ABSTRACT
Genomic data predict that, in addition to oxygen, the bacterial plant pathogen Ralstonia solanacearum can use nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), and nitrous oxide (N2O) as terminal electron acceptors (TEAs). Genes encoding inorganic nitrogen reduction were highly expressed during tomato bacterial wilt disease, when the pathogen grows in xylem vessels. Direct measurements found that tomato xylem fluid was low in oxygen, especially in plants infected by R. solanacearum. Xylem fluid contained ~25 mM NO3−, corresponding to R. solanacearum’s optimal NO3− concentration for anaerobic growth in vitro. We tested the hypothesis that R. solanacearum uses inorganic nitrogen species to respire and grow during pathogenesis by making deletion mutants that each lacked a step in nitrate respiration (ΔnarG), denitrification (ΔaniA, ΔnorB, and ΔnosZ), or NO detoxification (ΔhmpX). The ΔnarG, ΔaniA, and ΔnorB mutants grew poorly on NO3− compared to the wild type, and they had reduced adenylate energy charge levels under anaerobiosis. While NarG-dependent NO3− respiration directly enhanced growth, AniA-dependent NO2− reduction did not. NO2− and NO inhibited growth in culture, and their removal depended on denitrification and NO detoxification. Thus, NO3− acts as a TEA, but the resulting NO2− and NO likely do not. None of the mutants grew as well as the wild type in planta, and strains lacking AniA (NO2− reductase) or HmpX (NO detoxification) had reduced virulence on tomato. Thus, R. solanacearum exploits host NO3− to respire, grow, and cause disease. Degradation of NO2− and NO is also important for successful infection and depends on denitrification and NO detoxification systems.
IMPORTANCE
The plant-pathogenic bacterium Ralstonia solanacearum causes bacterial wilt, one of the world’s most destructive crop diseases. This pathogen’s explosive growth in plant vascular xylem is poorly understood. We used biochemical and genetic approaches to show that R. solanacearum rapidly depletes oxygen in host xylem but can then respire using host nitrate as a terminal electron acceptor. The microbe uses its denitrification pathway to detoxify the reactive nitrogen species nitrite (a product of nitrate respiration) and nitric oxide (a plant defense signal). Detoxification may play synergistic roles in bacterial wilt virulence by converting the host’s chemical weapon into an energy source. Mutant bacterial strains lacking elements of the denitrification pathway could not grow as well as the wild type in tomato plants, and some mutants were also reduced in virulence. Our results show how a pathogen’s metabolic activity can alter the host environment in ways that increase pathogen success.
INTRODUCTION
Diverse bacteria are capable of respiratory nitrate reduction, the membrane-bound conversion of nitrate (NO3−) to nitrite (NO2−), and denitrification, the stepwise enzymatic reduction of NO2− to nitrogen gas (N2) via nitric oxide (NO) and nitrous oxide (N2O). NO3− reduction and denitrification enable respiration in hypoxic or anaerobic environments because nitrogen oxides can serve as terminal electron acceptors (TEAs) in place of oxygen (O2) (1). Bacterial NO3− respiration and denitrification are major drivers of the global nitrogen (N) cycle, of nutrient losses from soils, and of climate change (2–4). Though it has been extensively investigated in mutualistic N-fixing bacteria, the role of inorganic N metabolism in the virulence of plant pathogens is largely unexplored (5, 6). This may be because energetic metabolism has been seen as biologically distinct from virulence. However, bacteria need energy to produce virulence factors and grow inside the host, which are both essential processes for pathogenesis (7–9). Studies of several animal-pathogenic bacteria reveal that the host environment induces microbial nitrate respiration and/or denitrification and that these bacterial inorganic N reductions affect virulence (10–12).
The soilborne plant-pathogenic bacterium Ralstonia solanacearum invades plants through their roots. It aggressively colonizes the xylem elements in its host’s vascular system, blocking water transport so that infected plants wilt and die (13). The bacterium thrives in plant xylem, quickly reaching densities upwards of 109 CFU/g of stem tissue (14). This rapid growth is puzzling, because xylem sap is relatively nutrient poor and, presumably, low in O2 (13, 15). R. solanacearum carries genes encoding three types of terminal oxidases (cytochrome bd oxidase, cytochrome c cbb3-type oxidase, and cytochrome c aa3-type oxidase). We previously found that one of the cbb3-type oxidases is required for normal microaerobic growth and contributes significantly to the virulence and multiplication of R. solanacearum during plant infection (16). While these observations indicate that the pathogen uses O2 during growth in the host, it is likely that R. solanacearum often encounters anaerobic conditions in its soil, rhizosphere, and plant habitats.
A preliminary analysis found that tomato xylem sap does contain significant levels of NO3−, which is an excellent TEA. In terms of redox potential, it is nearly as suitable as O2 (16, 17). Additionally, in response to pathogen infection, plants, like mammals, typically produce NO, another potential electron sink for denitrifying microbes (1, 18). NO can also limit microbial growth by damaging DNA, sequestering important metal cofactors, and inhibiting terminal oxidase function to arrest aerobic respiration (19–22). High NO levels in host tissue could possibly force a plant pathogen to either use a TEA other than O2 or halt growth.
The genome of R. solanacearum strain GMI1000 appears to encode a complete denitrification pathway, which includes a respiratory NO3− reductase (NarG), a NO2− reductase (AniA), a NO reductase (NorB), and a N2O reductase (NosZ). The genome also includes a gene for a predicted flavohemoglobin (HmpX) that can detoxify NO either aerobically, by converting it to NO3−, or anaerobically, by converting it to N2O. NorB can also convert NO anaerobically to N2O (5). A transcriptomic analysis of R. solanacearum gene expression early in bacterial wilt disease revealed that all of these genes were highly expressed during tomato pathogenesis (17).
Together, these observations suggested that this vascular pathogen may respire and generate energy using inorganic N species as TEAs in low-O2 and/or NO-rich microenvironments of plant xylem vessels. To test this hypothesis, we constructed a set of five single-gene deletion mutants of R. solanacearum strain GMI1000 that lacked narG, aniA, norB, nosZ, or hmpX. We analyzed the ability of these mutants to respire on NO3−, tolerate NO, grow in plants, and cause bacterial wilt disease. We found that although the bacterium respired on NO3− in the presence of O2, NO3− respiration contributed directly to growth only under low-oxygen or anaerobic conditions. NO2− did not support bacterial growth; indeed, NO2− inhibited growth in a dose-dependent fashion. Both HmpX and NorB detoxified NO under aerobic and microaerobic conditions, but only NorB could degrade NO under anaerobic conditions. During plant infection, R. solanacearum quickly consumed O2 and actively reduced inorganic N. NO3− respiration, denitrification, and aerobic NO detoxification all contributed to R. solanacearum’s growth in planta.
RESULTS
Ralstonia solanacearum expresses its inorganic nitrogen metabolic pathways in planta during bacterial wilt disease.
A recent transcriptomic study (17) found that predicted genes for NO3− respiration and denitrification were highly induced in R. solanacearum during tomato plant infection (Fig. 1A; see Table S1 in the supplemental material). The gene encoding the major catalytic subunit of the predicted respiratory NO3− reductase (NarG) was expressed at 30.6-fold-higher levels in planta than in culture in rich CPG medium. CPG is composed of peptone, casamino acids, glucose, and yeast extract (with small amounts of NO3−) (23). Similarly, genes predicted to encode the catalytic subunits of NO2− reductase (AniA), NO reductase (NorB), and N2O reductase (NosZ) were strongly induced in planta (86.8-fold, 51.3-fold, and 26.8-fold, respectively). In addition, the gene encoding the NO-detoxifying HmpX was also induced 43.2-fold during plant infection. The absolute expression values of these genes were among the highest in the bacterium’s in planta transcriptome (see Table S1 in the supplemental material), indicating that R. solanacearum is actively metabolizing these inorganic N species during growth in host plant xylem vessels.
FIG 1 .
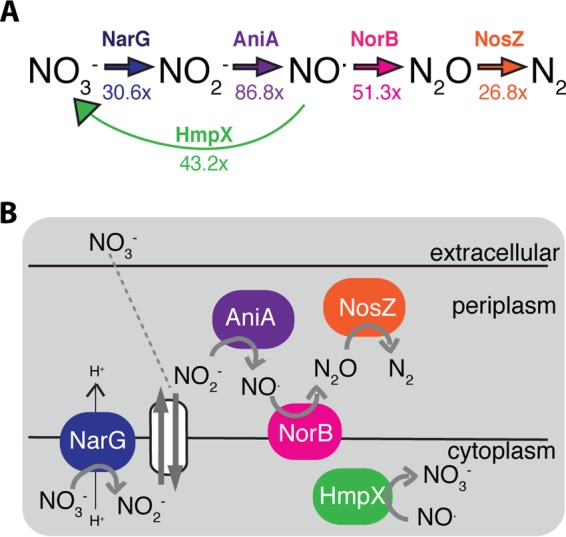
During tomato infection, Ralstonia solanacearum strain GMI1000 has high levels of expression of a complete nitrate respiration and denitrification pathway, including a nitric oxide detoxification system. (A) Inorganic N metabolic pathway with relevant enzyme shown above each reaction. The numbers below each reaction arrow indicate fold induction of the corresponding gene during plant infection relative to expression levels in culture (17). (B) Cellular context of NO3− respiration and denitrification. Following glycolysis and the citric acid cycle, electrons move through the electron transport chain to a terminal electron acceptor (TEA). In R. solanacearum, nitrate (NO3−), nitrite (NO2−), nitric oxide (NO.), and nitrous oxide (N2O) can potentially serve as TEAs. Data associated with each enzyme and its corresponding mutant are color coded consistently in all figures.
The general cellular context of the R. solanacearum denitrifying pathway and associated reactions, taken from genomic predictions, is shown in Fig. 1B (1). Briefly, NO3− can freely cross the outer cell membrane and is transported into the cytoplasm by NarK1 and/or NarK2 (24). In the cytoplasm, NarG reduces the NO3− to NO2−. This reaction pumps protons across the membrane into the periplasm, adding to the proton motive force generated in earlier steps of the electron transport chain. This proton motive force leads to ATP production. The NO3− importer exports NO2− from the cytoplasm to the periplasm, where AniA reduces it to NO. This reaction, along with the rest of the denitrification process, takes place in the periplasm and does not directly add to proton motive force. Instead, periplasmic reductase enzymes aid growth in some organisms by transferring electrons to their substrates, which allows for continued metabolic flow and continued proton motive force generation via early electron transport chain steps. In this way, NO is reduced by NorB. The resulting N2O is reduced by NosZ to dinitrogen gas (N2), which can freely dissipate into the extracellular environment.
To characterize the contributions of NO3− respiration, denitrification, and NO detoxification to R. solanacearum’s growth in culture, as well as to its growth and virulence in tomato plants, we generated five deletion mutant strains lacking the predicted narG, aniA, norB, nosZ, and hmpX genes, together with the corresponding complemented strains, with the exception of nosZ (see Table S2 in the supplemental material).
R. solanacearum uses narG-dependent nitrate respiration for growth under the low-oxygen conditions found in planta during disease.
Denitrification and NO3− respiration are typically low-O2-to-anaerobic behaviors (1). To define the O2 concentrations that support R. solanacearum NO3− respiration, we measured the growth of the bacterium supplied with NO3− in a range of atmospheric O2 levels (20.9, 15, 10, 5, 1, 0.5, 0.1, and 0% O2). To ensure maximum gas exchange, this growth assay was carried out in 10-ml cultures growing in 125-ml flasks. We used Van den Mooter (VDM) medium with 10 mM NO3−, which contains casamino acids, sodium succinate, magnesium sulfate, potassium phosphate, and KNO3 and supports respiration of inorganic nitrogen by R. solanacearum (25). To determine how much NO3−-supported growth was due to activity of the predicted NarG, we included the ΔnarG mutant and its complemented strain in this assay. All of the strains tested grew best when supplied with 15 or 10% O2 (Fig. 2A). However, narG was required for full growth at O2 levels below 1%. The growth defect of the ΔnarG strain was fully restored in the complemented ΔnarG strain.
FIG 2 .
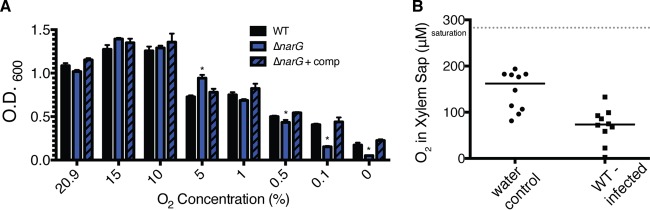
Nitrate respiration facilitates Ralstonia solanacearum growth at oxygen concentrations found in planta. (A) Culture densities (OD600) of wild-type (WT), ΔnarG, and complemented ΔnarG strains of R. solanacearum following 24 h of incubation in VDM medium with 10 mM NO3− at a range of oxygen concentrations. The WT strain grew better at 15% and 10% O2 than at 20.9% O2 (P = 0.0166 and 0.0206, respectively). Each bar represents the mean result from 3 biological replicates, and error bars indicate standard errors of the means. *, significantly different from the result for the WT at the same O2 concentration (P < 0.05, t test). (B) A Unisense multimeter microsensor was used to measure O2 concentrations in xylem sap from wilt-susceptible tomato plants following soil soak inoculation with either water or wild-type R. solanacearum. Each point represents data collected from a single plant. Ten plants per treatment were sampled at the time of symptom onset. Horizontal bars indicate median values. The gray dotted line indicates O2 saturation under these conditions. O2 concentrations in sap from healthy and infected plants were different (P = 0.0003, t test).
It has been postulated that bacteria experience a low-oxygen environment in plant xylem. To the best of our knowledge, however, the O2 levels in infected tomato plants have not been directly measured (15). To determine whether the plant xylem is a permissive environment for bacterial NO3− respiration and denitrification, we used a microprobe to measure the O2 content of tomato plant xylem sap. The xylem sap of healthy 3-week-old tomato plants contained 146.2 µM O2 on average (Fig. 2B). There was no difference between O2 measurements when the probe was inserted directly into xylem vessels or inserted into xylem sap accumulated on the surface of a newly cut stem (data not shown). We therefore took measurements from xylem sap accumulated on the cut stem surface, because this reduced the likelihood of breaking the delicate microprobe. Sap from healthy plants contained O2 concentrations similar to those in sterile, nonaerated liquid growth medium (data not shown). However, xylem sap from plants infected with wild-type R. solanacearum contained significantly less oxygen, with an average of 70.87 µM O2 (P = 0.0003, t test) (Fig. 2B). These values varied widely, ranging from 2.2 to 133 µM O2. The lower O2 concentrations were detected in plants with severe bacterial wilt symptoms, which harbored R. solanacearum populations of >109 CFU/g stem. The in planta data demonstrate that the bacterial wilt pathogen consumes O2 in host tissue and therefore experiences a low-O2 environment during plant infection, particularly at the later stages of disease. Although these in planta measurements are consistent with the in vitro findings described above, they cannot be directly compared. The probe measured molar concentrations of dissolved O2, while the controlled-O2 chamber used for the in vitro studies delivered a known percentage of atmospheric O2.
Reduction of nitrate, nitrite, and nitric oxide is required for growth of R. solanacearum at wild-type rates under anaerobic conditions.
We hypothesized that, in addition to NO3− respiration (Fig. 2A), the denitrification pathway of R. solanacearum is required for growth under anaerobic conditions in the presence of NO3−. We tested this by measuring the optical densities (ODs) of wild-type, ΔnarG, ΔaniA, ΔnorB, and ΔnosZ R. solanacearum strains following 24 h of stagnant anaerobic incubation in VDM medium with NO3− (Fig. 3A) (25). We included the ΔhmpX strain as a control because hmpX is not predicted to be involved in respiratory N metabolism but only in cytoplasmic NO consumption (26, 27). All of the strains tested reached endpoint optical densities below that of the wild-type parent strain, although the optical densities of the wild-type, ΔnosZ, and ΔhmpX strains were not significantly different. The ΔnarG, ΔaniA, and ΔnorB mutants grew less than the wild-type strain (P < 0.05, t test). The ΔnorB strain had the most drastic growth defect. Adding a complementing wild-type gene fully or partially restored anaerobic growth on NO3− to all deletion mutants. The complemented ΔaniA strain did not reach wild-type levels, but this strain did grow significantly better than its ΔaniA parent (P < 0.05, t test). Under aerobic conditions, all strains grew equally well, and the results were statistically indistinguishable (P > 0.05, t test) (Fig. 3B).
FIG 3 .
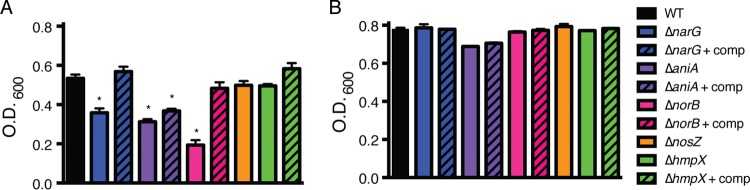
Nitrate, nitrite, and nitric oxide respiration contribute to R. solanacearum’s growth under anaerobic conditions. Growth of R. solanacearum strains was measured as the OD600 following 24 h of anaerobic (A) and aerobic (B) incubation in VDM medium with 10 mM NO3−. Bars show mean results for growth of 3 biological replicates, each containing 3 technical replicates. Error bars represent standard errors. *, significantly different from the result for the WT (P < 0.05, t test). Complementation did not fully restore the ability of all mutants to grow under these conditions, but each complemented strain grew better than its parent mutant (P < 0.05, t test).
Nitrate supports the growth of R. solanacearum in a dose-dependent manner, with an optimal concentration near that found in planta.
To understand whether NO3− respiration and denitrification directly or indirectly contribute to bacterial growth, we tested the ability of wild-type R. solanacearum to grow under microaerobic (0.1% O2) conditions in VDM medium with NO3− or NO2− concentrations ranging from 0 to 100 mM (Fig. 4A and 5A). While R. solanacearum grew no better on 10 µM or 100 µM NO3− than in the absence of NO3− (data not shown), the pathogen did grow better on NO3− at 1 mM (P = 0.047, repeated measures analysis of variance [ANOVA]), 10 mM (P = 0.0098, repeated measures ANOVA), 25 and 50 mM (P = 0.0021, repeated measures ANOVA), and 100 mM (P = 0.0151, repeated measures ANOVA). The bacterium grew optimally in culture at 25 to 50 mM NO3−. However, in 100 mM NO3−, the cultures had a much longer lag time, indicating that R. solanacearum is inhibited by high concentrations of either NO3− or its metabolic product, NO2−.
FIG 4 .
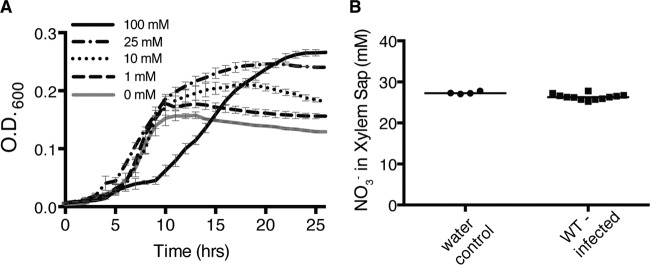
Nitrate directly supports the growth of R. solanacearum at biologically relevant concentrations. (A) Growth of wild-type R. solanacearum in 0.1% O2 in VDM medium with various NO3− concentrations. Data are mean results from 3 biological replicates. Growth in 0 mM NO3− was significantly different from growth in 1 mM, 10 mM, 25 mM, 50 mM, and 100 mM NO3− (P < 0.05, repeated measures ANOVA). Growth in 50 mM NO3− (not shown) was indistinguishable from growth in 25 mM NO3−. Error bars represent standard errors. (B) Twenty-one-day-old wilt-susceptible tomato plants were inoculated with either water or wild-type R. solanacearum. At symptom onset, plant xylem sap was collected, and NO3− was quantified in each sample using Unisense multimeter NOx and NO2− probes. Horizontal bars indicate mean values. Symbols indicate values from four plants inoculated with water and 12 plants inoculated with wild-type R. solanacearum.
FIG 5 .
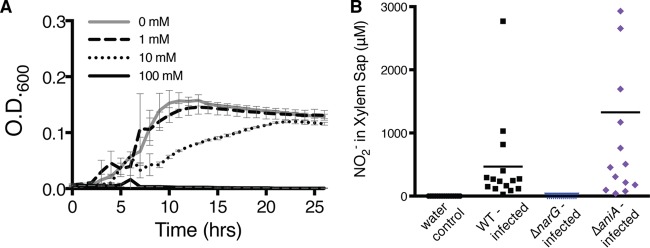
Nitrite inhibits R. solanacearum’s growth under low-oxygen conditions and is produced and consumed in planta via nitrate respiration and denitrification. (A) Growth of wild-type R. solanacearum in 0.1% O2 in VDM medium with various NO2− concentrations. Data are mean results from 3 biological replicates. Growth with 0 mM NO2− was significantly different from growth with 10 mM and 100 mM NO2− (P < 0.05, repeated measures ANOVA). Error bars represent standard errors. (B) NO2− concentrations in xylem sap from tomato plants infected with wild-type (WT), ΔnarG, or ΔaniA R. solanacearum or water-inoculated controls. At symptom onset, xylem sap was harvested and NO2− concentrations were determined using the Griess reaction. Horizontal bars indicate mean values, and each symbol represents the NO2− level in an individual plant. Xylem sap from plants infected with the ΔaniA mutant contained more NO2− than sap from plants infected with the WT (P = 0.0337, t test).
To connect these results to a relevant biological context, we measured NO3− in xylem sap from uninfected and R. solanacearum-infected tomato plants. Sap from both groups of plants contained 25 to 28 mM NO3− (Fig. 4B). This is similar to the NO3− concentration that best supported NO3−-respiratory-dependent growth of R. solanacearum in culture.
In contrast, adding NO2− did not enhance low-O2 growth of R. solanacearum at any level (Fig. 5A). In fact, cell growth was inhibited by 10 mM and 100 mM NO2−. This suggests that while NO3− respiration contributes directly to growth under oxygen limitation, denitrification only facilitates growth indirectly, by eliminating toxic levels of NO2− or by allowing the cell to release excess reducing power.
Nitrate is directly used as a terminal electron acceptor.
Adenylate energy charge measures bacterial metabolic activity as indicated by the available energy momentarily stored in the adenylate system (28). To determine the contribution of NO3− respiration to R. solanacearum’s energy pool, we measured the levels of ATP, ADP, and AMP in cells of ΔnarG, ΔaniA, ΔnorB, ΔnosZ, ΔhmpX, and wild-type R. solanacearum growing under denitrification-conducive conditions in VDM medium plus NO3−. Wild-type R. solanacearum cells had a relatively high energy charge (~0.8), suggesting normal metabolic flow (28). At ~0.6, the ΔnarG mutant had a significantly lower energy charge than its wild-type parent (P = 0.0103, t test) (Fig. 6). This suggests that the ΔnarG strain is metabolically impaired, as would be expected if NO3− is used directly as a TEA. In Escherichia coli, an energy charge of 0.6 to 0.7 indicates inhibition of cell growth (28). The ΔaniA and ΔnorB mutants had even lower average energy charges (~0.15 and ~0.09) than the ΔnarG mutant (P = 0.0021 and 0.0007, respectively, t test). These data suggest that these two strains are metabolically blocked and may be accumulating toxic levels of NO2− and NO. In E. coli, a reading below 0.5 is incompatible with metabolic flow and eventually results in cell death (28). The energy charges of the ΔnosZ and ΔhmpX mutants (both ~0.75) were not different from that of the wild-type strain (P = 0.0759 and 0.1015, respectively, t test). These results, along with the finding that narG is essential for microaerobic or anaerobic growth and NO2− production, indicate that R. solanacearum uses NO3− as a TEA.
FIG 6 .
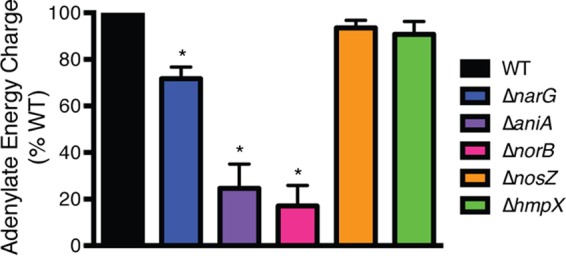
Relative energy charge levels indicate that R. solanacearum uses nitrate directly as a terminal electron acceptor. Cellular adenylate energy charge of wild-type and denitrification mutants incubated anaerobically in VDM medium with 10 mM NO3− for 20 h at 28°C was determined as described previously (28). Mean energy charges for each strain are shown as percentage of wild-type levels; data reflect 3 biological replicates, each with 3 technical replicates. Error bars show standard errors. *, significantly different from the WT result (P < 0.01, unpaired t test with Welch’s correction).
In culture, R. solanacearum produces and consumes nitrite under both aerobic and anaerobic conditions.
To determine whether R. solanacearum performs NO3− respiration and denitrification under aerobic conditions, we quantified the NO2− produced by aerobic cultures in VDM medium supplied with NO3− (Table 1). Wild-type cells produced NO2− but ΔnarG cells did not, indicating that NO3− reductase-mediated respiration is the source of the NO2− and that this reaction occurs when the bacterium grows aerobically. Cultures of the ΔaniA mutant accumulated more than twice as much NO2− as the wild type, consistent with the predicted NO2− reductase function of AniA. Interestingly, this result shows that NO2− respiration also occurs under aerobic conditions. However, inorganic N reduction did not contribute significantly to growth under aerobic conditions. We observed a similar pattern under anaerobic conditions (Table 1), although as expected, the cultures produced much more NO2− in the absence of O2 and, as previously described (Fig. 3), this did contribute to growth. N2 gas bubbles, which indicate complete denitrification, were present only when wild-type R. solanacearum grew anaerobically in liquid or semisolid medium containing NO3−. The ΔnarG, ΔaniA, ΔnorB, and ΔnosZ denitrification mutants did not produce gas bubbles under these conditions, but complementation restored gas production (data not shown).
TABLE 1 .
Nitrite and dinitrogen gas production under aerobic and anaerobic conditions
| Genotype | NO2− production [µM (±SEM)] under indicated conditionsa |
N2 production under anaerobic conditionsb |
|
|---|---|---|---|
| Aerobic | Anaerobic | ||
| WT | 43 (±8) | 113 (±29) | + |
| ΔnarG | 0 | 0 | − |
| ΔaniA | 113 (±14) | 9,066 (±976) | − |
NO2− concentrations were measured by Griess reaction after 20 h of growth under static anaerobic conditions or with shaking (225 rpm) under aerobic conditions in VDM medium with 10 mM NO3− at 28°C; the NO2− concentrations measured include any NO present. The starting OD600 for all strains was 0.08. Values in parentheses are standard errors of the means; the experiments included 3 biological replicates, each containing 3 technical replicates.
N2 was detected visually following static 28°C incubation of tubes containing liquid VDM medium with 10 mM NO3− inoculated with 10 µl of R. solanacearum strains at a range of cell densities. The same results were obtained when the assay was repeated in semisolid VDM medium with 10 mM NO3− and aerobic incubation at 28°C.
Wild-type R. solanacearum cultures produced visible gas bubbles within 2 h after inoculation at high cell densities (2.5 × 108 CFU/ml) into VDM medium with 30 mM NO3−. This medium was used because it supports R. solanacearum NO3− respiration and complete denitrification, ending in the production of N2 gas (25). Interestingly, NO2− production had not peaked at 2 HPI (hours postinoculation) but reached a maximum at 8 HPI (see Fig. S1 in the supplemental material).
R. solanacearum denitrifies and respires on nitrate during tomato infection.
To determine whether NO3− respiration and denitrification occur in the biologically relevant environment of a host plant, we measured NO2− in xylem sap from tomato plants inoculated with wild-type R. solanacearum, the ΔnarG mutant, the ΔaniA mutant, or water (Fig. 5B). Xylem sap from water control plants contained no detectable NO2−. Plants infected with wild-type R. solanacearum contained, on average, 466.6 µM NO2−. Xylem NO2− concentrations increased as pathogen populations in the stem increased and as disease severity increased. Sap from ΔaniA mutant-infected plants contained, on average, 1,327 µM NO2−, 2.8-fold more than plants infected by the wild type (P = 0.0337, t test). Sap from plants infected with the ΔnarG mutant contained no detectable NO2−. These results demonstrate that R. solanacearum produces NO2− via NarG-driven NO3− respiration during infection and that plants are not a source of NO2− in their xylem fluid.
R. solanacearum uses a denitrification enzyme, NorB, and the flavohemoglobin HmpX to detoxify nitric oxide.
Our results suggested that some steps in denitrification could detoxify potentially harmful inorganic N species, so we characterized the responses of wild-type, ΔaniA, ΔnorB, and ΔhmpX R. solanacearum strains to NO challenge. We challenged these NO metabolism-related R. solanacearum strains with NO and monitored recovery following NO exposure in a 96-well plate format with an automated plate reader in order to determine lag time and growth rate, measured as doubling time during log phase of each strain following exposure to NO. Strains lacking a gene involved in NO exposure recovery or detoxification would be expected to have a significantly prolonged lag time and, perhaps, a lowered growth rate or doubling time. Figure 7 shows the average growth doubling times (Fig. 7B) and lag times (Fig. 7A) of cultures treated with NO relative to those of untreated control cultures. Interestingly, NO had less effect on the culture lag times of the ΔaniA and ΔnorB denitrification mutants than on the lag time of the wild-type strain. It is likely that endogenous accumulation of NO2− and NO in these mutants primed their remaining detoxification systems and, thus, decreased their response time to exogenous NO. However, exogenous NO had a greater effect on culture doubling time in the ΔnorB and ΔhmpX strains than in the wild-type strain. This suggests that both denitrifying (NorB) and flavohemoglobin (HmpX) enzymes degrade exogenous NO.
FIG 7 .
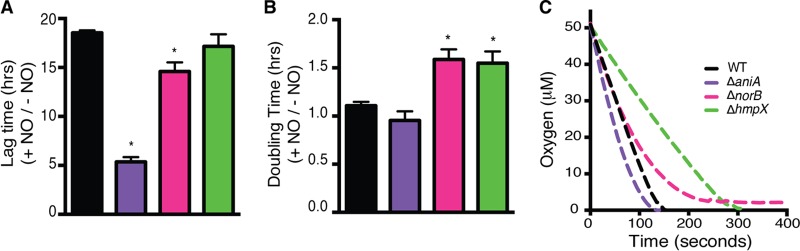
R. solanacearum detoxifies nitric oxide by denitrification under low-oxygen conditions and by HmpX under high-oxygen conditions. Overnight bacterial cultures in VDM medium without NO3− were pelleted, resuspended in fresh VDM medium without NO3−, and incubated for 4 h. Cultures were then diluted 1:10 in VDM medium with 10 mM NO3− and incubated statically for 2 h to induce the expression of denitrifying enzymes. One millimolar NO in the form of spermine NONOate was added to half of the wells, and the OD600 was read over 72 h. The growth of each strain with and without NO is shown as a ratio for lag time (A) and doubling time (B). *, significantly different from the WT result (P < 0.05, t test). The experiment was replicated 3 times with 3 technical replicates for each treatment; data from a representative assay are shown. Error bars indicate standard errors. (C) Oxidase inhibition assay. Overnight cultures were resuspended to uniform OD600 in fresh VDM medium with 10 mM NO3− and shaken aerobically for 3 h. One millimolar NO in the form of spermine NONOate was spiked into cultures, and O2 consumption was tracked until concentrations reached 0 or plateaued. Data are means of results from 3 biological replicates.
NO can inhibit bacterial terminal oxidases (19). To determine whether the slowed growth of the ΔnorB and ΔhmpX strains following NO exposure was due to oxidase inhibition, we directly measured O2 consumption in cultures challenged with 1 mM NO (Fig. 7C) (19). Both mutant strains consumed O2 significantly more slowly than wild-type R. solanacearum. Interestingly, the ΔnorB mutant was unable to consume the last ~3 µM O2, and although the ΔhmpX mutant consumed all of the O2, it did so at a much lower rate. In contrast, the ΔaniA mutant consumed O2 faster than the wild type. Together, these data suggest that NorB can detoxify NO under all O2 conditions, while HmpX only degrades NO aerobically (down to ~3 µM O2) but does so at a higher rate than NorB. This is in contrast to what was found in Vibrio fischerii, where Hmp functions under both aerobic and anaerobic conditions, while Nor functions only under anaerobic conditions (29). It seems likely that accumulating NO2− in cultures of the ΔaniA mutant activates NorB and/or HmpX. We speculate that NO2− binds NsrR, a predicted NO2−-responsive transcriptional regulator with a putative binding site upstream from norB, thus activating the expression of norB (30). HmpX may also be under the transcriptional control of NsrR. The R. solanacearum genome encodes a putative NsrR, but its function has never been investigated in this species. Alternatively, NsrR may not influence the expression of norB and/or hmpX and these genes may instead be controlled by alternative NO2−-responsive regulators. Posttranslational modifications, such as tyrosine nitration, may also play a role in the activation of NorB and/or HmpX function (31).
Nitrate respiration, denitrification, and nitric oxide detoxification all contribute to R. solanacearum’s growth in planta.
The results described above suggest that R. solanacearum can use its NarG-AniA-NorB-NosZ-HmpX enzymes to respire on NO3−, denitrify NO2−, and detoxify NO. In addition, we demonstrated that in tomato xylem vessels, the bacterium encounters levels of O2 and NO3− conducive to these processes. To determine whether metabolism of inorganic N species helps this pathogen colonize its host plants, we measured bacterial population sizes in tomato stems two and three days after inoculation through a cut leaf petiole. In addition to the growth of the wild-type strain, we also measured the growth of the ΔnarG, ΔaniA, ΔnorB, ΔnosZ, and ΔhmpX mutant strains and their corresponding complemented strains.
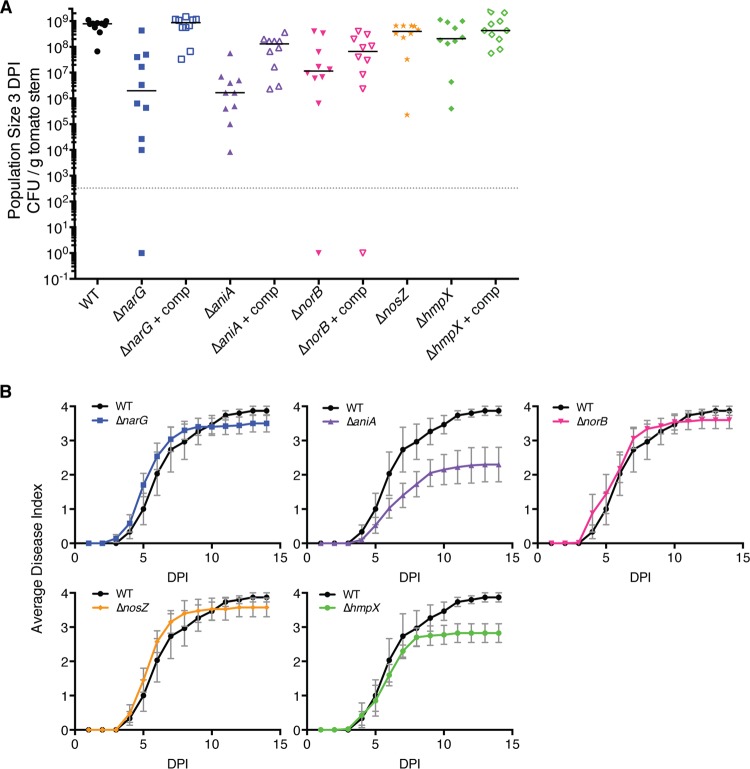
All five mutant strains reached lower population sizes in planta than wild-type R. solanacearum at both 2 DPI (days postinoculation) (data not shown) and 3 DPI (Fig. 8A). At 3 DPI, the wild-type population sizes in the tomato plants differed from those of the ΔnarG, ΔaniA, and ΔnorB mutants (P < 0.0001, t test), as well as from that of the ΔnosZ mutant (P = 0.0150). The average population size of the ΔhmpX mutant was smaller than that of the wild type, but this difference was not significant (P = 0.0602). Adding the wild-type gene to the ΔnarG mutant fully restored wild-type growth in planta. Complementing the ΔaniA and ΔnorB mutants measurably restored growth in planta, though these strains still grew significantly less than the wild type. The poor in planta growth of mutant strains suggests that NO3− respiration, denitrification, and NO detoxification all contribute to host xylem colonization by R. solanacearum.
FIG 8 .
Respiratory nitrogen metabolism and nitric oxide degradation contribute to bacterial wilt virulence and support pathogen growth in planta. Twenty-one-day-old tomato plants were inoculated with 500 CFU of the specified R. solanacearum strains through a cut leaf petiole. (A) Bacterial growth in planta. At 3 days postinoculation (DPI), the pathogen population size in each plant was determined by grinding and dilution plating a 0.1-g stem section centered at the site of inoculation. Ten plants were sampled per strain; each symbol represents the bacterial population size in a single plant, and the horizontal bars indicate the median population size for each strain. The gray dotted line indicates the limit of detection; samples containing no detectable bacteria were given a value of 1. The wild-type strain grew significantly better in planta than all mutants tested; complementation restored growth of mutants, although not always to wild-type levels. Compared to the WT result, the P values for each strain’s growth were as follows: ΔnarG, P < 0.0001; Δnarg + comp (complemented Δnarg mutant), P = 0.6371; ΔaniA, P < 0.0001; Δania + comp, P < 0.0001; ΔnorB, P < 0.0001; Δnorb + comp, P < 0.0001; ΔnosZ, P = 0.0150; ΔhmpX, P = 0.0602; and Δhmpx + comp, P = 0.411. (B) Virulence assay. Plants were rated daily for 14 days using a disease index of 0 to 4. Data presented are mean results from 3 to 4 independent assays, each containing 10 plants per strain. Error bars indicate standard errors of the means. Disease progress curves of the ΔaniA and ΔhmpX mutants were significantly different from those of the WT strain (P < 0.001, repeated measures ANOVA).
R. solanacearum needs nitrite reduction and nitric oxide detoxification for full virulence on tomato.
To assess the effects of inorganic N metabolism on bacterial wilt disease development, we measured the virulence of each mutant strain on tomato plants using the cut-petiole-inoculation assay described above. The ΔaniA and ΔhmpX mutants were significantly less virulent than the wild-type strain (P < 0.0001 and 0.0002, respectively, repeated measures ANOVA) (Fig. 8B). Although the ΔnarG, ΔnorB, and ΔnosZ mutants did not reach wild-type population sizes in tomato stems by 3 DPI, none of these mutants were reduced in virulence in this assay. Together, these data suggest that NO3− respiration, denitrification, and NO detoxification aid R. solanacearum in initial growth in the xylem but only NO2− reduction and NO detoxification are required for full virulence, at least when the bacteria are introduced directly into host xylem through a cut leaf petiole.
DISCUSSION
The goal of this study was to define the roles of inorganic N respiratory and detoxifying metabolism in R. solanacearum, especially during its pathogenic life inside plant host xylem. We recently showed that assimilation of NO3− contributes to R. solanacearum’s virulence and affects the production of extracellular polysaccharide, a virulence factor (32). We previously observed that a Tat secretion-defective mutant of K60, which cannot secrete its NO3− transporter or respire on NO3−, had reduced virulence on tomato, but this suggestive result did not prove that NO3− respiration itself contributes to bacterial wilt pathogenesis, as the Tat mutant was also defective in the secretion of other proteins unrelated to N metabolism (33). Little was known about NO3− reduction in this bacterium, possibly because Bergey’s Manual describes the species R. solanacearum as incapable of denitrification (34). However, comparative genomic analysis revealed that R. solanacearum strains in phylotypes I (Asia) and III (Africa) have complete denitrification pathways. Most strains in phylotypes II (Americas) and IV (Indonesia) have the three enzymes that allow them to reduce NO3− to N2O, but they lack the NosZ nitrous oxide reductase and, thus, cannot reduce N2O to dinitrogen gas (N2), the final step in a complete denitrification pathway (35, 36). These genomic data are supported by phenotypic analyses showing that most R. solanacearum strains can denitrify (25). Bergey’s mischaracterization apparently arose because they defined denitrification as the production of N2 gas bubbles during anaerobic growth on NO3− and they studied the type strain, K60, which belongs to phylotype II. N2 bubbles are only produced by the nosZ-carrying phylotype I and III strains.
Genes encoding NO3− respiration, NO detoxification, and denitrification were highly differentially expressed in planta, suggesting that these processes specifically adapt R. solanacearum to conditions in plant xylem tissue. We tested this hypothesis using a set of five defined deletion mutants that each lacked one of these strongly upregulated genes. Each mutant was characterized with respect to NO3− respiration, denitrification, and NO detoxification in culture; their phenotypes functionally confirmed the sequence-based gene annotations.
The ΔnarG mutant could not respire on NO3− and had significantly lower adenylate energy charge levels than its wild-type parent, indicating that R. solanacearum reduces NO3− to generate proton motive force and produce ATP. Additionally, wild-type R. solanacearum grew on NO3− in a dose-dependent fashion. The bacterium did not grow on NO2−, suggesting that it cannot use this molecule as an energy-generating TEA. In contrast, another betaproteobacterium, Neisseria meningitidis, can grow directly on NO2− (37). We observed low levels of background growth in VDM medium independent of NO3− and NarG function, suggesting that R. solanacearum has NO3−-independent ways to obtain energy under anaerobic conditions, possibly via amino acid fermentation or Stickland reactions (38). Various modifications in the composition of VDM medium did not eliminate this low-level growth. However, the background growth did not obscure the significant differences between mutant and wild-type strains under any of the conditions studied.
Because O2 is typically the preferred TEA, microbes tend to resort to NO3− respiration and denitrification only under anaerobic conditions (1). N. meningitidis, however, can denitrify aerobically when reactive nitrogen species (RNS) intermediates accumulate (39). Likewise, R. solanacearum reduced NO3−, NO2−, and NO in culture under both aerobic and anaerobic conditions. However, the reduction of these inorganic N species only supported bacterial growth under low O2 concentrations (<1%).
Though NO3−, NO2−, and NO reductases all functioned aerobically in R. solanacearum, the final step of denitrification, the reduction of N2O to N2 by NosZ, did not. This suggests that R. solanacearum NosZ only functions under complete anaerobiosis, as is true of Paracoccus denitrificans and Pseudomonas stutzeri (40, 41). Under anaerobic conditions, the growth of the ΔnosZ R. solanacearum strain was statistically indistinguishable from that of the wild type. Nonetheless, the ΔnosZ strain consistently reached lower optical densities than its wild-type parent. Though the ability to reduce N2O did not contribute greatly to R. solanacearum’s anaerobic growth, this consistent trend suggests that it may make a slight contribution. This could be biologically important for infection prior to xylem colonization. We are investigating this possibility further.
R. solanacearum reached different maximum readings for OD at 600 nm (OD600) in culture depending on the experimental conditions of the growth assays. To determine the optimal O2 concentration for growth, 10-ml cultures were shaken in 125-ml flasks to ensure maximum gas exchange. To determine the relative effects of anaerobiosis on the growth of the denitrifying mutants, the culture OD600 was measured after 24 h in 15-ml conical tubes under stagnant conditions. Finally, to determine the effects of differing nitrate concentrations on R. solanacearum, bacteria were cultured in 200-µl volumes in 96-well plates at 0.1% O2. Because of these differences in culture method, we compared growth trends and differences among strains, rather than the final OD600 endpoints.
Why do R. solanacearum NO3− respiration and denitrification pathways operate even in the presence of O2? First, the pathogen must rapidly adapt to major changes in O2 tension as it moves from soil to plant and between microhabitats within the plant. Before it colonizes xylem vessels, R. solanacearum forms microcolonies on root surfaces; such microcolonies formed by Pseudomonas aeruginosa are oxygen limited (42). In planta measurements showed that over the course of infection, R. solanacearum itself lowers the O2 content in xylem fluid. Active inorganic N metabolism may allow uninterrupted bacterial growth during transitions from an aerobic to an anaerobic habitat. Such transitions can be deleterious; for example, the metabolism of Dinoroseobacter shibae comes to a halt while denitrification is activated during the switch from aerobic to anaerobic respiration (43). Second, R. solanacearum may use denitrification to dissipate excess reducing power, as multiple Paracoccus species do with periplasmic nitrate reductases (44).
Third, R. solanacearum could use the denitrification pathway to protect itself from the damaging RNS NO2− and NO. These can be produced by the bacterium itself and, possibly, additionally by plant defense systems. They threaten the bacterium whether O2 is present or not. Although both NorB and HmpX could degrade NO in the presence of O2, HmpX was more efficient than NorB. However, HmpX was ineffective at less than 3 µM O2, like Pseudomonas aeruginosa’s flavohemoglobin, which functions only under aerobic conditions (45). In Salmonella enterica and Vibrio fischerii, Hmp is the main aerobic NO detoxification system (26, 29). Moraxella catarrhalis, which has no HmpX equivalent, relies on its NorB homolog for NO detoxification (46). R. solanacearum may have other minor NO detoxification systems, which would be evident in a ΔnorB ΔhmpX double mutant. For example, R. solanacearum GMI1000 expresses two potential cytochrome bd oxidases during disease (see Table S1 in the supplemental material); these can also act as NO detoxification systems and have been shown to contribute to virulence in Shigella flexneri (47, 48).
These explanations are not mutually exclusive, although our data directly support the detoxification hypothesis. In aerobic culture, exogenously supplied NO inhibited terminal oxidase function in the ΔnorB and ΔhmpX mutants, but strains that had intact norB and hmpX were unaffected (data not shown). Furthermore, in anaerobic culture, the ΔnorB strain had a low adenylate energy charge and grew very poorly on NO3−. This was presumably the result of toxic accumulation of endogenous NO, which could not be removed by HmpX under anaerobic conditions. When challenged with exogenous NO under low-O2 conditions, the ΔnorB strain had a longer doubling time than the wild type, confirming that this mutant is impaired in NO detoxification. Additionally, in both aerobic and anaerobic culture, the ΔaniA strain produced and accumulated NO2−, which inhibits R. solanacearum at concentrations above 1 mM. This led to an early decline in cell density, an overall lower growth endpoint, and a low adenylate energy charge. Taken together, the results of our experiments in culture suggest that inorganic N reduction and detoxification are needed to protect R. solanacearum from RNS toxicity under both anaerobic and aerobic conditions.
Genomic analyses suggest that R. solanacearum has a complex cascade of regulators that could modulate inorganic N metabolism, and several of these putative regulators are strongly expressed during wilt disease (17, 35). It appears that NO2− induces the expression of either norB or hmpX, because the ΔaniA strain, which accumulates NO2−, could detoxify NO and resist oxidase inhibition better than its wild-type parent. Our data support in silico predictions of transcriptional regulator binding sites that suggest norB expression could be controlled by NsrR, a NO2-responsive regulator (30). Additionally, we could not fully restore the ΔaniA mutant to wild-type growth with complementation constructs that included either the aniA gene plus its upstream regulatory region or the aniA gene plus the upstream regulatory region and two downstream putative signal peptide-encoding genes, RSp1501 and RSp1502. The upstream regulatory region may also modify norB gene expression, as in silico predictions suggest (30). The detrimental effects of a second copy of this regulatory region could explain this partial complementation. In a possibly analogous situation, complementation of the aniA homolog in Pseudomonas aeruginosa was reported to be “slightly toxic” (49). We also observed that NO3− respiration and denitrification occurred mainly during late log or early stationary phase, hinting that this function could be modulated by one of R. solanacearum’s two quorum-sensing systems, as is the case in P. aeruginosa (50, 51).
The concentrations of O2, NO, and NO2− are key regulatory signals for other denitrifying bacteria (1, 30, 52). Following detailed gene expression analyses in culture, biosensing fluorescent reporter constructs could be developed to nondestructively measure the levels of these compounds experienced by R. solanacearum over the course of plant infection. We speculate that bacteria aggregated on xylem vessel walls experience higher NO and NO2− levels than planktonic bacteria in flowing xylem fluid. It would also be interesting to determine how inorganic N respiration and detoxification affect biofilm formation, maintenance, and dispersal, which are dependent on inorganic N metabolism in P. aeruginosa (53, 54).
After exploring the roles of NO3− respiration, denitrification, and NO detoxification in R. solanacearum cells growing in culture, we characterized these metabolic behaviors in the biologically relevant plant environment. Our experiments in culture showed that the bacterium can use these pathways to grow in high NO3− concentrations over a range of O2 levels. These were similar to the NO3− and O2 levels that we detected in xylem sap from healthy and diseased tomato plants. NO2−, the product of NarG-mediated NO3− reduction, was present in sap from infected plants but not in sap from healthy plants. Furthermore, the NO2− concentration in sap increased with pathogen cell density and was dependent on narG and aniA function. Similarly, during macrophage infection, Mycobacterium tuberculosis uses NarG to produce NO2−, which influences the expression of many genes (55). This offers genetic and biochemical evidence that R. solanacearum reduces NO3− and NO2− in planta and indirectly suggests that the pathogen produces NO during infection. Attempts to use a microprobe to directly measure NO in xylem sap were unsuccessful, likely because both plant and microbial cells rapidly degrade this molecule, which is also chemically unstable (21). Notably, both NO2− and NO can affect plant defense signaling (56, 57). We are currently investigating the effects of R. solanacearum inorganic N metabolism on plant defense responses.
Direct measurements in plants infected by wild-type and mutant strains showed that R. solanacearum modifies its habitat by lowering the average O2 concentration in the xylem. This is likely the result of bacterial oxidative respiration, because during tomato infection, R. solanacearum had high expression levels of genes for several predicted oxidases, including high-affinity oxidases likely to be effective under hypoxic conditions (see Table S1 in the supplemental material). The O2 levels that we measured in xylem sap probably overestimate the amount of O2 available to the pathogen in microhabitats such as bacterial aggregates on xylem vessel walls. It has been hypothesized that during intestinal infection, Escherichia coli’s anaerobic metabolism fluctuates to efficiently scavenge O2 (58). We propose that R. solanacearum similarly consumes any available O2 in xylem vessels, thereby creating an environment that requires the pathogen to use alternate TEAs, such as NO3−, during pathogenesis. Alternatively, the two TEAs may be used simultaneously during infection.
In response to pathogens, plants produce NO, an early component of the defense signal transduction pathway (57). The results of our in vitro experiments indicate that NO can also directly inhibit R. solanacearum by interfering with terminal oxidase function and that the pathogen protects itself from NO by converting the toxic molecule into either N2O (via NorB) or NO3− (via HmpX, if O2 is present) (5). The plant pathogen Dickeya dadantii requires HmpX for full virulence on Saintpaulia plants (59). Because D. dadantii cannot produce NO via denitrification or any other known enzymatic pathway, it must use HmpX to detoxify plant-produced NO (60). Our results suggest that R. solanacearum produces NO in culture (via denitrification) and encounters NO during infection. Do plants produce NO in response to R. solanacearum, or is the NO presumably encountered by the bacterium during infection the result of bacterial metabolism? Additionally, does the pathogen experience inhibitory levels of NO during infection, and if so, has the pathogen adapted to make use of it? As shown above, R. solanacearum can use NO3− as a TEA to generate energy, so HmpX may play two synergistic roles by converting the host’s chemical weapon into an energy source. This may be of particular importance in NO3−-limited environments, such as in N-limited plants growing in low-input agriculture or natural ecosystems.
These data are consistent with the theory that plant pathogens nutritionally modify their host environment during infection (17, 61). One current paradigm is that the pathogen tricks its host into producing specific metabolites for the microbe’s benefit. For example, Salmonella enterica serotype Typhimurium can use as a TEA the compound tetrathionate, which is produced by the host following pathogen-triggered oxidative stress (62). Our results suggest that the host environment can also be shaped by the pathogen’s own metabolic activity in ways that increase pathogen success. We are currently pursuing broader metabolomic studies to better understand how this organism alters its host environment.
None of the denitrification or detoxification mutants grew as well in tomato stems as the wild-type parent. These growth defects may be due to the mutants’ inability to degrade toxic RNS (for ΔaniA, ΔnorB, ΔhmpX, and possibly, ΔnosZ strains) and an inability to access sufficient TEAs (for the ΔnarG strain). The partial redundancy of NorB and HmpX, which can both detoxify NO, may explain why the ΔnorB mutant does not have a bigger in planta growth defect and also has no virulence defect. Only the ΔaniA and ΔhmpX mutants had virulence defects following petiole inoculation. Because direct inoculation of bacteria into a cut leaf petiole strongly favors the pathogen, mutants must be seriously compromised to exhibit a virulence defect in this assay (63, 64). Thus, although the virulence defects of the ΔaniA and ΔhmpX mutants were relatively small, they are likely to be biologically relevant. Further experiments are needed to determine whether denitrification and N detoxification contribute to other steps in R. solanacearum pathogenesis, such as chemotaxis, root attachment and entry, biofilm formation and dispersal, and exit from necrotic tissue, as well as persistence in both plants and soil environments.
Taken together, these results suggest that, in host xylem, R. solanacearum experiences a high-inorganic N environment that is at least partly of its own making. To succeed under these conditions, R. solanacearum has adapted its N metabolism to take advantage of the opportunities offered by this resource and solve the problems posed by the associated growth-inhibitory RNS.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The Ralstonia solanacearum strains and plasmids used in these experiments are listed in Table S1 in the supplemental material. E. coli was grown in Luria-Bertani (LB) medium at 37°C, and R. solanacearum was cultured in CPG medium at 28°C (23) unless otherwise noted. Antibiotics were used as needed at the following concentrations: 15 µg/ml gentamicin, 25 µg/ml kanamycin, and 10 µg/ml tetracycline. A modified Van den Mooter (VDM) medium was used for low-oxygen and anaerobic growth (25). To decrease the complexity of this undefined medium, we used 0.5% (wt/vol) Casamino acids instead of yeast extract. VDM medium without NO3− was buffered to a pH of 6.2 with 10 mM morpholineethanesulfonic acid (MES) and supplemented with NO3− or NO2− as specified in the figure legends. Difco Laboratories (Detroit, MI), Sigma-Aldrich (St. Louis, MO), and Fisher Scientific (Hanover Park, IL) were our chemical suppliers.
Strain construction.
Clean deletion mutants of R. solanacearum strain GMI1000 lacking the complete open reading frame (ORF) of narG, aniA, norB, nosZ, or hmpX were generated via homologous recombination using targeted deletion constructs produced with splicing by overhang extension PCR (SOE PCR) as described previously (32). The corresponding complemented strains were constructed using the R. solanacearum genomic tools of Monteiro et al. (65). Briefly, the region of interest, including the native promoter, was amplified and inserted into the pENTR/D-TOPO vector (Invitrogen, Carlsbad, CA). Using Gateway technology, the region was then transferred to pRCT, which does not replicate in R. solanacearum (65). The complementing pRCT was then moved into the corresponding mutant via natural transformation (66), and antibiotic resistance was used to select strains that had incorporated a single copy of the complementing genomic region into the selectively neutral attTn7 site. PCR was used to confirm incorporation of the desired DNA fragment.
Growth assays.
To initially identify the oxygen levels at which nitrate respiration contributed to cellular multiplication, 9-ml amounts of liquid VDM medium containing 10 mM KNO3 were inoculated with 1-ml amounts of overnight cultures of R. solanacearum strains adjusted to an OD600 of 0.1 (~1 × 108 CFU/ml). The initial OD600 of the cultures was thus 0.01. The endpoint OD was measured following 24 h of incubation in an oxygen-controlled chamber (InVivo2 400; Ruskinn) set to a specified O2 concentration with N2 gas and with shaking at 225 rpm. The experiment was replicated three times.
Dose-dependent growth of R. solanacearum strains in 96-well plates on NO3− was measured continuously by a BioTek HT plate reader for 25 h in the Ruskinn InVivo2 400 at 0.1% O2. VDM broth supplemented with various concentrations of NO3− was inoculated with wild-type R. solanacearum GMI1000 to an initial OD600 of 0.01. Dose-dependent growth on NO2− was measured identically, with the exception that KNO2 was used in place of KNO3.
The endpoint growth of all deletion and complemented strains was assessed both under conditions of 0.1% O2 with shaking (data not shown) and under anaerobic conditions (Fig. 3) in an anaerobic jar (GasPak anaerobic system; BD). Each strain was cultured overnight in 5 ml of VDM broth with no added NO3− and incubated at 28°C with shaking at 225 rpm. These cultures were centrifuged at 6,000 rpm for 10 min and resuspended in sterile water to an OD600 of 0.1. One-milliliter amounts of these inocula were pipetted into 9-ml amounts of VDM broth containing 10 mM NO3−, bringing the initial OD600 to 0.01. The tubes were lightly capped, to allow gas exchange, and incubated in an anaerobic jar for 22 h at 28°C. The OD600 was then measured to determine the ability of each strain to grow without O2 under these denitrification-conducive conditions. The means and standard errors of data collected from five biological replicates (each including three technical replicates) are presented in Figure 3 for each strain.
Inorganic nitrogen and oxygen measurements.
The Griess reaction (67) was used to quantify the amount of NO2− (and indirectly, NO) produced by R. solanacearum cells under denitrification-supportive conditions. Overnight cultures of the indicated strains were set up as described above. Ten-milliliter amounts of VDM medium with 10 mM NO3− in 15-ml sterile conical tubes were inoculated with a final OD600 of 0.08 (Table 1) or 0.25 (see Fig. S1 in the supplemental material) of resuspended and washed bacterial cultures. The tubes were incubated anaerobically, in BD GasPak anaerobic systems, or aerobically, as specified. At indicated time points, cells were lysed. Briefly, cells were incubated at 95°C for 10 min, moved to −20°C for 10 min, and centrifuged for 1 min to collect cellular debris. Three hundred-microliter amounts of supernatants were transferred to 15-ml conical tubes containing 2.6-ml deionized water. One hundred microliters of Griess reagent (Molecular Probes, Inc.) was added to each tube. The OD548 was measured after 30 min of incubation at room temperature. NO2− was quantified by using standard curves generated with known concentrations of NO2−. The data presented represent the mean results and standard errors of three biological replications, each with three technical replicates. N2 production was qualitatively monitored. If denitrification went to completion, meaning that NosZ was reducing N2O, N2 gas bubbles could be seen in VDM medium with 10 mM NO3− when incubated at 28°C under stagnant conditions. Adding 0.25% noble agar to the VDM medium with 10 mM NO3− helped in dinitrogen visualization.
The Griess method was also used to chemically quantify NO2− (and, indirectly, NO) in infected and noninfected tomato plant xylem sap. Three-week-old tomato plants (bacterial wilt-susceptible cv. Bonny Best) were grown in 80 g of Sunshine Redi-earth professional plant growth mix (Sun Gro Horticulture, Agawarm, MA) and watered daily with Hoagland’s fertilizer, a commonly used nitrate-containing fertilizer. These plants were soil soak inoculated with 50 ml of the indicated R. solanacearum strains suspended in water to an OD600 of 0.1, giving a final inoculum of ~5 × 107 CFU/g soil (63). When symptoms appeared, plant xylem sap was harvested (32). Xylem sap was centrifuged to remove plant and bacterial cells, and 300 µl of the supernatant was subjected to the Griess reaction to determine the NO2− concentration. The NO2− (and NO) level in sap from one water-inoculated control plant was determined for each inoculated plant. The data presented are individual plant data, and horizontal bars indicate median values.
We directly measured the concentrations of NO3− and O2 in xylem sap from infected and noninfected tomato plants using a Unisense multimeter and the relevant Clark-type electrode probe (Unisense, Aarhus, Denmark). Plants were soil soak inoculated with R. solanacearum as described above. For NO3− measurements, the NOx (which measures NO3− and NO2−) and NO2− probes were used as recommended by the manufacturer. To determine NO3− measurements, NO2− probe data were subtracted from NOx data from each plant sample. Oxygen was measured using the OX-50 Unisense probe calibrated according to the manufacturer’s instructions. To minimize gas diffusion introduced through harvesting, these probes were inserted into xylem sap immediately as it accumulated on the cut stem of decapitated plants. Symptomatic plants were used for inorganic N and O2 quantification. For each wild-type R. solanacearum GMI1000-infected plant sampled, one water-inoculated control plant was also sampled. To validate probe function, 2 to 3 sets of probes were used for each assay.
Nitric oxide tolerance.
To determine the contribution of denitrification to NO tolerance and detoxification, the NO3− respiration, denitrification, and predicted NO detoxification mutant strains were subjected to NO challenge and their growth recovery was monitored. Each strain was cultured overnight in VDM medium with no added NO3−, the OD600 was adjusted to 0.3 (log phase), and the cultures were incubated for 4 additional hours at 28°C with shaking at 225 rpm. Twenty microliters of each culture was transferred to a well of a 96-well plate containing 160 µl of VDM medium with 10 mM NO3−. The plates were incubated statically at room temperature for 2 h to develop low O2 conditions and activate bacterial NO3− sensing. Then, wells were inoculated with either 20 µl of 0.01 M NaOH or 20 µl of 10 mM spermine NONOate (Cayman Chemical) in 0.01 M NaOH (10 mM spermine NONOate will release 20 mM NO with a half-life of 39 min at 37°C, pH 7.4; the rate of release is higher at lower pHs [we used 6.2] and lower at lower temperatures [we used 28°C]). The plates were immediately covered with breathable tape and placed in the BioTek HD plate reader. The OD600 was monitored for 65 h, and lag time and doubling time were calculated. The results are presented as the ratio of doubling time with NO challenge to doubling time with no NO and the ratio of lag time with NO challenge to lag time with no NO.
To measure the capacity of NO to inhibit R. solanacearum oxidase function, we spiked cultures with NO and measured O2 consumption as previously described (19). Overnight cultures were pelleted at 6,000 rpm for 10 min and resuspended in VDM medium with 10 mM NO3− to an OD600 of 0.5 (~5 × 108 CFU/ml). Cultures were shaken for 3 h aerobically at 225 rpm. Immediately prior to O2 quantification, a 1-ml aliquot was spiked with 500 µM spermine NONOate (=1 mM NO) and shaken vigorously to ensure O2 saturation. An O2 microprobe (Unisense, Aarhus, Denmark) was then inserted into the tube, and O2 (uM) was measured until the signal stabilized or reached 0. The rate of consumption of the last 50 µM O2 was measured.
ATP quantification and energy charge measurements.
R. solanacearum strains were cultured overnight in rich CPG medium, pelleted by centrifugation, and resuspended in liquid VDM medium with 10 mM NO3− to an OD600 of 1.0 (~1 × 109 CFU/ml). Two-milliliter amounts of these cultures were pipetted into sterile 15-ml conical tubes and incubated for 18 to 20 h anaerobically in jars as described above. Following incubation, 100 µl of each culture was used for population size determination via the dilution plating technique. One milliliter of the remaining culture was lysed, and ATP/ADP/AMP were extracted with hot ethanol. ADP was enzymatically converted to ATP with pyruvate kinase, and AMP was converted to ATP with pyruvate kinase and adenylate kinase as described (28). Once converted, total AMP, ADP, and ATP were quantified with luciferase using the Promega ATP Enliten kit. All enzymes and adenylate standards were purchased through Sigma-Aldrich (St. Louis, MO). Data were collected with a Centro XS3 LB 960 microplate luminometer (Berthold Technologies, Germany). Data represent the mean results of three biological replicates, each with three technical replicates and are presented as percentages of the wild-type energy charge, determined using the following formula: [(ATP) + 0.5(ADP)]/([ATP) + (ADP) + (AMP)].
In planta growth assays.
To determine the contribution of each deleted gene to R. solanacearum’s multiplication in host plant tissue, 21-day-old tomato plants were inoculated with 500 cells of the indicated bacterial strain via cut petiole as described above. Plants were grown and cared for as described above. Three days after inoculation, 0.1 g of plant tissue that included and surrounded the site of inoculation was harvested, ground, and subjected to dilution plating to determine the pathogen population size. Ten plants were sampled for each treatment.
Virulence assays.
To determine the contribution of each deleted gene to the virulence of R. solanacearum on wilt-susceptible tomato plants, 3-week-old Bonny Best plants were infected with 500 cells of the indicated bacterial strains via cut petiole inoculations as described previously (17). Plants were grown and cared for as described above. Plants were rated daily for symptom development over 2 weeks using a scale of 0 to 4, as follows: 0, no leaves wilted; 1, 1 to 25% of leaves wilted; 2, 26 to 50% of leaves wilted; 3, 51 to 75% of leaves wilted; and 4, 76 to 100% of leaves wilted. This experiment was repeated four times with 10 plants per treatment in each replicate.
SUPPLEMENTAL MATERIAL
Pattern of nitrite production and consumption by R. solanacearum under denitrifying conditions. Nitrate respiration of wild-type R. solanacearum growing anaerobically in VDM medium with 30 mM nitrate at 28°C was measured as nitrite produced, using the Griess reaction. Data shown are the means of 3 biological replicates, each containing 3 technical replicates; bars indicate standard errors. Download
Energy- and nitric oxide-related gene expression during tomato infection by R. solanacearum strain GMI1000.
Strains and plasmids used in this study.
ACKNOWLEDGMENTS
This research was funded by National Science Foundation grant IOS 1258082, USDA-Hatch project WIS01502, and the University of Wisconsin-Madison College of Agricultural and Life Sciences. B.L.D. was supported by a National Science Foundation predoctoral fellowship.
We gratefully acknowledge Jonathan M. Jacobs, Edward G. Ruby, Michael G. Thomas, Daniel Amador-Noguez, and Paul Weimer for helpful discussions.
Footnotes
Citation Dalsing BL, Truchon AN, Gonzalez-Orta ET, Milling AS, Allen C. 2015. Ralstonia solanacearum uses inorganic nitrogen metabolism for virulence, ATP production, and detoxification in the oxygen-limited host xylem environment. mBio 6(2):e02471-14. doi:10.1128/mBio.02471-14.
REFERENCES
- 1.Zumft WG. 1997. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornwell JC, Kemp WM, Kana TM. 1999. Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls, a review. Aquat Ecol 33:41–54. doi: 10.1023/A:1009921414151. [DOI] [Google Scholar]
- 3.Kirchman DL. 2012. Processes in microbial ecology. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 4.Conrad R. 1996. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole RK. 2005. Nitric oxide and nitrosative stress tolerance in bacteria. Biochem Soc Trans 33:176–180. doi: 10.1042/BST0330176. [DOI] [PubMed] [Google Scholar]
- 6.Lucinski R, Polcyn W, Ratajczak L. 2002. Nitrate reduction and nitrogen fixation in symbiotic association rhizobium-legumes. Acta Biochim Pol 49:537–546. [PubMed] [Google Scholar]
- 7.Cole JA. 2012. Legless pathogens: how bacterial physiology provides the key to understanding pathogenicity. Microbiology 158:1402–1413. doi: 10.1099/mic.0.059048-0. [DOI] [PubMed] [Google Scholar]
- 8.Barbier T, Nicolas C, Letesson JJ. 2011. Brucella adaptation and survival at the crossroad of metabolism and virulence. FEBS Lett 585:2929–2934. doi: 10.1016/j.febslet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Abu Kwaik Y, Bumann D. 2013. Microbial quest for food in vivo: “nutritional virulence” as an emerging paradigm. Cell Microbiol 15:882–890. doi: 10.1111/cmi.12138. [DOI] [PubMed] [Google Scholar]
- 10.Filiatrault MJ, Picardo KF, Ngai H, Passador L, Iglewski BH. 2006. Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth. Infect Immun 74:4237–4245. doi: 10.1128/IAI.02014-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, House AL, Autieri SM, Leatham MP, Lins JJ, Jorgensen M, Cohen PS, Conway T. 2007. Respiration of Escherichia coli in the mouse intestine. Infect Immun 75:4891–4899. doi: 10.1128/IAI.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Alst NE, Picardo KF, Iglewski BH, Haidaris CG. 2007. Nitrate sensing and metabolism modulate motility, biofilm formation, and virulence in Pseudomonas aeruginosa. Infect Immun 75:3780–3790. doi: 10.1128/IAI.00201-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peeters N, Guidot A, Vailleau F, Valls M. 2013. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol Plant Pathol 14:651–662. doi: 10.1111/mpp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schell MA. 2000. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol 38:263–292. doi: 10.1146/annurev.phyto.38.1.263. [DOI] [PubMed] [Google Scholar]
- 15.Pegg GF. 1985. Life in a black hole—the micro-environment of the vascular pathogen. Trans Br Mycol Soc 85:1–20. doi: 10.1016/S0007-1536(85)80151-0. [DOI] [Google Scholar]
- 16.Colburn-Clifford J, Allen C. 2010. A cbb3-type cytochrome C oxidase contributes to Ralstonia solanacearum R3bv2 growth in microaerobic environments and to bacterial wilt disease development in tomato. Mol Plant Microbe Interact 23:1042–1052. doi: 10.1094/MPMI-23-8-1042. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JM, Babujee L, Meng F, Milling A, Allen C. 2012. The in planta transcriptome of Ralstonia solanacearum: conserved physiological and virulence strategies during bacterial wilt of tomato. mBio 3(4):e00114-12. doi: 10.1128/mBio.00114-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. 2004. Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol 6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 19.Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J, Vázquez-Torres A. 2008. Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem 283:7682–7689. doi: 10.1074/jbc.M708845200. [DOI] [PubMed] [Google Scholar]
- 20.Stevanin TM, Ioannidis N, Mills CE, Kim SO, Hughes MN, Poole RK. 2000. Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalyzed by cytochromes bo' or bd, from nitric oxide. J Biol Chem 275:35868–35875. doi: 10.1074/jbc.M002471200. [DOI] [PubMed] [Google Scholar]
- 21.Hughes MN. 2008. Chemistry of nitric oxide and related species. Methods Enzymol 436:3–19. doi: 10.1016/S0076-6879(08)36001-7. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Urzúa E, Mills CE, White GP, Contreras-Zentella ML, Escamilla E, Vasudevan SG, Membrillo-Hernández J, Poole RK. 2003. Flavohemoglobin Hmp, but not its individual domains, confers protection from respiratory inhibition by nitric oxide in Escherichia coli. J Biol Chem 278:34975–34982. doi: 10.1074/jbc.M303629200. [DOI] [PubMed] [Google Scholar]
- 23.Hendrick CA, Sequeira L. 1984. Lipopolysaccharide-defective mutants of the wilt pathogen Pseudomonas solanacearum. Appl Environ Microbiol 48:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma V, Noriega CE, Rowe JJ. 2006. Involvement of NarK1 and NarK2 proteins in transport of nitrate and nitrite in the denitrifying bacterium Pseudomonas aeruginosa PAO1. Appl Environ Microbiol 72:695–701. doi: 10.1128/AEM.72.1.695-701.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayward AC, El-Nashaar HM, Nvdegger U, De Lindo LD. 1990. Variation in nitrate metabolism in biovars of Pseudomonas solanacearum. J Appl Bacteriol 69:269–280. doi: 10.1111/j.1365-2672.1990.tb01518.x. [DOI] [Google Scholar]
- 26.Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. 2006. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin hmp. J Biol Chem 281:28039–28047. doi: 10.1074/jbc.M605174200. [DOI] [PubMed] [Google Scholar]
- 27.Forrester MT, Foster MW. 2012. Protection from nitrosative stress: a central role for microbial flavohemoglobin. Free Radic Biol Med 52:1620–1633. doi: 10.1016/j.freeradbiomed.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Chapman AG, Fall L, Atkinson DE. 1971. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol 108:1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Dunn AK, Wilneff J, McFall-Ngai MJ, Spiro S, Ruby EG. 2010. Vibrio fischeri flavohaemoglobin protects against nitric oxide during initiation of the squid-Vibrio symbiosis. Mol Microbiol 78:903–915. doi: 10.1111/j.1365-2958.2010.07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodionov DA, Dubchak IL, Arkin AP, Alm EJ, Gelfand MS. 2005. Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput Biol 1:415–431. doi: 10.1371/journal.pcbi.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arasimowicz-Jelonek M, Floryszak-Wieczorek J. 2014. Nitric oxide: an effective weapon of the plant or the pathogen? Mol Plant Pathol 15:406–416. doi: 10.1111/mpp.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalsing BL, Allen C. 2014. Nitrate assimilation contributes to Ralstonia solanacearum root attachment, stem colonization, and virulence. J Bacteriol 196:949–960. doi: 10.1128/JB.01378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González ET, Brown DG, Swanson JK, Allen C. 2007. Using the Ralstonia solanacearum Tat secretome to identify bacterial wilt virulence factors. Appl Environ Microbiol 73:3779–3786. doi: 10.1128/AEM.02999-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrity G, Brenner DJ, Staley JT, Krieg NR, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H. 2005. Bergey’s manual of systematic bacteriology, 2nd ed, vol 2, part C Springer, New York, NY. [Google Scholar]
- 35.Remenant B, Coupat-Goutaland B, Guidot A, Cellier G, Wicker E, Allen C, Fegan M, Pruvost O, Elbaz M, Calteau A, Salvignol G, Mornico D, Mangenot S, Barbe V, Médigue C, Prior P. 2010. Genomes of three tomato pathogens within the Ralstonia solanacearum species complex reveal significant evolutionary divergence. BMC Genomics 11:379. doi: 10.1186/1471-2164-11-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remenant B, de Cambiaire JC, Cellier G, Jacobs JM, Mangenot S, Barbe V, Lajus A, Vallenet D, Medigue C, Fegan M, Allen C, Prior P. 2011. Ralstonia syzygii, the blood disease bacterium and some Asian R. solanacearum strains form a single genomic species despite divergent lifestyles. PLoS One 6:e24356. doi: 10.1371/journal.pone.0024356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock JD, Moir JW. 2005. Microaerobic denitrification in Neisseria meningitidis. Biochem Soc Trans 33:134–136. doi: 10.1042/BST0330134. [DOI] [PubMed] [Google Scholar]
- 38.Nisman B. 1954. The Stickland reaction. Bacteriol Rev 18:16–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock JD, Mahnane MR, Anjum MF, Shaw JG, Read RC, Moir JW. 2005. The pathogen Neisseria meningitidis requires oxygen, but supplements growth by denitrification. Nitrite, nitric oxide and oxygen control respiratory flux at genetic and metabolic levels. Mol Microbiol 58:800–809. doi: 10.1111/j.1365-2958.2005.04866.x. [DOI] [PubMed] [Google Scholar]
- 40.Bergaust L, van Spanning RJ, Frostegård A, Bakken LR. 2012. Expression of nitrous oxide reductase in Paracoccus denitrificans is regulated by oxygen and nitric oxide through FnrP and NNR. Microbiology 158:826–834. doi: 10.1099/mic.0.054148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honisch U, Zumft WG. 2003. Operon structure and regulation of the nos gene region of Pseudomonas stutzeri, encoding an ABC-type ATPase for maturation of nitrous oxide reductase. J Bacteriol 185:1895–1902. doi: 10.1128/JB.185.6.1895-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessel AK, Arshad TA, Fitzpatrick M, Connell JL, Bonnecaze RT, Shear JB, Whiteley M. 2014. Oxygen limitation within a bacterial aggregate. mBio 5(2):e00992. doi: 10.1128/mBio.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laass S, Kleist S, Bill N, Drüppel K, Kossmehl S, Wöhlbrand L, Rabus R, Klein J, Rohde M, Bartsch A, Wittmann C, Schmidt-Hohagen K, Tielen P, Jahn D, Schomburg D. 2014. Gene regulatory and metabolic adaptation processes of Dinoroseobacter shibae DFL12T during oxygen depletion. J Biol Chem 289:13219–13231. doi: 10.1074/jbc.M113.545004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson DJ. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology 146:551–571. [DOI] [PubMed] [Google Scholar]
- 45.Arai H, Hayashi M, Kuroi A, Ishii M, Igarashi Y. 2005. Transcriptional regulation of the flavohemoglobin gene for aerobic nitric oxide detoxification by the second nitric oxide-responsive regulator of Pseudomonas aeruginosa. J Bacteriol 187:3960–3968. doi: 10.1128/JB.187.12.3960-3968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Kinkel T, Martens-Habbena W, Stahl DA, Fang FC, Hansen EJ. 2011. The Moraxella catarrhalis nitric oxide reductase is essential for nitric oxide detoxification. J Bacteriol 193:2804–2813. doi: 10.1128/JB.00139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. 2011. The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta 1807:1398–1413. doi: 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giuffrè A, Borisov VB, Mastronicola D, Sarti P, Forte E. 2012. Cytochrome bd oxidase and nitric oxide: from reaction mechanisms to bacterial physiology. FEBS Lett 586:622–629. doi: 10.1016/j.febslet.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Zennaro E, Ciabatti I, Cutruzzola F, D’Alessandro R, Silvestrini MC. 1993. The nitrite reductase gene of Pseudomonas aeruginosa: effect of growth conditions on the expression and construction of a mutant by gene disruption. FEMS Microbiol Lett 109:243–250. doi: 10.1111/j.1574-6968.1993.tb06175.x. [DOI] [PubMed] [Google Scholar]
- 50.Denny TP. 2006. Plant pathogenic Ralstonia species, p 573–644. In Gnanamanickam SS (ed), Plant-associated bacteria. Springer, Utrecht, the Netherlands. [Google Scholar]
- 51.Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, Hilliard GM, Parvatiyar K, Kamani MC, Wozniak DJ, Hwang SH, McDermott TR, Ochsner UA. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Delivery Rev 54:1425–1443. doi: 10.1016/S0169-409X(02)00152-7. [DOI] [PubMed] [Google Scholar]
- 52.Zumft WG. 2002. Nitric oxide signaling and NO dependent transcriptional control in bacterial denitrification by members of the FNR-CRP regulator family. J Mol Microbiol Biotechnol 4:277–286. [PubMed] [Google Scholar]
- 53.Yoon MY, Lee KM, Park Y, Yoon SS. 2011. Contribution of cell elongation to the biofilm formation of Pseudomonas aeruginosa during anaerobic respiration. PLoS One 6:e16105. doi: 10.1371/journal.pone.0016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falsetta ML, McEwan AG, Jennings MP, Apicella MA. 2010. Anaerobic metabolism occurs in the substratum of gonococcal biofilms and may be sustained in part by nitric oxide. Infect Immun 78:2320–2328. doi: 10.1128/IAI.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunningham-Bussel A, Zhang T, Nathan CF. 2013. Nitrite produced by Mycobacterium tuberculosis in human macrophages in physiologic oxygen impacts bacterial ATP consumption and gene expression. Proc Natl Acad Sci U S A 110:E4256–E4265. doi: 10.1073/pnas.1316894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R, Xing X, Crawford N. 2007. Nitrite acts as a transcriptome signal at micromolar concentrations in Arabidopsis roots. Plant Physiol 145:1735–1745. doi: 10.1104/pp.107.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellin D, Asai S, Delledonne M, Yoshioka H. 2013. Nitric oxide as a mediator for defense responses. Mol Plant Microbe Interact 26:271–277. doi: 10.1094/MPMI-09-12-0214-CR. [DOI] [PubMed] [Google Scholar]
- 58.Jones SA, Gibson T, Maltby RC, Chowdhury FZ, Stewart V, Cohen PS, Conway T. 2011. Anaerobic respiration of Escherichia coli in the mouse intestine. Infect Immun 79:4218–4226. doi: 10.1128/IAI.05395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Favey S, Labesse G, Vouille V, Boccara M. 1995. Flavohaemoglobin HmpX: a new pathogenicity determinant in Erwinia chrysanthemi strain 3937. Microbiology 141:863–871. doi: 10.1099/13500872-141-4-863. [DOI] [PubMed] [Google Scholar]
- 60.Boccara M, Mills CE, Zeier J, Anzi C, Lamb C, Poole RK, Delledonne M. 2005. Flavohaemoglobin HmpX from Erwinia chrysanthemi confers nitrosative stress tolerance and affects the plant hypersensitive reaction by intercepting nitric oxide produced by the host. Plant J 43:226–237. doi: 10.1111/j.1365-313X.2005.02443.x. [DOI] [PubMed] [Google Scholar]
- 61.Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB. 2010. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Bäumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tans-Kersten J, Huang H, Allen C. 2001. Ralstonia solanacearum needs motility for invasive virulence on tomato. J Bacteriol 183:3597–3605. doi: 10.1128/JB.183.12.3597-3605.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flores-Cruz Z, Allen C. 2011. Necessity of OxyR for the hydrogen peroxide stress response and full virulence in Ralstonia solanacearum. Appl Environ Microbiol 77:6426–6432. doi: 10.1128/AEM.05813-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monteiro F, Solé M, van Dijk I, Valls M. 2012. A chromosomal insertion toolbox for promoter probing, mutant complementation, and pathogenicity studies in Ralstonia solanacearum. Mol Plant Microbe Interact 25:557–568. doi: 10.1094/MPMI-07-11-0201. [DOI] [PubMed] [Google Scholar]
- 66.Bertolla F, Van Gijsegem F, Nesme X, Simonet P. 1997. Conditions for natural transformation of Ralstonia solanacearum. Appl Environ Microbiol 63:4965–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. 1987. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pattern of nitrite production and consumption by R. solanacearum under denitrifying conditions. Nitrate respiration of wild-type R. solanacearum growing anaerobically in VDM medium with 30 mM nitrate at 28°C was measured as nitrite produced, using the Griess reaction. Data shown are the means of 3 biological replicates, each containing 3 technical replicates; bars indicate standard errors. Download
Energy- and nitric oxide-related gene expression during tomato infection by R. solanacearum strain GMI1000.
Strains and plasmids used in this study.