ABSTRACT
Streptococcus pyogenes (group A Streptococcus [GAS]) is an obligate human pathogen responsible for a spectrum of human disease states. Metallobiology of human pathogens is revealing the fundamental role of metals in both nutritional immunity leading to pathogen starvation and metal poisoning of pathogens by innate immune cells. Spy0980 (MntE) is a paralog of the GAS zinc efflux pump CzcD. Through use of an isogenic mntE deletion mutant in the GAS serotype M1T1 strain 5448, we have elucidated that MntE is a manganese-specific efflux pump required for GAS virulence. The 5448ΔmntE mutant had significantly lower survival following infection of human neutrophils than did the 5448 wild type and the complemented mutant (5448ΔmntE::mntE). Manganese homeostasis may provide protection against oxidative stress, explaining the observed ex vivo reduction in virulence. In the presence of manganese and hydrogen peroxide, 5448ΔmntE mutant exhibits significantly lower survival than wild-type 5448 and the complemented mutant. We hypothesize that MntE, by maintaining homeostatic control of cytoplasmic manganese, ensures that the peroxide response repressor PerR is optimally poised to respond to hydrogen peroxide stress. Creation of a 5448ΔmntE-ΔperR double mutant rescued the oxidative stress resistance of the double mutant to wild-type levels in the presence of manganese and hydrogen peroxide. This work elucidates the mechanism for manganese toxicity within GAS and the crucial role of manganese homeostasis in maintaining GAS virulence.
IMPORTANCE
Manganese is traditionally viewed as a beneficial metal ion to bacteria, and it is also established that most bacteria can tolerate high concentrations of this transition metal. In this work, we show that in group A Streptococcus, mutation of the mntE locus, which encodes a transport protein of the cation diffusion facilitator (CDF) family, results in accumulation of manganese and sensitivity to this transition metal ion. The toxicity of manganese is indirect and is the result of a failure of the PerR regulator to respond to oxidative stress in the presence of high intracellular manganese concentrations. These results highlight the importance of MntE in manganese homeostasis and maintenance of an optimal manganese/iron ratio in GAS and the impact of manganese on resistance to oxidative stress and virulence.
INTRODUCTION
Manganese is important for defense against oxidative stress, especially in Gram-positive bacteria (1, 2), and the acquisition of manganese has been shown to be crucial for survival and virulence of Streptococcus pneumoniae (3) and Streptococcus pyogenes (group A Streptococcus [GAS]) (4). Competition for transition metal ions has emerged as a significant component of the innate immune defense system against pathogens (5), and the ability of the host to withhold metal ions (zinc, iron, and manganese) from bacterial pathogens is an important aspect of nutritional immunity, defined here as the restriction of bacterial survival through control of available metal nutrients (6, 7). This is exemplified by the action of the host protein calprotectin, which exerts antimicrobial effects against bacterial pathogens via its sequestration of zinc and/or manganese (8).
In excess, the transition metal ions copper, zinc, and iron are toxic to the cell, and thus, bacteria have evolved sophisticated systems that sense excess transition metal ions and remove these by efflux from the cell (copper or zinc) or sequestration of excess metal ions inside the cell (iron and zinc) (9–12). In contrast to the other major first-row transition metal ions, manganese is usually regarded as being well tolerated by bacteria and can be accumulated to millimolar concentrations within Escherichia coli and many lactobacilli without any apparent deleterious effects on the cell (13, 14). However, recently it was observed that deletion of the manganese cation diffusion facilitator (CDF) protein, MntE, in S. pneumoniae resulted in increased hydrogen peroxide production and decreased virulence in a murine model of infection (15). Also, the high level of sensitivity of Neisseria gonorrhoeae to manganese in comparison to Neisseria meningitidis was correlated with the absence of a functional manganese efflux pump, MntX, in the former (16, 17). These observations suggest that in some bacteria, manganese efflux and homeostasis are important for cellular survival.
GAS is an obligate human pathogen responsible for a wide variety of diseases ranging from mild infections such as impetigo and pharyngitis to life-threatening invasive diseases such as streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis (18). There are more than 18 million cases of severe diseases caused by GAS infections which result in over 500,000 deaths each year (19). Since the 1980s, there has been an increase in the incidence of severe invasive GAS diseases, and despite sensitivity to penicillins, emerging resistance to macrolide antibiotic treatment has been reported (20). To date, there are no licensed vaccines against GAS (21). During infection, the host innate immune cells, such as neutrophils and macrophages, mobilize to the site of infection, to control GAS by phagocytosis (18). Recently, we showed that the CDF transporter, CzcD, is essential for efflux of zinc from GAS and is important for protection against neutrophil killing and virulence in a mouse model of infection (22), indicating that the innate immune system can use zinc as an antimicrobial agent. In this study, we characterize a second CDF family transporter in GAS, Spy0980, and describe its role in linking manganese and iron homeostasis with resistance to oxidative stress and virulence.
RESULTS
GAS Spy0980 is a homologue of S. pneumoniae MntE and functions as a manganese efflux pump.
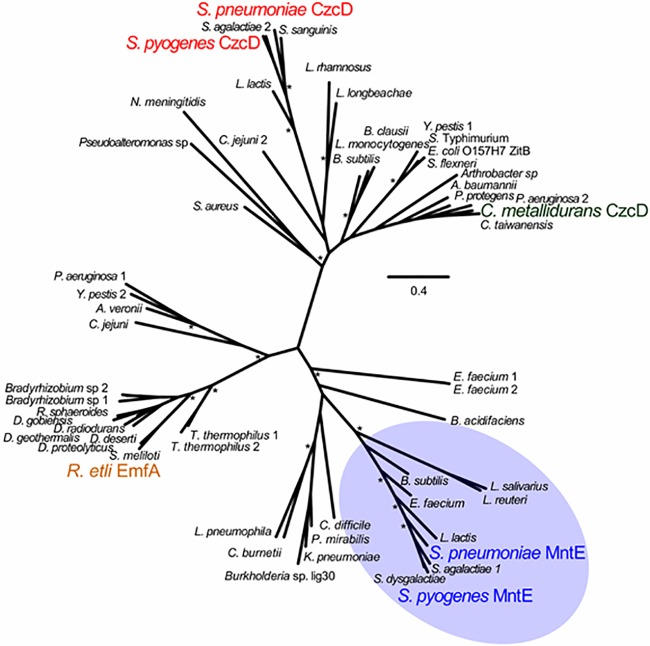
During our investigation of the GAS zinc efflux pump CzcD, we observed that a second CDF transporter, Spy0980, is encoded in the GAS genome. Spy0980 exhibited approximately 60% amino acid identity (NCBI BLASTp) to the manganese efflux pump MntE from S. pneumoniae, compared to only 20% amino acid identity to the zinc efflux pump CzcD from GAS, S. pneumoniae, or Cupriavidus metallidurans. Phylogenetic analysis revealed that the CzcD and MntE-related proteins clustered into distinct clades (Fig. 1). Hence, GAS Spy0980 was designated MntE and hypothesized to be involved in manganese efflux.
FIG 1 .
Phylogenetic clustering of 59 cation diffusion facilitator family protein sequences. Unrooted maximum-likelihood tree based on a ClustalO amino acid alignment. Key species possessing functionally characterized CzcD, EmfA, and MntE gene products are represented in green and red, brown, and blue, respectively. MntE homologues cluster together and are highlighted by blue shading. The scale bar indicates the number of amino acid substitutions per site under the WAG substitution model. Asterisks represent bootstrap values of greater than 90 (out of 100 replicates).
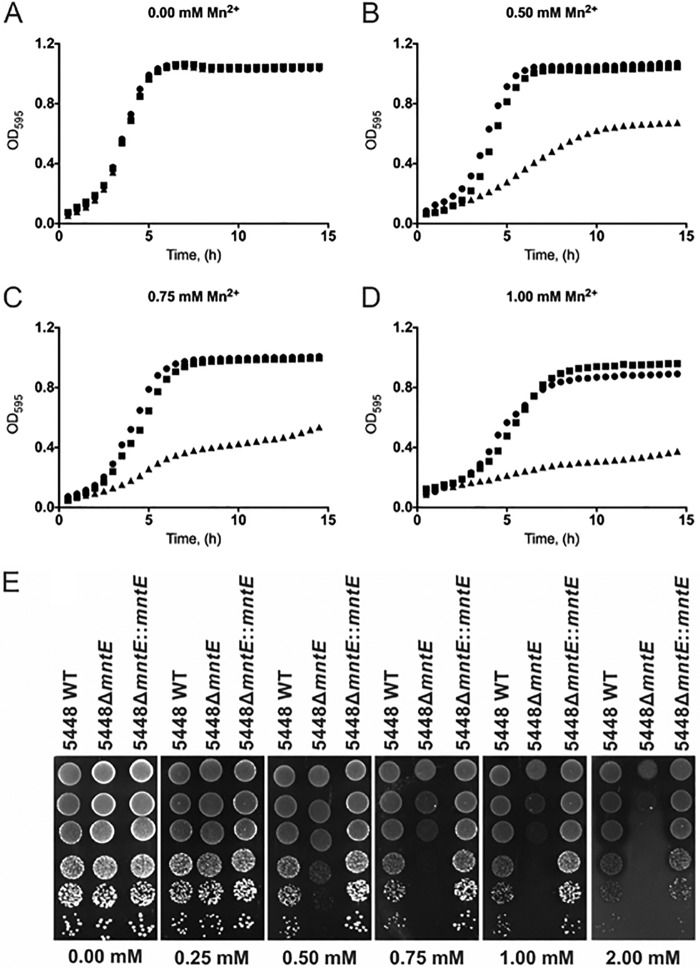
To investigate the role of mntE, an isogenic deletion mutant and complemented mutant were constructed in the well-characterized GAS M1T1 strain 5448 (23). In the absence of manganese, all three strains grew at comparable rates (Fig. 2A), but upon the addition of increasing concentrations of manganese, growth of the 5448ΔmntE mutant was inhibited (Fig. 2B to D). The defective growth phenotype in the 5448ΔmntE mutant was also observed on solid medium (Fig. 2E). Complementation of the deletion mutant fully restored the ability to grow in the presence of manganese (Fig. 2B to E), confirming that this phenotype is attributable to the mntE deletion. While other CDF proteins, such as CzcD and EmfA from S. pneumoniae, C. metallidurans, and Rhizobium etli, provide resistance to multiple metals (24–26), no differential inhibition of the 5448ΔmntE mutant was observed in the presence of cadmium, cobalt, copper, iron, nickel, and zinc (see Fig. S1 in the supplemental material). Hence, in GAS, MntE appears to be specific for manganese tolerance.
FIG 2 .
Comparison of the growth of 5448 wild-type GAS (WT), 5448ΔmntE, and 5448ΔmntE::mntE strains. (A to D) Growth curves of 5448 (circles), 5448ΔmntE (triangles), and 5448ΔmntE::mntE (squares) in Todd-Hewitt broth plus 1% yeast extract supplemented with increasing manganese concentrations (0.5 mM [B], 0.75 mM [C], and 1 mM [D]). The graph presents means of three independent experiments. (E) Drop test analysis of 5448, 5448ΔmntE, and 5448ΔmntE::mntE on THY agar without and with supplemented manganese (0.25, 0.50, 0.75, 1, and 2 mM). Cells were grown and adjusted to an OD600 of 0.6 and serially diluted, and 5-µl drops were spotted onto the plate from concentrations of 100 (top) to 10−5 (bottom).
To better understand the biochemical basis of the effect of manganese on the growth of the 5448ΔmntE mutant, we determined the intracellular content of manganese using inductively coupled plasma mass spectrometry (ICP-MS) for 5448 wild type (WT), 5448ΔmntE, and 5448ΔmntE::mntE grown in the absence or presence of 1 mM manganese. When grown in Todd-Hewitt broth supplemented with yeast extract (THY broth), the intracellular manganese contents remained identical for the 3 strains (P > 0.05) (Fig. 3A). However, the addition of manganese to the growth medium resulted in a significant accumulation of intracellular manganese in the 5448ΔmntE mutant compared to wild-type and complemented strains (P < 0.001) (Fig. 3A). This result is consistent with a role for MntE in manganese efflux.
FIG 3 .
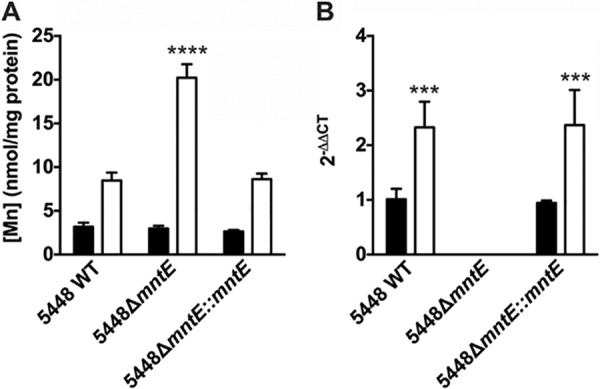
Comparison of the manganese accumulations and relative gene expression levels of 5448 wild-type GAS, 5448ΔmntE, and 5448ΔmntE::mntE strains. (A) Intracellular manganese analysis of 5448, 5448ΔmntE, and 5448ΔmntE::mntE grown on Todd-Hewitt agar plus 1% yeast extract in the absence (black bars) or presence (white bars) of 1 mM manganese. One-way ANOVA was performed comparing manganese accumulations of 5448 WT and 5448ΔmntE in the presence of manganese (****, P < 0.0001). (B) Gene expression of mntE in 5448 WT, 5448ΔmntE, and 5448ΔmntE::mntE grown in the absence (black bars) or presence (white bars) of 2 mM manganese. Expression of mntE was calculated using the 2−ΔΔCT method using proS as the reference gene. One-way ANOVA was performed comparing expression levels of mntE in 5448 wild type grown in THY broth versus 5448 wild type and 5448ΔmntE::mntE grown in THY broth supplemented with 2 mM manganese (***, P < 0.001). Graphs represent means and standard deviations of three independent experiments.
Additionally, we tested mntE relative gene expression levels in the presence of manganese. Expression of mntE in the wild type and complemented mutant is 2-fold higher when they are grown in the presence of 1 mM manganese than when grown in THY broth alone (P < 0.001) (Fig. 3B). Furthermore, mntE gene expression was not induced by other metal ions tested (Cd, Co, Cu, Fe, Ni, and Zn) (see Fig. S2 in the supplemental material), indicating that mntE gene expression is specifically induced by manganese.
Accumulation of intracellular manganese reduces tolerance of GAS to oxidative stress.
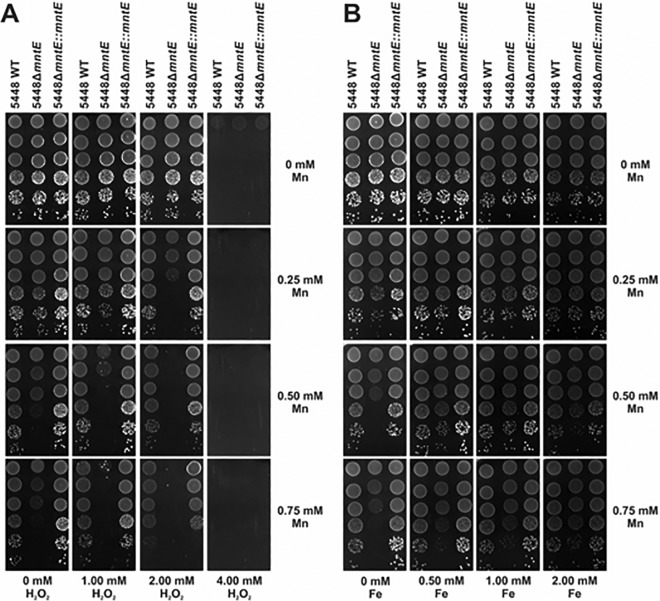
Previous studies have suggested that manganese plays a role in resistance of GAS to oxidative stress (4). We therefore examined the effect of manganese accumulation in 5448ΔmntE on tolerance to oxidative stress, using drop tests performed with increasing hydrogen peroxide and manganese concentrations. At sublethal concentrations of manganese, increasing hydrogen peroxide concentration resulted in reduced survival of the 5448ΔmntE mutant compared to wild-type and complemented strains (Fig. 4A).
FIG 4 .
Comparison of growth inhibition and rescue under oxidative and iron stress in combination with manganese. (A) Drop test analysis of 5448, 5448ΔmntE, and 5448ΔmntE::mntE on THY agar supplemented with increasing concentrations of manganese vertically (0, 0.25, 0.50, and 0.75 mM) and increasing concentrations of hydrogen peroxide horizontally (0, 1, 2, and 4 mM). (B) Drop test analysis of 5448, 5448ΔmntE, and 5448ΔmntE::mntE on THY agar supplemented with increasing concentrations of manganese vertically (0, 0.25, 0.50, and 0.75 mM) and increasing concentrations of iron horizontally (0, 0.5, 1, and 2 mM). Cells were grown and adjusted to an OD600 of 0.6 and serially diluted, and 5-µl drops were spotted onto the plate from concentrations of 100 (top) to 10−5 (bottom).
PerR mediates resistance to hydrogen peroxide stress in GAS (27). PerR can bind both iron and manganese, but when bound to iron, PerR forms a less stable repressor (Fig. 5A), and it is more sensitive to the presence of hydrogen peroxide (28, 29). As such, we hypothesized that addition of iron would rescue the growth defect of 5448ΔmntE in the presence of manganese. The addition of iron partially restored growth of 5448ΔmntE at otherwise growth-inhibitory concentrations of manganese (Fig. 4B). This suggested that the toxicity of manganese toward GAS might be exerted via a dysfunction in PerR-dependent regulation.
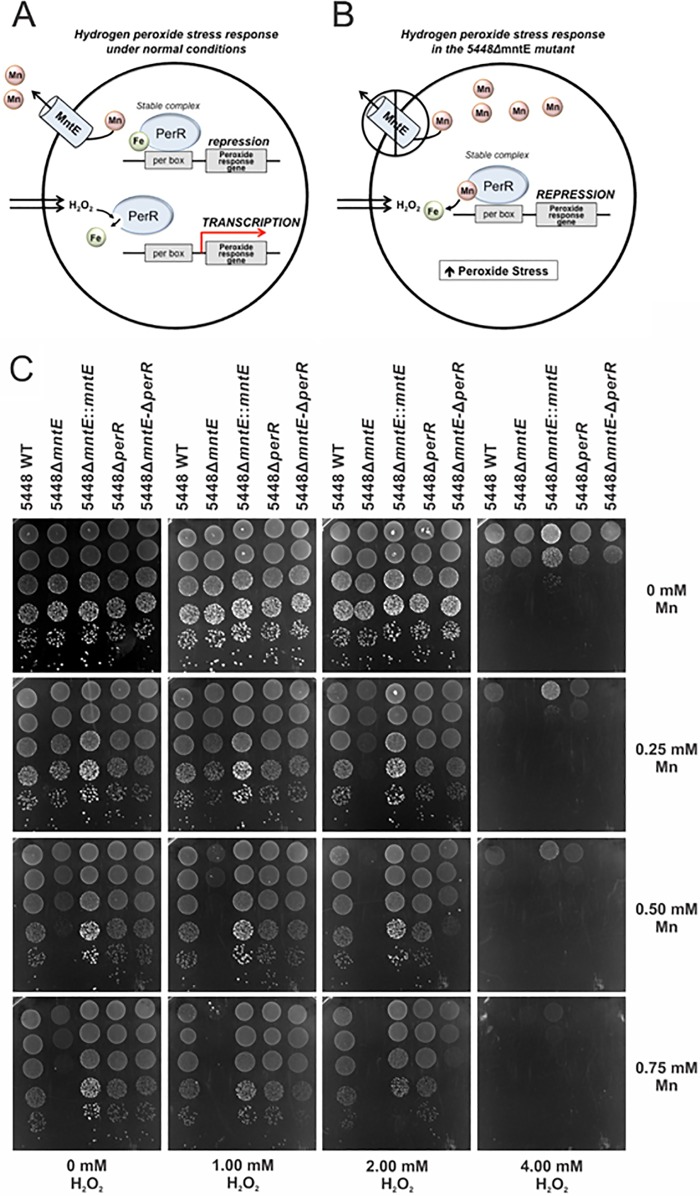
FIG 5 .
Proposed model of PerR-manganese displacement in GAS. (A) In wild-type 5448, excess manganese can exit the cell and PerR maintains iron as a cofactor. Under hydrogen peroxide stress, metal-catalyzed oxidation occurs to the iron cofactor, leading to damage of PerR, dissociation from Per boxes, and subsequent transcription of peroxide response genes. (B) In the 5448ΔmntE mutant, the presence of manganese and hydrogen peroxide stress results in non-redox-active manganese displacement of iron within PerR. This stabilizes the complex, resulting in continued repression of peroxide response genes. (C) Drop test analysis of 5448, 5448ΔmntE, 5448ΔmntE::mntE, 5448ΔperR, and 5448ΔmntE-ΔperR on THY agar supplemented with increasing concentrations of manganese vertically (0, 0.25, 0.50, and 0.75 mM) and increasing concentrations of hydrogen peroxide horizontally (0, 1, 2, and 4 mM). Cells were grown and adjusted to an OD600 of 0.6 and serially diluted, and 5-µl drops were spotted onto the plate from concentrations of 100 (top) to 10−5 (bottom).
Manganese efflux protects GAS against PerR-regulated oxidative stress.
In view of our findings, we have developed a model that describes the way in which manganese efflux would be critical for the PerR-regulated oxidative stress response. Under normal physiological conditions, the iron-loaded PerR responds to peroxide stress by dissociation from the Per box, inducing expression of peroxide-responsive genes (Fig. 5A). However, manganese-loaded PerR (similar to conditions experienced by the 5448ΔmntE mutant in the presence of manganese) results in stabilization of the PerR complex and inability to respond to peroxide stress (Fig. 5B). To test this model, we created a 5448ΔmntE-ΔperR double deletion mutant that we predicted would be unable to efflux manganese and yet would retain the ability to defend against peroxide stress. This is because the PerR regulon would be constitutively expressed, rescuing growth of the double mutant in the presence of manganese and peroxide. Drop test analysis with manganese and hydrogen peroxide illustrated that growth of the 5448ΔmntE-ΔperR double deletion mutant was restored to levels approaching those of the wild type (Fig. 5C). This result indicates that the mechanism of manganese toxicity is via the stabilization of the PerR complex, resulting in reduced ability to mount a response to oxidative stress in GAS.
Loss of manganese efflux in GAS results in decreased expression of the DNase Sda1 and increased killing by human neutrophils.
The key phage-encoded virulence determinant of M1T1 GAS, DNase Sda1 (30), is crucial for escape from neutrophil extracellular traps (NETs) (31) and is regulated by PerR (32). Western blot analysis demonstrated that Sda1 is uniformly secreted when the strains are grown in the absence of manganese (Fig. 6A). However, when the 5448ΔmntE mutant was grown in the presence of manganese, Sda1 production was reduced compared to the wild type and complemented mutant (Fig. 6A). These results suggest that the accumulation of manganese results in constitutive repression by PerR, thus affecting the expression of PerR-regulated genes such as sda1.
FIG 6 .
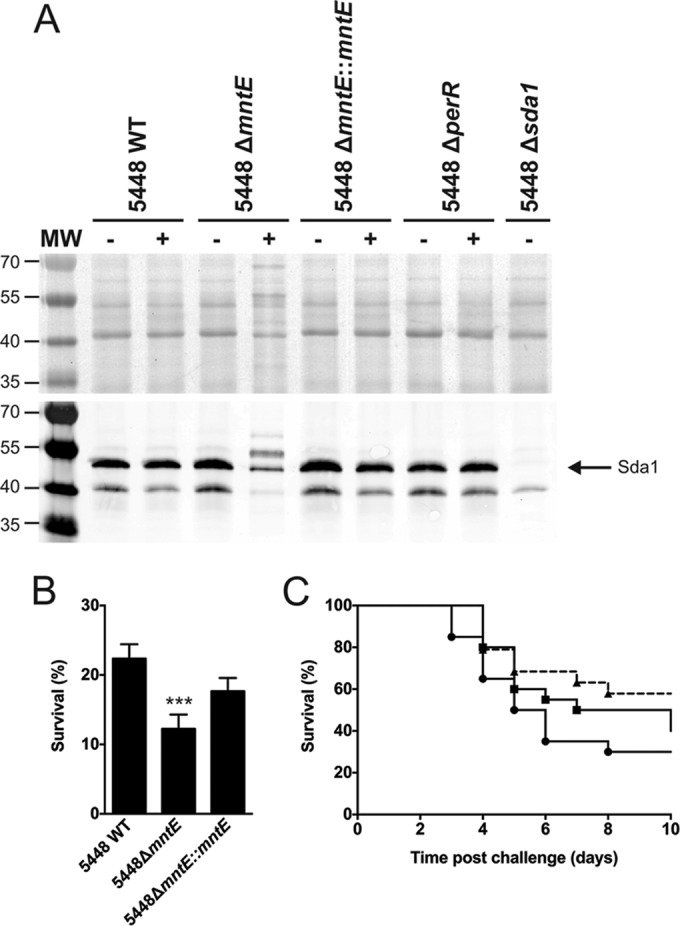
Effects of MntE on Sda1 expression, survival of GAS in a neutrophil killing assay, and virulence in a murine model of infection. (A) Wild-type 5448, 5448ΔmntE, 5448ΔmntE::mntE, 5448ΔperR, and 5448Δsda1 were grown in THY broth alone or THY broth supplemented with 0.5 mM manganese, and supernatant was harvested at mid-exponential growth phase. Cell supernatant proteins were trichloroacetic acid precipitated, run on an SDS-PAGE gel, and subjected to immunoblotting using anti-Sda1. MW, molecular weights in thousands. (B) Percent survival of 5448 wild type, 5448ΔmntE, and 5448ΔmntE::mntE following coculture with human neutrophils in vitro. One-way ANOVA was performed comparing 5448 wild type versus 5448ΔmntE (***, P < 0.001). The graph is representative of three independent experiments. (C) Survival of mice (WT and 5448ΔmntE::mntE, n = 20; 5448ΔmntE, n = 19) after subcutaneous challenge of transgenic humanized plasminogen AlbPLG1 C57BL/J6 mice with 5448 wild type (circles), 5448ΔmntE (triangles, dashed line), and 5448ΔmntE::mntE (squares). Infecting dose, 2 × 107 to 4 × 107 CFU. The Mantel-Cox log rank test was performed comparing 5448ΔmntE to 5448 wild type (P = 0.067) and 5448ΔmntE::mntE (P = 0.3369).
Neutrophils are recognized as the first line of host cellular innate immune defense against GAS, and the DNase Sda1 plays an important role in protecting GAS from neutrophil killing (33). Thus, we examined the level of resistance of 5448ΔmntE to neutrophil killing. 5448ΔmntE exhibited reduced survival compared to the 5448 wild type and complemented mutant (P < 0.001 and 0.05, respectively) (Fig. 6B).
Finally, we assessed the virulence of the 5448ΔmntE mutant using a transgenic humanized plasminogen mouse model of invasive GAS infection (34). Following subcutaneous infection, no significant difference (P = 0.067) was observed in murine survival between 5448 wild type and 5448ΔmntE (Fig. 6C). Taken together, these results indicate that there may be biological niche-specific differences in manganese requirements for GAS.
DISCUSSION
Manganese has been traditionally viewed as a beneficial metal ion for bacterial pathogens, particularly in Gram-positive streptococci (4, 35, 36). The acquisition of manganese is critical for the assembly of defenses against oxidative stress. This includes production of superoxide dismutase (SodA) and the protection of metalloenzymes against oxidative damage (37, 38). In order to acquire manganese, streptococcal species such as S. pneumoniae and GAS possess high-affinity inducible uptake systems. Expression of high-affinity manganese acquisition systems PsaABC in S. pneumoniae (39) and MtsABC in GAS (4) is under the control of the DtxR family protein MtsR (PsaR in S. pneumoniae) (40, 41), which ensures that expression of manganese uptake is repressed when there is sufficiency. However, our results indicate not only that manganese homeostasis is maintained by the control of manganese uptake but that an efflux pump for manganese, MntE, also plays an important role.
Since it is well known that manganese usually exerts antioxidant rather than pro-oxidant effects, it was not immediately obvious why this ion would be toxic to GAS. The answer appears to be linked to the mechanism of action of the peroxide-responsive repressor, PerR (42). In the model for the action of PerR, developed in Bacillus subtilis, this repressor can exist in two peroxide-responsive states depending on whether it binds ferrous iron or manganese at the reversible ion-binding site (43). The iron-loaded form of PerR is highly sensitive to hydrogen peroxide, and this leads to derepression of oxidative stress defense systems. In contrast, when manganese is present in the reversible binding site, PerR is more stable in the presence of hydrogen peroxide and thus continues to act as a repressor (29). This mechanism of action enables PerR to act as a sensor for hydrogen peroxide and also respond to the intracellular ratio of the pro-oxidant ferrous iron and antioxidant manganese. Our results are consistent with this model; when manganese is in excess in the 5448ΔmntE mutant, GAS is not able to defend against exogenously produced hydrogen peroxide (Fig. 5). Our observation that the 5448ΔmntE mutant is sensitive to manganese in the absence of externally added peroxide suggests that the MntE and PerR system also has a role in protection against production of hydrogen peroxide produced endogenously by GAS (44). Support for this model also comes from our observation that addition of ferrous iron restored resistance to manganese in the 5448ΔmntE mutant, consistent with the view that excess manganese leads to constitutive repression of PerR-regulated peroxide defenses. An additional secreted protein was observed in the 5448ΔmntE mutant in the presence of manganese (Fig. 6A). We hypothesize that the elevated expression of this protein results from the significant stress placed on the cell physiology of the 5448ΔmntE mutant under these conditions. MntE-type transporters are also present in other bacteria, but their impact on cell physiology may be different, depending on the regulatory networks that control the physiology of the bacterium. In S. pneumoniae, the mntE mutant was also sensitive to manganese and produced more hydrogen peroxide. The mutant exhibited reduced virulence in a mouse model of infection (15). However, S. pneumoniae lacks the PerR regulator, and so the influence of MntE on cell physiology and pathogenicity must be exerted via a distinct alternate mechanism.
During infection, GAS is subject to peroxide stress as a consequence of the action of innate immune cells such as neutrophils and macrophages (18). Thus, it is crucial that enzymes under PerR control involved in peroxide defense in GAS are derepressed. Additionally, other important virulence determinants such as Sda1 also appear to be regulated by PerR (32). Sda1 is a well-characterized GAS DNase responsible for the escape of this bacterium from DNA NETs (31, 33). This leads to the hypothesis that, during the adaptation of GAS to its human host, the sda1 gene has evolved to be under the control of PerR. Placing Sda1 expression under PerR control enables GAS to sense conditions where hydrogen peroxide is being produced and available manganese is low. Thus, the PerR regulon may be critical for the sensing of conditions of high neutrophil activity. In this scenario, the MntE efflux pump plays a key role as a modulator of PerR repressor activity since the absence of MntE leads to accumulation of manganese and failure to induce the PerR regulon. This model is consistent with the observation that in an ex vivo human neutrophil killing assay, the 5448ΔmntE deletion mutant is attenuated. On the other hand, in the subcutaneous infection model used in this study, there may be a niche-specific effect occurring, resulting in no significant difference between the virulence of the mutant and that of the WT. Loss of mntE in manganese-depleted and iron-replete conditions subcutaneously may lift selection against the 5448ΔmntE mutant compared to 5448 WT. Such conditions may result due to the action of calprotectin at the site of infection (45). Thus, manganese homeostasis in GAS as well as the ratio of manganese to iron in the bacterial cell may play a significant role in GAS pathogenesis and protection against oxidative stress.
It is also established that mutation of high-affinity manganese acquisition systems in S. pneumoniae (46) and GAS (4) leads to reduced growth and survival of these human pathogens in a mouse model of infection. Manganese may provide protection from oxidative stress through the displacement of redox-sensitive ferrous iron within key enzymes (38, 47), and manganese is also a cofactor in superoxide dismutase in bacterial pathogens such as S. pneumoniae (48) and GAS (37). Recently, it was also observed that in S. pneumoniae, zinc toxicity is a result of the inability of the bacteria to acquire manganese through the high-affinity manganese solute protein, PsaA (49). These observations indicate that there is a dynamic interplay of manganese, iron, and zinc at the host-pathogen interface. Bacterial pathogens are finely tuned to adjust their adaptive responses to altered availability of these transition metal ions via control of acquisition and efflux systems. This is likely to be a key factor in the regulation of virulence in GAS and other streptococci.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. pyogenes M1T1 clinical isolate 5448 (23) and isogenic derivatives were routinely grown on 5% horse blood agar or statically in liquid cultures at 37°C in Todd-Hewitt broth supplemented with 1% yeast extract (THY broth). Escherichia coli MC1061 was grown in Luria-Bertani medium (LB). Where required, erythromycin was used at 2 µg/ml (GAS) or 500 µg/ml (E. coli) and spectinomycin was used at 100 µg/ml (both GAS and E. coli). GAS isogenic mutants were constructed as previously described (31). All bacterial strains and plasmids are listed in Table S1 in the supplemental material.
DNA manipulation and genetic techniques.
GAS mutants 5448ΔmntE, 5448ΔperR, and 5448ΔmntE-ΔperR were constructed by deletion replacement. All PCR primer sequences are provided in Table S2 in the supplemental material. The 1-kb regions upstream of mntE and perR were amplified using primers mntE1 and mntE2 and primers perR1 and perR2, respectively. The 1-kb downstream regions were amplified using primers mntE3 and mntE4 and primers perR3 and perR4, respectively. The kanamycin cassette was amplified using primers kan-F and kan-R, while the spectinomycin cassette was amplified using primers spec-F and spec-R. For 5448ΔmntE, the three PCR fragments (5′- and 3′-flanking regions with kanamycin cassette) were joined together with primers mntE1 and mntE4, and for 5448ΔperR, the three fragments (5′- and 3′-flanking regions with spectinomycin cassette) were joined with primers perR1 and perR4. The subsequent fragment was cloned into the pHY304 shuttle vector (50) and transformed into E. coli MC1061. Electrotransformation and allelic replacement mutagenesis were undertaken using standard protocols (51, 52). Complementation of 5448ΔmntE was performed by marker rescue. The entire wild-type region of mntE, including the 1-kb upstream and downstream sequences, was amplified using primers mntE1 and mntE4. The construct was cloned into pHY304 via restriction ligation and transformed into E. coli MC1061. Electrotransformation and allelic replacement mutagenesis were undertaken as described above, and complemented strains were screened for the loss of kanamycin resistance. All strains were confirmed by DNA sequencing (Australian Equine Genome Research Centre, University of Queensland, Brisbane, Australia).
Phylogenetic analysis.
Phylogenetic analyses of 59 cation diffusion facilitator family protein sequences, selected on the basis of BLAST (NCBI) analysis against functionally characterized CDF family proteins, CzcD, EmfA, and MntE, were used to determine the clustering of the different CDF families. An amino acid alignment was generated using Clustal Omega v1.2.1 (53). A best-fit phylogenetic tree was estimated using PhyML v3.0 (54) based on the WAG substitution model with gamma correction of among-site rate variation. One hundred nonparametric bootstraps were applied.
Growth curve analyses.
Overnight cultures grown in THY broth were diluted to an optical density at 600 nm (OD600) of 0.05 in fresh THY broth supplemented with various amounts of manganese. The cells were statically grown in a 96-well microtiter plate, and OD595 was measured half-hourly using the FLUOstar Optima (BMG Labtech) plate reader at 37°C.
Drop test plate sensitivity assays.
Drop tests were performed as previously described (22). Antimicrobial agents tested were cobalt(II) (CoSO4⋅4H2O), copper(II) (CuSO4⋅5H2O), iron(II) (FeSO4⋅7H2O) and iron(III) [Fe(NO3)3⋅9H2O], manganese (MnSO4⋅4H2O), nickel(II) (NiSO4⋅6H2O), and zinc(II) (ZnSO4⋅7H2O). These assays were also repeated in the presence of hydrogen peroxide. Plates were photographically documented following overnight incubation at 37°C. Drop tests are representative of biological replicates performed on at least 3 separate occasions.
Intracellular metal concentration measurement.
Cells from an overnight THY agar plate, grown in the absence or presence of 1 mM MnSO4·4H2O, were resuspended, washed three times with phosphate buffer-0.25 M EDTA and three times with phosphate buffer, resuspended in 80% nitric acid, and incubated at 80°C for 24 h. The samples were then diluted to 2% nitric acid and submitted for inductively coupled plasmid mass spectrometry (ICP-MS) analysis at the School of Earth Sciences, University of Queensland. The final value was normalized to the amount of cells present by measuring the total protein content in accordance with the QuantiPro bicinchoninic acid (BCA) assay kit (Sigma) instructions.
Quantitative gene expression studies.
RNA was isolated from cells harvested under the desired growth phase grown in the presence or absence of 1 mM MnSO4·4H2O, in accordance with the RNeasy minikit (Qiagen) with the additional mechanical lysis step in lysing matrix B tubes (MP Biomedicals). The isolated RNA was DNase treated using the RNase-free DNase set (Qiagen) and quantified using a NanoDrop instrument (Thermo Scientific). One microgram of RNA was converted to cDNA using the SuperScriptIII first-strand synthesis system for reverse transcription-PCR (RT-PCR) (Invitrogen). Real-time reverse transcriptase PCR was performed using the primers specified in Table S2 in the supplemental material. The PCR was performed using SYBR green master mix (Applied Biosystems) according to the manufacturer’s instructions. All data were analyzed using the ViiA7 software (Applied Biosystems). Relative gene expression was calculated using the threshold cycle (2−ΔΔCT) method with proS as the reference gene (55). All reactions were performed in triplicate from 3 independently isolated RNA samples.
Sda1 Western blotting.
Detection of Sda1 by Western blotting from cell supernatants grown in the presence or absence of 0.5 mM MnSO4·4H2O to mid-logarithmic phase was performed using standard protocols (56).
Neutrophil killing assay.
GAS survival following incubation with human neutrophils ex vivo was assayed as previously described (31). Experiments were performed in triplicate using mid-logarithmic-phase (A600 = 0.4) GAS at a multiplicity of infection of 10:1 (GAS-to-neutrophil ratio).
Virulence of GAS in a humanized plasminogen transgenic mouse model.
Transgenic humanized plasminogen mice heterozygous for the human plasminogen transgene (AlbPLG1+/−) were infected with a dose of 2 × 107 to 4 × 107 CFU of either 5448, 5448ΔmntE, or 5448ΔmntE::mntE. Mice (n = 20 for 5448 WT and 5448ΔmntE::mntE, n = 19 for 5448ΔmntE) were subcutaneously infected with freshly prepared GAS strains in 100 µl of 1× phosphate-buffered saline (PBS), and virulence was assessed as previously described (31).
Ethics approval.
All animal experiments were conducted according to the Guidelines for the Care and Use of Laboratory Animals (National Health and Medical Research Council, Australia) and were approved by the University of Queensland Animal Ethics Committee. Human blood donation for use in neutrophil killing assays was conducted in accordance with the National Statement on Ethical Conduct in Human Research, complied with the regulations governing experimentation on humans, and was approved by the University of Queensland Medical Research Ethics Committee.
Statistical analyses.
Differences in intracellular metal ion concentration, relative gene expression, and neutrophil survival were analyzed using unpaired Student’s t test or 1-way analysis of variance (ANOVA) with Bonferroni’s posttest as necessary (GraphPad Prism 6). Murine survival curves were analyzed using the Mantel-Cox log rank test (GraphPad Prism 6).
SUPPLEMENTAL MATERIAL
Drop test analysis of 5448, 5448ΔmntE, and 5448ΔmntE::mntE on THY agar without and with supplemented cadmium (A), cobalt (B), copper (C), iron (D), nickel (E), and zinc (F). Cells were grown and adjusted to an OD600 of 0.6 and serially diluted, and 5-µl drops were spotted onto the plate from concentrations of 100 (top) to 10−5 (bottom). Download
Gene expression of mntE in 5448 WT, 5448ΔmntE, and 5448ΔmntE::mntE grown in THY broth alone (white bars) or in the presence (black bars) of 20 µM cadmium, 1 mM cobalt, 0.50 mM copper, 2 mM iron, 1 mM nickel, or 1 mM zinc. Expression of mntE was calculated using the 2−ΔΔCT method using proS as the reference gene. The graph represents means and standard deviations of three independent experiments. Download
List of strains and plasmids used in this study
List of primers used in this study
ACKNOWLEDGMENT
This research was supported by the National Health and Medical Research Council of Australia (grant 565526).
Footnotes
Citation Turner AG, Ong CY, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ. 2015. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio 6(2):e00278-15. doi:10.1128/mBio.00278-15.
REFERENCES
- 1.Papp-Wallace KM, Maguire ME. 2006. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre JD, Culotta VC. 2012. Battles with iron: manganese in oxidative stress protection. J Biol Chem 287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng HJ, McEwan AG, Paton JC, Jennings MP. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun 70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janulczyk R, Ricci S, Björck L. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun 71:2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stafford SL, Bokil NJ, Achard ME, Kapetanovic R, Schembri MA, McEwan AG, Sweet MJ. 2013. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci Rep 33:541–554. doi: 10.1042/BSR20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, Rathi S, Chazin WJ, Caprioli RM, Skaar EP. 2013. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun 81:3395–3405. doi: 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finney LA, O’Halloran TV. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 10.Hantke K. 2001. Bacterial zinc transporters and regulators. Biometals 14:239–249. doi: 10.1023/A:1012984713391. [DOI] [PubMed] [Google Scholar]
- 11.Valko M, Morris H, Cronin MT. 2005. Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 12.Nies DH, Silver S. 1995. Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol 14:186–199. doi: 10.1007/BF01569902. [DOI] [PubMed] [Google Scholar]
- 13.McEwan AG. 2009. New insights into the protective effect of manganese against oxidative stress. Mol Microbiol 72:812–814. doi: 10.1111/j.1365-2958.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- 14.Jakubovics NS, Jenkinson HF. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709–1718. [DOI] [PubMed] [Google Scholar]
- 15.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. 2009. Role of the manganese efflux system MntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol 72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis 190:136–147. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- 17.Veyrier FJ, Boneca IG, Cellier MF, Taha MK. 2011. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog 7:e1002261. doi: 10.1371/journal.ppat.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. 2014. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev 27:264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 20.Michos AG, Bakoula CG, Braoudaki M, Koutouzi FI, Roma ES, Pangalis A, Nikolopoulou G, Kirikou E, Syriopoulou VP. 2009. Macrolide resistance in Streptococcus pyogenes: prevalence, resistance determinants, and emm types. Diagn Microbiol Infect Dis 64:295–299. doi: 10.1016/j.diagmicrobio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Henningham A, Gillen CM, Walker MJ. 2012. Group A streptococcal vaccine candidates: potential for the development of a human vaccine. Curr Top Microbiol 368:207–242. doi: 10.1007/82_2012_284. [DOI] [PubMed] [Google Scholar]
- 22.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. 2014. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J Infect Dis 209:1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 23.Aziz RK, Pabst MJ, Jeng A, Kansal R, Low DE, Nizet V, Kotb M. 2004. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol Microbiol 51:123–134. doi: 10.1046/j.1365-2958.2003.03797.x. [DOI] [PubMed] [Google Scholar]
- 24.Von Rozycki T, Nies DH. 2009. Cupriavidus metallidurans: evolution of a metal-resistant bacterium. Antonie Van Leeuwenhoek 96:115–139. doi: 10.1007/s10482-008-9284-5. [DOI] [PubMed] [Google Scholar]
- 25.Cubillas C, Vinuesa P, Tabche ML, Davalos A, Vazquez A, Hernandez-Lucas I, Romero D, Garcia-de los Santos A. 2014. The cation diffusion facilitator protein EmfA of Rhizobium etli belongs to a novel subfamily of Mn(2+)/Fe(2+) transporters conserved in alpha-proteobacteria. Metallomics 6:1808–1815. doi: 10.1039/c4mt00135d. [DOI] [PubMed] [Google Scholar]
- 26.Kloosterman TG, van der Kooi-Pol MM, Bijlsma JJ, Kuipers OP. 2007. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol Microbiol 65:1049–1063. doi: 10.1111/j.1365-2958.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 27.Ricci S, Janulczyk R, Björck L. 2002. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect Immun 70:4968–4976. doi: 10.1128/IAI.70.9.4968-4976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JW, Helmann JD. 2006. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 29.Makthal N, Rastegari S, Sanson M, Ma Z, Olsen RJ, Helmann JD, Musser JM, Kumaraswami M. 2013. Crystal structure of peroxide stress regulator from Streptococcus pyogenes provides functional insights into the mechanism of oxidative stress sensing. J Biol Chem 288:18311–18324. doi: 10.1074/jbc.M113.456590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aziz RK, Ismail SA, Park HW, Kotb M. 2004. Post-proteomic identification of a novel phage-encoded streptodornase, Sda1, in invasive M1T1 Streptococcus pyogenes. Mol Microbiol 54:184–197. doi: 10.1111/j.1365-2958.2004.04255.x. [DOI] [PubMed] [Google Scholar]
- 31.Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, Simpson AJ, Buchanan JT, Chhatwal GS, Kotb M, Nizet V. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med 13:981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- 32.Wang CH, Chiang-Ni C, Kuo HT, Zheng PX, Tsou CC, Wang S, Tsai PJ, Chuang WJ, Lin YS, Liu CC, Wu JJ. 2013. Peroxide responsive regulator PerR of group A Streptococcus is required for the expression of phage-associated DNase Sda1 under oxidative stress. PLoS One 8:e81882. doi: 10.1371/journal.pone.0081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole JN, Barnett TC, Nizet V, Walker MJ. 2011. Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol 9:724–736. doi: 10.1038/nrmicro2648. [DOI] [PubMed] [Google Scholar]
- 34.Cole JN, McArthur JD, McKay FC, Sanderson-Smith ML, Cork AJ, Ranson M, Rohde M, Itzek A, Sun H, Ginsburg D, Kotb M, Nizet V, Chhatwal GS, Walker MJ. 2006. Trigger for group A streptococcal M1T1 invasive disease. FASEB J 20:1745–1747. doi: 10.1096/fj.06-5804fje. [DOI] [PubMed] [Google Scholar]
- 35.Ong CL, Potter AJ, Trappetti C, Walker MJ, Jennings MP, Paton JC, McEwan AG. 2013. Interplay between manganese and iron in pneumococcal pathogenesis: role of the orphan response regulator RitR. Infect Immun 81:421–429. doi: 10.1128/IAI.00805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogunniyi AD, Mahdi LK, Jennings MP, McEwan AG, McDevitt CA, Van der Hoek MB, Bagley CJ, Hoffmann P, Gould KA, Paton JC. 2010. Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J Bacteriol 192:4489–4497. doi: 10.1128/JB.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlach D, Reichardt W, Vettermann S. 1998. Extracellular superoxide dismutase from Streptococcus pyogenes type 12 strain is manganese-dependent. FEMS Microbiol Lett 160:217–224. doi: 10.1111/j.1574-6968.1998.tb12914.x. [DOI] [PubMed] [Google Scholar]
- 38.Sobota JM, Imlay JA. 2011. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci USA 108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marra A, Lawson S, Asundi JS, Brigham D, Hromockyj AE. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148:1483–1491. [DOI] [PubMed] [Google Scholar]
- 40.Lisher JP, Higgins KA, Maroney MJ, Giedroc DP. 2013. Physical characterization of the manganese-sensing pneumococcal surface antigen repressor from Streptococcus pneumoniae. Biochemistry 52:7689–7701. doi: 10.1021/bi401132w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merchant AT, Spatafora GA. 2014. A role for the DtxR family of metalloregulators in gram-positive pathogenesis. Mol Oral Microbiol 29:1–10. doi: 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenot A, King KY, Caparon MG. 2005. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol Microbiol 55:221–234. doi: 10.1111/j.1365-2958.2004.04370.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee JW, Helmann JD. 2006. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J Biol Chem 281:23567–23578. doi: 10.1074/jbc.M603968200. [DOI] [PubMed] [Google Scholar]
- 44.Seki M, Iida K, Saito M, Nakayama H, Yoshida S. 2004. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase and coupling with aerobic utilization of lactate. J Bacteriol 186:2046–2051. doi: 10.1128/JB.186.7.2046-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stríz I, Trebichavský I. 2004. Calprotectin—a pleiotropic molecule in acute and chronic inflammation. Physiol Res 53:245–253. [PubMed] [Google Scholar]
- 46.Eijkelkamp BA, Morey JR, Ween MP, Ong CL, McEwan AG, Paton JC, McDevitt CA. 2014. Extracellular zinc competitively inhibits manganese uptake and compromises oxidative stress management in Streptococcus pneumoniae. PLoS One 9:e89427. doi: 10.1371/journal.pone.0089427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anjem A, Imlay JA. 2012. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem 287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun 68:2819–2826. doi: 10.1128/IAI.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Couñago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O’Mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, McDevitt CA. 2014. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol 10:35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 50.Chaffin DO, Beres SB, Yim HH, Rubens CE. 2000. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J Bacteriol 182:4466–4477. doi: 10.1128/JB.182.16.4466-4477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol 16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 52.Sanderson-Smith ML, Dinkla K, Cole JN, Cork AJ, Maamary PG, McArthur JD, Chhatwal GS, Walker MJ. 2008. M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J 22:2715–2722. doi: 10.1096/fj.07-105643. [DOI] [PubMed] [Google Scholar]
- 53.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li WZ, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Venturini C, Ong CL, Gillen CM, Ben-Zakour NL, Maamary PG, Nizet V, Beatson SA, Walker MJ. 2013. Acquisition of the Sda1-encoding bacteriophage does not enhance virulence of the serotype M1 Streptococcus pyogenes strain sf370. Infect Immun 81:2062–2069. doi: 10.1128/IAI.00192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drop test analysis of 5448, 5448ΔmntE, and 5448ΔmntE::mntE on THY agar without and with supplemented cadmium (A), cobalt (B), copper (C), iron (D), nickel (E), and zinc (F). Cells were grown and adjusted to an OD600 of 0.6 and serially diluted, and 5-µl drops were spotted onto the plate from concentrations of 100 (top) to 10−5 (bottom). Download
Gene expression of mntE in 5448 WT, 5448ΔmntE, and 5448ΔmntE::mntE grown in THY broth alone (white bars) or in the presence (black bars) of 20 µM cadmium, 1 mM cobalt, 0.50 mM copper, 2 mM iron, 1 mM nickel, or 1 mM zinc. Expression of mntE was calculated using the 2−ΔΔCT method using proS as the reference gene. The graph represents means and standard deviations of three independent experiments. Download
List of strains and plasmids used in this study
List of primers used in this study