Abstract
The history of surgery for transposition of great arteries (TGA) has paralleled the history of cardiac surgery. In fact, it began before the birth of open heart surgery when the palliative Blalock–Hanlon septectomy was first performed in 1948. The atrial switch, which was an attempt to correct the physiology of transposition, had significant shortcomings. The arterial switch sought to address them. This has emerged as an anatomically as well as physiologically appropriate solution. Today we continue to pursue technical refinements as well as try to expand the indications of the arterial switch. This review traces the various milestones in this perpetual journey.
Keywords: Arterial switch, atrial switch, congenital heart disease, history of open-heart surgery, transposition of great arteries
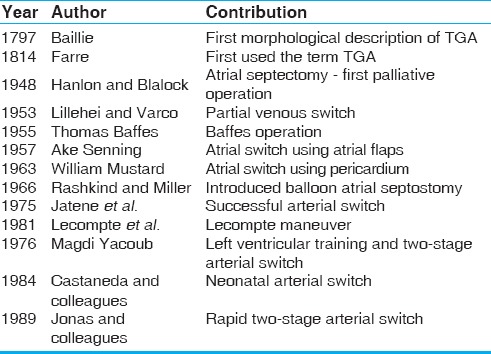
The human heart was considered a ‘surgical untouchable’ for most part of scientific history. The science and art of cardiac surgery has made rapid strides in the past 65 years. The surgery for transposition of great arteries (TGA) symbolises the manner in which cardiac surgery has progressed. The eventually successful solution in the form of the now popular arterial switch operation (ASO) was elucidated after multiple contributions from several surgical stalwarts and geniuses over more than 25 years [Table 1]. This review traces the various milestones along this journey.
Table 1.
Significant milestones in the evolution of surgery for transposition of great arteries (TGA)
The first morphological description of TGA can be credited to Baillie in 1797,[1] while the term ‘Transposition of Great Arteries’ was used for the first time by Farre in 1814.[2] Though the term ‘transposition’ was used aberrantly in literature till 1970, the ambiguity was resolved by Van Praagh et al., in 1971 who said that, it refers to the aorta and pulmonary artery (PA) being displaced across the ventricular septum.[3]
The first credit of surgical treatment of TGA goes to Alfred Blalock [Figure 1a] and his then resident, Rollins Hanlon [Figure 1b] from Johns Hopkins. In 1948, in a joint paper, they described the essence of successful surgical “treatment” of TGA, allowing effective mixing of both the parallel circulations. Analyzing 123 cases of TGA from different sources, they concluded that the presence of a ventricular septal defect was favorable for survival, followed by the presence of an atrial septal defect. A combination of the two defects was the most favorable situation.[4] Armed with this information they realized that, surgical creation of a mixing site would improve the longevity of these patients. As the heart lung machine was yet to be brought into clinical use, a closed heart procedure was the only possible answer. In the same article, they further described their canine experiments involving the redirection of pulmonary venous blood to the right atrial appendage or superior vena cava using special occlusive clamps through thoracotomy. It was found that redirection of the pulmonary venous blood to the SVC was better in terms of patency and had the potential to be evaluated further as a possible treatment modality for TGA. In 1950, they first reported the use of a surgically created atrial septal defect as a treatment of TGA called the ‘Blalock–Hanlon Septectomy’ [Figure 2].[5] Interestingly, like the Blalock–Taussig shunt (BTS), Blalock's assistant Vivien Thomas [Figure 1c] had conceived the idea and flawlessly executed it in animal models in the late 1940s. In spite of being a palliative procedure, this surgery laid the foundation of the surgical treatment of TGA and has been aptly described as an ‘act of surgical genius’.[6] With the advent of ‘balloon atrial septostomy (BAS), the Blalock–Hanlon septectomy eventually became redundant.
Figure 1.

Pioneers of transposition of great arteries (TGA) surgery. (a) Alfred Blalock (b) C Rollins Hanlon ((A and B) Reproduced with permission from: Konstantinov IE, Alexi-Meskishvili VV, Williams WG, Freedom RM, Van Praagh R. Atrial switch operation: Past, present, and future. Ann Thorac Surg. 2004 Jun;77(6):2250-8.) (c) Vivien Thomas (Reproduced with permission from: Cheng TO. Hamilton Naki and Christiaan Barnard versus Vivien Thomas and Alfred Blalock: Similarities and dissimilarities. Am J Cardiol. 2006 Feb 1;97(3):435-6.) (d) Thomas Baffes (right) with his mentor Willis Potts (left) (Reproduced with permission from: Mavroudis C, Backer CL, Siegel A, Gevitz M. Revisiting the Baffes operation: Its role in transposition of the great arteries. Ann Thorac Surg. 2014 Jan;97(1):373-7.) (e) Ake Senning (Reproduced with permission from: Tutarel O, Westhoff-Bleck M. Ake Senning. Clin Cardiol. 2009 Aug;32(8):E66-7.) (f) William T Mustard (Reproduced with permission from: Stoney WS. Evolution of cardiopulmonary bypass. Circulation. 2009 Jun 2;119(21):2844-53.) (g) Adib Dominos Jatene (Reproduced with permission from: Jacobs ML, Tchervenkov CI. Tribute to a patriarch: Adib Domingos Jatene,1929-2014. World J Pediatr Congenit Heart Surg. 2015 Jan;6(1):7-8.)
Figure 2.
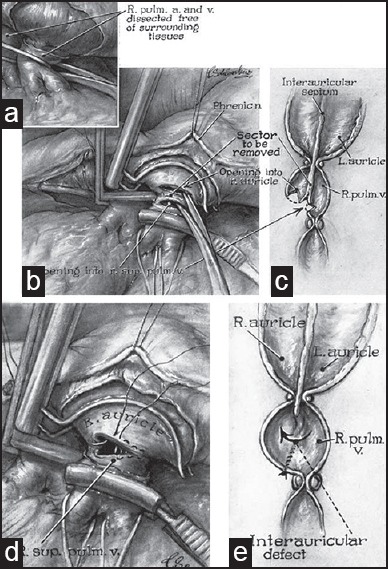
Blalock- Hanlon operation. (a) Right pulmonary artery and superior pulmonary vein dissected free from surrounding tissue. (b) After applying a special clamp, parallel incisions made on right atrium and pulmonary veins, area to be excised shown. (c) B in cross-section. (d) After excising septum, incision closed with sutures. (e) D in cross-section. L. auricle = Left auricle, R. auricle = Right auricle, R. pulm. a. and v.= Right pulmonary artery and vein, R. sup. pulm. v. = Right superior pulmonary vein (Reproduced with permission from: Blalock A, et al., Surg Gynecol Obstet; 1950;90:1-15.)
A historical review of surgical treatment of TGA will not be complete without acknowledging the role of BAS described for the first time by Rashkind and Miller from Philadelphia in 1966. The biggest advantage of BAS was, that an operation was not required and therefore, it allowed an effective palliation with relatively low risk in a precarious group of patients.[7] Later, Park et al., introduced a modification by including a small blade in the catheter tip to allow an effective septostomy in a thick septum.[8]
Edwards et al., (1964) noted that the atrial septectomy resulted in a predominantly right-to-left shunt than the desired left-to-right shunt, thereby increasing the pulmonary blood flow. This was identified to be the reason for poor results in younger patients, though relatively older patients seemed to do well. To circumvent this problem, they introduced a modification whereby the right pulmonary veins were redirected into the right atrium.[9]
In September 1953, Walton Lillehei and Richard Varco from University of Minnesota reported their attempts at surgical correction of TGA. In the first four patients, they anastomosed the right pulmonary veins to the right atrium. Two of these four patients survived. In the subsequent four patients, in addition, they also directed the inferior vena cava blood to the left atrium. Unfortunately, none of these patients survived.[10]
In the 1950s, Thomas Baffes was an inquisitive resident training under Dr Willis Potts [Figure 1d] at the Children's Memorial Hospital (currently Lurie Children's Hospital), Chicago. His rationale of attempting a TGA correction was in stark contrast to the arterial switch; ‘venous switch’. The perceived advantage was avoidance of a coronary transfer. In 1954-1955, Baffes approached Dr Potts who, after initial reluctance not only supported the idea and promised funds but also gave technical suggestions. In Baffes’ own words, “When I first broached to Dr Potts the idea of designing a partial venous correction for transposition of the great arteries, he puckered up his moustache, as he was wont to do, and lapsed into deep thought. After a while he said: ‘Transposition represents about half of the defects in cyanotic children coming into this hospital. We haven’t been able to do much for any of them, except those special ones having pulmonary stenosis with ventricular septal defects.‘ He thought a little longer; then his eyes twinkled with excitement. ‘Go to it! I’ll get the necessary funds.‘ Thus I began many happy years of inquiry and discovery under the guidance of the master surgeon” [11] As prosthetic grafts were not available commercially, they decided to use aortic homograft. The homograft was used to join the inferior vena cava to the left atrium and the right pulmonary veins were anastomosed to the right atrium [Figure 3]. After initially experimenting on dogs, the first successful procedure was performed on May 6, 1955 and reported in 1956; surprisingly by Baffes, as a sole author.[12] In 1960, 5-year results were described for 117 such patients with 29% survival.[13] Originally Dr. Baffes had planned to perform a second-stage procedure that would switch the superior vena cava with the left pulmonary veins, but this was never performed clinically. The Baffes operation remained the treatment option for infants with transposition over the next 10 years.[14]
Figure 3.
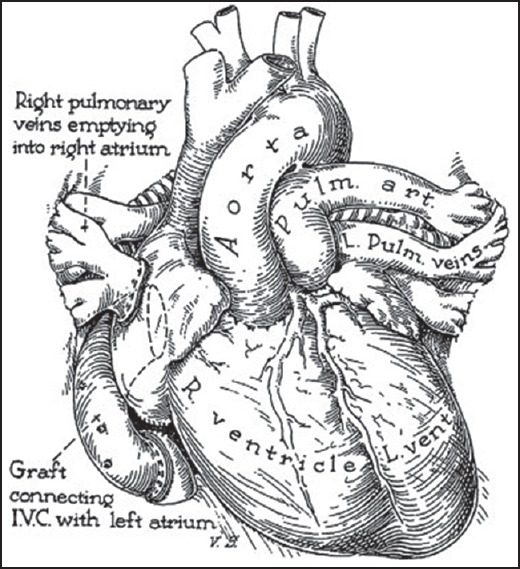
Baffes operation. Right pulmonary veins directed to right atrium. Inferior vena cava baffled to left atrium using a graft. L. Pulm. Veins = Left pulmonary veins, L. vent. = Left ventricle, Pulm. art. = Pulmonary artery, R. ventricle = Right ventricle. (Reproduced with permission from: Baffes TG, Surg Gynecol Obstet; 1956;102:227-33.)
The idea of attempting to correct TGA at the atrial level using a baffle can be attributed to Dr. Harold M Albert and is frequently referred to as the ‘Albert principle’ [Figure 4].[15] He suggested the use of ‘plastic prosthesis’ for the baffle, but used pericardium for his canine experiments.
Figure 4.
Albert operation. Right atrial view. Creation of bilobed flaps of interatrial septum (a) Each lobe used to cover the orifices of the vena cava. Remaining sides of the flaps sutured to the posterior wall of left atrium (b). View from right (c) and left (d) atrium. Use of graft instead of septum (e) (Reproduced with permission from: Albert HM, Surg Forum; 1955;5:74-7.)
Albert did not attempt this operation clinically. But this idea formed the basis of the atrial switch operation; specifically the Mustard procedure. Albert's mentor and guide was none other than Dr Willis Potts.
Creech et al., from New Orleans attempted an atrial switch in a 9-month-old boy in 1956 using a polyvinyl (Ivalon) baffle and extracorporeal circulation to transfer blood flow from the pulmonary veins into the tricuspid valve. A 100% saturation was achieved and the procedure was a technical success. Unfortunately the patient died 12 h later. An autopsy was performed, which revealed a nonobstructing prosthesis. Death was attributed to respiratory failure due to bronchial obstruction due to secretions and impaired ventilatory effort secondary to bilateral thoracotomy.[16]
On March 20, 1957, Alvin Merendino, from the University of Washington in Seattle, attempted an atrial switch using a premolded atrial septal prosthesis made of Ivalon using cardiopulmonary bypass in two patients. The first patient was a 6.5-year-old girl who died on table due to intractable ventricular fibrillation. This was possibly attributed to a repaired tear in the left ventricle (LV) created during redo sternotomy (the patient had undergone an open and shut sternotomy previously). The second patient was a 6-year-old girl who died few hours after surgery due to sudden bradycardia and apnea. This was attributed to a small missed ventricular septal defect. The prosthesis was found to be perfectly aligned.[17]
In 1957, Ake Senning [Figure 1e], who had trained under Dr. Clarence Crafoord, performed the first successful atrial switch using atrial flaps at the Karolinska Hospital, Stockholm, Sweden [Figure 5]. It was published in 1959, as a sole author. The same article also describes unsuccessful attempts at an arterial switch. The procedure was unsuccessful in the first two patients. The third case, a 9-year-old boy survived. A cardiac catheterization done 6 weeks following this procedure showed excellent results.[18] Using the same technique, Kirklin et al., operated on 11 patients at the Mayo clinic and reported four survivors.[19]
Figure 5.
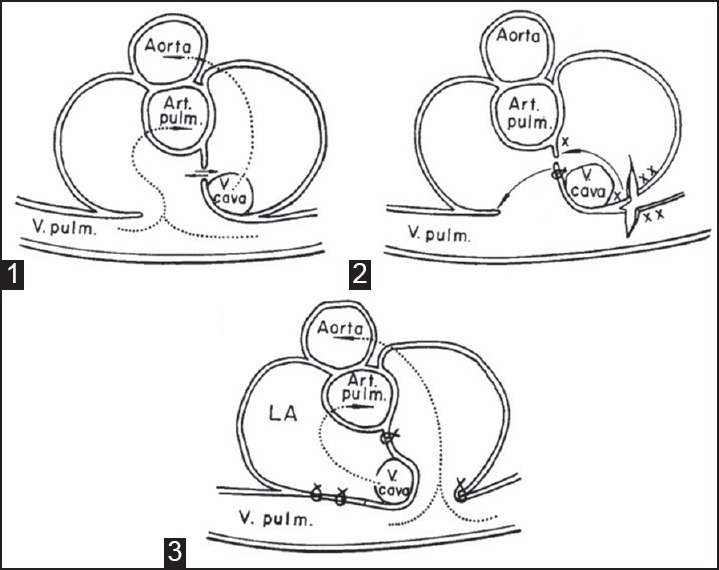
Senning operation. Preoperative (1), surgery (2), and b c postoperative (3); X and XX represent edges to be sutured. Arrows represent direction of blood flow. Art. pulm. = Pulmonary artery, LA = Left atrium, V. cava = Vena cava, V. pulm. = Pulmonary vein (Reproduced with permission from: Senning A, Surgery; 1959;45:966-80.)
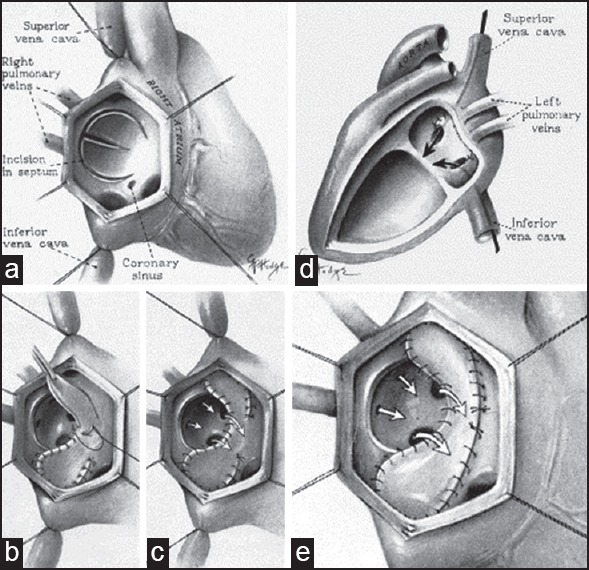
When asked to comment on use of an artificial material for atrial baffle, William T Mustard [Figure 1f] replied jokingly: “Use pericardium! The only excuse for using Dacron is if you drop the pericardium on the floor”.[14] On May 16, 1963, at the Hospital for Sick Children, Toronto, Mustard operated upon an 18-month-old girl who had previously undergone a Blalock–Hanlon operation [Figure 6]. He repaired the ventricular septal defect and then performed an atrial switch using an autologous pericardial baffle. The surgery was conceptually based on the Albert principle which Dr Mustard duly acknowledged. The patient recovered uneventfully and was healthy till last reported in 2001. In this article, Mustard stated: “In my manuscript I gave credit to Dr. Albert for his original principles; the only problem with these procedures was that they did not work”.[20]
Figure 6.
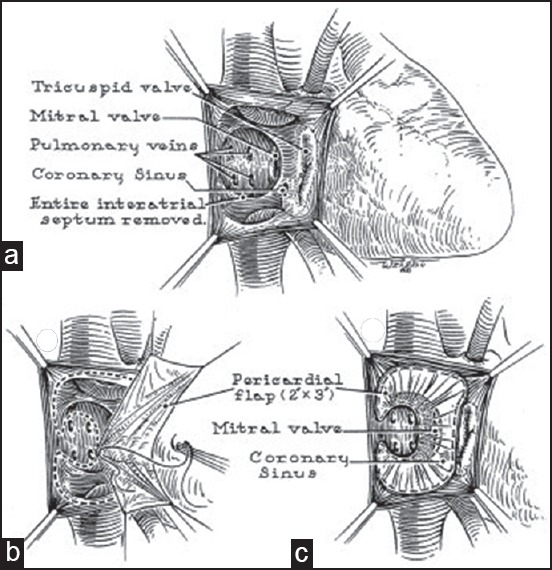
Mustard operation. Pericardial baffles are used to direct vena caval blood to mitral valve and pulmonary venous blood to tricuspid valve. (a) Atrial anatomy. (b) Suturing of pericardial baffle. (c) Completed procedure (Reproduced with permission from: Mustard WT, Surgery; 1964;55:469-72.)
The Senning operation was perceived as “although ingenious, technically extremely difficult in the infant or small child”. It was practically abandoned and for the next decade, the Mustard procedure was used universally by surgeons including Dr Senning himself.
However by 1975, the Mustard procedure revealed its shortcomings including baffle obstruction and lack of growth potential.[21] This led to a resurgence of the Senning procedure and it remained a favorite for the next decade.
Though the arterial switch was successfully added to the surgical armamentarium in 1975, multiple but seemingly unsuccessful attempts were already made by Mustard et al., in 1952 using monkey lung as an oxygenator,[22] Bailey et al., in 1952,[23] and also Senning in 1955.[18] The major difficulty perceived was the coronary transfer.
Adib Dominos Jatene [Figure 1g], a Brazilian surgeon of Lebanese origin performed successfully for the first time, a truly anatomical correction in the form of an ASO in 1975 at the University of Sao Paulo Heart Institute, Sao Paulo, Brazil. The first two patients were operated using profound hypothermia and total circulatory arrest. Coronary buttons were excised and the openings created were closed using homologous dura mater. After completing coronary transfer to the new site, the great vessels were transected; the ventricular septal defect was closed with a Dacron patch through a right ventriculotomy [Figure 7]. The first patient, an 11-day-old female was extubated 6 h after surgery, but expired on the 3rd postoperative day of renal failure. Necropsy showed a good anatomic correction. The second patient was a 40-day-old male who was successfully discharged 3 weeks after surgery. Jatene et al., stressed upon two technically important points — excising coronary buttons and transecting the great vessels away from the valves. Unfortunately the next five patients expired within a few hours after surgery. The findings were presented at the 56th Annual Meeting of the American Association of Thoracic Surgery at Los Angeles in April 1976 and its significance as a surgical milestone was immediately recognized.[24]
Figure 7.
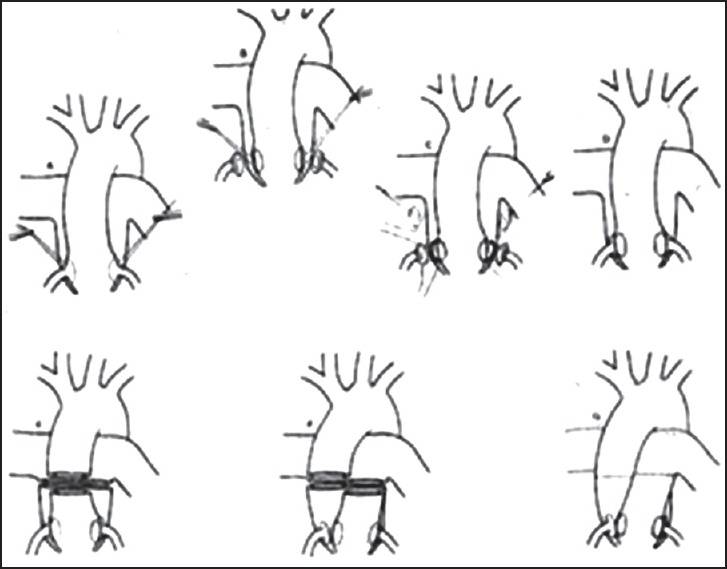
Jatene operation. Original sketch (Reproduced with permission from Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M et al. Anatomic correction of transposition of the great vessels. J Thorac CardiovascSurg. 1976 Sep;72(3):364-70.)
It was soon realized that a technically perfect coronary artery transfer was the most critical step in a successful ASO. As Jatene quotes “to divide, contrapose and then re-anastomose the great arteries is not a surgical problem. The major technical difficulty in this approach has been the transfer of coronary arteries” [24] Hence, a detailed understanding of the possible variations in the origin and course of coronaries and its surgical management was an essential step towards it.
Yacoub and Radley-Smith from Harefield Hospital, United Kingdom divided the possible coronary anatomy into five types (A-E) based on the origin, course, and branching pattern. They also described the method of surgical transfer of each type in 1978, thus providing newer insights and popularizing the operation further.[25]
The most widely accepted system of classifying coronary anatomy in TGA is called the Leiden convention. It was initially proposed by Gittenberger-DeGroot et al., working with Quaegebeur's group based in Leiden, Netherlands.[26] In this system, the aortic sinuses are numbered based on the perspective of an imaginary person looking from the aorta to the PA. This has further simplified the understanding and conduct of the ASO.
In 1981, Lecompte from Laennec Hospital, Paris, France described a technically important modification of surgically translocating the great vessels, thereby avoiding the use of prosthetic conduit. This is now popularly called the ‘Lecompte maneuver’. This greatly simplified the method of right ventricular outflow tract reconstruction during the ASO, besides providing a better anatomical lie for the coronary arteries and the newly reconstructed aortic anastomosis. This was a near revolution and nearly all surgeons round the world routinely perform this maneuver during the ASO.[27]
In 1984, Castaneda and colleagues from the Children's Hospital, Boston introduced the concept of the neonatal arterial switch describing their experience with 14 neonates. This emphasized the ability of the neonatal LV to successfully handle the systemic circulation.[28] Today neonatal arterial switch remains the treatment of choice for TGA with remarkably low mortality.
It was soon realized that the arterial switch did not give acceptable results after the 1st month of life. The inability of the LV to operate at a systemic pressure after regression of neonatal pulmonary hypertension was identified as the problem. Sir Magdi Yacoub from Harefield Hospital, United Kingdom proposed a two-stage repair by performing PA banding first, to train the LV followed by a second stage arterial switch several months or a year later. Thus, arose the concept of ‘left ventricular training’.[29]
The concept of rapid two-stage arterial switch for patients with TGA with intact septum presenting beyond neonatal period was introduced by Jonas and colleagues in 1989.[30] The concept was based on studies on molecular biology of cardiac hypertrophy of the then Chief of Cardiology of Boston Childrens’ Hospital; Dr Bernardo Nadal-Ginard. His work demonstrated that, in response to pressure load, there was a shift in the isoforms of heavy chain myosin and the changes were maximum at 48 h from the onset of pressure load.[31] In a rapid two-stage procedure, the first stage consists of pulmonary banding to provide the after-load with a modified BTS to provide a preload and improve the systemic oxygen saturation. This is followed by a corrective second stage arterial switch 5-7 days later.
The availability of mechanical circulatory support further allowed pushing the age limits of performing the arterial switch.[32] It was subsequently proved that the LV maintains its ability to support the systemic circulation well beyond the neonatal period. However, the time taken for the LV to undergo ‘reconditioning’ is a function of the age of surgery. If this period of temporary LV dysfunction is taken care of, using mechanical support like extracorporeal membrane oxygenation (ECMO), the age limit can be pushed to as much as 6 months. In addition, the ECMO supported approach also offers advantages of having to perform a single surgery, normoxemia, and progressive improvement in LV performance independent of the after-load and faster reconditioning period over the PA band-BTS-based two-stage approach.[33] The ECMO-based delayed arterial switch has been successfully reported in patients as old as 9 years.[34]
Yet another approach for the regressed LV includes an atrial switch with PA band in the same sitting to train the LV. This is later followed by takedown and a corrective arterial switch. This approach is not entirely new and has been tried before experimentally. However, it seems an attractive alternative in situations like regressed LV and nonavailability of ECMO and needs further evaluation.[35]
Surgical treatments of variants of TGA with left ventricular outflow tract obstruction evolved in parallel. Giancarlo Rastelli from Mayo clinic described the Rastelli procedure involving the use of a conduit for reconstruction of the pulmonary outflow tract.[36] Lack of growth of the conduit was perceived as a major shortcoming, giving way to the ‘REV’ (reéparation a la larat ventriculaire) procedure to reconstruct the pulmonary outflow tract without using a prosthetic conduit as an alternative to the Rastelli repair in 1980.[37] Two years later Dr. Hisashi Nikaidoh from Children's Medical Center, Dallas, Texas described aortic translocation with biventricular outflow tract reconstruction as another surgical option in patients in whom the native pulmonary valve was unusable as a future aortic valve.[38] The concept of anterior pulmonary root translocation was introduced by da Silva and colleagues from Brazil as an alternative to the Rastelli and REV procedures. The translocation is used to construct the right ventricular outflow tract after baffling the LV to the aorta. Thus a conduit was avoided and the native pulmonary valve was also used in the right ventricular outflow tract. Serial follow-up by this group has demonstrated growth of the native pulmonary annulus and freedom from gradients has been acceptable.[39]
As long-term results of arterial switch are being reported, newer sets of potential surgical problems are becoming evident. Progressive neo-aortic dilation (pulmonary valve at birth) has been observed. However, this dilation does not necessarily translate into aortic regurgitation (AR) and need for surgery in all patients. Older age at time of ASO, presence of ventricular septal defect, and previous PA banding have been found to be risk factors for AR.[40]
An alarming number of asymptomatic patients who have been evaluated by angiography have demonstrated coronary artery obstructions.[41] An even higher number have shown proximal eccentric intimal proliferation on evaluation with intravascular ultrasound.[42]
Right ventricular outflow tract obstruction (RVOTO) was recognized as a complication within the first decade of the ASO being performed.[28] It is one of the commonest causes of reintervention after ASO. The causes recognized include inadequate growth at the neo-pulmonary anastomotic site and inadequate mobilization of branch pulmonary arteries during the Lecompte maneuver. Though the number of reinterventions for RVOTO is going down as experience is accumulating, it has been identified as a cause for reduced physical performance.[43]
Apart from the Fontan palliation, very few surgical treatments have undergone such rapid evolution through various stages as that of TGA. History stands witness to a combination of brilliant innovation, technical ingenuity, and surgical expertise of multiple surgeons to whom this article is dedicated to. This overview shows that surgical techniques need continuous reappraisal and refinement. The quest to perform a perfect arterial switch remains a perpetual effort. The journey may have only just begun.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Baillie M. 2nd ed. London: Johnson and Nicol; 1797. The morbid anatomy of some of the more important parts of the human body; p. 38. [Google Scholar]
- 2.Farre JR. London: Longman, Hurst, Rees, Orme and Brown; 1814. Pathological researches. Essay 1: On malformations of the human heart; p. 28. [Google Scholar]
- 3.Van Praagh R, Perez-Trevino C, Lõpez-Cuellar M, Baker FW, Zuberbuhler JR, Quero M, et al. Transposition of the great arteries with posterior aorta, anterior pulmonary artery, subpulmonary conus and fibrous continuity between aortic and atrioventricular valves. Am J Cardiol. 1971;28:621–31. doi: 10.1016/0002-9149(71)90049-x. [DOI] [PubMed] [Google Scholar]
- 4.Hanlon CR, Blalock A. complete transposition of the aorta and the pulmonary artery: Experimental observations on venous shunts as corrective procedures. Ann Surg. 1948;127:385–97. doi: 10.1097/00000658-194803000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blalock A, Hanlon CR. The surgical treatment of complete transposition of the aorta and the pulmonary artery. Surg Gynecol Obstet. 1950;90:1–15. [PubMed] [Google Scholar]
- 6.Weldon CS. The Blalock-Hanlon operation. Ann Thorac Surg. 1987;43:448–9. doi: 10.1016/s0003-4975(10)62834-2. [DOI] [PubMed] [Google Scholar]
- 7.Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy. A palliative approach to complete transposition of the great arteries. JAMA. 1966;196:991–2. [PubMed] [Google Scholar]
- 8.Park SC, Zuberbuhler JR, Neches WH, Lenox CC, Zoltun RA. A new atrial septostomy technique. Cathet Cardiovasc Diagn. 1975;1:195–201. doi: 10.1002/ccd.1810010210. [DOI] [PubMed] [Google Scholar]
- 9.Edwards WS, Bargeron LM, Jr, Lyons C. Reposition of right pulmonary veins in transposition of great vessels. JAMA. 1964;188:522–3. doi: 10.1001/jama.1964.03060320044010. [DOI] [PubMed] [Google Scholar]
- 10.Lillehei CW, Varco RL. Certain physiological, pathological and surgical features of complete transposition of the great vessels. Surgery. 1953;34:376–400. [PubMed] [Google Scholar]
- 11.Baffes TG, Willis J. Potts: His contributions to cardiovascular surgery. Ann Thorac Surg. 1987;44:92–6. doi: 10.1016/s0003-4975(10)62371-5. [DOI] [PubMed] [Google Scholar]
- 12.Baffes TG. A new method for surgical correction of transposition of the aorta and pulmonary artery. Surg Gynecol Obstet. 1956;102:227–33. [PubMed] [Google Scholar]
- 13.Baffes TG, Lev M, Paul MH, Miller RA, Riker WL, De Boer A, et al. Surgical correction of the transposition of the great vessels: A five-year survey. J Thorac Cardiovasc Surg. 1960;40:298–309. [PubMed] [Google Scholar]
- 14.Konstantinov IE, Alexi-Meskishvili VV, Williams WG, Freedom RM, Van Praagh R. Atrial switch operation: Past, present, and future. Ann Thorac Surg. 2004;77:2250–8. doi: 10.1016/j.athoracsur.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Albert HM. Surgical correction of transposition of the great vessels. Surg Forum. 1955;5:74–7. [PubMed] [Google Scholar]
- 16.Creech O, Jr, Mahaffey DE, Sayegh SF, Sailors EL. Complete transposition of great vessels; A technique for intracardiac correction. Surgery. 1958;43:349–54. [PubMed] [Google Scholar]
- 17.Meredino KA, Jesseph JE, Herron PW, Thomas GI, Vetto RR. Interatrial venous transposition; A one-stage intracardiac operation for the conversion of complete transposition of the aorta and pulmonary artery to corrected transposition: Theory and clinical experience. Surgery. 1957;42:898–909. [PubMed] [Google Scholar]
- 18.Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959;45:966–80. [PubMed] [Google Scholar]
- 19.Kirklin JW, Devloo RA, Weidman WH. Open intracardiac repair of transposition of the great vessels: 11 cases. Surgery. 1961;50:58–66. [PubMed] [Google Scholar]
- 20.Mustard WT, Keith JD, Trusler GA, Fowler R, Kidd L. The surgical management of transposition of the great vessels. J Thorac Cadiovasc Surg. 1964;48:953–8. [PubMed] [Google Scholar]
- 21.Quaegebeur JM, Rohmer J, Brom AG. Revival of the Senning operation in the treatment of transposition of the great arteries. Preliminary report on recent experience. Thorax. 1977;32:517–24. doi: 10.1136/thx.32.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustard WT, Chute AL, Keith JD, Sirek A, Rowe RD, Vlad P. The surgical approach to transposition of the great vessels with extracorporeal circuit. Surgery. 1954;36:39–51. [PubMed] [Google Scholar]
- 23.Bailey CP, Cookson BA, Downing DF, Neptune WB. Cardiac surgery under hypothermia. J Thorac Surg. 1954;27:73–91. [PubMed] [Google Scholar]
- 24.Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72:364–70. [PubMed] [Google Scholar]
- 25.Yacoub MH, Radley-Smith R. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax. 1978;33:418–24. doi: 10.1136/thx.33.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gittenberger-de Groot AC, Sauer U, Oppenheimer-Dekker A, Quaegebeur J. Coronary arterial anatomy in transposition of the great arteries: A morphologic study. Pediatr Cardiol. 1983;4:15. [Google Scholar]
- 27.Lecompte Y, Zannini L, Hazan E, Jarreau MM, Bex JP, Tu TV, et al. Anatomic correction of transposition of the great arteries. J Thorac Cardiovasc Surg. 1981;82:629–31. [PubMed] [Google Scholar]
- 28.Castaneda AR, Norwood WI, Jonas RA, Colan SD, Sanders SP, Lang P. Transposition of the great arteries and intact ventricular septum: Anatomical repair in the neonate. Ann Thorac Surg. 1984;38:438–43. doi: 10.1016/s0003-4975(10)64181-1. [DOI] [PubMed] [Google Scholar]
- 29.Yacoub MH, Radley-Smith R, Maclaurin R. Two-stage operation for anatomical correction of transposition of the great arteries with intact interventricular septum. Lancet. 1977;1:1275–8. doi: 10.1016/s0140-6736(77)91317-4. [DOI] [PubMed] [Google Scholar]
- 30.Jonas RA, Giglia TM, Sanders SP, Wernovsky G, Nadal-Ginard B, Mayer JE, Jr, et al. Rapid, two-stage arterial switch for transposition of the great arteries and intact ventricular septum beyond the neonatal period. Circulation. 1989;80:I203–8. [PubMed] [Google Scholar]
- 31.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85:339–43. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mee RB, Harada Y. Retraining of the left ventricle with a left ventricular assist device (Bio-Medicus) after the arterial switch operation. J Thorac Cardiovasc Surg. 1991;101:171–3. [PubMed] [Google Scholar]
- 33.Kang N, de Leval MR, Elliott M, Tsang V, Kocyildirim E, Sehic I, et al. Extending the boundaries of the primary arterial switch operation in patients with transposition of the great arteries and intact ventricular septum. Circulation. 2004;110:II123–7. doi: 10.1161/01.CIR.0000138221.68312.33. [DOI] [PubMed] [Google Scholar]
- 34.Bisoi AK, Sharma P, Chauhan S, Reddy SM, Das S, Saxena A, et al. Primary arterial switch operation in children presenting late with d-transposition of great arteries and intact ventricular septum. When is it too late for a primary arterial switch operation? Eur J Cardiothorac Surg. 2010;38:707–13. doi: 10.1016/j.ejcts.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Sharma R. Pulmonary artery banding: Rationale and possible indications in the current era. Ann Pediatr Cardiol. 2012;5:40–3. [PMC free article] [PubMed] [Google Scholar]
- 36.Rastelli GC, McGoon DC, Wallace RB. Anatomic correction of transposition of the great arteries with ventricular septal defect and subpulmonary stenosis. J Thorac Cardiovasc Surg. 1969;58:545–52. [PubMed] [Google Scholar]
- 37.Lecompte Y, Neveux JY, Leca F, Zannini L, Tu TV, Duboys Y, et al. Reconstruction of the pulmonary outflow tract without prosthetic conduit. J Thorac Cardiovasc Surg. 1982;84:727–33. [PubMed] [Google Scholar]
- 38.Nikaidoh H. Aortic translocation and biventricular outflow tract reconstruction. A new surgical repair for transposition of the great arteries associated with ventricular septal defect and pulmonary stenosis. J Thorac Cardiovasc Surg. 1984;88:365–72. [PubMed] [Google Scholar]
- 39.da Silva JP, Baumgratz JF, da Fonseca L. Pulmonary root translocation in transposition of great arteries repair. Ann Thorac Surg. 2000;69:643–5. doi: 10.1016/s0003-4975(99)01387-9. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz ML, Gauvreau K, del Nido P, Mayer JE, Colan SD. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation. 2004;110:II128–32. doi: 10.1161/01.CIR.0000138392.68841.d3. [DOI] [PubMed] [Google Scholar]
- 41.Bonhoeffer P, Bonnet D, Piéchaud JF, Stümper O, Aggoun Y, Villain E, et al. Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. J Am Coll Cardiol. 1997;29:202–6. doi: 10.1016/s0735-1097(96)00433-0. [DOI] [PubMed] [Google Scholar]
- 42.Pedra SR, Pedra CA, Abizaid AA, Braga SL, Staico R, Arrieta R, et al. Intracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2005;45:2061–8. doi: 10.1016/j.jacc.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 43.Bové T, De Meulder F, Vandenplas G, De Groote K, Panzer J, Suys B, et al. Midterm assessment of the reconstructed arteries after the arterial switch operation. Ann Thorac Surg. 2008;85:823–30. doi: 10.1016/j.athoracsur.2007.10.043. [DOI] [PubMed] [Google Scholar]


