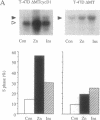
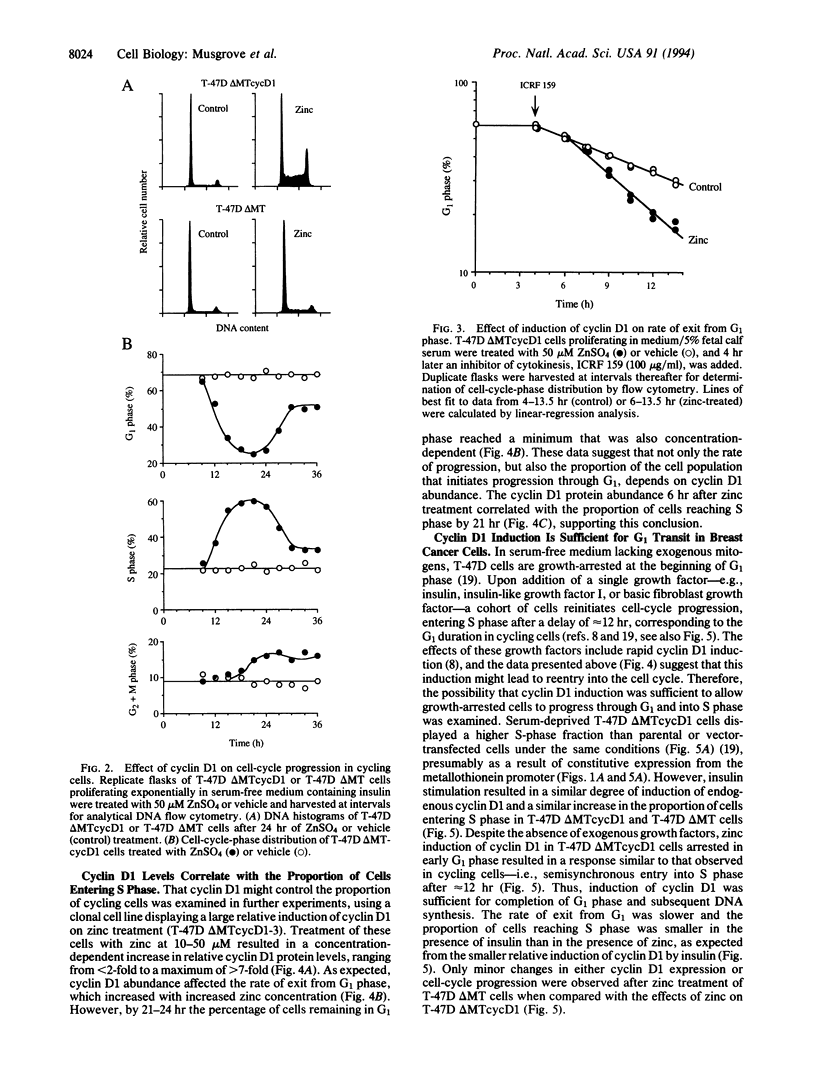
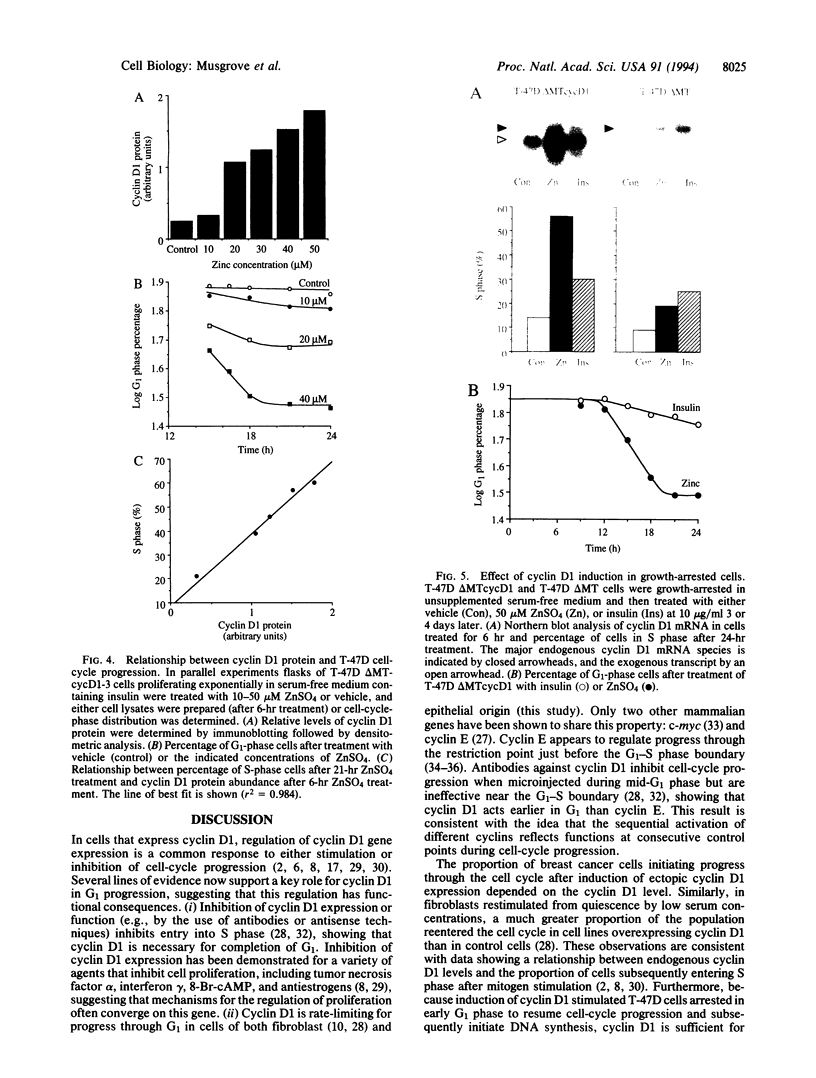
Abstract
The sequential transcriptional activation of cyclins, the regulatory subunits of cell-cycle-specific kinases, is thought to regulate progress through the cell cycle. Cyclins are therefore potential oncogenes, and cyclin D1 overexpression and/or amplification at its genomic locus, 11q13, are common features of several human cancers. Induction of cyclin D1 is an early response to mitogenic stimulation in several cell types, but the consequences of altered expression of this gene in human cells of epithelial origin remain undefined. We assessed the effects of alterations of cyclin D1 expression in human breast cancer cells by generating T-47D cells expressing human cyclin D1 under the control of a zinc-responsive metallothionein promoter. In cycling cells induction of cyclin D1 after zinc treatment resulted in an increase in the number of cells progressing through G1 and in the rate of transition from G1 to S phase, indicating that cyclin D1 is rate-limiting for progress through G1 phase. In cells arrested in early G1 phase after growth factor deprivation, zinc induction of cyclin D1 was sufficient for completion of the cell cycle, a process requiring growth factor stimulation in control cells. These data demonstrate a critical role for cyclin D1 in human breast cancer cell-cycle control and suggest that deregulated expression of cyclin D1 is likely to reduce dependence on normal physiological growth stimuli, thereby providing a growth advantage to tumor cells and a potential mechanism of resistance to endocrine therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajchenbaum F., Ando K., DeCaprio J. A., Griffin J. D. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993 Feb 25;268(6):4113–4119. [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Borg A., Sigurdsson H., Clark G. M., Fernö M., Fuqua S. A., Olsson H., Killander D., McGurie W. L. Association of INT2/HST1 coamplification in primary breast cancer with hormone-dependent phenotype and poor prognosis. Br J Cancer. 1991 Jan;63(1):136–142. doi: 10.1038/bjc.1991.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M. F., Sweeney K. J., Hamilton J. A., Sini R. L., Manning D. L., Nicholson R. I., deFazio A., Watts C. K., Musgrove E. A., Sutherland R. L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993 Aug;8(8):2127–2133. [PubMed] [Google Scholar]
- Cocks B. G., Vairo G., Bodrug S. E., Hamilton J. A. Suppression of growth factor-induced CYL1 cyclin gene expression by antiproliferative agents. J Biol Chem. 1992 Jun 15;267(17):12307–12310. [PubMed] [Google Scholar]
- Cross F. R. DAF1, a mutant gene affecting size control, pheromone arrest, and cell cycle kinetics of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4675–4684. doi: 10.1128/mcb.8.11.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly R. J., Harris W. H., Wang D. Y., Darbre P. D. Autocrine production of insulin-like growth factor II using an inducible expression system results in reduced estrogen sensitivity of MCF-7 human breast cancer cells. Cell Growth Differ. 1991 Sep;2(9):457–464. [PubMed] [Google Scholar]
- Dou Q. P., Levin A. H., Zhao S., Pardee A. B. Cyclin E and cyclin A as candidates for the restriction point protein. Cancer Res. 1993 Apr 1;53(7):1493–1497. [PubMed] [Google Scholar]
- Dulić V., Lees E., Reed S. I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992 Sep 25;257(5078):1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- Eisner J. M., Casey B. J. Malpractice, informed consent, and the use of low osmolality contrast media. Conn Med. 1988 Feb;52(2):87–91. [PubMed] [Google Scholar]
- Gaudray P., Szepetowski P., Escot C., Birnbaum D., Theillet C. DNA amplification at 11q13 in human cancer: from complexity to perplexity. Mutat Res. 1992 May;276(3):317–328. doi: 10.1016/0165-1110(92)90018-5. [DOI] [PubMed] [Google Scholar]
- Karn J., Watson J. V., Lowe A. D., Green S. M., Vedeckis W. Regulation of cell cycle duration by c-myc levels. Oncogene. 1989 Jun;4(6):773–787. [PubMed] [Google Scholar]
- Keyomarsi K., Pardee A. B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Lammie G. A., Peters G. Chromosome 11q13 abnormalities in human cancer. Cancer Cells. 1991 Nov;3(11):413–420. [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lidereau R., Callahan R., Dickson C., Peters G., Escot C., Ali I. U. Amplification of the int-2 gene in primary human breast tumors. Oncogene Res. 1988 Feb;2(3):285–291. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Motokura T., Keyomarsi K., Kronenberg H. M., Arnold A. Cloning and characterization of human cyclin D3, a cDNA closely related in sequence to the PRAD1/cyclin D1 proto-oncogene. J Biol Chem. 1992 Oct 5;267(28):20412–20415. [PubMed] [Google Scholar]
- Musgrove E. A., Hamilton J. A., Lee C. S., Sweeney K. J., Watts C. K., Sutherland R. L. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993 Jun;13(6):3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A., Lee C. S., Sutherland R. L. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991 Oct;11(10):5032–5043. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A., Sutherland R. L. Acute effects of growth factors on T-47D breast cancer cell cycle progression. Eur J Cancer. 1993;29A(16):2273–2279. doi: 10.1016/0959-8049(93)90221-z. [DOI] [PubMed] [Google Scholar]
- Musgrove E. A., Wakeling A. E., Sutherland R. L. Points of action of estrogen antagonists and a calmodulin antagonist within the MCF-7 human breast cancer cell cycle. Cancer Res. 1989 May 1;49(9):2398–2404. [PubMed] [Google Scholar]
- Nash R., Tokiwa G., Anand S., Erickson K., Futcher A. B. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988 Dec 20;7(13):4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M., Roberts J. M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993 Mar 26;259(5103):1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., van Tinteren H., Peterse J. L., Nunnink B., Thunnissen F. B., Devilee P., Cornelisse C. J., van de Vijver M. J., Mooi W. J. Amplification of genes within the chromosome 11q13 region is indicative of poor prognosis in patients with operable breast cancer. Cancer Res. 1992 Oct 1;52(19):5229–5234. [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L., Hall R. E., Pang G. Y., Musgrove E. A., Clarke C. L. Effect of medroxyprogesterone acetate on proliferation and cell cycle kinetics of human mammary carcinoma cells. Cancer Res. 1988 Sep 15;48(18):5084–5091. [PubMed] [Google Scholar]
- Sutherland R. L., Hall R. E., Taylor I. W. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res. 1983 Sep;43(9):3998–4006. [PubMed] [Google Scholar]
- Tsuda H., Hirohashi S., Shimosato Y., Hirota T., Tsugane S., Yamamoto H., Miyajima N., Toyoshima K., Yamamoto T., Yokota J. Correlation between long-term survival in breast cancer patients and amplification of two putative oncogene-coamplification units: hst-1/int-2 and c-erbB-2/ear-1. Cancer Res. 1989 Jun 1;49(11):3104–3108. [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K. A., Xiong Y., Beach D., Gilman M. Z. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Zhou D. J., Casey G., Cline M. J. Amplification of human int-2 in breast cancers and squamous carcinomas. Oncogene. 1988 Mar;2(3):279–282. [PubMed] [Google Scholar]