Abstract
Objective:
To assess the feasibility and outcome of laparoscopic Myomectomy and multiple layer closure of myoma bed for management of myomas at a tertiary care hospital.
Materials and Methods:
Four hundred and seventeen patients from September 2005 to September 2010 with large and moderate size myomas were managed by laparoscopic Myomectomy. Indications were subfertility, menorrhagia and abdominal mass. Pre-operative evaluation included history, clinical examination and sonographic mapping. Myomas were enucleated and retrieved laparoscopically. Myoma beds were sutured in multiple layers by endoscopic intracorporeal suturing.
Results:
Three hundred and fifteen patients presented with subfertility, 45 with menorrhagia and 57 with abdominal mass. The average maximum diameter of myoma was 9 cm. The mean duration of surgery was 120 min. The mean post-operative stay was 24 h. No intra-operative complication occurred and hospital course was uncomplicated. In one case, minilap incision was given for retrieval of myoma and suturing of the bed. Two patients had minor delayed wound healing of the morcellator port site. The patients did not report any complaints during follow-up except one patient who developed omental hernia at morcellator port site. There was no rupture of scar and very low adhesion scores in subsequent caesarian sections or second look scopies.
Conclusion:
With proper multilayer closure of the myoma bed, laparoscopic Myomectomy is feasible for moderate and even large myomas and has excellent outcomes.
Keywords: Better reproductive outcome, laparoscopic myomectomy, large myomas, multilayer closure
INTRODUCTION
Uterine leiomyoma is the most common tumor of female reproductive tract. An incidence of 20-25% in sexually active and 30-35% in all women irrespective of age has been reported.[1] The surgical management of myomas has advanced significantly, there has been a renewed interest in the removal of myoma alone, i.e., Myomectomy rather than hysterectomy, which was carried out historically. With the trends toward minimally invasive endoscopic surgery, procedures have been developed to accomplish Myomectomy via either laparoscopic or hysteroscopic route.[2] Just as these procedures have come up, they have met with several criticisms like lack of meticulous closure, inadequate hemostasis, chances of rupture in subsequent pregnancy and high-risk of adhesion formation. We report our experience of 417 cases of laparoscopic Myomectomy of large and moderate size myomas in a tertiary care hospital.
AIMS AND OBJECTIVE
In this article, our objective is to describe our technique of laparoscopic Myomectomy and multiple layer closure of myoma bed and discuss its outcomes and advantages, overcoming all the criticisms surrounding laparoscopic Myomectomy.
MATERIALS AND METHODS
In a retrospective study, conducted at our tertiary care hospital, we evaluated 417 patients in the past 5 years with large and moderate size myomas (more than 7 cm), largest being up to 17 cm producing an abdominal mass corresponding to 32 weeks size uterus.
Inclusion criteria included largely
Patients being treated for subfertility with myomas causing endometrial distortion or tubal occlusion with normal ovulation and normal male factor.
Patients with large myomas producing pressure symptoms.
Asymptomatic large myomas producing palpable abdominal mass up to the umbilicus or above.
Pre-operative evaluation included history, clinical examination, basic investigation for pre-anesthetic checkup and a detailed trans vaginal scan. Sonography included, mapping of myomas in number, size, and location and most importantly differentiating it from adenomyosis and adenomyoma.[3] Although, we carry out adenomyomectomy with almost identical technique, but still surgical preparedness is there and patient counseling is done accordingly.
In all cases, an informed consent was taken. Hemoglobin was optimized using iron sucrose injections. Extensive bowel and rectal preparation was carried out in each case. No patient received GnRH analog prior to surgery as it leads to loss of cleavage plane during surgery. All operations were performed under general anesthesia with inhalational anesthetics avoiding nitrous oxide as it causes bloating of bowel.[4]
Our innovative technique of port placement involved intra umbilical veress needle insertion without any incision and utilizing right upper 5 mm port for primary trocar insertion. The 10 mm port was optimized supra-umbilically, under direct vision of 5 mm telescope depending on the myoma size, which went even up to the xiphisternum in large myomas. All accessory ports were also made so that they remain above and out-side the biggest myoma. The most common port placement was: Two ipsilateral ports for enucleation and suturing, one contralateral port for the assistant anywhere between umbilicus and anterior superior iliac spine (depending on myoma size) and a supra pubic port for myoma screw. On peritoneal entry all pelvic and abdominal structures were inspected and other pathologies, if present, were noted. Pitressin was injected at the concentration of 20 units/200 ml between serosa and pseudo capsule of myoma to decrease the bleeding and for hydrodissection. Hysteroscopy was carried out in the meantime to see and treat any submucous myoma.
In most of the cases, we preferred to make a transverse incision over the most bulging part of the myoma with the harmonic scalpel taking care not to extend to cornual ends. In case of multiple myomas, we preferred to give single anterior or posterior incision in such a manner, that most myomas can be enucleated by a single incision from superficial to deepest locations. If incision was expected to extend too far than vertical incision was resorted to very rarely. Sharp dissection was carried out in the plane between myoma and the pseudo capsule with harmonic scalpel, coagulating, and cutting all vascular bridges, causing very minimal blood loss. Two myoma screws were used in case of very large myomas, one was kept in the constant position from the supra pubic port and the other kept changing to allow for traction near the working end. Myoma was finally enucleated from its bed by traction and counter traction. After enucleation, there was usually no bleeding, but if at all there was, then light minimal bipolar coagulation was carried out. After achieving adequate hemostasis, myoma bed was sutured in transverse, continuous and non-locking fashion in multiple layers. A suture of 45–50 cm length, no. 1–0 vicryl on a taper cut needle was loaded and introduced in the peritoneal cavity using the Clark Reich method of removing the 5 mm trocar. The angle knot was taken by passing the suture from upper and lower edge of myoma bed and an intracorporeal, tight surgeon's knot was secured. From this point continuous, non-locking suturing was carried out using needle holders in both hands for a much better grip. First layer of deep myometrium was thus completed and the same suture is used to take second layer of superficial myometrium. Third layer of serosa was taken, up to the starting point of the first layer at the angle and tied there. In very large myomas, which were very deep intramural and cause a symmetrical enlargement of the uterus, the myoma bed, which comes after Myomectomy was very deep. So, we preferred to go from the deepest myometrium working upwards layer by layer till a total reconstruction is carried out, and uterus appears absolutely in shape with only one knot on the surface. In very large myomas, we sutured in even up to six layers for proper approximation of the defect. While suturing all the layers, care was taken to take bites at equal distances and take adequate tissue to fully obliterate all dead space and give a neat look. To minimize the exposed thread volume, recently we have also applied subserosal baseball sutures in most superficial layer. In this way there was only one knot in the entire myoma bed minimizing the knot volume and decreasing the adhesion formation potential. Copious irrigation and lavage was carried out. Myomas were retrieved by Rotocut G1 morcellator (Karl storz, Germany) via 15 mm sleeve. Very large myomas were retrieved by 20 mm sleeve of Sawhle morcellator (Karl storz, Germany). Morcellator port site was meticulously closed by port closure needle and 20 mm site required rectus sheath closure [Figures 1–12]. All patients were up and about within 6 h of surgery, were orally allowed, started ambulation and deep breathing and leg raising exercise and were discharged after 24 h.
Figure 1.
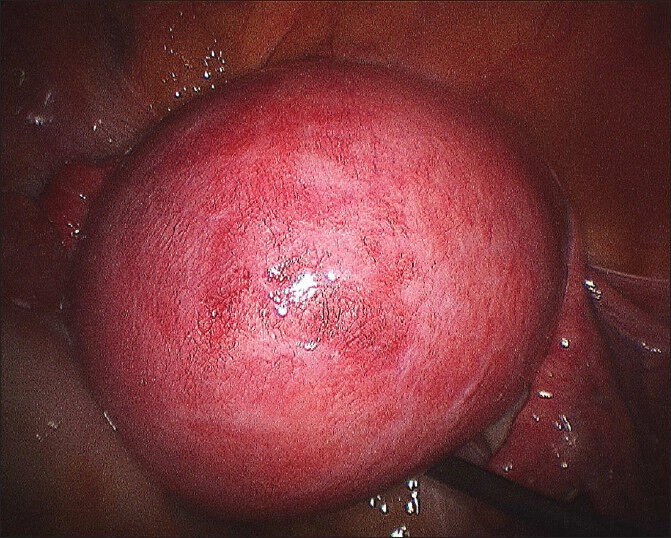
Big posterior wall myoma
Figure 12.
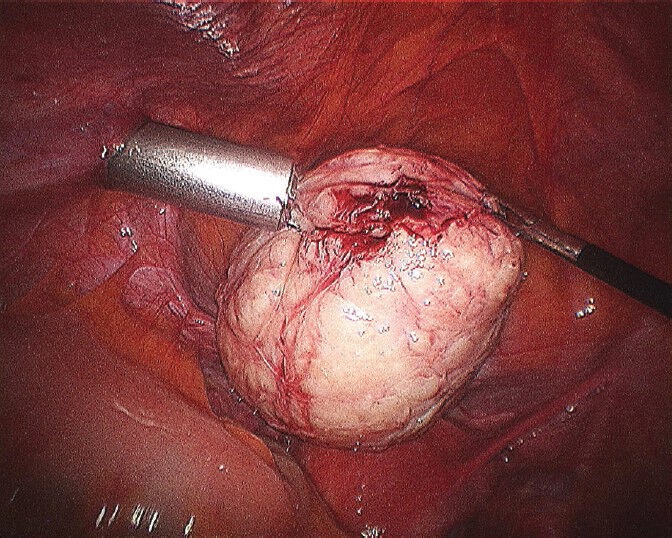
Completed third layer
Figure 2.
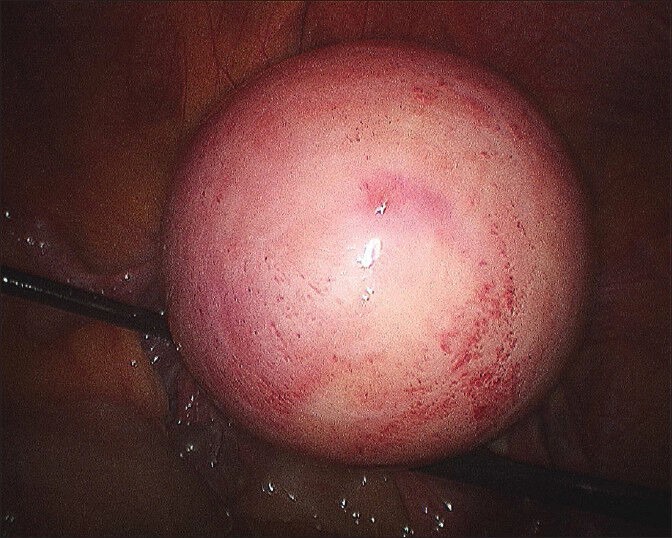
Last layer incorporating subserosa and serosa
Figure 3.
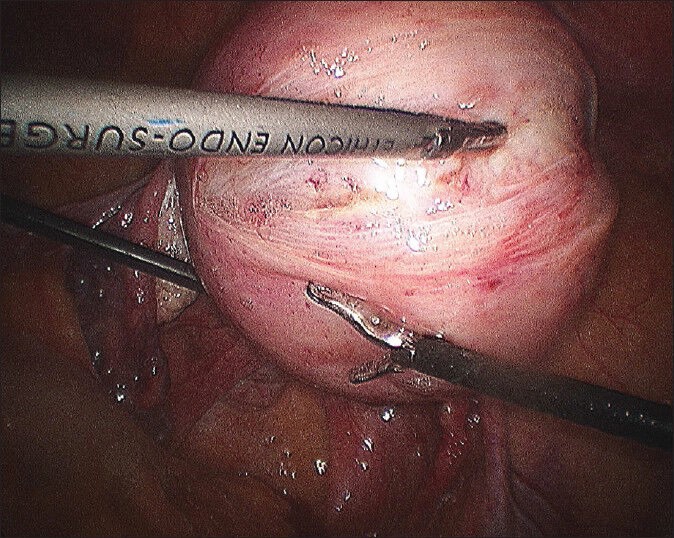
Final appearance of myoma bed sutured with single knot on the surface
Figure 4.
Morcellation of myoma in progress
Figure 5.
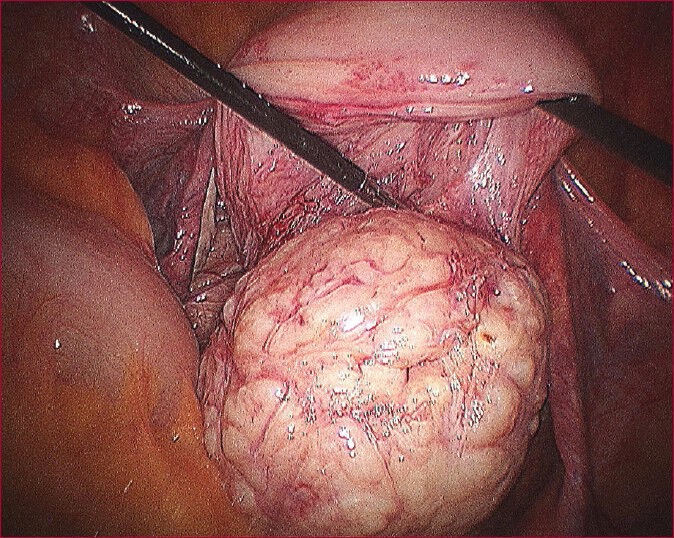
Blanched look of myoma after injecting diluted vasopressin
Figure 6.
Transverse incision over most bulging part of the myoma with harmonic ACE
Figure 7.
Enucleation of myoma
Figure 8.
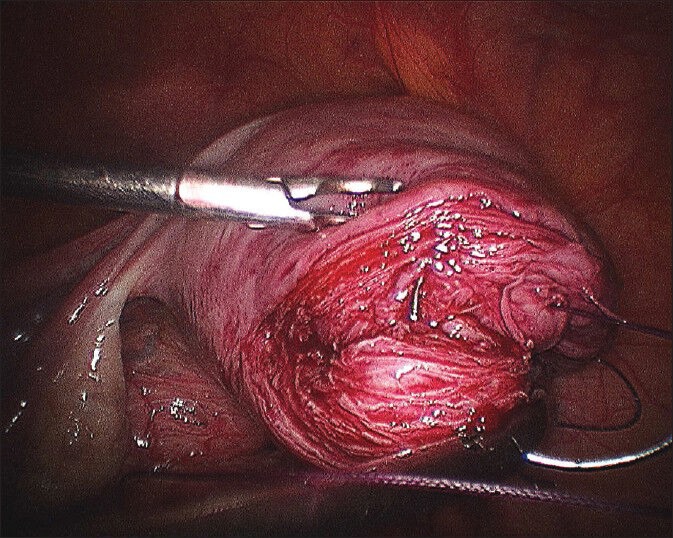
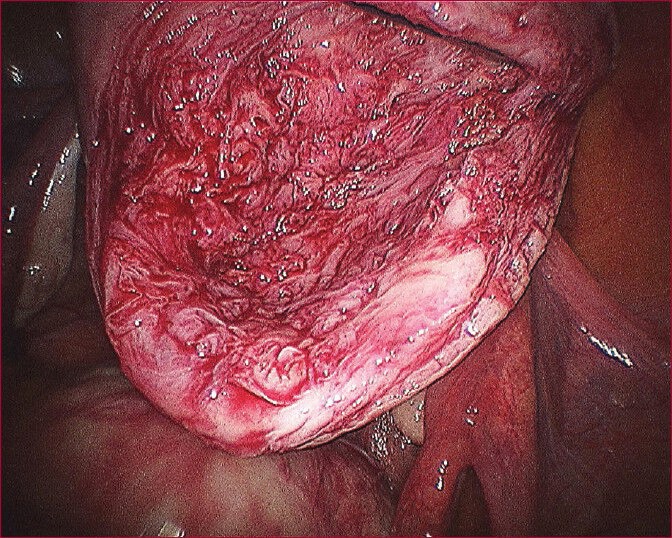
Myoma bed after myoma enucleation
Figure 9.
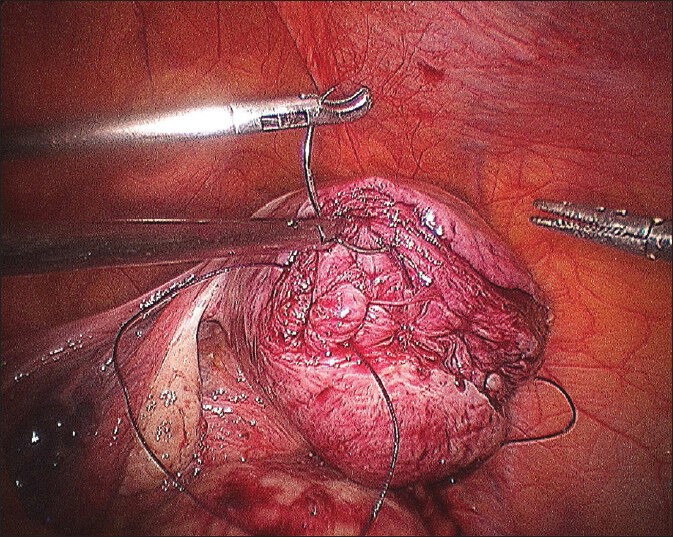
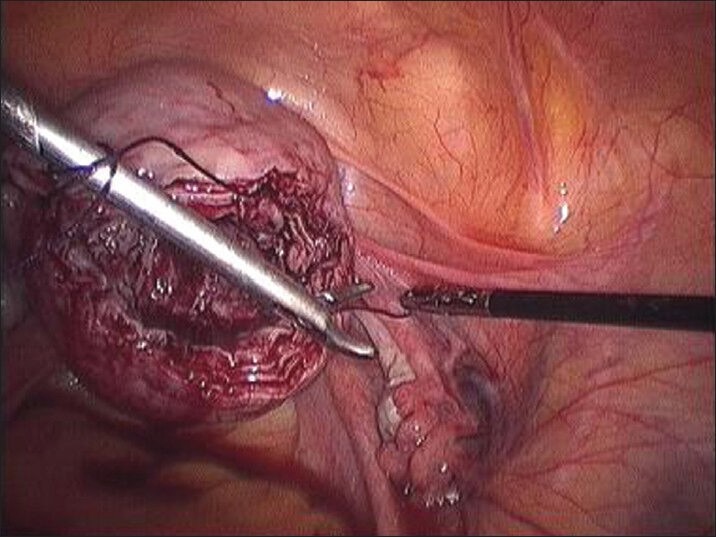
First stitch in deepest myometrium and knot being tied
Figure 10.
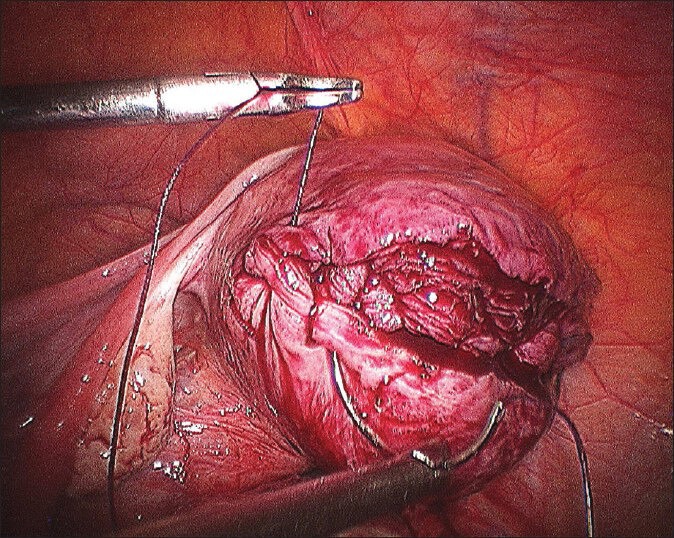
Second layer in progress
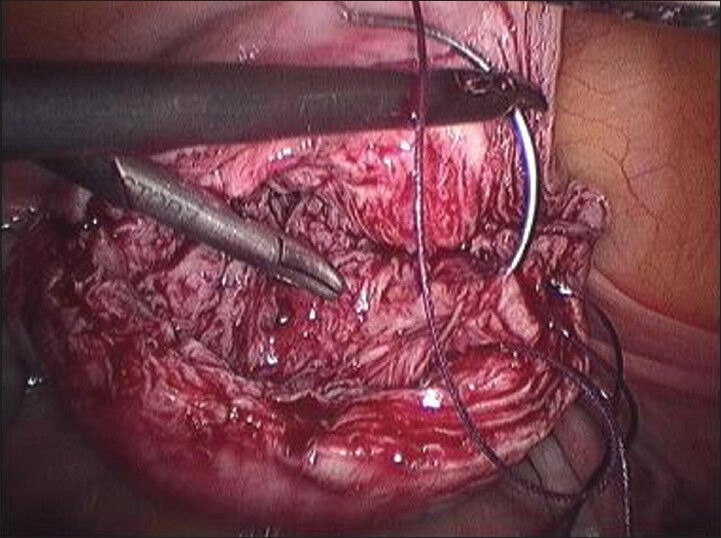
Figure 11.
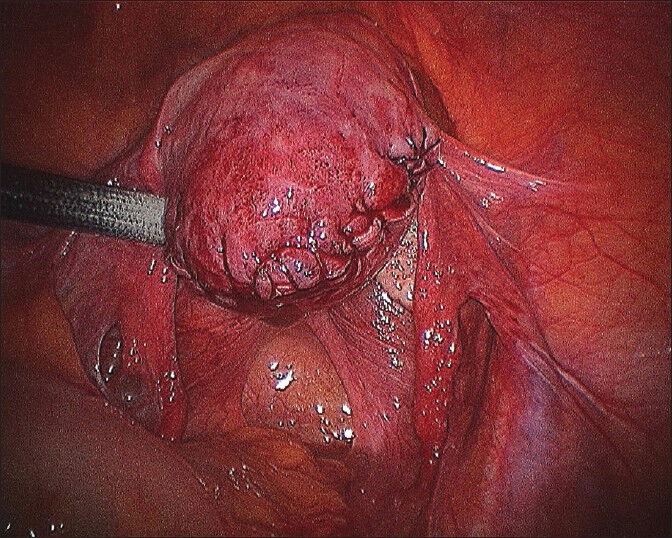
Third layer near completion
RESULTS
The mean age of patients was 28 ± 2 years. Three hundred and fifteen (75%) patients presented with sub fertility, 45 (11%) patients with menorrhagia and 57 (14%) patients with abdominal mass. The average maximum diameter of the myoma was 9 cm.
The mean duration of surgery was 120 min. No intra-operative complication occurred. Only in one case, minilap incision was given for retrieval of myomas and suturing of myoma bed. This was, in fact, a case of large uterus with multiple adenomyomas, which made a pure laparoscopic approach difficult due to lack of plane of cleavage, leading to more bleeding and difficult closure in adenomyotic tissue. Blood transfusion was required only in 25 (6%) patients with very large size myomas or multiple Myomectomy sites. There were no conversions to laparotomy ever and never a hysterectomy.
The mean post-operative stay was 24 h. All patients had an uneventful recovery. None of the patient had reactionary or late secondary hemorrhage.
Two patients reported delayed wound healing of the morcellator site and one patient developed omental hernia at morcellator site on long term follow-up.
Very low adhesions scores were observed in subsequent second look scopies carried out by us. Other colleagues also reported very low adhesions in patients they delivered by caesarian section.
Amongst the subgroup of patients who presented with infertility, 198 (63%) patients conceived after the surgery had an uneventful antenatal and intra natal course. No patient had rupture of Myomectomy scar. Minimal adhesions were noted in subsequent caesarean sections. Even the first Myomectomy we did conceived after 17 years of married life, just 1 month after Myomectomy. She continued pregnancy up to term and an elective caesarean was carried out at term, no adhesions or even depression at Myomectomy site was observed.
Recurrence of myomas was seen in 50 (12%) patients. Three (0.7%) patients out of these, who were around 35 years of age when Myomectomy was performed for menorrhagia, later underwent hysterectomy. Two (0.5%) patients desirous of pregnancy had a repeat Myomectomy carried out on account of recurrence of myoma during the study period.
DISCUSSION
In this era of minimally invasive endoscopic surgery, laparoscopic Myomectomy has become the order of the day. It offers several advantages over laparotomy including, less operative trauma and blood loss, reduced post-operative morbidity, shorter hospital stay and recovery time, earlier return to normal activity, fewer post-operative adhesions, better cosmesis, improved patient compliance and better pregnancy outcome. Furthermore, the magnification it provides allows careful micro surgical dissection, development of avascular planes and perfect hemostasis.[5]
Many surgeons still prefer to do open Myomectomy due to technical difficulties or lack of endo suturing skills. One of their major concerns is the risk of hemorrhage and uterine rupture in the subsequent pregnancies.[6] It has been reported that rupture can occur during the course of pregnancy or during delivery after removal of myomas.
However, in experienced surgeon's hands, with superior skill of endo suturing, laparoscopic Myomectomy is a very safe procedure and such complications are rare.
A long-term survey by Dubuisson, et al., found three cases of spontaneous uterine rupture in 15 pregnancies and only one occurred at the laparoscopic myomectomy site.[7] The strength of scar largely depends upon proper meticulous closure of the incision site. That is why multiple layer closure gives much better results than single or two layer closures. It also gives much better approximation of the defect and a neat finish in the end of procedure. Based on the clinical trials and case series, it would appear that the risk of uterine rupture during pregnancy is no higher than 1% when the Myomectomy incision is appropriately repaired.[8] We had no scar rupture even in very big myomas or multiple myomas.
Another complication, causing much concern is the subsequent adhesion formation. We, at our institute, perform continuous suturing of the myoma bed using no. 1-0 polygalactin suture on taper cut needle in multiple layers so that there is only one knot on the surface. The amount of adhesions formed per suture line is directly proportional to the number of knots and lesser the number of bulky knots lesser the adhesion scores. We have observed that this technique gives much better results than that of interrupted knotting leading to tremendous knot volume. However, this requires advanced suturing skills.
Retrieval of large myoma also poses technical difficulties to most of the surgeons. Morcellators have made this task much simpler.[9] As the myoma is progressively morcellated and the uterine size reduced, additional space is created for the optimum movement of instruments. The 12 or 15 mm claw forceps of the morcellator offers better grip and steady traction. This tremendously reduces the operating time and reduces the technical difficulty of the procedure. We took extreme care to remove myomas in longer chips to reduce the morcellation time. Exact count of myomas and location of their parking in the abdomen was kept to avoid misplacing any myoma. Such misplaced myomas are a common cause of Iatrogenic myomas as reported by Nezhat et al. in their study.[10]
The only complications we observed were that of delayed wound healing of the morcellator site in two patients. This was probably due to jagged margins created by repeated manipulations at the morcellator site. We thus, recommend that one should avoid using towel clips to avoid gas escape around morcellator site, which starts off in prolonged morcellation time.
Another safer and relatively simpler procedure in the hands of experienced laparoscopist as well as novices is laparoscopic assisted minilap myomectomy.[11] In this procedure initially laparoscopy is performed giving incision over myoma with the ultrasonic shears. Myoma is partly enuclated by traction of myoma screw. A 3-4 cm minilap pfannenstiel incision is given. Through this minilap incision enucleation, morcellation and suturing of beds is carried out of all multiple and big myomas. Then minilap incision is sutured back, laparoscope re-inserted and thorough suction irrigation and lavage carried out. However, it should be planned pre-operatively and there is no sudden conversion on table from laparoscopic to laparoscopic assisted Myomectomy.
Our experience demonstrates the feasibility of dealing with even large size myoma successfully laparoscopically and we encourage multiple layer closure to decrease the dead space and avoidance of hematoma formation. And in turn, achieve good reproductive outcome.
CONCLUSION
Laparoscopic Myomectomy is a safe and favorable alternative to open Myomectomy as it offers several advantages over laparatomy with minimal complications.[12] It is equally feasible for even large size myomas in experienced hands with advance suturing skills. Multilayer closure of the myoma bed in continuous, non-locking fashion gives excellent reproductive outcomes in terms of very low adhesion scores and negligible risk of scar rupture in subsequent pregnancies. However, one should appropriately select the myoma size according to one's suturing skills, instruments available and experience.
Footnotes
Source of Support: Equipment, Karl Storz, Germany.
Conflict of Interest: None declared.
REFERENCES
- 1.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil Steril. 1981;36:433–45. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 2.Hurst BS, Matthews ML, Marshburn PB. Laparoscopic myomectomy for symptomatic uterine myomas. Fertil Steril. 2005;83:1–23. doi: 10.1016/j.fertnstert.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Fedele L, Bianchi S, Dorta M, Arcaini L, Zanotti F, Carinelli S. Transvaginal ultrasonography in the diagnosis of diffuse adenomyosis. Fertil Steril. 1992;58:94–7. [PubMed] [Google Scholar]
- 4.Alexander GD, Noe FE, Brown EM. Anesthesia for pelvic laparoscopy. Anesth Analg. 1969;48:14–8. [PubMed] [Google Scholar]
- 5.Reich H, Thompson KA, Nataupsky LG, Grabo TN, Sekel L. Laparoscopic myomectomy: An alternative to laparotomy myomectomy or hysterectomy? Gynecol Endosc. 1997;6:7–12. [Google Scholar]
- 6.Dubuisson JB, Chapron C, Levy L. Difficulies and complications of laparoscopic Myomectomy. J Gynecol Surg. 1996;12:159–65. [Google Scholar]
- 7.Dubuisson JB, Fauconnier A, Deffarges JV, Norgaard C, Kreiker G, Chapron C. Pregnancy outcome and deliveries following laparoscopic myomectomy. Hum Reprod. 2000;15:869–73. doi: 10.1093/humrep/15.4.869. [DOI] [PubMed] [Google Scholar]
- 8.Sinha R, Sundaram M. Laparoscopic management of large myomas. J Gynecol Endosc Surg. 2009;1:73–82. doi: 10.4103/0974-1216.71611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha RY, Hegde A, Warty N, Jain R. Laparoscopic devascularization of uterine myomata followed by enucleation of the myomas by direct morcellation. J Am Assoc Gynecol Laparosc. 2004;11:99–102. doi: 10.1016/s1074-3804(05)60023-0. [DOI] [PubMed] [Google Scholar]
- 10.Nezhat C, Kho K. Iatrogenic myomas: New class of myomas? J Minim Invasive Gynecol. 2010;17:544–50. doi: 10.1016/j.jmig.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: A report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39–44. [PubMed] [Google Scholar]
- 12.Miller CE, Davies S, Johnston M. Atlanta, GA: Presented at the 27th Annual Meeting of the American Association of Gynecologic Laparoscopists, Miller CE, Laparoscopic Myomectomy with uterine reconstruction is a safe surgical procedure; 1998. Nov 10-15, [Google Scholar]


