ABSTRACT
Choline is a water-soluble nutrient essential for human life. Gut microbial metabolism of choline results in the production of trimethylamine (TMA), which upon absorption by the host is converted in the liver to trimethylamine-N-oxide (TMAO). Recent studies revealed that TMAO exacerbates atherosclerosis in mice and positively correlates with the severity of this disease in humans. However, which microbes contribute to TMA production in the human gut, the extent to which host factors (e.g., genotype) and diet affect TMA production and colonization of these microbes, and the effects TMA-producing microbes have on the bioavailability of dietary choline remain largely unknown. We screened a collection of 79 sequenced human intestinal isolates encompassing the major phyla found in the human gut and identified nine strains capable of producing TMA from choline in vitro. Gnotobiotic mouse studies showed that TMAO accumulates in the serum of animals colonized with TMA-producing species, but not in the serum of animals colonized with intestinal isolates that do not generate TMA from choline in vitro. Remarkably, low levels of colonization by TMA-producing bacteria significantly reduced choline levels available to the host. This effect was more pronounced as the abundance of TMA-producing bacteria increased. Our findings provide a framework for designing strategies aimed at changing the representation or activity of TMA-producing bacteria in the human gut and suggest that the TMA-producing status of the gut microbiota should be considered when making recommendations about choline intake requirements for humans.
IMPORTANCE
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide, and increased trimethylamine N-oxide (TMAO) levels have been causally linked with CVD development. This work identifies members of the human gut microbiota responsible for both the accumulation of trimethylamine (TMA), the precursor of the proatherogenic compound TMAO, and subsequent decreased choline bioavailability to the host. Understanding how to manipulate the representation and function of choline-consuming, TMA-producing species in the intestinal microbiota could potentially lead to novel means for preventing or treating atherosclerosis and choline deficiency-associated diseases.
INTRODUCTION
A major role played by the intestinal microbiota is to aid in the harvest of nutrients from the diet (1). Dietary components that are not readily absorbed in the small intestine serve as growth substrates for members of the gut microbiota, which in turn can modify the bioavailability and nutritional properties of those same dietary components (1). For example, many of the beneficial effects associated with consumption of whole grains, vegetables, and fruits are at least in part mediated by end products of microbial metabolism, including short-chain fatty acids (e.g., butyrate) and phenolic acids (e.g., protocatechuic acid) (2–6). Likewise, gut microbes can also convert otherwise beneficial dietary compounds, such as choline, into metabolites that are detrimental to human health (7–10).
Choline is required for a wide range of biological activities, including maintaining the structural integrity of cell membranes, supporting cholinergic neurotransmission, and donating methyl groups in a number of biosynthetic reactions (11). Although choline is synthesized endogenously, this synthesis does not meet the levels necessary for optimal health (11). Previous studies have established that gut microbial metabolism of choline results in the production of trimethylamine (TMA) (12–14). Once TMA is absorbed by the host, it is further metabolized by flavin monooxygenases 1 and 3 (FMO1 and FMO3) in the host liver to generate trimethylamine-N-oxide (TMAO) (8, 15, 16).
Recent human studies have established that the levels of TMAO in serum are positively correlated with impaired renal function, colorectal cancer, and cardiovascular disease (CVD) (8, 10, 17, 18). TMAO exacerbates atherosclerosis in a genetic knockout mouse model, in part by promoting forward cholesterol transport and by inhibiting reverse cholesterol transport (8, 10, 19, 20). In addition, TMAO exacerbates impaired glucose tolerance, obstructs hepatic insulin signaling, and promotes adipose tissue inflammation of mice maintained on a high-fat high-sugar diet (21).
Subsequent experiments with the choline-degrading sulfate-reducing bacterium Desulfovibrio desulfuricans (i) revealed a pathway that involves a radical C–N bond cleavage of choline to generate TMA and acetaldehyde and (ii) identified a gene cluster encoding this activity (22). This cluster includes cutC, which encodes a glycyl radical enzyme with choline trimethylamine-lyase activity; cutD, which encodes a glycyl radical-activating protein; and genes encoding proteins involved in the assembly of microcompartments which may sequester the acetaldehyde generated as a by-product during TMA production (22, 23). However, the diversity of gut microbes that contribute to TMA production in humans and the impact of these species on serum TMAO levels and choline bioavailability remain unknown.
In this study, we used ultrahigh-pressure liquid chromatography coupled with tandem mass spectrometry (uHPLC-MS/MS) to identify human gut isolates able to convert choline into TMA. Follow-up gnotobiotic mouse experiments characterized the relative contributions of these microbes, host diet, and host factors (e.g., genotype) to choline bioavailability and TMAO accumulation.
RESULTS AND DISCUSSION
In vitro screening reveals human gut isolates able to generate TMA from choline.
Seventy-nine isolates representing six phyla found in the human intestinal tract (i.e., Bacteroidetes [21 strains], Firmicutes [36 strains], Actinobacteria [8 strains], Proteobacteria [12 strains], Verrucomicrobia [1 strain], and Lentisphaerae [1 strain]; see Table S1 in the supplemental material) were tested in vitro for choline consumption and TMA production from choline under anaerobic conditions. All strains were inoculated in a diluted gut medium (Table S2) supplemented with 15 mM choline and incubated for 24 h in a 96-well plate at 37°C (24). Cell-free supernatants were derivatized, diluted, and analyzed using uHPLC coupled to mass spectrometry on a high-resolution mass spectrometer (Thermo Scientific Q Exactive) (22). We identified eight species representing two different phyla (Firmicutes and Proteobacteria) and six genera that showed significant choline consumption and TMA accumulation: Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Escherichia fergusonii, Proteus penneri, Providencia rettgeri, and Edwardsiella tarda (Table S1 and Table 1). These strains generated TMA only if the medium was supplemented with choline. We confirmed that TMA was derived from choline by inoculating cultures of TMA-producing strains with labeled choline [choline chloride-(trimethyl-d9)], which resulted in the appearance of labeled TMA (trimethyl-d9) (see Fig. S1 in the supplemental material). These organisms consumed more than 60% of the choline provided in the growth media, unlike strains that did not make TMA (Table S1). None of the identified TMA-producing strains generated TMA from l-carnitine, another quaternary amine linked to TMAO accumulation, under the same test conditions (Table 1) (10).
TABLE 1 .
Bacterial strains used to colonize germ-free mice
| Strains introduced into germ-free micea |
In vitro TMA production in the presence of b: |
||||
|---|---|---|---|---|---|
| Bacterial strain | “Core” | “Core plus C. sporogenes” | “Core plus all” | Choline | l-Carnitine |
| Bacteroides caccae ATCC 43185 | √ | √ | √ | − | − |
| Bacteroides ovatus ATCC 8483 | √ | √ | √ | − | − |
| Bacteroides thetaiotaomicron VPI-5482 | √ | √ | √ | − | − |
| Collinsella aerofaciens ATCC 25986 | √ | √ | √ | − | − |
| Eubacterium rectale ATCC 33656 | √ | √ | √ | − | − |
| Anaerococcus hydrogenalis DSM 7454 | ○ | ○ | √ | + | − |
| Clostridium asparagiforme DSM 15981 | ○ | ○ | √ | + | − |
| Clostridium hathewayi DSM 13749 | ○ | ○ | √ | + | − |
| Clostridium sporogenes ATCC 15579 | ○ | √ | √ | + | − |
| Edwardsiella tarda ATCC 23685 | ○ | ○ | √ | + | − |
| Escherichia fergusonii ATCC 35469 | ○ | ○ | √ | + | − |
| Proteus penneri ATCC 35198 | ○ | ○ | √ | + | − |
| Providencia rettgeri DSM 1131 | ○ | ○ | √ | + | − |
Symbols: √, species present in the community; ○, species not present in the community.
Symbols: +, TMA produced; −, TMA not produced.
Seven of the eight identified species encode components of the choline utilization TMA-producing pathway described above (see Fig. S2 in the supplemental material), including cutC, cutD, and genes encoding proteins involved in assembly of microcompartments. In contrast, E. tarda strain 23685 produces TMA from choline but does not appear to contain these genes in the published draft genome, raising the possibility that it encodes a novel mechanism of choline metabolism. Different strains of the Edwardsiella tarda species varied in their ability to consume choline and generate TMA. While E. tarda ATCC 23685 strain generated TMA from choline, ATCC 15947 strain did not, suggesting that the ability of microbes to convert choline to TMA is a strain-specific metabolic trait that may be acquired via lateral gene transfer.
We also identified two species—Providencia alcalifaciens and Providencia rustiganii—predicted to encode key components of the choline utilization TMA-producing pathway that did not generate TMA in our original screen (see Table S1 and Fig. S2 in the supplemental material) (22). Further uHPLC-MS/MS analysis of these two organisms grown individually in Hungate tubes confirmed that they do not accumulate TMA after 24 h of incubation in the medium mentioned above, despite reaching high cell densities; however, P. rustiganii showed TMA accumulation after 72 h of incubation, whereas P. alcalifaciens did not generate TMA under any of the tested conditions (Table S1). Altogether, these results highlight the importance of functional studies when inquiring about the metabolic activities of a microbe and suggest that phylogeny is a poor predictor of microbial TMA production from choline.
Colonization with TMA-producing bacteria modulates TMAO accumulation in gnotobiotic mice.
Germ-free mouse models are of critical value for characterizing the properties and functions of gut microbes. We tested whether introducing defined changes in the composition of the gut microbiota can modulate cecal TMA and serum TMAO levels. Three groups of adult germ-free male C57BL/6J mice (5 mice/group) were orally gavaged with the following microbial mixtures: (i) the “core” community which included five species that do not produce TMA from choline in vitro, Collinsella aerofaciens, Bacteroides caccae, Bacteroides ovatus, Bacteroides thetaiotaomicron, and Eubacterium rectale (Table 1); (ii) the “core plus C. sporogenes” community that added one TMA producer (C. sporogenes) to the “core” community mixture; and (iii) the “core plus all” community that included the “core” community plus the eight TMA-producing species listed in Table 1. All mice were fed a purified diet containing 1% (wt/wt) choline (Harlan TD.140179; see Table S3 for diet composition) for a week before and 2 weeks after colonization. At sacrifice, serum, feces, and cecal contents were collected for analyses of metabolite concentration and microbial community composition.
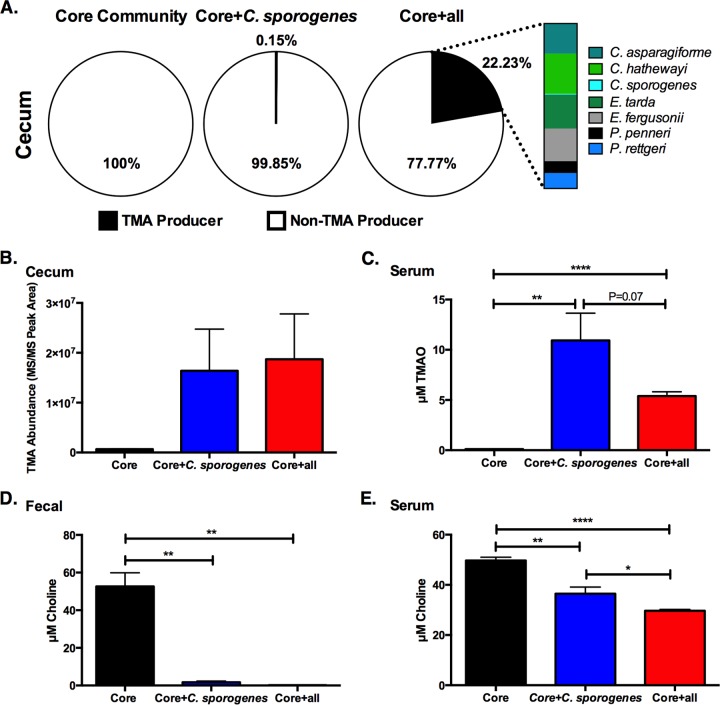
Figure 1A shows microbial community composition in the three groups of mice as determined by COPRO-Seq (community profiling by sequencing) analysis of cecal contents. With the exception of A. hydrogenalis, all species introduced into the mice were detected in cecal samples at a sequencing depth of ≥10,000 reads/sample, which allows us to detect microbes that represent at least 0.1% of the community. uHPLC-MS/MS analysis indicates that colonization with the “core” community did not result in the accumulation of TMA or TMAO (Fig. 1B and C). Addition of the TMA-producing species C. sporogenes, which represented only 0.15% ± 0.01% (average ± standard error of the mean [SEM]) of the cecal community, resulted in the significant reduction of fecal choline, accumulation of TMA in the cecum, and appearance of TMAO in serum (Fig. 1B to D). Colonization with the “core plus all” community resulted in >100-fold increase in the relative abundance of TMA-producing bacteria in the distal gut compared to “core plus C. sporogenes” community (Fig. 1A). Despite this, cecal levels of TMA, fecal levels of choline, and serum levels of TMAO were not significantly different between these two groups of mice (Fig. 1B to D). These results demonstrate a causal link between gut microbial TMA-producing status and TMAO accumulation in vivo and suggest that other factors (e.g., host genotype) besides the abundance of TMA-producing bacteria may account for differences in TMAO accumulation (25).
FIG 1 .
Colonization with TMA-producing bacteria affects the levels of choline and TMAO in serum. (A) COPRO-Seq (community profiling by sequencing) analysis of cecal contents from male mice colonized with (i) the “core” community, (ii) the “core plus C. sporogenes” community, and (iii) the “core plus all” community. The pie charts depict the combined abundance of TMA-producing species and non-TMA-producing species in the community. The color bar chart (right) shows the partial contribution of each TMA producer to the total TMA-producing fraction in “core plus all” community. A. hydrogenalis was not detectable in the cecal samples of mice colonized with the “core plus all” community (10,000 to 60,000 reads/sample). (B to E) TMA abundance (in arbitrary units) in cecum (B), serum levels of TMAO (C), fecal levels of choline (D), and (E) serum levels of choline in mice colonized with various communities. Values are averages plus standard errors of the means (SEMs) (error bars) (4 or 5 animals in each experimental group). Values that were significantly different by an unpaired two-tailed Student’s t test are indicated by a bar and asterisk as follows: *, P value of <0.05; **, P value of <0.01; ****, P value of <0.0001. Similar results were observed in adult female mice (i.e., TMA and TMAO levels were detected only when animals were colonized with TMA-producing bacteria).
Colonization with TMA-producing bacteria decreases levels of choline available to the host.
As mentioned above, colonization with TMA-producing bacteria results in a dramatic decrease in the abundance of choline in feces relative to mice colonized with only the “core” community (Fig. 1D). COPRO-seq analysis revealed that TMA-consuming bacteria were present in the small intestines of mice (the main site of choline absorption) (see Fig. S3, top row, in the supplemental material). We therefore tested whether colonization with TMA-producing species modulates choline bioavailability to the host. We measured serum levels of choline in the three groups of mice described above. Results disclosed a significant decrease in the serum levels of choline as the relative abundance of TMA producers increased (Fig. 1E). Similar trends were also seen in experiments performed in female mice (Fig. S4).
Significant reduction in choline consumption leads to organ dysfunction and failure along with an increased risk of the development of heart disease, cancer, and liver disease (11, 26). Currently, only an estimated 10% of the U.S. population consistently meets or exceeds the daily recommended intake of choline established by the Institute of Medicine (11). Furthermore, the composition of the gut microbiota and the representation of choline-consuming TMA-producing bacteria are not currently considered when developing daily recommended values at the population level. While further experimentation is required to determine whether the reduction in the bioavailability of choline observed here recapitulates the biochemical and pathological manifestations of choline deficiency, our findings suggest that determining personalized values could be required for optimal health for individuals.
Host gender affects FMO activity and TMAO accumulation in gnotobiotic mice colonized with the same microbiota.
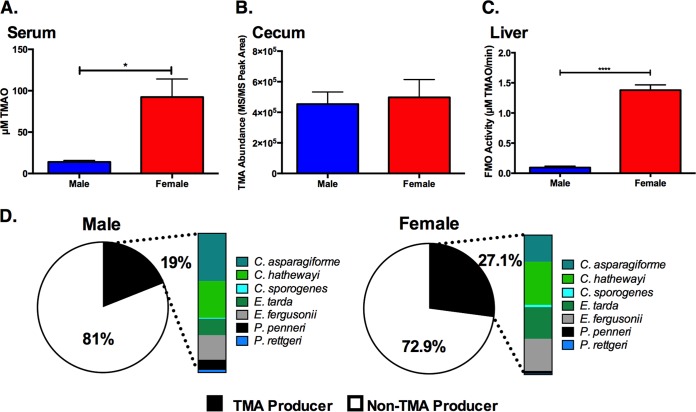
We examined TMAO accumulation in serum samples obtained from adult male and female gnotobiotic mice colonized with the “core plus all” community. uHPLC-MS/MS analysis of serum revealed that TMAO accumulated at significantly higher levels in females than in their male counterparts (Fig. 2A). Although females harbored increased levels of TMA-producing bacteria, cecal levels of TMA were not significantly different between the two groups (Fig. 2B and D). As mentioned above, microbiota-derived TMA is further metabolized by flavin monooxygenases (FMO) in the host liver to generate TMAO (8, 15, 16). FMO activity measurements in hepatic tissue homogenates indicated significantly higher enzymatic activity in females than in males (Fig. 2C). These data suggest that gender-associated differences in TMAO accumulation are likely not derived from higher levels of microbiota-generated TMA (Fig. 2B), but rather higher FMO activity in females, consistent with previous studies in conventionally raised mice which demonstrated that FMO3 is expressed at higher levels in females than in males (8, 27).
FIG 2 .
Gender modulates TMAO accumulation in serum. (A to D) Serum TMAO levels (A), cecal TMA abundance (B), total hepatic FMO enzymatic activity levels (C), and microbial community composition in cecal contents from male and female adult NMRI mice that were colonized with the “core plus all” community (Table 1) (D) and maintained on a choline-supplemented diet (4 or 5 mice in each experimental group). Samples with less than 10,000 reads were not used for analysis. COPRO-Seq results shown in the pie charts are the average abundance of TMA-producing species and non-TMA-producing species in the community. The color bar charts show the partial contribution of each TMA producer to the total TMA-producing fraction. Statistical significance was calculated by an unpaired two-tailed Student’s t test and indicated by a bar and asterisks as follows: *, P value of <0.05; ****, P value of <0.0001.
Host genotype and community composition modulate FMO activity and TMAO accumulation in female mice.
We compared serum TMAO levels in two strains of mice (C57BL/6J and NMRI) colonized with the same “core plus all” community. uHPLC-MS/MS analysis disclosed similar levels of TMAO among males of the two strains (P > 0.1) (see Fig. S5 in the supplemental material). In contrast, NMRI females showed a 2-fold increase in serum TMAO levels compared to their C57BL/6J counterparts (Fig. 3A). Similar differences in TMAO levels were observed between C57BL/6J and NMRI adult females colonized with the “core plus C. sporogenes” community (Fig. 3A). Consistent with these findings, hepatic FMO enzyme activity assays showed higher FMO activity in NMRI females than in C57BL/6J females (Fig. 3B).
FIG 3 .

Host genotype and community composition modulate FMO activity and serum TMAO levels. (A and B) Serum TMAO levels (A) and total hepatic FMO enzymatic activity levels (B) measured in adult female C57BL/6J and NMRI mice colonized with the “core plus C. sporogenes” community and with the “core plus all” community(average plus SEM; 3 to 6 animals in each experimental group). Significance was calculated by an unpaired two-tailed Student’s t test as follows: *, P value of <0.05; ****, P value of <0.0001.
Measurements of serum TMAO levels as a function of microbiota composition revealed that both strains of female mice colonized with the “core plus all” community accumulated lower serum levels of TMAO and exhibited lower hepatic FMO activity than their counterparts colonized with the “core plus C. sporogenes” community (Fig. 3A and B). A similar trend in serum TMAO levels was observed in male mice colonized by these two communities (Fig. 1C). These results suggest that gut microbiota composition affects TMAO levels, independently of TMA production, and that at least one species present in the “core plus all” community reduces FMO activity.
Expression of the main FMO enzyme involved in TMAO production from TMA, FMO3, is induced by bile acids via a mechanism that involves the farnesoid X receptor (FXR) (27). Specifically, cholic acid stimulates FMO3 expression (27). Genome analysis for members of the “core plus all” community, using the curated database for metabolic pathways MetaCyc, disclosed that Clostridium hathewayi carries genes that encode proteins (3-α-hydroxysteroid dehydrogenase/carbonyl reductase and 3-oxo-cholyl-coenzyme A [CoA] oxidoreductase) predicted to be involved in the metabolism of cholic acid that were not detected in members of the “core plus C. sporogenes” community (28). Thus, it is plausible that the decreased levels of TMAO detected in sera of mice colonized with the “core plus all” community (Fig. 1C and Fig. 3A) are caused by increased microbial degradation of cholic acid, which would result in lower levels of fmo3 expression (27).
Dietary choline is necessary for TMA production but does not impact the abundance of TMA-producing bacteria in a low-complexity gut microbial consortium.
To determine the impact of dietary choline on community composition and serum levels of TMAO, adult male C57BL/6J germ-free mice were inoculated by oral gavage with the “core plus all” community. Mice were maintained for 2 weeks on either the 1% (wt/wt) choline diet described above or the same diet formulated without choline (i.e., choline-deficient diet; see Table S4 in the supplemental material). uHPLC-MS/MS analysis of samples collected at the time of sacrifice showed that mice with choline in their diet showed detectable levels of TMAO in their serum, whereas mice fed the choline-deficient diet did not (Fig. 4A). There were no significant differences in the total abundance of TMA-producing bacteria in the cecum in the two groups of mice (24.7% ± 2.2% for the choline-deficient mice and 24.1% ± 1.8% for the mice given choline [average ± SEM]) despite significant changes in the relative abundance of specific TMA-producing species (C. hathewayi and P. rettgeri; P < 0.05 by Student’s t test) (Fig. 4B). Both C. hathewayi and P. rettgeri increased in their abundance in response to dietary choline together with C. asparagiforme (P = 0.10), whereas E. tarda (P = 0.06), and E. fergusonii (P = 0.09) showed decreased abundance, although these changes did not reach statistical significance in our experiments. These results suggest that dietary choline is not necessary for colonization of choline-consuming TMA-producing bacteria and that dietary choline does not seem to provide these species with a major fitness advantage, at least in our simplified gnotobiotic mouse model of the human gut ecosystem.
FIG 4 .

Dietary choline is required for TMAO accumulation. (A) Levels of TMAO in serum from adult male C57BL/6J mice colonized with the “core plus all” community (Table 1) and fed a 1% (wt/wt) choline-supplemented diet or a choline-deficient diet for 2 weeks after colonization. Data shown are averages plus SEMs (3 mice per group). Similar results were observed when the experiment was conducted in NMRI mice (4 or 5 mice per group; see Fig. S6 in the supplemental material). (B) COPRO-Seq analysis of cecal contents from the mice described above for panel A. Samples included in the analysis have >10,000 reads. The pie charts depict combined abundance of TMA-producing species and non-TMA-producing species in the community. The color bar charts show the partial contribution of each TMA producer to the total TMA-producing fraction. Values that were statistically significant (P value of <0.01) by an unpaired two-tailed Student’s t test are indicated (**).
Altogether, the presented results highlight the multiple factors, i.e., microbial, host, and environmental factors, that modulate metabolism of choline to TMAO. Future studies aimed at understanding how to manipulate the representation of choline-consuming TMA-producing bacteria in the gut microbiota or at identifying species that modulate host conversion of TMA to TMAO might lead to novel interventions for preventing or treating atherosclerosis and/or choline deficiency-associated diseases.
MATERIALS AND METHODS
Growth medium.
All bacteria were grown on Mega Medium (see Table S2 in the supplemental material) (24). This medium was filter sterilized and stored in a Coy anaerobic chamber (5% H2, 20% CO2, and 75% N2) at least 24 h prior to use.
Gnotobiotic husbandry.
All experiments involving mice were performed using protocols approved by the University of Wisconsin—Madison Animal Care and Use Committee. Both C57BL/6J and NMRI strains were maintained in a controlled environment in plastic flexible film gnotobiotic isolators under a strict 12-h light cycle and received sterilized water and food ad libitum. Experimental diets were sterilized by irradiation. Table S3 and Table S4 in the supplemental material show the compositional information for the choline-supplemented and choline-deficient diets used in our experiments. Sterility of germ-free animals was assessed by incubating freshly collected fecal samples under aerobic and anaerobic conditions using standard microbiology methods.
Gnotobiotic mouse colonization.
Strains used to colonize mice were grown as monocultures on Mega Medium agar plates anaerobically for 48 to 72 h at 37°C. Single colonies were then inoculated into 3 ml of Mega Medium and grown anaerobically for 36 h at 37°C. After 36 h, strains belonging to the same treatment group were combined in an equal volume ratio in a Hungate tube. Germ-free 6- to 16-week-old mice were inoculated by oral gavage with ~0.2 ml of mixed bacterial culture inside the gnotobiotic isolator, using a mix of 5, 6, or 13 strains as shown in Table 1. Mice were maintained on the experimental diet for a week before and for 2 weeks after colonization. The mice were then sacrificed, and their intestinal contents were immediately collected, frozen, and stored at −80°C.
uHPLC-MS/MS analysis of metabolites.
Twenty-five microliters of frozen bacterial cultures were inoculated into 1 ml of Mega Medium supplemented with 15 mM choline chloride (Sigma-Aldrich) in a 96-well deep-well plate sealed with sterile foil, and incubated anaerobically for 48 h at 37°C. Cell culture supernatants were harvested by centrifugation at 4°C and then derivatized according to published methods with minor modifications to accommodate the large number of samples being run in parallel (22). Samples were filtered through a 0.2-µm filter (Millipore) and diluted with uHPLC-grade H2O. Supernatant used to measure choline was not derivatized before being filtered with a 0.2-µm filter and diluted 1:10,000 using uHPLC-grade H2O. Samples were analyzed using a uHPLC coupled to a high-resolution mass spectrometer (Thermo Scientific Q Exactive) (see “uHPLC-MS/MS parameters” below).
Serum samples were prepared for analysis by precipitating proteins with 4 volumes of ice-cold methanol spiked with 2.5 µM deuterium-labeled choline and TMAO internal standards. Samples were centrifuged at 18,213 × g at 4°C for 3 min. The recovered supernatants were diluted 1:1 in uHPLC-grade water prior to screening. Feces and intestinal contents were homogenized with a 40:40:20 mixture of ice-cold acetonitrile, methanol, and water (20 µl/mg of sample). Samples were centrifuged for 5 min at 4°C at 7,227 × g, and supernatants were prepared as described above for TMA or diluted 1:10,000 for choline quantification.
Liver homogenate samples were prepared and incubated according to previously published methods with minor modifications (27). Briefly, protein was extracted from liver samples in radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) spiked with a protease inhibitor cocktail (catalog no. 97036-010; VWR) and quantified using a Bradford assay kit (Bio-Rad) after a 1:100 dilution in double-distilled water (ddH2O). Determination of FMO enzymatic activity was conducted in 250-µl reaction mixtures containing 1 mg protein homogenate, 100 µM TMA, and 100 µM NADPH in 10 mM HEPES (pH 7.4). The reactions were quenched with 100 µl acetonitrile after 1, 5, 15, 60, and 120 min of incubation at 37°C. FMO activity was determined by calculating the conversion rate of TMA to TMAO during the first 5 min of incubation.
uHPLC-MS/MS parameters.
After sample preparation, identification and quantitation of TMA, TMAO, and choline was performed using a uHPLC (Dionex 3000) coupled to a high-resolution mass spectrometer (Thermo Scientific Q Exactive). Liquid chromatography separation was achieved on a Dikma Bio-Bond C4 column (150 mm by 2.1 mm; 3-µm particle size) using a 7-min isocratic gradient (50:50 methanol [MeOH]−water, 5 mM ammonium formate, and 0.1% formic acid). A heated electrospray ionization interface, working in positive mode, was used to direct column eluent to the mass spectrometer. Quantitation of TMA, D9-TMA, TMAO, and D9-TMAO was performed via targeted MS/MS using the following paired masses of parent ions and fragments: TMA (146.118 and 118.0865), D9-TMA (155.1740 and 127.1434), TMAO (76.0762 and 58.0659), and D9-TMAO (85.1318 and 68.1301). Quantitation of choline and d9-choline was performed in full-MS scan mode by monitoring their exact masses: 104.1075 and 113.1631, respectively.
COPRO-Seq analysis.
Bacterial communities resulting from inoculation of germ-free animals were analyzed using Illumina sequencing according to the COPRO-Seq (community profiling by sequencing) method (29). In short, DNA isolated from contents of the intestine via bead beating was used to prepare libraries for shotgun Illumina sequencing. Five hundred nanograms of DNA from each sample was fragmented by sonication and subjected to enzymatic blunting and adenine tailing. Customized Illumina adapters containing maximally distant 8-bp bar codes were ligated to the poly(A)-tailed DNA. Gel-extracted DNA (size selection ~250 to 300 bp) was amplified by PCR using primers and cycling conditions recommended by Illumina. Purified PCR products were submitted to the UW-Madison Biotechnology Center for a single end 50-bp Illumina MiSeq run. Results were processed using the software pipeline detailed by McNulty et al. (29).
Data deposition.
The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo) under accession number GSE63461.
SUPPLEMENTAL MATERIAL
Microbial conversion of labeled choline chloride-(trimethyl-d9) results in accumulation of labeled TMA (trimethyl-d9). Strains identified to consume choline and produce TMA in the initial screen were incubated with labeled choline [choline chloride-(trimethyl-d9)]. Data shown are averages of labeled TMA (trimethyl-d9) abundance (MS/MS peak area) ± SEMs (n = 3) for strains used in the “core plus all” community (Table 1). Download
Gene neighborhoods containing genes predicted to be involved in TMA production from choline. (A) Putative choline utilization gene cluster identified in D. desulfuricans. (B) BLAST analysis identifies components of this pathway in nine human gut strains screened in vitro. All strains except for P. alcalfaciens generated TMA from choline using an in vitro assay (see Table S1 in the supplemental material). All gene neighborhoods are diagrammed on the forward strand for simplified visual comparison. Gene size, position, and identification were determined by comparison of multiple genome annotations. Download
TMA-producing bacteria are detected in the small and large intestines of gnotobiotic mice. COPRO-Seq analysis of luminal contents collected from the small intestines, ceca, and large intestines from mice colonized with (i) the “core” community, (ii) the “core plus C. sporogenes” community, and (iii) the “core plus all” community. The pie charts depict combined abundance of TMA-producing species and non-TMA-producing species in the community. Samples with less than 10,000 reads were not used for analysis of the cecum or large intestine. Samples with less than 1,000 reads were not used for small intestine samples. A lower cutoff for small intestine samples was necessary, given the high levels of host (Mus musculus) DNA detected in these samples. Download
TMA producer abundance modulates the bioavailability of choline to the host in female mice. Serum levels of choline measured in female mice colonized with the “core plus C. sporogenes” or “core plus all” community (average ± SEM; 4 or 5 animals in each experimental group). Significance was calculated using an unpaired two-tailed Student’s t test and indicated as follows: *, P value of <0.05; **, P value of <0.01. Download
C57BL/6J and NMRI male mice exhibit comparable serum levels of TMAO. Serum TMAO levels were measured in adult male C57BL/6J and NMRI mice colonized with the “core plus all” community (average ± SEM; 3 to 6 animals in each experimental group). Significance (P value of >0.1) was calculated using an unpaired two-tailed Student’s t test. Download
Dietary choline is required for TMAO accumulation in NMRI mice. (A) TMAO levels in sera obtained from adult male NMRI mice colonized with the “core plus all” community (Table 1) fed a diet supplemented with 1% (wt/wt) choline or a choline-deficient diet for 2 weeks after colonization. Data shown are average ± SEM (n = 4 to 5). (B) COPRO-Seq analysis of cecal contents from the mice described above for panel A. Samples included in the analysis have >10,000 reads. The pie charts depict combined abundance of TMA-producing species and non-TMA-producing species in the community. The color bar charts show the partial contribution of each TMA producer to the total TMA-producing fraction. Significance was calculated using an unpaired two-tailed Student’s t test and indicated as follows: ****, P value of <0.0001. Download
Bacterial strains used in the high-throughput screen.
Composition of Mega Medium.
Choline supplemented diet (1% wt/wt).
Choline-deficient diet.
ACKNOWLEDGMENTS
We thank David Stevenson for technical support and Nate McNulty for assistance with COPRO-Seq analysis pipeline. We are indebted to Jeffrey I. Gordon, Dave O’Donnell, Maria Karlson, Janaki Guruge, and Sabrina Wagoner.
The project described was supported by the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS) grants UL1TR000427 and KL2TR000428 (F.E.R.).
Footnotes
Citation Romano KA, Vivas EI, Amador-Noguez D, Rey FE. 2015. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6(2):e02481-14. doi:10.1128/mBio.02481-14.
REFERENCES
- 1.Tremaroli V, Bäckhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 3.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, Gordon JI. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. 2011. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. 2007. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr 137:2043–2048. [DOI] [PubMed] [Google Scholar]
- 7.Zeisel SH, daCosta KA, Youssef M, Hensey S. 1989. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J Nutr 119:800–804. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeth RA, Wang Z, Levison BS, Buffa JA, Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. 2013. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel SH, da Costa K-A. 2009. Choline: an essential nutrient for public health. Nutr Rev 67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisel SH, Wishnok JS, Blusztajn JK. 1983. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther 225:320–324. [PubMed] [Google Scholar]
- 13.De la Huerga J, Popper H. 1951. Urinary excretion of choline metabolites following choline administration in normals and patients with hepatobilary disease. J Clin Invest 30:463–470. doi: 10.1172/JCI102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward HR. 1960. Anaerobic degradation of choline. III. Acetaldehyde as an intermediate in the fermentation of choline by extracts of Vibrio cholinicus. J Biol Chem 235:3592–3596. [PubMed] [Google Scholar]
- 15.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. 2015. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker JR, Chaykin S. 1962. The biosynthesis of trimethylamine-N-oxide. J Biol Chem 237:1309–1313. doi: 10.1016/0006-3002(60)90062-7. [DOI] [PubMed] [Google Scholar]
- 17.Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. 2015. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng T-YD, Miller JW, Green R, Lane DS, Beresford SAA, Caudill MA. 2014. Plasma choline metabolites and colorectal cancer risk in the women’s health initiative observational study. Cancer Res 74:7442–7452. doi: 10.1158/0008-5472.CAN-14-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjørndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. 1 December 2014. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. 2014. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. 2014. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng 118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Craciun S, Balskus EP. 2012. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A 109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thibodeaux CJ, van der Donk WA. 2012. Converging on a mechanism for choline degradation. Proc Natl Acad Sci U S A 109:21184–21185. doi: 10.1073/pnas.1219534110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, Gordon JI. 2011. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A 108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller CA, Corbin KD, da Costa K-A, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O’Connor A, Zeisel SH. 2014. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr 100:778–786. doi: 10.3945/ajcn.114.087692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strilakou AA, Lazaris AC, Perelas AI, Mourouzis IS, Douzis IC, Karkalousos PL, Stylianaki AT, Pantos CI, Liapi CA. 2013. Heart dysfunction induced by choline-deficiency in adult rats: the protective role of l-carnitine. Eur J Pharmacol 709:20–27. doi: 10.1016/j.ejphar.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. 2013. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, Holland TA, Keseler IM, Kothari A, Kubo A, Krummenacker M, Latendresse M, Mueller LA, Ong Q, Paley S, Subhraveti P, Weaver DS, Weerasinghe D, Zhang P, Karp PD. 2014. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 42:D459–D471. doi: 10.1093/nar/gkt1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, Chervaux C, Knights D, Lozupone CA, Knight R, Duncan AE, Bain JR, Muehlbauer MJ, Newgard CB, Heath AC, Gordon JI. 2011. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med 3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microbial conversion of labeled choline chloride-(trimethyl-d9) results in accumulation of labeled TMA (trimethyl-d9). Strains identified to consume choline and produce TMA in the initial screen were incubated with labeled choline [choline chloride-(trimethyl-d9)]. Data shown are averages of labeled TMA (trimethyl-d9) abundance (MS/MS peak area) ± SEMs (n = 3) for strains used in the “core plus all” community (Table 1). Download
Gene neighborhoods containing genes predicted to be involved in TMA production from choline. (A) Putative choline utilization gene cluster identified in D. desulfuricans. (B) BLAST analysis identifies components of this pathway in nine human gut strains screened in vitro. All strains except for P. alcalfaciens generated TMA from choline using an in vitro assay (see Table S1 in the supplemental material). All gene neighborhoods are diagrammed on the forward strand for simplified visual comparison. Gene size, position, and identification were determined by comparison of multiple genome annotations. Download
TMA-producing bacteria are detected in the small and large intestines of gnotobiotic mice. COPRO-Seq analysis of luminal contents collected from the small intestines, ceca, and large intestines from mice colonized with (i) the “core” community, (ii) the “core plus C. sporogenes” community, and (iii) the “core plus all” community. The pie charts depict combined abundance of TMA-producing species and non-TMA-producing species in the community. Samples with less than 10,000 reads were not used for analysis of the cecum or large intestine. Samples with less than 1,000 reads were not used for small intestine samples. A lower cutoff for small intestine samples was necessary, given the high levels of host (Mus musculus) DNA detected in these samples. Download
TMA producer abundance modulates the bioavailability of choline to the host in female mice. Serum levels of choline measured in female mice colonized with the “core plus C. sporogenes” or “core plus all” community (average ± SEM; 4 or 5 animals in each experimental group). Significance was calculated using an unpaired two-tailed Student’s t test and indicated as follows: *, P value of <0.05; **, P value of <0.01. Download
C57BL/6J and NMRI male mice exhibit comparable serum levels of TMAO. Serum TMAO levels were measured in adult male C57BL/6J and NMRI mice colonized with the “core plus all” community (average ± SEM; 3 to 6 animals in each experimental group). Significance (P value of >0.1) was calculated using an unpaired two-tailed Student’s t test. Download
Dietary choline is required for TMAO accumulation in NMRI mice. (A) TMAO levels in sera obtained from adult male NMRI mice colonized with the “core plus all” community (Table 1) fed a diet supplemented with 1% (wt/wt) choline or a choline-deficient diet for 2 weeks after colonization. Data shown are average ± SEM (n = 4 to 5). (B) COPRO-Seq analysis of cecal contents from the mice described above for panel A. Samples included in the analysis have >10,000 reads. The pie charts depict combined abundance of TMA-producing species and non-TMA-producing species in the community. The color bar charts show the partial contribution of each TMA producer to the total TMA-producing fraction. Significance was calculated using an unpaired two-tailed Student’s t test and indicated as follows: ****, P value of <0.0001. Download
Bacterial strains used in the high-throughput screen.
Composition of Mega Medium.
Choline supplemented diet (1% wt/wt).
Choline-deficient diet.