Abstract
Objective:
Evaluation of the efficacy and toxicity of split-course accelerated hyperfractionated irradiation (CHA-CHA) as a sole treatment for advanced head and neck (H&N) cancer patients.
Methods:
We enrolled 101 patients (39 in CHA-CHA and 37 in conventional (Conv.) arm completed the treatment). The CHA-CHA arm patients were irradiated twice a day, 7 days a week, using a fraction dose (fd) of 1.6 Gy up to 64 Gy with an 8-day gap in midterm. Patients in the control (Conv.) arm group were irradiated with a fd of 2 Gy, five times a week to a total dose of 72–74 Gy in the overall treatment time of 50–53 days. Quality of life (QOL) and acute mucosal reaction were evaluated during radiotherapy (RT). After RT, we followed the effect of treatment, QOL, performance status and adverse effects of radiation. For statistical analysis mainly a hierarchical multilevel modelling was used.
Results:
QOL was most deteriorated in the CHA-CHA arm; the CHA-CHA scheme also caused a relatively stronger acute injury. There were no significant differences in late adverse effects. In the CHA-CHA arm in 35% and in Conv. arm in 30% of patients, disease was controlled during follow-up. Tumour regression 1 year after the treatment was significantly better in the CHA-CHA arm. However, the overall survival rate analysis did not show significant difference between both arms.
Conclusion:
Despite differences in treatment results, we cannot conclude that split-course accelerated hyperfractionated irradiation is superior to conventionally fractionated RT as a sole treatment for advanced H&N cancer patients.
Advances in knowledge:
Obtained results in the context of published data support the statement that altered fractionations alone do not give an advantage for advanced H&N cancer patients.
There are, and have been, many attempts to create a clear and effective treatment modality for advanced inoperable head and neck (H&N) cancers. The most common are different combinations of chemoradiotherapy, or different schedules of altered and usually intensive radiation treatment. It is clear that intense dose delivery combined with a high total dose (TD) and short treatment time gives a higher probability of tumour destruction; unfortunately, it is also connected with a higher, usually unacceptable, risk of normal tissue damage. One of the causes of radiation toxicity in such cases is the inability of sufficient repair of healthy tissue damage.
The reason for this is the intensity of dose delivery and the lack of time for repopulation of normal cells and proper repair. On the other hand, the accelerated repopulation of squamous cancer cells starts 4 weeks after radiotherapy (RT); therefore, radiation treatment should be completed in that time. Considering the aforementioned facts, we tried to construct a very intense and short RT schedule allowing for normal tissue (mainly mucosa) repair. We went back to the old concept of split-course RT and combined it with accelerated, continuous, intense hyperfractionation.
Finally, we proposed twice-a-day irradiation, using fraction doses of 1.6 Gy, 7 days a week up to a TD of 32 Gy, an 8-day gap and the repetition of such course. Using this schedule, the treatment was completed exactly within 4 weeks to avoid repopulation of accelerated tumour clonogens and the 8-day gap in the midterm allowed for normal tissue repair.
PURPOSE
The aim of this study was to evaluate the efficacy and toxicity of split-course accelerated hyperfractionated irradiation (CHA-CHA) as a sole treatment for advanced H&N cancer patients.
METHODS AND MATERIALS
Material
Phase III of the trial was accepted by the local ethical committee on 31 January 2003.
Into the study patients were enrolled suffering from advanced squamous cell H&N cancers (T2N3, T3N03 and T4N0-3), excluding nasopharyngeal cancers. All patients started RT between March 2003 and September 2009. The collection of data was completed in December 2012.
This trial was designed in 1998, when it was not so clear as to whether combined radiochemotherapy was the best treatment modality for advanced H&N cancer patients. We recruited, for this trial, addicted patients (heavy drinkers, heavy smokers) who (owing to their habits) were not the most appropriate candidates for combined treatment; therefore, we considered that altered fractionation alone as the only possibility of radical treatment.
In the protocol of this study, we calculated the number of patients who could be recruited on the basis of epidemiological data. During that time about 500 new pharynx, larynx and oral cavity cancer patients per year were reported in our region. On the basis of our department's experience (Radiotherapy Department, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch, Gliwice, Poland), we knew that about 15 of them, who had severe addictions and were in the advanced stage of disease, but who were still suitable for potentially radical treatment, would be referred to our department. We calculated that during 5 years we could collect 75 patients; however, owing to the high dropout rate during the enrollment time, we decided to prolong this period to 6 years to complete this study.
101 patients (78 males and 23 females) suffering from advanced H&N cancer were enrolled into the study. Their age varied from 42 to 73 years (mean, 57 years). 91 patients had a good performance status (PS—Zubrod scale), 9 had a PS 2 and 1 had a PS 3. The locations of cancers are presented in Table 1.
Table 1.
Locations of cancers in the whole randomized group of patients
| Location | Larynx | Tongue—mobile part | Oropharynx | Palate tonsil | Hypopharynx | Floor of the mouth | Buccal mucosa | Base of the tongue |
|---|---|---|---|---|---|---|---|---|
| Number | 26 | 20 | 19 | 17 | 9 | 6 | 3 | 1 |
87 patients had T4; 12, T3; and 2, T2 stage of disease. 28 patients had N3; 58, N2 (35, N2c; 12, N2b; 4, N2a); 12, N1; and 3, N0 nodal status.
In 47 cases, histopathological diagnosis was described as carcinoma planoepitheliale, in 33 as carcinoma planoepitheliale keratodes and in 21 as akeratodes.
The differentiation of the cancer was unspecified in 45 cases; in 12 it was assessed as grade (G)I, in 41 as GII and in 3 as GIII.
15 patients had chondrolysis and 9 osteolysis. In all 15 cases of chondrolysis involved larynx cartilages (in 12 cases primary larynx and in 3 hypopharynx cancers). In eight cases, osteolysis appeared in the mandible (two floor of the mouth, three tonsil, three oropharynx and two buccal mucosa cancers) and in one case in the hyoid bone (larynx cancer).
In 21 cases, cachexia was described.
63 patients drank alcohol (the majority had a problem with alcohol addiction). Negation of alcohol drinking by 13 patients was rather doubtful in the context of their behaviour during RT. 92 patients smoked cigarettes.
All patients were inoperable owing to the stage of disease or comorbidities, or did not agree to surgery.
8 of the randomized patients did not start treatment, and 17 did not complete the treatment leaving 76 patients who completed the planned treatment.
Out of the eight patients who did not start RT, three resigned from participation in the study after randomization, one came to our department 2 months later with too advanced disease and was referred to palliative treatment. Two did not start RT owing to a very fast local progression after randomization. One had severe dyspnoea and could not lay on the treatment couch, and in the last case purulent fistula developed.
From the group of 17 patients who did not complete RT, 8 were randomized to the CHA-CHA arm and 9 to the conventional (Conv.) arm.
In the CHA-CHA arm, two patients resigned from study participation after the first part of the treatment and were irradiated conventionally. In three cases, the treatment was stopped owing to PS deterioration. In one case, PS deteriorated, but treatment was continued using a 2-Gy fraction dose. In one case, the treatment was stopped owing to a very high mucosal reaction, and one patient died during the treatment.
In the Conv. arm, three patients died during the treatment. In three cases, RT was stopped owing to PS deterioration, one patient resigned from treatment continuation, one patient had cardiac infarction and one had haemorrhaging during the treatment.
Finally, the treatment was completed for 76 patients (63 males, 13 females), and this group was considered as the analysed material. 39 patients were treated in CHA-CHA and 37 in Conv. arm.
The age of patients in this group varied from 42 to 73 years. Means for CHA-CHA and Conv. arms were 57.3 years and 55.8 years, respectively.
The particular locations of cancers are shown in Table 2.
Table 2.
Locations of cancers in the evaluated patient group
| Location | Larynx | Tongue—mobile part | Oropharynx | Palate tonsil | Hypopharynx | Floor of the mouth | Buccal mucosa | Base of the tongue |
|---|---|---|---|---|---|---|---|---|
| CHA-CHA (n) | 11 | 10 | 5 | 5 | 5 | 2 | 1 | 0 |
| Conv. (n) | 7 | 6 | 9 | 7 | 2 | 3 | 2 | 1 |
CHA-CHA, split-course accelerated hyperfractionated irradiation; Conv., conventional.
Gross tumour volume (GTV) varied in the CHA-CHA arm 42.5–622.3 ccm (mean, 213.4 ccm) and from 45.1 to 573.8 ccm (mean, 182.3 ccm) in Conv. arm.
The detailed characteristics of patients and tumours in both arms are shown in Tables 3 and 4.
Table 3.
Characteristics of patients (numbers of evaluated patients)
| Treatment arm | Performance status (Zubrod scale) |
Chondrolysis | Osteolysis | Cachexia | Smoking | Alcohol drinking | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| CHA-CHA | 34 | 5 | 0 | 7 | 1 | 9 | 38 | 33 |
| Conv. | 36 | 0 | 1 | 3 | 4 | 5 | 32 | 30 |
CHA-CHA, split-course accelerated hyperfractionated irradiation; Conv., conventional.
Table 4.
Characteristics of tumours (numbers of evaluated patients)
| Treatment arm | TNM |
Grading |
H-P |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 | T3 | T4 | N0 | N1 | N2a | N2b | N2c | N2x | N3 | GI | GII | GIII | Unspecified | Carcinoma keratodes | Carcinoma akeratodes | Unspecified (SCC) | |
| CHA-CHA | 1 | 4 | 34 | 3 | 4 | 2 | 4 | 13 | 2 | 11 | 6 | 17 | 0 | 16 | 17 | 9 | 13 |
| Conventional | 0 | 5 | 32 | 0 | 7 | 0 | 5 | 17 | 0 | 10 | 3 | 15 | 1 | 18 | 9 | 6 | 22 |
CHA-CHA, split-course accelerated hyperfractionated irradiation; H-P, histopathological diagnosis; SCC, squamous cell cancer.
The haematological status of the analysed patient group is shown in Table 5.
Table 5.
The haematological status of the analysed group of patients at the treatment start
| Blood parameters | Hb (mg%) | Ht (%) | RBC (mln) | WBC | PLT |
|---|---|---|---|---|---|
| Range and mean | 9.6–15.9 (13) | 26.6–45.5 (38) | 3.1–5.5 (4) | 5600–16,700 (9900) | 80,000–918,000 (326,300) |
HB, haemoglobin; Ht, haematocrit; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
Method
Patients were included into a particular trial arm using a previously randomly generated list of 0–1 numbers (following this patients were connected with consecutive numbers on the list).
In the first (evaluated) CHA-CHA treatment arm, patients were irradiated (primary tumour and enlarged lymphatic nodes) twice a day (with a minimal gap of 6 h), 7 days a week, using 1.6 Gy per fraction to 32 Gy (10 days) and after an 8-day gap, the cycle was repeated to a TD of 64 Gy. The overall treatment time (OTT) was 28 days.
We wanted to avoid an irradiation of elective regions in a very intense manner as to not enhance adverse effects and to worsen a tolerance of the treatment. Because we can treat the “supraclavicular field” separately, we decided to use Conv. fractionations where irradiation had an elective character. The electively irradiated supraclavicular and lower neck area were treated conventionally (2 Gy per fraction, once a day, five times a week to 50 Gy). The maximal accepted dose delivered to the spinal cord was 38.4 Gy.
We contoured three clinical target volumes (CTVs) as well as three planning target volumes (PTVs). CTV1 comprised anatomical groups of neck lymph nodes (except supraclavicular—if not affected by metastases) and GTV +1.5 cm of margin (owing to the stage of disease in the majority of cases, these volumes were connected). PTV1 was created by adding 1 cm of margin to CTV1. In the CHA-CHA arm, this volume was irradiated during the first 4 days of treatment twice a day, next only in the morning to a dose of 38.4 Gy. CTV2 comprised neck lymph nodes excluding nodes of posterior chain and supraclavicular (if not involved) and GTV +1.5 cm of margin, and PTV2 comprised CTV2 +1 cm of margin. These volumes were irradiated during evening fractions from Days 5 to 8 and from 11 to 18 to a TD of 57.6 Gy. CTV3 was GTV +1.5 margin and PTV3 was CTV3 +1 cm of margin. These boosted volumes were used during the second daily fraction in Days 9, 10 and 19, 20.
Patients in the control arm were irradiated conventionally, 2 Gy per fraction, five times a week up to a TD of 72 Gy or 74 Gy (T3-4N2-4) in the OTT of 50–53 days. Electively treated volumes were irradiated to 50 Gy. The maximal accepted dose delivered to the spinal cord was 45 Gy.
The Conv. arm PTV1 was irradiated to a dose of 44 Gy. Next, the PTV2 was irradiated to 60 Gy and PTV3 to 72–74 Gy.
30 patients (16 in CHA-CHA and 14 in Conv. arm) were irradiated using conformal RT (CRT) and 46 (23 in CHA-CHA and 23 in Conv. arm) using the intensive-modulated radiation therapy technique.
In vivo dosimetry was an obligatory procedure at the beginning of each new field irradiation start (when the CRT technique was used). At the same time, portal images using the electronic portal imaging device were obtained.
The body weight and haematological values were checked during the radiation treatment.
The course of acute mucosal radiation reaction during the radiation treatment was analysed using the Dische1 scale.
Patients were controlled 2 and 4 weeks after their treatment completion, then every month up to 6 months after the treatment, then every 2 months up to 18 months and then every half year to the end of the follow-up period.
During the follow-up examinations, the effect of treatment, quality of life (QOL), PS and adverse effects of radiotherapy of the mucosa up to the third month were evaluated using the Dische1 scale and later on the mucosa and skin using the LENT-SOMA scale were evaluated.2
QOL was evaluated at the beginning, at the end of RT and then according to the follow-up (FU) schedule. The general EORTC QLQ-C30 and QLQ H&N35 modules were used (in QLQ H&N35 module, acronyms of particular scales starting from the letters HN).3 The agreement for the questionnaires used was given on 11 December 2005 by European Organisation for Research and Treatment of Cancer (EORTC) Data Center, The QOL Unit.
Percentages of patients with different degrees of regressions during FU in both evaluated groups, as well as, in subgroups with and without unplanned gaps were calculated.
Statistical analysis
The characteristics of data distribution were checked using the Shapiro–Wilk test.
The statistically significant differences in QLQ scores between the beginning and the end of RT (CHA-CHA and Conv.) have been established using Student's t-test for paired samples.
In the statistical analysis, hierarchical (multilevel) modelling has also been conducted, which allowed for the variance in the outcome variable (QOL scores) to be analysed at multiple hierarchical levels (i.e. time of observation and treatment methods).4 To test whether the scores were different between the two radiotherapies (CHA-CHA and Conv.), a mixed effects' model with random intercepts and fixed effects of the interaction between the time of observation and treatment methods was applied; this computation was performed in “nlme” package.5,6
Differences between haematological values at the beginning and at the end of RT for the whole analysed group and both treatment arms, as well as, values of acute mucosal radiation reaction 1 and 3 months after RT completion were checked using Student's t-test and Wilcoxon math-pair test (for dependent values).
The differences between CHA-CHA and Conv. in acute mucosal radiation reaction for the first 7 consecutive weeks after treatment start were established using Wilcoxon's test.
Grades of late radiation effects of the mucosa and skin were compared using Student's t-test for dependent samples.
A percentage of patients with complete regressions (CRs) and the number of patients who died in both treatment arms were calculated.
Hierarchical (multilevel) modelling was used for evaluation of tumour response.
The Kaplan–Meier overall survival (OS) analysis for both treatment arms was performed and compared using the log-rank test.
RESULTS
Follow-up
In the whole analysed group, FU ranged from 72.2 months (mean, 12.5 months). The means of FU (months) in CHA-CHA and Conv. arms were 12.9 and 12.2, respectively.
Treatment gaps
In 15 cases, unplanned gaps during RT course appeared.
In CHA-CHA arm, nine patients had unplanned gaps that lasted from 1 to 9 days (mean, 3.2 days). In three cases, gaps were owing to temporary PS deterioration, in one owing to bleeding from the primary tumour and in five, owing to the individual patient's decision (usually owing to addiction, mainly alcohol or unexplained).
In Conv. RT arm, only six patients had treatment gaps that lasted from 1 to 7 days (mean, 2.7 days). In one case, the gap was caused by a pronounced, badly tolerated radiation adverse effect and in five cases by the patient's decision.
Quality of life
Owing to limited data over a longer period, we analysed data during the RT and up to 12 months after the treatment completion. In the majority of evaluated scales of both questionnaires, QOL was more deteriorated in the CHA-CHA arm (larger dose intensity and higher acute radiation reaction).
The statistically significant differences in QLQ scores between the beginning and the end of RT are presented in Table 6.
Table 6.
The statistically significant differences in QLQ scores between the beginning and the end of radiotherapy
| RT method | Scale | Score difference (%) | p-value | Confidence interval 95% |
|---|---|---|---|---|
| CHA-CHA | Nausea and vomiting (NV) | −22 | 0.022 | (−40%, −4%) |
| Pain (HNPA) | −35 | 0.001 | (−53%, −18%) | |
| Swallowing (HNSW) | −31 | 0.01 | (−54%, −9%) | |
| Senses problems (HNSE) | −35 | 0.004 | (−56%, −13%) | |
| Opening mouth (HNOM) | −38 | 0.004 | (−62%, −14%) | |
| Sticky saliva (HNSS) | −38 | 0.01 | (−67%, −10%) | |
| Conventional RT | Appetite loss (AP) | −37 | 0.02 | (−67%, −6%) |
| Constipation (CO) | −37 | 0.03 | (−69%, −4%) | |
| Pain (HNPA) | −28 | 0.03 | (−53%, −4%) | |
| Senses problems (HNSE) | −43 | 0.001 | (−63%, −24%) | |
| Speech problems (HNSP) | −25 | 0.03 | (−47%, −3%) |
CHA-CHA, split-course accelerated hyperfractionated irradiation; RT, radiotherapy.
Following the results given in Table 6, it can be seen that a higher score of the reported QLQ scores is given at the end of therapy. The differences are more pronounced for the QLQ H&N35 module.
The statistically significant differences in QOL between CHA-CHA and Conv. RT during a 12-month period are reported specifically in Table 7.
Table 7.
The statistically significant differences (interactions) for specific scales between CHA-CHA and conventional radiotherapy
| Scale | Parameter | Mean | p-value | Standard error |
|---|---|---|---|---|
| Social functioning (SF) | Intercept | 78.78 | <0.0001 | 5.95 |
| Time | −1.34 | 0.01 | 0.52 | |
| RT (conventional RT vs CHA-CHA) | −11.62 | 0.3 | 9.93 | |
| Interaction (time and RT) | 2.35 | 0.023 | 1.02 | |
| Pain (PA) | Intercept | 39.43 | <0.0001 | 6.23 |
| Time | 0.48 | 0.53 | 0.75 | |
| RT (conventional RT vs CHA-CHA) | 30.68 | 0.006 | 10.11 | |
| Interaction (time and RT) | −2.9 | 0.046 | 1.44 | |
| Appetite loss (AP) | Intercept | 30.04 | <0.0001 | 5.88 |
| Time | 1.03 | 0.2 | 0.85 | |
| RT (conventional RT vs CHA-CHA) | 25.15 | 0.02 | 10.00 | |
| Interaction (time and RT) | −4.8 | 0.006 | 1.72 | |
| Financial difficulties (FI) | Intercept | 37.12 | <0.0001 | 8.39 |
| Time | 2.34 | 0.002 | 0.71 | |
| RT (conventional RT vs CHA-CHA) | 11.68 | 0.4 | 13.75 | |
| Interaction (time and RT) | −3.14 | 0.03 | 1.38 | |
| Pain killers (HNPK) | Intercept | 20.44 | 0.02 | 8.70 |
| Time | 1.35 | 0.3 | 1.16 | |
| RT (conventional RT vs CHA-CHA) | −10.6 | 0.5 | 14.75 | |
| Interaction (time and RT) | 5.42 | 0.03 | 2.43 |
CHA-CHA, split-course accelerated hyperfractionated irradiation; RT, radiotherapy.
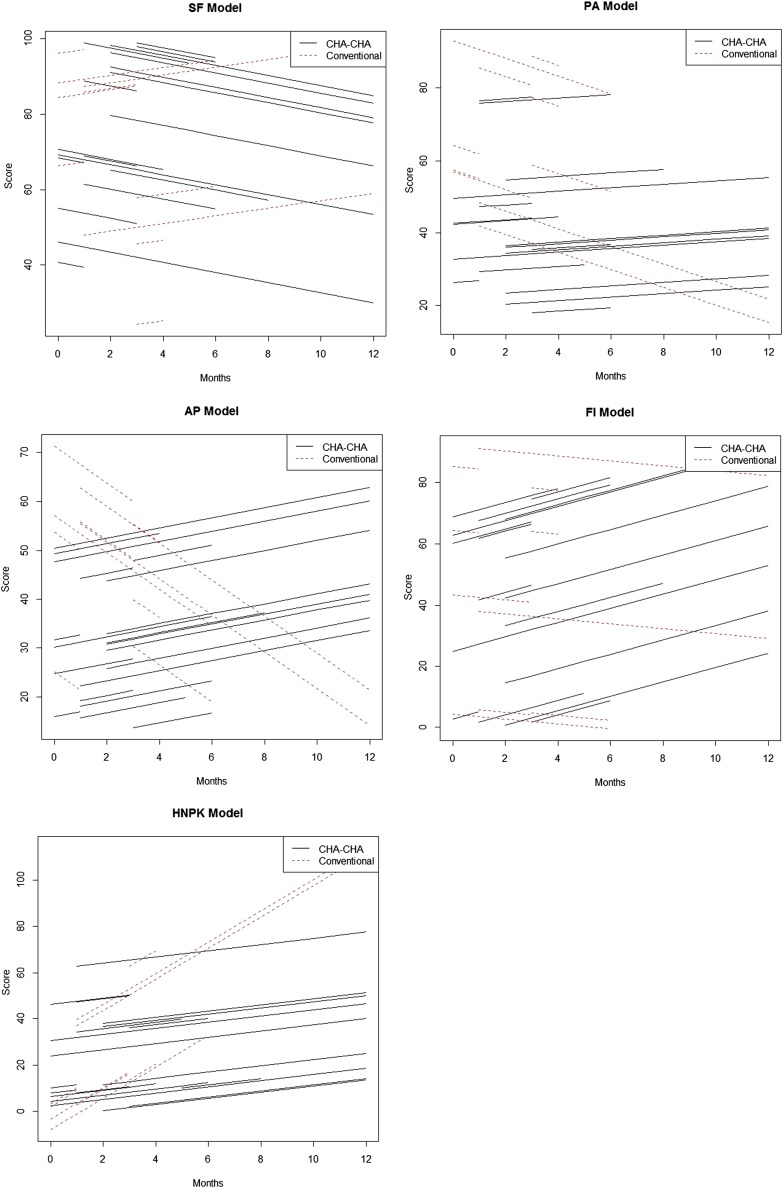
The statistical interpretation of the results given in Table 7 is as follows: on average, at the time of 0, the social functioning (SF) rate for a patient was expected to be nearly 79% (this coefficient is statistically significant as the standard error is relatively small, resulting in a small p-value). Under a linear assumption, for each month reached, an average patient was also losing 1.3% of SF score. The coefficient of the main effect of the Conv. is negative, 11.6%, indicating that this method has a lower average SF score at the beginning of observation compared with CHA-CHA treatment; however, the difference is statistically insignificant. Whereas, the coefficient of the interaction term between time and RT term is significant and that for each increment of 1 month, Conv. will have a higher average SF rate of 2.4% than CHA-CHA. The interpretation of other results given in Table 7 is analogous. The QLQ modelled outcomes (for significant scales) are presented graphically on Figure 1.
Figure 1.
The QLQ modelled outcomes—significant differences for particular scales. AP, appetite loss; FI, financial difficulties; PA, pain; SF, social functioning. HPNK, pain killers.
Treatment toxicity
The body weight of irradiated patients changed during the treatment. In the majority of cases, patients lost weight. In the CHA-CHA arm, the weight change varied between −11 and +7 kg (mean, −3.9 kg); 13 patients from this group had a weight increase. In the Conv. arm, the body weight varied from −11.5 to +5 kg (mean, −1.8 kg) and in 15 cases, the body weight increased.
For the whole analysed group of patients, except the platelet count, statistically significant differences between haematological values at the beginning and at the end of radiation treatment were found (all values decreased). Results are presented in Table 8.
Table 8.
The haematological status of the analysed group of patients at the treatment completion and results of comparison between haematological status at the beginning and the end of the radiotherapy
| Blood parameters | Hb (mg%) | Ht (%) | RBC (mln) | WBC | PLT |
|---|---|---|---|---|---|
| Range and mean | 7.5–14.9 (11.8) | 23.8–42.7 (34.8) | 3.0–5.9 (4.0) | 3200–12,900 (6600) | 86,100–721,000 (311,080) |
| Used test | Student’s t-test | Student’s t-test | Student’s t-test | Wilcoxon | Wilcoxon |
| p-value | 0.000 | 0.000001 | 0.02 | 0.000 | 0.12 |
HB, haemoglobin; Ht, haematocrit; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
Similar analysis was performed for both evaluated subgroups. As in the whole group, both subgroups had all values decreased during RT. Except for the platelet count in the Conv. arm, the remaining differences were statistically significant (Table 9).
Table 9.
Results of comparison between haematological status at the beginning and the end of the radiotherapy in both study arms
| Blood parameters | Hb | Ht | RBC | WBC | PLT |
|---|---|---|---|---|---|
| CHA-CHA | |||||
| Used test | Student’s t-test | Student’s t-test | Wilcoxon | Student’s t-test | Student’s t-test |
| p-value | 0.00006 | 0.00060 | 0.00800 | 0.00004 | 0.02 |
| Conventional | |||||
| Used test | Student’s t-test | Student’s t-test | Student’s t-test | Wilcoxon | Wilcoxon |
| p-value | 0.0005 | 0.0004 | 0.048 | 0.0002 | 0.9 |
CHA-CHA, split-course accelerated hyperfractionated irradiation; HB, haemoglobin; Ht, haematocrit; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
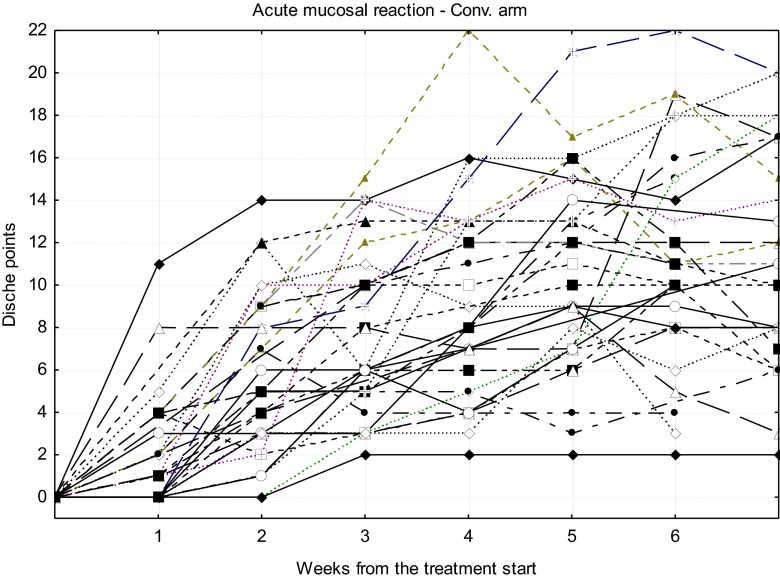
Courses of acute radiation reaction for particular patients treated in CHA-CHA and Conv. arm are shown in Figures 2 and 3, respectively.
Figure 2.
Courses of acute mucosal radiation reaction for particular patients treated in the split-course accelerated hyperfractionated irradiation (CHA-CHA) arm.
Figure 3.
Courses of acute mucosal radiation reaction for particular patients treated in the conventional (Conv.) arm.
The differences between CHA-CHA and Conv. in acute mucosal radiation reaction for the first 7 consecutive weeks are presented in Table 10.
Table 10.
Comparison of acute mucosal radiation reaction courses of both radiotherapy schedules (medians of Dische scores)
| Week | CHA-CHA | Conventional | p-value |
|---|---|---|---|
| 1 | 5.0 | 1.0 | 0.0010 |
| 2 | 9.5 | 5.0 | 0.0030 |
| 3 | 13.0 | 6.0 | <0.0001 |
| 4 | 13.5 | 8.0 | 0.0003 |
| 5 | 13.5 | 11.5 | 0.0600 |
| 6 | 13.0 | 11.0 | 0.5000 |
| 7 | 10.5 | 11.0 | 0.8000 |
CHA-CHA, split-course accelerated hyperfractionated irradiation.
Based on the outcomes given in Table 10, it can be seen that there is a statistically significant difference in medians of the Dische scores during the first 4 weeks of treatment (in the fifth week, the difference is on the border of the statistical significance); the CHA-CHA scheme causes relatively stronger acute injury than the Conv. Graphical presentations of the medians are plotted in Figure 4.
Figure 4.
The course of acute mucosal reaction intensity (medians) during 7 weeks from radiotherapy start for both treatment arms. CHA-CHA, split-course accelerated hyperfractionated irradiation; Conv., conventional.
The intensity of acute mucosal radiation reaction decreased rapidly after the completion of radiation treatment. In the CHA-CHA group, the mean was 10.5, 3 weeks after RT end; in the Conv. arm, at this time (time of RT end), the mean of mucosal reaction was 11 points. 1 and 3 months later, the means of acute mucosal reactions for CHA-CHA and Conv. arms were 8.8, 4.4 (p = 0.02) and 5.9, 3.2 (p = 0.2), respectively.
Late effects assessed in the LENT-SOMA scale were not pronounced in both groups. The largest was noted for mucosa 2 years after treatment (mean, 4.3 points). In other periods, the mean of late reaction (for mucosa as well as for skin) did not exceed two points. No statistically significant difference was found between late radiation effects of mucosa and skin; however, for observations 1 year after treatment and longer, a late injury of the skin was greater for the Conv. arm (p = 0.06).
Treatment results
In the CHA-CHA arm in 35% of patients, disease was controlled after RT—CR and lack of metastasis (only 3 patients from CHA-CHA arm failed owing to distant metastases). During further observation, two patients from this group relapsed. 23 patients in this treatment arm died at the end of data collection.
In the Conv. arm, disease was controlled in 30% of cases (also only three patients from this arm failed from distant metastases). Also in this group, two patients relapsed later on. 25 patients died at the end of data collection.
Detailed information concerning percentage of patients with local progression, stagnation, partial regression (PR) and CR in particular arms of the study, evaluated 1, 3, 6 and 12 months after treatment completion, is presented in Table 11. Owing to the kind of evaluated (usually addicted) patients and the stage of disease, they did not fit the follow-up scheme precisely; therefore, data in this table should be interpreted very carefully—different patients were followed in different periods.
Table 11.
Percentage of patients with local progression (P), stagnation (S), partial regression (PR) and complete regression (CR) in particular arms of the study, evaluated 1, 3, 6 and 12 months after treatment completion
| Treatment arm | CHA-CHA |
Conventional |
||||||
|---|---|---|---|---|---|---|---|---|
| Time of control (months) | 1 | 3 | 6 | 12 | 1 | 3 | 6 | 12 |
| P (%) | 3.2 | 11 | 14.3 | 13.3 | 0 | 4 | 13.3 | 10 |
| S (%) | 3.2 | 0 | 0 | 0 | 4.4 | 4 | 0 | 0 |
| PR (%) | 91.4 | 63 | 33.3 | 20 | 69.6 | 56 | 20 | 20 |
| CR (%) | 3.2 | 26 | 52.4 | 66.7 | 26 | 36 | 66.7 | 70 |
CHA-CHA, split-course accelerated hyperfractionated irradiation.
According to information from the municipal registry, all deaths in the evaluated group were related to cancer disease.
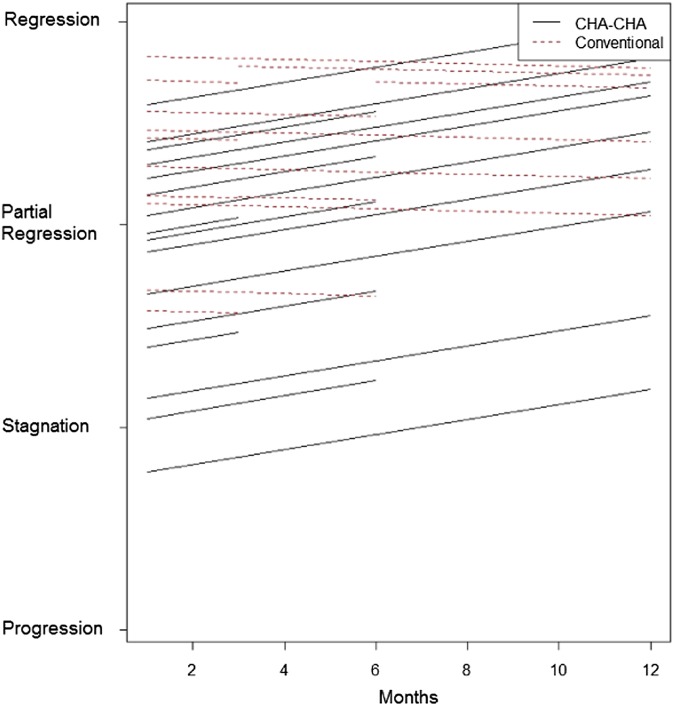
The hierarchical multilevel regression showed a statistically significant difference in tumour response between both treatment arms during the first 12 months (tumour regression was better in the CHA-CHA arm) (Table 12, Figure 5).
Table 12.
Tumour response during 12-month-long follow-up (hierarchical multilevel modelling)
| Parameter | Mean | p-value |
|---|---|---|
| Intercept | 1.92 | <0.0001 |
| Time (months) | 0.04 | 0.04 |
| Conventional vs CHA-CHA | 0.41 | 0.06 |
| Interaction | −0.04 | 0.15 |
CHA-CHA, split-course accelerated hyperfractionated irradiation.
Figure 5.
The tumour response in both treatment arms during first 12 months (hierarchical multilevel regression). CHA-CHA, split-course accelerated hyperfractionated irradiation.
This effect disappears when considered in 2- and 3-year periods. For a 3-year long observation, a significant correlation between time and tumour response exists (longer time–better regression) (p = 0.049).
The OS analysis did not show any significant difference between both arms (p = 0.2) (Figure 6).
Figure 6.
Overall survival (OS) in both treatment arms. CHA-CHA, split-course accelerated hyperfractionated irradiation; Conv., conventional.
The relatively small subgroup with unplanned gaps (15 patients) makes an analysis of gap impact on treatment result difficult. We checked the percentage of PR and CR in both subgroups 1 and 6 months after RT completion. 1 month after RT in the subgroup without gaps, PR was 82.2% and CR 11.1% of patients. In the subgroup with unplanned gaps, PR was 77.8% and CR 22.2% of patients. 6 months after RT in the subgroup without gaps, PR was 30.3% and CR 57.6% of patients. In the subgroup with gaps, none had PR and 66.6% had CR. On the basis of these results, we can conclude that in the analysed group of patients unplanned gaps had no significant impact on tumour regression.
Salvage treatment
In the vast majority of cases in which CR was not reached, salvage treatment was not possible (stage of the disease, PS, delivered radiation dose, second malignancy, massive dissemination) or patients disagreed to it. In eight cases, a secondary treatment was performed. Two patients were operated on 18 months after RT completion owing to persistent nodal disease. Others were operated on owing to nodal relapse 2.5 years after RT and then re-irradiated with a fraction dose (fd) of 1.8 Gy to a TD of 50.4 Gy for neck lymph nodes. Two patients were re-irradiated with palliative intention (20 Gy delivered in 2 Gy fractions), one was treated with superficial palliative hyperthermia alone (6 times for 1 h, 43 °C) for persistent lymph nodes, one with extracranial palliative radiosurgery for local relapse and one suffering from persistent nodal metastases was treated with palliative chemotherapy and then palliative RT combined with superficial hyperthermia (20 Gy a 2 Gy + 4 times for 1 h, 43 °C).
DISCUSSION
In the past years, a lot of studies concerning hypofractionated, accelerated RT of patients suffering from advanced H&N cancers have been published. We can divide them into three groups: radiochemotherapy, RT alone and their combination with surgery. The majority of publications refer to the first mentioned option.7–15 Less frequent were attempts of RT as an alone utility.16–20 Some individual publications describe a combination of RT and surgery.21,22
There is very limited data concerning QOL after such therapies. Ringash21 reported on the course of QOL after hyperfractionated, accelerated RT for locally advanced H&N cancer followed by neck dissection. He described a decrease of QOL after RT completion and then a slow recovery during the first year, which is in agreement with the results obtained by us. A detailed comparison is not possible owing to the different scales used.
Acute mucosal effects during intense treatment are always more pronounced than in cases of Conv. treatment. Despite that, the course of CHA-CHA acute radiation reaction was previously described as acceptable.18,23,24 Again, different scales were used in the literature. Among publications regarding RT alone, Cvek et al17 described 51% and Yoney et al20 24% of Grade 3 acute mucositis (RTOG/EORTC).
We did not observe any severe late adverse effects. Similar results are described by Cvek et al;17 after hyperfractionated accelerated RT with integrated boosts, no 3 or 4 grade toxicity was observed (RTOG/EORTC). Saunders et al19 described a small, but significant reduction in epidermal and mucosal late adverse effects in the arm treated with accelerated hyperfractionation in comparison with Conv. treatment. On the other hand, Allen et al7 described a 30% of grade 3 late toxicity after accelerated hyperfractionated chemoradiation (5-fluorouracil cisplatin) and Yoney et al20 a 5% grade 4 injury for hyperfractionated accelerated RT alone.
The OS rate in our study was worse than those described in other publications. The 2-year OS in the analysed group was 50% for CHA-CHA and only 27% for the Conv. arm. Other authors reported larger percentages of 2-year OS: 55%, 60%, 71% and 74% for hyperfractionated accelerated RT with concomitant integrated boost,17 hyperfractionated accelerated RT combined with chemotherapy,15 neoadjuvant chemotherapy followed by concurrent hyperfractionated radiation therapy and sensitizing chemotherapy9 and hyperfractionated accelerated RT plus cisplatin,12 respectively. Some authors described an even better 3-year OS: 57% and 60% for split-course hyperfractionated accelerated radiochemotherapy14 and accelerated hyperfractionated RT concurrent with carboplatin and paclitaxel,8 respectively (OS 17% in the CHA-CHA arm). The 5-year OS in our group was 11% for CHA-CHA and 9% for Conv. in comparison with 25% reported by Yoney et al20 in small (20 patient) groups of locally advanced H&N cancers treated with hyperfractionated accelerated RT. Better OS after hyperfractionated RT of advanced H&N cancer patients was reported by Beitler et al16 than with Conv., continuous accelerated and accelerated with split. Interpretation of obtained results concerning OS in the context of others publications is very complex; it is extremely difficult to compare advanced H&N cancer patients. The only device is TNM system, which is not volumetric and therefore is not ideal for comparison purposes. The second issue influencing the cure probability is addiction. Heavy drinking and smoking in older age patients in connection with cancer (and its treatment) can significantly deteriorate PS and lead to death even without relapse. Looking at the aforementioned results, it is clearly seen that a better result is only attainable after a combined treatment (accelerated hyperfractionation + chemotherapy).8,9,12,14,15 The only exception is a small retrospective study by Yoney et al20—25% of 5-year OS.
On the other hand, in the CHA-CHA arm, the disease was controlled (CR and lack of metastasis) in 35% and this percentage was larger than in the Conv. arm (30%). These numbers are slightly higher than those described in the literature. Majumder et al11 reported 15%, 26.3% and 23.8% of CR after chemotherapy combined with Conv. RT, RT delivered six times a week and hyperfractionation for advanced H&N cancer patients, respectively.
The next issue is a lack of difference between treatment results of both evaluated treatment arms, and what is not consistent with expectations. There may be three main reasons for such situations: larger toxicity in CHA-CHA, a lack of balanced observation of GTV (213.4 ccm in CHA-CHA and 182.3 ccm in Conv.) and, finally, treatment gaps—more numerous (9 vs 6) and longer [3.2 vs 2.7 days (means)] in the CHA-CHA arm.
CONCLUSIONS
A greater percentage of patients with tumour control and better tumour regression during the 1-year period was in the CHA-CHA arm. However, in the context of a more pronounced mucosal radiation reaction, the poorer QOL during the follow-up in this group, and considering the lack of a significant difference in OS, we cannot conclude that CHA-CHA is superior to conventionally fractionated RT as a sole treatment for advanced H&N cancer patients.
FUNDING
This article is funded by Maria Sklodowska-Curie memorial Cancer Center and Institute of Oncology, Gliwice Branch, Gliwice, Poland.
REFERENCES
- 1.Dische S. The uniform reporting of treatment-related morbidity. Semin Radiat Oncol 1994; 4: 112–18. doi: 10.1053/SRAO00400112 [DOI] [PubMed] [Google Scholar]
- 2.LENT SOMA scales for all anatomic sites. Int J Radiat Oncol Biol Phys 1995; 31: 1049–91. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76. [DOI] [PubMed] [Google Scholar]
- 4.Raudenbush S, Bryk A. Hierarchical linear models: applications and data analysis methods. 2nd edn. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 5.Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 6.Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. Version 3.1–113. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 7.Allen AM, Elshaikh M, Worden FP, Bradford CR, Teknos TN, Chepeha DB, et al. Acceleration of hyperfractionated chemoradiation regimen for advanced head and neck cancer. Head Neck 2007; 29: 137–42. doi: 10.1002/hed.20495 [DOI] [PubMed] [Google Scholar]
- 8.Carter DL, Asmar L, Barrera D, Caracandas J, Dakhil JS, McCracken D, et al. Favorable survival observed after carboplatin, paclitaxel, and concurrent accelerated hyperfractionated radiotherapy for treatment of locally advanced head and neck carcinoma. Invest New Drugs 2008; 26: 473–81. [DOI] [PubMed] [Google Scholar]
- 9.Finnegan V, Parsons JT, Greene BD, Sharma V. Neoadjuvant chemotherapy followed by concurrent hyperfractionated radiation therapy and sensitizing chemotherapy for locally advanced (T3–T4) oropharyngeal squamous cell carcinoma. Head Neck 2009; 31: 167–74. [DOI] [PubMed] [Google Scholar]
- 10.Kuhnt T, Sandner A, Wendt T, Engenhart-Cabillic R, Lammering G, Flentje M, et al. Phase I trial of dose-escalated cisplatin with concomitant cetuximab and hyperfractionated-accelerated radiotherapy in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 2010; 21: 2284–9. [DOI] [PubMed] [Google Scholar]
- 11.Majumder D, Choudhury K, Das P, Kundu S, Mitra D. Different fractionation schedules of radiotherapy in locally advanced head and neck malignancy: a prospective randomized study to compare the results of treatment and toxicities of different protocols. South Asian J Cancer 2013; 2: 31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuyts S, Dirix P, Clement PM, Poorten VV, Delaere P, Schoenaers J, et al. Impact of adding concomitant chemotherapy to hyperfractionated accelerated radiotherapy for advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2009; 73: 1088–95. doi: 10.1016/j.ijrobp.2008.05.042 [DOI] [PubMed] [Google Scholar]
- 13.Sadat F, Wienke A, Dunst J, Kuhnt T. Survival of patients with head and neck cancer. Impact of physical status and comorbidities. Strahlenther Onkol 2012; 188: 62–70. doi: 10.1007/s00066-011-0009-8 [DOI] [PubMed] [Google Scholar]
- 14.Stadler P, Putnik K, Kreimeyer T, Sprague LD, Koelbl O, Schäfer C. Split course hyperfractionated accelerated radio-chemotherapy (SCHARC) for patients with advanced head and neck cancer: influence of protocol deviations and hemoglobin on overall survival, a retrospective analysis. BMC Cancer 2006; 7: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welz H, Pöttgen C, Abu Jawad J, Wierlemann A, Wittig A, Stüben G, et al. Hyperfractionated accelerated radiotherapy versus conventional fractionation both combined with chemotherapy in patients with locally advanced head and neck carcinomas: a retrospective analysis of a monoinstitutional series. Oncology 2009; 76: 405–12. [DOI] [PubMed] [Google Scholar]
- 16.Beitler JJ, Zhang Q, Fu KK, Trotti A, Spencer SA, Jones CU, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014; 89: 13–20. doi: 10.1016/j.ijrobp.2013.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cvek J, Kubes J, Skacelikova E, Otahal B, Kominek P, Halamka M, et al. Hyperfractionated accelerated radiotherapy with concomitant integrated boost of 70-75 Gy in 5 weeks for advanced head and neck cancer. A Phase I dose escalation study. Strahlenther Onkol 2012; 188: 666–70. [DOI] [PubMed] [Google Scholar]
- 18.Miszczyk L, Wozniak G, Tarnawski R, Maciejewski B. Split-course accelerated hyperfractionation (CHA-CHA) for advanced head and neck cancers—preliminary results. Neoplasma 2005; 52: 143–9. [PubMed] [Google Scholar]
- 19.Saunders MI, Rojas AM, Parmar MK, Dische S; CHART Trial Collaborators. Mature results of a randomized trial of accelerated hyperfractionated versus conventional radiotherapy in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010; 77: 3–8. [DOI] [PubMed] [Google Scholar]
- 20.Yoney A, Akboru H, Kandemir O, Unsal M. Hyperfractionated accelerated radiotherapy in locally advanced head and neck cancers. Onkologie 2007; 30: 479–84. doi: 10.1159/0000104489 [DOI] [PubMed] [Google Scholar]
- 21.Ringash J, Lockwood G, O'Sullivan B, Warde P, Bayley A, Cummings B, et al. Hyperfractionated, accelerated radiotherapy for locally advanced head and neck cancer: quality of life in a prospective Phase I/II trial. Radiother Oncol 2008; 87: 181–7. doi: 10.1016/j.radonc.2007.12.028 [DOI] [PubMed] [Google Scholar]
- 22.Waldron J, Warde P, Irish J, Pintilie M, Sellmann S, Bayley A, et al. A dose escalation study of hyperfractionated accelerated radiation delivered with integrated neck surgery (HARDWINS) for the management of advanced head and neck cancer. Radiother Oncol 2008; 87: 173–80. doi: 10.1016/j.radonc.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 23.Miszczyk L, Tarnawski R, Woźniak G, Maciejewski B. Evaluation of mucosal acute radiation injury during accelerated-hyperfractionated split course radiotherapy (CHA-CHA) in advanced head and neck neoplasms. [In Polish.] Otolaryngol Pol 2002; 56: 543–9. [PubMed] [Google Scholar]
- 24.Tukiendorf A, Miszczyk L, Bojarski J. Damped sinusoidal function to model acute irradiation in radiotherapy patients. Phys Med 2013; 29: 513–19. doi: 10.1016/j.ejmp.2012.12.004 [DOI] [PubMed] [Google Scholar]