Abstract
Background:
Although exercise has been addressed as an adjuvant treatment for anxiety, depression and cancer-related symptoms, limited studies have evaluated the effectiveness of exercise in patients with lung cancer.
Methods:
We recruited 116 patients from a medical centre in northern Taiwan, and randomly assigned them to either a walking-exercise group (n=58) or a usual-care group (n=58). We conducted a 12-week exercise programme that comprised home-based, moderate-intensity walking for 40 min per day, 3 days per week, and weekly exercise counselling. The outcome measures included the Hospital Anxiety and Depression Scale and the Taiwanese version of the MD Anderson Symptom Inventory.
Results:
We analysed the effects of the exercise programme on anxiety, depression and cancer-related symptoms by using a generalised estimating equation method. The exercise group patients exhibited significant improvements in their anxiety levels over time (P=0.009 and 0.006 in the third and sixth months, respectively) and depression (P=0.00006 and 0.004 in the third and sixth months, respectively) than did the usual-care group patients.
Conclusions:
The home-based walking exercise programme is a feasible and effective intervention method for managing anxiety and depression in lung cancer survivors and can be considered as an essential component of lung cancer rehabilitation.
Keywords: anxiety, depression, exercise, lung cancer, symptoms
The proportion of lung cancer survivors is growing worldwide (Forman et al, 2013) and in Taiwan (Ministry of Health and Welfare, 2013). Patients with lung cancer frequently experience psychological problems, such as anxiety and depression. The prevalence rates of anxiety and depression in lung cancer survivors are 34% and 33%, respectively (Hopwood and Stephens, 2000). Lung cancer is a highly symptomatic disease, involving symptoms such as pain, fatigue, sleep disturbance, shortness of breath and dry mouth during the treatment period and in the follow-up stage (Wang et al, 2010; Pan et al, 2012). Anxiety, depression and cancer-related symptoms might limit the functional ability and impair the quality of life (QOL) in lung cancer survivors.
Although exercise provides several benefits for patients with cancer, the effects of exercise on anxiety, depression and cancer-related symptoms have been inconsistently reported in various studies. As demonstrated by three previous studies, exercise can reduce both anxiety and depression (Mehnert et al, 2011), only anxiety (Blacklock et al, 2010), or only depression (Midtgaard et al, 2011) in patients with breast cancer and other cancers. However, four previous studies have indicated that exercise intervention is ineffective in reducing both anxiety and depression in the patient population with breast, lung, colorectal and other cancers (Temel et al, 2009; Duijts et al, 2012; Jacobsen et al, 2013; Lin et al, 2014). Regarding cancer-related symptoms, a study with a small sample size demonstrated that exercise can reduce the symptoms of advanced lung cancer such as coughing and dyspnoea (Temel et al, 2009). Conversely, two other studies have indicated that exercise has no effect on cancer-related symptoms such as fatigue and sleep disturbance in patients with breast, colorectal or ovarian cancer (Dodd et al, 2010; Lin et al, 2014). These studies have involved the use of various exercise modalities (e.g., brisk walking, running, cycling, swimming and resistance training), including various exercise modes with varying frequency, intensity and duration, thereby resulting in inconsistent conclusions.
Schmitz et al (2010) suggested that patients with cancer must perform moderate-intensity aerobic exercises such as walking and cycling for 20–60 min per session for 3–5 days per week to improve mood and QOL and reduce fatigue (Schmitz et al, 2010). Walking is strongly recommended for patients with pulmonary diseases because it is involved in most activities of daily living (ADLs) (American College of Sports Medicine, 2006). In addition, walking is the most preferred exercise of patients with lung cancer because it is flexible and can be performed alone in Taiwanese patients with lung cancer (Lin et al, 2013).
A home-based, moderate-intensity walking programme can reduce anxiety (Mock et al, 1997; Courneya et al, 2003), depression (Courneya et al, 2003) and the severity of cancer-related symptoms (Mock et al, 1997; Courneya et al, 2003; Mock et al, 2005; Chang et al, 2014) in patients with breast and colorectal cancers; however, research on the effects of home-based walking exercise on patients with lung cancer remains limited. The current study aimed to determine the effectiveness of a 12-week home-based walking-exercise programme in managing anxiety, depression and the severity of cancer-related symptoms in Taiwanese patients with lung cancer. We hypothesised the walking-exercise group to be superior to the usual-care group regarding patient-rated anxiety, depression and the severity of cancer-related symptoms. According to a thorough literature review, this is the first study that investigates the psychological effects of home-based walking exercise on patients with lung cancer.
Materials and Methods
Research design and study sample
We conducted a parallel, randomised, controlled and single-centre trial with a home-based walking-exercise group and a usual-care group. Participants were recruited from a medical centre in northern Taiwan with the following eligibility criteria: patients who were diagnosed with primary lung cancer, were aged ⩾18 years, were able to speak Mandarin or Taiwanese and who exhibited no cognitive impairment. A patient was excluded if he or she (1) had engaged in regular exercise (150 min per week, moderate intensity) or had received cognitive behaviour therapy in the past 6 months; (2) had been diagnosed with congestive heart disease; (3) had been diagnosed with lower limb orthopaedic diseases that restrict walking ability; or (4) had been diagnosed with repeated onset of depression. The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital (Taipei, Taiwan).
Procedure
When the patients visited a physician at the outpatient department of this hospital and agreed to participate in the study, they were referred to the researcher, who explained the study procedure. After the participants received an explanation and provided informed consent, they underwent a baseline assessment (T1) at the hospital. The researchers used a computer to generate a randomisation list featuring four-person blocks before performing the experiments; the lists of the various groups were then sealed inside opaque envelops. During pretest data collection, one of the researchers opened the envelopes and randomly assigned participants to the walking-exercise or the usual-care group. Follow-up questionnaires were distributed by post during the third (T2) and sixth (T3) months. On study completion, the usual-care group participants received a walking-exercise booklet by post.
Intervention
The exercise programme was a 12-week home-based, moderate-intensity walking-exercise programme consisting of 40 min per session, 3 sessions per week, and weekly exercise counselling. During the enrolment period, we offered a walking-exercise booklet to the participants, and used its contents to instruct the patients regarding the mode, intensity and frequency of exercise; pulse-rate measurement; Borg's rating of perceived exertion (RPE) scale (6–20) (Borg, 1998); prevention of sports injuries; and the time point of terminating the exercise. Each participant was instructed to achieve a target heart rate of 60–80%, based on the Karvonen method (Karvonen et al, 1957), and a score of 13–15 on the RPE scale. In addition, the aforementioned researcher conducted weekly telephone interviews with the participants to discuss their exercise experiences and remind them to complete the exercise records. When a participant had not exercised for a minimum of 3 days because of reasons other than physical illness, the exercise programme content was reviewed with the participant, and he or she was encouraged to continue exercising. Because we contacted the participants to teach them the walking exercise, and also collected data during the follow-up periods, blinding of both the patients and the researcher was impossible. To compensate, three data (T1, T2 and T3) key-in procedures were performed by a research assistant to obscure patient identification data (e.g., names) before submitting the data to the researchers for subsequent statistical analyses. In addition, to control the research quality, the progress of this study was reported annually to the Institutional Review Board of Taipei Veterans General Hospital (Taipei, Taiwan). Project aspects, such as research design and intervention measures, were not modified during the research period.
Study measures
The patients' demographic data, including age, sex, educational attainment, employment status and marital status, were obtained using a baseline questionnaire. The cancer stage and current treatment records were acquired from the baseline medical records of the patients. The primary outcomes were anxiety and depression, as assessed using the Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983), that comprises seven items each for anxiety and depression. Each item of the anxiety subscale (HADS-A) and the depression subscale (HADS-D) was scored on a 4-point scale from 0 (not at all) to 3 (very much so). Higher scores indicated higher anxiety or depression levels. Subscale scores of ⩾11 were considered to indicate a ‘definite case', whereas subscale scores of 8 to 10 and 0 to 7 were considered to be a ‘suspicious case' and a ‘noncase', respectively, of anxiety or depression (Zigmond and Snaith, 1983). This scale is a reliable tool, and is widely used to assess patients with cancer (Feinstein et al, 2010; Duijts et al, 2012). We anticipated that the effects of exercise on anxiety and depression would be observable during the third month (T2) after intervention.
The secondary outcomes comprised the severity of cancer-related symptoms including pain, fatigue, nausea, sleep disturbance, sadness, shortness of breath, difficulty remembering, poor appetite, drowsiness, dry mouth, distress, vomiting and numbness that were assessed using the Taiwanese version of the MD Anderson Symptom Inventory (MDASI-T) (Lin et al, 2007). MDASI-T comprises 13 single-item measures of symptom intensity in the past 24 h, each of which is scored on an 11-point scale from 0 (not at all) to 10 (as bad as you can imagine). The symptom severity score was an average of these 13 symptom items: higher scores indicated greater symptom intensity. The questionnaire was validated for a sample of Taiwanese patients with cancer, which exhibited a strong Cronbach's α, with an internal consistency of 0.89 and a test–retest reliability of 0.97 (Lin et al, 2007). The MDASI-T scale is a reliable tool and has been used to assess Taiwanese patients with cancer (Chang and Lin, 2009; Pan et al, 2012).
Statistical analysis and sample size calculation
Based on the results from the eight participants in the pilot study, the mean (±s.d.) HADS-A scores of the walking-exercise and usual-care groups were 3.50 (3.42) and 5.75 (3.78), respectively, after the 12-week home-based walking exercise programme. Using the G*Power software (version 3.1.0) (Erdfelder et al, 2009) for repeated measures, and with a significance level of 0.05, an effect size of 0.30, a power of 0.8 and a correlation of 0.8, the sample size was estimated as 40 patients for each group. Based on an assumed dropout rate of 30%, 104 patients were considered adequate.
We used an intention-to-treat (ITT) approach. All statistical analyses were conducted using the IBM Statistical Package for the Social Sciences (SPSS) (Version 20) for Windows (IBM, Somers, NY, USA). We used t-test or χ2-test to determine the differences in the demographic data and the disease characteristics or outcome variables at baseline between the non-dropout patients (measured at all three time points) and the dropout patients (measured at one or two time points only). We used descriptive statistics to analyse the demographic data and disease characteristics as well as anxiety, depression and cancer-related symptom severity. We compared the baseline values for the demographic data, disease characteristics and outcome measures between the walking-exercise and usual-care groups by using the t-test or the χ2-test.
We used a general linear model to evaluate the mean and s.d. values, and the differences between the group outcomes (anxiety, depression and cancer-related symptom severity) at the baseline, third month and sixth month, and to model the outcomes (anxiety, depression and cancer-related symptom severity) as a function of the main effect (group differences) and main time effect. Both stability and repeated relationship analyses were conducted using generalised estimation equations (GEE). We added an interaction term (group difference × time) to each model to investigate the cooperative effect of exercise and time. The changes in study outcome values (anxiety, depression and cancer-related symptom severity) from baseline to follow-up periods (third and sixth months) were expressed in both the walking-exercise and usual-care groups. We used the general linear model to model the outcomes (number of patients with anxiety and depression according to the clinical cutoff point) as a function of the main effect (group differences). Both stability and repeated relationship analyses were conducted using GEE. An interaction term (group difference × time) was added to each model to investigate the interactive effects of exercise and time. All the tests involved a two-sided significance level of α=0.05.
Trial Registry: www.chictr.org; Identifier: ChiCTR-TRC-14004756.
Results
Participant characteristics
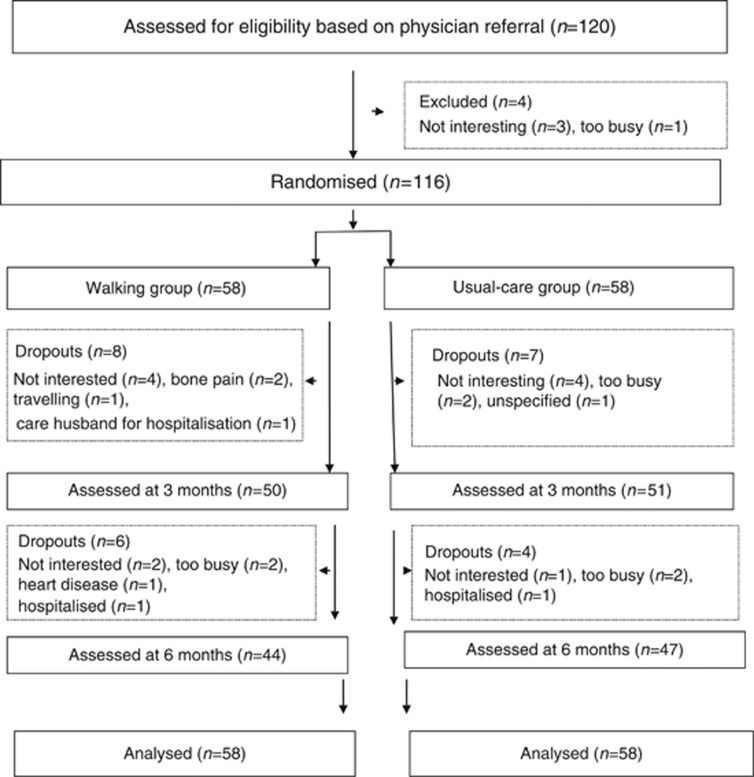
Participants were recruited from March 2010 to November 2012. As shown in Figure 1, 120 eligible patients from the outpatient department were referred by two physicians for study enrolment. After face-to-face consultations during which the study protocols and aims were explained to the patients in the hospital, four patients were excluded because they were uninterested or too busy to participate. As shown in Table 1, the sample comprised 116 patients (54 men and 62 women) aged 37–88 years, with a mean age of 64.16±10.89 years. Most participants were married and unemployed; 65% of participants had stage 1 lung cancer, and 54% had undergone operations. No statistically significant differences emerged in the baseline demographic data and disease characteristics between the walking and the usual-care groups (P>0.050). In addition, no significant differences emerged in the amount of baseline physical activity between both groups.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram showing the flow of participants through the trial.
Table 1. Demographic data and disease characteristics for all participants categorised based on the two groups (n=116).
| Variables | Walking-exercise group, n=58 | Usual-care group, n=58 | P-valuea |
|---|---|---|---|
|
Age (years) | |||
| Mean (s.d.) | 64.76 (11.28) | 63.57 (10.54) | 0.559 |
|
Education (years) | |||
| Mean (s.d.) | 10.66 (4.73) | 10.62 (4.66) | 0.969 |
|
Sex (n, %) | |||
| Male | 26 (44.8) | 28 (48.3) | 0.710 |
| Female | 32 (55.2) | 30 (51.7) | |
|
Employment (n, %) | |||
| No | 42 (72.4) | 34 (58.6) | 0.118 |
| Yes | 16 (27.6) | 24 (41.4) | |
|
Marital status (n, %) | |||
| Married | 48 (82.8) | 48 (82.8) | 1 |
| Not married/single | 10 (17.2) | 10 (17.2) | |
|
Cancer stage (n, %) | |||
| 1 | 34 (58.6) | 41 (70.7) | 0.681 |
| 2 | 5 (8.6) | 4 (6.9) | |
| 3 | 6 (10.4) | 5 (8.6) | |
| 4 | 5 (8.6) | 4 (6.9) | |
| Unknown | 8 (13.8) | 4 (6.9) | |
|
Current treatment (n, %) | |||
| No treatment | 19 (32.8) | 17 (29.3) | 0.889 |
| Operation | 30 (51.8) | 33 (57.0) | |
| Chemotherapy | 1 (1.7) | 0 (0.0) | |
| Radiotherapy | 2 (3.4) | 2 (3.4) | |
| Target therapy | 4 (6.9) | 5 (8.6) | |
| Chemotherapy and radiotherapy | 2 (3.4) | 1 (1.7) | |
The P-values are based on χ2 analyses for categorical variables and t-tests for continuous variables.
Missing value analysis
Measurements were recorded at 3 time points: the measurements for 91 (78.4%) participants (non-dropout patients) were recorded at all 3 time points, but for 25 participants (dropout patients), the measurements were recorded at 1 or 2 time points only, after which the patients dropped out. The dropout patients were older (68.14 vs 62.90 years, P=0.026) and exhibited higher baseline depression scores (6.57 vs 4.77, P=0.023) than did the non-dropout patients.
Overall, 14 (24.1%) and 11 (19.0%) participants dropped out in the walking and usual-care groups, respectively, but the difference that was observed in the dropout rates between the two groups (x2=0.450, P=0.498) was nonsignificant. The total attrition rate was 21.6%, and the reasons for dropping out are presented in Figure 1.
Protocol adherence
Of the 58 patients in the walking-exercise group, 26 (44.8%) completed the 12-week programme, and 36 (62%) completed at least half of the 12-week programme. The mean value of the number of completed walking-exercise sessions was 21.48 (s.d.=15.80). The exercise adherence rate was 59.7%. The participants did not complete the entire session because they either dropped out (n=10), perceived discomfort (n=12), lacked motivation (n=6) or were too busy (n=4). No exercise-related adverse effects were observed in the walking-exercise group during the study period.
Intervention effects on anxiety, depression and cancer-related symptoms
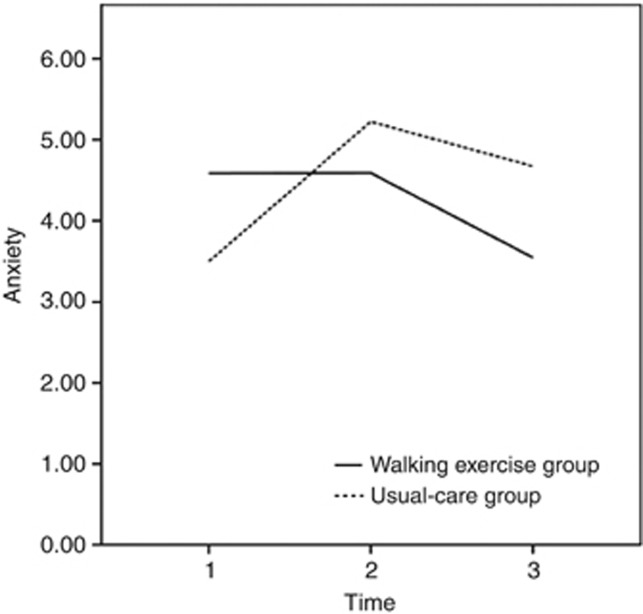
The mean anxiety scores at baseline were 4.59 (s.d.=3.46) and 3.50 (s.d.=3.91) in the walking-exercise and usual-care groups, respectively; no significant intergroup differences were observed (t=1.47, P=0.144; Table 2). The mean anxiety score of the walking-exercise group declined by 1.04 points between the baseline and the sixth month and was compared using the general linear model; however, this change was not statistically significant. In contrast, the mean anxiety score of the usual-care group at the third month (mean difference=1.72, P=0.012) increased significantly from baseline, and then remained stable until the sixth month (Table 2). The anxiety scores of the walking-exercise group declined by 0.63 points at the third month and by 1.03 points at the sixth month compared with the corresponding scores of the usual-care group, although these differences were not statistically significant (Table 2). Furthermore, a significant interaction term (group difference × time) of the model at the third month (β=−1.80, P=0.009) and sixth month (β=−2.18, P=0.006) verified that the walking exercise programme effectively reduced anxiety levels over time (Table 3 and Figure 2).
Table 2. Intention-to-treat analysis: mean and s.d. values and outcome differences between both groups at baseline and at third and sixth months according to the general linear model.
|
Baseline |
Thirrd month |
Sixth month |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome by group | No. of patients | Mean | s.d. | No. of patients | Mean | s.d. | Difference between groups (95% CI) | P-value | No. of patients | Mean | s.d. | Difference between groups (95% CI) | P-value |
|
Anxiety | |||||||||||||
| Walking group | 58 | 4.59 | 3.46 | 50 | 4.59 | 2.63 | −0.63 | 0.379 | 44 | 3.55 | 3.34 | −1.03 | 0.168 |
| Usual-care groupa | 58 | 3.50 | 3.91 | 51 | 5.22 | 4.01 | (−2.04; 0.78) | 47 | 4.57 | 3.96 | (−2.49;0.43) | ||
|
Depression | |||||||||||||
| Walking group | 58 | 5.67 | 3.57 | 50 | 4.92 | 3.14 | −2.45 | 0.001* | 44 | 4.41 | 3.79 | −1.68 | 0.035* |
| Usual-care groupa | 58 | 4.74 | 3.73 | 51 | 7.37 | 4.52 | (−3.95; −0.95) | 47 | 6.09 | 4.11 | (−3.23; −0.12) | ||
|
Symptoms | |||||||||||||
| Walking group | 58 | 1.93 | 1.45 | 50 | 1.50 | 1.27 | −0.58 | 0.053 | 44 | 1.54 | 1.68 | −0.21 | 0.512 |
| Usual-care groupa | 58 | 2.04 | 1.48 | 51 | 2.08 | 1.65 | (−1.18; 0.01) | 47 | 1.74 | 1.55 | (−0.82; 0.41) | ||
Abbreviation: CI=confidence interval.
*P <0.05.
Usual-care group is reference group.
Table 3. Results of the generalised linear modela regarding the effects of walking exercise on anxiety, depression and cancer-related symptoms (n=116).
| Variables | β | s.e. | P-value |
|---|---|---|---|
|
Anxiety | |||
| Group (WG vs UG) | 1.09 | 0.68 | 0.110 |
| Time | |||
| Baseline | Reference | ||
| Third month | 1.75 | 0.52 | 0.001* |
| Sixth month | 1.07 | 0.53 | 0.042* |
| Group × time | |||
| Group × baseline | Reference | ||
| Group × third month | −1.80 | 0.69 | 0.009* |
| Group × sixth month | −2.18 | 0.79 | 0.006* |
|
Depression | |||
| Group (WG vs UG) | 0.93 | 0.67 | 0.166 |
| Time | |||
| Baseline | Reference | ||
| Third month | 2.66 | 0.59 | 0.00001* |
| Sixth month | 1.44 | 0.58 | 0.013* |
| Group × time | |||
| Group × baseline | Reference | ||
| Group × third month | −3.31 | 0.83 | 0.00006* |
| Group × sixth month | −2.57 | 0.88 | 0.004* |
|
Cancer-related symptoms | |||
| Group (WG vs UG) | −0.11 | 0.27 | 0.679 |
| Time | |||
| Baseline | Reference | ||
| Third month | −0.03 | 0.15 | 0.819 |
| Sixth month | −0.38 | 0.18 | 0.039* |
| Group × time | |||
| Group × baseline | Reference | ||
| Group × third month | −0.43 | 0.22 | 0.050 |
| Group × sixth month | −0.03 | 0.30 | 0.910 |
Abbreviations: β=modelling coefficient; UG=usual-care group; WG=walking-exercise group.
*P<0.05.
Using generalised estimation equations for repeated measurements and exchangeable correlation structure.
Figure 2.
Changes in anxiety subscale scores at baseline and at the third and sixth months according to the general linear model.
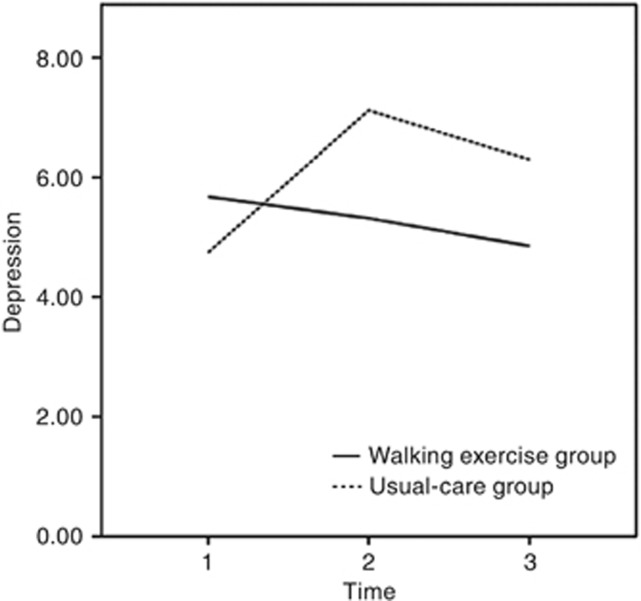
The mean depression scores at baseline were 5.67 (s.d.=3.57) and 4.74 (s.d.=3.73) in the walking-exercise and usual-care groups, respectively. No significant intergroup differences were observed (t=1.37, P=0.173). The mean depression scores of the walking-exercise group gradually declined from baseline to the third and sixth months (Table 2), although these changes revealed no statistical significance in these scores. However, the mean depression scores of the usual-care group at the third month increased significantly from baseline (mean difference=2.63, P=0.0004), revealing a marginally significant increase at sixth month from baseline (mean difference=1.35, P=0.071; Table 2). In addition, significant differences were observed at the third and sixth months between the walking and the usual-care groups (mean difference=−2.45, P=0.001; mean difference=−1.68, P=0.035, respectively; Table 2). Furthermore, a significant interaction term (group difference × time) of the model at the third (β=−3.31, P=0.00006) and sixth (β=−2.57, P=0.004) months revealed that the participants engaging in walking exhibited further reductions in depression over time. These results indicated that the walking-exercise programme effectively reduced depression (Table 3 and Figure 3).
Figure 3.
Changes in depression subscale scores at baseline and at the third and sixth months according to the general linear model.
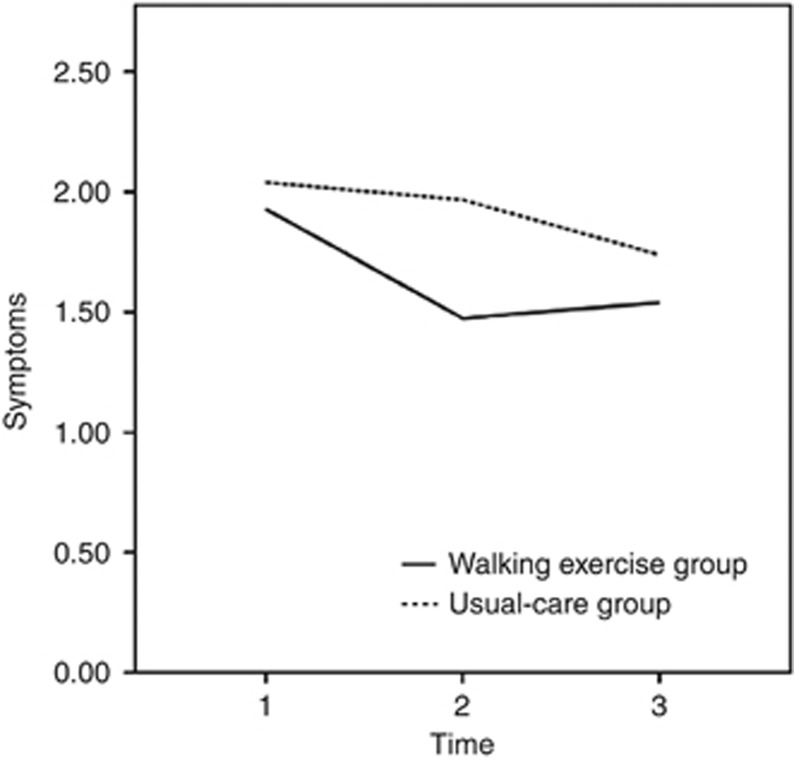
At baseline, the mean symptom scores were 1.93 (s.d.=1.45) and 2.04 (s.d.=1.48) for the walking-exercise and usual-care groups, respectively. Because the value of presentation was skewed, the median and interquartile ranges (IQRs) were considered instead of the mean and s.d. values. The median symptom scores were 1.62 (IQR=0.90–3.10) and 2.11 (IQR=0.83–2.92) for the walking-exercise and the usual-care groups, respectively. No significant intergroup differences were observed (t=−0.41, P=0.683). Moreover, the mean symptom score of the walking-exercise group declined from the baseline to the third month (mean difference=0.43, P=0.137) and remained stable from the third to the sixth month (mean difference=0.04, P=0.896). However, the mean symptom scores of the usual-care group at the third (mean difference=0.04, P=0.886) and sixth (mean difference=0.30, P=0.313) months remained unaltered from the baseline (Table 2). A marginally significant difference was observed between both the groups at the third month (1.50 vs 2.08 points, P=0.053; Table 2). However, the model of the interaction term (group difference × time) at the third month was marginally significant (β=−0.43, P=0.050) but did not achieve statistical significance at the sixth month (β=−0.03, P=0.910) (Table 3 and Figure 4). Detailed information is presented in Tables 2 and 3.
Figure 4.
Changes in symptom severity at baseline and at the third and sixth months according to the general linear model.
Intervention effects on anxiety and depression according to the clinical cutoff point
A cutoff point of 8 was used to investigate the clinical significance of the observed changes; the walking-exercise and usual-care groups revealed similar numbers of definite and suspicious (scores ⩾8) anxiety cases at baseline (13 vs 8, P=0.288). Although a significant difference was observed from the baseline to the third month (a decrease of 4 patients in the walking-exercise group vs an increase of 9 patients in the usual-care group, P=0.014), no significant differences were observed from the baseline to the sixth month (a decrease of 8 patients in the walking-exercise group vs no decrease in the usual-care group, P=0.118). A significant interaction term (group difference × time) in the GEE model (Wald x2=6.04, P=0.049) verified that the walking-exercise programme effectively reduced the number of patients with anxiety over time (Table 4).
Table 4. Effects of walking exercise on the Hospital Anxiety and Depression Scale (HADS) outcome variables according to the clinical cutoff point at baseline and at third and sixth months (general linear model).
|
Pretest,a
case number (%) |
Third month, case number (%) |
Sixth month, case number (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome variables | ⩾8 | <8 | ⩾8 | <8 | Wald χ2 | P-value of group × time | ⩾8 | <8 | Wald χ2 | P-value of group × time | Overall of Wald χ2 | Overall P-value of group × time |
| Anxiety | 6.04 | 0.014* | 2.45 | 0.118 | 6.04 | 0.049* | ||||||
| Walking-exercise group | 13 (22.4) | 45 (77.6) | 9 (18.4) | 40 (81.6) | 5 (11.4) | 39 (88.6) | ||||||
| Usual-care groupa | 8 (13.8) | 50 (86.2) | 17 (34.7) | 32 (65.3) | 8 (17.0) | 39 (83.0) | ||||||
| Depression | 10.26 | 0.001* | 1.28 | 0.257 | 10.74 | 0.005* | ||||||
| Walking-exercise group | 18 (31.0) | 40 (69.0) | 10 (20.4) | 39 (79.6) | 13 (29.5) | 31 (70.5) | ||||||
| Usual-care groupa | 14 (24.1) | 44 (75.9) | 23(46.9) | 26(53.1) | 16 (34.0) | 31 (66.0) | ||||||
Using generalised estimation equations for repeated measurements and (AR1) correlation structure.
*P<0.05.
Reference group.
Similarly, when the cutoff point of 8 was used to investigate the clinical relevance of the observed changes, both the walking-exercise and usual-care groups revealed a similar number of definite and suspicious (scores ⩾8) depression cases at baseline (18 vs 14, P=0.406). Moreover, although a significant difference was observed from the baseline to the third month (a decrease of 8 patients vs an increase of 9 patients, P=0.001), no significant differences were observed from the baseline to the sixth month (a decrease of 5 patients vs an increase of 2 patients, P=0.257). The significant interaction term (group difference × time) in the GEE model (Wald x2=10.74, P=0.005) indicated that the walking-exercise programme effectively reduced the number of patients with depression over time (Table 4).
Discussion
This is the first study to investigate the psychological effects of a home-based walking-exercise programme on patients with lung cancer. The trial results support the hypothesis that a home-based walking exercise exerts positive effects on anxiety and depression in patients with lung cancer. These results are consistent with those obtained in previous studies on patients with colorectal and breast cancer (Mock et al, 1997; Courneya et al, 2003; Mehnert et al, 2011). In addition, our results indicate that the psychological benefits of exercise observed in other cancer populations apply to patients with lung cancer as well. However, a previous study (Duijts et al, 2012) conducted a 12-week, home-based, self-directed exercise programme (e.g., swimming and running) for patients with breast cancer and reported negative results; this may have been caused by 36% lower exercise adherence rates. Similarly, another study (Jacobsen et al, 2013) adopted a home-based walking exercise programme that included a 12-min video and an exercise booklet, and failed to reduce anxiety and depression in patients with cancer undergoing chemotherapy. We suggest that researchers implement weekly telephonic interviews and personal discussions with patients to increase their adherence rate, and also offer face-to-face instructions instead of videos when conducting a home-based exercise intervention study.
Regarding exercise intensity, because of the constraints of our research budget, we used both the heart rate and Borg's RPE that were measured by the patients themselves; however, accurate heart rate detection can be challenging for elderly people. We suggest that a future study with a larger budget use watch-like heart rate monitors to facilitate precise heart rate detection; this would reduce the measurement error when patients measure their own heart rate as well as the patient load for heart rate measurement, particularly on elderly patients. Moreover, in this study, three patients presented with postoperative dyspnoea; therefore, they could not endure moderate-intensity exercises during the first and second weeks of the walking-exercise programme, but adapted gradually after the second week. A previous study reported that light-intensity exercise has an antianxiety effect on breast cancer survivors (Blacklock et al, 2010). Therefore, in future studies, moderate-intensity exercise should be modified to light-intensity exercise for patients with dyspnoea.
Regarding the effects of exercise on symptom severity, no substantial reduction was observed in the symptoms. This result is consistent with the results of two previous studies (Dodd et al, 2010; Lin et al, 2014), wherein neither supervised exercise nor home-based exercise alleviated the cancer-related symptoms in patients with breast, colorectal and ovarian cancer. Although no significant difference was observed in the severity of symptoms between the two groups over time, the symptom scores of the walking-exercise group were marginally significantly lower than those of the usual-care group (1.50 vs 2.08 points, respectively, P=0.053) in the third month. A possible reason that symptoms were not relieved in this study is the floor effect, because the symptom scores of the participants averaged 2.0 points at baseline, indicating mild symptoms.
In the walking-exercise group, 14 (24.1%) patients dropped out, and 11 (19.0%) patients in the usual-care group dropped out. The patients who dropped out exhibited higher depression scores than did the non-dropout patients. This finding is similar to that of a study on patients enrolled in a cardiac rehabilitation programme; the depression status served as a predictor of the number of exercise sessions completed and the dropout rate (Glazer et al, 2002). We suggest that health-care providers conduct regular follow-ups of the depression status of patients, and encourage them to continue exercising when conducting an exercise programme.
The strengths of this trial include it being the first home-based walking-exercise, randomised controlled trial to focus on Taiwanese patients with lung cancer; the 97% recruitment rate; and the adoption of a feasible and flexible exercise programme. However, this study had several limitations. First, the registration was retrospective and may have created risk when changing the study design and analysis after data collation. To control the research quality, the progress of this study was reported annually to the Institutional Review Board of Taipei Veterans General Hospital (Taipei, Taiwan). Project aspects such as research design and intervention measures were not modified during the research period. Second, although lack of blinding is inevitable in this type of study, it may have induced a placebo effect on the patients and an observational bias among the researchers; therefore, the outcome assessors should be trained in standardised testing procedures to reduce bias. Third, three patients reported that they could not endure the moderate-intensity exercises during the first and second weeks; this negatively affected the intervention fidelity and possibly diluted the intervention effect. Other study limitations included the use of a single centre to recruit patients, the lack of blinding and the heterogeneity of this patient population. This home-based walking-exercise programme warrants adoption in lung cancer rehabilitation because of its connection with ADLs.
In summary, this paper is the first to document evidence regarding the feasibility and effectiveness of walking-exercise training programmes in patients with lung cancer. The study results suggest that home-based walking exercise can reduce anxiety and depression, and elucidate the critical role of exercise in the rehabilitation of patients with cancer. Health-care team members must comprehend and consider exercise as a supportive care intervention for lung cancer survivors.
Acknowledgments
We thank all participants in this study. This study was supported by NSC 100-2314-B-038-012-MY3(3-3).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- American College of Sports Medicine 2006ACSM's Guidelines for Exercise Testing and Prescription7th edn.Lippincott Williams & Wilkins: Philadelphia [Google Scholar]
- Blacklock R, Rhodes R, Blanchard C, Gaul C. Effects of exercise intensity and self-efficacy on state anxiety with breast cancer survivors. Oncol Nurs Forum. 2010;37 (2:206–212. doi: 10.1188/10.ONF.206-212. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg's Perceived Exertion and Pain Scales. Human Kinetics: Champaign, IL; 1998. [Google Scholar]
- Chang JA, Lin CC. A longitudinal study of the role of patient-reported outcomes on survival prediction of palliative cancer inpatients in Taiwan. Support Care Cancer. 2009;17 (10:1285–1294. doi: 10.1007/s00520-009-0583-9. [DOI] [PubMed] [Google Scholar]
- Chang NW, Lin KC, Lee SC, Chan JYH, Lee YH, Wang KY. Effects of an early postoperative walking exercise programme on health status in lung cancer patients recovering from lung lobectomy. J Clin Nursing. 2014;23 (23-24:3391–3402. doi: 10.1111/jocn.12584. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM, Quinney HA, Fields ALA, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care. 2003;12 (4:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Cho MH, Miaskowski C, Painter PL, Paul SM, Cooper BA, Duda J, Krasnoff J, Bank KA. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs. 2010;33 (4:245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijts SF, van Beurden M, Oldenburg HS, Hunter MS, Kieffer JM, Stuiver MM, Gerritsma MA, Menke-Pluymers MB, Plaisier PW, Rijna H, Lopes Cardozo AM, Timmers G, van der Meij S, van der Veen H, Bijker N, de Widt-Levert LM, Geenen MM, Heuff G, van Dulken EJ, Boven E, Aaronson NK. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012;30 (33:4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41 (4:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Feinstein MB, Krebs P, Coups EJ, Park BJ, Steingart RM, Burkhalter J, Logue A, Ostroff JS. Current dyspnea among long-term survivors of early-stage non-small cell lung cancer. J Thorac Oncol. 2010;5 (8:1221–1226. doi: 10.1097/JTO.0b013e3181df61c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Waminathan R, Ferlay J.(eds) (2013. Cancer Incidence in Five Continents, Vol. X (electronic version). Retrieved 7 July 2014, from http://ci5.iarc.fr . [DOI] [PubMed]
- Glazer KM, Emery CF, Frid DJ, Banyasz RE. Psychological predictors of adherence and outcomes among patients in cardiac rehabilitation. J Cardiopulm Rehabil. 2002;22 (1:40–46. doi: 10.1097/00008483-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18 (4:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Phillips KM, Jim HSL, Small BJ, Faul LA, Meade CD, Thompson L, Williams CC, Jr, Loftus LS, Fishman M, Wilson RW. Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psychooncology. 2013;22 (6:1229–1235. doi: 10.1002/pon.3122. [DOI] [PubMed] [Google Scholar]
- Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn. 1957;35 (3:307–315. [PubMed] [Google Scholar]
- Lin CC, Chang AP, Cleeland CS, Mendoza TR, Wang XS. Taiwanese version of the M. D. Anderson symptom inventory: symptom assessment in cancer patients. J Pain Symptom Manage. 2007;33 (2:180–188. doi: 10.1016/j.jpainsymman.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Lin KY, Shun SC, Lai YH, Liang JT, Tsauo JY. Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nurs. 2014;37 (2:E21–E29. doi: 10.1097/NCC.0b013e3182791097. [DOI] [PubMed] [Google Scholar]
- Lin YY, Lai YF, Lu HI, Lai YL, Lin CC. Physical activity preferences among patients with lung cancer in Taiwan. Cancer Nurs. 2013;36 (2:155–162. doi: 10.1097/NCC.0b013e31825f4db1. [DOI] [PubMed] [Google Scholar]
- Mehnert A, Veers S, Howaldt D, Braumann KM, Koch U, Schulz KH. Effects of a physical exercise rehabilitation group program on anxiety, depression, body image, and health-related quality of life among breast cancer patients. Onkologie. 2011;34 (5:248–253. doi: 10.1159/000327813. [DOI] [PubMed] [Google Scholar]
- Midtgaard J, Stage M, Møller T, Andersen C, Quist M, Rørth M, Herrstedt J, Vistisen K, Christiansen B, Adamsen L. Exercise may reduce depression but not anxiety in self-referred cancer patients undergoing chemotherapy. Post-hoc analysis of data from the 'Body & Cancer' trial. Acta Oncol. 2011;50 (5:660–669. doi: 10.3109/0284186X.2010.543145. [DOI] [PubMed] [Google Scholar]
- Ministry of Health and Welfare 2013. Taiwan Cancer registry annual report, 2010. Retrieved 7 July 2014, from http://www.hpa.gov.tw/BHPNet/Web/Stat/StatisticsShow.aspx?No=201305060001 .
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, Quitasol W, Mitchell S, Chakravarthy A, Gage I. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24 (6:991–1000. [PubMed] [Google Scholar]
- Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, Stewart KJ, Cameron L, Zawacki K, Podewils LJ, Cohen G, McCorkle R. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14 (6:464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- Pan HH, Lin KC, Ho ST, Liang CY, Lee SC, Wang KY. Factors related to daily life interference in lung cancer patients: a cross-sectional regression tree study. Eur J Oncol Nurs. 2012;16 (4:345–352. doi: 10.1016/j.ejon.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley & Sons: New York; 1987. [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, Von Gruenigen VE, Schwartz AL. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42 (7:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- Temel JS, Greer JA, Goldberg S, Vogel PD, Sullivan M, Pirl WF, Lynch TJ, Christiani DC, Smith MR. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4 (5:595–601. doi: 10.1097/JTO.0b013e31819d18e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, Behav, Immun. 2010;24 (6:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67 (6:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]