Abstract
Objective:
To explore the diagnostic value of quantitative contrast-enhanced (CE) ultrasonography for crush injury in the hind limb muscles of rabbits.
Methods:
A total of 120 New Zealand white rabbits were randomized to receive compression on the left hind limb for either 2 h (n = 56) or 4 h (n = 56) to induce muscle crush injury. Another eight animals were not injured and served as normal controls. CE ultrasonography parameters such as peak intensity (PI), ascending slop, descending slop and area under curve (AUC) were measured at 0.5, 2, 6 and 24 h and 3, 7 and 14 days after decompression.
Results:
Compared with the uninjured muscles, reperfusion of the injured muscles showed early and high enhancement in CE ultrasonography images. The time-intensity curve showed a trend of rapid lift and gradual drop. The PI and AUC values differed significantly among the three groups and were positively correlated with serum and tissue biomarkers. Rabbits of the 4-h compression group showed significantly higher PI and AUC values, and serum and tissue parameters than the 2-h compression group at each time points.
Conclusion:
CE ultrasonography can effectively detect muscle crush injury and monitor dynamic changes of the injured muscles in rabbits. PI and AUC are promising diagnostic parameters for this disease.
Advances in knowledge:
CE ultrasonography might play an important role in the pre-hospital and bedside settings for the diagnosis of muscle crush injury.
Muscle crush injury usually occurs during earthquakes, collapse of buildings and heavy whip beatings, and often induces crush syndrome if not treated promptly. Crush injury is estimated to account for 3–20% of all injuries during natural disasters, and the lower limbs are the most frequently affected.1 Limb crush injury and its complications are life threatening and the most frequent cause of disability and death after earthquakes.2
The mortality rate in patients with crush syndrome can be as high as 21%, which is the most dangerous complication of all injuries during disasters.3 Crush syndrome can cause acute kidney injury and acute osteofascial compartment syndrome (AOCS), which are the most life-threatening complications. AOCS has a 47% mortality, and unrecognized AOCS can leave a patient with non-viable limbs requiring amputation.4 Severe muscle crush injury can also result in multiple organ dysfunction syndrome, acute respiratory distress syndrome, disseminated intravascular coagulation and severe arrhythmia.5 Early diagnosis of muscle crush injury and correct assessment of its severity are critical for good prognosis of patients. However, bedside and pre-hospital diagnosis of crush injury still lacks effective methods.
Typical muscle crush injury and related AOCS are usually diagnosed with clinical symptoms, but the sensitivity of this method is very low.6,7 Impaired microcirculation is the initial pathological change of crushed muscles.4 A variety of imaging methods have been used to examine reperfusion of the extremities and therefore detect the presence of muscle crush injury, such as CT, MRI and ultrasonography.8–11 However, the equipment of CT and MRI is large and inconvenient for bedside or pre-hospital settings or in situ care at the trauma scene. On the contrary, ultrasonography devices can be light, portable and convenient for bedside or traumatic scenes. Ultrasonography also has no radiation. Conventional ultrasonography has been used to determine limb muscle crush injury, rhabdomyolysis and AOCS during the 2008 Sichuan earthquake in China.12 However, the sensitivity of conventional ultrasonography is low for the diagnosis of extremity crush injury, and its detecting ability of microvascular perfusion is also very poor.13
Gas-filled microbubbles can significantly augment the back scattered signals and do not leak out of the blood vessel and therefore are used as a contrast agent for Doppler ultrasonography to trace the bloodstream. Contrast-enhanced (CE) ultrasonography has been successfully used to measure microcirculation of the skeletal muscles, such as measurement of muscle perfusion after exercise, and evaluation of muscle perfusion in inflammatory myopathy or peripheral arterial disease.14–16 However, application of CE ultrasonography in the assessment of microcirculation perfusion in muscle crush injury has rarely been reported.17
In this study, microcirculation of extremities that underwent crush injury were evaluated using CE ultrasonography to investigate the values of CE ultrasonography in diagnosing limb crush injury.
METHODS AND MATERIALS
Animal grouping
A total of 120 healthy New Zealand white rabbits, including 60 males and 60 females, were used in this study. The animals were 1.0–1.5 years old, weighing 2.5–3.3 kg. The animals were obtained from the Laboratory Animal Center of Chinese PLA General Hospital, Beijing, China, and were given a 72-h acclimation before the experiments. The rabbits were randomly divided into 3 groups: the control group (n = 8), 2-h compression group (n = 56) and 4-h compression group (n = 56). The study protocol was approved by the Ethics Committee of Chinese PLA General Hospital. All experiments were performed in accordance with the Revised Guide for the Care and Use of Laboratory Animals.18
Animal modelling
The rabbits were anesthetized with pentobarbital sodium 30 mg kg−1 injected into the external ear vein. The general anaesthesia was maintained by injecting 10 mg kg−1 of pentobarbital sodium every 2 h throughout the compression process. The left hind leg was shaved and wrapped up with a cotton pad (5 × 25 cm), then covered by a balloon cuff. The balloon cuff was connected to a pressure meter and aired to maintain a pressure of 40 kPa for 2 or 4 h in the two experiment groups. Two ear vein catheters were placed for CE ultrasonography and venous blood collection, respectively.
Ultrasonography
At 0.5, 2.0, 6.0 and 24.0 h and 3, 7 and 14 days after decompression, conventional ultrasonography and CE ultrasonography were performed by using the IU22 ultrasonic diagnostic instrument (Philips Medical System, Bothell, WA) with an L12-5 transducer (5–12 MHz). The ultrasonography contrast agent was SonoVue® (0.025 ml kg−1; Bracco, Milan, Italy) and was administered with a quick bolus through the ear vein. The agent consists of stabilized microbubbles containing inert gas (sulphur hexafluoride SF6) covered by a phospholipid membrane. The agent can provide 8 μl ml−1 of sulphur hexafluoride microbubbles (SF6) after reconstitution with 5 ml of saline.19 The scan settings, including the gain, scanning depth and time gain control, were optimized for each target region independently. The focus was set to the deeper aspect of the lesion being examined.
Conventional ultrasonography was performed before CE ultrasonography detection. Greyscale imaging was used to observe the echo and continuity of the subcutaneous tissue and muscle texture, the echo character of the injured area and the scope of the fluid sonolucent area in the lesions. Colour Doppler ultrasound was used to detect the blood flow signals in the lesions and their velocity and spectrum in the arteries.
All CE ultrasonography images and cines were reviewed by two experienced readers blinded to the study design. For each rabbit, CE ultrasonography images were obtained and recorded as double-frame pictures with multiple cine-loops for offline analysis. The images showed the enhancement patterns and the echogenicity changes, which were used to analyse the microcirculation perfusion using QLAB software (Philips Medical System). For each image, the area of 5 × 5 mm square with the highest enhancement in the crushed region was selected as the region of interest (ROI), which was kept away from large vessels. Then time–intensity curves for the ROI of the crushed region were created automatically. CE ultrasonography parameters, including peak intensity (PI), ascending slop (AS), descending slop (DS) and area under curve (AUC) for each ROI were sampled three times from the time–intensity curve, and the mean value was calculated.
Tissue oedema
At each time point, eight rabbits from each of the two compression groups were sacrificed with intravenous injection of 50 ml air. The biceps femoris muscle of the left hind leg was harvested, weighed and placed in a drying oven at 55 °C until a constant weight was obtained. Muscle oedema was determined by calculating the wet to dry weight ratio (W/D).
Biochemistry tests
Blood samples were obtained from the ear vein and centrifuged at 3000 rpm for 10 min at 4 °C. The plasma levels of creatine kinase (CK) and lactate dehydrogenase (LDH) were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits. The biceps femoris was chopped into 1 × 0.5 cm cubes for the measurement of malondialdehyde (MDA) content and myeloperoxidase (MPO) activity using the ELISA method.
Histopathology
The muscle specimens were fixed in 10% neutral buffered formalin and embedded within paraffin. The tissue blocks were cut into 4-μm thick sections and stained with haematoxylin and eosin. Two pathologists who were blinded to the study design examined the tissue sections.
Statistical analysis
All variables were presented as mean ± standard variation. The data were analysed by SPSS® v. 11.0 (SPSS Inc., Chicago, IL). Differences between groups were analysed using one-way analysis of variance followed by Tukey's post hoc test. A p < 0.05 was considered statistically significant.
RESULTS
Baseline data
Crush injuries were successfully created in the two compression groups. Among the 112 rabbits that underwent crush injury, 8 died from overdose of anaesthetics or complications of muscle injury within 14 days and 104 survived until their sacrifice. The eight rabbits that died were not included in the statistical analysis.
Muscle wet to dry weight ratio
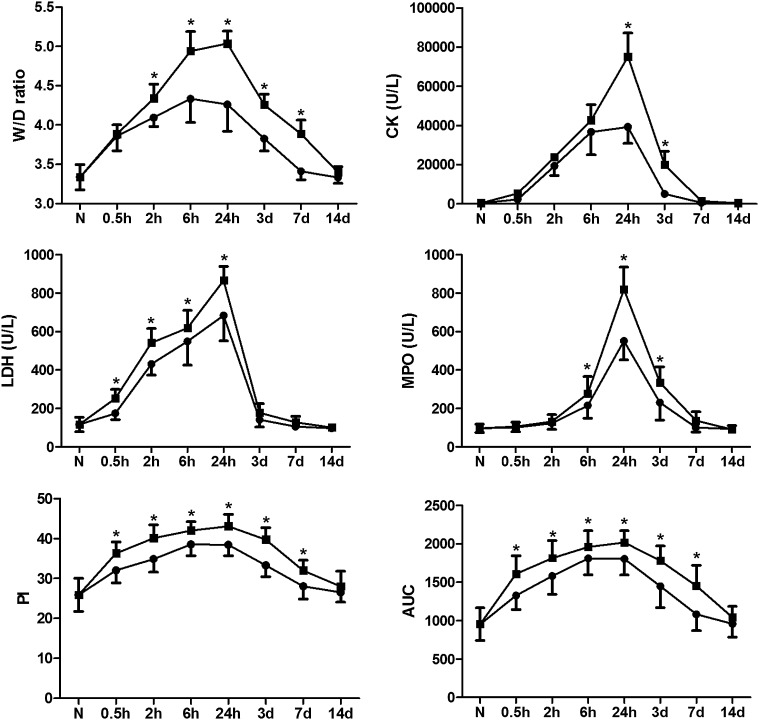
Crush injury caused significant oedema in all left hind legs. The W/D ratios of the two compression groups were significantly higher than that of the control group at each time point (all p < 0.01). The 4-h compression group showed significantly higher W/D ratios than the 2-h compression group (Figure 1; all p < 0.01).
Figure 1.
Comparison of wet to dry weight ratio (W/D) ratio, plasma levels of creatine kinase (CK), lactate dehydrogenase (LDH), myeloperoxidase (MPO) measurements, peak intensity (PI) and area under curve (AUC) between the 2-h and 4-h compression groups. *p < 0.05, vs the 2-h compression group. •, 2-h compression group; ■, 4-h compression group; d, days; h, hours; N, normal controls.
Biochemistry results
The plasma levels of CK, LDH, MPO and MDA measurements in the two compression groups were significantly higher than that in the control group at each time point (all p < 0.01). The 4-h compression group showed significantly higher levels of CK, LDH, MPO and MDA measurements than the 2-h compression group (Figure 1; all p < 0.01).
Ultrasonography
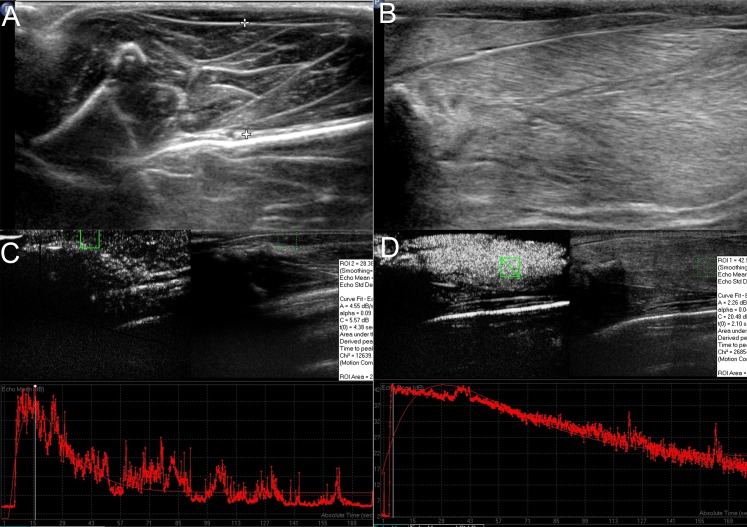
Conventional ultrasonography showed oedema in the subcutaneous tissue of the injured muscles, with vague muscle texture and cloudy or ground-glass-like lesions. Most of the injured lesions showed hyperechogenicity with unclear boundary and uneven strength. Fluid sonolucent areas with spindle shape or irregular shape were also seen in the injured muscles (Figure 2b).
Figure 2.
(a, c) Conventional ultrasonography and contrast-enhanced (CE) ultrasonography images of the normal muscle. (b) Conventional ultrasonography of the injured muscles showed subcutaneous oedema with vague muscle texture and cloudy or ground-glass-like lesions. (d) CE ultrasonography showed quick enhancement and slow washout in the injured muscles. The time–intensity curve showed a trend of rapid lift and gradual drop, indicating fast washin and slow washout in the crushed muscles. ROI, region of interest.
CE ultrasonography showed quick enhancement and slow washout in the injured muscles in comparison with the normal muscles. The intensities of enhancement in the injured muscles were significantly higher than that of the normal muscle in the arterial phase. The time–intensity curve showed a trend of rapid lift and gradual drop, indicating fast washin and slow washout in the crushed muscles (Figure 2d). Four CE ultrasonography parameters were measured from the time–intensity curve: PI, AS, DS and AUC.
Diagnostic values of contrast-enhanced ultrasonography parameters
PI and AUC of muscles in the two compression groups were significantly higher than those in the control group at each time point except at Day 14 (all p < 0.01). PI and AUC values of the 2-h compression group were significantly lower than that of the 4-h compression group (Figure 1; all p < 0.01). Both PI and AUC showed positive correlations with various biochemistry parameters in the two compression groups (Table 1). Significantly higher AS and lower DS were seen in the two compression groups than that in the control group at each time point except at Day 14 (all p < 0.01). AS and DS did not differ significantly between the two compression groups (all p > 0.05).
Table 1.
| Correlations between peak intensity (PI) and various biochemistry parameters in the two compression groups | |||||
|---|---|---|---|---|---|
| Correlation coefficient | PI/AUC | PI/CK | PI/LDH | PI/MDA | PI/MPO |
| PI in the 2-h compression group (n = 61) | |||||
| r | 0.97 | 0.81 | 0.84 | 0.82 | 0.70 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| PI in the 4-h compression group (n = 59) | |||||
| r | 0.97 | 0.75 | 0.78 | 0.82 | 0.63 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
AUC, area under curve; CK, creatine kinase; LDH, lactate dehydrogenase; MDA, malondialdehyde; MPO, myeloperoxidase.
| Correlations between area under curve (AUC) and various biochemistry parameters in the two compression groups | |||||
|---|---|---|---|---|---|
| Correlation coefficient | AUC/PI | AUC/CK | AUC/LDH | AUC/MDA | AUC/MPO |
| AUC in the 2-h compression group (n = 61) | |||||
| r | 0.97 | 0.83 | 0.84 | 0.82 | 0.70 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| AUC in the 4-h compression group (n = 59) | |||||
| r | 0.97 | 0.73 | 0.77 | 0.81 | 0.63 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
CK, creatine kinase; LDH, lactate dehydrogenase; MDA, malondialdehyde; MPO, myeloperoxidase; PI, peak intensity.
Histopathological findings
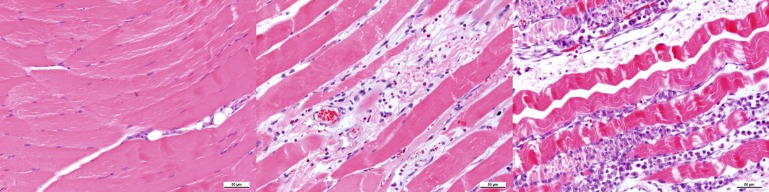
The crushed muscles showed inflammatory cell infiltration, intercellular oedema, cloudy swelling of the myocytes, cell gap changing, cell degeneration and cell bunchy necrosis. Intercellular oedema and cell gap oedema were obvious at 6 and 24 h post compression. Inflammatory cell infiltration and muscular necrosis occurred from 6 h. Abundant inflammatory cells and necrotic muscular cells began to occur from 24 h (Figure 3).
Figure 3.
Haematoxylin and eosin staining of the biceps femoris muscle of the left hind leg. Left, uncrushed normal muscle. Middle, abundant inflammatory cell infiltration and necrotic muscular cells were seen at 24 h after decompression. Right, muscular cell necrosis, fibroblasts hyperplasia and inflammatory cell infiltration were seen at 72 h after decompression. Scale bar, 50 μm.
DISCUSSION
There are several established animal models of skeletal muscle crush injury. A simple rubber tourniquet on the hind limb can create crush injury,20 but the pressure is difficult to be controlled accurately. Hind limb can be pressed by heavy blocks to create crush injury,21 but the injury is not homogeneous. The modelling method used in this study can accurately control the pressure and injury area, which is around the hind limb muscle and is homogeneous.17 In this study, the two compression groups showed similar biochemical and pathological changes in the injured muscles, suggesting good consistency and reproducibility of the disease model.
In this study, higher enhancement and higher intensities were found by CE ultrasonography in the injured muscles than in the normal muscles. The microbubbles vanished slower in the injured muscles than in the normal muscles. The time–intensity curve showed a trend of rapid lift and gradual drop. It was suggested that the blood volume was increased in the injured muscles compared with the normal muscles. And the washout of microbubbles from the injured muscles was slower than that from normal muscles.
There are several possible reasons for the CE ultrasonography findings in this study. First, the prolonged ischaemic injury led to metabolic product accumulation in the injured muscles. This resulted in metabolic regulation and opening of microvessels to clear away these products.22,23 Second, the venules were damaged and occluded by the accumulated blood cells and microthrombi in the injured muscles. But the arteries were less affected.24 This resulted in obstructed venous blood flow and less affected arterial blood perfusion, leading to congested microcirculation in the injured muscles. Third, damaged endothelial cells and increased vascular permeability caused the microbubbles to exudate into the tissue space.
Compared with a previous study by Lv et al,17 this study examined the effects of two different compressions on muscles. The observation time was 14 days, which was much longer than the previous 72 h. In addition, we examined more biochemical and CE ultrasonography parameters and therefore obtained a better understanding of the pathology of muscle crush injury.
There are limitations in this study. First, the rabbit hind limb muscle accounts for a much lower proportion of the whole body than that of humans. Hence, caution should be used when extrapolating these results to clinical applications. Second, the severity of muscle injury in this study was not adequate for observing muscle perfusion. Third, only one crush injury was created in each animal. This does not reflect the reality in natural disasters that crush injuries are commonly combined with fracture and arterial injuries.
In conclusion, CE ultrasonography can effectively detect muscle crush injury and monitor the dynamic changes of this disease process. PI and AUC are promising diagnostic CE ultrasonography parameters. CE ultrasonography might play an important role in the pre-hospital and bedside settings for the diagnosis of muscle crush injury.
REFERENCES
- 1.Gonzalez D. Crush syndrome. Crit Care Med 2005; 33(1 Suppl): S34–41. [DOI] [PubMed] [Google Scholar]
- 2.Sever MS, Vanholder R, Lameire N. Management of crush-related injuries after disasters. N Engl J Med 2006; 354: 1052–63. doi: 10.1056/NEJMra054329 [DOI] [PubMed] [Google Scholar]
- 3.Ersoy A, Yavuz M, Usta M, Ercan I, Aslanhan I, Güllülü M, et al. Survival analysis of the factors affecting in mortality in injured patients requiring dialysis due to acute renal failure during the Marmara earthquake: survivors vs non-survivors. Clin Nephrol 2003; 59: 334–40. [DOI] [PubMed] [Google Scholar]
- 4.Mabvuure NT, Malahias M, Hindocha S, Khan W, Juma A. Acute compartment syndrome of the limbs: current concepts and management. Open Orthop J 2012; 6: 535–43. doi: 10.2174/1874325001206010535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman JL, Shen MC. Rhabdomyolysis. Chest 2013; 144: 1058–65. doi: 10.1378/chest.12-2016 [DOI] [PubMed] [Google Scholar]
- 6.Malik AA, Khan WS, Chaudhry A, Ihsan M, Cullen NP. Acute compartment syndrome—a life and limb threatening surgical emergency. J Perioper Pract 2009; 19: 137–42. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma 2002; 16: 572–7. [DOI] [PubMed] [Google Scholar]
- 8.Partovi S, Karimi S, Jacobi B, Schulte AC, Aschwanden M, Zipp L, et al. Clinical implications of skeletal muscle blood-oxygenation-level-dependent (BOLD) MRI. Magma 2012; 25: 251–61. doi: 10.1007/s10334-012-0306-y [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Fang ZJ, Liu F, Fu P, Tao Y, Li ZY, et al. Magnetic resonance imaging and magnetic resonance angiography in severe crush syndrome with consideration of fasciotomy or amputation: a novel diagnostic tool. Chin Med J (Engl) 2011; 124: 2068–70. [PubMed] [Google Scholar]
- 10.Zhang LY, Ding JT, Wang Y, Zhang WG, Deng XJ, Chen JH. MRI quantitative study and pathologic analysis of crush injury in rabbit hind limb muscles. J Surg Res 2011; 167: e357–63. doi: 10.1016/j.jss.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 11.Moratalla MB, Braun P, Fornas GM. Importance of MRI in the diagnosis and treatment of rhabdomyolysis. Eur J Radiol 2008; 65: 311–15. doi: 10.1016/j.ejrad.2007.03.033 [DOI] [PubMed] [Google Scholar]
- 12.Su BH, Qiu L, Fu P, Luo Y, Tao Y, Peng YL. Ultrasonic appearance of rhabdomyolysis in patients with crush injury in the Wenchuan earthquake. Chin Med J (Engl) 2009; 122: 1872–6. [PubMed] [Google Scholar]
- 13.Lamminen AE, Hekali PE, Tiula E, Suramo I, Korhola OA. Acute rhabdomyolysis: evaluation with magnetic resonance imaging compared with computed tomography and ultrasonography. Br J Radiol 1989; 62: 326–30. [DOI] [PubMed] [Google Scholar]
- 14.Krix M, Krakowski-Roosen H, Amarteifio E, Fürstenberger S, Delorme S, Kauczor HU, et al. Comparison of transient arterial occlusion and muscle exercise provocation for assessment of perfusion reserve in skeletal muscle with real-time contrast-enhanced ultrasound. Eur J Radiol 2011; 78: 419–24. [DOI] [PubMed] [Google Scholar]
- 15.Krix M, Weber MA, Kauczor HU, Delorme S, Krakowski-Roosen H. Changes in the micro-circulation of skeletal muscle due to varied isometric exercise assessed by contrast-enhanced ultrasound. Eur J Radiol 2010; 76: 110–16. [DOI] [PubMed] [Google Scholar]
- 16.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol 2009; 53: 2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv F, Tang J, Luo Y, Ban Y, Wu R, Tian J, et al. Contrast-enhanced ultrasound assessment of muscle blood perfusion of extremities that underwent crush injury: an animal experiment. J Trauma Acute Care Surg 2013; 74: 214–19. [DOI] [PubMed] [Google Scholar]
- 18.Bayne K. Revised guide for the care and use of laboratory animals available. American Physiological Society. Physiologist 1996; 39: 199, 208–11. [PubMed] [Google Scholar]
- 19.Morel DR, Schwieger I, Hohn L, Terrettaz J, Llull JB, Cornioley YA, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol 2000; 35: 80–5. [DOI] [PubMed] [Google Scholar]
- 20.Murata I, Ooi K, Sasaki H, Kimura S, Ohtake K, Ueda H, et al. Characterization of systemic and histologic injury after crush syndrome and intervals of reperfusion in a small animal model. J Trauma 2011; 70: 1453–63. doi: 10.1097/TA.0b013e31820ca00a [DOI] [PubMed] [Google Scholar]
- 21.Akimau P, Yoshiya K, Hosotsubo H, Takakuwa T, Tanaka H, Sugimoto H. New experimental model of crush injury of the hindlimbs in rats. J Trauma 2005; 58: 51–8. [DOI] [PubMed] [Google Scholar]
- 22.Barrett EJ, Rattigan S. Muscle perfusion: its measurement and role in metabolic regulation. Diabetes 2012; 61: 2661–8. doi: 10.2337/db12-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 2004; 97: 393–403. doi: 10.1152/japplphysiol.00179.2004 [DOI] [PubMed] [Google Scholar]
- 24.Vollmar B, Westermann S, Menger MD. Microvascular response to compartment syndrome-like external pressure elevation: an in vivo fluorescence microscopic study in the hamster striated muscle. J Trauma 1999; 46: 91–6. [DOI] [PubMed] [Google Scholar]