.Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2006; 98: 1528–37. [DOI] [PubMed] [Google Scholar]
- 2.Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol 2009; 91: 4–15; discussion 1–3. doi: 10.1016/j.radonc.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 3.Broniscer A, Ke W, Fuller CE, Wu J, Gajjar A, Kun LE. Second neoplasms in pediatric patients with primary central nervous system tumors: the St. Jude Children’s Research Hospital experience. Cancer 2004; 100: 2246–52. [DOI] [PubMed] [Google Scholar]
- 4.Little MP, de Vathaire F, Shamsaldin A, Oberlin O, Campell S, Grimaud E, et al. Risks of brain tumour following treatment for cancer in childhood: modification by genetic factors, radiotherapy and chemotherapy. Int J Cancer 1998; 78: 269–75. [DOI] [PubMed] [Google Scholar]
- 5.Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973-2002. Int J Cancer 2007; 121: 2233–40. doi: 10.1002/ijc.22827 [DOI] [PubMed] [Google Scholar]
- 6.Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst 2011; 27: 445–53. doi: 10.1007/s00381-011-1390-4 [DOI] [PubMed] [Google Scholar]
- 7.Salminen E, Pukkala E, Teppo L. Second cancers in patients with brain tumors—impact of treatment. Eur J Cancer 1999; 35: 102–5. [DOI] [PubMed] [Google Scholar]
- 8.Müller HL, Gebhardt U, Warmuth-Metz M, Pietsch T, Sörensen N, Kortmann RD. Meningioma as second malignant neoplasm after oncological treatment during childhood. Strahlenther Onkol 2012; 188: 438–41. [DOI] [PubMed] [Google Scholar]
- 9.Pettorini BL, Park YS, Caldarelli M, Massimi L, Tamburrini G, Di Rocco C. Radiation-induced brain tumors after central nervous system irradiation in childhood: a review. Childs Nerv Syst 2008; 24: 793–805. [DOI] [PubMed] [Google Scholar]
- 10.Galloway TJ, Indelicato DJ, Amdur RJ, Morris CG, Swanson EL, Marcus RB. Analysis of dose at the site of second tumor formation after radiotherapy to the central nervous system. Int J Radiat Oncol Biol Phys 2012; 82: 90–4. doi: 10.1016/j.ijrobp.2010.10.062 [DOI] [PubMed] [Google Scholar]
- 11.Diallo I, Haddy N, Adjadj E, Samand A, Quiniou E, Chavaudra J, et al. Frequency distribution of second solid cancer locations in relation to the irradiated volume among 115 patients treated for childhood cancer. Int Radiat Oncol Biol Phys 2009; 74: 876–83. doi: 10.1016/j.ijrobp.2009.01.040 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 13.Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst 2002; 94: 1555–63. [DOI] [PubMed] [Google Scholar]
- 14.You SH, Lyu CJ, Kim DS, Suh CO. Second primary brain tumors following cranial irradiation for pediatric solid brain tumors. Childs Nerv Syst 2013; 29: 1865–70. doi: 10.1007/s00381-013-2098-4 [DOI] [PubMed] [Google Scholar]
- 15.Galloway TJ, Indelicato DJ, Amdur RJ, Swanson EL, Morris CG, Marcus RB. Favorable outcomes of pediatric patients treated with radiotherapy to the central nervous system who develop radiation-induced meningiomas. Int J Radiat Oncol Biol Phys 2011; 79: 117–20. [DOI] [PubMed] [Google Scholar]
- 16.Duffner PK, Krischer JP, Horowitz MC, Cohen ME, Burger PC, Friedman HS, et al. Second malignancies in young children with primary brain tumors following treatment with prolonged postoperative chemotherapy and delayed irradiation: POG study. Ann Neurol 1998; 44: 313–16. [DOI] [PubMed] [Google Scholar]
- 17.Hall EJ, Phill D. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int Radiat Oncol Biol Phys 2006; 65: 1–7. [DOI] [PubMed] [Google Scholar]