ABSTRACT
Direct, mediator-free transfer of electrons between a microbial cell and a solid phase in its surrounding environment has been suggested to be a widespread and ecologically significant process. The high rates of microbial electron uptake observed during microbially influenced corrosion of iron [Fe(0)] and during microbial electrosynthesis have been considered support for a direct electron uptake in these microbial processes. However, the underlying molecular mechanisms of direct electron uptake are unknown. We investigated the electron uptake characteristics of the Fe(0)-corroding and electromethanogenic archaeon Methanococcus maripaludis and discovered that free, surface-associated redox enzymes, such as hydrogenases and presumably formate dehydrogenases, are sufficient to mediate an apparent direct electron uptake. In genetic and biochemical experiments, we showed that these enzymes, which are released from cells during routine culturing, catalyze the formation of H2 or formate when sorbed to an appropriate redox-active surface. These low-molecular-weight products are rapidly consumed by M. maripaludis cells when present, thereby preventing their accumulation to any appreciable or even detectable level. Rates of H2 and formate formation by cell-free spent culture medium were sufficient to explain the observed rates of methane formation from Fe(0) and cathode-derived electrons by wild-type M. maripaludis as well as by a mutant strain carrying deletions in all catabolic hydrogenases. Our data collectively show that cell-derived free enzymes can mimic direct extracellular electron transfer during Fe(0) corrosion and microbial electrosynthesis and may represent an ecologically important but so far overlooked mechanism in biological electron transfer.
IMPORTANCE
The intriguing trait of some microbial organisms to engage in direct electron transfer is thought to be widespread in nature. Consequently, direct uptake of electrons into microbial cells from solid surfaces is assumed to have a significant impact not only on fundamental microbial and biogeochemical processes but also on applied bioelectrochemical systems, such as microbial electrosynthesis and biocorrosion. This study provides a simple mechanistic explanation for frequently observed fast electron uptake kinetics in microbiological systems without a direct transfer: free, cell-derived enzymes can interact with cathodic surfaces and catalyze the formation of intermediates that are rapidly consumed by microbial cells. This electron transfer mechanism likely plays a significant role in various microbial electron transfer reactions in the environment.
INTRODUCTION
Direct electron transfer between a microbial cell and a solid surface in its environment is a recently discovered phenomenon and proposed to occur in a variety of microorganisms and environments (1–13). Direct electron transfer involves physical contact between a microbial cell (e.g., by extracellular components and/or appendages, covalently bound to a cell) and a redox-active surface, where the electron transfer occurs without a diffusible, extracellular compound as an intermediate (13). Direct electron transfer between a cell and a redox-active surface can serve two physiological functions: (i) to access spatially distant or insoluble electron acceptors, such as during reductive mineral dissolution, or (ii) to access electron donors, such as in Fe(0) corrosion and microbial electrosynthesis. The redox potential of the surface relative to the corresponding catabolic substrate determines whether microorganisms can take up cathodic electrons for cellular metabolism or donate electrons derived from cellular metabolism to an anodic surface. Most research has focused on anodic electron transport from microbial cells to redox-active surfaces in Shewanella and Geobacter species. In Shewanella oneidensis, MR-1 nanowire-like outer membrane and periplasmic extensions in concert with outer membrane multiheme cytochromes MtrC and OmcA transfer electrons from a cell to surfaces (14–16). Geobacter species have been proposed to conduct electrons via pilus-like structures called nanowires and cytochromes associated with these pili (17–20). These molecular mechanisms have been inferred to operate also for the uptake of cathodic electrons into these species (10, 16, 20, 21). In addition, recent studies identified cable-like structures as an explanation for how Desulfobulbus species couple spatially separated biochemical processes in sediments through direct electron transfer from one cell to another over centimeter-scale distances (7, 8). However, the mechanistic basis for this electron transfer is unknown.
A direct uptake of surface-derived electrons into a cell at relatively low redox potentials (ca. −400 to −800 mV; all potentials are reported here versus the standard hydrogen electrode) has been postulated for several microorganisms and is largely based on indirect evidence from studies in a variety of environmental systems, including natural and artificial habitats (1, 22–26). Direct electron uptake by microorganisms is also thought to play a significant role in biocorrosion and microbial electrosynthesis.
Microbially influenced Fe(0) corrosion in anoxic environments is a major concern for the long-term stability of iron and steel installations, and the yearly economic loss caused by this process is estimated to be in the billion dollar range (27, 28). Microbial electrosynthesis, on the other hand, is thought to be a promising green technology to sustainably produce commodity chemicals or transportation fuels from cathodic electrons and CO2 and to achieve independence from fossil fuels (29). From a metabolic perspective, microbially influenced Fe(0) corrosion and microbial electrosynthesis are very similar processes where free, surface-derived electrons at low redox potentials (<−400 mV) are funneled into microbial metabolism. Corrosion of metallic iron [Fe(0) → Fe2+ + 2e−] releases electrons at a potential of E0'= −470 mV (30). Thus, in anoxic, aqueous environments, protons can oxidize elemental iron and molecular hydrogen is formed at low rates by an exergonic, purely chemical reaction (2H+ + 2e− → H2; E0'= −414 mV). However, microorganisms, in particular sulfate-reducing bacteria, enhance anaerobic corrosion rates of elemental iron, and several mechanisms have been proposed over the last decades (11, 31–34). Widely accepted mechanisms for iron corrosion have included the biogenic sulfide formation resulting in accelerated corrosion rates as well as cathodic depolarization by biogenic hydrogen consumption and destruction of a passivating hydrogen layer (31–34). However, subsequent studies disproved the cathodic depolarization theory by showing that some exclusively hydrogenotrophic microorganisms do not enhance corrosion rates by scavenging chemically formed H2 (11, 12). In addition, cytochrome- and hydrogenase-dependent hydrogen shuttling has been proposed previously but was not proven to take place in intact microbial culture (35, 36). Recently, a mechanism called “electrical microbially influenced corrosion” involving the direct uptake of electrons by microorganisms has been proposed to explain the high corrosion rates observed in recently isolated, highly corrosive strains (3, 11, 12, 30, 37). In this mechanism, biologically facilitated hydrogen formation was attributed to a rerouting of reducing equivalents to a side branch of cellular metabolism due to an imbalance of electron uptake from Fe(0) and electron consumption during sulfate reduction (11).
Similarly, direct electron uptake has been suggested to play a role for various electrosynthetic microorganisms, such as methanogens during electromethanogenesis (13, 24, 38, 39) and different homoacetogens during electrosynthesis of multicarbon compounds (e.g., Clostridium and Sporomusa species) (29, 40). During microbial electrosynthesis, electrons are typically supplied to these hydrogenotrophic microorganisms by a cathode poised at −400 to −700 mV (6, 13, 29, 40, 41). Electrons at these potentials are suitable for microbial reduction of CO2 to organic compounds, including CH4, acetate, or more industrially desirable end products requiring the genetic engineering of microbes (4, 13, 29, 40). At these low redox potentials, hydrogen, formate, and carbon monoxide formation at a cathodic graphite surface is thermodynamically favored but kinetically limited. Thus, the abiotic rates of formation of these molecules are too slow to explain the observed product formation rates (6, 24). As in microbially influenced Fe(0) corrosion (27), the molecular mechanism of microbial electron uptake in electrosynthesis is unknown (13, 29, 39), but an involvement of hydrogenases and related redox coenzymes (e.g., ferredoxins and cytochromes) has been considered (42, 43). Adsorption of redox-active enzymes to the cathodic surface would decrease the overpotential and allow rapid formation of small molecules, whose formation is kinetically hindered at the electrode surfaces commonly used.
Here, we investigated the electron uptake mechanism of the methanogenic archaeon Methanococcus maripaludis and the homoacetogenic bacterium Sporomusa sphaeroides, which both have been known to mediate electrical microbially influenced Fe(0) corrosion (3, 37, 44) as well as electrosynthesis (6, 13). Based on genetic data, a recent study excluded hydrogen as an essential intermediate for electromethanogenesis by M. maripaludis (13). Because of the lack of measurable intermediates besides hydrogen under electromethanogenic conditions and the elimination of H2 based on genetic data, this study could not resolve between a direct and an indirect electron uptake mechanism. In the present study, we experimentally resolved this issue and provide conclusive evidence that the apparent direct electron uptake is based on the rapid cycling of small metabolites, such as H2 or formate. Formation of these metabolites is mediated by extracellular redox-active enzymes sorbed to the redox-active surface, which provides the missing mechanistic explanation for the observed high electron transfer rates.
RESULTS
Corrosion experiments.
Previous experiments showed that the rates of electron uptake from graphite cathodes by M. maripaludis were enhanced in cells after the preculture had entered stationary phase (13). Moreover, M. maripaludis MM901 wild-type cells formed CH4 from Fe(0) at a rate of 514 ± 149 nmol electron equivalents (eeq) h−1, which is about 10 times faster than expected based on the hydrogen formation rate in sterile medium (abiotic control; 50 ± 2.8 nmol eeq h−1) (Fig. 1a; Table 1). However, when a hydrogenase deletion mutant of M. maripaludis (strain MM1284, lacking all catabolic and an anabolic hydrogenase [45]) was used in Fe(0) corrosion experiments, rates of methane formation were more than one order of magnitude lower (9.4 ± 3.6 nmol eeq h−1) than in experiments using the wild type (Fig. 1a; Table 1). This observation pointed toward a significant involvement of hydrogenases in electron uptake by M. maripaludis MM901 wild type, despite the very low background abiotic hydrogen formation rates in sterile controls. Thus, the rate enhancement could be due to a component of the spent culture medium (SCM) which is released, e.g., by lysed cells, and that component could facilitate electron uptake by M. maripaludis. To test this hypothesis, we prepared spent cell-free culture medium by passing stationary-phase cell cultures, typically used for biocorrosion or electromethanogenesis experiments, through a 0.2-µm-pore-size sterile filter and examined the effect of the filtrate alone on Fe(0) corrosion. When Fe(0)-containing vials were amended with MM901 spent cell-free culture medium, molecular hydrogen and formate were formed. The rates of formation of H2 (274 ± 49 nmol eeq h−1) and formate (49 ± 17 nmol eeq h−1) corresponded to an electron release from Fe(0) granules that was 6 to 7 times faster than in abiotic medium controls (51 ± 3.1 nmol eeq h−1) (Fig. 1b and c; Table 1). Spent, cell-free culture supernatant from the hydrogenase deletion mutant MM1284 did produce H2 from Fe(0) at a rate comparable to that of the abiotic control (51 ± 7.8 nmol eeq h−1 and 50 ± 2.8 nmol eeq h−1, respectively). However, formate accumulated at low rates in MM1284 SCM-amended vials (16 ± 14 nmol eeq h−1) (Fig. 1b and c). Formation rates of H2 and/or formate in both wild-type MM901 and the mutant MM1284 were approximately equivalent to methanogenesis rates catalyzed by intact cells based on electron equivalents (Fig. 1; Table 1). Thus, we hypothesized that hydrogenases or formate dehydrogenase released from cells was adsorbed to the Fe surface and may have facilitated molecular hydrogen evolution or formate formation from Fe(0)-derived electrons. Indeed, heat inactivation of spent, cell-free culture medium completely abolished the formation of H2 and formate (see Fig. S1 in the supplemental material), consistent with enzymatic reactions. No accumulation of products was observed in spent, cell-free culture medium controls lacking Fe(0) granules (see Fig. S1).
FIG 1 .
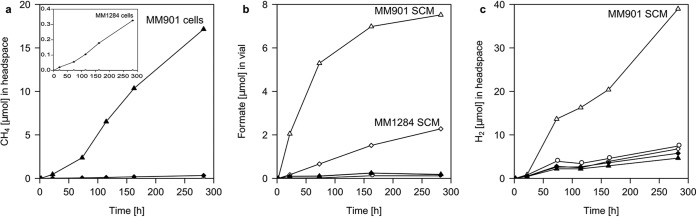
Methane, hydrogen, and formate formation during Fe(0) corrosion by M. maripaludis wild type (MM901) cells, hydrogenase deletion mutant (MM1284) cells, and respective spent cell-free culture medium with Fe(0) granules [0.4 g Fe(0) and 15 ml medium in 60-ml serum vials]. (a) Methane formation by MM901 cells (inset, MM1284 cells); no methane was formed in SCM-amended vials or medium controls; (b) formate formation by SCM of strains MM901 and MM1284; formate was barely detectable with living cells or in medium controls; (c) hydrogen formation by spent culture medium of strain MM901. Triangles, MM901; diamonds, MM1284; circles, medium control; open symbols, SCM; filled symbols, living cells.
TABLE 1 .
Hydrogen, formate, and methane formation rates in Fe(0) corrosion experiments in nmol electron equivalents/ha
| Experimental condition | Formate | H2 | CH4 |
|---|---|---|---|
| MM901 cells with Fe | 1 ± 0.2 | 514 ± 149 | |
| MM901 SCM with Fe | 49 ± 17 | 274 ± 115 | |
| MM1284 cells with Fe | 1 ± 0.4 | 40 ± 4 | 9.4 ± 3.6 |
| MM1284 SCM with Fe | 16 ± 14 | 51 ± 7.8 | |
| Abiotic with Fe | 0.9 ± 0.3 | 50 ± 2.8 |
Values are the means from 5 replicate experiments ± standard deviations (reported as electron equivalents required to form these compounds from their oxidized precursors H+ or CO2).
Electron uptake in bioelectrochemical reactors.
To study the observed electron transfer by surface-sorbed enzymes under more controlled conditions, we conducted electromethanogenic experiments in bioelectrochemical reactors. Experiments previously performed with intact M. maripaludis cells (13), pointing to an H2-independent mechanism of uptake of cathodic electrons, were repeated using cell-free spent culture medium rather than intact cells in this study. As expected, spent cell-free culture medium of wild-type MM901 cells enhanced formate and hydrogen formation at a graphite cathode poised to −600 mV (Fig. 2a and b). Spent cell-free culture medium of the hydrogenase deletion mutant MM1284 did not facilitate hydrogen formation, and only formate accumulated in the bioelectrochemical reactors. The observed rates of H2 and formate formation were sufficient to explain electromethanogenesis rates obtained previously under identical conditions with intact cells (13) (Table 2). The rates of spent cell-free culture medium-catalyzed formation of H2 and formate did not increase when the potential was lowered by 100 mV to −700 mV, indicating the surface-associated enzymatic reactions proceeded at the maximum rate already at −600 mV (Fig. 2a and b). Interestingly, methane formation from cathodic electrons by intact cells of strain MM1284 also did not increase with a decrease in cathodic potential in previous experiments (13). In contrast, the abiotic rate of H2 formation dramatically increased with a decrease in cathode potential. In the absence of intact cells, no methane formation was observed (see Fig. S2 in the supplemental material). Congruent with the above-reported Fe(0) corrosion experiments, the catalytic activity of spent cell-free culture medium was heat and proteinase sensitive (see Fig. S3 in the supplemental material). Notably, when the electrode was switched to function as an anode by setting the potential to −200 mV, the observed H2- and formate-forming reactions were reversed, as current was generated upon the addition of hydrogen or formate (see Fig. 3). Under those conditions, current was generated due to enzymatic oxidation of H2 and formate (E0' =−414 mV and −420 mV, respectively). Thus, bioelectrochemical as well as genetic evidence indicated that sorbed enzymes, such as hydrogenases and formate dehydrogenase, can take up electrons from cathodic surfaces and catalyze the formation of small soluble molecules that are catabolic substrates for hydrogenotrophic microorganisms.
FIG 2 .
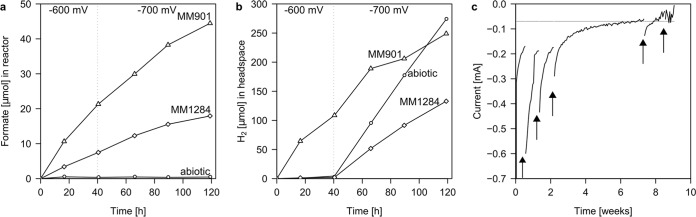
Hydrogen and formate formation by spent cell-free culture medium of M. maripaludis wild-type (MM901) and hydrogenase deletion mutant (MM1284) strains on graphite cathodes. (a) Formate formation on graphite cathodes; (b) hydrogen formation on graphite cathodes. Triangles, MM901; diamonds, MM1284; circles, medium control. Electrode potentials as indicated. (c) Surface attachment and long-term stability of the catalytic activity of MM901 SCM at a cathode potential of −600 mV. Above-background current consumption was observed for several weeks in a bioelectrochemical reactor amended with cell-free spent medium of strain MM901. Current consumption increased after the entire medium was exchanged in the cathode chamber (indicated by arrows). This current increase was likely due to replenished substrate supply (CO2 and H+) and indicates surface attachment of the catalytic activity. Background current consumption without SCM is indicated by a gray line.
TABLE 2 .
Hydrogen, formate, and methane formation rates using a graphite cathode poised at −600 mV in nmol electron equivalents/ha
| Experimental condition | Formate | H2 | CH4 |
|---|---|---|---|
| MM901 cells | 8 | 3040 | |
| MM901 SCM | 1040 ± 260 | 1800 ± 440 | |
| MM1284 cellsa | 20 | 320 | |
| MM1284 SCM | 360 ± 300 | 52 ± 26 | |
| Abiotic | 18 | 80 | 1.6 |
Data published elsewhere (9). Values obtained in this study are the means from 3 replicate experiments ± standard deviations reported as electron equivalents required to form these compounds from their oxidized precursors H+ or CO2.
FIG 3 .
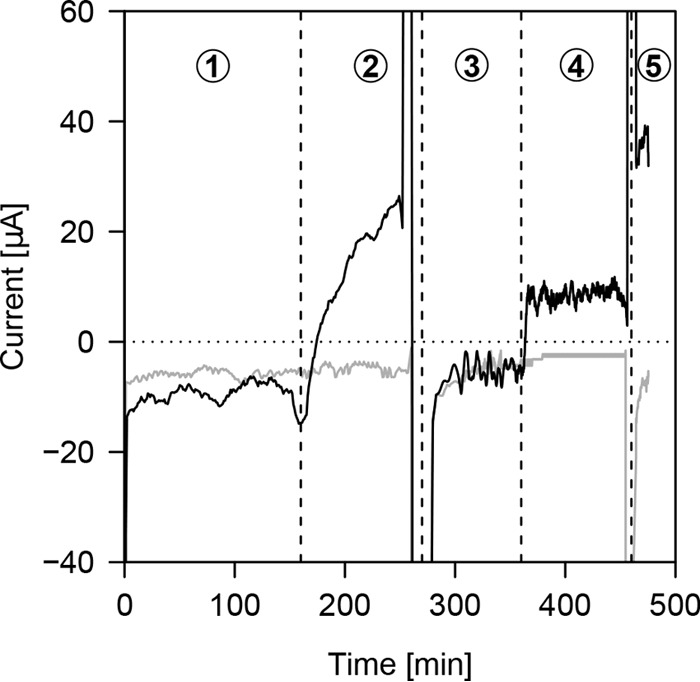
Reversibility of spent cell-free culture medium-mediated hydrogen and formate formation in bioelectrochemical reactors at −200 mV. Low background current consumption is observed in an N2-CO2 (80/20) atmosphere in SCM-amended (solid black line) and uninoculated medium (gray line) (1). Upon addition of 10% H2, current is generated only in the presence of SCM (2), current returns to background after replacing the gas phase with N2-CO2 (3), current is generated upon addition of 100 mM formate only in the presence of SCM (4), and replacing the gas phase by H2-CO2 (80/20) further increases current generation by SCM (5). Current spikes between treatments are due to disconnection of the electrochemical reactors from the potentiostat during handling.
Next, we sought to investigate the stability of this spent cell-free culture medium-catalyzed formation of H2 and formate at a cathode and to test whether the catalytic activity remains associated with the electrode surface. We therefore operated reactors inoculated with only spent cell-free culture medium from the M. maripaludis wild type over a period of 2 months. We exchanged the medium in the reactors repeatedly to remove any soluble compound not attached to the electrode surface. Even after several weeks of operation and repeated medium exchanges, current consumption of the spent cell-free culture medium reactors was higher than the background current consumption (Fig. 2c), suggesting that the catalytic activity is relatively stable and tightly associated with the electrode. Interestingly, replacing the medium increased the current for a few days, indicating rate limitation by a medium component.
To examine whether this mechanism of sorbed enzymes acting in electron uptake is also operating in other microorganisms, we performed electrochemical experiments using Sporomusa sphaeroides. This homoacetogen has been described to corrode Fe(0) as well as to perform electrosynthesis of acetate on graphite electrodes (3, 6). While we could not observe enhanced iron corrosion with intact cells or cell-free culture medium (data not shown), cell-free culture medium did enhance hydrogen formation when added to an electrochemical reactor at a poised electrode potential of −500 mV (Fig. 4). This effect was abolished when the spent medium was heat inactivated. In reactors inoculated with intact cells, hydrogen first accumulated at rates identical to the rates in cell-free culture medium reactors but then was consumed and reached a steady-state partial pressure of about 30 to 40 Pa. Only intact cells formed acetate in the electrochemical system (Fig. 4).
FIG 4 .
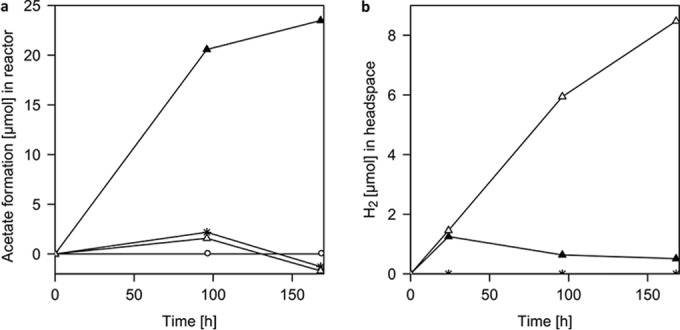
Acetate and hydrogen formation by S. sphaeroides cells and cell-free spent culture medium on graphite cathodes poised at −500 mV. (a) Only intact cells of S. sphaeroides (filled triangles) form acetate. (b) Cell-free spent culture medium (SCM; open triangles) and intact cells (filled triangles) form H2 initially, but H2 accumulates to higher concentrations only without intact cells. Open circles, medium control; stars, heat-inactivated SCM controls.
DISCUSSION
The results presented here demonstrate that an apparent direct electron uptake can be explained by redox-active enzymes sorbed to a redox-active surface catalyzing the formation of small compounds. These small compounds, such as H2 or formate, then function as soluble electron donors in microbial catabolism. Redox-active enzymes, such as hydrogenases and formate dehydrogenase, can be present in cell-free spent medium, adsorb, and subsequently interact with a cathodic surface. This finding is significant because a direct mechanism of electron uptake is frequently postulated, e.g., during anaerobic Fe(0) corrosion (11, 30, 37) or in bioelectrosynthetic systems (3, 13, 24, 29, 39). A direct mechanism has been proposed in particular when (i) the rate of the biologically mediated process is higher than the abiotic formation rate of potential substrates for cellular metabolism and (ii) the concentrations of these compounds in abiotic controls are very low and would not enable (fast) cellular metabolism. However, our study showed that the choice of the “abiotic control” can significantly affect data interpretation and, subsequently, the conclusions drawn from these data. In the case of M. maripaludis, using cell-free spent culture medium as the abiotic control (i.e., only the cells were removed from the experimental setup) resulted in formation of metabolizable intermediates at high rates; when using fresh medium that was never inoculated with cells as the abiotic control, such metabolites were undetectable (formate) or formed only at low rates (H2) (13). By using the latter abiotic control, postulating the existence of a direct electron uptake mechanism offers a tempting explanation for the discrepancies in the observed electron transfer rates. While our findings do not exclude other electron transfer mechanisms during electrosynthesis and biocorrosion, they certainly emphasize the need for proper controls and careful data interpretation.
This study also provides an explanation for the hydrogenase-independent mechanism of electron uptake proposed for M. maripaludis previously (13). Electromethanogenesis of the hydrogenase deletion strain is a result of the enzymatic formation of formate as a soluble intermediate on the cathode, whereas the high electromethanogenesis rates observed for wild-type M. maripaludis are due to enzymatic formation of both H2 and formate on the cathodic surface. The formation rates of potential intermediates of methanogenesis (H2 and formate) presented here are sufficient to explain electromethanogenesis rates reported previously for intact M. maripaludis cells under identical conditions (13). Although metals, sulfides, and biologically assembled cofactors deposited on the electrode surface might facilitate formation of reduced compounds (46), the proteinase and heat sensitivity of the process indicates that indeed intact enzymes are catalyzing these reactions. Furthermore, cell-free spent medium of the hydrogenase deletion strain MM1284 did not catalyze hydrogen formation, corroborating the claim that these enzymes are the catalytically active compound in the cell-free culture medium.
The ability of various purified redox enzymes to accept electrons directly from cathodic surfaces has been repeatedly demonstrated (47–50), and the particular involvement of cell-free hydrogenases in metal corrosion has been proposed for decades (35, 44, 51, 52). Hydrogenase-coated electrodes have been known and investigated for a long time (50, 53), and formate dehydrogenase of Syntrophobacter fumaroxidans has been shown to catalyze CO2 reduction on a graphite electrode (48). In addition, carbon monoxide dehydrogenase catalyzed CO2 reduction effectively on an electrode (49). Paradoxically, although these very enzymes are present in all corrosive and electrosynthetic microbes investigated so far, these enzymes have not been shown to be at the core of an electron uptake mechanism. Generally, free enzymes are thought to be mostly unstable and therefore may not always have been taken into consideration as a possible mechanism for the generation of soluble molecules on redox-active surfaces—especially when direct electron transfer offered an exciting alternative explanation. However, hydrogenases can be quite stable (51), especially when immobilized to electrodes (54). Furthermore, it was shown that the lifetime of the catalytic activity of enzymes is extended in crude culture supernatant compared to that in purified enzyme preparations (55). Notably, extracellular enzymes have been shown to be stable in sediment and soil environments, especially when attached to minerals or complex organic substances (56–59). Therefore, our observations that hydrogenase and formate dehydrogenase were active even after several weeks of continuous operation of the bioelectrochemical reactor are consistent with these previous observations. This opens the possibility for sustained extracellular enzyme-mediated electron transfer in a variety of environments where conductive minerals like pyrite or graphite are present.
The here-identified mechanism of electron uptake via sorbed redox-active enzymes requires that these typically cytoplasmic enzymes are released from cells. Loss of cellular integrity and subsequent enzyme release can be caused by various factors and can occur at multiple stages and to different extents during microbial life (60, 61), e.g., growth phase, starvation, transfer of cultures, stirring, and exposure to high Fe ion concentrations in iron corrosion experiments or to low redox potentials in electrochemical or Fe(0) corrosion experiments could all cause cell lysis. Consistent with this reasoning, the use of filtered culture medium did not result in increased currents or hydrogen formation in a study with an unknown hydrogenotrophic methanogen (24), whereas another study using filtered spent medium concluded the absence of soluble mediators but failed to explain the distinct increase in current consumption at potentials lower than −700 mV relative to fresh, unused medium (62). However, generally few studies used filtered culture medium in experiments. A variability in cell lysis is also consistent with reports that not all M. maripaludis strains facilitate Fe(0) corrosion (11, 37). Microbial biofilms are also known to induce metabolic stress in inhabiting cells, and (induced) cell lysis has long been considered part of biofilm development (63). Thus, electrochemical systems that rely on microbial biofilms on electrodes could be particularly prone to lytic release and cumulative sorption of redox-active enzymes to an electrode surface. Thus, enzymes in biofilms could potentially explain frequently observed high activities of direct electron uptake in these systems.
In contrast to previous studies (3, 6), we did not observe a significant increase in Fe(0) corrosion by Sporomusa sphaeroides or significant electrosynthetic properties under our experimental conditions. This discrepancy could be explained by the absence of a preestablished biofilm that was present in previous electrosynthesis studies (6). However, cell-free spent culture medium increased hydrogen evolution rates significantly under electrosynthetic conditions compared to those of the abiotic or heat-inactivated controls. This indicates that enzymes are present in cell-free spent culture supernatant and can also play a role in electron uptake by homoacetogens during electrosynthesis. Thus, the extent of Fe(0) corrosion or electrosynthesis rates might depend on the amount of intracellular enzymes released under the respective experimental conditions. Besides different experimental conditions, another explanation for the different experimental outcome could be an intrinsic differential propensity of strains to lyse. Furthermore, the physical properties and the preferential orientation of the enzymes on the Fe(0) or electrode surfaces might cause significant differences in electron transfer efficiency among different strains. All together, these findings emphasize that the potential for biocorrosion not only might depend strongly on the experimental conditions in each study or environmental system but also might be a species- or strain-specific trait.
Collectively, our data show that corrosiveness and electrosynthetic properties of certain microbial organisms might not depend on the presence of a specialized direct electron uptake apparatus but rather (i) on the quantity and stability of cellular redox-active enzymes released under the conditions investigated (e.g., due to cell lysis; especially in biofilms), (ii) on whether these enzymes can bind to and interact with the cathodic surface, and (iii) on whether the enzymes can be reduced by a cathodic surface. At this point, it is unclear to what extent a release of such enzymes is accidental or biologically controlled, e.g., by a sacrificial subpopulation. The importance of a sacrificial subpopulation has been described previously during intestinal inflammation by Salmonella Typhimurium (64). Given the universal distribution of low-redox-potential enzymes in microorganisms, the ubiquity of cell lysis (actively induced or passive due to environmental stresses) leading to the release of these enzymes, and the fact that surface-bound enzymes can remain active for weeks, enzyme-mediated electron transfer might play a significant role in enhancing electron transfer reactions, such as Fe(0) corrosion or bioelectrosynthesis. In addition, a role of extracellular enzymes within microbial communities or syntrophic cooperations can be suspected.
MATERIALS AND METHODS
Analytical procedures.
H2 was measured using a reductive trace gas detector as described previously (13). Methane and high concentrations of hydrogen were measured using a gas chromatograph (equipped with both a thermal conductivity detector [TCD] and a flame ionization detection [FID] detector). Formate and acetate were measured via high-performance liquid chromatography (HPLC) as described previously (13). To allow comparisons of electron uptake rates during the formation of different compounds, H2 concentrations are sometimes reported here as electron equivalents (eeq) by multiplying the measured H2 concentrations of the compounds by the number of electrons required for its formation (2 µmol eeq/μmol H2 and 8 µmol eeq/μmol CH4 or acetate).
Microbial strains, cultivation, and experimental setup.
M. maripaludis was routinely cultured on DSMZ medium 141, omitting Na-acetate, yeast extract, and Trypticase but containing 200 mM formate and buffered with 200 mM morpholinepropanesulfonic acid (MOPS) as described previously (13). M. maripaludis strain MM901 (a derivative of strain S2 lacking uracil phosphoribosyl transferase [UPT]) was used as a wild-type strain, and strain MM1284 was used as a mutant strain, where all six catabolic hydrogenase genes were deleted (45). Hydrogenases have been deleted by Costa et al. (45) using markerless in-frame deletions (65, 66).
Sporomusa sphaeroides strain dsm2875 was routinely cultured on DSMZ medium 311 omitting Casitone, resazurin, betaine, and cysteine but buffered with 100 mM MOPS. Twenty millimoles betaine or a gas phase of H2-CO2 (80/20) was added as a carbon and energy source.
Stationary-phase cultures were flushed excessively for 30 min with N2-CO2 (80/20) to remove volatile compounds like H2, CH4, and sulfide before experiments were inoculated, as described previously (13). Amounts indicated in the Results section of the flushed cultures were transferred to the experiment vessels using one-way plastic syringes. Cell-free spent culture medium (SCM) was prepared by filter sterilizing the stationary-phase culture through two stacked syringe filters (surfactant-free cellulose acetate [SFCA] membrane, 0.2-µm pore size; Thermo Scientific, Santa Clara, CA) using a 20- or 60-ml syringe.
Corrosion experiments with M. maripaludis were carried out in 60-ml serum vials containing 15 ml medium, a gas phase of N2-CO2 (80/20), and 0.3 to 0.4 g Fe(0) granules (1 to 2 mm, 99.98% purity metals basis; Alfa Aesar, Ward Hill, MA) with 1.5 ml inoculum as indicated. The medium used was DSMZ medium 141 without a carbon source and buffered with 100 mM MOPS buffer. Abiotic controls were amended with 1.5 ml of fresh growth medium. Corrosion experiments with S. sphaeroides were carried out in the same way but with vials containing 20 ml medium. The medium was DSMZ medium 311 without an additional carbon source and modified as described above and amended with 0.5 g Fe(0) granules. A total of 0.5 ml of an equal mixture of two stationary-phase cultures, one grown on betaine and one grown on H2-CO2 (80/20), or 0.5 ml SCM was used as the inoculum. A total of 0.5 ml fresh medium and heat-inactivated SCM were used as controls. The formation of H2 and formate was followed over time, and SCM-amended treatments were compared to the abiotic vials. Corrosion experiments were carried out in five independent replicates per condition.
For experiments on graphite electrodes, 6 to 10 ml of SCM was inoculated into 90 ml medium in each 150-ml cathode chamber, and the bioelectrochemical cell was incubated overnight to allow the cellular materials to sorb to the electrode before the potential was applied, as described previously (13). The medium used in the bioelectrochemical reactors in experiments with M. maripaludis was modified DSMZ medium 141 without a carbon source and buffered with 100 mM MOPS buffer, and the medium used for S. sphaeroides experiments was modified DSMZ medium 311 without a carbon source and buffered with 100 mM MOPS buffer. The headspace of the electrochemical cells was flushed with N2-CO2 (80/20). The formation of H2 and formate was followed over time, and SCM-amended treatments were compared to the abiotic reactors. Bioelectrochemical experiments were carried out in three independent replicates per condition.
Identification of the active ingredient of SCM.
To identify whether the catalytic activity observed in SCM was due to active enzymes or due to other cell-derived compounds, SCM activity was tested after different treatments. SCM was either heat inactivated overnight at 90°C or at 98°C for at least 20 min, treated with pronase (final concentration of 1.0 mg/ml pronase [Calbiochem, EMD Chemicals, Inc., San Diego, CA], overnight at 37°C) or left untreated.
To prove the reversibility of the catalytic activity, electrodes were poised at a potential of −200 mV (ca. 200 mV more positive than the hydrogen equilibrium potential of H2 and formate [E0' =−414 mV and −420 mV, respectively]), and current formation from the oxidation of formate or H2 was measured.
SUPPLEMENTAL MATERIAL
Hydrogen and formate formation during Fe(0) corrosion of M. maripaludis wild-type (MM901) cell-free spent medium with iron granules (0.4 g iron and 15 ml medium in a 60-ml serum vial). (a) Formate formation by spent culture medium of strain MM901 and controls; no formate was detected after heat inactivation of SCM, in medium controls with Fe(0), or in SCM controls without Fe(0). (b) Hydrogen formation by spent culture medium of strain MM901 and controls. Triangles, strain MM901 SCM; circles, medium control with Fe(0); stars, heat-inactivated MM901 SCM; squares, SCM control without Fe(0). Download
Methane formation in bioelectrochemical experiments with spent, cell-free medium at the indicated potentials. No methane was formed in SCM-amended bioelectrochemical reactors. Download
Formate and hydrogen formation in bioelectrochemical reactors amended with spent cell-free medium of the wild-type M. maripaludis strain MM901 at −600 mV and in controls. (a) Formate formation by untreated SCM, proteinase-inactivated SCM (final concentration of 1 mg/ml pronase [Calbiochem, EMD Chemicals, Inc., San Diego, CA], overnight at 37°C), heat-inactivated SCM (90°C, overnight), and medium controls. (b) Hydrogen formation by the same treatments as in panel a. Triangles, strain MM901 SCM; circles, medium control; stars, heat-inactivated MM901 SCM; squares, proteinase-treated SCM. Download
ACKNOWLEDGMENTS
This work was supported by the Global Climate and Energy Program (GCEP) grant awarded to A.M.S.
We thank Bruce E. Logan, Svenja Lohner, Ann Lesnefsky, and Maeva Fincker for useful discussions.
We thank John A. Leigh and his group for providing the M. maripaludis strains.
Footnotes
Citation Deutzmann JS, Sahin M, Spormann AM. 2015. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 6(2):e00496-15. doi:10.1128/mBio.00496-15.
REFERENCES
- 1.Kato S, Hashimoto K, Watanabe K. 2012. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci U S A 109:10042–10046. doi: 10.1073/pnas.1117592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato S, Hashimoto K, Watanabe K. 2012. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ Microbiol 14:1646–1654. doi: 10.1111/j.1462-2920.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 3.Kato S, Yumoto I, Kamagata Y. 2015. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol 81:67–73. doi: 10.1128/AEM.02767-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabaey K, Rozendal RA. 2010. Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat Rev Micro 8:706–716. doi: 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- 5.Lovley DR. 2011. Powering microbes with electricity: direct electron transfer from electrodes to microbes. Environ Microbiol Rep 3:27–35. doi: 10.1111/j.1758-2229.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 6.Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, Snoeyenbos-West OL, Lovley DR. 2011. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol 77:2882–2886. doi: 10.1128/AEM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, Meyer RL, Kjeldsen KU, Schreiber L, Gorby YA, El-Naggar MY, Leung KM, Schramm A, Risgaard-Petersen N, Nielsen LP. 2012. Filamentous bacteria transport electrons over centimetre distances. Nature 491:218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M. 2010. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- 9.Bond DR, Lovley DR. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory KB, Bond DR, Lovley DR. 2004. Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6:596–604. doi: 10.1111/j.1462-2920.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 11.Dinh HT, Kuever J, Mussmann M, Hassel AW, Stratmann M, Widdel F. 2004. Iron corrosion by novel anaerobic microorganisms. Nature 427:829–832. doi: 10.1038/nature02321. [DOI] [PubMed] [Google Scholar]
- 12.Enning D, Venzlaff H, Garrelfs J, Dinh HT, Meyer V, Mayrhofer K, Hassel AW, Stratmann M, Widdel F. 2012. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14:1772–1787. doi: 10.1111/j.1462-2920.2012.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohner ST, Deutzmann JS, Logan BE, Leigh J, Spormann AM. 2014. Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J 8:1673–1681. doi: 10.1038/ismej.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirbadian S, Barchinger SE, Leung KM, Byun HS, Jangir Y, Bouhenni RA, Reed SB, Romine MF, Saffarini DA, Shi L, Gorby YA, Golbeck JH, El-Naggar MY. 2014. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc Natl Acad Sci U S A 111:12883–12888. doi: 10.1073/pnas.1410551111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci U S A 103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto A, Nakamura R, Hashimoto K. 2011. In-vivo identification of direct electron transfer from Shewanella oneidensis MR-1 to electrodes via outer-membrane OmcA–MtrCAB protein complexes. Electrochim Acta 56:5526–5531. doi: 10.1016/j.electacta.2011.03.076. [DOI] [Google Scholar]
- 17.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 18.Vargas M, Malvankar NS, Tremblay PL, Leang C, Smith JA, Patel P, Snoeyenbos-West O, Nevin KP, Lovley DR. 2013. Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. mBio 4:e00105-00113. doi: 10.1128/mBio.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay PL, Summers ZM, Glaven RH, Nevin KP, Zengler K, Barrett CL, Qiu Y, Palsson BO, Lovley DR. 2011. A c-type cytochrome and a transcriptional regulator responsible for enhanced extracellular electron transfer in Geobacter sulfurreducens revealed by adaptive evolution. Environ Microbiol 13:13–23. doi: 10.1111/j.1462-2920.2010.02302.x. [DOI] [PubMed] [Google Scholar]
- 20.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 21.Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR. 2011. Towards electrosynthesis in Shewanella: energetics of reversing the Mtr pathway for reductive metabolism. PLoS One 6:e16649. doi: 10.1371/journal.pone.0016649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita M, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR. 2011. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2:e00159-00111. doi: 10.1128/mBio.00159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Rotaru A, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. 2012. Promoting direct interspecies electron transfer with activated carbon. Energy Environ Sci 5:8982–8989. doi: 10.1039/c2ee22459c. [DOI] [Google Scholar]
- 24.Villano M, Aulenta F, Ciucci C, Ferri T, Giuliano A, Majone M. 2010. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour Technol 101:3085–3090. doi: 10.1016/j.biortech.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 25.Rotaru A, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, Zengler K, Wardman C, Nevin KP, Lovley DR. 2014. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci 7:408–415. doi: 10.1039/C3EE42189A. [DOI] [Google Scholar]
- 26.Rotaru AE, Shrestha PM, Liu F, Markovaite B, Chen S, Nevin KP, Lovley DR. 2014. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl Environ Microbiol 80:4599–4605. doi: 10.1128/AEM.00895-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enning D, Garrelfs J. 2014. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80:1226–1236. doi: 10.1128/AEM.02848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beech IB, Sunner J. 2007. Sulphate-reducing bacteria and their role in corrosion of ferrous materials. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 29.Lovley DR, Nevin KP. 2013. Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr Opin Biotechnol 24:385–390. doi: 10.1016/j.copbio.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Venzlaff H, Enning D, Srinivasan J, Mayrhofer KJJ, Hassel AW, Widdel F, Stratmann M. 2013. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros Sci 66:88–96. doi: 10.1016/j.corsci.2012.09.006. [DOI] [Google Scholar]
- 31.Von Wolzogen Kühr CAH, van der Vlugt LS. 1934. The graphitization of cast iron as an electrochemical process in anaerobic soils. Water 18:147–165. [Google Scholar]
- 32.Butlin KR, Adams ME, Thomas M. 1949. Sulphate-reducing bacteria and internal corrosion of ferrous pipes conveying water. Nature 163:26–27. doi: 10.1038/163026a0. [DOI] [PubMed] [Google Scholar]
- 33.Wanklyn JN, Spruit CJP. 1952. Influence of sulphate-reducing bacteria on the corrosion potential of iron. Nature 169:928–929. doi: 10.1038/169928b0.14941089 [DOI] [Google Scholar]
- 34.Spruit CJ, Wanklyn JN. 1951. Iron-sulphide ratios in corrosion by sulphate-reducing bacteria. Nature 168:951–952. doi: 10.1038/168951a0. [DOI] [PubMed] [Google Scholar]
- 35.Van Ommen Kloeke F, Bryant RD, Laishley EJ. 1995. Localization of cytochromes in the outer membrane of Desulfovibrio vulgaris (Hildenborough) and their role in anaerobic biocorrosion. Anaerobe 1:351–358. doi: 10.1006/anae.1995.1038. [DOI] [PubMed] [Google Scholar]
- 36.Da Silva S, Basséguy R, Bergel A. 2004. Electron transfer between hydrogenase and 316L stainless steel: identification of a hydrogenase-catalyzed cathodic reaction in anaerobic mic. J Electroanal Chem 561:93–102. doi: 10.1016/j.jelechem.2003.07.005. [DOI] [Google Scholar]
- 37.Uchiyama T, Ito K, Mori K, Tsurumaru H, Harayama S. 2010. Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76:1783–1788. doi: 10.1128/AEM.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng S, Xing D, Call DF, Logan BE. 2009. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol 43:3953–3958. doi: 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- 39.Beese-Vasbender PF, Grote J-P, Garrelfs J, Stratmann M, Mayrhofer KJ. 2015. Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. Bioelectrochemistry 102:50–55. doi: 10.1016/j.bioelechem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Lovley DR, Nevin KP. 2011. A shift in the current: new applications and concepts for microbe-electrode electron exchange. Curr Opin Biotechnol 22:441–448. doi: 10.1016/j.copbio.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Nevin KP, Woodard TL, Franks AE, Summers ZM, Lovley DR. 2010. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1(2):e00103-10. doi: 10.1128/mBio.00103-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Aulenta F, Villano M, Angenent LT. 2011. Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol 102:324–333. doi: 10.1016/j.biortech.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Sydow A, Krieg T, Mayer F, Schrader J, Holtmann D. 2014. Electroactive bacteria—molecular mechanisms and genetic tools. Appl Microbiol Biotechnol 98:8481–8495. doi: 10.1007/s00253-014-6005-z. [DOI] [PubMed] [Google Scholar]
- 44.Mori K, Tsurumaru H, Harayama S. 2010. Iron corrosion activity of anaerobic hydrogen-consuming microorganisms isolated from oil facilities. J Biosci Bioeng 110:426–430. doi: 10.1016/j.jbiosc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Costa KC, Lie TJ, Jacobs MA, Leigh JA. 2013. H2-independent growth of the hydrogenotrophic methanogen Methanococcus maripaludis. mBio 4:e00062-00013. doi: 10.1128/mBio.00062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yates MD, Siegert M, Logan BE. 2014. Hydrogen evolution catalyzed by viable and non-viable cells on biocathodes. Int J Hydrogen Energy 39:16841–16851. doi: 10.1016/j.ijhydene.2014.08.015. [DOI] [Google Scholar]
- 47.Armstrong FA, Hirst J. 2011. Reversibility and efficiency in electrocatalytic energy conversion and lessons from enzymes. Proc Natl Acad Sci U S A 108:14049–14054. doi: 10.1073/pnas.1103697108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reda T, Plugge CM, Abram NJ, Hirst J. 2008. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. Proc Natl Acad Sci U S A 105:10654–10658. doi: 10.1073/pnas.0801290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkin A, Seravalli J, Vincent KA, Ragsdale SW, Armstrong FA. 2007. Rapid and efficient electrocatalytic CO2/CO interconversions by Carboxydothermus hydrogenoformans CO dehydrogenase I on an electrode. J Am Chem Soc 129:10328–10329. doi: 10.1021/ja073643o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaropolov AI, Karyakin AA, Varfolomeev SD, Berezin IV. 1984. Mechanism of H2-electrooxidation with immobilized hydrogenase. Bioelectrochem Bioenerg 12:267–277. doi: 10.1016/0302-4598(84)87009-9. [DOI] [Google Scholar]
- 51.Chatelus C, Carrier P, Saignes P, Libert MF, Berlier Y, Lespinat PA, Fauque G, Legall J. 1987. Hydrogenase activity in aged, nonviable Desulfovibrio vulgaris cultures and its significance in anaerobic biocorrosion. Appl Environ Microbiol 53:1708–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laishley EJ, Bryant RD. 2003. Electron flow in ferrous biocorrosion, p 252–260. In Ljungdahl LG, Adams MW, Barton LL, Ferry JG, Johnson MK (ed), Biochemistry and physiology of anaerobic bacteria. Springer Verlag, New York, NY. [Google Scholar]
- 53.Vincent KA, Parkin A, Armstrong FA. 2007. Investigating and exploiting the electrocatalytic properties of hydrogenases. Chem Rev 107:4366–4413. doi: 10.1021/cr050191u. [DOI] [PubMed] [Google Scholar]
- 54.Karyakin AA, Morozov SV, Karyakina EE, Varfolomeyev SD, Zorin NA, Cosnier S. 2002. Hydrogen fuel electrode based on bioelectrocatalysis by the enzyme hydrogenase. Electrochem Commun 4:417–420. doi: 10.1016/S1388-2481(02)00335-1. [DOI] [Google Scholar]
- 55.Sané S, Jolivalt C, Mittler G, Nielsen PJ, Rubenwolf S, Zengerle R, Kerzenmacher S. 2013. Overcoming bottlenecks of enzymatic biofuel cell cathodes: crude fungal culture supernatant can help to extend lifetime and reduce cost. Chemsuschem 6:1209–1215. doi: 10.1002/cssc.201300205. [DOI] [PubMed] [Google Scholar]
- 56.Nannipieri P, Kandeler E, Ruggiero P. 2002. Enzyme activities and microbiological and biochemical processes in soil, p 1–33. In Burns RG, Dick RP (ed), Enzymes in the environment. Marcel Dekker, New York, NY. [Google Scholar]
- 57.Tabatabai M, Dick W, Burns R, Dick R. 2002. Enzymes in soil: research and developments in measuring activities, p 567–596. In Burns RG, Dick RP (ed), Enzymes in the environment. Marcel Dekker, New York, NY. [Google Scholar]
- 58.Tate R., III 2002. Microbiology and enzymology of carbon and nitrogen cycling, p 227–248. In Burns RG, Dick RP (ed), Enzymes in the environment. Marcel Dekker, New York, NY. [Google Scholar]
- 59.Nosrati K, Govers G, Ahmadi H, Sharifi F, Amoozegar MA, Merckx R, Vanmaercke M. 2011. An exploratory study on the use of enzyme activities as sediment tracers: biochemical fingerprints? Int J Sediment Res 26:136–151. doi: 10.1016/S1001-6279(11)60082-6. [DOI] [Google Scholar]
- 60.Bayles KW. 2014. Bacterial programmed cell death: making sense of a paradox. Nat Rev Microbiol 12:63–69. doi: 10.1038/nrmicro3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. 2006. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet 2:e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marshall CW, Ross DE, Fichot EB, Norman RS, May HD. 2012. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl Environ Microbiol 78:8412–8420. doi: 10.1128/AEM.02401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayles KW. 2007. The biological role of death and lysis in biofilm development. Nat Rev Microbiol 5:721–726. doi: 10.1038/nrmicro1743. [DOI] [PubMed] [Google Scholar]
- 64.Ackermann M, Stecher B, Freed NE, Songhet P, Hardt WD, Doebeli M. 2008. Self-destructive cooperation mediated by phenotypic noise. Nature 454:987–990. doi: 10.1038/nature07067. [DOI] [PubMed] [Google Scholar]
- 65.Costa KC, Wong PM, Wang T, Lie TJ, Dodsworth JA, Swanson I, Burn JA, Hackett M, Leigh JA. 2010. Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc Natl Acad Sci U S A 107:11050–11055. doi: 10.1073/pnas.1003653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore BC, Leigh JA. 2005. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J Bacteriol 187:972–979. doi: 10.1128/JB.187.3.972-979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hydrogen and formate formation during Fe(0) corrosion of M. maripaludis wild-type (MM901) cell-free spent medium with iron granules (0.4 g iron and 15 ml medium in a 60-ml serum vial). (a) Formate formation by spent culture medium of strain MM901 and controls; no formate was detected after heat inactivation of SCM, in medium controls with Fe(0), or in SCM controls without Fe(0). (b) Hydrogen formation by spent culture medium of strain MM901 and controls. Triangles, strain MM901 SCM; circles, medium control with Fe(0); stars, heat-inactivated MM901 SCM; squares, SCM control without Fe(0). Download
Methane formation in bioelectrochemical experiments with spent, cell-free medium at the indicated potentials. No methane was formed in SCM-amended bioelectrochemical reactors. Download
Formate and hydrogen formation in bioelectrochemical reactors amended with spent cell-free medium of the wild-type M. maripaludis strain MM901 at −600 mV and in controls. (a) Formate formation by untreated SCM, proteinase-inactivated SCM (final concentration of 1 mg/ml pronase [Calbiochem, EMD Chemicals, Inc., San Diego, CA], overnight at 37°C), heat-inactivated SCM (90°C, overnight), and medium controls. (b) Hydrogen formation by the same treatments as in panel a. Triangles, strain MM901 SCM; circles, medium control; stars, heat-inactivated MM901 SCM; squares, proteinase-treated SCM. Download


