Abstract
Background:
Carcinoid heart disease is a complication of metastatic neuroendocrine tumours (NETs). We sought to identify factors associated with echocardiographic progression of carcinoid heart disease and death in patients with metastatic NETs.
Methods:
Patients with advanced non-pancreatic NETs and documented liver metastases and/or carcinoid syndrome underwent prospective serial clinical, biochemical, echocardiographic and radiological assessment. Patients were categorised as carcinoid heart disease progressors, non-progressors or deceased. Multinomial regression was used to assess the univariate association between variables and carcinoid heart disease progression.
Results:
One hundred and thirty-seven patients were included. Thirteen patients (9%) were progressors, 95 (69%) non-progressors and 29 (21%) patients deceased. Baseline median levels of serum N-terminal pro-brain natriuretic peptide (NT-proBNP) and plasma 5-hydroxyindoleacetic acid (5-HIAA) were significantly higher in the progressors. Every 100 nmol l−1 increase in 5-HIAA yielded a 5% greater odds of disease progression (OR 1.05, 95% CI: 1.01, 1.09; P=0.012) and a 7% greater odds of death (OR 1.07, 95% CI: 1.03, 1.10; P=0.001). A 100 ng l−1 increase in NT-proBNP did not increase the risk of progression, but did increase the risk of death by 11%.
Conclusions:
The biochemical burden of disease, in particular baseline plasma 5-HIAA concentration, is independently associated with carcinoid heart disease progression and death. Clinical and radiological factors are less useful prognostic indicators of carcinoid heart disease progression and/or death.
Keywords: neuroendocrine tumours, carcinoid heart disease, progression
Neuroendocrine tumours (NETs) have a heterogeneous natural history. They often follow an indolent course, progressing slowly over many years (Bhattacharyya et al, 2007). However, the development of carcinoid heart disease is associated with adverse clinical outcomes (Fox and Khattar, 2004). Cardiac involvement is characterised by right-sided valvular dysfunction, which can progress to right ventricular dilatation and failure. Serotonin is implicated in the development of carcinoid heart disease (Robiolio et al, 1995; Gustafsson et al, 2008), however high circulating levels of serotonin have a limited specificity for cardiac involvement and it is likely that there are other contributing factors to the pathogenesis of carcinoid heart disease.
Carcinoid heart disease has a significant impact on patient morbidity and mortality. Although survival from carcinoid heart disease is likely to have improved in recent years owing to the introduction and increasingly widespread use of somatostatin analogues lowering plasma serotonin concentrations, there is lack of contemporary survival data from patients diagnosed within last decade to confirm this assumption. Pellikka et al (1993) demonstrated a mean survival of 1.6 years in those with cardiac involvement compared with 4.6 years in those without. Furthermore, Westberg et al (2001) found that carcinoid heart disease was the main predictor of prognosis in patients with midgut carcinoid syndrome. As moderate to severe right ventricular dilatation and New York Heart Association (NYHA) class III–IV symptoms are associated with increased mortality, it is important to screen patients to identify carcinoid heart disease as early as possible (Møller et al, 2005).
To date, few authors have reported factors associated with the progression of carcinoid heart disease or defined which measure of progression is most useful. Møller et al (2003), in a study of 71 patients with carcinoid syndrome and 32 patients referred directly for valve replacement, demonstrated that progression of cardiac involvement, in 35% of patients, was associated with higher urinary 5-hydroxyindoleacetic acid (5-HIAA) levels and was more likely in patients who had received chemotherapy. More recently, Bhattacharyya et al (2011) demonstrated a 17.5% progression rate in patients with carcinoid syndrome over a median follow-up duration of 29 months. Independent predictors of the development or progression of carcinoid heart disease were urinary 5-HIAA levels greater than 300 μmol per24 h and three or more daily episodes of facial flushing. In both of these studies, carcinoid heart disease progression was defined as a 25% or more deterioration in the echocardiographic score of the patient. A third study, which defined progression of carcinoid heart disease as a score increase of greater than twice the s.d. of the mean intraobserver variability, also demonstrated an association between post-therapy 5-HIAA levels and the progression of carcinoid heart disease (Denney et al, 1998). A prospective study of 80 patients with carcinoid syndrome, followed up for 26 months, demonstrated a similar progression rate of 20%, with a further 20% of patients developing carcinoid heart disease during the study period but the authors did not clearly define what was meant by ‘progression' of carcinoid heart disease, or investigate potential factors associated with progression in this cohort (Mansencal et al, 2010).
It is not known how frequently NET patients should be screened for carcinoid heart disease, with consensus guidelines recommending ‘regular' echocardiography (Pape et al, 2012). Furthermore, there is uncertainty surrounding how frequently to scan those with established carcinoid heart disease to monitor for disease progression. Identification of factors associated with the development and progression of carcinoid heart disease may aid development of more specific, evidence-based guidelines both for screening, monitoring and management of the disease.
We were unable to identify any previous studies that have assessed the progression of carcinoid heart disease in a population of patients with liver metastases, with or without the carcinoid syndrome. The purpose of this observational cohort study was therefore to prospectively identify the clinical, biochemical and radiological characteristics that are associated with the development and progression of carcinoid heart disease and overall survival in a population of patients with metastatic NETs and/or carcinoid syndrome.
Materials and methods
Recruitment
We aimed to recruit >100 patients based on a number of factors including: (i) a conservative estimate of 20% incidence of carcinoid heart disease in our patient population; (ii) the cumulative total of patients attending each of the tertiary referral centres; and (iii) the availability of a dedicated Cardiology Research Registrar with expertise in echocardiography to recruit and study the patients.
Participants
All patients with non-pancreatic NETs, liver metastases and/or carcinoid syndrome, who visited the outpatient department of one of four tertiary referral NET centres (University Hospital Aintree NHS Trust, Liverpool, Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, The Christie Hospital NHS Foundation Trust, Manchester and Huddersfield Royal Infirmary, Yorkshire) between April 2009 and September 2013 were eligible to participate in this observational cohort study. The exclusion criteria included the presence of prosthetic heart valves or inadequate echocardiographic windows precluding comprehensive assessment of valve leaflet morphology, mobility and thickening. Liverpool Research Ethics Committee approved the study (reference 09/H1005/40), and all patients gave written informed consent.
Clinical assessment of disease severity
Patients' symptoms were assessed at the time of echocardiography, with specific questions regarding frequency of flushing, diarrhoea, wheezing, breathlessness and/or ankle swelling. Carcinoid syndrome was defined as episodes of cutaneous flushing, diarrhoea or wheezing. Symptomatic progression was defined as >50% increase in the number of daily flushing episodes or bowel movements compared with the previous visit, as used in a similar previous study (Bhattacharyya et al, 2011).
Biochemical assessment of disease severity
Venous (non-fasting) blood samples were taken on the day of baseline echocardiography and at the time of subsequent scans to determine the concentrations of N-terminal pro brain natriuretic peptide (NT-proBNP) and 5-HIAA. Plasma and serum were separated by centrifugation (3500 rpm) and stored at −80 °C until further analysis.
Biochemical assays
Our group's previous work has validated the use of plasma 5-HIAA and serum NT-proBNP as sensitive and specific biomarkers for the presence of carcinoid heart disease (Dobson et al, 2013). Thus, serum NT-proBNP, upper limit of normal (ULN) 146 ng l−1 (Hess et al, 2005), (electrochemiluminescence technology on the fully automatic Elecsys analyser) with intra-assay precision below 4% and an inter-assay precision below 5% at concentrations above 70 pg ml−1 (Roche Diagnostics, Rotkreuz, Switzerland) was measured in 133 patients. Plasma 5-HIAA, ULN 118 nmol l−1, LC-MS/MS method (comparable to that used by Tellez et al, 2013 with QuanLynx software (Waters, Watford, UK) with an inter-assay coefficient of variation of 2.6–9.8% and a intra-assay variation of 2–4.7%) (Miller et al, 2010) was measured in 128 patients. All biochemical measurements were made without knowledge of the clinical status of the patient. Biochemical progression was defined as >50% increase in NT-proBNP or plasma 5-HIAA from the baseline value.
Radiological assessment of disease severity
Baseline contrast enhanced computed tomography (CT) imaging of the chest, abdomen and pelvis was performed in all patients and repeated at 12 monthly intervals. All CT scans were reviewed by radiologists with expertise in the assessment of NETs, and radiological progression was defined in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST) guidelines (Eisenhauer et al, 2009).
Echocardiographic assessment of disease progression
Trans-thoracic echocardiography image acquisition and analysis was performed by one of two experienced operators, using a GE Vivid 7 or Vivid Q machine (2.5 MHz phased array transducer, Horten, Norway). Valve anatomy and function were assessed in the parasternal long and short axes, and apical 4 chamber, 2 chamber and long axes. Evaluation included two-dimensional, M-mode, pulsed and continuous wave Doppler and pulsed-wave tissue Doppler imaging. Video loops were acquired triggered to the ECG (three cardiac cycles) and saved digitally for subsequent offline analysis (Echopac V9.01). Echocardiograms were performed at 12 monthly intervals, or sooner if clinically indicated.
Trans-thoracic echocardiography image analysis and interpretation
A diagnosis of carcinoid heart disease was decided by the operator based on consensus guidelines (Plockinger et al, 2009). Carcinoid heart disease was defined as thickening and reduced excursion of the four valvular leaflets, cusps and chordae, with possible consequent retraction, shortening and fixation of leaflets or cusps (Plockinger et al, 2009). Valve regurgitation and stenosis was quantified according to American College of Cardiology Guidelines (Bonow et al, 2006). Tricuspid stenosis was quantified according to the mean gradient across the valve (mild 1-5 mm Hg, moderate 5–8 mm Hg, severe >8 mm Hg). Pulmonary stenosis was quantified according to the maximum gradient across the valve (mild <25 mm Hg, moderate 25–50 mm Hg and severe >50 mm Hg). Regurgitation was quantified using a composite of Doppler (colour Doppler jet width and spectral density using continuous wave Doppler) and two-dimensional imaging (volume loading of the chambers). Right ventricular size and function was assessed according to the American Society of Echocardiography guidelines (Rudski et al, 2010). This enabled calculation of a previously validated echocardiographic score (Bhattacharyya et al, 2008) incorporating assessment of all four cardiac valves. Leaflet thickening, mobility and morphology, valvular regurgitation and stenosis, and right ventricular size and function were graded, with higher scores indicating more severe valvular pathology. The score ranges from 0–66, with no agreed threshold as to what defines carcinoid heart disease.
Reproducibility of echocardiographic interpretation
To ensure inter-observer agreement a randomly selected sub-group of 80 echocardiographic studies (58%) were cross-checked by a different observer blinded to the initial echocardiographic score. With a maximum score of 66, scores within two points were considered to be concordant. In 70 studies (88%) the assigned scores were concordant, whereas in the remaining 10 studies there was a discrepancy of >2 points, (mean discrepency 0.234). Where there was score discordance, a third independent observer analysed the echocardiographic study, blinded to the other scores. An average of the two most concordant scores was then used for these 10 echocardiographic studies.
Categorisation of progression vs non-progression vs death
Progression of carcinoid heart disease was defined as an increase in the degree of tricuspid regurgitation, and/or an increase in the degree of tricuspid leaflet thickening or immobility. Using this definition patients were classified as progressors or non-progressors. Patients who died prior to a second echocardiogram, in whom assessment of progression was not possible, were categorised as deceased. Those who died after their second scan were classified as either progressors or non-progressors. Where a subject died prior to the second echocardiographic assessment, the interval between first assessment and date of death was recorded. We noted the presence or absence of carcinoid heart disease at baseline.
Statistical analysis
Comparisons between continuous variables in the carcinoid heart disease and the non-carcinoid heart disease group were made using the Mann–Whitney U-test, as our data did not satisfy the assumption of equal variances for the two-sample t-test. Categorical variables were compared using the χ2- test, or Fisher's exact test where cell counts were insufficient. Multinomial regression was used to assess the univariate association between a number of variables and (simultaneously) disease progression and death. Statistical analyses were performed using Stata/IC 12.0 software (StataCorp LP, College Station, TX, USA). A P-value of <0.05 was considered statistically significant.
Results
Demographics
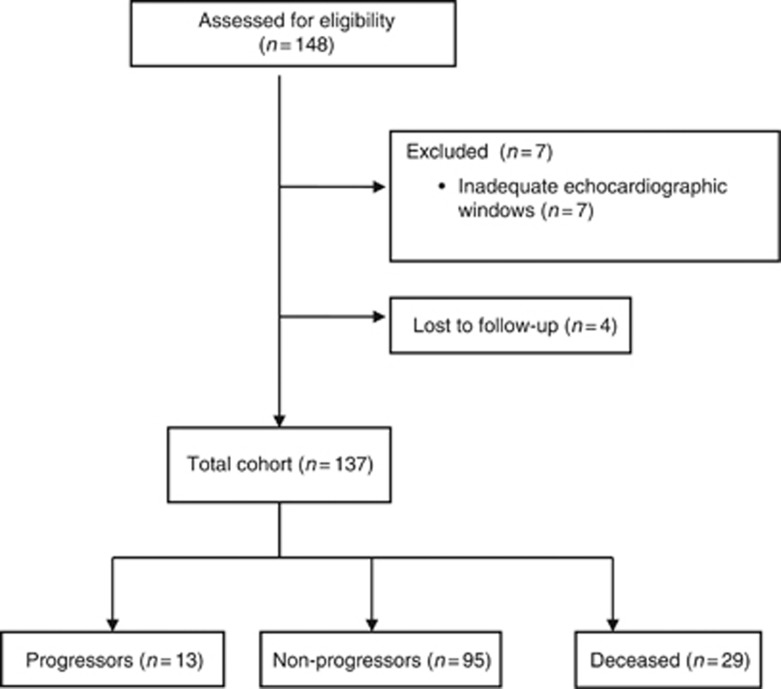
One hundred and forty-eight patients were prospectively recruited. Four patients were lost to follow up and seven patients were excluded owing to inadequate echocardiographic windows precluding clear visualisation of all valves, resulting in a total of 137 patients studied (see Figure 1). The median duration of follow-up from first scan to last scan or death was 27 months (interquartile range 12–37), with a total of 2862 patient years. Twenty-six patients (19%) had carcinoid heart disease at the beginning of the study.
Figure 1.
Consort flow diagram.
Clinical variables
During the follow-up period, nine patients had echocardiographic evidence of progression of carcinoid heart disease and four patients developed de novo cardiac involvement. Twenty-nine patients died in the first year of follow-up, without undergoing a second echocardiogram. The baseline characteristics of our patient population are illustrated in Table 1. There were no significant differences in age, gender or primary tumour site between the three groups. Disease duration was significantly shorter in the deceased group (15 months, P=0.005) but there was no difference in duration of disease between progressors and non-progressors (52 vs 64 months, P=0.554). Tumour grade differed significantly between the groups (P<0.0001), with a higher proportion of grade 2 (intermediate) tumours in the progressor group, and a higher proportion of grade 3 (high grade) tumours in the deceased group. The origin of the primary tumour in those with grade 3 disease was small bowel (one patient), stomach (one patient) and recto-sigmoid (two patients). All of these patients had hepatic metastases.
Table 1. Baseline characteristics of patients.
| Variable | Progression of carcinoid heart disease (n=13) | No progression of carcinoid heart disease (n=95) | Died prior to 2nd echo (n=29) | P-value |
|---|---|---|---|---|
|
Demographics | ||||
| Age (years)a | 68±13 | 67±10 | 70±11 | 0.112 |
| Male sex (no %) | 5 (39%) | 55 (58%) | 16 (55%) | 0.417 |
| Follow-up (months)b | 29 (13–41) | 27 (12–37) | — | 0.887 |
|
Clinical characteristics | ||||
|
Tumour grade (no %) | ||||
| Grade 1 | 1 (8%) | 55 (58%) | 9 (31%) | 0.007 |
| Grade 2 | 3 (23%) | 7 (7%) | 2 (7%) | |
| Grade 3 | 0 | 0 | 4 (14%) | |
| Unknown | 9 (69%) | 33 (35%) | 14 (48%) | |
| Duration of disease (months)b | 52 (27–138) | 64 (40–88) | 15 (9–59) | 0.005 |
|
Site of primary tumour | ||||
| Small bowel | 8 (62%) | 71 (75%) | 19 (66%) | 0.126 |
| Large bowel | 1 (8%) | 7 (7%) | 1 (3%) | |
| Lung | 0 | 1 (1%) | 2 (7%) | |
| Other | 1 (8%) | 1 (1%) | 3 (10%) | |
| Unknown | 3 (24%) | 15 (16%) | 4 (14%) | |
| Liver metastases | 11 (85%) | 80 (84%) | 27 (93%) | 0.473 |
| Carcinoid syndrome | 12 (92%) | 63 (66%) | 21 (72%) | 0.151 |
| Baseline carcinoid heart disease | 9 (70%) | 5 (5%) | 12 (41%) | <0.001 |
| Baseline echocardiographic score | 9 (7.5–14) | 3 (1–5) | 5 (2.5–14.5) | <0.001 |
| Baseline NT-proBNP (ng l−1) | 267 (108–578) | 84 (29–224) | 401 (116–978) | 0.001 |
| Baseline 5-HIAA (nmol l−1) | 2247 (807–2939) | 316 (138–661) | 1221 (167–437) | 0.009 |
|
Therapeutic intervention | ||||
| SSA therapy | 12 (92%) | 78 (82%) | 17 (59%) | 0.012 |
| Primary tumour resection | 6 (46%) | 63 (66%) | 7 (24%) | <0.001 |
| Resection of hepatic metastases | 1 (8%) | 9 (10%) | 2 (7%) | 0.903 |
| Interferon | 0 | 8 (8%) | 2 (7%) | 0.547 |
| Chemotherapy | 0 | 10 (10%) | 6 (21%) | 0.111 |
| Targeted radionuclide therapy | 4 (31%) | 27 (28%) | 4 (14%) | 0.259 |
| Chemo-embolisation | 1 (8%) | 11 (12%) | 0 | 0.154 |
| Radio-frequency ablation | 0 | 6 (6%) | 0 | 0.25 |
Mean±s.d.
Median and interquartile range, SSA somatostatin analogue. Significant P-values (<0.05) are indicated in bold.
Worsening of carcinoid syndrome symptoms was associated with progression of carcinoid heart disease; 85% of those with symptom deterioration demonstrated progression of carcinoid heart disease compared with 5% of those with no symptom deterioration (P<0.001, Table 2). Although worsening of symptoms was independently associated with progression of carcinoid heart disease, (OR 99, 95% CI 17–573, P<0.001, Table 3) the wide confidence interval implies some uncertainty in this estimate.
Table 2. Association of variables with progression of carcinoid heart disease.
| Variable | Progression of carcinoid heart disease (n=13) | No progression of carcinoid heart disease (n=95) | P-value |
|---|---|---|---|
| Symptomatic deteriorationa | 11 (85%) | 5 (5%) | <0.001 |
| NT-proBNP progressionb | 8 (62%) | 24 (31%) | 0.04 |
| Plasma 5-HIAA progressionb | 6 (46%) | 14 (18%) | 0.035 |
| Radiological progressionc | 4 (31%) | 35 (37%) | 0.461 |
>50% increase in the number of daily flushing episodes or bowel movements compared with the previous visit.
>50% increase from the baseline value.
in accordance with RECIST (Response Evaluation Criteria in Solid Tumors) guidelines. Significant P-values (<0.05) are indicated in bold.
Table 3. Multinomial logistic regression analysis.
| Variable | Unit change | Group | OR (95% CI) | P-value |
|---|---|---|---|---|
| Age (years) | 5 years 5 years | Progression Death | 1.09 (0.82–1.45) 1.16 (0.94–1.43) | 0.570 0.167 |
| Disease duration (months) | 100 months 100 months | Progression Death | 0.99 (0.36–2.72) 0.22 (0.07–0.73) | 0.986 0.013 |
| Symptom deterioration | — | Progression | 99 (17–573) | <0.001 |
| Baseline NT-proBNP (ng l−1) | 100 units 100 units | Progression Death | 1.04 (0.92–1.18) 1.11 (1.02–1.21) | 0.486 0.014 |
| Baseline 5-HIAA (nmol l−1) | 100 units 100 units | Progression Death | 1.05 (1.01–1.09) 1.07 (1.03–1.10) | 0.012 0.001 |
| Radiological increase in tumour bulk | — | Progression | 0.76 (0.22–2.66) | 0.670 |
Significant P-values (<0.05) are indicated in bold.
The numbers of patients who underwent primary tumour resection or received somatostatin receptor analogues were significantly different between the groups (see Table 1). There was, however, no difference in the proportions of patients undergoing hepatic resection, trans-arterial chemo-embolisation, targeted radionuclide therapy, radio-frequency ablation or chemotherapy between the groups. The odds of death in those who had not had a primary tumour resection was ∼3.7 times greater than in those who had undergone a primary resection (OR 3.72, 95% CI: 1.60–8.69; P<0.002) but primary resection did not increase the odds of carcinoid heart disease progression (OR 2.30, 95% CI: 0.71–7.40; P=0.164).
Biochemical variables
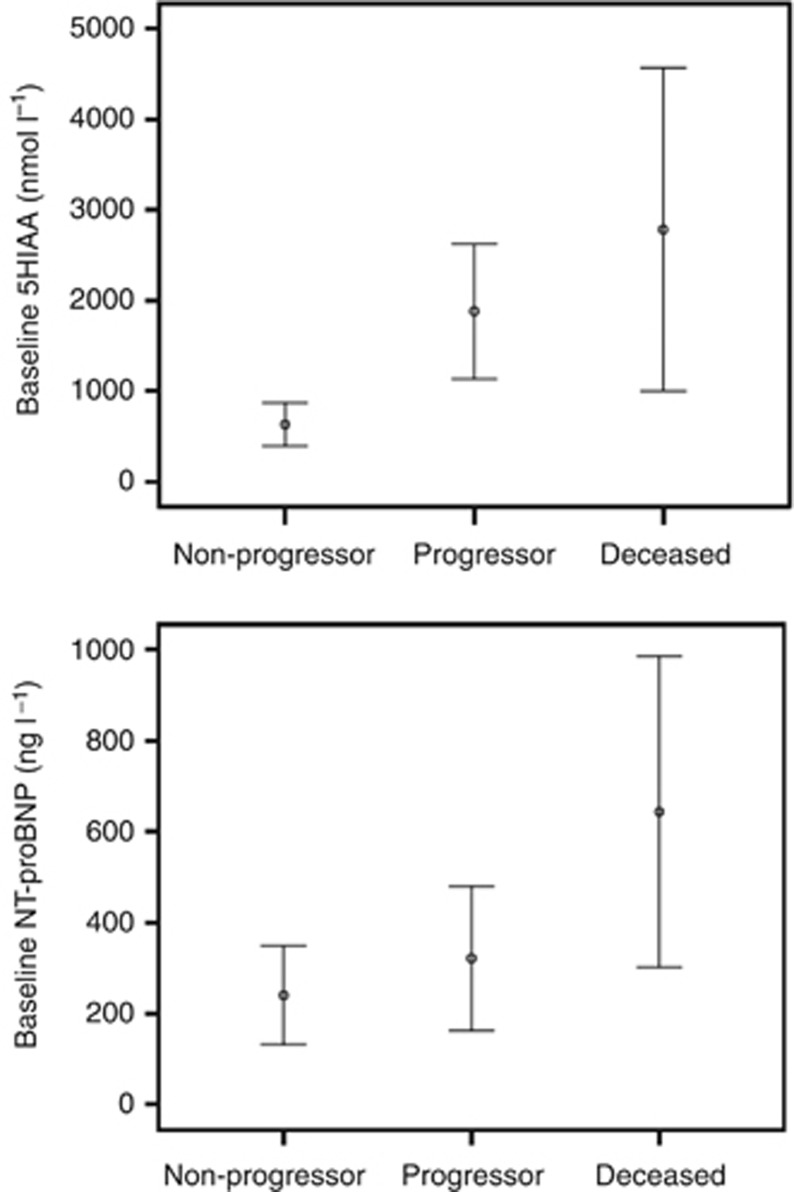
Baseline plasma 5-HIAA and NT-proBNP concentrations were significantly different between the groups (Table 1 and Figure 2), with the highest NT-proBNP levels seen in the deceased group and the highest 5-HIAA concentrations in the progressors. Baseline 5-HIAA concentration was significantly associated with disease progression: every 100 nmol l−1 increase in 5-HIAA yielded a 5% greater odds of disease progression (OR 1.05, 95% CI: 1.01, 1.09; P=0.012) and 7% higher odds of death before second follow-up (OR 1.07, 95% CI: 1.03, 1.10; P=0.001, see Table 3). The proportion of patients with NT-proBNP or 5-HIAA progression was significantly higher in the progressors compared with the non-progressors (62% vs 31%, P=0.04 and 46% vs 18%, P=0.035, respectively, see Table 2).
Figure 2.
Baseline plasma 5-HIAA and serum NT-proBNP concentrations according to patient group.
Radiological variables
Increase in tumour bulk was not associated with progression of carcinoid heart disease; 10% of those with stable disease demonstrated progression of carcinoid heart disease compared with 13% of those with an increase in tumour bulk (P=0.669), OR 0.76 (95% CI 0.22–2.66, P=0.670, Table 3).
Carcinoid heart disease score
Increasing echocardiographic score was an independent predictor of both carcinoid heart disease progression and death. A five-point increase in the score was associated with an odds ratio of 2.95 (95% CI 1.71–5.09, P<0.005) for carcinoid heart disease progression and 2.66 (95% CI 1.63–4.35, P<0.005) for death.
Death
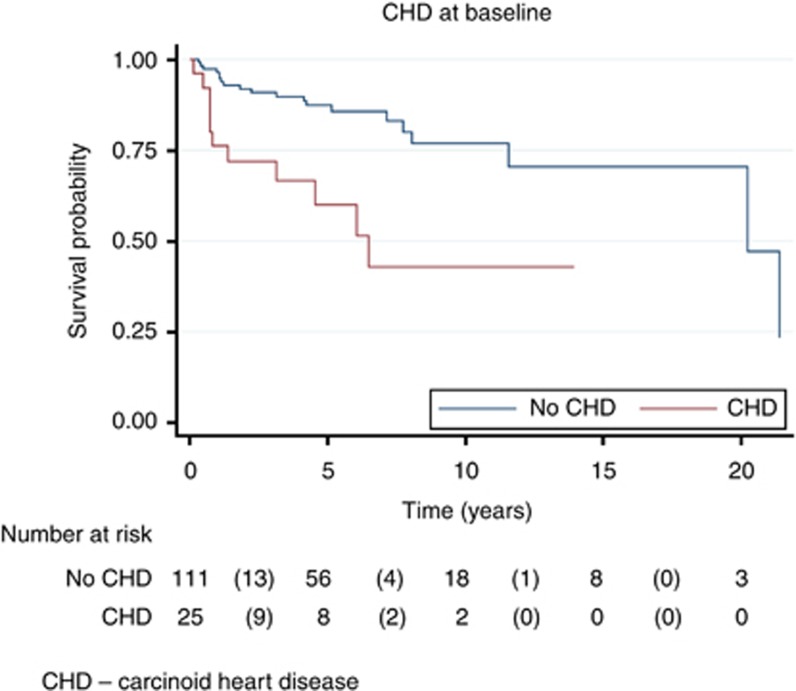
The deceased patients had significantly shorter durations of disease (15 months vs 64 months in the non-progressors and 52 months in the progressors, P=0.005). They also had histologically more aggressive tumours (14% high-grade vs 0% in the other groups, P<0.001). Of the 29 deceased patients, 12 (41%) had carcinoid heart disease. In a univariate Cox proportional hazard model, the risk of death in those with carcinoid heart disease at baseline was significantly greater than in those without carcinoid heart disease (hazard ratio 3.61, 95% CI (1.69–7.69), P=0.001). Figure 3 shows Kaplan–Meier survival estimates according to presence or absence of baseline carcinoid heart disease.
Figure 3.
Effect of baseline carcinoid heart disease on survival. Abbreviation: CHD=Carcinoid heart disease.
Discussion
In this large, prospective, observational cohort study we have demonstrated that biochemical variables, in particular baseline plasma 5-HIAA concentration, are independently associated with carcinoid heart disease progression in patients with metastatic NETs and have greater prognostic value than clinical or radiological variables.
Our finding of the value of plasma 5-HIAA concentration in the prediction of carcinoid heart disease progression is consistent with results from Bhattacharyya et al (2011) and Møller et al (2003) who demonstrated similar associations between carcinoid heart disease progression and urinary 5-HIAA concentrations. Serotonin is a major biochemical mediator of carcinoid heart disease (Gustafsson et al, 2005; Hutcheson et al, 2011) and therefore the measurement of its main metabolite, 5-HIAA, is a logical biomarker to measure. Plasma measurement of 5-HIAA is easier for the patient, and correlates well with the more traditional 24 h urinary measurement (Tellez et al, 2013). We have also demonstrated that both NT-proBNP and 5-HIAA are independently associated with death in patients with metastatic NETS and/or carcinoid syndrome. An elevated NT-proBNP concentration may reflect other factors known to increase risk of death such as NYHA class III–IV or right ventricular dilatation. Biochemical measurement is, however, a more objective measurement than attempting to evaluate NYHA class, which is notoriously difficult to estimate (Goldman et al, 1981).
Patients with symptomatic deterioration were more likely to demonstrate carcinoid heart disease progression than those with stable symptoms. This finding is similar to that of Bhattacharyya et al (2011) and is likely to be due to higher serotonin levels in the patients, with worsening symptoms of the carcinoid syndrome. Use of somatostatin analogue therapy was more common in the progressors, which may be a reflection of more advanced disease in this patient group.
We found no relationship between radiological progression and progression of carcinoid heart disease. However, resection of the primary tumour was more common in the non-progressors than in the progressors or deceased groups. This may support previous findings suggesting that a reduction in tumour burden by hepatic resection may decrease the risk of progression of carcinoid heart disease through the reduction in circulating hormone levels (Bernheim et al, 2008).
The rate of progression of carcinoid heart disease is difficult to estimate owing to the number of deaths in our study (no progression data available for these patients). The progression rate could be as low as 10% (13/137) or as high as 31% (42/137), but is likely to fall somewhere within this range, in keeping with the progression rates of similar studies (Møller et al, 2003; Bhattacharyya et al, 2011).
There are several limitations to our study to acknowledge. First is the lack of a standardised definition of carcinoid heart disease progression, although this can be applied to all similar studies. As the echocardiographic score assesses both sides of the heart, an increase in the score could be due to coexisting pathology such as degenerative mitral valve disease, and therefore using an absolute increase in the echocardiographic score as the determinant of carcinoid heart disease progression would lead to an overestimate of the number of patients demonstrating progression. For this reason we used a clinical definition. We also acknowledge the relatively short duration of follow-up in our study and the heterogeneity of the study population with differences in tumour biology/grade, types and duration of treatment modalities and duration of diagnosis. This limitation may be overcome in larger, perhaps national studies, in which common therapeutic pathways and algorithms may be adopted and longer-term outcome available. Finally, the cause of death was unknown and it is uncertain in all individuals whether death was attributable to NET disease progression, to carcinoid heart disease progression or to an unrelated cause.
The findings of this study suggest that it is the biochemical or hormonal burden of disease, rather than radiological extent or duration of disease that dictates development and progression of carcinoid heart disease. This implies that any treatments, medical (e.g. somatostatin analogues) or surgical (resection of primary tumour) that reduce secretion of vasoactive substances may be protective against the development and progression of carcinoid heart disease.
Acknowledgments
We would like to thank Mr Daniel Lythgoe and Ms Rebecca Asher at the Liverpool Cancer-Research UK Centre for their help with the statistical analysis. Aside from the above grant, this research did not receive any other specific grant from any funding agency in the public, commercial or not-for-profit sector.
Dr DJ Cuthbertson declares an investigator-initiated research grant from Ipsen. The remaining authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bernheim AM, Connolly HM, Rubin J, Møller JE, Scott CG, Nagorney DM, Pellikka PA. Role of hepatic resection for patients with carcinoid heart disease. Mayo Clin Proc. 2008;83 (2:143–150. doi: 10.4065/83.2.143. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid heart disease. Circulation. 2007;116 (24:2860–2865. doi: 10.1161/CIRCULATIONAHA.107.701367. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J. Usefulness of N-terminal pro-brain natriuretic peptide as a biomarker of the presence of carcinoid heart disease. Am J Cardiol. 2008;102 (7:938–942. doi: 10.1016/j.amjcard.2008.05.047. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Toumpanakis C, Chilkunda D, Caplin ME, Davar J. Risk factors for the development and progression of carcinoid heart disease. Am J Cardiol. 2011;107 (8:1221–1226. doi: 10.1016/j.amjcard.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Bonow RO, Carabello BA, Kanu C, de Leon AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B, Guidelines ACoCAHATFoP, Anesthesiologists SoC, Interventions SfCAa, Surgeons SoT ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114 (5:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- Denney WD, Kemp WE, Anthony LB, Oates JA, Byrd BF. Echocardiographic and biochemical evaluation of the development and progression of carcinoid heart disease. J Am Coll Cardiol. 1998;32 (4:1017–1022. doi: 10.1016/s0735-1097(98)00354-4. [DOI] [PubMed] [Google Scholar]
- Dobson R, Burgess MI, Banks M, Pritchard DM, Vora J, Valle JW, Wong C, Chadwick C, George K, Keevil B, Adaway J, Ardill JE, Anthoney A, Hofmann U, Poston GJ, Cuthbertson DJ. The association of a panel of biomarkers with the presence and severity of carcinoid heart disease: a cross-sectional study. PLoS One. 2013;8 (9:e73679. doi: 10.1371/journal.pone.0073679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45 (2:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Fox DJ, Khattar RS. Carcinoid heart disease: presentation, diagnosis, and management. Heart. 2004;90 (10:1224–1228. doi: 10.1136/hrt.2004.040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64 (6:1227–1234. doi: 10.1161/01.cir.64.6.1227. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM. Carcinoid heart disease. Int J Cardiol. 2008;129 (3:318–324. doi: 10.1016/j.ijcard.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen U, Waldum H. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111 (12:1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- Hess G, Runkel S, Zdunek D, Hitzler WE. Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): a study in blood donors. Clin Chim Acta. 2005;360 (1-2:187–193. doi: 10.1016/j.cccn.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease–it was meant 2B. Pharmacol Ther. 2011;132 (2:146–157. doi: 10.1016/j.pharmthera.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansencal N, Mitry E, Bachet JB, Rougier P, Dubourg O. Echocardiographic follow-up of treated patients with carcinoid syndrome. Am J Cardiol. 2010;105 (11:1588–1591. doi: 10.1016/j.amjcard.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Miller AG, Brown H, Degg T, Allen K, Keevil BG. Measurement of plasma 5-hydroxyindole acetic acid by liquid chromatography tandem mass spectrometry–comparison with HPLC methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878 (7-8:695–699. doi: 10.1016/j.jchromb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Møller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348 (11:1005–1015. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]
- Møller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112 (21:3320–3327. doi: 10.1161/CIRCULATIONAHA.105.553750. [DOI] [PubMed] [Google Scholar]
- Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, Arnold R, Denecke T, Plöckinger U, Salazar R, Grossman A, participants BCC ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95 (2:135–156. doi: 10.1159/000335629. [DOI] [PubMed] [Google Scholar]
- Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, Kvols LK. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87 (4:1188–1196. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- Plockinger U, Gustafsson B, Ivan D, Szpak W, Davar J. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: echocardiography. Neuroendocrinology. 2009;90 (2:190–193. doi: 10.1159/000225947. [DOI] [PubMed] [Google Scholar]
- Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92 (4:790–795. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23 (7:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Tellez MR, Mamikunian G, O'Dorisio TM, Vinik AI, Woltering EA. A single fasting plasma 5-HIAA value correlates with 24-hour urinary 5-HIAA values and other biomarkers in midgut neuroendocrine tumors (NETs) Pancreas. 2013;42 (3:405–410. doi: 10.1097/MPA.0b013e318271c0d5. [DOI] [PubMed] [Google Scholar]
- Westberg G, Wängberg B, Ahlman H, Bergh CH, Beckman-Suurküla M, Caidahl K. Prediction of prognosis by echocardiography in patients with midgut carcinoid syndrome. Br J Surg. 2001;88 (6:865–872. doi: 10.1046/j.0007-1323.2001.01798.x. [DOI] [PubMed] [Google Scholar]