ABSTRACT
The capacity of subinhibitory levels of antibiotics to modulate bacterial virulence in vitro has recently been brought to light, raising concerns over the appropriateness of low-dose therapies, including antibiotic prophylaxis for recurrent urinary tract infection management. However, the mechanisms involved and their relevance in influencing pathogenesis have not been investigated. We characterized the ability of antibiotics to modulate virulence in the uropathogens Staphylococcus saprophyticus and Escherichia coli. Several antibiotics were able to induce the expression of adhesins critical to urothelial colonization, resulting in increased biofilm formation, colonization of murine bladders and kidneys, and promotion of intracellular niche formation. Mice receiving subinhibitory ciprofloxacin treatment were also more susceptible to severe infections and frequent recurrences. A ciprofloxacin prophylaxis model revealed this strategy to be ineffective in reducing recurrences and worsened infection by creating larger intracellular reservoirs at higher frequencies. Our study indicates that certain agents used for antibiotic prophylaxis have the potential to complicate infections.
IMPORTANCE
Antibiotics are the mainstay treatment for bacterial infections; however, evidence is emerging that argues these agents may have off-target effects if sublethal concentrations are present. Most studies have focused on changes occurring in vitro, leaving questions regarding the clinical relevance in vivo. We utilized a murine urinary tract infection model to explore the potential impact of low-dose antibiotics on pathogenesis. Using this model, we showed that subinhibitory antibiotics prime uropathogens for adherence and invasion of murine urothelial tissues. These changes in initial colonization promoted the establishment of chronic infection. Furthermore, treatment of chronically infected mice with subtherapeutic ciprofloxacin served to exacerbate infection. A part of these changes was thought to be due to suppression of mucosal immunity, as demonstrated through reductions in cytokine secretion and migration of leukocytes into the urinary tract. This work identifies novel risk factors associated with antibiotic therapy when dosing strategies fall below subtherapeutic levels.
INTRODUCTION
The use of antibiotics to treat and prevent bacterial infections has made an unprecedented impact on improving human health. However, recent studies demonstrating the capacity of antibiotics to modify bacterial phenotypes at levels below the MIC are challenging the paradigm that their effects are benign and have raised the possibility that they may actually enhance bacterial virulence. Transcriptional analyses suggest that subinhibitory levels of antibiotics induce the differential expression of as many as 5 to 10% of the genome, modulated through the activities of both general stress responses and specific signaling systems (1–5), including the enhancement of toxin and biofilm production by potential human pathogens (3, 6–14). In addition, antibiotics, including macrolides and fluoroquinolones, are now recognized for their capacity to suppress proinflammatory host responses (15, 16). Thus, we hypothesize that modulation of gene expression in both the host and the pathogen during prophylactic treatment with a low dose of an antibiotic could alter the course of bacterial pathogenesis. Clinical studies have suggested that subinhibitory antibiotic therapies and dosing strategies are risk factors for severe complication of infection, risk of breakthrough infection, and future recurrence episodes (17–21). Although the etiology of therapy failure in many of these cases remains unknown, we propose that inappropriate antibiotic treatment may be a contributing factor. The objective of our study was to explore this phenomenon in the context of a subinhibitory dosing regimen, namely, antibiotic prophylactic management for recurrent urinary tract infection (rUTI).
UTIs are very common, accounting for 10.5 million outpatient and emergency room visits during 2007 in the United States alone (22). They are a significant cause of morbidity in women throughout their life span and in infant boys and older men, with serious sequelae, including treatment failure, frequent recurrences, pyelonephritis with sepsis, and renal damage in young children (23, 24); however, upwards of 44% of women will experience a recurrence, with ~10% of these caused by a relapse of the original index episode strain (25). Such recurrent or persistent infections are generally managed by using long-term, low-dose prophylactic regimens. In general, UTIs can be readily treated with short-course antibiotic therapy (~3 to 7 days). However, following therapy, recurrent or persistent infections may develop that are often managed using long-term, low-dose prophylactic regimens. While prophylaxis is usually effective in reducing symptoms during its application (26), it does not alter the long-term risk of recurrence, as infection rates return to pretreatment levels following therapy (27) and pathogens become more resistant to future treatment (28). Thus, it is critical to understand the effectiveness of antimicrobial prophylaxis in managing recurrent infections and whether the benefits of such treatments outweigh the risks of promoting resistance and potential increased virulence.
Uropathogenic Escherichia coli (UPEC) and Staphylococcus saprophyticus collectively cause ~95% of all uncomplicated UTIs (23, 29) and are often associated with recurrent and chronic disease necessitating long-term prophylaxis management. Type 1 fimbriae and uro-adherence factors (Uafs) are critical in establishing UPEC and S. saprophyticus UTIs, respectively (30, 31). In particular, type 1 fimbriae are important in promoting the invasion of superficial umbrella cells on the luminal surface of the bladder epithelium and formation of intracellular bacterial communities, whereby UPEC rapidly replicates within the cytoplasm during acute infection to establish a foothold in the bladder (32–37). While the fate of intracellular S. saprophyticus is unknown, intracellular UPEC proliferation results in the formation of large, multicellular aggregates, dubbed intracellular bacterial communities (IBCs) (32). Within this niche, uropathogens are able to subvert host immune cells and rapidly build up in numbers, thus facilitating the progression toward chronic infection in mice. In addition, UPEC has been shown to be able to survive antibiotic therapy by forming dormant quiescent intracellular reservoirs (QIRs) that can serve as seeds for recurrent infection (38–41).
We hypothesized that subinhibitory antibiotic-dependent virulence modulation and immune suppression in vitro would translate to actual changes in disease outcome in vivo. Thus, we investigated various classes of subinhibitory antibiotics for their capacity to influence bacterial virulence traits in two uropathogenic clinical isolates, S. saprophyticus 15305 and E. coli UTI89. We also attempted to characterize the signaling networks involved with these responses in UPEC by using expression analyses. Changes in virulence and pathogenesis were assessed in vivo by inoculating ciprofloxacin-primed mice (who had received 1/4 the minimal inhibitory dose of ciprofloxacin) with S. saprophyticus or UPEC and determining acute and chronic infection characteristics, including bacterial persistence within the urinary tract and establishment of intracellular bacterial reservoirs. The effect of inadequate (with 1/50 the empirical therapeutic dose) ciprofloxacin dosing in chronically infected or resolved mice was also investigated, in addition to characterizing immune responses in this host. Lastly, the efficacy of prophylaxis was assessed for its ability to prevent persistent UTIs and clear UPEC from host urine and tissues in addition to the intracellular niche.
RESULTS
Subinhibitory antibiotics induce adhesin expression and biofilm formation in uropathogens.
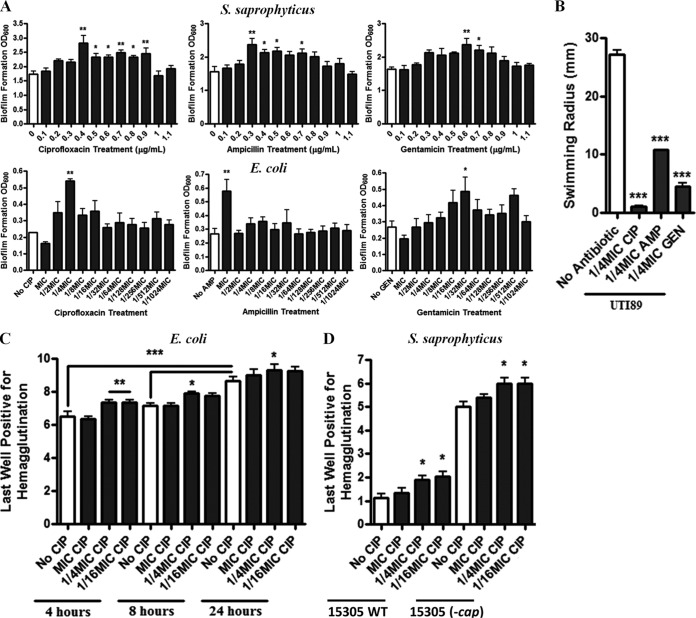
Challenge of both S. saprophyticus and UPEC with subinhibitory ciprofloxacin, ampicillin, or gentamicin resulted in denser biofilm production (Fig. 1A). In addition, enhanced planktonic aggregation was observed with both organisms (see Fig. S1 in the supplemental material). For UPEC, motility was suppressed (Fig. 1B), as determined in a soft agar motility assay, and type 1 fimbriation was increased, as determined via a guinea pig erythrocyte hemagglutination assay (Fig. 1C). Similarly, subinhibitory antibiotics increased sheep erythrocyte hemagglutination with S. saprophyticus, suggesting increased exposure or the presence of adhesin uro-adherence factor A (UafA) (Fig. 1D). Since capsular polysaccharide can interfere with UafA-ligand interactions, hemagglutination was also evaluated in an acapsular strain to discern whether our observations were due to increased UafA expression or decreased capsular polysaccharide abundance. We found that subinhibitory antibiotics also increased hemagglutination in the acapsular strain, indicative of increased UafA surface exposure (Fig. 1D).
FIG 1 .
Subinhibitory antibiotics prime uropathogens for colonization. (A) S. saprophyticus 15305 and E. coli UTI89 biofilm formation in the presence of various antibiotic concentrations at 24 h. (B) Swimming motility of E. coli UTI89 in the presence of subinhibitory antibiotic concentrations. (C) Type 1 fimbria-dependent hemagglutination of E. coli UTI89 exposed to various subinhibitory concentrations of ciprofloxacin over time. (D) UafA-dependent hemagglutination of S. saprophyticus 15305 and the acapsular C1 strain following exposure to various concentrations of ciprofloxacin. Means from at least three independent experiments are shown. Significance was determined using a one-way ANOVA and Dunnett’s or Bonferroni’s multiple comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Antibiotic-induced adhesin expression is SOS dependent in UPEC.
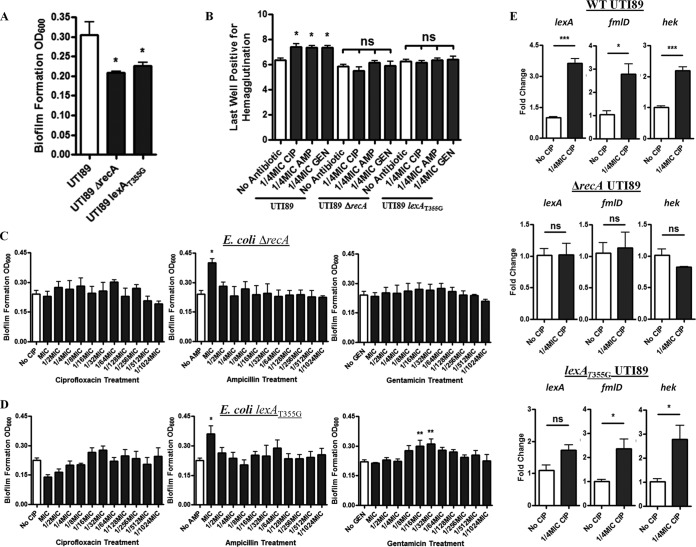
RNA sequencing analysis confirmed significant induction of both a fimH-like adhesin, fmlD (4.84-fold), and the suspected uropathogenic adhesin/invasin hek (2.47-fold) (Table 1). Interestingly, changes in type 1 fimbrial transcripts were not observed via RNA sequencing, nor were any changes observed in the proportion of the population that had the invertible fim promoter in the ON orientation (data not shown). As the SOS-responsive elements lexA and recA were also highly induced with treatment (4.08- and 3.37-fold, respectively), we assessed their contributions to adhesin expression and biofilm formation by using SOS-deficient E. coli UTI89 strains. These strains demonstrated a modest suppression in biofilm formation (Fig. 2A) and type 1 fimbria-dependent hemagglutination (Fig. 2B) and did not respond to subinhibitory ciprofloxacin or gentamicin treatment (Fig. 2C and D). Expression analysis using quantitative PCR (qPCR) confirmed fmlD and hek induction with ciprofloxacin challenge in wild-type UPEC; however, transcription was not altered in the recA-deficient strain (Fig. 2E). Ampicillin increased biofilm mass in all strains tested, but only at concentrations near the MIC (Fig. 2C and D).
TABLE 1 .
Subinhibitory ciprofloxacin induces urothelium adhesin gene expressiona
| Gene | Fold change (log2) | Median effect size | Functional annotation |
|---|---|---|---|
| fmlD | 2.275 | 1.891 | Fimbrial, FimH-like protein, mannose binding |
| Hek | 1.304 | 3.475 | Hek adhesin/virulence factor |
| lexA | 2.03 | 5.635 | Repressor of LexA |
| recA | 1.751 | 3.75 | DNA repair, SOS induction |
Significant changes in differentially expressed transcripts of interest in E. coli UTI89 after 4-h exposure to 1/4 MIC ciprofloxacin.
FIG 2 .
SOS activation is essential for antibiotic-primed adhesion. (A) Biofilm formation of E. coli UTI89 and SOS-deficient strains after 24 h. (B) Type 1 fimbria-dependent hemagglutination of wild-type and SOS-deficient strains of E. coli UTI89. (C and D) Biofilm formation of E. coli SOS-deficient strains ΔrecA (C) and lexAT355G (D) after 24 h in the presence of various antibiotics at subinhibitory concentrations. (E) Quantitative real-time PCR analysis of wild-type and SOS-deficient strains of E. coli UTI89; the reported data are the fold changes in expression of lexA, hek, and fmlD after 4 h of exposure to 1/4 MIC ciprofloxacin. Means from at least three independent experiments are shown. Significance was determined using a one-way ANOVA and Dunnett’s multiple comparison test. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Subinhibitory antibiotics prime uropathogens for urothelial colonization.
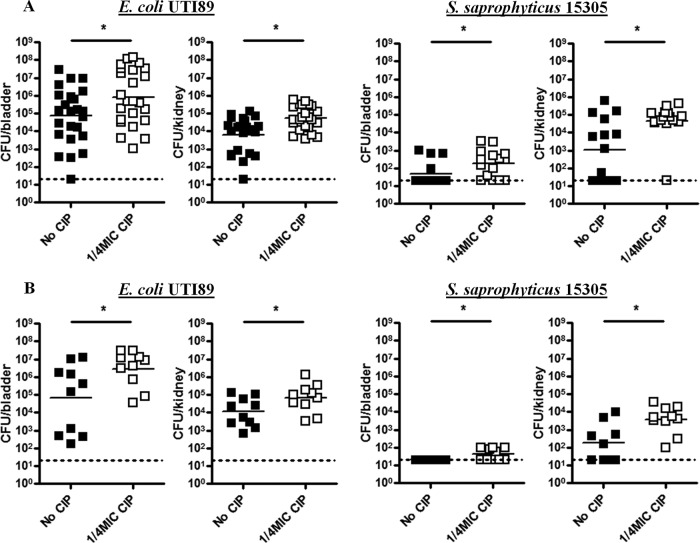
Ciprofloxacin priming prior to infection resulted in higher overall bladder and kidney titers for both S. saprophyticus 15305 and E. coli UTI89 at 24 h postinfection (p.i.) (Fig. 3A). Almost all murine kidneys inoculated with primed uropathogens lacked signs of bacterial clearance. Ciprofloxacin priming also resulted in increased risk of severe UTI, manifesting as persistent bacteriuria, high bacterial bladder titer, and chronic inflammation, an outcome referred to as chronic cystitis, at 14 days p.i. (Fig. 3B). Chronic cystitis represents unchecked luminal bacterial replication and is defined histologically by urothelial hyperplasia and submucosal lymphoid aggregates, a histological pattern similar to that seen in humans experiencing chronic UTI (33, 42, 43). The bladder colonization threshold for assessing the risk of chronic cystitis is the presence of E. coli UTI89 loads of >104 CFU/ml at 14 days p.i. or later (such data do not exist for S. saprophyticus) (44). Considering this threshold, priming resulted in 100% of mice presenting with a high risk of chronic E. coli UTI89 cystitis. Although there are no established infection thresholds for S. saprophyticus 15305, it is noteworthy that 100% of mice inoculated with untreated pathogen completely resolved their infection, while 50% maintained low, but measurable, bladder titers with ciprofloxacin priming. Among primed S. saprophyticus 15305-treated mice, 100% maintained at least some level of infectious kidney titer, while in control groups only 60% exhibited the same result.
FIG 3 .
Antibiotic priming improves uropathogen colonization. S. saprophyticus 15305 and E. coli UTI89 titers in C3H/HeN mouse bladders and kidneys at 24 h (A) or 14 days (B) postinfection. The dotted line indicates the limit of detection. Means from two independent experiments are shown. Significance was determined using the Mann-Whitney test (Gaussian approximation). *, P < 0.05.
Antibiotic-driven colonization promotes tissue invasion and IBC formation.
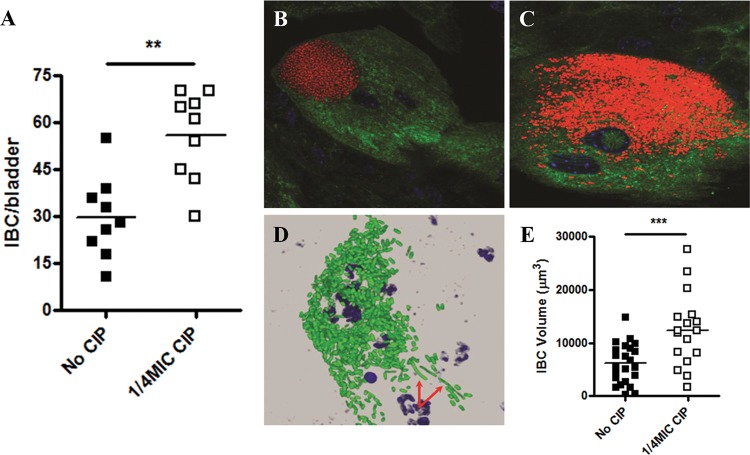
Changes in IBC formation during the early stages of infection were assessed for E. coli UTI89 by staining for lacZ expression using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). This technique indicated significantly more IBCs in ciprofloxacin-primed groups, indicative of more numerous urothelial invasion events (Fig. 4A). IBC morphology was further explored using a green fluorescent protein (GFP)-expressing E. coli UTI89 strain and confocal microscopy. Appearance of untreated E. coli UTI89 was unremarkable, as IBCs demonstrated characteristic tight, globular clustering (Fig. 4B). However, examination of ciprofloxacin-primed E. coli UTI89 revealed larger, diffuse IBCs with atypical morphology (Fig. 4C). Early filamentation was also noted in several of the IBCs belonging to the ciprofloxacin-treated E. coli UTI89 group (Fig. 4D). Volumetric analysis of the IBCs confirmed that ciprofloxacin priming significantly increased overall size 2.0-fold (Fig. 4E). The ability for ciprofloxacin to induce filamentation was investigated further, as this process might contribute to observed early IBC evacuation, which has been shown to lead to spreading of the infection to neighboring cells (45). Cultures of E. coli UTI89 were treated with subinhibitory ciprofloxacin and imaged using transmission electron microscopy (TEM). Control E. coli UTI89 cells were unremarkable, appearing to be ~1 to 2 µm in length (see Fig. S2A in the supplemental material). Most cells in the ciprofloxacin treatment group appeared similar in nature; however, ~10% of the overall population presented with the observed filamenting phenotype, sometimes increasing to >10 µm in length (see Fig. S2B).
FIG 4 .
Antibiotic priming increases the invasive capacity of UPEC. (A) E. coli UTI89 IBC quantity at 6 h p.i. of C3H/HeN bladders. (B and C) Representative confocal images of control (B) and antibiotic-pretreated (C) E. coli UTI89 IBCs in C3H/HeN mouse bladders 6 h p.i. (red, GFP/UTI89; blue, Syto9/nuclei; green, WGA/cell). (D) Early pathogen evacuation from an IBC via filamentation (arrows) in a ciprofloxacin-pretreated sample. (E) Volumetric analysis of confocal images is depicted for both control and antibiotic-pretreated IBCs. Means from at least two independent experiments are shown. Significance was determined using a one-way ANOVA, Dunnett’s multiple comparison test, or the Mann-Whitney test. *, P < 0.05; **, P < 0.01.
Subtherapeutic ciprofloxacin dosing induces recurrence and severe infection in mice.
The effects of oral subinhibitory ciprofloxacin therapy were considered in two groups of mice that had been previously inoculated with E. coli UTI89 and had either (i) naturally resolved their infection (described as a <104 CFU/ml bacterial urine load >30 days p.i.) or (ii) maintained chronic urine titers (described as >104 CFU/ml for >30 days p.i.). Prior to the initiation of oral antibiotic therapy, the urine titers for all mice were measured over 3 days in the absence of antibiotics to determine the baseline bacterial burden. Supplementation of ciprofloxacin into drinking water at 1/50 the empirical therapeutic concentration caused 80% of previously resolved mice to develop significant infection (>104 CFU/ml) after 3 to 6 days (Fig. 5A). Although 1/25 the empirical therapeutic dose had no effect, 1/10 the empirical therapeutic dose resulted in retraction of bacterial urine loads to the limit of detection, suggesting that the observed antibiotic-dependent increases were not due to the appearance of resistant mutants (data not shown). In chronically infected mice, supplementation with 1/50 the empirical therapeutic dose for 3 days significantly increased mean UPEC urine titers by 26-fold (Fig. 5B). Again, treatment with 1/10 the therapeutic dose was sufficient to decrease bacteriuria to the limit of detection (data not shown).
FIG 5 .
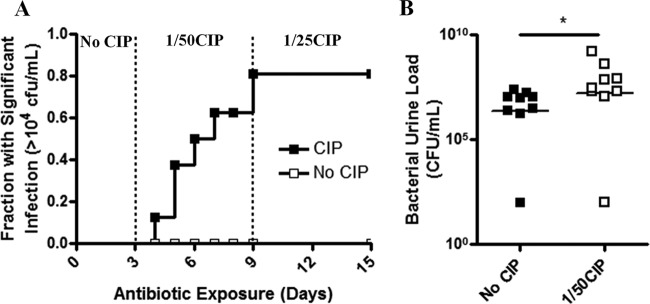
Subtherapeutic ciprofloxacin increases bacterial burden and recurrence frequency. (A) The fraction of resolved C3H/HeN mice presenting with clinically significant (>104 CFU/ml) bacterial urine titers following subtherapeutic ciprofloxacin dosing over time (n = 10 mice). The antibiotic dosing period is indicated on the upper x axis as fractions of the therapeutic dose. (B) Urine titers of chronically infected mice receiving either no antibiotic or subtherapeutic ciprofloxacin for 3 days. Means from at least two independent experiments are shown. Significance was determined using a log rank test (panel A; P = 0.0131) or Mann-Whitney test (panel B; P = 0.0142).
Ciprofloxacin modulates human and murine mucosal immune responses.
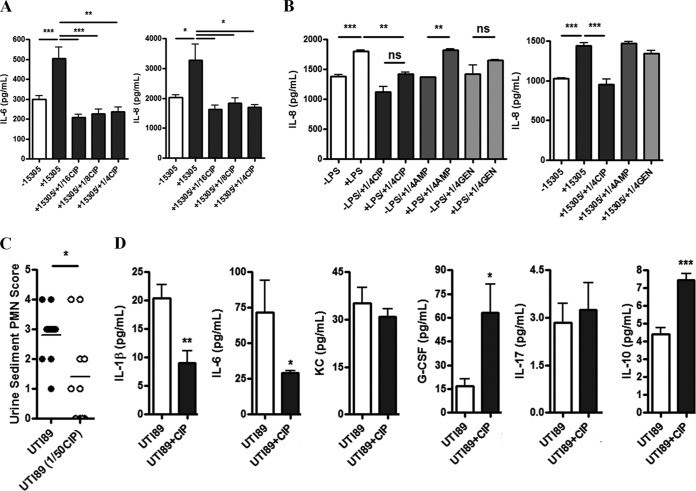
Luminex profiling demonstrated induction of the cytokines interleukin-6 (IL-6) and IL-8 in T24 bladder cells infected with S. saprophyticus 15305, but this induction was suppressed to below baseline levels with the application of subinhibitory ciprofloxacin (Fig. 6A). IL-8 secretion in human 5637 bladder cells treated with S. saprophyticus 15305 or lipopolysaccharide (LPS) was also reduced by ciprofloxacin, slightly by gentamicin, but not affected by ampicillin, as determined in an enzyme-linked immunosorbent assay (ELISA) (Fig. 6B). Thus, we hypothesized that these observed immunomodulatory effects suppress the secretion of proinflammatory mediators and decrease immune cell infiltrate in vivo. Urine sediment polymorpohonuclear leukocytes (PMNs) taken 3.5 h after UPEC inoculation was reduced in mice receiving subtherapeutic ciprofloxacin (Fig. 6C). Ciprofloxacin-dependent modulation of cytokine secretion was assessed using a Luminex bead-based multiplex assay of extracted bladder tissue homogenates. A total of seven urinary-relevant cytokines were assessed and included IL-1β, IL-6, keratinocyte chemoattractant (KC, also called IL-8), granulocyte colony-stimulating factor (G-CSF), IL-17, IL-10, and tumor necrosis factor alpha (TNF-α) (46). All produced reliable profiles with the exception of TNF-α (data not shown). Changes in IL-17 secretion were unremarkable in either the control or ciprofloxacin treatment groups (Fig. 6D). Similarly, KC was not significantly impacted by ciprofloxacin, although there was a trend of reduced secretion when antibiotic was present (Fig. 6D). The proinflammatory mediators IL-1β and IL-6 were suppressed, while secretion of the anti-inflammatory cytokine IL-10 was increased (Fig. 6D). In contrast, the proinflammatory cytokine G-CSF was significantly increased in the presence of ciprofloxacin and E. coli UTI89 infection (Fig. 6D).
FIG 6 .
Ciprofloxacin suppresses urinary cytokine secretion and PMN infiltration. (A) IL-6 and IL-8 cytokine expression profiles for T24 bladder cells infected with S. saprophyticus 15305 exposed to various subinhibitory levels of ciprofloxacin. (B) Release of IL-8 from 5637 bladder cells following treatment with either LPS or S. saprophyticus 15305 in the presence of 1/4 MIC of either ciprofloxacin, ampicillin, or gentamicin, as indicated. (C) PMN counts collected from murine urine sediments 3.5 h after E. coli UTI89 inoculation. Mice were treated with or without 1/50 the subtherapeutic ciprofloxacin concentration in their drinking water. (D) Murine bladder cytokine secretion 3.5 h p.i. with E. coli UTI89 in the presence or absence of 1/50 the subtherapeutic ciprofloxacin concentration in drinking water. Means from at least two independent experiments are shown. Significance was determined using a one-way ANOVA and Bonferroni’s multiple comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Prophylactic intervention is ineffective in curtailing recurrence and increases intracellular UPEC burden.
The possibility that low-dose prophylaxis promotes virulence and enhances pathogenesis was investigated in the C57BL/6J model of rUTI, where frequent recurrent infections originate from QIRs (33, 39, 47). Following inoculation with nonprimed UPEC, changes in bacterial urine load were assessed at least every 2 days to track the course of infection using a novel prophylaxis therapy model. Establishment of infection after 24 h and response to trimethoprim-sulfamethoxazole (“co-trimoxazole”) intervention were observed (see Fig. S3 in the supplemental material). The following 7-day antibiotic-free period revealed dynamic changes in E. coli UTI89 urine titers, with frequent recurrences observed. Initiation of prophylactic therapy did not appear to improve the frequency of infection resolution over the 7-day treatment period, as 2/20 mice in this group presented with clinically significant bacteriuria while 3/20 in the untreated group did so at the study’s end (Fig. 7A). Both bladder and kidneys demonstrated greater bacterial loads in the prophylaxis group, although this difference was not significant (Fig. 7B). However, comparison of bladder titers revealed prophylaxis significantly increased the presence of low-level bladder CFU as determined via ex vivo gentamicin protection; this was likely indicative of more numerous QIRs (Fig. 7C).
FIG 7 .
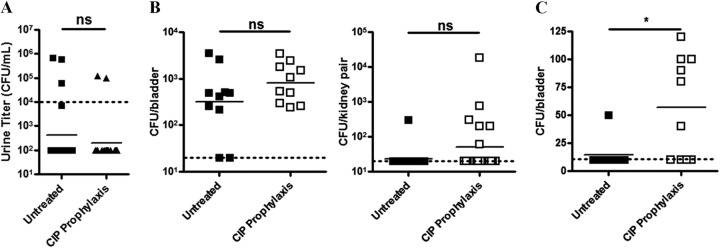
Low-dose ciprofloxacin therapy increases intracellular UPEC reservoirs. (A and B) E. coli UTI89 titers in C57BL/6 mouse urine (A) or bladders and kidneys (B) following a 1-week prophylactic treatment regimen with either ciprofloxacin or vehicle. (C) Gentamicin protection assays indicated the presence of intracellular E. coli reservoirs. The dotted line indicates the limit of detection. Means from at least two independent experiments are shown. Significance was determined using an unpaired, two-tailed t test. ns, not significant; *, P = 0.0148.
DISCUSSION
Subinhibitory antibiotics promote SOS-dependent virulence factor expression.
We have demonstrated that subinhibitory antibiotics enhance adherence and biofilm production in uropathogens. However, unlike previous studies, which focused on single pathogen-antibiotic combinations, we found that several agents targeting distinct cellular processes were capable of similar effects in unrelated pathogens and that these changes translated to enhanced virulence in vivo. Thus, in addition to increasing the risk of resistant infection, subinhibitory antibiotic therapy may also pose an additional risk of promoting the expression of virulence factors. We further characterized the nature of adherence and determined that adhesins critical to uropathogenesis were induced in both S. saprophyticus 15305 and E. coli UTI89 by subinhibitory concentrations of antibiotics. RNA sequencing of subinhibitory ciprofloxacin-treated UPEC revealed that fmlD and hek induction coincided with SOS activation. Further phenotypic and qPCR investigations of strains deficient in SOS revealed the importance of this stress response system to adhesin gene expression and enhanced adherence, as deficient strains no longer responded to treatment. This finding provides a general mechanism by which diverse classes of antibiotics might enhance virulence via a system essentially ubiquitous across bacterial species. Investigations further characterizing the poorly established role of the SOS in virulence regulation are warranted (48).
Subinhibitory ciprofloxacin priming enhances pathogenesis in a murine model of UTI.
Previous results have demonstrated that antibiotics are capable of priming organisms for downstream colonization, primarily through the upregulation of adhesins (49–51). In mice, ciprofloxacin treatment prior to infection shifted the equilibrium toward infection persistence in bladders and kidneys. This effect was most dramatic in the kidneys, where both uropathogens showed no signs of clearance. The results imply that infection severity could be worsened by the application of ciprofloxacin prophylactically, a practice that is widely conducted (52–54). S. saprophyticus 15305 poorly colonized the bladders of mice but showed a similar infective capacity as E. coli UTI89 in the kidneys. The absence of UafA ligand in the mouse bladder likely accounts for this (55–57). C3H/HeN mice inoculated with 107 CFU of E. coli UTI89 and then presenting with >104 CFU/ml at 14 days p.i. are known to be at very high risk of chronic UTI (44). The increased titers observed following ciprofloxacin priming suggested that these organisms were better equipped to colonize the host than were the untreated counterparts. The host-pathogen interactions during the first 24 h p.i. are thought to play a significant role in infection establishment or resolution, suggesting that increased titers at this time have some predictive value in determining the severity of UTI (33). The data obtained at 14 days p.i. with E. coli UTI89 validated this finding, as ciprofloxacin priming prior to inoculation was sufficient to increase the frequency of chronic cystitis to 100%, compared to 60% in untreated groups. While no such data exist that describe the infection thresholds of S. saprophyticus 15305, it is noteworthy that 100% of mice resolved infection when inoculated with untreated S. saprophyticus 15305, while 50% of ciprofloxacin-primed inoculated mice maintained low-grade bladder colonization. Although life-long infection carriage needs to be evaluated in this model, the data are highly suggestive that organism virulence is enhanced by the presence of subinhibitory antibiotic levels, such that there is a direct impact on persistence within host tissues.
The capacity for ciprofloxacin to promote chronic UPEC infections is likely dependent on its ability to induce invasion of urothelial cells. Type 1 fimbriae are known to be critical in internalization and IBC formation (58–60); their expression in response to ciprofloxacin and subsequent chronic infection development is likely paramount. Although changes in type 1 fimbria transcript abundance was not observed, posttranscriptional modification or capsule downregulation and enhanced FimH exposure might contribute to the observed changes in antibiotic-induced hemagglutination (61, 62). Increased numbers of IBCs are associated with an increased risk of chronic cystitis (36). The enhanced IBC-forming capacity observed in ciprofloxacin-primed E. coli UTI89 indicated that establishment of an intracellular niche is an important step in developing chronic infection in the C3H/HeN model of chronic cystitis. Ciprofloxacin also altered the morphology of IBCs, resulting in a dispersed phenotype compared to that of untreated controls. The characteristics of these IBCs, such as increased numbers of bacterial filaments, are reminiscent of those observed during later stages of infection (63), suggesting that ciprofloxacin priming alters IBC development, resulting in an earlier dispersal and fluxing of the bacteria from the IBC biomass and their spread to neighboring cells. Ciprofloxacin-triggered filamentation could thus hasten the spread to distal tissues before exfoliation can occur, promoting the establishment of quiescent intracellular reservoirs in the bladder that can serve as seeds for recurrent UTI (39). Thus, ciprofloxacin-induced changes in E. coli UTI89 might contribute to increased urothelial adherence, immune evasion, and invasion potential (45, 64). These processes might further help uropathogens subvert aspects of early host immunity by rapidly gaining access to the intracellular environment, and at a higher frequency. Combined, alterations in these pathogenic mechanisms are likely responsible for driving the dynamics of the host-pathogen equilibrium in favor of bacterial persistence.
Subtherapeutic ciprofloxacin augments infection severity and recurrence risk in chronically infected and resolved mice.
Two experimental groups were evaluated for the influence of subtherapeutic ciprofloxacin treatment on (i) the worsening of prognosis in chronically infected mice and (ii) the predisposition of previously inoculated but resolved mice for clinically significant recurrences. In both cases, subtherapeutic ciprofloxacin was associated with increased E. coli UTI89 urine titers. The effects of subtherapeutic ciprofloxacin dosing on modulating cytokine expression could, in part, account for increased pathogen urine load in chronically infected mice (discussed below). However, the ability of ciprofloxacin to trigger clinically significant recurrences in resolved mice was unexpected and might occur through reemergence of E. coli from QIRs. As fluoroquinolones inhibit phosphodiesterase activity in mammalian cells (65, 66), a resulting accumulation of cyclic AMP has been proposed to trigger exocytosis of intracellular UPEC (67). Although chronically infected mice were housed in separate cages from resolved mice, cross-infection between mice experiencing recurrences within the same cage cannot be ruled out. Interestingly, there were mice that never experienced recurrences with ciprofloxacin therapy, indicating urinary clearance and providing an argument against cross-infection. These findings are the first to directly associate subtherapeutic antibiotic therapy with increased infection risk in vivo. If translatable to the clinic, noncompliance with antibiotic prescriptions, or failure to follow strict intraoperative redosing guidelines, could contribute to infection complications (19, 20).
Ciprofloxacin modulates aspects of host immunity.
Ciprofloxacin was found to have an immunomodulatory effect in urothelial tissues, which might account for the changes in bacterial urine titers observed in mice during subtherapeutic treatment. The effect was noted in both human bladder epithelial cell lines in addition to murine bladder tissues extracted following infection. Low doses of ciprofloxacin were sufficient to depress the release of IL-6 and IL-8 in T24 and 5637 bladder cells, suggesting that residual levels left over following therapy might predispose to infection later on. IL-6 and IL-8 are both important proinflammatory mediators for host cell immunity during UTIs, and suppression of either cytokine has important implications on neutrophil chemotaxis to sites of infection (68–70). This was confirmed in mice, where there were significantly depressed PMN infiltrates in urine sediments when animals were treated with subtherapeutic ciprofloxacin. In addition to IL-6 and IL-8 suppression in vivo, the proinflammatory mediator IL-1β, produced by activated macrophages, is an important early response element to UPEC infection, and this cytokine was suppressed with ciprofloxacin treatment. Alternatively, the anti-inflammatory cytokine IL-10 was upregulated with infection and ciprofloxacin presence. IL-10 is important in urothelial protection during UTI as it limits macrophage activation (71–73). Interestingly, ciprofloxacin dramatically increased the expression of G-CSF. This cytokine increases neutrophil migration from the bone marrow, and UPEC is known to trigger its upregulation during UTIs (46). G-CSF presence could be effective in increasing levels of circulating neutrophils; however, the local suppression of chemotactic cytokines in the bladder would diminish the effect at sites of infection. As serum was not collected from these mice, ciprofloxacin augmentation of systemic responses was not assessed. G-CSF also has immunomodulatory effects on macrophages and attenuates IL-1β production, leading to less efficient bacterial clearance, which further corroborates our findings (66, 74, 75). The capacity for ciprofloxacin to induce one cytokine and suppress another while having no effect on others might depend on the augmentation of immune populations and their activity within the bladder during infection. Further characterization of cytological profiles of immune population changes in response to ciprofloxacin would corroborate this hypothesis.
Ciprofloxacin prophylaxis is not associated with improved outcome and increases the intracellular bladder reservoir in mice.
We were able to demonstrate therapeutically relevant effects of ciprofloxacin treatment on UPEC pathogenesis using a murine model of prophylaxis. The results indicated that the effect of prophylaxis on bacterial urine loads was negligible and not effective in preventing recurrence risk. Further assessment of sacrificed mice for augmentation in bladder and kidney E. coli UTI89 loads revealed a trend toward higher titers when ciprofloxacin was provided. The most important finding from these studies was in revealing the propensity for prophylaxis to enhance UPEC tissue invasion in bladders. These results mirror clinical observations, in that while prophylaxis might assist in decreasing UTI symptoms, they inexorably do not alter the long-term risk of recurrence and may in fact encourage future episodes by promoting the persistence or accumulation of intracellular reservoirs (28, 52, 76). The presence of intracellular UPEC has been associated with recurrence risk in both humans (77) and mice (36) in the past. Further studies are warranted to determine the longevity of these antibiotic-induced bacterial reservoirs and whether they result in future recurrence episodes.
Clearly, there is a need to reevaluate the effectiveness of prophylactic strategies in patients with highly recurrent UTIs, especially when treatment length is short or when individuals are noncompliant. The findings presented herein likely extend beyond recurrent UTI management, raising important questions regarding the appropriateness of even well-defined antibiotic therapeutic approaches in other disease milieux. For example, empirical therapy for emergent bacteremia may compromise some patients when pathogens are resistant and thus exposed to only subinhibitory antibiotic levels. The issues to overcome may include ensuring appropriate Gram-positive and -negative coverage, but also only using agents which are known to not induce the release of potentially deadly toxins. This study highlights the need to validate and optimize current regimens and ensure their use is based upon empirical evidence of efficacy rather than anecdotal observation, theory, or instinct on what is thought to work.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Organisms were grown in lysogeny broth (LB) supplemented with antibiotics when appropriate. MIC determinations were performed via the broth microdilution method as per CLSI protocols. Enumeration of bacterial CFU was performed using LB agar (Bacto agar; BD) plates prepared as per the manufacturer’s instructions and supplemented with antibiotics when required. Cultures were grown statically unless stated otherwise. If shaking was implemented, it was done so at 200 rpm. The UPEC strain used in this study was a kanamycin-resistant derivative of the human cystitis isolate UTI89: attHK022::COM-GFP (E. coli UTI89) (78). S. saprophyticus 15305 was purchased from the American Type Culture Collection (Manassas, VA). SOS-deficient strains E. coli PAS0209 (ΔrecA) and PAS0211 (lexAT355G; also referred to as lexAG85D or the Ind− mutant) derived from the UTI89 background were provided by Sheryl Justice (The Research Institute at Nationwide Children’s Hospital, Columbus, OH). The acapsular S. saprophyticus C1 strain derived from the 15305 background was provided by Toshiko Ohta (Graduate School of Comprehensive Human Science, University of Tsukuba, Tsukuba, Japan).
Biofilm formation assays.
Overnight (24-h) cultures of S. saprophyticus 15305 or E. coli strains UTI89, ΔrecA, and lexAT355G grown in LB were subcultured 1,000-fold in fresh medium. The wells of a 96-well plate were prepared by loading various subinhibitory concentrations of either ciprofloxacin, ampicillin, gentamicin, or vehicle in 100 µl of LB. Organisms were added at 10 µl per well, and plates were sealed and then incubated for either 24 or 48 h at 37°C. If incubation periods continued for longer than 24 h, wells were washed and replaced with fresh LB containing the appropriate concentration of antibiotic. Following these incubation periods, wells were washed, stained with crystal violet, and decolorized with 70% ethanol. Biofilm abundance was determined by measuring the optical density at 600 nm (OD600).
In vitro human cell line assays.
Please refer to the further description of our methods provided in the supplemental material for details used regarding our in vitro human cell line assays..
Hemagglutination titers.
Alsevers guinea pig red blood cells (RBCs; Colorado Serum Company, Denver, CO), for E. coli hemagglutination, or sheep RBCs (Fisher Scientific), for S. saprophyticus hemagglutination, were prepared as previously described (79, 80). E. coli UTI89, ΔrecA, or lexAT355G and S. saprophyticus 15305 or C1 were subcultured 1,000-fold from overnight (24-h) cultures into fresh LB medium containing 1/4 MIC of relevant antibiotics or vehicle for 4 h. Bacteria were then pelleted at 6,500 rpm and resuspended in phosphate-buffered saline (PBS) to an OD600 of 1.0. One milliliter of the resulting suspension was transferred to a 1.5-ml microcentrifuge tube and centrifuged at 6,500 rpm for 2 min using a Sorvall Legend Microbiology 21 centrifuge (Thermo Scientific) to pellet. Supernatants were then aspirated, and pellets were resuspended in 100 µl of PBS. V-bottom, 96-well plates were prepared by transferring 25 µl of PBS into each well. Additionally, 4% (wt/vol) α-methyl-d-+-mannopyranoside (Sigma) was prepared in a second plate in a similar manner and served as a measure of mannose-resistant hemagglutination. In duplicate, 25 µl of each suspension was transferred to the first column of the plate and serially diluted 2-fold into each subsequent well to create a dilution gradient of organisms. Twenty-five microliters of the prepared blood suspension was added per well and mixed by gently tapping the plate. Plates were then sealed, covered, and placed at 4°C for 2 to 3 h prior to analysis. Titers were read by determining the last well to display RBC agglutination.
Soft agar motility assay.
Soft agar plates (0.3% agar) were prepared by loading with either 1/4 MIC levels of antibiotic or vehicle as previously described (81). E. coli UTI89, ΔrecA, or lexAT355G was subcultured 1,000-fold in fresh LB without antibiotic, incubated at 30°C with shaking (200 rpm) for 4 h, and adjusted to an OD600 of 1.0 before being spot plated onto the surface of antibiotic-loaded or unloaded soft agar plates. Organisms were also pretreated with 1/4 MIC levels of relevant antibiotics for 4 h prior to plating on unloaded soft agar plates. The radius of the resulting swimming zone (in millimeters) was measured 24 h postincubation.
Mouse infection protocols.
Murine infections models (82) routinely made use of 6- to 8-week-old female C3H/HeN or C57BL/6 mice obtained from Harlan (Harlan Sprague Dawley Inc., Indianapolis, IN) or Jackson (Jackson Laboratory, Bar Harbor, ME), respectively. All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals under Animal Welfare Assurance A3381-01 at the Washington University School of Medicine, St. Louis, MO. The Washington University School of Medicine Animal Study Committee approved animal protocol 20120216 (expiration, 01/11/2016).
E. coli UTI89 and S. saprophyticus 15305 used for infections were inoculated into 10 ml LB directly from 80°C freezer stocks, grown statically overnight at 37°C, subcultured (100-fold) in fresh LB medium with or without 1/4 MIC ciprofloxacin, and grown for another 4 h at 37°C (83). These cultures were then centrifuged for 10 min at 3,000 × g, resuspended in 10 ml of PBS, and diluted to an OD600 of 0.35 (~2.0 × 108 CFU/ml). Fifty microliters of this suspension (~1 × 107 to 2 × 107 CFU) was used to inoculate the bladders of mice via transurethral catheterization as previously described (82). Urine bacterial load (titer) was determined via serial dilution in PBS and spot plating (10 µl per drop, 50 µl total per dilution, 1 plate per sample) during the course of infection where indicated. At the indicated times, mice were sacrificed, and bladders and kidneys were aseptically removed and processed for microscopy or mechanically homogenized for CFU titration.
The effect of subtherapeutic ciprofloxacin dosing on the murine response to UTI was investigated in previously infected mice. The animals were initially infected with a dose (~108 CFU) of E. coli UTI89 and were left for at least 30 days to either spontaneously resolve infection or develop persistent high-titer bacteriuria (>104 CFU/ml for ≥28 days p.i.), which is an accurate indicator of the presence of chronic cystitis (44). The urine titers of mice were additionally monitored for 3 days prior to initiation of subtherapeutic ciprofloxacin supplementation. The empirical therapeutic ciprofloxacin dose in mice was estimated based on human data (40 µg/ml ciprofloxacin administered daily). Ciprofloxacin was supplemented into the drinking water for ad libitum consumption, and water intake was monitored and did not differ between antibiotic-supplemented and control groups, indicating ciprofloxacin was well tolerated and did not alter palatability (data not shown). Dosing ranges of <1/25 the empirical therapeutic dose were found to not significantly decrease bacterial urine titers over a 3-day period (data not shown). Following optimization of dosing parameters, such that UPEC was not negatively influenced by the level of ciprofloxacin present, 1/50 the empirical ciprofloxacin therapeutic dose was supplemented into the drinking water and replaced each day for 3 to 6 days. The urine titer of each mouse was monitored daily for changes as described elsewhere. At either 3 or 6 days, the dose was further increased to 1/25 the empirical therapeutic dose for another 3- to 6-day period, with urine titers again determined daily. Lastly, the dose was increased further to 1/10 the empirical therapeutic dose for 3 days to clear infection and ensure that spontaneous development of ciprofloxacin-resistant mutants did not occur.
Evaluation of intracellular bacterial populations within the bladders.
E. coli UTI89 IBC enumeration, visualization, and volumetrics were performed using lacZ staining and confocal microscopy as previously described (36, 84). For IBC enumeration, animals were sacrificed 6 h p.i., and bladders were excised, bisected longitudinally, and placed onto a silicone bladder pinning pad. They were fixed with 3% paraformaldehyde (Sigma), washed thrice with 2 mM MgCl2 (Sigma), 0.01% sodium deoxycholate (Sigma), and 0.02% Nonidet P-40 (Roche, Mississauga, ON, Canada) in PBS. Staining was performed using 25 mg/ml X-Gal (Sigma) and a solution containing 1 mM potassium ferrocyanide and 1 mM potassium ferricyanide (Sigma). After an incubation period of 16 h at 30°C, bladders were visualized under an Olympus SZX12 dissecting microscope (Olympus, America, Center Valley, PA).
For IBC visualization, infections and bladder fixation were conducted as described elsewhere, with the exception that E. coli UTI89 carrying the GFP-expressing plasmid pANT4 was utilized. Fixed bladders were washed and counterstained with nuclear ToPro3 (Molecular Probes) and recombinant wheat germ agglutinin (r-WGA) to outline superficial umbrella cells (1:700 dilution for each). Bladders were imaged using a Zeiss LSM 510 Meta laser scanning inverted confocal microscope (Thornwood, NY). IBCs were rendered in three-dimensions (3D) via reconstructive z-stacking of images, and volumes were determined by using Volocity 4 image analysis software (PerkinElmer, Waltham, MA).
Murine and human tissue cytokine profiling and urine sediment analysis.
Mice were supplemented with 1/50 the empirical ciprofloxacin therapeutic dose or vehicle for 3 days, then inoculated with 107 nontreated E. coli UTI89 and sacrificed at 3.5 h p.i. Bacterial urine load and immune cell infiltrate were determined at the time of sacrifice, and bladders and kidneys were excised and snap-frozen in liquid nitrogen for future cytokine profiling. Murine urine immune cell sediment analysis was carried out using the CytoPro 7620 cytocentrifuge (Wescor, Logan, UT) as per the manufacturer’s instructions. Stained urine sediments were examined by light microscopy on an Olympus BX51 light microscope, and the average number of PMN per 400× magnification field (high-powered field) was calculated by counting at least five fields and using a semiquantitative scoring system as previously described (44).
The cytokine expression profiles for murine bladders were determined using an xMAP fluorescent bead-based technology (Luminex Corporation, Austin, TX). Cytokines were liberated from tissues by using homogenization in extraction buffer containing 20 mM Tris-HCl (pH 7.5; Sigma), 150 mM NaCl (Sigma), 1-mM phenylmethylsulfonyl fluoride (PMSF; Sigma), 0.05% Tween 20 (Sigma), and a protease inhibitor cocktail (100-fold dilution; Roche). Conversely, supernatants from previous in vitro human T24 tissue cell line experiments were also extracted and analyzed. Total protein levels were assessed using a bicinchoninic acid (BCA) kit (Thermo Scientific) as per the manufacturer’s instructions and diluted when required. Levels of cytokines IL-1β, IL-6, KC, G-CSF, IL-17, IL-10, and TNF-α were measured using multiplexed immunoassay kits according to the manufacturer’s instructions (Bio-Rad Laboratories, Inc., Hercules, CA). Cytokine levels (in picograms per milliliter) were automatically calculated from standard curves using Bio-Plex Manager software (v. 4.1.1; Bio-Rad).
ELISAs were used to determine IL-8 expression levels in human 5637 bladder cells. Infections were carried out as previously described, with the exceptions that LPS (Sigma) was added at 5 µg/ml in place of S. saprophyticus 15305 and ampicillin and gentamicin were also tested at 1/4 MIC levels in addition to ciprofloxacin. Four hours post-5637 cell infection, supernatants were extracted and cytokines were quantified using the human CXCL8/IL-8 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) as per the manufacturer’s instructions.
Model of ciprofloxacin prophylaxis.
C57BL/6 mice were inoculated as previously described with ~107 CFU of nonprimed E. coli UTI89. Infections were allowed to develop for 24 h prior to initiation of a 3-day regimen of normal co-trimoxazole therapy (270 µg/ml replaced daily; Qualitest Pharmaceuticals, Huntsville, AL) (40). Following therapy cessation, mice were provided a 7-day rest period prior to initiation of ciprofloxacin prophylaxis. They then received either a typical prophylactic dose of ciprofloxacin (1/4 the empirical therapeutic dose; 100 µl of a 1-µg/ml solution) or vehicle (distilled H2O) daily via oral gavage for 7 days. Following prophylactic therapy, animals were sacrificed, and kidneys and bladders were excised for determinations of bacterial titers. In some cases, ex vivo gentamicin protection assays were conducted on bladders for enumeration of the intracellular population. Urine was taken at least every 2 days throughout the course of the experiment to allow tracking of infection.
Gene expression analyses.
For details on the methods used for the gene expression analyses, please refer to the supplemental material.
Statistical analyses.
Statistical analyses were conducted using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Significance was determined using one-way analysis of variance (ANOVA) and Dunnett’s multiple comparison test or Bonferroni’s multiple comparison test, a Mann-Whitney test with Gaussian approximation, a log-rank test, or a two-tailed or unpaired t test. Each test used for the various experiments is described in detail in the corresponding figure legend. Statistical analyses for the differential abundance of mapped mRNA reads from RNA sequencing were conducted by the ALDEx2 R package version 2.0.6 (85, 86). A log2 median effect size (calculated by using ALDEx, as the mean ratio of the difference between groups versus the maximum difference within groups) of at least 1.5 (representing at least a 2.25-fold-greater difference between groups versus within groups) was required for genes to be considered differentially expressed. In addition, a >1% overlap in the distributions between the two conditions was permitted for inclusion (85, 87). This approach ensures that the within-group variance is consistently and substantially smaller than the between-group difference when small sample sizes are used.
SUPPLEMENTAL MATERIAL
Subinhibitory ciprofloxacin promotes bacterial aggregation. (A to D) Representative images showing adherence and aggregation of S. saprophyticus 15305 (A and B) (scanning electron microscopy) and E. coli UTI89 (C and D) (transmission electron microscopy) and the effect of 1/4 MIC ciprofloxacin treatment (B and D) on these processes. (E and F) Average planktonic cluster size (number of bacteria/aggregate) of S. saprophyticus 15305 (E) and E. coli UTI89 (F) following exposure to 1/4 MIC ciprofloxacin, ampicillin, gentamicin, or no antibiotic. Means from at least three independent experiments are shown. Significance was determined using a one-way ANOVA and Dunnett’s multiple comparison test (*, P < 0.05; **, P < 0.01). Download
Subinhibitory ciprofloxacin induces UPEC filamentation. Representative TEM images of nontreated (A) and 1/4 MIC ciprofloxacin-treated (B) E. coli UTI89 filamentation. The first panel depicts individual and clustered rod and coccobacillus E. coli, while the second panel reveals filamenting and nonfilamenting organisms. Magnification for each micrograph is indicated. Download
Daily bacterial urine loads during low-dose ciprofloxacin treatment. Two experimental replicates were performed for E. coli UTI89 urine titers in C57BL/6 mice treated prophylactically with ciprofloxacin (red) or vehicle (water; black) via daily gavage. Each line represents the bacterial urine load for an individual mouse. Axis labels indicate the start of co-trimoxazole (TMP/SMX) therapy and the period of prophylaxis dosing. The dotted line indicates the cutoff for clinically significant (>104 CFU/ml) infection. Download
Supplemental experimental procedures Download
ACKNOWLEDGMENTS
We thank Sheryl Justice for providing the SOS-deficient strains, Shannon Seney for conducting Luminex cytokine quantitation assays, and David Carter for preparing the libraries for RNA sequencing.
Lee W. Goneau was supported by a Frederick Banting and Charles Best Canadian Institutes of Health Research Doctoral Research Award.
Footnotes
Citation Goneau LW, Hannan TJ, MacPhee RA, Schwartz DJ, Macklaim JM, Gloor GB, Razvi H, Reid G, Hultgren SJ, Burton JP. 2015. Subinhibitory antibiotic therapy alters recurrent urinary tract infection pathogenesis through modulation of bacterial virulence and host immunity. mBio 6(2):e00356-15. doi:10.1128/mBio.00356-15.
REFERENCES
- 1.Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol 9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Goh EB, Yim G, Tsui W, McClure J, Surette MG, Davies J. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci U S A 99:17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 4.Kelley WL. 2006. Lex marks the spot: the virulent side of SOS and a closer look at the LexA regulon. Mol Microbiol 62:1228–1238. doi: 10.1111/j.1365-2958.2006.05444.x. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Xu L, Wang J, Wen Y, Vuong C, Otto M, Gao Q. 2005. Conversion of Staphylococcus epidermidis strains from commensal to invasive by expression of the ica locus encoding production of biofilm exopolysaccharide. Infect Immun 73:3188–3191. doi: 10.1128/IAI.73.5.3188-3191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. 2012. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob Agents Chemother 56:3277–3282. doi: 10.1128/AAC.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumitrescu O, Boisset S, Badiou C, Bes M, Benito Y, Reverdy ME, Vandenesch F, Etienne J, Lina G. 2007. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother 51:1515–1519. doi: 10.1128/AAC.01201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goerke C, Köller J, Wolz C. 2006. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother 50:171–177. doi: 10.1128/AAC.50.1.171-177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernodle DS, McGraw PA, Barg NL, Menzies BE, Voladri RK, Harshman S. 1995. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis 172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 10.Nerandzic MM, Donskey CJ. 2011. Effect of ceftobiprole treatment on growth of and toxin production by Clostridium difficile in cecal contents of mice. Antimicrob Agents Chemother 55:2174–2177. doi: 10.1128/AAC.01612-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto MP, Martin E, Badiou C, Lebrun S, Bes M, Vandenesch F, Etienne J, Lina G, Dumitrescu O. 2013. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 68:1524–1532. doi: 10.1093/jac/dkt073. [DOI] [PubMed] [Google Scholar]
- 13.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 14.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culić O, Eraković V, Parnham MJ. 2001. Anti-inflammatory effects of macrolide antibiotics. Eur J Pharmacol 429:209–229. doi: 10.1016/S0014-2999(01)01321-8. [DOI] [PubMed] [Google Scholar]
- 16.Dalhoff A, Shalit I. 2003. Immunomodulatory effects of quinolones. Lancet Infect Dis 3:359–371. doi: 10.1016/S1473-3099(03)00658-3. [DOI] [PubMed] [Google Scholar]
- 17.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med 31:2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 18.Madden T, Doble A, Aliyu SH, Neal DE. 2011. Infective complications after transrectal ultrasound-guided prostate biopsy following a new protocol for antibiotic prophylaxis aimed at reducing hospital-acquired infections. BJU Int 108:1597–1602. doi: 10.1111/j.1464-410X.2011.10160.x. [DOI] [PubMed] [Google Scholar]
- 19.Miliani K, L’Hériteau F, Astagneau P, INCISO Network Study Group. 2009. Non-compliance with recommendations for the practice of antibiotic prophylaxis and risk of surgical site infection: results of a multilevel analysis from the INCISO Surveillance Network. J Antimicrob Chemother 64:1307–1315. doi: 10.1093/jac/dkp367. [DOI] [PubMed] [Google Scholar]
- 20.Namias N, Harvill S, Ball S, McKenney MG, Salomone JP, Civetta JM. 1999. Cost and morbidity associated with antibiotic prophylaxis in the ICU. J Am Coll Surg 188:225–230. doi: 10.1016/S1072-7515(98)00287-7. [DOI] [PubMed] [Google Scholar]
- 21.Patel U, Dasgupta P, Amoroso P, Challacombe B, Pilcher J, Kirby R. 2012. Infection after transrectal ultrasonography-guided prostate biopsy: increased relative risks after recent international travel or antibiotic use. Bju Int 109:1781–1785. doi: 10.1111/j.1464-410X.2011.10561.x. [DOI] [PubMed] [Google Scholar]
- 22.Schappert SM, Rechtsteiner EA. 2011. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics, Hyattsville, MD: Vital Health Stat 13(169):1–38. [PubMed] [Google Scholar]
- 23.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 24.Gupta K, Hooton TM, Stamm WE. 2001. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med 135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ikäheimo R, Siitonen A, Heiskanen T, Kärkkäinen U, Kuosmanen P, Lipponen P, Mäkelä PH. 1996. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 26.RIVUR Trial Investigators, Hoberman A, Greenfield SP, Mattoo TK, Keren R, Mathews R, Pohl HG, Kropp BP, Skoog SJ, Nelson CP, Moxey-Mims M, Chesney RW, Carpenter MA. 2014. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med 370:2367–2376. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickel JC. 2005. Practical management of recurrent urinary tract infections in premenopausal women. Rev Urol 7:11–17. [PMC free article] [PubMed] [Google Scholar]
- 28.Conway PH, Cnaan A, Zaoutis T, Henry BV, Grundmeier RW, Keren R. 2007. Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials. JAMA 298:179–186. doi: 10.1001/jama.298.2.179. [DOI] [PubMed] [Google Scholar]
- 29.Raz R, Colodner R, Kunin CM. 2005. Who are you—Staphylococcus saprophyticus? Clin Infect Dis 40:896–898. doi: 10.1086/428353. [DOI] [PubMed] [Google Scholar]
- 30.Buchanan K, Falkow S, Hull RA, Hull SI. 1985. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol 162:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keith BR, Maurer L, Spears PA, Orndorff PE. 1986. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun 53:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 33.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. 2012. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 35.Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. 2015. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci U S A 112:E871–E880 doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. 2011. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun 79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz DJ, Conover MS, Hannan TJ, Hultgren SJ. 2015. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog 11:e1004599. doi: 10.1371/journal.ppat.1004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blango MG, Mulvey MA. 2010. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother 54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mysorekar IU, Hultgren SJ. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A 103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilling JD, Lorenz RG, Hultgren SJ. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun 70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivick KE, Mobley HL. 2010. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun 78:568–585. doi: 10.1128/IAI.01000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansson S, Hanson E, Hjälmås K, Hultengren M, Jodal U, Olling S, Svanborg-Edén C. 1990. Follicular cystitis in girls with untreated asymptomatic or covert bacteriuria. J Urol 143:330–332. [DOI] [PubMed] [Google Scholar]
- 43.Schlager TA, LeGallo R, Innes D, Hendley JO, Peters CA. 2011. B cell infiltration and lymphonodular hyperplasia in bladder submucosa of patients with persistent bacteriuria and recurrent urinary tract infections. J Urol 186:2359–2364. doi: 10.1016/j.juro.2011.07.114. [DOI] [PubMed] [Google Scholar]
- 44.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. 2010. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Justice SS, Hunstad DA, Seed PC, Hultgren SJ. 2006. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc Natl Acad Sci U S A 103:19884–19889. doi: 10.1073/pnas.0606329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingersoll MA, Kline KA, Nielsen HV, Hultgren SJ. 2008. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol 10:2568–2578. doi: 10.1111/j.1462-5822.2008.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schilling JD, Mulvey MA, Hultgren SJ. 2001. Dynamic interactions between host and pathogen during acute urinary tract infections. Urology 57:56–61. doi: 10.1016/S0090-4295(01)01130-X. [DOI] [PubMed] [Google Scholar]
- 48.Li B, Smith P, Horvath DJ Jr., Romesberg FE, Justice SS. 2010. SOS regulatory elements are essential for UPEC pathogenesis. Microbes Infect 12:662–668. doi: 10.1016/j.micinf.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Bisognano C, Vaudaux P, Rohner P, Lew DP, Hooper DC. 2000. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother 44:1428–1437. doi: 10.1128/AAC.44.6.1428-1437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denève C, Bouttier S, Dupuy B, Barbut F, Collignon A, Janoir C. 2009. Effects of subinhibitory concentrations of antibiotics on colonization factor expression by moxifloxacin-susceptible and moxifloxacin-resistant Clostridium difficile strains. Antimicrob Agents Chemother 53:5155–5162. doi: 10.1128/AAC.00532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasigade JP, Moulay A, Lhoste Y, Tristan A, Bes M, Vandenesch F, Etienne J, Lina G, Laurent F, Dumitrescu O. 2011. Impact of sub-inhibitory antibiotics on fibronectin-mediated host cell adhesion and invasion by Staphylococcus aureus. BMC Microbiol 11:263. doi: 10.1186/1471-2180-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, Young L. 2006. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics 117:626–632. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 53.Garnica M, Nouér SA, Pellegrino FL, Moreira BM, Maiolino A, Nucci M. 2013. Ciprofloxacin prophylaxis in high risk neutropenic patients: effects on outcomes, antimicrobial therapy and resistance. BMC Infect Dis 13:356. doi: 10.1186/1471-2334-13-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattoo TK. 2009. Are prophylactic antibiotics indicated after a urinary tract infection? Curr Opin Pediatr 21:203–206. doi: 10.1097/MOP.0b013e3283257d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King NP, Beatson SA, Totsika M, Ulett GC, Alm RA, Manning PA, Schembri MA. 2011. UafB is a serine-rich repeat adhesin of Staphylococcus saprophyticus that mediates binding to fibronectin, fibrinogen and human uroepithelial cells. Microbiology 157:1161–1175. doi: 10.1099/mic.0.047639-0. [DOI] [PubMed] [Google Scholar]
- 56.Kline KA, Ingersoll MA, Nielsen HV, Sakinc T, Henriques-Normark B, Gatermann S, Caparon MG, Hultgren SJ. 2010. Characterization of a novel murine model of Staphylococcus saprophyticus urinary tract infection reveals roles for Ssp and SdrI in virulence. Infect Immun 78:1943–1951. doi: 10.1128/IAI.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuoka E, Tanaka Y, Kuroda M, Shouji Y, Ohta T, Tanaka I, Yao M. 2011. Crystal structure of the functional region of Uro-adherence factor A from Staphylococcus saprophyticus reveals participation of the B domain in ligand binding. Protein Sci 20:406–416. doi: 10.1002/pro.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hadjifrangiskou M, Gu AP, Pinkner JS, Kostakioti M, Zhang EW, Greene SE, Hultgren SJ. 2012. Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. J Bacteriol 194:6195–6205. doi: 10.1128/JB.01012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright KJ, Seed PC, Hultgren SJ. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol 9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 61.Brook I, Hausfeld JN. 2006. In vitro effects of penicillin and telithromycin on the expression of Streptococcus pneumoniae capsule. J Antimicrob Chemother 58:678–679. doi: 10.1093/jac/dkl261. [DOI] [PubMed] [Google Scholar]
- 62.Schembri MA, Blom J, Krogfelt KA, Klemm P. 2005. Capsule and fimbria interaction in Klebsiella pneumoniae. Infect Immun 73:4626–4633. doi: 10.1128/IAI.73.8.4626-4633.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A 101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. 2008. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol 6:162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- 65.Blaine TA, Pollice PF, Rosier RN, Reynolds PR, Puzas JE, O’Keefe RJ. 1997. Modulation of the production of cytokines in titanium-stimulated human peripheral blood monocytes by pharmacological agents. The role of cAMP-mediated signaling mechanisms. J Bone Joint Surg Am 79:1519–1528. [DOI] [PubMed] [Google Scholar]
- 66.Ono Y, Ohmoto Y, Ono K, Sakata Y, Murata K. 2000. Effect of grepafloxacin on cytokine production in vitro. J Antimicrob Chemother 46:91–94. doi: 10.1093/jac/46.1.91. [DOI] [PubMed] [Google Scholar]
- 67.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. 2007. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 68.Hang L, Haraoka M, Agace WW, Leffler H, Burdick M, Strieter R, Svanborg C. 1999. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol 162:3037–3044. [PubMed] [Google Scholar]
- 69.Hannan TJ, Roberts PL, Riehl TE, van der Post S, Binkley JM, Schwartz DJ, Miyoshi H, Mack M, Schwendener RA, Hooton TM, Stappenbeck TS, Hansson GC, Stenson WF, Colonna M, Stapleton AE, Hultgren SJ. 2014. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1:46–57. doi: 10.1016/j.ebiom.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hedges S, Agace W, Svensson M, Sjögren AC, Ceska M, Svanborg C. 1994. Uroepithelial cells are part of a mucosal cytokine network. Infect Immun 62:2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. 1991. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duell BL, Carey AJ, Tan CK, Cui X, Webb RI, Totsika M, Schembri MA, Derrington P, Irving-Rodgers H, Brooks AJ, Cripps AW, Crowley M, Ulett GC. 2012. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol 188:781–792. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 73.Mosser DM. 2003. The many faces of macrophage activation. J Leukoc Biol 73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 74.Boneberg EM, Hartung T. 2002. Granulocyte colony-stimulating factor attenuates LPS-stimulated IL-1β release via suppressed processing of proIL-1β, whereas TNF-α release is inhibited on the level of pro-TNF-α formation. Eur J Immunol 32:1717–1725. doi:. [DOI] [PubMed] [Google Scholar]
- 75.Kim SO, Sheikh HI, Ha SD, Martins A, Reid G. 2006. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol 8:1958–1971. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 76.Enzler MJ, Berbari E, Osmon DR. 2011. Antimicrobial prophylaxis in adults. Mayo Clin Proc 86:686–701. doi: 10.4065/mcp.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, Hultgren SJ. 2007. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun 75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wright KJ, Seed PC, Hultgren SJ. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun 73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuroda M, Yamashita A, Hirakawa H, Kumano M, Morikawa K, Higashide M, Maruyama A, Inose Y, Matoba K, Toh H, Kuhara S, Hattori M, Ohta T. 2005. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc Natl Acad Sci U S A 102:13272–13277. doi: 10.1073/pnas.0502950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salit IE, Gotschlich EC. 1977. Hemagglutination by purified type I Escherichia coli pili. J Exp Med 146:1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Croze OA, Ferguson GP, Cates ME, Poon WC. 2011. Migration of chemotactic bacteria in soft agar: role of gel concentration. Biophys J 101:525–534. doi: 10.1016/j.bpj.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat Protoc 4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goneau LW, Yeoh NS, MacDonald KW, Cadieux PA, Burton JP, Razvi H, Reid G. 2014. Selective target inactivation rather than global metabolic dormancy causes antibiotic tolerance in uropathogens. Antimicrob Agents Chemother 58:2089–2097. doi: 10.1128/AAC.02552-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. 2013. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One 8:e67019. doi: 10.1371/journal.pone.0067019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandes AD, Reid JN, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. 2014. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2:15. doi: 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Macklaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB. 2013. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 1:12. doi: 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subinhibitory ciprofloxacin promotes bacterial aggregation. (A to D) Representative images showing adherence and aggregation of S. saprophyticus 15305 (A and B) (scanning electron microscopy) and E. coli UTI89 (C and D) (transmission electron microscopy) and the effect of 1/4 MIC ciprofloxacin treatment (B and D) on these processes. (E and F) Average planktonic cluster size (number of bacteria/aggregate) of S. saprophyticus 15305 (E) and E. coli UTI89 (F) following exposure to 1/4 MIC ciprofloxacin, ampicillin, gentamicin, or no antibiotic. Means from at least three independent experiments are shown. Significance was determined using a one-way ANOVA and Dunnett’s multiple comparison test (*, P < 0.05; **, P < 0.01). Download
Subinhibitory ciprofloxacin induces UPEC filamentation. Representative TEM images of nontreated (A) and 1/4 MIC ciprofloxacin-treated (B) E. coli UTI89 filamentation. The first panel depicts individual and clustered rod and coccobacillus E. coli, while the second panel reveals filamenting and nonfilamenting organisms. Magnification for each micrograph is indicated. Download
Daily bacterial urine loads during low-dose ciprofloxacin treatment. Two experimental replicates were performed for E. coli UTI89 urine titers in C57BL/6 mice treated prophylactically with ciprofloxacin (red) or vehicle (water; black) via daily gavage. Each line represents the bacterial urine load for an individual mouse. Axis labels indicate the start of co-trimoxazole (TMP/SMX) therapy and the period of prophylaxis dosing. The dotted line indicates the cutoff for clinically significant (>104 CFU/ml) infection. Download
Supplemental experimental procedures Download