Abstract
Background
Liver transplantation regularly requires transfusion of red blood cells (RBCs), plasma, and platelets. Compared to fresh frozen plasma (FFP) from single blood donors, solvent/detergent-treated plasma (SD-plasma) pooled from several hundred blood donors has advantages with respect to pathogen reduction, standardized content of plasma proteins, and significantly reduced risk of transfusion related lung injury and allergic/immunologic adverse reactions. However, SD-plasma has been suspected to increase the incidence of hyperfibrinolysis and thromboembolic events.
Study Design and Methods
We investigated the transfusion practices, hyperfibrinolysis parameters, and thrombosis outcomes in 195 consecutive adult primary liver transplants in our center using SD-plasma (Octaplas) as the exclusive source of plasma.
Results
Perioperatively, median (interquartile range) 4 (1 to 9) RBC-units, 10 (4 to 18) plasma-bags, and 0 (0 to 2) platelet-units were transfused. Hyperfibrinolysis defined as LY30 ≤ 7.5% was detected in 12/138 thrombelastography-monitored patients (9%). These patients received significantly more RBCs, plasma, and platelets than did patients without hyperfibrinolysis. Thrombotic graft complications were observed in three patients (2%). Pulmonary embolism was not observed in any patient.
Conclusion
SD-plasma is a safe plasma product for liver transplant recipients, and the incidences of hyperfibrinolysis and thromboembolic events are not significantly different from those seen in centers using FFP.
Keywords: Liver transplantation, Solvent/detergent-treated plasma, Hyperfibrinolysis, Thromboembolism
Significant perioperative bleeding and transfusions of plasma, red blood cells (RBCs), and platelets are considered part of the normal clinical course of orthotopic liver transplantation (OLT).1 By far the two most common plasma products available today are fresh frozen plasma (FFP) from single blood donors and solvent/detergent (SD)-treated pooled plasma. Whereas FFP does not undergo any kind of pathogen inactivation, the SD treatment secures pathogen reduction.2–4 Due to dilution and possible neutralization of the responsible antibodies, SD-plasma has a markedly lower rate of allergic/immunologic reactions including no reported cases of transfusion related acute lung injury (TRALI) despite over 10 million units transfused.5,6 Further, SD-plasma can be regarded as a biopharmaceutical product with standardized amounts of coagulation factors as compared to FFP where there is great bag-to-bag variation.7 Accordingly, SD-plasma has gained increased popularity during the last two decades and has partly replaced FFP in Canada, Mexico, and many European countries. One disadvantage of SD-plasma is that the SD-process inevitably reduces the levels of α2-antiplasmin and protein S, and could increase the risk of hyperfibrinolysis8 or thromboembolic events such as pulmonary embolism during liver transplantation.9 In addition to the track record of SD-plasma in Europe during the last two decades, both randomized controlled trials10–12 and critical reviews4,6 have suggested that these risks are largely theoretical. US SD-plasma was first introduced in 1998, but production was terminated in 2002–2003 in part due to thromboembolic complications not observed with European SD-plasma. Quality differences between European and US SD-plasma that can be responsible for these adverse effects have since been documented.13,14 Based on newer hemovigilance data, the adverse events incidence per 10,000 units of FFP compared to SD-plasma is: 0.29 versus 0.00 for TRALI; 34.48 versus 2.92 for severe allergic reactions; 153.85 versus 10.53 for all allergic reactions. To our knowledge, no such adverse events data have been detailed for liver transplant patients, nor have incidence data been published for hyperfibrinolysis or thromboembolism.15 Recently the US Food and Drug Administration (FDA) licensed the European SD-plasma, OctaplasLG (Octapharma AG, Lachen, Switzerland), for clinical use in the United States. Taking the costs of adverse effects of plasma products into account, a recent report suggests that replacing FFP with OctaplasLG may be cost effective.16
In Norway, the SD-plasma product Octaplas has been the only plasma product transfused since 1993. A quality assurance study to investigate transfusion load and eventual side effects after liver transplantation was undertaken in the period from 2005 to 2009 to explore the consequences of using this product in the liver transplantation unit serving the whole Norwegian population of 5×106 inhabitants. Special emphasis was placed on possible hyperfibrinolysis and thromboembolism.
Materials and Methods
Study Population
The study was approved by the institution’s personal protection ombudsman (overseer) as a quality assurance study. One hundred and ninety five patients (113 males, 82 females) underwent primary liver transplantation in a 4-year period from 2005. The median age was 52 years, ranging from 20 to 73 years. Baseline characteristics of the 195 patients are presented in Table 1, and the diagnoses are presented in Table 2.
Table 1.
Preoperative blood values in 195 liver transplant recipients transplanted from 2005 to 2009.
| Preoperative | Median (range) |
|---|---|
| Hemoglobin (g/dL) | 11.8 (6.7–16.8) |
| Blood platelets (×109/L) | 135 (13–753) |
| International Normalized Ratio1 (INR) | 1.4 (0.6–5.6) |
| Albumin (g/L) | 33 (15–79) |
| Bilirubin (μM) 1 | 48 (4–771) |
| Creatinine (μM) 1 | 69 (20–581) |
| MELD-score1 | 11 (5–42) |
The MELD (Model of End Stage Liver Disease) –score was calculated using INR, bilirubin, and creatinine
Table 2.
Distribution (n) of diagnoses in 195 liver transplant recipients from 2005 to 2009.
| Diagnosis | Number |
|---|---|
| Cirrhotic liver disease | 83 |
| Post hepatitis B or C | 23 |
| Alcoholic | 19 |
| Biliary | 17 |
| Autoimmune | 9 |
| Cryptogenic | 9 |
| Other | 6 |
| Primary sclerosing cholangitis | 53 |
| Malignant liver disease | 28 |
| Hepatocellular carcinoma | 12 |
| Secondary liver tumors | 12 |
| Biliary carcinoma | 4 |
| Acute liver failure | 19 |
| Other | 12 |
Surgical Procedure
The inferior caval vein saving piggy back technique was used in 184/195 transplantations, and veno-venous bypass with clamping of the inferior caval vein was used in 11 cases. The order of sewing the obligate anastomoses was equal in all cases; hepatic vein, portal vein, hepatic artery, and finally bile ducts. All patients had at least one central venous catheter, and the majority had two, since using a pulmonary float catheter is part of our standard procedure. The transplantation team was stable in the investigated period and consisted of six liver transplant surgeons and eight anesthesiologists. We aimed at a central venous pressure not higher than 10 mmHg during the hepatectomy.
Transfusion Practice
At the start of the surgical procedure, four units of RBCs were available for immediate transfusion. The patients were administered RBCs if their preoperative hemoglobin was lower than 9 to 10 g/dL. International Normalized Ratio (INR) values higher than 2.0 were in most cases corrected with plasma or, in cases of severe coagulopathy and/or volume overload, with prothrombin complex (factors II, VII, IX, and X, as well as proteins C and S) from various manufacturers. Platelets were most often transfused if the preoperative count was below 30–40 ×109/L, and the patient had a bleeding tendency assessed clinically and/or by thromboelastography (TEG). We did not routinely administer any antifibrinolytic agent. In general, 1 to 2 g of tranexamic acid was given intravenously if the patient had a bleeding tendency, and if the TEG findings indicated hyperfibrinolysis as well. Rarely, this dose was given without performing TEG. Until 2007, patients with severe liver failure were occasionally administered aprotinin as continuous intravenous infusion from the start of the procedure to prevent hyperfibrinolysis. Low concentrations of fibrinogen were most often corrected with plasma or with fibrinogen concentrate (Haemocomplettan, CSL Behring, Marburg, Germany), which has largely replaced cryoprecipitate at our institution. A postoperative hemoglobin concentration of 7 (8) to 10 g/dL was targeted.
Identification of Transfused Blood Products
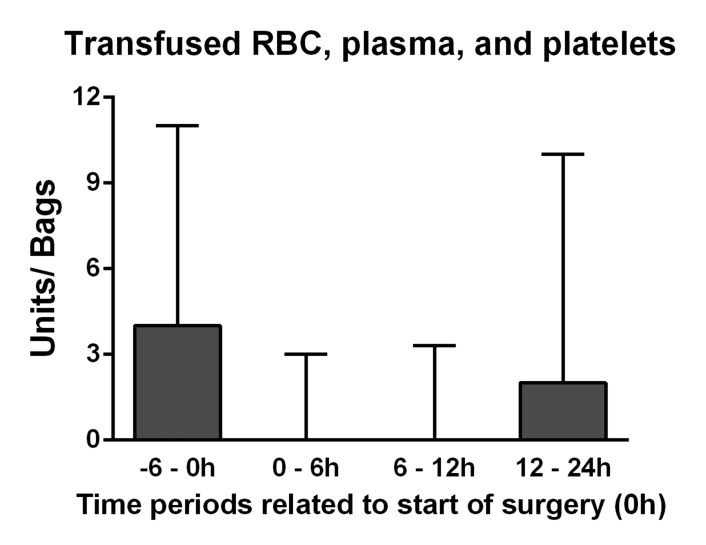
The transfused bags of RBC, plasma, and platelets were identified by an advanced computerized and author independent search in our institution’s blood bank registry. The time points of delivering the blood products were related to start of surgery (T0), and the perioperative period was defined as units delivered during 6 hours before and until 24 hours after T0 (Figure 1); an exact delimitation of the intraoperative period was not possible, as products delivered during 6 hours before T0 often were transfused intraoperatively. Approximate volumes of the units of RBCs and platelets are 250 mL, whereas one bag of plasma contains 200 mL. Intraoperative cell salvage was used infrequently, and such instances were not reported.
Figure 1.
Delivered units of red blood cell/platelets and bags of plasma related to time point of start of surgery (0). The perioperative period reached from 6 hours before start of surgery until 24 hours after. Data are presented as median (upper quartile).
Detection of Hyperfibrinolysis
The indication for thromboelastography-monitoring (TEG, Haemonetics, Braintree, MA) was based on clinical evaluation for each case. Intraoperatively, 619 TEG-measurements were performed in 138 of the 195 patients. On average, 4.5 measurements were performed in each analyzed patient, ensuring that the different stages during the procedure were covered. The normal values given in the manufacturer’s manual, and as used by others,17 were applied for interpretation, and hyperfibrinolysis was accordingly considered present if “LY30%” (ie, rate of fibrinolysis at 30 minutes) was 7.5 % or higher.
Identification of Thromboembolic Complications
The patients’ medical records were searched in a computerized and author independent way for episodes of pulmonary embolism as well as hepatic artery, portal and hepatic vein thrombosis.
Statistical Analyses
Data from different groups were compared by the Kruskal-Wallis and Mann-Whitney U- tests, and for the latter test the P-values were Bonferroni adjusted according to the number of compared groups. Correlation analyses were performed with the Spearman’s rho test. The presented P-values are 2-sided, and P ≤ 0.05 was considered significant. The statistical analyses were performed using SPSS 18.0 (IBM, Chicago, IL).
Results
Study Population
The overall 1- and 3-year survival rates were 92% and 87%, respectively. One patient with acute liver failure died during the transplantation procedure due to surgical complications, with bleeding from the inferior caval vein; the death was not considered to be an adverse event related to the transfusions. In the studied population, neither TRALI nor other severe immunological or pulmonary reactions occurred that could be related to the use of blood products. There was no evidence of transfusion-transmitted infection.
Transfusion Practice
Perioperatively, a median of four units of RBC (interquartile range [IQR] 1–9), ten bags of plasma (IQR 4–18), and zero units of platelets (IQR 0–2) were transfused. There was a strong statistical correlation between transfused bags of plasma and units of RBC (r = 0.85, P < 0.001) and to lesser degree between plasma and platelets (r = 0.60, P < 0.001). The number of transplantations increased each year from 30 in 2005 to 64 in 2008, but the increased experience with the procedure and changes in patient mix only lead to a slight, non-significant reduction in the transfusion rates for all blood products (data not shown).
Prior to the perioperative period, 40 patients received one or more units of RBC, 37 received plasma, and 19 were administered platelets. Median (IQR) was 0 (0) for all. After the perioperative period, lasting for a median of 27 (IQR 23–32) days, median (IQR) 1 (0–4) unit of RBC, 0 (0–4) bags of plasma, and 0 (0) units of platelets were transfused.
Hyperfibrinolysis
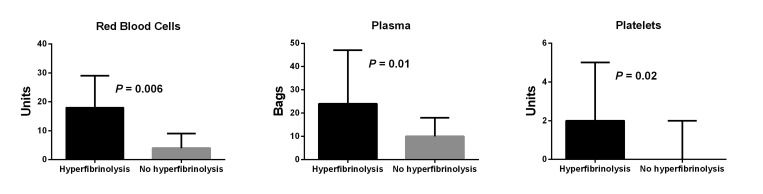
Hyperfibrinolysis was detected in 12 of the 138 (9%) patients in which TEG was performed, and LY30 had a median value of 52%, ranging from 8% to 88%. Median LY30 in the remaining samples was 0%. In four cases, hyperfibrinolysis was detected at baseline. These patients were in a severe medical condition with a median Model of End stage Liver Disease (MELD)-score of 24, ranging from 14 to 29, significantly higher than the other patients (P = 0.04). All had received plasma in the range from 10 to 20 bags before TEG-analyses. Hyperfibrinolysis was detected in the anhepatic period in six patients, and in two cases shortly after reperfusion of the portal vein. In eight cases a complete normalization of LY30 was observed following administration of tranexamic acid. In one of the cases in which hyperfibrinolysis was detected shortly after graft reperfusion, LY30 normalized spontaneously. Patients with intraoperatively detected hyperfibrinolysis received significantly increased numbers of RBC, plasma, and platelet units. This was the case not only in the perioperative period as shown in Figure 2, but also in the period later than 24 hours after start of surgery, when significantly more RBC (median [IQR] 3.5 [2–9] vs. 1 [0–4] units, P = 0.02), plasma (4 [0.5–13.5] vs. 0 [0–4] bags, P = 0.005), and platelets (1.5 [0–3] vs. 0 [0–0] units, P = 0.01) were transfused. No differences were found before the perioperative period (data not shown). Three of the TEG-monitored patients were administered aprotinin, and hyperfibrinolysis was not detected in any of these. The 12 patients with hyperfibrinolysis were hospitalized for a median (IQR) of 41 (29–52) days, which was significantly longer than the other patients who stayed for a median (IQR) of 29 (25–36) days (P = 0.009).
Figure 2.
Transfused red blood cells, plasma, and platelets in 12 patients with intraoperatively detected hyperfibrinolysis compared to 126 patients without hyperfibrinolysis. Data are presented as median and upper quartile.
Thromboembolism
The patient who had hyperfibrinolysis that normalized without administration of any antifibrinolytic agent was diagnosed with thromboses of the hepatic artery, portal vein, and hepatic vein postoperatively. This patient was known to have activated protein-C resistance due to a factor V Leiden mutation, and was only administered 2000 units of unfractionated heparin intraoperatively due to a bleeding tendency assessed clinically and by TEG. In another patient who had thrombosis of the hepatic artery, and in one patient with portal vein thrombosis, no obvious reasons for the thrombotic events were found. None of these patients had been administered tranexamic acid or aprotinin. Additionally, two patients had occluded hepatic arteries due to a surgery-related anatomical twist but no evidence of thrombosis. In no patient was pulmonary embolism observed.
Discussion
Our results on 195 consecutive liver transplant recipients are in accord with previous observations10,11 and suggest that SD-plasma administered as Octaplas is safe for patients undergoing liver transplantation. The relatively low concentrations of α2-antiplasmin and protein S in Octaplas, compared to products that have not undergone pathogen reduction, such as FFP, do not seem to have a major clinical impact. It should be noted that Octaplas in its current form is currently being replaced in several countries by OctaplasLG, a further prion inactivated product with higher concentrations of α2-antiplasmin.18 We propose that possible disadvantages with Octaplas are well compensated for by pathogen reduction,3,4 markedly decreased risk of TRALI,5,6 standardized plasma protein content,7 and reduced frequency of allergic/immunologic adverse effects.6 The incidence of TRALI in Norway is approximately 0.2 cases per 10,000 transfusions. RBCs and platelets have been implicated, but so far no cases have been reported that implicate SD-plasma in TRALI after transfusion of more than 750,000 bags.19 In centers using FFP, the incidence of TRALI caused by FFP alone has been reported to be 0.29.15
Hyperfibrinolysis was detected in 9% of the patients monitored with TEG. Even if aprotinin disguised hyperfibrinolysis in three patients, the incidence would not have been higher than 11%. We cannot acquit SD-plasma as source of the hyperfibrinolysis, but since this rate is similar to those described in reports from centers using standard FFP as their source of plasma,8,20 other causes emerge as well likely. The four patients with hyperfibrinolysis at baseline had significantly higher MELD-scores than the other patients, and severe liver disease is a known risk factor for developing hyperfibrinolysis. The major fibrinolytic enzyme is plasmin, which is converted from plasminogen. The plasminogen to plasmin conversion is driven by activators such as tissue plasminogen activator (t-PA), urokinase plasminogen activator, and activated factor XII. Anti-activators t-PA inhibitors such as plasminogen activator inhibitor, plasmin inhibitor, and thrombin-activatable fibrinolysis inhibitor usually prevent hyperfibrinolysis. Since both activators and anti-activators are simultaneously down regulated in liver insufficiency, hyperfibrinolysis is not present. However, this down regulated state is considered more vulnerable, and intercurrent conditions like peritonitis or transfusion-requiring bleeding episodes may induce hyperfibrinolysis.21,22 The high transfusion rates and longer hospital stays in patients with hyperfibrinolysis compared to other patients strengthens the seriousness of this condition. While tranexamic acid is effective in this setting, at least as judged by TEG,23 it does not resolve all such coagulopathies.
The frequency of thrombotic graft episodes that could not be related to surgical-anatomical circumstances was 2%, and no episode of pulmonary embolism was detected. This compares favorably to results with FFP.9 Thus, our data support our clinical experience with Octaplas being safe with regard to occurrence of thromboembolic complications. Also, 1–2 g tranexamic acid administered because of hyperfibrinolysis does not seem to be associated with increased numbers of thromboembolic events in our study of liver transplants.
With regard to transfusions, three important limitations must be considered in this retrospective quality assurance study. First, since this is a report from a center solely using Octaplas as plasma source, no direct comparison between SD-plasma and FFP was undertaken. Accordingly, we cannot tell if SD-plasma is overall safer than FFP, or if it may cause more episodes of hyperfibrinolysis and thromboembolic complications. Second, the method used to identify the time points of administered blood products is not internationally established. Care should be taken when comparing our transfusion data to those from other centers, especially data from prospective studies. Third, the method does not allow an accurate measurement of blood loss during each stage of the transplantation procedure (eg, preoperative, anhepatic, post reperfusion, postoperative). However, the low amount of blood products issued from T0 and 12 hours intraoperatively indicates low blood consumption in the anhepatic and post reperfusion periods (Figure 1), which is in agreement with a recent Canadian study.1
Conclusion
This quality assurance study comprising all adult patients in Norway that underwent primary liver transplantation between 2005 and 2009 supports the idea that the pooled SD-treated plasma Octaplas is an acceptable plasma product for liver transplant recipients. Additional studies are needed to ensure its safety profile in comparison to FFP.
Acknowledgements
We are indebted to Karl Saebjorn Kjollesdal for extraction of data from the Hospital database and Ada Krogh for extraction of data from the Blood Bank database.
References
- 1.Massicotte L, Denault AY, Beaulieu D, Thibeault L, Hevesi Z, Nozza A, Lapointe R, Roy A. Transfusion Rate for 500 Consecutive Liver Transplantations: Experience of One Liver Transplantation Center. Transplantation 2012;93:1276–1281. [DOI] [PubMed] [Google Scholar]
- 2.Prince AM, Horowitz B, Brotman B. Sterilisation of hepatitis and HTLV-III viruses by exposure to tri(n-butyl)phosphate and sodium cholate. Lancet 1986;1:706–710. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz B, Bonomo R, Prince AM, Chin SN, Brotman B, Shulman RW. Solvent/detergent-treated plasma: a virus-inactivated substitute for fresh frozen plasma. Blood 1992;79:826–831. [PubMed] [Google Scholar]
- 4.Solheim BG, Seghatchian J. Update on pathogen reduction technology for therapeutic plasma: an overview. Transfus ApherSci 2006;35:83–90. [DOI] [PubMed] [Google Scholar]
- 5.Bux J. Antibody-mediated (immune) transfusion-related acute lung injury. Vox Sang 2011;100:122–128. [DOI] [PubMed] [Google Scholar]
- 6.Hellstern P, Solheim BG. The Use of Solvent/Detergent Treatment in Pathogen Reduction of Plasma. Transfus Med Hemother 2011;38:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeck H, Hellstern P. In vitro characterization of solvent/detergent-treated human plasma and of quarantine fresh frozen plasma. Vox Sang 1998;74Suppl 1:219–223. [DOI] [PubMed] [Google Scholar]
- 8.de Jonge J, Groenland TH, Metselaar HJ, Ijzermans JN, van Vliet HH, Visser L, Tilanus HW. Fibrinolysis during liver transplantation is enhanced by using solvent/detergent virus-inactivated plasma (ESDEP). Anesth Analg 2002;94: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Matsusaki T, Dai F, Tanaka KA, Donaldson JB, Hilmi IA, Wallis MJ, Planinsic RM, Humar A. Pulmonary thromboembolism during adult liver transplantation: incidence, clinical presentation, outcome, risk factors, and diagnostic predictors. Br J Anaesth 2012;108:469–477. [DOI] [PubMed] [Google Scholar]
- 10.Williamson LM, Llewelyn CA, Fisher NC, Allain JP, Bellamy MC, Baglin TP, Freeman J, Klinck JR, Ala FA, Smith N, Neuberger J, Wreghitt TG. A randomized trial of solvent/detergent-treated and standard fresh-frozen plasma in the coagulopathy of liver disease and liver transplantation. Transfusion 1999;39:1227–1234. [DOI] [PubMed] [Google Scholar]
- 11.Bartelmaos T, Chabanel A, Leger J, Villalon L, Gillon MC, Rouget C, Gomola A, Denninger MH, Tardivel R, Naegelen C, Courtois F, Bardiaux L, Giraudeau B, Ozier Y. Plasma transfusion in liver transplantation: a randomized, doubleblind, multicenter clinical comparison of three virally secured plasmas. Transfusion 2013;53:1135–1145. [DOI] [PubMed] [Google Scholar]
- 12.Bindi ML, Miccoli M, Marietta M, Meacci L, Esposito M, Bisa M, Mozzo R, Mazzoni A, Baggiani A, Scatena FFilipponi F, Biancofiore G. Solvent detergent vs. fresh frozen plasma in cirrhotic patients undergoing liver transplant surgery: a prospective randomized control study. Vox Sang 2013;105:137–143. [DOI] [PubMed] [Google Scholar]
- 13.Solheim BG, Hellstern P. Composition, efficacy, and safety of S/D-treated plasma. Transfusion 2003;43:1176–1178. [DOI] [PubMed] [Google Scholar]
- 14.Salge-Bartels U, Breitner-Ruddock S, Hunfeld A, Seitz R, Heiden M. Are quality differences responsible for different adverse reactions reported for SD-plasma from USA and Europe? Transfus Med 2006;16:266–275. [DOI] [PubMed] [Google Scholar]
- 15.Svae TE, Heger A, Biesert L, Neisser-Svae A, Frenzel W. Solvent detergent plasma. In: Bertolini J, Goss N, Curling J, eds. Production of plasma proteins for therapeutic use. Hoboken, NJ: John Wiley & Sons; 2012. 345–357. [Google Scholar]
- 16.Huisman EL, van Eerd MC, Ouwens JN, de Peuter MA. Cost-effectiveness and budget impact study of solvent/detergent (SD) treated plasma (octaplasLG®) versus fresh-frozen plasma (FFP) in any patient receiving transfusion in Canada. Transfus Apher Sci 2013. May 23. pii S1473-0502(13)00147-X. 10.1016/j.transci.2013.04.045. [Epub ahead of print] [DOI] [PubMed]
- 17.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88:312–319. [DOI] [PubMed] [Google Scholar]
- 18.Lawrie AS, Green L, Canciani MT, Mackie IJ, Peyvandi F, Scully MA, Machin SJ. The effect of prion reduction in solvent/detergent-treated plasma on haemostatic variables. Vox Sang 2010;99:232–238. [DOI] [PubMed] [Google Scholar]
- 19.Steinsvag CT, Espinosa A, Flesland O. Surveillance of blood in Norway 2011. Adverse outcomes of transfusion. Available at: http://www.kunnskapssenteret.no/Publikasjoner/