ABSTRACT
There have been many studies on the relationship between nonpathogenic bacteria and human epithelial cells; however, the bidirectional effects of the secretomes (secreted substances in which there is no direct bacterium-cell contact) have yet to be fully investigated. In this study, we use a transwell model to explore the transcriptomic effects of bacterial secretions from two different nonpathogenic Escherichia coli strains on the human colonic cell line HCT-8 using next-generation transcriptome sequencing (RNA-Seq). E. coli BL21 and W3110, while genetically very similar (99.1% homology), exhibit key phenotypic differences, including differences in their production of macromolecular structures (e.g., flagella and lipopolysaccharide) and in their secretion of metabolic byproducts (e.g., acetate) and signaling molecules (e.g., quorum-sensing autoinducer 2 [AI-2]). After analysis of differential epithelial responses to the respective secretomes, this study shows for the first time that a nonpathogenic bacterial secretome activates the NF-κB-mediated cytokine-cytokine receptor pathways while also upregulating negative-feedback components, including the NOD-like signaling pathway. Because of AI-2’s relevance as a bacterium-bacterium signaling molecule and the differences in its secretion rates between these strains, we investigated its role in HCT-8 cells. We found that the expression of the inflammatory cytokine interleukin 8 (IL-8) responded to AI-2 with a pattern of rapid upregulation before subsequent downregulation after 24 h. Collectively, these data demonstrate that secreted products from nonpathogenic bacteria stimulate the transcription of immune-related biological pathways, followed by the upregulation of negative-feedback elements that may serve to temper the inflammatory response.
IMPORTANCE
The symbiotic relationship between the microbiome and the host is important in the maintenance of human health. There is a growing need to further understand the nature of these relationships to aid in the development of homeostatic probiotics and also in the design of novel antimicrobial therapeutics. To our knowledge, this is the first global-transcriptome study of bacteria cocultured with human epithelial cells in a model to determine the transcriptional effects of epithelial cells in which epithelial and bacterial cells are allowed to “communicate” with each other only through diffusible small molecules and proteins. By beginning to demarcate the direct and indirect effects of bacteria on the gastrointestinal (GI) tract, two-way interkingdom communication can potentially be mediated between host and microbe.
INTRODUCTION
With approximately 1014 bacterial cells (1) populating the human gastrointestinal (GI) tract, scientific investigations have uncovered that interkingdom interactions play an important role in maintaining homeostasis (2–4). However, the normal microbiome can also elicit a dysregulated immune response that can be a source of pathogenicity in inflammatory bowel diseases, most commonly Crohn’s disease and ulcerative colitis. In the GI tract, intestinal epithelial cells (IECs), which are an important part of the innate immune system, act as a bridge to the adaptive immune system through their expression and secretion of inflammatory cytokines. IECs initiate this mechanism through pathogen-associated molecular pattern (PAMP) receptors, such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD) receptors, which recognize bacterial products, such as lipopolysaccharides (LPSs), flagella, and peptidoglycan. These receptors activate signaling pathways, mainly through the transcription factor NF-κB, that culminate in the production of cytokines (5–7). As the first point of contact, IECs are continuously exposed to huge numbers of eubacteria (1010 to 1012 cells per g) in the colon (8) and therefore play an important role in bacterium-host communication (9–11).
An understanding of the mechanisms of response and communication between the secretomes of epithelial cells and bacteria can aid in the understanding of the evolutionary biology of signal development as well as interventional design strategies for maintaining homeostasis (Fig. 1) (11, 12). Moreover, signals that coordinate phenomena among bacteria (e.g., quorum sensing [QS]) and signals that mediate bacterium-IEC interactions are of particular interest, as these communication networks are involved in pathogenesis and the progression of disease (10, 13, 14). Commensurate with the need to understand this interkingdom communication, there have been many studies exploring the effects of nonpathogenic, commensal strains of bacteria on human cells (15–19). However, most of these involved direct bacterium-IEC interaction, and those that investigated the secretome did not determine a global transcriptomic or proteomic response, leaving the effects of bacterial secretions to be largely unexplored. We have characterized the effects of the Escherichia coli secretome, which is well represented in the colon (20), through the use of a transwell that separates bacteria from epithelial cells while allowing small molecules and proteins to pass, and we have employed transcriptome sequencing (RNA-Seq) because it provides several advantages over DNA microarrays, including lower background noise, an absolute transcript count, and higher resolution (21). By determining the global transcriptomic response of IECs to bacterial incubations in a system that allows only indirect contact, we can then more closely investigate the commonalities of interkingdom communication.
FIG 1 .
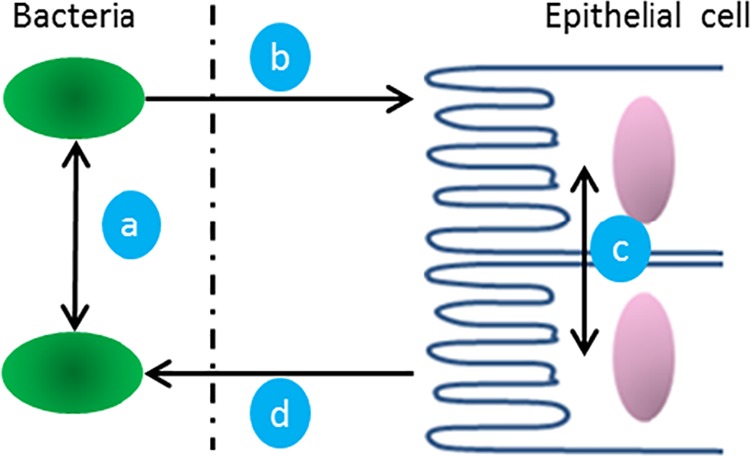
Interkingdom communication between the microbiome and the host in the GI tract. (a) Quorum-sensing (QS) molecules coordinate actions among bacteria. (b) Secretomes of bacteria, including QS molecules, affect the host's cellular machinery. (c) Epithelial cells secrete signals to neighboring and distant cells through signaling molecules. (d) Soluble factors secreted by the host affect bacteria.
In this work, we exposed nonpathogenic strains of two Gram-negative, group A E. coli strains, BL21 and W3110, grown in the upper chamber of a transwell, to the IEC line HCT-8, cultured in a monolayer beneath the transwell. BL21, a B strain derivative, and W3110, a K-12 strain derivative, have significantly different transcriptomes and proteomes, leading to important phenotypic differences (22, 23). Our investigations show that the secretome of either BL21 or W3110 activated the cytokine-cytokine receptor pathway (e.g., interleukin 8 [IL-8] and tumor necrosis factor [TNF]), while also upregulating the negative-feedback regulators in NF-κB and NOD-like signaling pathways, namely, the NF-κB α subunit (Iκβα) and TNFAIP3, respectively. The upregulation of cytokines that activate the immune system as well as negative-feedback regulators that reduce the transcription of these cytokines may be part of the normal physiological response using a negative-feedback loop (24), without which uncontrolled stimulation of inflammatory cytokines would lead to damaging inflammation in the host (2, 24).
The role of AI-2 was investigated further by incubating the in vitro-synthesized signal molecule at various concentrations and time periods with IECs in follow-up studies. The inflammatory cytokine IL-8, which plays an important role in attracting neutrophils, was found to be initially upregulated at all concentration levels of AI-2 tested (50, 150, and 400 µM) at 6 and 12 h postaddition. IL-8 was subsequently significantly reduced at all concentrations relative to levels in the control after 24 h. These data support a hypothesis that AI-2 is an IEC signaling molecule and that bacterial secretions, including AI-2, may have an initial transcriptional inflammatory response that is downregulated through alternative mechanisms, possibly including the negative regulators NF-κB-α and TNFAIP3.
RESULTS
The secretomes of BL21 and W3110 cause differential gene expression in HCT-8 cells.
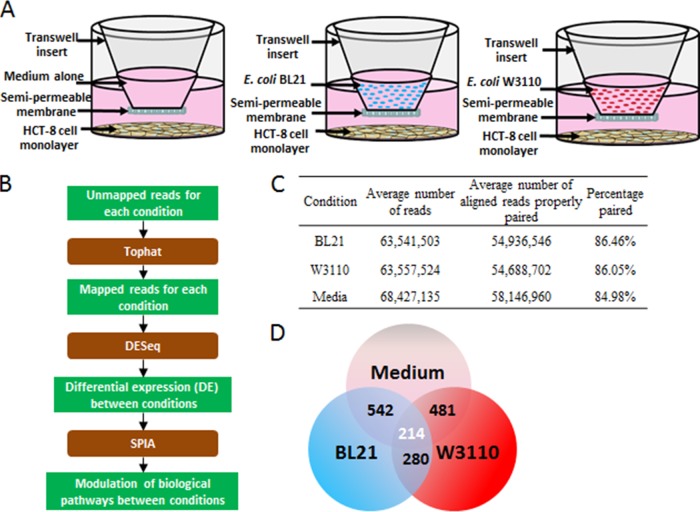
In this study, we explored the transcriptomic changes of coincubations of BL21 and W3110 in a transwell model with the IEC line HCT-8. We chose a coincubation model, instead of using conditioned medium, because bacteria themselves are affected by secretable molecules from mammalian cells, and we chose to include any such cross talk (25, 26). Toward this end, overnight cultures of BL21 and W3110 were reinoculated in fresh medium in the upper chamber of the transwell, and blank medium alone was used as a negative control (Fig. 2A). The 0.4 µM transwell does not allow measurable amounts of bacteria to pass through the upper chamber (verified through optical density measurements of the lower chamber) but is large enough to allow metabolites and signaling molecules to pass. After 6 h of coincubation, both bacterial strains reached similar cell densities (optical density at 600 nm [OD600] of ~1 [data not shown]), and IECs appeared visibly intact, with a cell viability assay showing less than 5% cell death (data not shown). The RNA of the HCT-8 cells was extracted, and cDNA libraries were created from polyadenylated RNA.
FIG 2 .
(A) HCT-8 epithelial cells were grown to confluence and then incubated with BL21, W3110, or medium alone in the upper chamber of a transwell. After 6 h of incubation, the RNAs of the epithelial cells were extracted and sequenced. (B) Downstream RNA-Seq pipeline for analysis of sequencing data (brown boxes indicate the open-source program). (C) Results of mapping HCT-8 NGS transcripts to a RefSeq annotated human genome, hg19. Five biological replicates were performed with the software TopHat. (D) Genes differentially expressed as determined by using the software DESeq. There were 542 DE genes between HCT-8 cells incubated with BL21 and those incubated with blank medium, and 481 DE genes between HCT-8 cells incubated with W3110 and those incubated with blank medium. We found 280 DE genes between HCT-8 cells incubated with BL21 compared to those incubated with W3110. Of the 542 DE genes in incubations with BL21 compared to blank medium and 481 DE genes in incubations with W3110 compared to blank medium alone were 214 differentially expressed genes common to incubations with either bacteria compared to blank medium.
The cDNA libraries from each condition were sequenced via NGS (Next Generation Sequencing) and then analyzed (see Materials and Methods) with downstream statistical software (Fig. 2B). We performed five biological replicates, each constituting an average of over 60 million 100-bp paired-end reads mapping to hg19, a RefSeq annotated human genome (Fig. 2C). Mapping sequenced reads to the genome was performed using TopHat (27) (which uses a built-in alignment tool) and Bowtie (28) (which maps the cDNA reads to the reference genome). TopHat then aligns reads that did not initially align because of a splicing event and discards reads that cannot be aligned. The aligned reads were input into the open-source software DESeq (29), which was used to determine significantly differentially expressed (DE) genes (Benjamini-Hochberg-adjusted P values were below 0.05).
DESeq results indicated that BL21 and W3110 caused 542 and 481 differentially expressed genes to be up- or downregulated compared to their expression in blank medium, and 280 were differentially expressed between BL21 and W3110 bacterial incubations. Common to the 542 genes that were differentially expressed in incubations of BL21 compared with blank medium alone and the 481 genes that were differentially expressed in W3110 compared with blank medium alone were 214 differentially expressed genes (Fig. 2D). A closer examination of differentially expressed transcriptional levels between the three direct comparisions illustrate that the majority of differentially expressed fold changes were small-magnitude (<2-fold) differences (Table 1). With five biological replicates, we were able to determine significant differential gene expression between conditions that displayed these small differences (for complete differential-expression data, see Tables S1 to S3 in the supplemental material). Additionally, we selected 8 genes that spanned a wide range of expression for quantitative PCR (qPCR) verification and measured transcriptional levels with qPCR, which showed a high degree of correlation, as expected (Fig. S1).
TABLE 1 .
DE genes in HCT-8 cells in incubations with BL21, W3110, or medium alonea
| Incubation conditions | No. of upregulated genes |
No. of downregulated genes |
Total no. of DE genesb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 < FC < 1.5 | 1.5 < FC < 2 | FC > 2 | Total | 1 < FC < 1.5 | 1.5 < FC < 2 | FC > 2 | Total | ||
| BL21 to medium | 154 | 45 | 42 | 241 | 262 | 26 | 13 | 301 | 542 |
| W3110 to medium | 154 | 28 | 21 | 203 | 166 | 26 | 86 | 278 | 481 |
| BL21 to W3110 | 66 | 33 | 126 | 225 | 39 | 14 | 2 | 55 | 280 |
FC, fold change.
DE is determined using the open-source software DESeq. All genes listed have a Benjamini-Hochberg-adjusted P of <0.05.
BL21 and W3110 activate the cytokine-cytokine receptor pathway.
The biological implications of these differentially expressed genes were determined using signaling pathway impact analysis (SPIA) software (30). SPIA uses overrepresentation analysis (the prevalence of genes different from all background genes), functional class scoring (the similarity of functions of genes differentially expressed), and pathway topology (a priori knowledge of signaling pathways) to identify activated or inhibited pathways (see Fig. S2 in the supplemental material).
Since epithelial cells are often damaged through extracellular stimuli, they often initiate inflammation through the release of cytokines (31). The cytokine-cytokine interaction pathway is regulated through the chemokine and NF-κB pathways, and as expected, these pathways were activated in both bacterial incubations (Table 2). The Toll-like receptor (TLR) pathway is not listed in Table 2, as the pathway was not activated. It has been shown that TLR receptors in colonic IECs, unlike other types of epithelial cells, develop tolerance after exposure to PAMPs, such as LPS and lipotechnoic acid (LTA) (32, 33), and activate the TLR pathway only after being primed with interferon gamma (IFN-γ) (34).
TABLE 2 .
Activated or inactivated pathways of DE genesa
| Incubation conditions | Interaction, KEGG pathway, or disease | P valueb | Status |
|---|---|---|---|
| BL21 to medium | Cytokine-cytokine receptor interaction | 9.27E–06 | Activated |
| Chemokine signaling pathway | 4.06E–04 | Activated | |
| Osteoclast differentiation | 3.58E–03 | Activated | |
| NF-κB signaling pathway | 1.10E–02 | Activated | |
| HTLV-I infection | 1.18E–02 | Activated | |
| Chagas disease | 4.10E–02 | Activated | |
| NOD-like receptor signaling pathway | 5.48E–02 | Inhibited | |
| W3110 to medium | Cytokine-cytokine receptor interaction | 4.26E–05 | Activated |
| Chemokine signaling pathway | 1.81E–04 | Activated | |
| NOD-like receptor signaling pathway | 1.93E–04 | Inhibited | |
| HTLV-I infection | 2.67E–04 | Activated | |
| Epstein-Barr virus infection | 4.10E–04 | Activated | |
| NF-κB signaling pathway | 7.94E–04 | Activated | |
| Osteoclast differentiation | 5.47E–02 | Activated |
DE genes were input into SPIA (signaling pathway impact analysis) software to determine activated or inactivated pathways. Incubations of BL21 compared to those with medium alone resulted in the modulation of seven annotated KEGG pathways, and incubations of W3110 compared to those with medium alone also resulted in the modulation of seven annotated KEGG pathways. Common to both sets were the activation of the cytokine-cytokine receptor interaction, the chemokine signaling pathway, osteoclast differentiation, the NF-κB signaling pathway, human T-lymphotropic virus type I (HTLV-I) infection, and the inactivation of the NOD-like receptor signaling pathway.
P value is a Bonferroni-adjusted global P value.
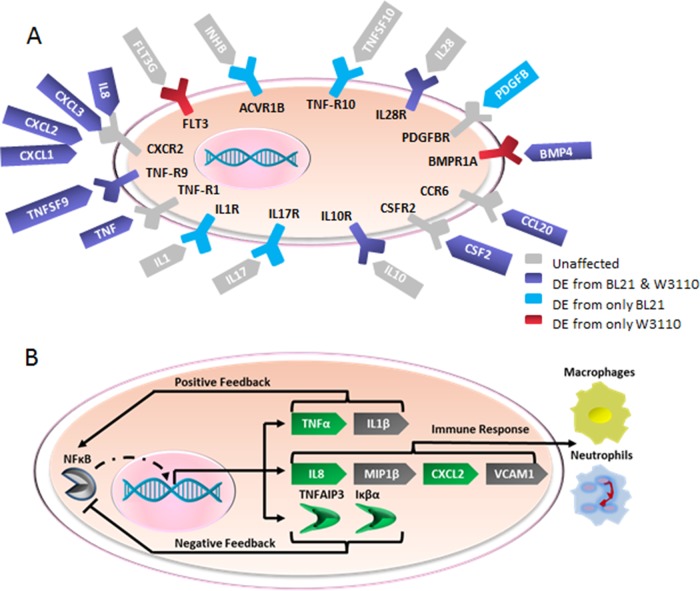
A closer investigation of the cytokine network found that 10 cytokines were significantly differentially expressed in one sample or the other (Fig. 3A). All of these cytokines were upregulated, except BMP4, which is responsible for the regeneration of epithelial cells. The upregulation of a granulocyte/macrophage colony-stimulating factor (CSF2) stimulates stem cells to produce granulocytes (neutrophils, eosinophils, and basophils) and monocytes. The CXC cytokines that were upregulated (CXCL1, CXCL2, CXCL3, IL-8) are chemotactic for neutrophils, and all of the CXC chemokines upregulated act as agonists for the same receptor. TNF, TNFSF9, and TNFRSF9 were upregulated and act as proapoptotic signals or receptors, as well as promote leukocyte chemotaxis through the induction of proinflammatory cytokines (35). CCL20, a CC motif cytokine, is weakly chemotactic for neutrophils and strongly attractive for lymphocytes. Taken together, the cytokines act collectively to induce the activation and long-term survival of neutrophils. This upregulation indicates that bacterial secretions have caused the IEC to signal the adaptive immune response due to the secretomes of these nonpathogenic bacteria.
FIG 3 .
(A) Activation of the cytokine-cytokine receptor interaction pathway in incubations with BL21 or W3110. The schematic shows cytokines (ovals) and cytokine receptors (polygons) upregulated only by incubation with BL21 (blue) or only by incubation with W3110 (red). Incubations with either E. coli strain (purple) or with no change in regulation by either E. coli strain (gray) is also shown. (B) Schematic of genes involved in the canonical NF-κB pathway, adapted from the KEGG. Gene expression levels upregulated (green) and unaffected (gray) by incubations with both BL21 and W3110 compared to expression levels in medium alone are shown.
BL21 and W3110 activate the NF-κB pathway and its negative-feedback components.
The NF-κB pathway is an integral part of the immune response and functions as a protein complex that controls DNA transcription. To prevent uncontrolled inflammation, it is thought that the negative-feedback mechanisms associated with PAMP receptor activation are upregulated to suppress the overproduction of inflammatory cytokines (36). Consistently with this hypothesis, the canonical NF-κB pathway was activated in both bacterial incubations, and the negative-feedback components (i.e., the Iκβα inhibitor) were upregulated as well (Fig. 3B).
The function of the NF-κB pathway is controlled by the inhibitor of kappa-B kinase (IKK) complex, which consists of NF-κB essential modulator (NEMO), IKK-α, and IKK-β. The IKK complex phosphorylates the Iκβα inhibitor, which causes its proteosomal degradation. The degradation of the Iκβα inhibitor leads to the free movement of NF-κB into the nucleus and subsequent initiation of gene transcription. In both the BL21 and W3110 incubations, the end products of the canonical NF-κB pathway were upregulated (inflammatory cytokines IL-8, TNF-α, and CXCL2), while the end products of the atypical NF-κB pathway (e.g., apoptosis regulator Bcl-XL) were unchanged. This indicates that the bacterial secretomes stimulated the HCT-8 immune response through the canonical NF-κB pathway and that possible microenvironmental conditions, such as hypoxia, which activate the atypical NF-κB pathway (37) did not elicit an immune response.
Critically, Iκβα inhibitor, which is integral to the negative feedback in the NF-κB pathway, was upregulated in both the BL21 and W3110 incubations. Additionally, the NOD-like receptor pathway was inhibited in both pathways, with its negative-feedback response regulator, TNFAIP3, also upregulated in both bacterial samples. NOD-like receptors (NLRs) act as cytosolic sensors and, once activated, subsequently activate a receptor-interacting protein (RIP). TNFAIP3 acts as the negative regulator of RIP, thereby quenching the signaling cascade despite the continued presence of agonists of NLRs (38). TNFAIP3 has also been shown to be a critical negative-feedback regulator of the NF-κB pathway (39). The activation of the NF-κB pathway and the negative-feedback regulators Iκβα inhibitor and TNFAIP3 suggest that components of the bacterial secretions act as a stimulus to the immune system and that the epithelial cells have coincidently upregulated the negative-feedback components to prevent uncontrolled inflammation from this nonpathogenic encounter.
Upregulation of gene expression by bacterial secretomes does not translate into increased cytokine protein expression.
Using a 12-cytokine multianalyte enzyme-linked immunosorbent assay (ELISA) kit, we surveyed two of the upregulated cytokines from incubations with BL21 and W3110 (TNF and IL-8) as well as 10 other cytokines involved in inflammation. We found that while inflammatory cytokine gene expression was upregulated at the transcriptional level, there was no concomitant increase in secretion (see Fig. S3 in the supplemental material). This finding is supported by Kamada et al., who similarly used a transwell model and found that IL-8 secretion was unchanged in the IEC line HCT-15 when cells were incubated with E. coli K-12 strain DH10β for 4 h (18). These results indicate that the transcriptomic upregulation is quenched either posttranslationally or through the upregulation of negative-feedback mechanisms, such as NF-κB and NOD-like signaling pathways.
BL21 and W3110 cause differential expression in genes responsible for tissue structure.
Since the bacterial secretome includes components such as LPS, an activator of osteoclastogenesis, to enhance bone resorption in both in vitro and in vivo studies (40, 41), differential expression of genes responsible for tissue structure was expected. Both BL21 and W3110 resulted in the activation of the osteoclast differentiation signaling pathway. CTSK, an end product of this pathway, was upregulated in incubations with both BL21 and W3110 and encodes the protein cathepsin K, a protease that breaks down elastin, gelatin, and collagen, which are critical components of bone and cartilage. Furthermore, in the cytokine-cytokine receptor pathway, the downregulation of BMP4 induces increased epithelial stem cell renewal. Collectively, these transcriptional differences indicate that the epithelial cells have been insulted by the bacterial secretomes, causing the upregulation of genes responsible for cell renewal.
Strain-specific differentially expressed genes.
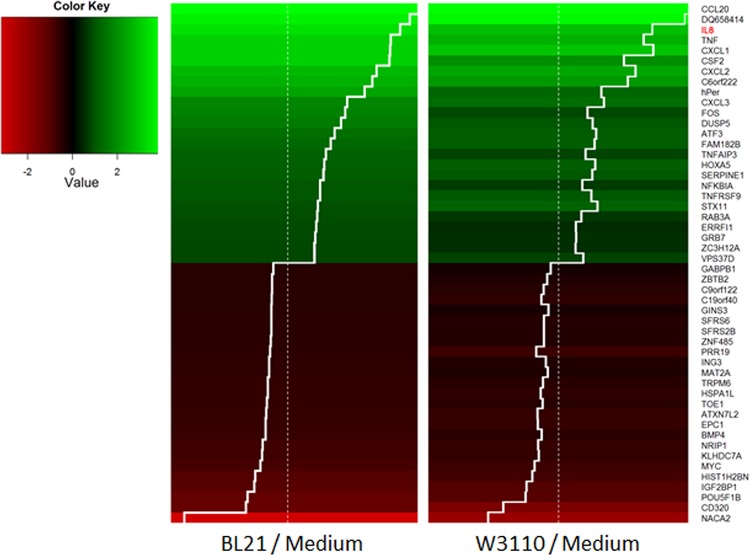
BL21 and W3110 are, respectively, derivatives of the B and K-12 strains of E. coli, which comprise the majority of all laboratory strains. Despite the similarity of their genomes, B strains and K-12 strains show marked phenotypic differences. B strains grow faster in minimal medium and have lower acetate production (22, 23). Furthermore, while B cells produce smaller amounts of intracellular proteases (e.g., Lon, ClpA, and ClpP), they secrete higher total levels of extracellular proteins, mainly through their type II secretion pathway. K-12 strains have higher levels of expression of genes for heat shock proteins and flagella, which provide motility, and they more ably survive stress insults (e.g., osmolarity and pH) than W3110 (22). A closer investigation into the differential regulation caused by each strain illustrates that they affect the directionality (i.e., upregulation or downregulation) of their gene expression in similar manners. Of the 214 DE genes that are common in incubations of BL21 compared to blank media and W3110 compared to blank media, 100% of them were regulated in the same manner (i.e., upregulated or downregulated). Figure 4 shows a heatmap of the two strains organized by the 25 most up- and downregulated genes in the BL21 coincubation. The similarity in gene expression to that in incubations with W3110 was striking, with many cytokines being the most upregulated genes.
FIG 4 .
Heatmap of the 25 most upregulated (green) and downregulated (red) genes in HCT-8 in incubations with BL21 compared to their levels of expression in medium alone and in incubations with W3110 compared to blank medium. The trace line (white) indicates the direction and extent of differential expression. Differential expression levels are similar between incubations of BL21 and W3110, and 100% of differential expression is regulated in the same manner (i.e., up- or downregulated). DESeq was used to identify differential expression, and all genes listed have a Benjamini-Hochberg-adjusted P of <0.05. Cytokines, including IL-8 (red text), were among the genes most upregulated.
While both strains showed similarity in fold change expression levels and directionality of regulation, we found that the amplitude of the up- and/or downregulation was higher in BL21 incubations. This trend was also subtly revealed by more carefully considering the results from Fig. 2C. Of the 214 DE genes that were common to incubations of BL21 compared to blank medium and W3110 compared to blank medium, 96 were upregulated, and 76 of these (79.1%) were more upregulated in the BL21 sample. Of the 118 genes downregulated in common, 75 (63.5%) were more downregulated in the BL21 sample. There were 280 DE genes between incubations of BL21 compared to W3110 (Fig. 2D), and of these genes, 225 (80.3%) were differentially expressed to a greater amplitude in incubations with BL21. Of particular importance is the gene for IL-8, a proinflammatory cytokine, as it shows greater abundance in incubations with BL21 than with W3110 (Fig. 5). These expression level differences indicate that secretions from BL21 induce a greater epithelial cell response than secretions from W3110. Importantly, cell densities of inocula were identical, as were the final optical densities (data not shown).
FIG 5 .
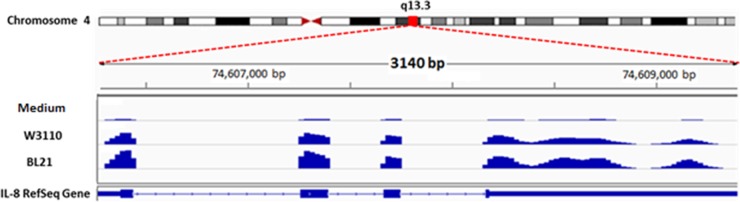
NGS sequenced reads mapped the annotated IL-8 gene as visualized in the Integrative Genome Viewer (IGV). The IL-8 gene is shown at the bottom, with four exons separated by three introns. Each read is represented by a blue square, and the abundance of reads under each condition (BL21, W3110, or medium alone) is shown. HCT-8 incubations with W3110 show a greater abundance of IL-8 transcription than incubations with medium alone, while incubations with BL21 illustrate higher levels than W3110. One representative replicate sample of each condition is shown.
We then sought to investigate the cause of the greater perturbation caused by BL21 than by W3110. Because of the use of the transwell, the phenotypic differences that require direct interaction can be ignored, and we can focus on secretable substances. One possible candidate, LPS, is more highly expressed in BL21 than in W3110 (22), and it is well known that LPS has inflammatory effects on cytokines (42, 43) and, through them, activates NF-κB in colonic IECs (44). However, in colonic epithelial cells, the addition of the cytokine IFN-γ to IECs is needed to express myeloid differentiation protein 2 (MD-2), which is required for LPS responsiveness (34, 42). Furthermore, priming of IFN-γ with subsequent LPS exposure shows a transient upregulation of IL-8 that returns to baseline levels after 6 h, which is the time period used in this study. On the other hand, BL21 produced much more extracellular AI-2 than W3110 (~35 µM compared to 8 µM [see Fig. S4 in the supplemental material]), and BL21 cells do not express the ABC transporter for uptake of the quorum-sensing signal molecule autoinducer 2 (AI-2) or the intracellular kinase that sequesters AI-2 inside the cell (45). The fact that BL21 showed much better the effect of AI-2 on colonic cells is of particular interest not only because the highest concentrations of bacteria are found in the gut but also because eubacteria are almost entirely concentrated in this area of the GI tract (8). Furthermore, the LuxS/AI-2 production system is highly conserved among the eubacteria (46–48); therefore, we chose to investigate the effect of AI-2 on IECs. While we have shown that the robust transcriptional responses of epithelial cells to BL21 and W3110 are similar, the slightly greater amplitude shift in BL21 may be caused by the much higher levels of AI-2 in BL21. We then sought to tease out this small effect from the overall systematic responses elicited from the secretome.
AI-2 initiates upregulation of inflammatory cytokines before downregulation.
Bacteria secrete and detect small molecules or autoinducers to coordinate gene expression in a cell density-dependent manner (known as quorum sensing [QS]). These QS molecules are produced throughout the eubacterial hierarchy and influence characteristics such as swarming motility, biofilm formation, and virulence, among others (reviewed in references 49 to 52). The terminal synthase for one prevalent autoinducer, AI-2, has been found in over 80 species (46, 47).
Studies have shown both beneficial and deleterious effects of QS molecules on human epithelial cells. N-3-(Oxododecanoyl)-l-homoserine lactone (OdDHL) produced by Pseudomonas aeruginosa induces apoptosis in many mammalian cell types (53–55), while indole has been found to decrease inflammation in IECs by attenuating IL-8 production, reducing TNF-α-mediated NF-κB activation, and tightening cell junctions (4). Investigations into interkingdom effects of AI-2 on human cells have been limited to one study, where Bryan et al. performed microarray studies of alveolar cells exposed to AI-2 at 50 µM and found only 4 genes with over 2-fold changes in expression (56).
We chose to investigate the effect of AI-2 directly on IECs and have performed a time course analysis using a range of AI-2 concentrations: 50, 150, and 400 µM. It must be noted that the levels of AI-2, or any other quorum-sensing metabolite, are unknown in the GI tract. However, indole has been found in human feces at concentrations ranging from ~50 to 1,100 µM (57, 58), and interkingdom studies have used a range of concentrations from 0.4 to 250 µM for the AI-1 molecule OdDHL (54–56). In our study, 50 µM AI-2 was chosen, as it is the concentration used in the only previous interkingdom study (56) and is a level approximating that used in our coincubation studies with BL21, which exposed the HCT-8 cells to much higher levels of AI-2 than those cells incubated with W3110 (Fig. S4). One hundred fifty micromoles represents the upper limit reached by standard LB cultures of E. coli BL21 (59). Finally, since it has been shown that higher concentrations of eubacteria can populate the colon than can be reached in vitro (8) and that QS molecules can reach much higher levels in biofilms (~600 µM) (60), we also selected 400 µM AI-2 as a possible representation of high local QS molecule concentrations.
Thus, we exposed HCT-8 cells to 50, 150, and 400 µM AI-2 for 6, 12, and 24 h. We performed AI-2 assays on samples after 24 h and found that significant quantities of AI-2 were still present (data not shown). After the RNA was harvested, we found that IL-8, a proinflammatory cytokine that is chemotactic to neutrophils, was moderately upregulated, with the average fold changes for all three concentrations totaling 2.29 and 1.69 at the 6- and 12-h time points, respectively, before being downregulated (−1.98) compared to their expression in blank medium for all three concentrations ranges at 24 h (Fig. 6). This trend was consistent with that of the secretomes and was found at all 3 concentrations. Interestingly, the same trend but with lower amplitudes was found for TNF and CSF2 at some concentrations (Fig. S5). It is hypothesized that the interplay between host and the microbiota is tightly regulated and that microbial metabolites induce changes in the host signaling pathways, which are restored through negative-feedback loops (24). The initial upregulation of IL-8 expression levels with exposure of BL21 and W3110 to the HCT-8 cells, followed by abatement to lower levels, is consistent with this hypothesis.
FIG 6 .
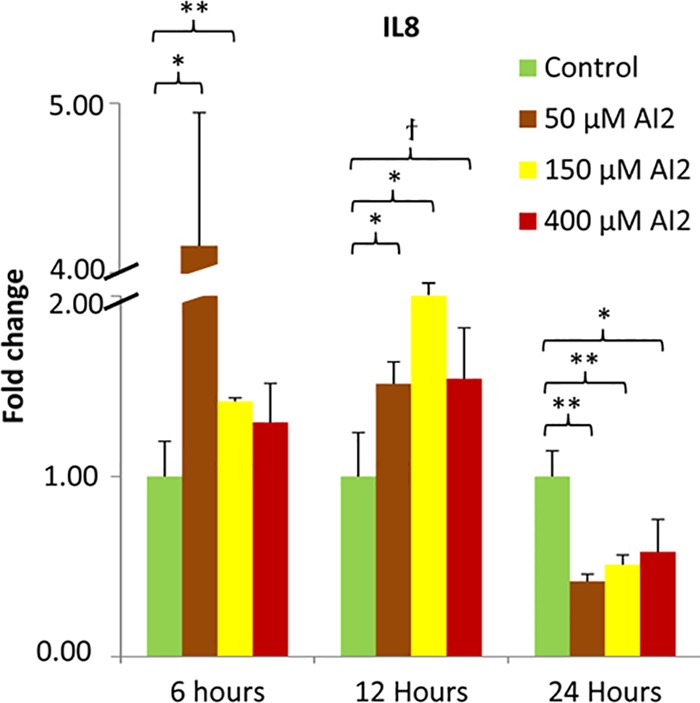
HCT-8 cells were incubated with AI-2 at 50, 150, and 400 μM for 6, 12, and 24 h; expression levels are normalized to those with medium alone. At early times (6 and 12 h), incubations with AI-2 result in an upregulation of IL-8 gene expression compared to expression in medium alone, while at 24 h, IL-8 expression levels are downregulated compared to levels in medium alone. qPCR fold level changes are shown. †, P < 0.10; *, P < 0.05; **, P < 0.01.
DISCUSSION
Investigations into interkingdom communication in the GI tract can aid in treatment for diseases such as inflammatory bowel disease, which arises from the immune system, causing inflammation from commensal bacteria, and colorectal cancer, which is believed to be promoted through chronic inflammation. In this study, we have shown for the first time that bacterial secretions from nonpathogenic E. coli cells upregulated a number of proinflammatory pathways in IECs, leading to the transcription of cytokines involved in recruiting leukocytes, particularly neutrophils. The activation of biological-defense-related pathways from secretions of two different strains of E. coli, BL21 and W3110, illustrate that direct contact from flagella, membrane-bound proteins, or secretion systems are not necessary to induce an immunological response from IECs. That is, we have shown that E. coli secretions cause the upregulation of proinflammatory cytokines through the activation of the mediation pathway NF-κB, indicating that the immune response was elicited through bacterial secretions.
Our results also show that the negative-feedback components of the NF-κB pathways (Iκβα inhibitor) and NOD-like receptor pathways (TNFAIP3) were upregulated in the HCT-8 cells, indicating a negative-feedback loop to control the upregulation of cytokine gene expression from nonpathogenic E. coli. Iκβα inhibitor acts to block the canonical and atypical NF-κB pathways, and its upregulation directly inhibits the transcription of cytokines. TNFAIP3 is a negative regulator of the NOD-like receptor pathway, the intracellular sensing mechanism corollary to the extracellular TLR sensing mechanism. The inhibition of the NOD-like pathway suggests a response to block the signaling cascade of bacterial products that were transported into the mammalian environment. The upregulation of these negative-feedback components may suggest that IECs are preventing the physiological response from developing into a pathological response.
While both bacteria elicited similar responses, BL21 appeared to cause greater perturbations in HCT-8 cells. As noted above, phenotypic differences between BL21 and W3110 include flagella, LPS, heat shock proteins, metabolic byproduct secretions, and AI-2 production. Our investigations into the interkingdom effects of AI-2 revealed a moderate, but significant, upregulation in IL-8 at both 6 and 12 h, followed by a significant downregulation at 24 h. Like the results from the full secretome, this may indicate that AI-2 as a single signal molecule has an inflammatory effect but that after some period of modulation, the IEC inflammation is controlled through negative feedback to prevent a pathological response to a nonpathogenic stimulus.
In conclusion, while it may be expected that bacterial secretomes affect IECs and immune function in the gut, our study has demonstrated that a bacterial-bacterial signaling molecule also influences the same. That is, IECs evidently “listen in” on the communication between bacteria that reside in the lumen and alter their behavior based on these signaling phenomena. Further exploration of the effects of bacterial soluble factors on IECs will aid in the understanding of microbial disease, and modulation of existing interkingdom signaling networks may result in novel methods to combat infections.
MATERIALS AND METHODS
HCT-8 incubations with bacteria.
HCT-8 cells were plated in 6-well culture plates (Fisher Scientific) at a seeding density of 750,000 cells per well (375,000 cells/ml) in 10% (vol/vol) horse serum RPMI 1640 medium (ATCC). The culture was grown to confluence for 48 h at 37°C in the presence of 5% CO2 humidified air. A 0.4-µm transwell (Becton, Dickinson) was placed in each culture plate, and BL21 (2.6% overnight culture) or W3110 (2.6% overnight culture) in 1.5 ml of RPMI 1640 medium was added. RPMI 1640 medium alone was added as a negative control. The coculture was then incubated for 6 h at 37°C in the presence of 5% CO2 humidified air. After incubation, the transwell and enclosed medium in the upper chamber were discarded, and the medium of the lower chamber was removed and harvested for the Vibrio harveyi BB170 AI-2 activity assay and ELISAs. The RPMI 1640 medium is supplemented with phenol red, and there was no change in color in the lower chamber, indicating that there were no significant pH changes during incubation. RNA was extracted with the RNAqueous kit (Invitrogen), and eluted RNA was stored at −80°C until thawed for sequencing and qPCR.
HCT-8 incubations with AI-2.
HCT-8 cells were plated and cultured in similar manners as described above. Synthetic AI-2 (10 mM) in water was generously provided by the Sintim Research Group. AI-2 at 50, 150, and 400 µM in 2 ml of fresh RPMI 1640 medium was incubated with HCT-8 cells for 6, 12, and 24 h.
AI-2 activity assay.
After incubation for 6 h under the respective conditions, the media of the HCT-8 cells were harvested and tested for the presence of AI-2 by inducing luminescence in Vibrio harveyi reporter strain BB170, a procedure outlined by Bassler and coworkers (61). Briefly, BB170 was grown for 16 h with shaking at 30°C in Autoinducer Bioassay (AB) medium and kanamycin, diluted 1:5,000 in fresh AB medium and kanamycin, and aliquoted to sterile 12- by 75-mm tubes (Fisher Scientific). The medium of each condition was added to a final concentration of 10% (vol/vol) to these tubes. Luminescence was measured by quantifying light production with a luminometer, and obtained values were in the linear range. Values represent fold changes from the negative control. All conditions were analyzed in triplicate.
qPCR.
RNA was synthesized to cDNA using the BIO-73005 SensiFAST SYBR Hi-ROX one-step kit. For the selected candidate genes, primers were taken from the literature or designed using PrimerQuest (primers are listed in Table S4 in the supplemental material). Βeta-2-microglobulin (β2M) was used as a housekeeping gene, and qPCR was performed on the 7900HT real-time PCR system (Applied Biosystems) with thermal conditions of 10 min at 45°C, 2 min at 95°C, and 40 cycles of 5 s at 95°C and 20 s at 60°C. The relative gene expression level of each target gene was then normalized to the mean expression of β2M in each group. The control for each gene expression sample set was selected to be the 0 µM AI-2 samples at each time point. Fold change was calculated using the change in threshold cycle (ΔΔCT) comparative method. Data from all the studies were analyzed using analysis of variance. Samples were tested in triplicate, and standard deviations are reported (n = 3).
RNA downstream analysis.
Each sample’s reads were aligned to the RefSeq annotated human genome hg19 using the software TopHat (27). These read abundances were then output into DESeq (29), an open-source program in R that analyzes the statistical significance of differential expression. The abundances of sequenced reads, “counts” of each gene, were input into DESeq, a software that uses variance, transcript abundance, and fold change to determine differential expression, normalized by the size of each sample’s cDNA library. A modified Fisher exact test with data fit to a negative binomial distribution of the DESeq package was used to identify the differentially expressed genes. Differentially expressed genes were output to SPIA (30) to evaluate pathway activation.
ELISA.
Cell culture supernatants of HCT-8 cells in transwell incubations with BL21, W3110, and medium alone were harvested and subsequently assayed with the human inflammatory cytokine multianalyte ELISArray kit MEH-004A (Qiagen).
SUPPLEMENTAL MATERIAL
qPCR validation of RNA-Seq results show high correlation for a range of expression levels. Download
Significantly differentially expressed genes and expression levels from DESeq are input into SPIA (signaling pathway impact analysis) to determine activation and inhibition of entire biological pathways. Download
Multianalyte ELISAs were performed on cell culture supernatants of HCT-8 cells in transwell coincubations with BL21, W3110, and medium alone. Download
AI-2 concentration levels in the HCT-8 supernatant from incubations with BL21 and W3110. Download
HCT-8 cells were incubated with AI-2 at 50, 150, and 400 µM for 6, 12, and 24 h. Download
Expression level differences between incubations with BL21 and medium alone determined by DESeq software.
Expression level differences between incubations with W3110 and medium alone determined by DESeq software.
Expression level differences between incubations with W3110 and BL21 determined by DESeq software.
Primers used for SYBR green qPCR.
ACKNOWLEDGMENTS
Funding was provided by the Defense Threat Reduction Agency (DTRA; grant HDTRA1-13-1-00037), the Office of Naval Research (grant N000141010446), the National Science Foundation (grants CBET 1160005 and CBET 1264509), and the R. W. Deutsch Foundation.
Footnotes
Citation Zargar A, Quan DN, Carter KK, Guo M, Sintim HO, Payne GF, Bentley WE. 2015. Bacterial secretions of nonpathogenic Escherichia coli elicit inflammatory pathways: a closer investigation of interkingdom signaling. 6(2):e00025-15. doi:10.1128/mBio.00025-15.
REFERENCES
- 1.Sekirov I, Russell SL, Antunes LC, Finlay BB. 2010. Gut microbiota in health and disease. Physiol Rev 90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LW, Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bansal T, Alaniz RC, Wood TK, Jayaraman A. 2010. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A 107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 6.Inohara N, Nuñez G. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 7.Creagh EM, O’Neill LA. 2006. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Nord CE, Kager L, Heimdahl A. 1984. Impact of antimicrobial agents on the gastrointestinal microflora and the risk of infections. Am J Med 76:99–106. doi: 10.1016/0002-9343(84)90250-X. [DOI] [PubMed] [Google Scholar]
- 9.Lyte M. 2013. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog 9:e1003726. doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis MM, Sperandio V. 2011. A complex relationship: the interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol 4:133–138. doi: 10.1038/mi.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes DT, Sperandio V. 2008. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. 2004. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet 20:292–299. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasko DA, Sperandio V. 2010. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 15.Borruel N, Casellas F, Antolín M, Llopis M, Carol M, Espíin E, Naval J, Guarner F, Malagelada JR. 2003. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am J Gastroenterol 98:865–870. doi: 10.1111/j.1572-0241.2003.07384.x. [DOI] [PubMed] [Google Scholar]
- 16.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. 2004. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol 5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 17.Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. 2000. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 47:79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada N, Maeda K, Inoue N, Hisamatsu T, Okamoto S, Hong KS, Yamada T, Watanabe N, Tsuchimoto K, Ogata H, Hibi T. 2008. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect Immun 76:214–220. doi: 10.1128/IAI.01193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammers KM, Helwig U, Swennen E, Rizzello F, Venturi A, Caramelli E, Kamm MA, Brigidi P, Gionchetti P, Campieri M. 2002. Effect of probiotic strains on interleukin 8 production by HT29/19A cells. Am J Gastroenterol 97:1182–1186. doi: 10.1111/j.1572-0241.2002.05693.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Medina M, Aldeguer X, Lopez-Siles M, González-Huix F, López-Oliu C, Dahbi G, Blanco JE, Blanco J, Garcia-Gil LJ, Darfeuille-Michaud A. 2009. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflamm Bowel Dis 15:872–882. doi: 10.1002/ibd.20860. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Gerstein M, Snyder M. 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon SH, Han MJ, Jeong H, Lee CH, Xia XX, Lee DH, Shim JH, Lee SY, Oh TK, Kim JF. 2012. Comparative multi-omics systems analysis of Escherichia coli strains B and K-12. Genome Biol 13:R37. doi: 10.1186/gb-2012-13-5-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon S, et al. 2009. Genomics, biological features, and biotechnological applications of Escherichia coli B: “is B for better?!,” p 1–17. In Lee S (ed), Systems biology and biotechnology of Escherichia coli. Springer, Amsterdam, Netherlands. [Google Scholar]
- 24.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baruch M, Belotserkovsky I, Hertzog BB, Ravins M, Dov E, McIver KS, Le Breton YS, Zhou Y, Cheng CY, Chen CY, Hanski E. 2014. An extracellular bacterial pathogen modulates host metabolism to regulate its own sensing and proliferation. Cell 156:97–108. doi: 10.1016/j.cell.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendall MM, Gruber CC, Parker CT, Sperandio V. 2012. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. mBio 3:e00050-12. doi: 10.1128/mBio.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. BioInformatics 25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol 11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarca AL, Draghici S, Khatri P, Hassan SS, Mittal P, Kim JS, Kim CJ, Kusanovic JP, Romero R. 2009. A novel signaling pathway impact analysis. Bioinformatics 25:75–82. doi: 10.1093/bioinformatics/btn577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swamy M, Jamora C, Havran W, Hayday A. 2010. Epithelial decision makers: in search of the epimmunome. Nat Immunol 11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. 2001. Decreased expression of toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 33.Naik S, Kelly EJ, Meijer L, Pettersson S, Sanderson IR. 2001. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J Pediatr Gastroenterol Nutr 32:449–453. doi: 10.1097/00005176-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki M, Hisamatsu T, Podolsky DK. 2003. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun 71:3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, Park JE, Park HY, Kwack K, Choi BK, Kwon BS, Oh GT. 2010. CD137 (4–1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation 121:1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 36.Liew FY, Xu D, Brint EK, O’Neill LA. 2005. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol 5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 37.Perkins ND. 2007. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 38.Ma A, Malynn BA. 2012. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12:774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vereecke L, Beyaert R, van Loo G. 2009. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol 30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Hausmann E, Raisz LG, Miller WA. 1970. Endotoxin: stimulation of bone resorption in tissue culture. Science 168:862–864. doi: 10.1126/science.168.3933.862. [DOI] [PubMed] [Google Scholar]
- 41.Ishihara Y, Nishihara T, Maki E, Noguchi T, Koga T. 1991. Role of interleukin-1 and prostaglandin in in vitro bone resorption induced by Actinobacillus actinomycetemcomitans lipopolysaccharide. J Periodontal Res 26:155–160. doi: 10.1111/j.1600-0765.1991.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 42.Angrisano T, Pero R, Peluso S, Keller S, Sacchetti S, Bruni CB, Chiariotti L, Lembo F. 2010. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol 10:172. doi: 10.1186/1471-2180-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A 90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 45.Studier FW, Studier FW, Daegelen P, Lenski RE, Maslov S, Kim JF. 2009. Understanding the differences between genome sequences of Escherichia coli B strains REL606 and BL21(DE3) and comparison of the E. coli B and K-12 genomes. J Mol Biol 394:653-680. doi: 10.1016/j.jmb.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Quan DN, Bentley WE. 2012. Gene network homology in prokaryotes using a similarity search approach: queries of quorum sensing signal transduction. PLoS Comput Biol 8:e1002637. doi: 10.1371/journal.pcbi.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira CS, de Regt AK, Brito PH, Miller ST, Xavier KB. 2009. Identification of functional LsrB-like autoinducer-2 receptors. J Bacteriol 191:6975–6987. doi: 10.1128/JB.00976-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Wang L, Hashimoto Y, Tsao CY, Wood TK, Valdes JJ, Zafiriou E, Bentley WE. 2006. A stochastic model of Escherichia coli AI-2 quorum signal circuit reveals alternative synthesis pathways. Mol Syst Biol 2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng WL, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayaraman A, Wood TK. 2008. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng 10:145–167. doi: 10.1146/annurev.bioeng.10.061807.160536. [DOI] [PubMed] [Google Scholar]
- 51.Ahmer BM. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol Microbiol 52:933–945. doi: 10.1111/j.1365-2958.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 52.Walters M, Sperandio V. 2006. Quorum sensing in Escherichia coli and salmonella. Int J Med Microbiol 296:125–131. doi: 10.1016/j.ijmm.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 53.Mota LJ, Cornelis GR. 2005. The bacterial injection kit: type III secretion systems. Ann Med 37:234–249. doi: 10.1080/07853890510037329. [DOI] [PubMed] [Google Scholar]
- 54.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun 71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shiner EK, Terentyev D, Bryan A, Sennoune S, Martinez-Zaguilan R, Li G, Gyorke S, Williams SC, Rumbaugh KP. 2006. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell Microbiol 8:1601–1610. doi: 10.1111/j.1462-5822.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 56.Bryan A, Watters C, Koenig L, Youn E, Olmos A, Li G, Williams SC, Rumbaugh KP. 2010. Human transcriptome analysis reveals a potential role for active transport in the metabolism of Pseudomonas aeruginosa autoinducers. Microbes Infect 12:1042–1050. doi: 10.1016/j.micinf.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karlin DA, Mastromarino AJ, Jones RD, Stroehlein JR, Lorentz O. 1985. Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J Cancer Res Clin Oncol 109:135–141. doi: 10.1007/BF00391888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuccato E, Venturi M, Di Leo G, Colombo L, Bertolo C, Doldi SB, Mussini E. 1993. Role of bile acids and metabolic activity of colonic bacteria in increased risk of colon cancer after cholecystectomy. Dig Dis Sci 38:514–519. doi: 10.1007/BF01316508. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J, Pei D. 2008. A LuxP-based fluorescent sensor for bacterial autoinducer II. ACS Chem Biol 3:110–119. doi: 10.1021/cb7002048. [DOI] [PubMed] [Google Scholar]
- 60.Charlton TS, de Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2:530–541. doi: 10.1046/j.1462-2920.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- 61.Bassler BL, Greenberg EP, Stevens AM. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol 179:4043–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qPCR validation of RNA-Seq results show high correlation for a range of expression levels. Download
Significantly differentially expressed genes and expression levels from DESeq are input into SPIA (signaling pathway impact analysis) to determine activation and inhibition of entire biological pathways. Download
Multianalyte ELISAs were performed on cell culture supernatants of HCT-8 cells in transwell coincubations with BL21, W3110, and medium alone. Download
AI-2 concentration levels in the HCT-8 supernatant from incubations with BL21 and W3110. Download
HCT-8 cells were incubated with AI-2 at 50, 150, and 400 µM for 6, 12, and 24 h. Download
Expression level differences between incubations with BL21 and medium alone determined by DESeq software.
Expression level differences between incubations with W3110 and medium alone determined by DESeq software.
Expression level differences between incubations with W3110 and BL21 determined by DESeq software.
Primers used for SYBR green qPCR.