Abstract
Background:
Metaplastic breast carcinoma (MBC) is a rare type of breast cancer that has basal-like characteristics and is perceived to have poorer prognosis when compared with conventional no specific type/ductal carcinomas (ductal/NST). However, current data on MBC are largely derived from small case series or population-based reports. This study aimed to assess the clinicopathological features and outcome of MBC identified through an international multicentre collaboration.
Methods:
A large international multicentre series of MBC (no=405) with histological confirmation and follow-up information has been included in this study. The prognostic value of different variables and outcome has been assessed and compared with grade, nodal status and ER/HER2 receptor-matched ductal/NST breast carcinoma.
Results:
The outcome of MBC diagnosed in Asian countries was more favourable than those in Western countries. The outcome of MBC is not different from matched ductal/NST carcinoma but the performance of the established prognostic variables in MBC is different. Lymph node stage, lymphovascular invasion and histologic subtype are associated with outcome but tumour size and grade are not. Chemotherapy was associated with longer survival, although this effect was limited to early-stage disease. In this study no association between radiotherapy and outcome was identified. Multivariate analysis of MBC shows that histologic subtype is an independent prognostic feature.
Conclusions:
This study suggests that MBC is a heterogeneous disease. Although the outcome of MBC is not different to matched conventional ductal/NST breast carcinoma, its behaviour is dependent on the particular subtype with spindle cell carcinoma in particular has an aggressive biological behaviour. Management of patients with MBC should be based on validated prognostic variables.
Keywords: breast cancer, metaplastic carcinoma, prognosis, outcome, race, tumour type, grade
Mammary parenchymal cells show a high degree of phenotypic plasticity, which is seen in both benign and malignant lesions (Smith and Taylor 1969; Spagnolo and Shilkin 1983; Kaufman et al, 1984; Wang et al, 2001; Rosen 2009; van Deurzen et al, 2011). Conventional (ductal/no specific type) invasive breast carcinoma occasionally shows minor components of metaplastic elements with squamous and/or mesenchymal appearances (Kaufman et al, 1984). However, when the metaplastic components form a significant proportion (usually >10%, although some authors have used different cutoffs including <10% (Downs-Kelly et al, 2009), ⩾20% (Gwin et al, 2010) or ⩾50% (Yamaguchi et al, 2010)) the term metaplastic breast carcinoma (MBC) is used. Although MBC is rare comprising 0.3%–1.5% of breast cancer, it is recognized to be a heterogeneous group of tumours with multiple subtypes reflecting variable histological appearances (Hennessy et al, 2006; Tse et al, 2006; Pezzi et al, 2007; Yamaguchi et al, 2010; Tseng and Martinez, 2011; Reis-Filho et al, 2012). There is a perception that MBC is an aggressive tumour with poor outcome. This is mainly based on previous studies of MBC that included either a limited number of cases or a population-based database without histological confirmation and with different clinical stages (i.e., stage I to IV (Hennessy et al, 2006)) and ethnicity (Foschini et al, 1993; Rayson et al, 1999; Hennessy et al, 2006; Tse et al, 2006; Luini et al, 2007; Pezzi et al, 2007; Jung et al, 2010; Yamaguchi et al, 2010; Bae et al, 2011; Tseng and Martinez 2011; Lee et al, 2012; Lester et al, 2012; Reis-Filho et al, 2012). In addition, some molecular studies have shown some shared characteristics between MBC and the aggressive high grade basal-like class of ductal carcinoma (Reis-Filho et al, 2006). As a consequence, the outcome and prognostic risk stratification of patients with MBC remain uncertain. Clinicopathological variables that are well-validated in conventional invasive breast carcinoma may behave differently in MBC. Therefore, in this large international multicentre study of MBC with histological confirmation and long-term follow-up, we aimed to assess the prognostic value of the different clinicopathological variables and determine its outcome compared with grade, node and receptor matched conventional invasive ductal carcinoma of no special type.
Materials and methods
This study included three case series. The first series comprises MBC diagnosed at and identified from the files of six institutions in Europe including the United Kingdom (three institutions; Nottingham, Leeds and Leicester), The Netherlands, Switzerland and Spain (n=313). The second series comprises MBC identified from the files of three institutions from Asia including Singapore and Hong Kong (two institutions; n=92). These series consist of cases diagnosed between 1991 and 2012. Cases were reviewed by a consultant pathologist in each centre to confirm the initial diagnosis and to assess the neoplastic cell phenotype/morphology. Criteria for diagnosis of MBC were as previously published (Rosen, 2009; Reis-Filho et al, 2012). MBC was defined by the presence of non-glandular epithelial (squamous) or mesenchymal (spindle or matrix producing) elements associated with DCIS or conventional mammary type invasive carcinoma. In the occasional cases lacking a conventional carcinomatous element, the diagnosis was confirmed using immunohistochemical markers of epithelial differentiation. MBC histologic subtypes included the following: spindle (including sarcomatoid and pleomorphic), squamous, mixed squamous and spindle and matrix producing types (Rosen, 2009; Reis-Filho et al, 2012). Variables assessed and collected include tumour histological subtype, proportion of each metaplastic component, histological grade and its components (tubule formation, pleomorphism and mitotic count), degree of cellularity, presence and degree of tumour necrosis and presence, grade and extent of associated ductal carcinoma in situ (DCIS). Clinicopathological characteristics including tumour size, total number of lymph nodes and number of positive nodes, lymphovascular invasion, hormone receptor and HER2 status were obtained from the database whenever available. Clinical and outcome data including menopausal status, treatment performed including local (surgical and radiotherapy) and systemic therapy (endocrine therapy and chemotherapy), development of local, regional and distant recurrence and time to events, survival status, survival time and cause of death were collected from patients' notes. Breast cancer-specific survival (BCSS) was defined as the interval between the operation and death from (or with) breast cancer, death being scored as an event, and patients who died from other causes or were still alive were censored at the time of last follow-up (Rakha, 2013). Out of the 405 MBC, 41 cases were excluded as follows: cases presented as metastatic (within 2 months of presentation; n=5), recurrent (n=14) or contralateral (n=4) breast cancer, cases received neoadjuvant chemotherapy or conventional mammary carcinoma with ⩽10% metaplastic component (n=18). The clinicopathological features of the remaining 364 MBC are shown in Table 1. Complete follow-up data of MBC after exclusion of ineligible cases was available for 285 cases.
Table 1. Clinicopathological features of metaplastic breast carcinoma (n=364).
| Variables | MBC |
|---|---|
|
Time of diagnosisa | |
| Before 2005 | 43% |
| At or after 2005 | 57% |
| Mean age in years (range) |
60 (27–96) |
|
Menopausal status | |
| Pre/perimenopausal | 32% |
| Postmenopausal |
68% |
|
Type of surgery | |
| Breast conserving surgery | 41% |
| Mastectomy |
59% |
|
Tumour Size | |
| TNM pT1 | 23% |
| TNM pT2 | 53% |
| TNM pT3&4 |
24% |
|
Focality | |
| Localised | 89% |
| Multifocal |
11% |
|
LN stage | |
| 1 (LN negative) | 71% |
| 2 (1–3 positive nodes) | 19% |
| 3 (>3 positive nodes) |
10% |
|
Invasive carcinoma grade | |
| 1 | 2% |
| 2 | 23% |
| 3 |
75% |
|
Mitotic counts | |
| 1 | 9% |
| 2 | 19% |
| 3 |
72% |
|
Degree of nuclear pleomorphism | |
| Mild | 2% |
| Moderate | 7% |
| Marked |
91% |
|
Associated conventional invasive carcinoma | |
| Yes | 57% |
| No |
43% |
|
Proportion of metaplastic elements | |
| 11%-50% | 32% |
| 51%-90% | 24% |
| >90% |
44% |
|
Degree of cellularity | |
| High | 59% |
| Intermediate | 31% |
| Low | 2% |
| Heterogeneous |
8% |
|
Associated DCIS | |
| Yes | 42% |
| No |
58% |
|
Lymphovascular invasion | |
| Negative | 79% |
| Positive |
21% |
|
Oestrogen receptor | |
| Negative | 93% |
| Positive |
7% |
|
Progesterone receptor | |
| Negative | 94% |
| Positive |
6% |
|
HER2 | |
| Negative | 99% |
| Positive |
1% |
| Radiotherapy |
69% |
| Chemotherapy | 65% |
Abbreviations: DCIS=ductal carcinoma in situ; MBC=metaplastic breast carcinoma.
Cases were split at 2005 based on the number of cases, time of diagnosis and the length of follow-up period.
The third series is a control group (n=285) of age, histological grade, lymph node stage, oestrogen receptor (ER) and HER2 status matched conventional invasive ductal/NST primary breast carcinomas identified from the well-defined Nottingham primary operable (⩽5 cm) breast cancer series (n=1950) that has been described in previous publications (Rakha et al, 2007, 2008, 2009, 2011).
This study was approved by the Nottingham Research Ethics Committee.
Statistical analysis
Survival curves were produced using the Kaplan–Meier method and were compared using log rank tests. Survival rates are presented with their 95% confidence intervals. Multivariate analyses were conducted using Cox proportional hazard regression models. The clinicopathological variables were compared using contingency tables and χ2-tests. All comparisons were two-sided and a p-value of <0.05 was considered significant.
Results
All MBC patients were female, of whom 70% had axillary clearance and 30% had lymph node (LN) sample or sentinel node biopsy. Median LN number was 9 (range 1–46). Thirty percent showed metastatic (positive) nodes that were mainly of low number (median=2). Forty-five percent of the positive nodes contained deposits of metaplastic elements as either pure (25%) or mixed with conventional carcinomas (20%), the remainder were involved by conventional adenocarcinoma of ductal/NST type. Diagnosis of MBC was based on the presence of non-glandular (squamous and/or mesenchymal including matrix producing) differentiation associated with conventional-type carcinomatous element (<90%) that was identified in 57% of cases and/or DCIS that was identified in 42% of cases (Table 1). More mixed spindle and squamous (37%) and spindle (28%) presented at an advanced stage (pT3&4) than squamous (21%) and matrix producing (18%) carcinomas but this different was not significant (P=0.17).
Of the whole series, 276 (76%) were from Western countries and 88 (24%) from Asian countries. There was a significant difference between MBC diagnosed in Western countries and Asian countries with frequent mastectomy and higher histological grade tumours with more squamous and less spindle carcinoma subtypes in Asian countries (Table 2).
Table 2. Comparison between MBC diagnosed in the Western countries and Asian countries.
| Western series | Asian series | P-value | |
|---|---|---|---|
| Mean age in years (range) |
61 (27–96) |
57 (32–85) |
0.017 |
|
Type of surgery | |||
| Breast conserving surgery | 95 (50) | 21 (24) | <0.001 |
| Mastectomy |
96 (50) |
67 (76) |
|
|
Tumour Size | |||
| TNM pT1 | 46 (24) | 16 (19) | 0.797 |
| TNM pT2 | 100 (52) | 47 (55) | |
| TNM pT3&4 |
45 (24) |
22 (26) |
|
|
Tumour subtypes | |||
| Spindle cell | 95 (34) | 21 (24) | 0.001 |
| Squamous | 47 (17) | 30 (34) | |
| Mixed squamous and spindle | 36 (13) | 13 (15) | |
| Matrix producing | 80 (29) | 24 (27) | |
| Fibromatosis-likea |
18 (7) |
0 (0) |
|
|
Associated conventional carcinoma | |||
| Yes | 147 (58) | 73 (88) | <0.001 |
| No |
105 (42) |
10 (12) |
|
|
Invasive carcinoma grade | |||
| 1 | 4 (1) | 2 (2) | 0.009 |
| 2 | 71 (26) | 7 (10) | |
| 3 |
196 (73) |
64 (88) |
|
|
LN stage | |||
| 1 (LN negative) | 131 (73) | 49 (68) | 0.118 |
| 2 (1–3 positive nodes) | 35 (19) | 12 (16) | |
| 3 (>3 positive nodes) |
14 (8) |
12 (16) |
|
|
Lymphovascular invasion | |||
| Negative | 150 (78) | 68 (81) | 0.596 |
| Positive |
42 (22) |
16 (19) |
|
| Radiotherapy |
123 (68) |
30 (50) |
0.038 |
| Chemotherapy | 114 (61) | 28 (81) | 0.022 |
Fibromatosis-like is a recently recognized subtype is a low grade spindle carcinoma diagnosed mainly in Nottingham a part of a consultation service. Follow-up was available for two cases only; therefore, they were grouped with spindle MBC.
Outcome analysis
During the period of follow-up (maximum 244 months, interquartile range 56), 65 patients developed recurrent disease and 95 patients died (55 of BC and 40 of other causes). No difference in the outcome was detected between recent (at or after 2005) and old (before 2005) cases (X2=0.08, P=0.779). When cases were stratified based on the centre of diagnosis, a significant difference in the outcome between centres was found (X2=21.35, P=0.011). There was a significant difference in the outcome between MBC diagnosed in Western countries and those diagnosed in Asian countries (X2=8.95, DF=1, P=0.003). However, when locally advanced cases were excluded this difference was no longer significant (X2=2.71, DF=1, P=0.099 and Table 3). Figure 1 shows the outcome of MBC from Western and Asian countries as compared with the control group. Therefore, further analysis of prognostic markers in MBC was performed with consideration to countries of origin (Western versus Asian) and stage of the disease (with and without stage pT3&4 tumours).
Table 3. Cumulative survival of metaplastic carcinoma (including western and Asian subgroups) after exclusion of advanced-stage cases compared with early-stage conventional NST carcinoma.
| Interval start time in months | Number of patients entering interval | Number exposed to risk | Cumulative proportion surviving at end of interval |
|---|---|---|---|
|
MBC | |||
| 30 | 285 | 242 | 0.85 |
| 60 | 162 | 137 | 0.77 |
| 90 | 98 | 78 | 0.74 |
| 120 | 54 | 46 | 0.72 |
| 150 |
35 |
27 |
0.67 |
|
Western | |||
| 30 | 145 | 122 | 0.88 |
| 60 | 84 | 70 | 0.78 |
| 90 | 48 | 36 | 0.76 |
| 120 | 23 | 20 | 0.72 |
| 150 |
15 |
11 |
0.59 |
|
Asian | |||
| 30 | 63 | 53 | 0.96 |
| 60 | 40 | 36 | 0.85 |
| 90 | 27 | 22 | 0.85 |
| 120 | 16 | 14 | 0.85 |
| 150 |
10 |
8 |
0.85 |
|
IDC NST | |||
| 30 | 284 | 282 | 0.91 |
| 60 | 254 | 254 | 0.80 |
| 90 | 222 | 210 | 0.76 |
| 120 | 188 | 169 | 0.74 |
| 150 | 144 | 116 | 0.71 |
Abbreviations: IDC NST=invasive ductal carcinoma of no special type; MBC=metaplastic breast carcinoma.
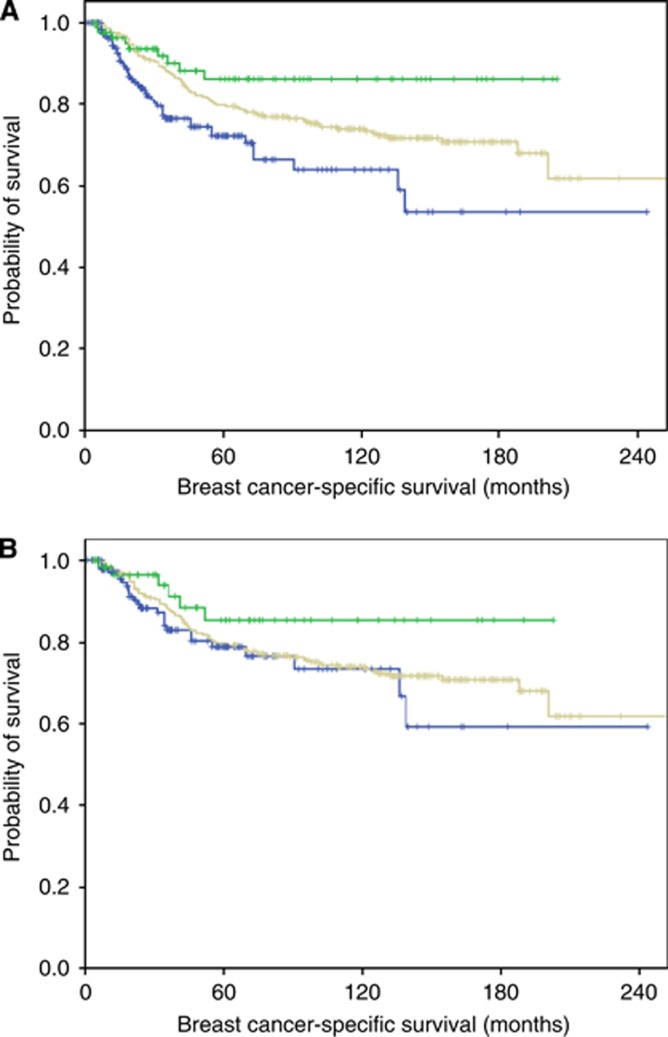
Figure 1.
Correlation between MBC diagnosed in Western and Asian countries and outcome. (A) Comparison of breast cancer-specific survival (BCSS) of patients with metaplastic breast carcinoma diagnosed Asian countries (upper green curve; 88 patients) and Western countries (lower blue curve; 197 patients) as compared with the control group of conventional ductal carcinoma (middle grey curve; 285 patients) (X2=12.1, DF=2, P=0.002). (B) Comparison of BCSS of same groups after exclusion of locally advanced cases (tumours >stage T2); Asian patients (upper green curve; 63 patients) and Western patients (lower blue curve; 145 patients as compared with the control group of conventional ductal carcinoma (middle grey curve; 285 patients) (X2=2.9, DF=2, P=0.237). A full color version of this figure is available at the British Journal of Cancer journal online.
Prognostic markers in MBC
No association with outcome in terms of BCSS and DFI was found in MBC regarding patients' age, menopausal status, histological grade; even when analysed as low and high grade (grade 1/2 versus 3) or any of its components (pleomorphism, mitosis or tubule formation) when analysed separately. Similarly no association between outcome and the degree of tumour cellularity, presence of tumour necrosis or coexistence of conventional-type carcinoma or DCIS was found (P>0.05).
There was a significant association between lymph node stage (Figure 2A) and presence of lymphovascular invasion and BCSS (X2=15.8, DF=2, P<0.0001 and X2=7.6, DF=2, P=0.006, respectively) but not with DFI. The same associations were observed in the Western and Asian subgroups with or without inclusion of locally advanced (pT3&4) cases. TNM pT stage was correlated with outcome (X2=10.3, P=0.006 and X2=11.5, P=0.003 for BCSS and DFI, respectively); however, when pT3&4 cases were excluded, these associations were no longer significant.
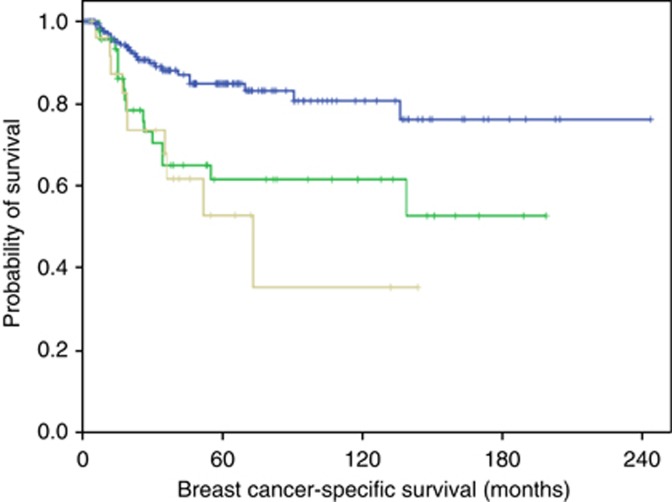
Figure 2.
Association between lymph node stage in MBC and breast cancer-specific survival (node negative (180 patients)=upper blue, node positive 1–3 (46 patients)=middle green and node positive >3 (26 patients)=lower grey) (X2=15.8, DF=2, P<0.0001). The same association was observed in Western and Asian subgroups with or without inclusion of locally advanced cases. A full color version of this figure is available at the British Journal of Cancer journal online.
There was an association between MBC histologic subtypes and BCSS and DFI (X2=13.9, P=0.008 and X2=6.7, P=0.01, respectively). The difference in the outcome between matrix producing and squamous carcinomas was significant and both were associated with a better prognosis while spindle and mixed spindle and squamous were associated with the worst prognosis and their outcome was not statistically different (Figure 3). These associations were maintained after exclusion of locally advanced cases (X2=7.4, P=0.006 and X2=6.2, P=0.01 for BCSS and DFI, respectively). Chemotherapy was associated with longer survival (X2=4.1, P=0.045) but, when locally advanced cases were excluded, this association lost its significance. No associations between radiotherapy and outcome were found. Table 4 shows multivariate cox regression analysis of MBC with and without locally advanced cases. This indicates that MBC subtype is an independent prognostic variable associated with BCSS and DFI.
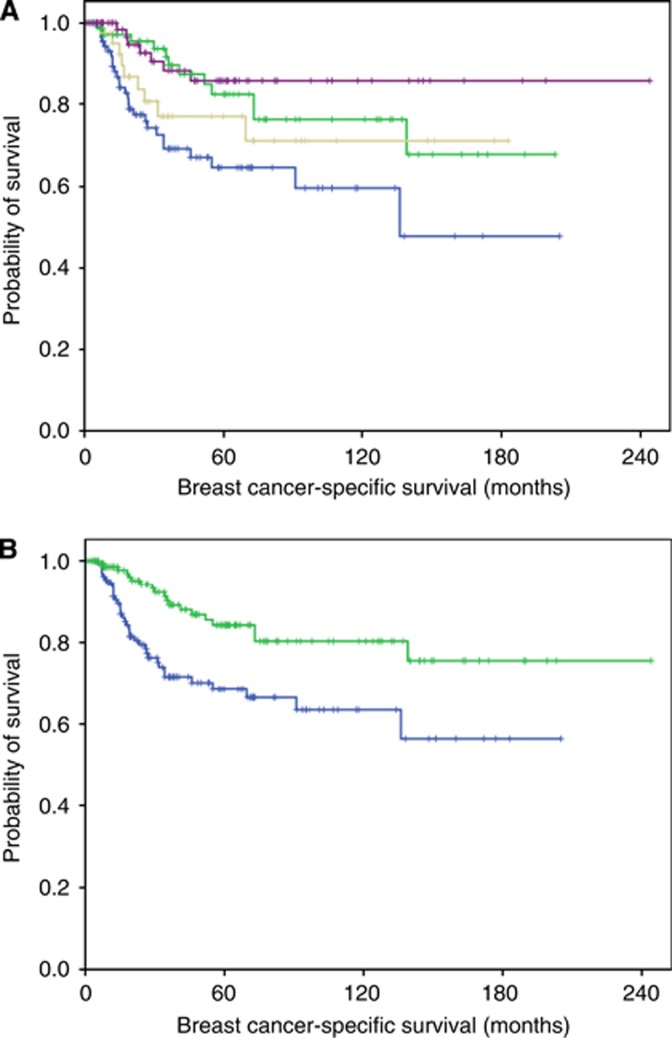
Figure 3.
Association between MBC histologic subtype and outcome. (A) Association between breast cancer-specific survival and MBC histologic subtypes (matrix producing carcinoma (77 cases; upper purple), squamous (74 patients; upper middle green), mixed squamous and spindle (41 patients; lower middle grey) and spindle carcinoma (91 patients; lower blue) (X2=13.9, DF=3, P=0.008). (B) Association between breast cancer-specific survival and MBC histologic subtype analysed as two groups (upper green; matrix producing and squamous combined (151 cases) and lower blue; spindle and mixed spindle and squamous (132 patients); X2=10.8, DF=1, P=0.001). A full color version of this figure is available at the British Journal of Cancer journal online.
Table 4. Multivariate analysis of variables associated with outcome in metaplastic breast carcinoma with and without inclusion of locally advanced cases.
|
All MBC cases |
MBC after excluding pT3&4 |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
95%CI |
|
|
95%CI |
|
|||
| Hazard ratio | Lower | Upper | P-value | Hazard ratio | Lower | Upper | P-value | |
|
Breast cancer-specific survival | ||||||||
| TNM pT stage | 2.067 | 1.175 | 3.637 | 0.012 | 1.529 | 0.519 | 4.501 | 0.441 |
| LN stage | 1.463 | 0.808 | 2.649 | 0.209 | 2.156 | 1.044 | 4.455 | 0.038 |
| LVI | 1.666 | 0.649 | 4.278 | 0.289 | .984 | 0.258 | 3.746 | 0.981 |
| Chemotherapy | 0.305 | 0.143 | 0.650 | 0.002 | .359 | 0.140 | 0.920 | 0.033 |
| MBC subtype |
1.061 |
0.927 |
1.213 |
0.391 |
1.201 |
1.030 |
1.399 |
0.019 |
|
Disease-free interval | ||||||||
| TNM pT stage | 1.409 | 0.865 | 2.294 | 0.169 | 1.662 | 0.645 | 4.287 | 0.293 |
| LN stage | 1.284 | 0.739 | 2.229 | 0.375 | 1.486 | 0.750 | 2.944 | 0.256 |
| LVI | 1.393 | 0.545 | 3.558 | 0.488 | 0.593 | 0.144 | 2.438 | 0.468 |
| Chemotherapy | 0.570 | 0.288 | 1.127 | 0.106 | 0.605 | 0.273 | 1.341 | 0.216 |
| MBC subtype | 1.173 | 1.041 | 1.322 | 0.009 | 1.313 | 1.146 | 1.504 | 0.0001 |
Abbreviations: LN=lymph node; LVI=lymphovascular invasion; MBC=metaplastic breast carcinoma; TNM=tumour node metastasis.
Discussion
Metaplastic carcinoma of the breast (MCB) remains as a poorly characterised subtype of breast cancer. Although MBC is rare, its recognition as a discrete entity is increasing (Pezzi et al, 2007; Tseng and Martinez, 2011; Lee et al, 2012). Most of the prognostic studies of MBC have been small and with conflicting results or included non-validated cases from population based databases (Foschini et al, 1993; Rayson et al, 1999; Hennessy et al, 2006; Tse et al, 2006; Luini et al, 2007; Pezzi et al, 2007; Jung et al, 2010; Yamaguchi et al, 2010; Bae et al, 2011; Tseng and Martinez 2011; Lee et al, 2012; Lester et al, 2012; Reis-Filho et al, 2012). In this large international multicentre series of histologically confirmed cases of MBC we have critically assessed the prognostic value of known prognostic variables and determine the outcome in comparison with matched conventional breast carcinoma of ductal/no specific type.
This study identified some factors that may explain the conflicting data published to date regarding clinicopathological and outcome data. MBC diagnosed in Asian countries showed longer survival compared with those diagnosed in Western countries. This can be partly explained by the over-representation of locally advanced cases in Western countries, other epidemiological factors or environmental determinants akin to those observed in the stomach and upper gastrointestinal cancer (Gill et al, 2003). Other factors may include differences in treatment protocols. In this study, mastectomy rates and systemic chemotherapy use were higher in the Asian series.
The over-representation of metastatic advanced and locally advanced cases in some series may be one of the reasons for the reported poor outcome of MBS as the outcome in our series improved when these cases were excluded. Outcome analysis revealed that MBC is associated with shorter survival compared with matched conventional carcinoma. However, when analysis was restricted to early-stage cases (pT1&2) the outcome was not different to stage matched conventional carcinoma. Consistent with our findings, some authors have reported that, although MBC is associated with poor prognostic indicators, its outcome is comparable to matched conventional breast carcinomas (Beatty et al, 2006). When known prognostic variables in conventional breast carcinoma were analysed in the context of MBC, we found that lymph node stage and lymphovascular invasion were significant predictors of outcome. However, no association between histological grade or its components (mitosis, tubule formation and pleomorphism) (Rakha et al, 2008) or the Trojani grading system of sarcoma (mitosis, necrosis and differentiation) (Trojani et al, 1984) and outcome was detected. A finding that may represent the nature of the tumour with transdifferentiation of the malignant epithelial mammary tissue to a different histologic type. Tumour size also was not a significant prognostic factor.
One key observation in the current study is that the different subtypes of MBC are associated with distinct outcome. In this series, matrix producing carcinoma was associated with the best outcome while spindle and mixed spindle and squamous carcinomas were associated with the worst outcome and this was an independent prognostic variable (Beatty et al, 2006; Nayak et al, 2013). The better outcome of matrix producing carcinoma compared with other subtypes of MBC may be a reflection of its smaller primary tumour size and less frequent nodal metastasis and lymphovascular invasion (data not shown). In this study, it should be noted that few cases of fibromatosis-like metaplastic carcinoma with linked outcome data were included and no low-grade adenosquamous subtypes, which we consider as a distinct entity, were included in this study. Both of these subtypes are recognized to have an excellent outcome (Van Hoeven et al, 1993; Gobbi et al, 1999).
Chemotherapy was associated with better outcome, although the effect was limited in early stage cases. Some authors have reported that systemic therapy may be less effective in MBC (Rayson et al, 1999; Gibson et al, 2005). In this study no association between radiotherapy and outcome was identified. Although Tseng and Martinez (2011) reported that radiotherapy is associated with improved overall survival I MBC, they included historical cases diagnosed from 1988 in their analysis and only 39% received RT with apparent low 10-year survival (53%). The effect of chemotherapy and radiotherapy on the outcome is best assessed in a focused randomized clinical trial.
This study has limitations. It is a retrospective study with the possibility of selection bias. Although the histological diagnosis of MBC was reviewed by breast pathologists, this was carried out locally in each institution with no central pathology review.
In conclusion, this study provides evidence-based data that MBC is a heterogeneous disease encompassing biologically different tumour classes with variable outcome. Although the behaviour of MBC overall is not different to matched conventional forms of ductal/NST invasive breast carcinoma, the pattern of relevant prognostic variables in MBC is different from the spectrum of well-established variables in conventional breast carcinoma. Tumour histological subtype of MBC provides independent prognostic information. Both observations should be considered when managing MBC patients.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, Kim S, Choe JH, Lee JE, Kim JH, Kim JS, Nam SJ, Yang JH. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011;126:471–478. doi: 10.1007/s10549-011-1359-8. [DOI] [PubMed] [Google Scholar]
- Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer: clinical significance. Am J Surg. 2006;191:657–664. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Downs-Kelly E, Nayeemuddin KM, Albarracin C, Wu Y, Hunt KK, Gilcrease MZ. Matrix-producing carcinoma of the breast: an aggressive subtype of metaplastic carcinoma. Am J Surg Pathol. 2009;33:534–541. doi: 10.1097/PAS.0b013e31818ab26e. [DOI] [PubMed] [Google Scholar]
- Foschini MP, Dina RE, Eusebi V. Sarcomatoid neoplasms of the breast: proposed definitions for biphasic and monophasic sarcomatoid mammary carcinomas. Semin Diagn Pathol. 1993;10:128–136. [PubMed] [Google Scholar]
- Gibson GR, Qian D, Ku JK, Lai LL. Metaplastic breast cancer: clinical features and outcomes. Am Surg. 2005;71:725–730. [PubMed] [Google Scholar]
- Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol. 2003;21:2070–2076. doi: 10.1200/JCO.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Gobbi H, Simpson JF, Borowsky A, Jensen RA, Page DL. Metaplastic breast tumors with a dominant fibromatosis-like phenotype have a high risk of local recurrence. Cancer. 1999;85:2170–2182. doi: 10.1002/(sici)1097-0142(19990515)85:10<2170::aid-cncr11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Gwin K, Wheeler DT, Bossuyt V, Tavassoli FA. Breast carcinoma with chondroid differentiation: a clinicopathologic study of 21 triple negative (ER-, PR-, Her2/neu-) cases. Int J Surg Pathol. 2010;18:27–35. doi: 10.1177/1066896909332732. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz TA, Babiera G, Hortobagyi GN, Valero V. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17:605–613. doi: 10.1093/annonc/mdl006. [DOI] [PubMed] [Google Scholar]
- Jung SY, Kim HY, Nam BH, Min SY, Lee SJ, Park C, Kwon Y, Kim EA, Ko KL, Shin KH, Lee KS, Park IH, Lee S, Kim SW, Kang HS, Ro J. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120:627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- Kaufman MW, Marti JR, Gallager HS, Hoehn JL. Carcinoma of the breast with pseudosarcomatous metaplasia. Cancer. 1984;53:1908–1917. doi: 10.1002/1097-0142(19840501)53:9<1908::aid-cncr2820530917>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, Lee KS, Lee S, Kim SW, Kang HS, Ko KL, Ro J. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65:441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- Lester TR, Hunt KK, Nayeemuddin KM, Bassett RL, Jr., Gonzalez-Angulo AM, Feig BW, Huo L, Rourke LL, Davis WG, Valero V, Gilcrease MZ. Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Breast Cancer Res Treat. 2012;131:41–48. doi: 10.1007/s10549-011-1393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, Maisonneuve P, Vento AR, Viale G. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- Nayak A, Wu Y, Gilcrease MZ. Primary squamous cell carcinoma of the breast: predictors of locoregional recurrence and overall survival. Am J Surg Pathol. 2013;37:867–873. doi: 10.1097/PAS.0b013e3182877569. [DOI] [PubMed] [Google Scholar]
- Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14:166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- Rakha EA. Pitfalls in outcome prediction of breast cancer. J Clin Pathol. 2013;66:458–464. doi: 10.1136/jclinpath-2012-201083. [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25:4772–4778. doi: 10.1200/JCO.2007.12.2747. [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, Blamey RW, Ellis IO. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet JS, Akslen LA, Evans AJ, Blamey R, Reis-Filho JS, Foulkes WD, Ellis IO. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–2310. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, Macmillan D, Ellis IO. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2011;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. 1999;10:413–419. doi: 10.1023/a:1008329910362. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Lakhani SR, Gobbi H, Sneige N.Eds. (2012Metaplastic carcinoma WHO Classification of Tumours of the Breast. IARC press: Lyon [Google Scholar]
- Reis-Filho JS, Milanezi F, Steele D, Savage K, Simpson PT, Nesland JM, Pereira EM, Lakhani SR, Schmitt FC. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- Rosen PP. Rosen's Breast Pathology. Lippincott Williams & Wilkins: Philadelphia; 2009. [Google Scholar]
- Smith BH, Taylor HB. The occurrence of bone and cartilage in mammary tumors. Am J Clin Pathol. 1969;51:610–618. doi: 10.1093/ajcp/51.5.610. [DOI] [PubMed] [Google Scholar]
- Spagnolo DV, Shilkin KB. Breast neoplasms containing bone and cartilage. Virchows Arch A Pathol Anat Histopathol. 1983;400:287–295. doi: 10.1007/BF00612190. [DOI] [PubMed] [Google Scholar]
- Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- Tse GM, Tan PH, Putti TC, Lui PC, Chaiwun B, Law BK. Metaplastic carcinoma of the breast: a clinicopathological review. J Clin Pathol. 2006;59:1079–1083. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WH, Martinez SR. Metaplastic breast cancer: to radiate or not to radiate. Ann Surg Oncol. 2011;18:94–103. doi: 10.1245/s10434-010-1198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurzen CH, Lee AH, Gill MS, Menke-Pluijmers MB, Jager A, Ellis IO, Rakha EA. Metaplastic breast carcinoma: tumour histogenesis or dedifferentiation. J Pathol. 2011;224:434–437. doi: 10.1002/path.2872. [DOI] [PubMed] [Google Scholar]
- Van Hoeven KH, Drudis T, Cranor ML, Erlandson RA, Rosen PP. Low-grade adenosquamous carcinoma of the breast. A clinocopathologic study of 32 cases with ultrastructural analysis. Am J Surg Pathol. 1993;17:248–258. doi: 10.1097/00000478-199303000-00005. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori I, Tang W, Yang Q, Nakamura M, Nakamura Y, Sato M, Sakurai T, Kennichi K. Metaplastic carcinoma of the breast: p53 analysis identified the same point mutation in the three histologic components. Mod Pathol. 2001;14:1183–1186. doi: 10.1038/modpathol.3880456. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Horii R, Maeda I, Suga S, Makita M, Iwase T, Oguchi M, Ito Y, Akiyama F. Clinicopathologic study of 53 metaplastic breast carcinomas: their elements and prognostic implications. Hum Pathol. 2010;41:679–685. doi: 10.1016/j.humpath.2009.10.009. [DOI] [PubMed] [Google Scholar]