Abstract
Objective
Atrial fibrillation (AF) often progresses from paroxysmal or persistent to more sustained forms, but the rate and predictors of AF progression in clinical practice are not well described.
Methods
Using the Outcomes Registry for Better Informed Treatment of AF, we analysed the incidence and predictors of progression and tested the discrimination and calibration of the HATCH (hypertension, age, TIA/stroke, chronic obstructive pulmonary disease, heart failure) and CHA2DS2VASc scores for identifying AF progression.
Results
Among 6235 patients with paroxysmal or persistent AF at baseline, 1479 progressed, during follow-up (median 18 (IQR 12–24) months). These patients were older and had more comorbidities than patients who did not progress (CHADS2 2.3±1.3 vs 2.1±1.3, p<0.0001). At baseline, patients with AF progression were more often on a rate control as opposed to a rhythm control strategy (66 vs 56%, p<0.0001) and had higher heart rate (72(64–80) vs 68(60–76) bpm, p<0.0001). The strongest predictors of AF progression were AF on the baseline ECG (OR 2.30, 95% CI 1.95 to 2.73, p<0.0001) and increasing age (OR 1.16, 95% CI1.09 to 1.24, p<0.0001, per 10 increase), while patients with lower heart rate (OR 0.84, 95% CI 0.79 to 0.89, p<0.0001, per 10 decrease ≤80) were less likely to progress. There was no significant interaction between rhythm on baseline ECG and heart rate (p=0.71). The HATCH and CHA2DS2VASc scores had modest discriminatory power for AF progression (C-indices 0.55 (95% CI 0.53 to 0.58) and 0.55 (95% CI 0.52 to 0.57)).
Conclusions
Within 1.5 years, almost a quarter of the patients with paroxysmal or persistent AF progress to a more sustained form. Progression is strongly associated with heart rate, and age.
Introduction
The progressive nature of atrial fibrillation (AF) is a well-described phenomenon in both animal models and clinical medicine.1–3 Over time, the paroxysms of AF often become longer in duration and more frequent and many patients eventually progress to permanent AF.1 In experimental models, initiation of AF leads to both electrophysiologic and structural remodelling that promote the maintenance and persistence of AF.2 3 The definition of progression varies, but is usually defined either as a transition from a self-terminating to a non-self-terminating form (ie, paroxysmal to persistent or permanent) or any transition to a more sustained form of AF (ie, either paroxysmal to persistent/permanent or persistent to permanent).4–9 Although clinical trials have failed to demonstrate the superiority of a rhythm control strategy (ie, the ultimate treatment goal is restoration and maintenance of sinus rhythm) over a rate control strategy,10 11 the presence of sinus rhythm is associated with improved outcomes and function.12 Likewise, progression of AF has been shown to be associated with increased risks of hospitalisation and thromboembolic events.6
Observational studies suggest that the rate of AF progression ranges between 10% and 20% per year depending on the patient selection, duration of follow-up and the definition of progression.4–9 Scoring systems have also been developed to predict AF progression, but their discriminatory capabilities are not well validated. The objective of this study was (1) to determine the incidence and predictors of AF progression in a large, contemporary cohort, (2) to determine whether or not risk factors for progression are similar in patients with paroxysmal and persistent AF and (3) to test existing predictive models of AF progression.
Methods
The ORBIT-AF study is a contemporary registry of outpatients in the USA with AF managed by a variety of providers. Its design has been described in detail elsewhere.13 Briefly, a nationally representative sample of sites was invited to participate and an adaptive design was used to ensure provider and geographic heterogeneity. Consecutive patients with AF, meeting all the inclusion criteria (≥18 years, electrocardiographic evidence of AF, providing informed consent) and none of the exclusion criteria (life expectancy <6 months or AF secondary to reversible conditions) were enrolled. For the purpose of this analysis, patients with first-detected or permanent AF at baseline, a history of AV nodal ablation or missing follow-up or data for AF type in follow-up were excluded.
Data collection included demographics, past medical history, type of AF and prior interventions, ongoing antithrombotic therapy, vital signs, laboratory studies, electrocardiographic findings and echocardiographic findings. Participating sites were instructed to record the patient's heart rate (ascertained from ECG or from a physical examination) at baseline after a 5-min resting period. In ORBIT-AF, follow-up data collection occurs at 6-month intervals. Assessment of AF type was made by the site investigator according to consensus definitions, and updated with each follow-up.
Paroxysmal AF was defined as recurrent AF episodes that terminated spontaneously within 7 days; persistent AF was defined as recurrent AF that was sustained for more than 7 days; permanent AF was defined as continuous AF in which the presence of the AF was accepted by the patient and physician.14
Statistical analyses
Progression in AF was considered as a binary outcome (either ‘the same or better’ or ‘worsening’). ‘Worsening’ was defined as either paroxysmal AF at baseline becoming persistent or permanent at any follow-up visit or persistent AF at baseline becoming permanent at any follow-up visit. We compared the characteristics of patients with and without AF progression. Continuous variables were presented as medians (IQR) and differences across two groups were assessed using the Wilcoxon rank-sum test. Categorical variables were presented as counts (proportions) and differences across two groups were assessed using the χ2 test.
We identified predictors of AF progression using pooled logistic regression, in order to account for differential follow-up duration. Also referred to as a proportional odds model for discrete time, this method essentially fits a logistic regression for the binary occurrence of event, at each 6-month follow-up, and combines the results to provide a single OR for the effect of covariates. As in a Cox proportional hazards model, individuals contributed all available follow-up information and were censored (removed from the risk set) when follow-up was lost. To account for the correlation in the same site, we incorporated generalised estimating equations. Results were reported using ORs with 95% CIs and p values. The final regression model for the AF progression was developed from candidate variables (see online supplementary appendix) using backward selection, with an α for exclusion of 0.05. Due to the importance of baseline AF type (ie, paroxysmal vs persistent), it was included in the final model regardless of selection. All continuous variables were tested for linearity, and non-linear relationships were accounted for using linear splines. To explore whether predictors of AF progression differ based on baseline AF type, we tested for interactions. We provided separate risk estimates of the identified predictors among patients with baseline paroxysmal AF and among patients with baseline persistent AF when an interaction was found to be present. Missing data on the covariates used in the modelling were handled using multiple imputations.15 16 Combined results from the five imputed data sets were used in the calculation of the final risk estimates and SEs.15 16
In order to test the ability of the HATCH score (hypertension+(age>75 years)+(TIA/stroke)×2+chronic obstructive pulmonary disease+(congestive heart failure)×2)6 to predict AF progression, we evaluated discrimination and calibration in our population. For comparability, we matched the derivation of the HATCH score: including only patients with paroxysmal AF and evaluating only 12-month follow-up. Discrimination of the model (ie, whether or not a given patient is correctly identified as a progressor) was measured by the c-index and by comparing the distribution of HATCH scores between people who did and did not progress. Calibration was evaluated by comparing the observed progression rates among the low, medium and high-risk categories to those predicted by the HATCH model. For comparison, we also evaluated the CHA2DS2-VASc score for discrimination of progression.17
All statistical analyses of the aggregate, de-identified data were performed by the Duke Clinical Research Institute using SAS software (V.9.3, SAS Institute, Cary, North Carolina, USA). All p values were two sided. The ORBIT-AF Registry is approved by the Duke Institutional Review Board, and all participating sites obtained institutional review board approval pursuant to local requirements. All subjects provided written, informed consent.
Results
Cohort formation and characteristics
The entire baseline ORBIT-AF population included 10 132 patients enrolled between 29 June 2010 and 9 August 2011 from 176 sites. For the purpose of the current analysis, we excluded 3897 patients: 3040 due to first-detected or permanent AF at baseline, 114 due to previous AV nodal ablation, 733 due to missing follow-up, and 10 due to missing data for AF type. This yielded a final study population of 6235 patients from 170 sites.
Overall, 1479 patients (24%) demonstrated progression of their AF during a median of 18 (IQR 12–24) months follow-up. Of these patients, 1032 (70%) had paroxysmal AF at baseline, while 447 (30%) had persistent. Among patients with paroxysmal AF who progressed during follow-up, 556 (54%) progressed to persistent AF while the remainder progressed to permanent (n=476, 46%). The rate of progression at 18 months, estimated by KM methods that account for differential follow-up, was 23% (95% CI 22% to 24%). Table 1 shows the baseline characteristics in the overall study population and by AF progression. Compared with patients without AF progression, patients with AF progression were older (75 (67–82) vs 73 (65–81) years, p<0.0001) and more often of white race (92% vs 90%, p=0.0014). They had more comorbidity, with a higher prevalence of hypertension, COPD, congestive heart failure, valvular disease and sinus node dysfunction. Patients with subsequent AF progression had higher heart rates (72 (64–80) vs 68 (60–76) bpm, p<0.0001) at baseline and larger left atria on echocardiography (4.5 (4.0–5.0) vs 4.2 (3.8–4.8) cm, p<0.0001) in univariate analyses.
Table 1.
Baseline characteristics by AF progression
| Overall | No AF progression | AF progression | p Value | |
|---|---|---|---|---|
| (N=6235) | (N=4756) | (N=1479) | ||
| Age (years) | 74 (65–81) | 73 (65–81) | 75 (67–82) | <0.0001 |
| Male | 57 | 56 | 57 | 0.56 |
| Race | ||||
| White | 91 | 90 | 92 | 0.0014 |
| Black or African American | 4.4 | 5.0 | 2.6 | |
| Hispanic | 3.5 | 3.4 | 3.8 | |
| Other | 1.4 | 1.5 | 1.3 | |
| Private insurance | 28 | 28 | 24 | 0.0087 |
| Medical history | ||||
| Hypertension | 82 | 81 | 85 | 0.0004 |
| Diabetes | 28 | 28 | 29 | 0.65 |
| Obstructive sleep apnoea | 19 | 18 | 19 | 0.41 |
| Coronary artery disease | 35 | 35 | 37 | 0.071 |
| Congestive heart failure | 29 | 27 | 35 | <0.0001 |
| Significant valvular disease | 22 | 21 | 25 | 0.0004 |
| Sinus node dysfunction | 18 | 17 | 21 | 0.0001 |
| Prior cerebrovascular events | 15 | 15 | 16 | 0.22 |
| Anaemia | 17 | 17 | 16 | 0.17 |
| Chronic obstructive pulmonary disease | 15 | 15 | 17 | 0.0157 |
| Gastrointestinal bleeding | 8.5 | 8.6 | 8.2 | 0.61 |
| Cognitive impairment or dementia | 2.7 | 2.8 | 2.4 | 0.39 |
| Frailty | 4.9 | 4.6 | 6.1 | 0.0178 |
| BMI (kg/m2) | 29 (25–34) | 29 (25–34) | 30 (25–35) | 0.070 |
| Heart rate (bpm) | 69 (61–78) | 68 (60–76) | 72 (64–80) | <0.0001 |
| Systolic blood pressure (mm Hg) | 126 (116–138) | 126 (117–138) | 125 (116–136) | 0.098 |
| Diastolic blood pressure (mm Hg) | 72 (66–80) | 72 (66–80) | 72 (66–80) | 0.83 |
| Calculated creatinine clearance (mL/min per 1.73 m2) | 71 (51–100) | 72 (52–101) | 68 (50–96) | 0.0099 |
| LVEF≥50% | 73 | 74 | 70 | <0.0001 |
| Left atrial diameter (cm) | 4.3 (3.8–4.8) | 4.2 (3.8–4.8) | 4.5 (4.0–5.0) | <0.0001 |
Continuous variables are presented as median and IQR.
AF, atrial fibrillation; BMI, body mass index; bpm, beats per min.
Atrial fibrillation characteristics at baseline
AF history and characteristics are summarised in table 2. Patients with AF progression during follow-up had longer history of AF and were more likely to have persistent AF (30% vs 23%, p<0.0001). Patients with AF progression more frequently were in AF on their baseline ECG (58 vs 34%, p<0.0001). They were more likely to have undergone cardioversion (37 vs 30%, p<0.0001), but were less likely to have undergone catheter ablation of AF at baseline (5.5% vs 7.7%, p=0.0053). Patients with subsequent AF progression were less likely to be treated with a rhythm control strategy at baseline (34% vs 44%, p<0.0001) and were less likely to be on an antiarrhythmic agent (30% vs 41%, p<0.0001). Their CHADS2 score was higher compared with patients without AF progression (2.3±1.3 vs 2.1±1.3, p<0.0001) and they were more likely to be on oral anticoagulation therapy.
Table 2.
Atrial fibrillation (AF) history by AF progression
| Overall | No AF progression | AF progression | p Value | |
|---|---|---|---|---|
| (N=6235) | (N=4756) | (N=1479) | ||
| AF type | ||||
| Paroxysmal | 76 | 77 | 70 | <0.0001 |
| Persistent | 24 | 23 | 30 | |
| Family history of AF | 15 | 15 | 15 | 0.53 |
| Duration of AF diagnosis (months) | 42 (18–85) | 41 (17–82) | 49 (21–92) | <0.0001 |
| AF on most recent ECG | 40 | 34 | 58 | <0.0001 |
| EHRA symptom level | ||||
| No symptoms | 36 | 34 | 39 | 0.0015 |
| Mild | 47 | 47 | 44 | |
| Severe | 15 | 16 | 14 | |
| Disabling | 2.1 | 2.3 | 1.5 | |
| CHADS2 risk groups | ||||
| 0 | 7.8 | 8.6 | 5.2 | <0.0001 |
| 1 | 24 | 24 | 22 | |
| ≥2 | 68 | 67 | 72 | |
| Prior treatment | ||||
| Oral anticoagulation therapy | 81 | 79 | 88 | <0.0001 |
| Antiarrhythmic drug | 53 | 53 | 51 | 0.26 |
| Prior cardioversions | 32 | 30 | 37 | <0.0001 |
| Prior catheter ablation of AF | 7.2 | 7.7 | 5.5 | 0.0053 |
| Current treatment | ||||
| Oral anticoagulation therapy | 73 | 70 | 83 | <0.0001 |
| β blockers | 63 | 62 | 66 | 0.0059 |
| Calcium channel blockers | 30 | 30 | 32 | 0.29 |
| Digoxin | 0 | 19 | 26 | <0.0001 |
| Antiarrhythmic drug | 38 | 41 | 30 | <0.0001 |
| Rhythm strategy | 42 | 44 | 34 | <0.0001 |
Continuous variables are presented as median and inter-quartile range.
AF, atrial fibrillation; EHRA, European Heart Rhythm Association.
Factors associated with atrial fibrillation progression
Factors independently associated with progression of AF type after adjustment is summarised in table 3. In addition to presence of AF on the most recent ECG at baseline (OR 2.30, 95% CI 1.95 to 2.73, p<0.0001), the factors with the strongest association with a higher likelihood of AF progression were increasing age (OR 1.16, 95% CI 1.09 to 1.24, p<0.0001; per 10 year increase), heart failure (OR 1.61, 95% CI 1.26 to 2.06, p=0.0002; New York Heart Association (NYHA) class III/IV vs no heart failure) and left atrial enlargement (OR 1.35, 95% CI 1.14 to 1.59, p=0.0004; moderate enlargement vs no enlargement). In contrast, the factor with the strongest association with a lower likelihood of AF progression was a lower heart rate (OR 0.84 per 10 bpm decrease below 80 bpm, 95% CI 0.79 to 0.89, p<0.0001). The C-index of the model presented in table 3 was 0.67 (95% CI 0.66 to 0.69), and the C-index for the model adjusted for only heart rate, AF on the ECG at baseline and baseline AF type was 0.64 (95% CI 0.63 to 0.66).
Table 3.
Predictors of AF progression
| Risk factor | Adjusted OR (95% CI) | t Value | p Value |
|---|---|---|---|
| AF or atrial flutter on baseline ECG | 2.30 (1.95 to 2.73) | 9.66 | <0.0001 |
| Heart Rate ≤80, bpm (per 10 decrease) | 0.84 (0.70 to 0.89) | 5.60 | <0.0001 |
| Age, years (per 10 increase) | 1.16 (1.09 to 1.24) | 4.48 | <0.0001 |
| NYHA class III/IV vs no heart failure | 1.61 (1.26 to 2.06) | 3.78 | 0.0002 |
| Moderate left atrial enlargement vs no enlargement | 1.35 (1.14 to 1.59) | 3.52 | 0.0004 |
| NYHA class II vs no heart failure | 1.38 (1.15 to 1.65) | 3.51 | 0.0005 |
| African American vs white | 0.56 (0.40 to 0.78) | −3.49 | 0.0005 |
| Mild left atrial enlargement vs no enlargement | 1.24 (1.07 to 1.42) | 2.93 | 0.0034 |
| Severe left atrial enlargement vs no enlargement | 1.30 (1.07 to 1.59) | 2.61 | 0.0101 |
| Anaemia* | 0.80 (0.67 to 0.97) | −2.30 | 0.0215 |
| Prior valve replacement or repair | 1.25 (1.03 to 1.52) | 2.22 | 0.0267 |
| NYHA class I vs no heart failure | 1.23 (1.02 to 1.48) | 2.18 | 0.0291 |
| Weight, kg (per 10 increase) | 1.03 (1.00 to 1.06) | 2.15 | 0.0317 |
| Persistent AF vs paroxysmal AF | 0.95 (0.74 to 1.22) | −0.40 | 0.6926 |
| Hispanic vs white | 1.04 (0.49 to 2.21) | 0.10 | 0.9201 |
| Other race vs white | 0.99 (0.62 to 1.60) | −0.02 | 0.9815 |
OR and 95% CI are attained by combining results from the five imputed data sets.
*Significant interaction between risk factor and baseline AF type.
AF, atrial fibrillation; NYHA, New York Heart Association.
Moreover, the addition of treatment variables (eg, rhythm or rate control, prior cardioversion, medications) to the original model led to similar results (C-index 0.67 for full model (95% CI 0.66 to 0.69)). Notably, rhythm or rate control strategy was not independently associated with the rate of AF progression (see online supplementary Appendix). There was no significant interaction between rhythm at most recent ECG and heart rate on AF progression any of the associations (p=0.71).
Factors associated with progression in paroxysmal versus persistent AF
All but one of the risk factors had similar impact in patients with paroxysmal and persistent AF at baseline, the only exception being the influence of anaemia (adjusted OR 0.73, 95% CI 0.59 to 0.90, p=0.0031 vs OR 1.02, 95% CI 0.79 to 1.32, p=0.89. p Value for interaction=0.0273), which was stronger in patients with paroxysmal AF.
Model-based progression prediction—HATCH and CHA2DS2VASc scores
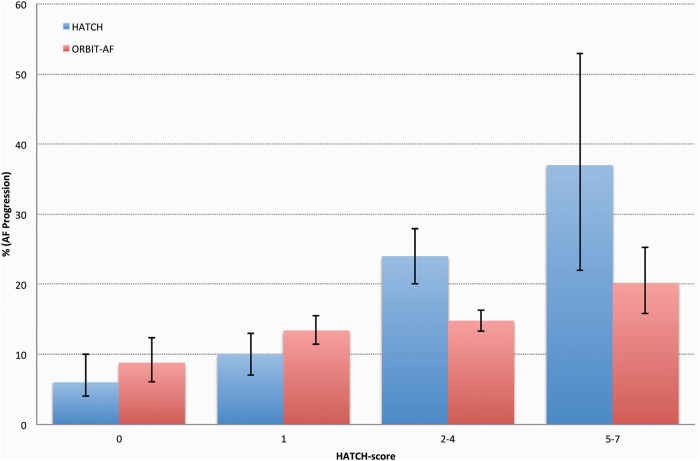
We calculated HATCH scores in the subset of patients (n=3958) with (1) paroxysmal AF at baseline and (2) available 12-month follow-up.6 The median age was 74 years (IQR 66–81) and comorbidities included in the HATCH score were frequently present (hypertension 82%, TIA/stroke 15%, COPD 15% and congestive heart failure 26%). The distribution of HATCH score risk groups by AF progression is summarised in table 4. The HATCH score was modestly higher in the 565 (14%) patients with AF progression during the 12-month follow-up (mean±SD; 2.5±1.6 vs 2.2±1.5, p<0.0001), and the ability to discriminate the risk was low (C-index=0.5524, 95% CI 0.5274 to 0.5774). The AF progression rates by HATCH score risk groups are illustrated in figure 1. Similarly, the CHA2DS2-VASc score was higher in patients with AF progression during 12-month follow-up (mean±SD; 4.0±1.7 vs 3.7±1.7, p=0.0003). The C-index of the CHA2DS2-VASc score for the prediction of AF progression was 0.5468 (95% CI 0.5218 to 0.5717).
Table 4.
Descriptive of AF progression across four HATCH score group among patients with paroxysmal AF at baseline and 12 month follow-up
| HATCH score* | Overall N=3958 |
No AF progression N=3393 |
AF progression N=565 |
||||
|---|---|---|---|---|---|---|---|
| Score | Risk | N | (%) | N | (%) | N | (%) |
| 0 | Very low | 341 | (8.6) | 311 | (9.2) | 30 | (5.3) |
| 1 | Low | 1128 | (29) | 977 | (29) | 151 | (27) |
| 2–4 | Moderate | 2192 | (55) | 1868 | (55) | 324 | (57) |
| 5–7 | High | 297 | (7.5) | 237 | (7.0) | 60 | (11) |
*HATCH score=Hypertension+(Age >75 years)+(TIA or stroke)×2+Chronic obstructive pulmonary disease+(congestive heart failure)×2.6
AF, atrial fibrillation; HATCH, hypertension, age, TIA/stroke, chronic obstructive pulmonary disease, heart failure.
Figure 1.
Percentage of atrial fibrillation (AF) progression across four HATCH score group among patients with paroxysmal AF at baseline and 12 month follow-up. HATCH score=Hypertension+(Age >75 years)+(TIA or stroke)×2+Chronic obstructive pulmonary disease+(congestive heart failure)×2.6 The corresponding percentages in the original by de Vos et al6 are shown for comparison. The error bars represent the 95% CI. AF, atrial fibrillation; HATCH, hypertension, age, TIA/stroke, chronic obstructive pulmonary disease, heart failure.
Discussion
In this study of AF progression in a nationwide cohort of more than 6000 patients, there are three major findings. First, nearly a quarter of patients with paroxysmal or persistent AF progress to a more sustained form of AF within 18 months. Second, apart from the presence of AF on the most recent ECG at baseline, the factors with the strongest associations with AF progression were higher heart rate, heart failure, and age. Finally, both the HATCH and the CHA2DS2-VASc score provided poor discrimination for AF progression in this patient population.
The observed rate of progression in the current analysis (23% over an 18-month period) is consistent with similar, but smaller studies, which consistently have shown progression rates of about 14–18% at 1 year.6 8 9 The one notable exception is the Canadian Registry of Atrial Fibrillation (CARAF), which enrolled 757 patients with paroxysmal AF.4 The progression rate to chronic AF (defined as ECG documentation of AF on two consecutive ECGs at least a week apart) was only 8.6% during the first year, although a slow but steady increase was observed thereafter (about 25% at 5 years). It may be, that their careful measures to exclude patients with potentially chronic AF at baseline (patients who were observed to be in AF at baseline and were without documentation of return to sinus rhythm up to the 3-month visit were considered chronic and excluded) may have led to a lower observed rate of progression.4
Delineation of factors associated with AF progression can help identify patients who may be at risk for developing more advanced forms of AF. Several of the factors independently associated with AF progression in the current study are well known and consistent with prior investigations (eg, increasing age, heart failure and left atrial enlargement).4–9 However, these data also demonstrate an association between increasing heart rate and an increased likelihood of AF progression. In contrast, the CARAF study, which enrolled patients at their initial presentation of AF (ie, all patients were in AF at baseline), suggested that higher heart rates during AF were associated with a lower rate of progression.4 Direct comparison of the two studies is hampered by the fact that patients included in the present study could be in either sinus rhythm or AF at baseline and at any stage of the disease. These differences are in part illustrated by the mean heart rate at baseline, which was substantially higher in CARAF (124 bpm) compared with our study (69 bpm). Notably, the association between heart rate and AF progression was exclusively seen within the normal range (ie, below 80 bpm) in the present study. Again, it bears emphasising that the association between heart rate and AF progression was preserved regardless of whether the patient was in sinus rhythm or AF. The RACE II trial suggested that lenient rate control is non-inferior to strict rate control to prevent major clinical events. However, this study was small and had a wide non-inferiority margin. Our findings, while hypothesis generating, suggest that heart rate control in patients with paroxysmal and persistent AF may impact AF progression. Alternatively, heart rate may be associated with other (unmeasured) factors that influence the likelihood of AF progression such as autonomic tone. This hypothesis should be tested in future clinical studies.1 18
Finally, it should be noted that while numerous factors were associated with AF progression, the discriminatory power of the model was principally driven only by two factors: AF on the baseline ECG and heart rate.
Patients with persistent AF were not more likely to progress than patients with paroxysmal AF, in contrast with the findings in RECORD-AF.7 Moreover, factors associated with AF progression were largely the same in patients with paroxysmal and persistent AF, indicating that the overall mechanisms of progression are similar. The single exception was anaemia, which was associated with a lower progression rate in patients with paroxysmal AF. The explanation to this association is not obvious and could represent a chance finding.
We attempted to determine if risk scores can be used to identify patients at risk for progression. Unfortunately, our data suggest that such efforts are mildly successful at best. Previously, De Vos et al,6 investigated progression in patients with paroxysmal AF in the Euro Heart Survey. During the 1-year follow-up, 15% of the patients progressed to persistent or permanent AF, with heart failure, age, previous TIA/stroke, COPD and hypertension as independent predictors of progression.6 These predictors were combined into a scoring model, the HATCH score, which was shown to discriminate individuals who would or would not progress during follow-up with clinically meaningful accuracy.6
Compared to the original cohort from the Euro Heart Survey, ORBIT-AF patients were older and had more comorbidity. Therefore, patients in ORBIT-AF had substantially higher HATCH scores, such that two-thirds of the patients were moderate-to-high risk. Although patients in ORBIT-AF with a higher HATCH score were slightly more likely to progress in their AF, the discriminatory power of HATCH score added little value over simply flipping a coin and was only marginally superior to using CHA2DS2-VASc score. This highlights the challenges of predicting AF progression and the need for better measures of disease progression. Recent data suggests that left atrial scar quantification via cardiac magnetic resonance may have an important role in this regard.19
These data are derived from a voluntary, observational study and thus are susceptible to the limitations inherent in such methods. These include both selection and reporting biases. Despite the use of stringent adjustment techniques with a wide variety of covariates, we cannot exclude the possibility that unmeasured confounding may influence our findings. As per the study protocol, ECGs were recorded in the case report form every 6 months and consequently, more detailed electrocardiographic data or quantitative AF burden were not available.
Conclusions
Almost a quarter of the patients with paroxysmal or persistent AF progressed to a more sustained form during a median of 18 months follow-up. The factors with the strongest association with AF progression were AF on baseline ECG, heart rate, age and the presence of heart failure. While a rate or rhythm control strategy was not independently associated with AF progression, lower heart rate and/or stricter rate control may be of importance to prevent AF progression. Finally, identification of patients at risk for progression remains challenging given the discriminatory power of current models, highlighting the need for better measures of AF disease progression.
Key messages.
What is already known about this subject?
It is well known that atrial fibrillation (AF) often progresses from paroxysmal or persistent to more sustained forms, but the rate and predictors of AF progression in clinical practice are not well described. Scoring systems, like the hypertension, age, TIA/stroke, chronic obstructive pulmonary disease, heart failure (HATCH) score, have been developed to predict AF progression, but their discriminatory capabilities are not well validated.
What does this study add?
Heart rate at baseline was identified as a potentially important predictor of AF progression. The results of our analyses show that neither HATCH nor CHA2DS2VAS scores predict AF progression with clinically meaningful accuracy.
How might this impact on clinical practice?
While a rate or rhythm control strategy is not independently associated with AF progression, lower heart rate may influence or delay AF progression. Finally, identification of patients at risk for progression remains challenging given the discriminatory power of current models, highlighting the need for better measures of AF disease progression.
Supplementary Material
Footnotes
Contributors: FH, BAS and JPP contributed to the conception and design of the study and to the data analyses and interpretation. SK and LT contributed to the acquisition of data and to data analyses and interpretation. JAR, KWM, BJG, GCF, GVN, PC, JVF, PRK and EDP contributed to the conception and design of the study. All authors contributed to preparation of the manuscript and approved the final version. All of the authors take full responsibility for the data herein, and have read and approved the final manuscript.
Funding: The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, New Jersey, USA.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Duke Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 2.Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. 10.1161/01.CIR.92.7.1954 [DOI] [PubMed] [Google Scholar]
- 3.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. 10.1016/S0008-6363(02)00258-4 [DOI] [PubMed] [Google Scholar]
- 4.Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149:489–96. 10.1016/j.ahj.2004.09.053 [DOI] [PubMed] [Google Scholar]
- 5.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation 2007;115:3050–6. 10.1161/CIRCULATIONAHA.106.644484 [DOI] [PubMed] [Google Scholar]
- 6.de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. 10.1016/j.jacc.2009.11.040 [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Breithardt G, Crijns H, et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF. J Am Coll Cardiol 2011;58:493–501. 10.1016/j.jacc.2011.03.034 [DOI] [PubMed] [Google Scholar]
- 8.De Vos CB, Breithardt G, Camm AJ, et al. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial Fibrillation cohort: clinical correlates and the effect of rhythm-control therapy. Am Heart J 2012;163:887–93. 10.1016/j.ahj.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 9.Zhang YY, Qiu C, Davis PJ, et al. Predictors of Progression of Recently Diagnosed Atrial Fibrillation in REgistry on Cardiac Rhythm DisORDers Assessing the Control of Atrial Fibrillation (RecordAF)-United States Cohort. Am J Cardiol 2013;112:79–84. 10.1016/j.amjcard.2013.02.056 [DOI] [PubMed] [Google Scholar]
- 10.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33. 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 11.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834–40. 10.1056/NEJMoa021375 [DOI] [PubMed] [Google Scholar]
- 12.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509–13. 10.1161/01.CIR.0000121736.16643.11 [DOI] [PubMed] [Google Scholar]
- 13.Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J 2011;162:606–12.e1. 10.1016/j.ahj.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2012;9:632–96.e21. 10.1016/j.hrthm.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 15.Enders CK. Applied missing data analysis. New York; London: Guilford, 2010. [Google Scholar]
- 16.Rubin DB. Multiple imputation for nonresponse in surveys. New York; Chichester: Wiley, 1987. [Google Scholar]
- 17.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 18.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–73. 10.1056/NEJMoa1001337 [DOI] [PubMed] [Google Scholar]
- 19.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. 10.1001/jama.2014.3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.