Abstract
Background
The aim of this study was to investigate the effects of arginine in the development of atherosclerosis in rats fed a high-fat diet supplemented with arginine and to evaluate the role of CD36 in this process.
Material/Methods
A total of 40 Sprague-Dawley rats were randomly assigned to 4 groups: control group, fat diet group, simvastatin group, and arginine group. They were fed for 12 weeks and were then sacrificed. Immunohistochemical CD36 expression and pathology was investigated in the aorta; CD36 expression in mononuclear cells was detected by Western blot and RT-PCR.
Results
The thickness of the aortal intima, media, and I/M significantly decreased in the arginine group rats compared with those in the fat diet group (P<0.05). CD36 expression was up-regulated in rats in the fat diet group compared with the control group and was down-regulated in rats in the arginine group compared with rats in the fat diet group.
Conclusions
The addition of arginine has a significant effect on reducing rat atherosclerosis development, which may be attributed to both the down-regulation of CD36 expression in rat aortic endothelial and blood mononuclear cells and the NO pathway.
MeSH Keywords: Antigens, CD36; Arginine; Atherosclerosis
Background
Atherosclerosis (AS) is a significant pathophysiologic cause of cardiovascular events. Reasons for the occurrence and development of AS include lipid aggregation, endothelial injury, and inflammation [1]. In humans, endothelial dysfunction is one of the first detectable vascular alterations in the evolution of atherosclerosis, and its presence also correlates well with future cardiovascular events [2]. Endothelial injury is one of the primary pathogenesis in the initiation and progression of atherosclerosis [1]. Nitric oxide (NO) is a potential regulating factor of protected internal environment homeostasis and prevented apoptosis [3]. NO generated continuously can prevent apoptosis of endothelial cells and protect endothelial cells from damage [4], which is beneficial to prevent occurrence and development of AS [5]. During injury, the bioavailability of NO is enhanced, which can protect endothelial function [6]. Arginine is the main precursor of NO in the vascular endothelium. Arginine supplementation protects endothelial function [7], which reduces the occurrence and development of atherosclerosis. Therefore, arginine may reduce the development of atherosclerosis in rats fed a high-fat diet by protecting endothelial function. The present study, in which rats were fed a high-fat diet supplemented with arginine and their vasculum was detected, confirms this hypothesis. CD36 can adhere to and assist macrophages in phagocytizing oxygenized low-density lipoproteins (oxLDLs). Macrophages change into foam cells, which form the core of AS. CD36 plays an important part in AS plaque formation. Arginine potentially contributes to the reduction of platelet aggregation and the adhesion of mononuclear cells to the endothelium in hypercholesterolemic subjects [8]. This study aimed to define the role of CD36 in AS rats treated with arginine by detecting the expression of CD36. Simvastatin is currently accepted for curing atherosclerosis in clinical practice [9]. Simvastatin is used in many clinical studies, so we choose Simvastatin as the positive control lipid-lowering drug. Simvastatin was used as the positive control group of drug intervention in this study.
Material and Methods
Animals
Twenty adult male and 20 adult female Sprague-Dawley rats, aged 8 weeks and weighing 200±20 g, were supplied by the Shanghai Super B&K Laboratory Animal Co., Ltd., Shanghai, China. All rats were housed under standard conditions at 25°C with a 12/12 h light-dark cycle and free access to water. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Fudan University.
Rat model, groups, and drug intervention
The rats were randomly divided into 4 groups: control group (10), fat diet group (10), simvastatin group (10), and arginine group (10). Control group rats were fed with fat-free food for 12 weeks; the other 3 groups rats were fed a high-fat diet (94.3% basal feed +3% lard (purchased in the market) +2% cholesterol (Shanghai Technical Service Industry and Biological Engineering Co., Ltd., Shanghai, China) +0.5% sodium cholate (Shanghai Technical Service Industry and Biological Engineering Co., Ltd., Shanghai, China) +0.2% propylthiouracil (Shanghai Zhaohui Pharmaceutical Co., Ltd., Shanghai, China) and were intramuscularly injected with vitamin D3 (Harbin MT Agricultural Veterinary Medicine Co., Ltd., Harbin, China) (3×105 u/kg/moon) for 12 weeks. The arginine group rats were given arginine hydrochloride injections (20 ml: 5 g, Shanghai Sine Jin Zhu Pharmaceutical Co., Ltd., Shanghai, China) by gavage (1 g/kg/d). The dosage is chosen according to its usual dosage 10 g/day in 60 kilogram human and conversion formula of human-rat 1:6.17. The simvastatin group rats were given distilled water of the same volume with the simvastatin (Hangzhou MSD Pharmaceutical Co., Ltd., Hangzhou, China) by gavage (4 mg/kg/d). The dosage is chosen according to its usual dosage of 40 mg/day in 60-kilogram humans and conversion formula of human-rat 1:6.17. The control and fat diet group rats were given distilled water of the same volume for 12 weeks by gavage. After 12 weeks of gavage, all rat groups were anesthetized. Rat blood and aortas were collected.
Aortic pathology
The aortic root and arch were fixed with 10% formalin. Then, routine dehydration, paraffin embedding, HE staining, and microscopic examination were performed. The aortic lesions were recorded.
Immunohistochemistry
Routine paraffin sectioning, dewaxing, and hydration using 3% hydrogen peroxide were performed to remove endogenous peroxidase. Approximately 50 μl (1:100) of rabbit anti-rat CD36 antibody (Wuhan EIAab Science Co., Ltd., Wuhan, China) was added, and the mixture was incubated at 25°C overnight. Then, approximately 50 μl of goat anti-rabbit IgG antibody (Wuhan EIAab Science Co., Ltd., Wuhan, China) was added, and the mixture was incubated at 37°C for 60 min and colored using diaminobenzidine (Tiangen Biotech Co., Ltd., Beijing, China). The sample was dyed with hematoxylin and dehydrated with gradient alcohol and xylene.
Western blotting
Mononuclear cells were extracted from venous blood by density gradient centrifugation.
Mononuclear cells were lysed in buffer and ethylene diamine tetraacetic acid (EDTA). Proteins were extracted from cell lysates. Equal amounts of protein were electrophoresed on a 10% SDS-polyacrylamide gel and electrophoretically transferred to polyvinylidene fluoride membranes. After blocking with 2% BSA in Tris-buffered saline at room temperature for 1 h, the membranes were incubated with an antibody against rabbit anti-rat CD36 antibody (Wuhan EIAab Science Co., Ltd., Wuhan, China) overnight at 4°C. The membranes were then washed with PBS and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Wuhan EIAab Science Co., Ltd., Wuhan, China) for 1 h at room temperature. Immunoblotting was detected by chemiluminescence detection.
RT-PCR
Total RNA from mononuclear cells was isolated using Trizol-A+ Reagent (Tiangen Biotech Co., Ltd., Beijing, China) and cDNA was synthesized with 4 μl total RNA using the Quantscript RT Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s protocol. PCR resulted in specific amplificates according to the cDNA using SuperReal PreMix (SYBR Green) (Tiangen Biotech Co., Ltd., Beijing, China). The reactions were run using the following program: 95°C for 15 min, 40 cycles of 95°C for 10 s, and 60°C for 32 s. The sequences of primers specific for CD36 (sense: 5′-aggaagtggcaaagaatagcag-3′, antisense: 5′-acagacagtgaaggctcaaaga-3′) and beta-actin (sense: 5′-CCCATCTATGAGGGTTACGC-3′, antisense: 5′-TTTAATGTCACGCACGATTT-3) were synthesized by Sangon Biotech (Shanghai, China).
Statistical analysis
The overall response to increasing concentrations of TGF-b1 in monocytic cells was tested using a regression analysis. Comparisons of cells obtained from the same donor and incubated either under control conditions or in the presence of TGF-b1 were made using the matched-pairs signed rank Wilcoxon test.
Stata7.0 software was used to conduct statistical analyses. The results were expressed as the means ±SEM. The data among the several groups were analyzed by one-factor analysis of variance and the Bonferroni method for multiple comparisons or the Kruskal-Wallis rank test. In all analyses, statistical significance was set at P<0.05. Image measurement data were analyzed with Image Pro-Plus image analysis software.
Results
The pathological changes of aortas
After 12 weeks of being fed a high-fat diet, aortal intimal thickening and lipid deposition in the middle media developed in rats in the fat diet and arginine groups, but not those in the control and simvastatin groups. The thickness of aortal intima, media, and I/M significantly decreased in arginine group rats compared with the fat diet group rats (P<0.05, Table 1, Figure 1).
Table 1.
The thickness measurements (μm) of aortal intima, media and I/M in rats of each group.
| Group | Thickness of aortal intima | Thickness of media | Ratio of aortal intima and media (I/M) |
|---|---|---|---|
| Control | 11.5±1.3 | 125.2±5.6 | 0.09±0.02 |
| Fat diet | 172.6±4.4* | 285.4±8.4* | 0.60±0.03* |
| Simvastatin | 18.2±2.4** | 153.2±7.9** | 0.12±0.02** |
| Arginine | 51.5±3.8*,** | 163.5±6.6** | 0.31±0.03*,** |
P<0.05, vs. control group;
P<0.05, vs. fat diet group.
Figure 1.
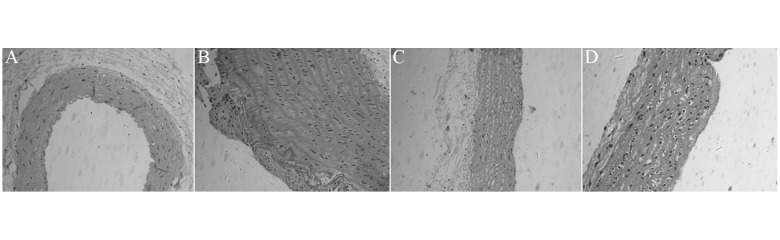
Representative photomicrograph of aortic pathological section in each group rats, HE staining, (100×). (A) Control group; (B) fat diet group; (C) simvastatin group; (D) arginine group.
CD36 expression
CD36 expression with immunohistochemistry (membrane and cytoplasm of positive cells were stained brown) was up-regulated in fat diet group rats compared to the control group and down-regulated in arginine and simvastatin group rats compared with fat diet group rats (Figure 2).
Figure 2.
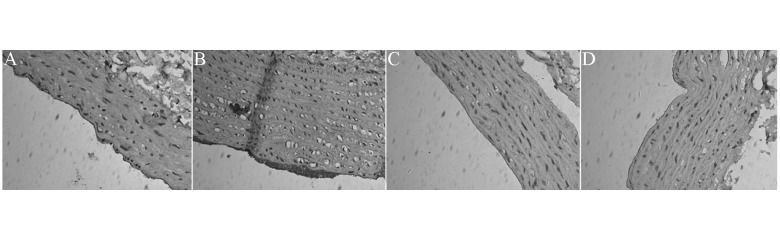
Representative photomicrograph of CD36 expression with immunohistochemistry in each group rats (200×). (A) Control group; (B) fat diet group; (C) simvastatin group; (D) arginine group.
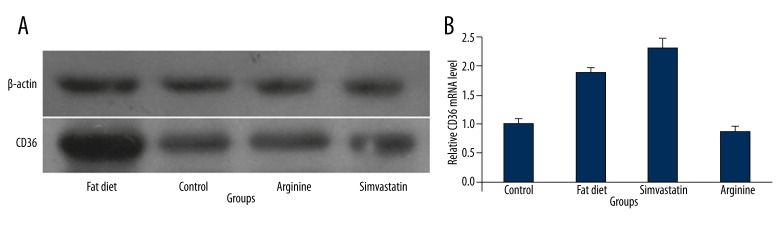
The CD36 mRNA expression of mononuclear cells was up-regulated in fat diet group rats compared with the control group and down-regulated in arginine group rats compared with the fat diet group. However, the CD36 mRNA expression was up-regulated in simvastatin group rats compared with both the control group and the fat diet group (Figures 3, 4B). The CD36 protein expression of mononuclear cells was up-regulated in fat diet group rats compared with the control group. No significant difference was found in the CD36 protein expression of mononuclear cells among rats in the control, arginine, and simvastatin groups (Figure 4A).
Figure 3.
Real-time RT-PCR amplification curves of β-actin RNA and CD36 mRNA. This shows that there is no pollution and interference of physical factors in the specimen. The standard is distributed uniformly and its gradient is normal.
Figure 4.
(A) CD36 protein expression of mononuclear cells with Western blot analysis in each group. (B) The ratio of CD36 mRNA expression and β-actin RNA expression with RT-PCR in each group.
Discussion
AS is a significant pathophysiologic cause of cardiovascular events. Clinical manifestations include myocardial infarction, aortic stenosis, aortic aneurysm, cerebral ischemia, cerebral hemorrhage, ischemic nephropathy, ischemic enteropathy, and peripheral artery disease [1]. The complex causes of AS development include lipid accumulation, endothelial damage, and inflammation. Low-dose folic acid supplementation has a beneficial effect on blood lipids [10]. Adiponectin may protect the aorta from atherosclerosis injury by reducing lesion formation size in the aortic root and reducing TC, TG, and LDL-C in serum [11]. Vascular endothelial injury is a main pathogenesis of AS development. Vascular endothelial injury can cause increased endothelial permeability for lipoproteins and other plasma components. The normal regulative balance of the vascular endothelium is altered, including a reduction of NO synthesis and secretion, increased endothelin release, increased expression of endothelial adhesion molecules, and disorder of endothelial antithrombotic function [1]. Endothelial dysfunction is one of the first early and detectable vascular changes of AS. Endothelial dysfunction is also closely associated with the presence of additional cardiovascular events [6]. Endothelial dysfunction can promote the formation of atherosclerotic plaques and the development of AS [2]. NO is not only a vasodilator substance, but it can also prevent platelet adhesion and aggregation, prevent leukocyte adhesion and migration to the arterial wall, and inhibit smooth muscle cell proliferation, which is crucial for AS development. In circulation, NO is highly permeable, and a small molecule generated from arginine is catalyzed by NOS. Arginine is the main precursor of NO in vascular endothelium. The addition of exogenous arginine can enhance the bioavailability of NO.
Research by Javanmard et al. [12] showed that the use of an arginine diet can prevent vascular fatty streak formation induced by a fat diet, elevation of von Willebrand factor (vWF), and endothelial damage. An arginine diet also can improve the bioavailability of NO. Fiorito et al. [7] demonstrated that arginine supplementation has an appropriate endothelial protective effect in C57BL/6J mice by increasing endothelial progenitor cells (EPCs) and vascular endothelial growth factor (VEGF). Many experiments [13–16] have indicated that arginine supplementation can repair endothelial function, improve cardiac function, reduce the incidence of arrhythmias, and relieve ischemia-reperfusion injury by NO in many diseases associated with endothelial dysfunction. There is no significant difference in the efficacy of intravenous or oral arginine [17]. In this study, the thickness of aortal intima, media, and I/M significantly decreased in arginine group rats compared with fat diet group rats. The results show that a high-fat diet with simultaneous oral arginine supplementation can reduce the development of atherosclerosis, which may be related to the arginine-NO pathway that reduces vascular endothelial inflammation and protects endothelial function, thereby reducing the development of atherosclerosis.
CD36 antigen is widely present in many different types of cells, such as macrophages, monocytes, platelets, and endothelial cells [18]. Oxidized low-density lipoprotein (oxLDL) can be adhered to and phagocytized by CD36 into macrophages, which eventually become foam cells. Foam cells, which constitute the core infrastructure of AS, play an important role in the formation of plaque [19,20]. A variety of AS-induced inflammatory cytokines, such as macrophage colony-stimulating factor (MCSF), γ-interferon (IFN-γ), and interleukin-10 (L-10), may increase the expression of CD36 [21,22]. Animal experiments showed that pitavastatin can decrease the transcription of macrophages and Th1 cell surface CD36 mRNA and the expression of surface protein [23]. In hypercholesterolemic patients given oral atorvastatin for 6 days, the CD36 expression on the platelet surface was observed to be significantly reduced [24]. Arginine plays a potential beneficial role in reducing platelet aggregation and monocyte and endothelial cell adhesion for hypercholesterolemic patients [8]. It has been confirmed that arginine can inhibit the development of rat artery atherosclerosis [25]. In addition to the known NO pathway, the effects of arginine on CD36 expression should be studied as a possible mechanism.
This experiment studied CD36 protein expression in the rat aorta in each group. CD36 protein expression was up-regulated in fat diet group rats compared with control group rats and down-regulated in arginine group rats compared with fat diet group rats. The same results were observed in rat blood mononuclear cell experiments. The expression of CD36 was detected using both Western blot and quantitative real-time PCR in this experiment. According to the real-time PCR results, the CD36 mRNA expression of rat blood mononuclear cells was up-regulated in fat diet group rats compared with control group rats and down-regulated in arginine group rats compared with fat diet group rats. Similar expression levels of CD36 mRNA were detected in rats in both the arginine and control groups. The experimental pathology confirmed that arginine can reduce atherosclerosis in fat diet rats and reduce CD36 expression in rat blood mononuclear cells and aorta. Therefore, it can be concluded that arginine can reduce the occurrence and development of atherosclerosis by reducing CD36 expression in fat diet rat blood mononuclear cells and aorta. In simvastatin group rats, the CD36 mRNA expression was up-regulated, but the CD36 protein expression was down-regulated compared with the fat diet group. Therefore, Simvastatin may have an anti-atherosclerosis effect by intervening in some mechanism in the CD36mRNA downstream channel to influence expression of CD36 protein, not by down-regulating CD36mRNA expression. Arginine may reduce platelet aggregation and monocyte and endothelial cell adhesion and provides anti-oxidative and anti-inflammatory properties to reduce the generation of inflammatory cytokines, which reduces CD36 expression in blood mononuclear cells and aorta. This finding needs further experimental research to identify the mechanisms and pathways through which arginine affects the expression of CD36.
Conclusions
Supplementation with arginine can alleviate atherosclerosis occurrence in high-fat diet rats. In addition to NO, the mechanism of this phenomenon may be associated with the down-regulation of CD36 expression in the endothelia of the aorta and monocytes in blood circulation.
Footnotes
Conflict of interest
All authors have no conflict of interest regarding this paper.
Source of support: This study is supported by Jinshan Science and Technology Committee, Shanghai, China (2009-3-02)
Reference
- 1.Andreoli TE, Carpenter CC, Griggs RC. Cecil Essentials of Medicine. Peking University Medical Press; Peking: 2008. [Google Scholar]
- 2.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 3.Li CQ, Wogan GN. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2005;226:1–15. doi: 10.1016/j.canlet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Tricot O, Mallat Z, Heymes C, et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–53. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 5.Napoli C, Ignarro LJ. Nitric oxide-releasing drugs. Annu Rev Pharmacol Toxicol. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- 6.Flammer AJ, Lüscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 7.Fiorito C, Balestrieri ML, Crimi E, et al. Effect of L-arginine on circulating endothelial progenitor cells and VEGF after moderate physical training in mice. Int J Cardiol. 2008;126:421–23. doi: 10.1016/j.ijcard.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Kawano H, Motoyama T, Hirai N, et al. Endothelial dysfunction in hypercholesterolemia is improved by L-arginine administration: possible role of oxidative stress. Atherosclerosis. 2002;161:375–80. doi: 10.1016/s0021-9150(01)00671-2. [DOI] [PubMed] [Google Scholar]
- 9.Toth PP, Ballantyne CM, Davidson MH, et al. Changes in prescription patterns before and after reporting of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression trial (ENHANCE) results and expected effects on low-density lipoprotein-cholesterol reduction. J Clin Lipidol. 2012;6:180–91. doi: 10.1016/j.jacl.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Mierzecki A, Kłoda K, Bukowska H, et al. Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors. Med Sci Monit. 2013;19:733–39. doi: 10.12659/MSM.889087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Pu H, Ma C, et al. Adiponectin abates atherosclerosis by reducing oxidative stress. Med Sci Monit. 2014;20:1792–800. doi: 10.12659/MSM.892299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javanmard SH, Gheisari Y, Soleimani M, et al. Effect of L-arginine on circulating endothelial progenitor cells in hypercholesterolemic rabbits. Int J Cardiol. 2010;143:213–16. doi: 10.1016/j.ijcard.2008.11.203. [DOI] [PubMed] [Google Scholar]
- 13.Chin-Dusting JP, Willems L, Kaye DM. L-Arginine transporters in cardiovascular disease: A novel therapeutic target. Pharmacol Ther. 2007;116:428–36. doi: 10.1016/j.pharmthera.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Gad MZ, Abu El Maaty MA, El-Maraghy SA, et al. Investigating the cardio-protective abilities of supplemental L-arginine on parameters of endothelial function in a hypercholesterolemic animal model. J Nutr Sci Vitaminol (Tokyo) 2014;60:145–51. doi: 10.3177/jnsv.60.145. [DOI] [PubMed] [Google Scholar]
- 15.Dhar I, Dhar A, Wu L, Desai K. Arginine attenuates methylglyoxal- and high glucose-induced endothelial dysfunction and oxidative stress by an endothelial nitric-oxide synthase-independent mechanism. J Pharmacol Exp Ther. 2012;342:196–204. doi: 10.1124/jpet.112.192112. [DOI] [PubMed] [Google Scholar]
- 16.Sourij H, Meinitzer A, Pilz S, et al. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis. 2011;218:220–25. doi: 10.1016/j.atherosclerosis.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Shen W, Bai YY, Zhang XX, Wang YF. L-Arginine improves endothelial function of brachial artery. J Clin Cardiol. 2006;22:653–55. [Google Scholar]
- 18.Matsumoto K, Hirano K, Nozaki S, et al. Expression of macrophage (Mphi) scavenger receptor, CD36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor gamma, which regulates gain of Mphilike phenotype in vitro, and its implication in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:1027–32. doi: 10.1161/01.atv.20.4.1027. [DOI] [PubMed] [Google Scholar]
- 19.Robertson S, Colombo ES, Lucas SN, et al. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci. 2013;134:304–11. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014;46:e99. doi: 10.1038/emm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubic T, Lorenz RL. Downregulated CD36 and oxLDL up take and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of inter-leukin210. Cardiovasc Res. 2006;69:527–35. doi: 10.1016/j.cardiores.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira V, van Dijk KW, Groen AK, et al. Macrophage specific inhibition of NF-kappaB activation reduces foam-cell formation. Atherosclerosis. 2007;192:283–90. doi: 10.1016/j.atherosclerosis.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Zhou X, Yokoyama T, et al. Pitavastatin downregulates expression of the macrophage type B scavenger receptor, CD36. Circulation. 2004;109:790–96. doi: 10.1161/01.CIR.0000112576.40815.13. [DOI] [PubMed] [Google Scholar]
- 24.Puccetti L, Sawamura T, Pasqui AL, et al. Atorvastatin reduces platelet2oxi-dized2 LDL receptor ex ression in hypercholesterolaemic patients. Eur J Clin Invest. 2005;35:47–51. doi: 10.1111/j.1365-2362.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- 25.Aji W, Ravalli S, Szabolcs M, et al. L-arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation. 1997;95:430–37. doi: 10.1161/01.cir.95.2.430. [DOI] [PubMed] [Google Scholar]