Abstract
This study describes the epidemiology of congenital amelia (absence of limb/s), using the largest series of cases known to date. Data were gathered by 20 surveillance programs on congenital anomalies, all International Clearinghouse for Birth Defects Surveillance and Research members, from all continents but Africa, from 1968 to 2006, depending on the program. Reported clinical information on cases was thoroughly reviewed to identify those strictly meeting the definition of amelia. Those with amniotic bands or limb-body wall complex were excluded. The primary epidemiological analyses focused on isolated cases and those with multiple congenital anomalies (MCA). A total of 326 amelia cases were ascertained among 23,110,591 live births, stillbirths and (for some programs) elective terminations of pregnancy for fetal anomalies. The overall total prevalence was 1.41 per 100,000 (95% confidence interval: 1.26–1.57). Only China Beijing and Mexico RYVEMCE had total prevalences, which were significantly higher than this overall total prevalence. Some under-registration could influence the total prevalence in some programs. Liveborn cases represented 54.6% of total. Among monomelic cases (representing 65.2% of nonsyndromic amelia cases), both sides were equally involved, and the upper limbs (53.9%) were slightly more frequently affected. One of the most interesting findings was a higher prevalence of amelia among offspring of mothers younger than 20 years. Sixty-nine percent of the cases had MCA or syndromes. The most frequent defects associated with amelia were other types of musculoskeletal defects, intestinal, some renal and genital defects, oral clefts, defects of cardiac septa, and anencephaly.
Keywords: amelia, epidemiology, prevalence, frequency, ICBDSR
INTRODUCTION
Amelia (from Greek: α ‘without, lack of’, plus μέλοζ ‘limb’) is a congenital anomaly characterized by the complete absence of one or more limbs. According to the classification suggested by Frantz and O’Rahilly [1961] or Swanson [1976], amelia constitutes a specific group among the terminal transverse reduction defects of the limbs.
Some Historical Aspects
Limb defects have always attracted general attention, and the earliest known written records are extremely ancient. Their descriptions appear on clay tablets found at Nineveh in the archives of the Assyrian king Ashurbanipal (668–626 BC), referring to 62 different human limb defects. Probably, the first patient of known identity reported with amelia was born in 1575 in Switzerland [Sonderegger, 1927; Czeizel et al., 1994]. Since then, many other individual cases have been reported.
Embryology of the Limbs
Human limb development initiates on the 26th day after fertilization for the upper limb and the 28th day for the lower limb, and extends until day 56 [Sadler, 2009]. The appendicular skeleton develops from the lateral plate mesoderm (split into paraxial and somatic). Activation of the mesenchymal cells of the lateral mesodermal plate causes an outgrowth of the limb buds, which become visible as outpocketings from the ventrolateral body wall. Each tissue (cartilage, bone, and muscle) arises through several mechanisms of differentiation. In the limb bud, mesenchyme, derived from the somatic layer of the lateral plate mesoderm is the source of the skeletal components that will form the bones and connective tissues of the limb. Mesenchyme derived from the myotomes of the paraxial mesoderm forms the muscular component [Moore and Persaud, 2008]. The mesenchymal core is covered by a layer of cuboidal ectoderm which becomes thickened at the distal rim of the limb bud to form the apical ectodermal ridge (AER) on the 33rd day. This AER exerts an inductive influence on the underlying mesenchyme [Summerbell, 1974]. Subjacent to the AER, a vascular channel can be found that is essential for the integrity of the AER and for continued limb outgrowth. Mesenchyme adjacent to the AER remains as a population of undifferentiated, rapidly proliferating cells, whereas cells located farther away from the influence of the AER begin to differentiate into cartilage and muscle.
According to the progress zone model, a cell’s proximodistal identity is determined by the length of time spent in the distal limb region termed the “progress zone.” By 6 weeks, the hand and foot plates are apparent. Development of the feet is similar to that of the hands, but starts approximately 2 days later. As the limb bud grows, apoptosis in the AER separates the ridge into five parts and indentations become apparent in the hand and foot plate. During the 7th and the 8th weeks of human development the digits can be recognized. The hand and foot plates become separated from the proximal segment of the limb by a circular constriction which becomes the wrist and ankle. Later, a second constriction at the level of the elbow and knee divides the proximal portion into two segments, so that the main segments of the limb (proximal stylopod, middle zeugopod, and distal autopod) can be distinguished.
By the 6th week of development the first hyaline cartilage in the limbs can be recognized. The skeleton of the limbs is formed as a hyaline cartilage precursor which ossifies by the end of the embryonic period. Primary ossification centers are present in all long bones of the limbs by the 12th week of development.
Molecular Embryology and Genetics
The genetic processes that control development of the limbs are complicated and still not fully understood. Some genes or gene families and molecular genetic factors are known to be involved in growth and differentiation of the developing limb [Barham and Clarke, 2008], a process which is spatially and temporally coordinated. The products of those genes act as signals to turn on other genetic pathways. Some influence the initiation and patterning of both the forelimb and the hindlimb, but others are differentially expressed in the developing forelimb and hindlimb. In Table I, the main genes or gene families involved in limb development are summarized, and other details are provided by Bermejo-Sánchez et al. [in press] in this issue of the journal. Apart from the action of these genetic factors, retinoic acid (RA) levels must be carefully controlled during limb bud development since both high and low levels have been associated with developmental abnormalities. RA up-regulates the Hox genes in the limb fields. It also stimulates Sonic Hedgehog (SHH) up-regulation, influences the creation of the zone of polarizing activity (ZPA), controls the condensation and differentiation of chondroblasts and coordinates chondrocyte maturation, osteoblast differentiation, and bone formation.
TABLE I.
Summary of the Molecular Embryology of the Limbs
| Genes or gene families | Function |
|---|---|
| Pitx1 | This belongs to an expanding family of bicoid-related vertebrate homeobox genes. It encodes a transcription factor that is expressed throughout the developing hindlimb, but not in forelimb buds. Pitx1 is not essential for hindlimb development, and if it is knocked out, the hindlimb will develop, but with a morphology similar to that of a forelimb |
| T-box genes | This is a family of transcription factors. Tbx4 and Tbx5 are expressed in the forelimb and hindlimb, respectively. The temporal expression patterns of Tbx5, Tbx4, and Pitx1 suggest they play an important role in programming the identity of the developing limb. Ectopically expressed Tbx5 can induce expression of the forelimb marker Hoxd9 and repress the hindlimb marker Hoxc9 [Rodríguez-Esteban et al., 1999; Takeuchi et al., 1999]. If Tbx5 is knocked out or inactivated, complete failure of formation of any elements of the forelimb occurs. Tbx5 interacts with Fgf and Wnt to initiate outgrowth of the limb bud [Agarwal et al., 2003; Rallis et al., 2003]. Tbx5 and Tbx4 activate fibroblast growth factor-10 (Fgf10) in the forelimb and hindlimb, respectively |
| Fgf family | FGF10 signals the ectoderm to induce Fgf8, which is instrumental in the formation of the AER at the tip of the developing limb bud. FGF10 promotes Fgf8 expression, and FGF8 promotes Fgf10 expression in a positive feedback loop, regulated by the Wnt signaling pathway [Agarwal et al., 2003]. If Fgf10 is knocked out in mice, no limb develops [Min et al., 1998]. Fgf4 is expressed at the dorsal end of the limb bud AER. Fgf4 and Fgf8 expression stimulates and maintains the rapid growth of the progress zone and prevents the local mesenchymal cells from differentiating into chondrocytes [Vogel et al., 1996]. Tissue proximal to the progress zone, being no longer influenced by the AER, becomes influenced by bone morphogenetic proteins (BMP) causing condensation and differentiation of the mesenchymal cells into groups of chondrocytes |
| R-fng (radical fringe) | This is expressed in the dorsal half of the limb and restricts the AER to the distal tip of the developing limb, by causing expression of Serrate-2, which defines the border of the AER [Laufer et al., 1997]. Engrailed-1 suppresses the expression of R-fng and therefore Serrate-2 and influence the formation of the AER |
| Hox-A and Hox-D clusters | These control patterning and hence morphology of the developing limb in the human embryo. The Meis1/2, Hoxa11, and Hoxa13 expression domains mark the three proximodistal territories (stylopod—Meis1/2, zeugopod—Hoxa11, and autopod—Hoxa13) [Bénazet and Zeller, 2009]. In the stylopod stage, Hoxd-9 and Hoxd-10 express during the formation of the humerus. In the zeugopod stage, Hoxd-9, Hoxd-10, Hoxd-11, Hoxd-12, and Hoxd-13 overlap in their expression to form the radius/tibia and the ulna/fibula. In the autopod stage, Hoxa-12, Hoxa-13, Hoxd-10, Hoxd-11, Hoxd-12, and Hoxd-13 express to form the developing hand and foot |
| Hoxb-8 | Hoxb-8 and retinoic acid act on the posterior mesoderm to initiate the ZPA in the posterior border of the limb, close to the AER and adjacent to the body wall [Charite et al., 1994; Scadding, 1999] |
| SHH (Sonic Hedge hog) | This controls the development of the antero-posterior axis [Riddle et al., 1993]. Shh stimulates Fgfs in the AER, and Fgfs in AER activate Shh in the ZPA, to develop more than one axis |
| Wnt7a | This maintains the Shh signal once it has been initiated. The regulated expression or suppression of Wnt7a controls patterning in the dorso-ventral axis. It also influences anterior–posterior patterning by promoting Shh expression in the ZPA [Tickle, 2003]. Mutation of WNT7A has been found related with tetra-amelia [Eyaid et al., 2011] |
| BMP (Bone morphogenetic proteins) | They induce the formation of bone and cartilage. BMP2, BMP4, and BMP7 are found in the developing mesoderm and the AER, and have important roles in skeletal development. BMPs are expressed in response to the Shh signal pathway. BMP2 plays a key role in osteoblast differentiation and induction of bone formation. BMP4 regulates the formation of limbs from the mesoderm, and BMP7 is important in osteoblast differentiation. BMP2 and BMP7, under the influence of Shh play a crucial role in digit identity and formation [Barham and Clarke, 2008] |
| Sox9 | This initiates the condensation and differentiation of chondroblasts in the embryonic limb. Cartilage fails to develop in limbs where Sox9 is inactivated [Foster, 1996; Akiyama et al., 2002] |
| Cbfa1 | This transcription factor regulates chondrocyte maturation and osteoblast differentiation |
AER, apical ectodermal ridge; ZPA, zone of polarizing activity.
Regarding the genetic aspects of amelia, it usually occurs as a sporadic event. Brent and Holmes [1988] noted the more restricted etiologies for amelia compared with the broader categories of limb reduction defects. Amelia is not generally considered to be of genetic origin [Lenz, 1980]. In the study of Froster-Iskenius and Baird [1990], no evidence for familial recurrence was observed. Although it may occur with additional congenital anomalies, amelia is an infrequent feature in genetic syndromes. For example, if one introduces “amelia” (affecting upper or lower limbs) as a search criterion in the Winter–Baraitser Dysmorphology Database [Winter and Baraitser, 2010] and the OMIM (Online Mendelian Inheritance in Man) database [OMIM, 2011] combined, the result is a list of only 31 syndromes meeting the search criterion (Table II). Some of these are known to be caused by mutations in specific genes, such as WNT3 in Tetra-amelia (OMIM 273395), or IRF6 in popliteal pterygium syndrome. Table II also includes the chromosome location and responsible gene for those syndromes where these are known.
TABLE II.
Syndromes or Defined Phenotypes Presenting With Amelia [Winter and Baraitser, 2010; OMIM, 2011]
| Syndrome or defined phenotype | OMIM number, or Refs. | Location | Human gene/locus |
|---|---|---|---|
| Amelia, anorectal, and genital atresia | Ghosh and Gupta [2004] | — | — |
| CHILD (congenital hemidysplasia, ichthyosis, limb defects) | 308050 | Xq28 | NSDHL |
| Cloacal extrophy and limb defects | Sawaya et al. [2010] | — | — |
| Diaphragmatic hernia limb anomalies | Lai et al. [2010] | — | — |
| Disorganization-like | 223200 | — | — |
| DK-phocomelia | 223340 | — | — |
| Femur-fibula-ulna (FFU) complex | 228200 | — | — |
| Fetal alcohol syndrome | Pauli and Feldman [1986] | — | — |
| Fetal bifonazole | Linder et al. [2010] | — | — |
| Fetal cocaine | Marles et al. [2003] | — | — |
| Fetal thalidomide | Lenz [1961, 1962], McCredie and Willert [1999] | — | — |
| Fibular aplasia, oligodactyly, camptomelia | 246570 | — | — |
| Glass—ear anomalies, clefting, limb reduction defects | Glass et al. [1994] | — | — |
| LL syndrome—amelia, upper limb defects | Lazjuk et al. [1976] | — | — |
| Maternal diabetes syndrome | Martínez-Frías [1994] | — | — |
| McKusick—cataract, unilateral limb defects | 246000 | — | — |
| Michaud—autosomal recessive amelia | 601360 | — | — |
| Microgastria—upper limb anomalies | 156810 | — | — |
| Ohdo—tetraamelia, facial abnormalities, mental retardation | 273390 | — | — |
| Popliteal pterygium syndrome | 119500 | 1q32.3-q41 | IRF6 |
| Ratan—limb defects, imperforate anus, ventricular septal defect | Ratan et al. [2005] | — | — |
| Roberts (pseudothalidomide) syndrome | 268300 | 8p21.1 | ESCO2 |
| Schinzel—phocomelia and additional anomalies | 276820 | 3p25 | WNT7A |
| Splenogonadal fusion-limb defects | 183300 | — | — |
| Steinfeld—holoprosencephaly, limb defects | 184705 | — | — |
| Upper limb amelia, male pseudohermaphroditism | Ohro et al. [1998] | — | — |
| Urioste—limb deficiency, vertebral hypersegmentation, absent thymus | Urioste et al. [1996], Martínez-Frías et al. [1997b] | — | — |
| VACTERL (vertebral, anal, cardiac, tracheo-esophageal, renal and limb defects) | 192350 | 2q31.1 | HOXD13 |
| XK-aprosencephaly | 207770 | — | — |
| Yim—amelia, hydrocephalus, iris coloboma, cleft lip/palate | Kariminejad et al. [2009] | — | — |
Pathogenesis
It has been established that there are at least three mechanisms by which limb deficiencies can occur: (a) failure of formation of the limb anlage in the very early stages of embryo development, which can be the result of errors in the genetic control of limb development, or an insult during blastogenesis [Froster-Iskenius and Baird, 1990; Martínez-Frías et al., 1997a]; (b) intra-uterine amputation from amniotic bands [Tadmor et al., 1997]; and (c) disruption of the developing arterial supply to the limb [Hoyme et al., 1982; Weaver, 1998]. Regarding the first mechanism, the processes that take place for the formation of the limbs, and the genes controlling or affecting those processes, have been explained above in detail. With respect to amniotic bands, there is evidence that they can form a constriction around the developing limb that interferes with its growth, resulting in degrees of damage from a minor constriction band around a limb that is otherwise normal to complete transverse amputation. Disruption of the developing arterial supply may cause severe ischemia of the limb bud, producing the anomaly also with variable degrees of severity and associated lesions. Such disruption of the arterial supply can be the consequence of uterine artery occlusion, or exposure to factors which diminish the blood flow at the uterine/placental unit, such as cocaine or other vasoconstrictive agents, or those causing vasculitis or infectious arteritis, or vaginal bleeding. Moreover, some abnormalities of the placental–fetal unit (observed in cases of placental insufficiency, twin arterial–arterial or arterial–venous anastomoses, amnion rupture, or umbilical cord obstruction), or an abnormal fetal unit (due to disruption of newly formed vessels, or external compression of blood vessels, embolic events, premature ablation of transient vessels, or aberrant regulation of vessel formation) could have an effect. In fact, placental vascular anastomoses between the placentas in twins, which are more frequent in monozygotic twinning, have been related to amelia by altering the arterial supply [Phelan et al., 1998].
Epidemiology
Data on the prevalence of amelia are scarce, and most published articles on this congenital defect are single case reports or limited series. Moreover, in some studies cases of amelia were not analyzed separately from other transverse limb reduction defects or from phocomelia (which is characterized by the absence of the intermediate segments of the limb with the distal segments being present, and is reviewed in this issue of the journal [Bermejo-Sánchez et al., in press]). As can be observed from the few published studies providing data on this condition (Table III), amelia has a low prevalence ranging from 0.95 per 100,000 births [Källén et al., 1984] to 1.71 per 100,000 births [Castilla et al., 1995]. However, the prevalence of amelia among stillbirths (SB) (varying from 34.56 per 100,000 [Martínez-Frías et al., 1997a] to 79.05 per 100,000 [Froster and Baird, 1993]) was reported to be at least 30.9 times higher than that among live births (LB) (Table III). In the study of Castilla et al. [1995], 34% (n = 50) of the amelia cases were SB, this figure being much higher than in the study of Martínez-Frías et al. [1997a] (16.7%, n = 18).
TABLE III.
Prevalence of Amelia From Various Published Studies
| Study | Prevalence | Population/sample |
|---|---|---|
| Referred to total births | ||
| Castilla et al. [1995] | 1.71 per 100,000 births | 2,917,074 births |
| Evans et al. [1994] | 1.02 per 100,000 births | 1,575,904 births |
| Källén et al. [1984] | 0.95 per 100,000 births | 1,368,024 births |
| Martínez-Frías et al. [1997a] | 1.50 per 100,000 births | 1,198,580 births |
| Mastroiacovo et al. [1992] | 1.50 per 100,000 births | 9,848,000 births |
| Referred to live births | ||
| Bod et al. [1983] | 0.53 per 100,000 LB | 561,915 LB |
| Froster-Iskenius and Baird [1990] | 1.48 per 100,000 LB | 1,213,913 LB |
| Martínez-Frías et al. [1997a] | 1.12 per 100,000 LB | 1,333,879 LB |
| Birch-Jensen [1949] | 0.2a per 100,000 LB | Nonspecified number of LB |
| Referred to stillbirths | ||
| Martínez-Frías et al. [1997a] | 34.56 per 100,000 SB | 8,680 SB |
| Froster and Baird [1993] | 79.05 per 100,000 SB | 7,590 SB |
LB, live births; SB: stillbirths.
Includes only amelia of the upper limb.
Amelia affected the upper and lower limbs equally in the study of Froster-Iskenius and Baird [1990], and 11.1% (n = 18) of liveborn cases had both the upper and lower limbs affected. However, in the study of Martínez-Frías et al. [1997a], globally, the lower limbs were affected in 72.2% of cases (n = 18).
Regarding laterality, according to data of Froster-Iskenius and Baird [1990] (n= 18), bilateral amelia occurred in 22% of cases, left-sided defects occurred in 50%, and right-sided defects occurred in 28%; this difference between left- and right-sided defects was not statistically significant. In the study of Martínez-Frías et al. [1997a], 16.7% (n = 18) of cases were bilateral, 33.3% had the left side involved and 50% the right one; most cases (83.3%) had absence of one limb, and three (16.7%) had absence of two limbs. Amelia involved a single limb in 58% (n = 24) of cases with anomalies in other organ systems in addition to amelia in the study of Evans et al. [1994].
The sex ratio in the study of Froster-Iskenius and Baird [1990] (11 males to 7 females) was not significantly different from the one among LB in the general population of British Columbia during the study period. However, according to the data of Martínez-Frías et al. [1997a], there was a small excess of females affected (7 males to 9 females), although this ratio was not significantly different from that found by Froster-Iskenius and Baird [1990] or from that of the general population in Spain (1.06 males to 1 female) [Martínez-Frías et al., 1997a]. According to data derived from the World Health Organization (WHO) database, the proportion of male newborns, although subject to geographical variation, is approximately 51% [Parazzini et al., 1998]. Nevertheless, the small excess of females in the study of Martínez-Frías et al. [1997a] could be due just to small numbers.
Regarding other characteristics of infants with amelia, some were studied by Martínez-Frías et al. [1997a]. The birth weight and gestational age of amelia cases were significantly lower than among the healthy controls. The mean birth weight of amelia cases was below the 3rd centile for the mean gestational age (35.47 weeks), which could be expected due to the absence of the limb(s). Breech and other non-cephalic presentations at birth were more frequent among cases (46.7%, n = 18) than among controls (3.9%, n = 25,086). The percentage of a single umbilical artery was also significantly higher (57.1%, n = 7) than among controls (1.1%, n = 14,482).
None of the cases included in the study of Froster-Iskenius and Baird [1990] (n = 18) had a family member registered with a limb anomaly, although the brother of a stillborn index patient had imperforate anus, and a cousin had meningomyelocele with hydrocephaly, which the authors interpreted as a possible familial recurrence of an early disturbance of development. There were three further cases with apparently unrelated defects among their relatives.
Associated Defects
In the study of Froster-Iskenius and Baird [1990], up to 61% of the LB (n= 18) and 100% of the SB amelia cases (n = 6) also had associated defects. The prevalence with which malformations in other organ systems were present in liveborn individuals with amelia was not different from that in cases with all types of limb reduction defects (348 out of 659). The most frequently occurring additional malformation among amelia cases was omphalocele (six LB and three SB), which occurred together with neural tube defects in two cases, and with absent diaphragm but no neural tube defect in three cases. One LB case and two SB had anencephaly. Similarly, the kidney was absent unilaterally in two LB and two SB. Cleft lip (with or without cleft palate) also occurred in two LB and two SB cases with amelia. All these prevalences are much higher than expected.
In the study of Evans et al. [1994], 56.3% (n = 16) of the cases had defects in other organs, a percentage which was slightly lower than in the study of Froster-Iskenius and Baird [1990], and much lower than in the one by Castilla et al. [1995] (72%, n = 50). According to data of Evans et al. [1994], there was a high prevalence of body wall defects, anencephaly, and cleft lip among the amelia cases. These associations were also reported by Froster-Iskenius and Baird [1990] and Mastroiacovo et al. [1992].
The study by Martínez-Frías et al. [1997a] (n = 18) reported no cases with omphalocele but noted renal anomalies in 27.8% of cases, body wall defects also in 27.8%, neural tube defects in 16.7%, cleft lip (with or without cleft palate) in 11.1%, and diaphragmatic defects in 11.1% of the cases.
Rosano et al. [2000] found that the total prevalence of amelia combined with other major congenital anomalies was 0.77 per 100,000 births (0.08 among LB, 0.62 among SB, and 0.08 among elective terminations of pregnancy for fetal anomalies (ETOPFA)). Those authors found significant associations with gastroschisis, unilateral kidney dysgenesis, severe defects of genitalia, ring constriction-amniotic bands, omphalocele, and anorectal atresia.
Risk Factors and Prevention
We failed to find additional published studies that specifically focused on risk factors for amelia. Since amelia has been described in several infants exposed to thalidomide, from studies on this drug, it was concluded that the sensitive period for producing amelia extends from days 24 to 29 after fertilization for the upper limbs and days 27 to 31 for the lower limbs [Brent and Holmes, 1988]. In the study of Martínez-Frías et al. [1997a], the proportion of infants with amelia whose mothers had vaginal bleeding during pregnancy (41.2%, n = 18) was significantly higher than that among control infants (11.1%, n = 25,048; P = 0.001); parental ages did not significantly differ from the ones observed among controls.
There are limited published data on the prevention of amelia. However, there is some suggestion that maternal periconceptional multivitamin use may be associated with a lower risk for transverse limb deficiencies [Yang et al., 1997], and for limb defects in general [Botto et al., 2004; Czeizel, 2004].
In order to expand on the limited information on the epidemiology of amelia, we conducted a descriptive analysis of prevalence data collected on this congenital defect reported by surveillance programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). In this analysis, we examined the variation in total prevalence by program and by selected maternal and infant characteristics.
METHODS
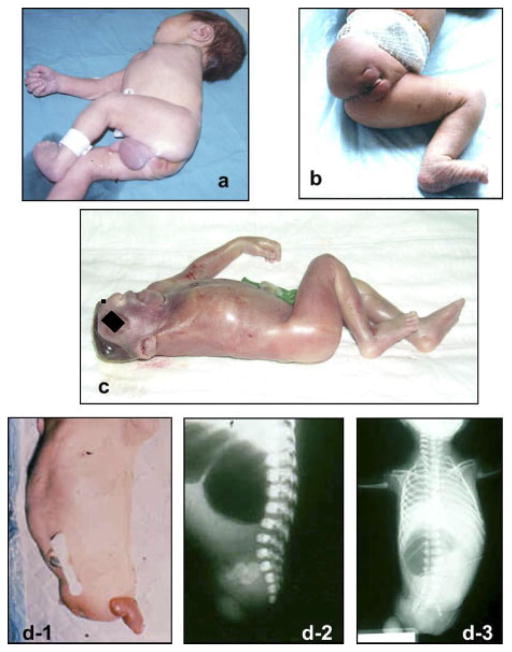
Data were derived from the 20 surveillance programs for congenital anomalies listed in Table IV, all of which are members of the ICBDSR [2011a,b]. The data represented 23 countries and 4 continents (all but Africa). Two countries have three or more programs, and one (ECLAMC-Estudio Colaborativo Latino-Americano de Malformaciones Congénitas) includes data from 10 different South American countries. A total of 23,110,591 births, including LB, SB and, for some programs, ETOPFA, were surveyed from 1968 to 2006, although the study period was variable among programs. For each population, the number of births and the maternal age distribution were reported. Programs were asked to provide de-identified information on the cases, following a common protocol, including data on phenotype, results of any genetic testing, and selected demographic and prenatal information, as it is explained in detail in the article by Castilla and Mastroiacovo [in press] in this issue of the journal. Local scrutiny of the cases was performed by the most qualified dysmorphologist involved in each surveillance program, using all the available documentation. This means that he/she tried to confirm that the proximal humerus or femur were absent in cases with clinical amelia. Additionally, the collected data for this study were furthermore reviewed by three of the authors (E.B-S., M-L.M-F., and P.M.), who corresponded with the participating program directors when needed to identify those cases strictly meeting the case definition of amelia (complete absence of one or more limbs) to be included in this study. The study protocol underlined that only cases with complete absence should be included. Figure 1 illustrates several amelia cases, showing total absence of a limb. Amputations in the context of amniotic bands or limb-body wall complex (LBWC) were not included. In fact, there may be an etiologic distinction between amelia combined with gross body wall defects and amelia in cases with no gross body wall defect [Mastroiacovo et al., 1992].
TABLE IV.
Total Prevalence of Amelia in 20 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| Surveillance program | Period | Births | Total number of cases | % of Total cases that were SB | % of Total cases that were ETOPFAa | Total prevalence per 100,000 births | 95% CI |
|---|---|---|---|---|---|---|---|
| Canada Alberta | 1980–2005 | 1,062,483 | 17 | 23.5 | 41.2 | 1.60 | 0.93–2.56 |
| USA Utah | 1997–2004 | 380,706 | 2 | 50.0 | 0 | 0.53 | 0.06–1.90 |
| USA Atlanta | 1968–2004 | 1,283,999 | 20 | 25.0 | 31.6 | 1.56 | 0.95–2.41 |
| USA Texas | 1996–2002 | 2,054,788 | 30 | 16.7 | 13.3 | 1.46 | 0.99–2.08 |
| Mexico RYVEMCE | 1978–2005 | 1,058,885 | 25 | 44.0 | NP | 2.36 | 1.53–3.49 |
| South America ECLAMC | 1982–2006 | 4,556,173 | 52 | 34.6 | NP | 1.14 | 0.85–1.50 |
| Finland | 1993–2004 | 713,494 | 9 | 0 | 44.4 | 1.26 | 0.58–2.39 |
| Northern Netherlands | 1981–2003 | 369,658 | 3 | 0 | 0 | 0.81 | 0.17–2.37 |
| Germany Saxony–Anhalt | 1980–2004 | 355,184 | 2 | 50.0 | 50.0 | 0.56 | 0.07–2.03 |
| Slovak Republic | 2000–2005 | 318,257 | 6 | 16.7 | 0 | 1.89 | 0.69–4.10 |
| France Central East | 1979–2004 | 2,500,214 | 46 | 2.2 | 63.0 | 1.84 | 1.35–2.45 |
| Italy North East | 1981–2004 | 1,186,497 | 5 | 0 | 20.0 | 0.42 | 0.14–0.98 |
| Italy Emilia Romagna | 1982–2004 | 558,176 | 9 | 0 | 22.2 | 1.61 | 0.74–3.06 |
| Italy Tuscany | 1992–2004 | 336,744 | 4 | 0 | 0 | 1.19 | 0.32–3.04 |
| Italy Campania | 1992–2004 | 643,962 | 3 | 0 | 33.3 | 0.47 | 0.10–1.36 |
| Italy Sicily | 1991–2002 | 216,257 | 4 | 0 | 25.0 | 1.85 | 0.50–4.74 |
| Spain ECEMC | 1980–2004 | 2,045,751 | 15 | 13.3 | NR | 0.73 | 0.41–1.21 |
| Israel | 1975–2005 | 151,562 | 3 | 0 | 33.3 | 1.98 | 0.41–5.78 |
| China Beijing | 1992–2005 | 1,927,622 | 47 | 44.7 | NR | 2.44 | 1.79–3.24 |
| Australia Victoria | 1983–2004 | 1,390,179 | 24 | 58.3 | 25.0 | 1.73 | 1.11–2.57 |
| Total | 23,110,591 | 326 | 25.8 | 19.0a | 1.41 | 1.26–1.57 |
ECEMC, Estudio Colaborativo Español de Malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino-Americano de Malformaciones Congénitas; RYVEMCE, Registroy Vigilancia Epidemiológica de Malformaciones Congénitas; SB, stillbirths; ETOPFA, elective termination of pregnancy for fetal anomaly; CI, confidence interval; NP, not permitted; NR, not reported.
The percentage computed on the 16 programs registering ETOPFA is 33.2% (n = 62/187).
Figure 1.
Clinical photographs of some amelia cases, showing total absence of a limb; (a) amelia of the upper left limb; (b) amelia of the right lower limb; (c) amelia of the right upper limb combined with anencephaly; (d-1, d-2, and d-3) amelia of a lower limb combined with phocomelia of the contralateral lower limb (Courtesy of Dr. A. Sanchis, Dr. S. Martínez, Dr. I. Arroyo Carrera, and Dr. E. Burón).
The total prevalence of amelia was estimated for each program (LB + SB + ETOPFA cases divided by all LB + SB) with 95% confidence intervals (CI) calculated using the Poisson distribution. More details on the statistical methodology used in this project are provided by Castilla and Mastroiacovo [in press] in this issue.
Cases included in the analyses were classified as: (1) isolated if amelia was the only defect present, and (2) multiple congenital anomalies (MCA) if unrelated defects were present in addition to amelia. There were 101 cases with isolated amelia and 218 with amelia in MCA. The remaining seven cases had known syndromes and were excluded from these analyses since their cause is already known or suspected.
Distributions for categorical variables were compared with χ2 tests or Fisher’s exact tests. Prevalence ratios with corresponding 95% CI were calculated for 5-year maternal age groups relative to the reference age group of mothers younger than 20 years. The risk of developing amelia with associated malformations compared with isolated amelia cases in relation to different variables was examined with odds ratios (ORs) and their 95% CI; the adjusted ORs (aORs) were obtained after adjustment for tertiles of percentage of MCA cases (a new variable was created from the percentage of MCA cases in each program, so that each program was assigned a value for this variable depending on the corresponding tertile, and the adjustment was made for that new variable). We conducted the logistic regression analyses of variables using Stata (Statistics/Data Analysis) Special Edition 8.0. P-values lower than 0.05 were considered statistically significant. Additional information on the methodology, variables, data gathered and analyses for this study are detailed in the article by Castilla and Mastroiacovo [in press].
RESULTS
A total of 326 cases with amelia were detected among a total of 23,110,591 births (LB, SB and, for some programs, ETOPFA), for an overall total prevalence of 1.41 per 100,000 (95% CI: 1.26–1.57). This estimates that there was at least one case with amelia in every 63,694–79,365 births. Among the total amelia cases, 54.6% were LB infants, 25.8% were SB, and 19.0% were ETOPFA. In 0.6% the pregnancy outcome was not specified.
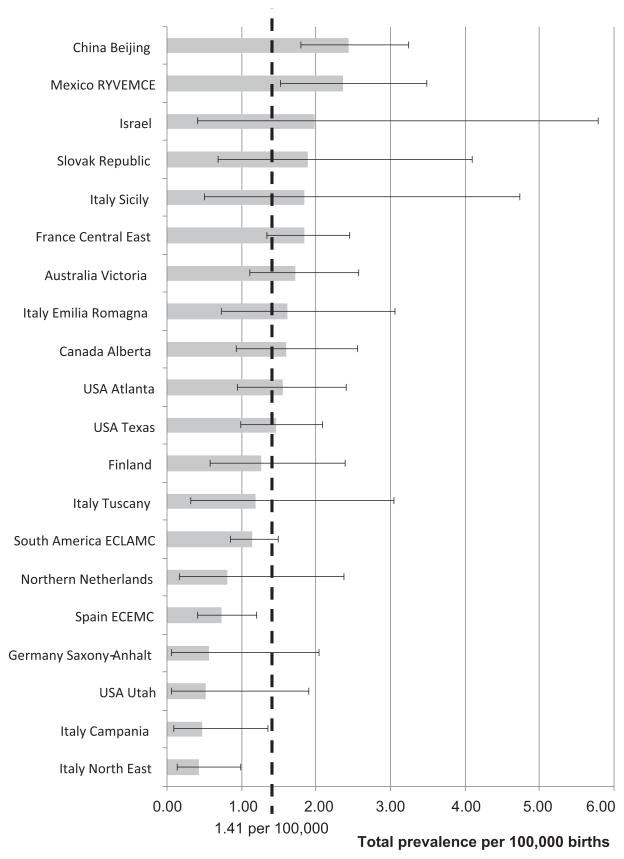
Table IV shows the participating surveillance programs and specifies the study period, number of births surveyed, number of amelia cases, percentage of SB, percentage of ETOPFA, total prevalence, and 95% CI. Four programs contributed approximately 50% of the cases (South America ECLAMC, China Beijing, France Central East, and USA Texas). Figure 2 presents the total prevalence and 95% CI for each program, compared with the overall total prevalence. Total prevalence for individual programs differed significantly from the overall total estimate for a lower estimate in Italy North East (0.42 per 100,000; CI: 0.14–0.98; P = 0.0008), Italy Campania (0.47 per 100,000; CI: 0.10–1.36; P = 0.02), and Spain ECEMC (Spanish Collaborative Study of Congenital Malformations) (0.73; CI: 0.41–1.21; P = 0.035), and a higher estimate in China Beijing (2.44; CI: 1.79–3.24; P = 0.0004) and Mexico RYVEMCE (Registro y Vigilancia Epidemiológica de Malformaciones Congénitas) (2.36; CI: 1.53–3.49; P = 0.011).
Figure 2.
Total prevalence of amelia per 100,000 births (bar) and 95% confidence interval (bracketed line) by surveillance program, and overall total prevalence (dotted line), in 20 surveillance programs of the International Clearinghouse for Birth Defects Surveillance and Research.
Regarding the distribution of the cases by clinical presentation, 101 (31.0%) had isolated amelia and 218 (66.9%) had MCA. Seven (2.1%) had different syndromes: one case with Brachmann-de Lange syndrome (OMIM: 122470) [OMIM, 2011], two with Roberts syndrome (OMIM: 268300), one with FFU (femur-fibula-ulna) syndrome (OMIM: 228200), one with trisomy 13, and two with the particular phenotype combining severe limb defects, vertebral hypersegmentation and mirror polydactyly, with suggested autosomal recessive inheritance [Urioste et al., 1996; Martínez-Frías et al., 1997b].
Among the nonsyndromic cases 65.2% were monomelic, with absence of only one limb, and 32.6% were dimelic (Table V). Only one case had absence of three limbs, and four cases (1.7% of the total) had absence of all four limbs. Among those monomelic cases, each side was affected with equal frequency, with the upper limbs affected slightly more frequently than the lower (53.9% vs. 46.1%). Among dimelic cases, the upper limbs were affected more often than the lower (61.8% vs. 30.3%).
TABLE V.
Distribution of Nonsyndromica Amelia Cases by Number of Affected Limbs, Upper/Lower Limb Involvement and Laterality of the Defect, Among 20 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| N | % | % of Total cases | |
|---|---|---|---|
| Monomelic | |||
| Upper right | 38 | 25.0 | |
| Upper left | 44 | 28.9 | |
| Lower right | 38 | 25.0 | |
| Lower left | 32 | 21.1 | |
| Total monomelic | 152 | 100 | 65.2 |
| Dimelic | |||
| Upper/upper | 47 | 61.8 | |
| Lower/lower | 23 | 30.3 | |
| Upper/Lower | 6 | 7.9 | |
| Total dimelic | 76 | 100 | 32.6 |
| Trimelic | 1 | — | 0.4 |
| Tetramelic | 4 | — | 1.7 |
| Total (specified) | 233 | — | 100 |
Syndromic cases (n = 7) were excluded for this and the following analyses.
Table VI depicts some characteristics of the 319 nonsyndromic cases with amelia (101 isolated and 218 with MCA). Overall, cases were more often male (52.4%) than female (34.5%) with 8.8% having indeterminate sex and 4.4% with sex not stated. Among the isolated cases, the male to female ratio (1.74, 61 males to 35 females) was slightly higher (no statistical difference) than among cases with MCA (1.41, 106 males to 75 females). With respect to birth outcomes, most cases (53.9%) were LB, reaching 61.4% and 50.5% among isolated and MCA cases, respectively. Regarding birth weight of nonsyndromic liveborn cases, a high proportion of them (40.7%) weighed 2,500 g or more; cases with MCA were more likely to weigh between 1,500 and 2,499 g or less than 1,500 g, than those with isolated amelia. Most amelia cases (56.4%) were born at term, but cases with MCA were more likely to be born before 32 weeks (31.8% vs. 6.5% among isolated). Multiple deliveries accounted for 7.8% of nonsyndromic cases. The distribution by maternal age showed that the most numerous maternal age group was that of mothers aged 20–24 years (31.3% of all cases). A high percentage of missing data for previous parity, previous spontaneous abortions, parental age difference, and years of maternal education made these variables difficult to study.
TABLE VI.
Characteristics of Nonsyndromica Cases With Amelia and by Clinical Phenotype Among 20 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| Variables | All casesa (n = 319)a
|
Cases with isolated amelia (n = 101)
|
Cases with amelia and MCA (n = 218)
|
|||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Sex | ||||||
| Male | 167 | 52.4 | 61 | 60.4 | 106 | 48.6 |
| Female | 110 | 34.5 | 35 | 34.7 | 75 | 34.4 |
| Indeterminate | 28 | 8.8 | 0 | 0.0 | 28 | 12.8 |
| Missing data | 14 | 4.4 | 5 | 5.0 | 9 | 4.1 |
| Outcome | ||||||
| Live births | 172 | 53.9 | 62 | 61.4 | 110 | 50.5 |
| Stillbirths | 84 | 26.3 | 23 | 22.8 | 61 | 28.0 |
| ETOPFA | 62 | 19.4 | 16 | 15.8 | 46 | 21.1 |
| Missing data | 1 | 0.3 | 0 | 0.0 | 1 | 0.5 |
| Birth weight among live births (g) | ||||||
| <1,500 | 26 | 15.1 | 6 | 9.7 | 20 | 18.2 |
| 1,500–2,499 | 58 | 33.7 | 18 | 29.0 | 40 | 36.4 |
| ≥2,500 | 70 | 40.7 | 36 | 58.1 | 34 | 30.9 |
| Missing data | 18 | 10.5 | 2 | 3.2 | 16 | 14.5 |
| Gestational age among live births (weeks) | ||||||
| < 32 | 39 | 22.7 | 4 | 6.5 | 35 | 31.8 |
| 33–36 | 29 | 16.9 | 8 | 12.9 | 21 | 19.1 |
| ≥37 | 97 | 56.4 | 46 | 74.2 | 51 | 46.4 |
| Missing data | 7 | 4.1 | 4 | 6.5 | 3 | 2.7 |
| Previous parity | ||||||
| 0 | 71 | 22.3 | 32 | 31.7 | 39 | 17.9 |
| 1 | 102 | 32.0 | 25 | 24.8 | 77 | 35.3 |
| ≥2 | 49 | 15.4 | 19 | 18.8 | 30 | 13.8 |
| Missing data | 97 | 30.4 | 25 | 24.8 | 72 | 33.0 |
| Previous spontaneous abortions | ||||||
| 0 | 124 | 38.9 | 44 | 43.6 | 80 | 36.7 |
| ≥1 | 27 | 8.5 | 11 | 10.9 | 16 | 7.3 |
| Missing data | 168 | 52.7 | 46 | 45.5 | 122 | 56.0 |
| Plurality | ||||||
| Single | 278 | 87.1 | 93 | 92.1 | 185 | 84.9 |
| Twin | 24 | 7.5 | 4 | 4.0 | 20 | 9.2 |
| Triplet | 1 | 0.3 | 0 | 0.0 | 1 | 0.5 |
| Missing data | 16 | 5.0 | 4 | 4.0 | 12 | 5.5 |
| Maternal age | ||||||
| <20 | 38 | 11.9 | 11 | 10.9 | 27 | 12.4 |
| 20–24 | 100 | 31.3 | 31 | 30.7 | 69 | 31.7 |
| 25–29 | 81 | 25.4 | 26 | 25.7 | 55 | 25.2 |
| 30–34 | 60 | 18.8 | 25 | 24.8 | 35 | 16.1 |
| ≥35 | 22 | 6.9 | 4 | 4.0 | 18 | 8.3 |
| Missing data | 18 | 5.6 | 4 | 4.0 | 14 | 6.4 |
| Parental age difference | ||||||
| Mother same age or older | 32 | 10.0 | 14 | 13.9 | 18 | 8.3 |
| Mother 1–2 years younger | 26 | 8.2 | 11 | 10.9 | 15 | 6.9 |
| Mother 3–4 years younger | 30 | 9.4 | 14 | 13.9 | 16 | 7.3 |
| Mother >4 years younger | 26 | 8.2 | 6 | 5.9 | 20 | 9.2 |
| Missing data | 205 | 64.3 | 56 | 55.4 | 149 | 68.3 |
| Maternal education (years) | ||||||
| <9 | 27 | 8.5 | 10 | 9.9 | 17 | 7.8 |
| ≥9 | 70 | 21.9 | 20 | 19.8 | 50 | 22.9 |
| Missing data | 222 | 69.6 | 71 | 70.3 | 151 | 69.3 |
Syndromic cases (n = 7) were excluded from analysis.
Figure 3 shows the prevalence ratios and corresponding 95% CIs for maternal age groups relative to the reference age group of mothers younger than 20 years. There was a statistically significant decreasing trend (P = 0.0026) in the prevalence with advancing maternal age, with the three maternal age groups of 25–29, 30–34, and 35–39 years having statistically significant lower prevalences of amelia compared with the reference group.
Figure 3.
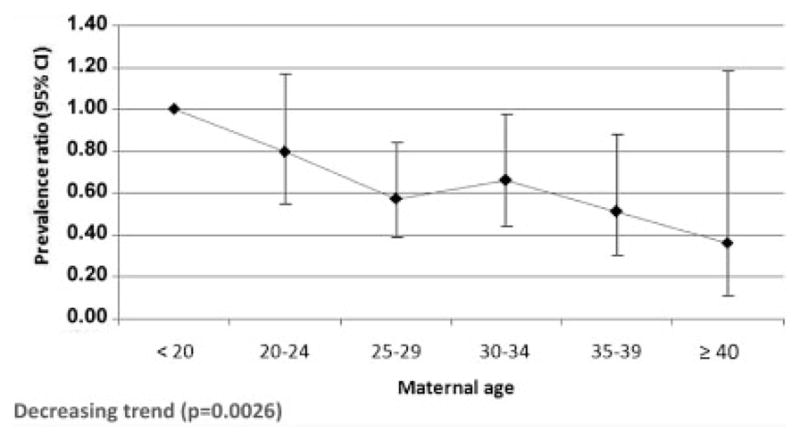
Prevalence ratios for maternal age groups relative to the reference age of <20 years with corresponding 95% CIs for amelia in 18 surveillance programs★ of the International Clearinghouse for Birth Defects Surveillance and Research (syndromic cases excluded). ★Cases and births excluded for the following programs because no births by maternal age were available: China Beijing <1997 and >2003, Germany Saxony–Anhalt <1991, Italy Emilia Romagna <1985, Italy North East, Italy Sicily.
Table VII summarizes the comparison of possible factors or variables associated with MCA versus isolated cases using only data from surveillance programs with less than 20% of missing data values. The analyses were adjusted for tertiles of percentage of MCA cases observed in each program. Among MCA cases, there were statistically significantly higher risks for SB (aOR = 5.18; 95% CI: 1.70–15.73) and ETOPFA (aOR = 3.09; 95% CI: 1.41–6.79), and for premature birth (gestational age <32 weeks: aOR = 5.40, 95% CI: 1.61–18.08; gestational age 32–36 weeks: aOR = 3.17, 95% CI: 1.13–8.92). There were no statistically significant differences for previous parity and previous spontaneous abortions. Twins were associated with MCA (aOR = 2.95), although this result was almost marginally statistically significant. Regarding the comparison of maternal age groups and parental age difference, no statistically significant result was found (Table VII).
TABLE VII.
Crude and Adjusted Odds Ratios (OR) With 95% Confidence Intervals (95% CI) for the Association of Various Characteristics Among Multiple Congenital Anomalies Cases (Cases) Versus Isolated Cases (Controls) of Amelia Reported by 20 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| Crude OR | 95% CI | Adjusted OR (aOR)a | 95% CI | |||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 1.00 | Referent | 1.00 | Referent | ||
| Female | 1.23 | 0.74 | 2.05 | 1.21 | 0.71 | 2.05 |
| Outcome | ||||||
| Live births | 1.00 | Referent | 1.00 | Referent | ||
| Stillbirths | 4.81 | 1.70 | 13.66 | 5.18 | 1.70 | 15.73 |
| ETOPFA | 2.56 | 1.26 | 5.20 | 3.09 | 1.41 | 6.79 |
| Birth weight among live births (g) | ||||||
| <1,500 | 3.53 | 1.26 | 9.84 | 2.63 | 0.81 | 8.47 |
| 1,500–2,499 | 2.35 | 1.14 | 4.87 | 1.64 | 0.74 | 3.65 |
| ≥2,500 | 1.00 | Referent | 1.00 | Referent | ||
| Gestational age among live births (weeks) | ||||||
| <32 | 7.89 | 2.60 | 23.91 | 5.40 | 1.61 | 18.08 |
| 32–36 | 2.37 | 0.96 | 5.86 | 3.17 | 1.13 | 8.92 |
| ≥37 | 1.00 | Referent | 1.00 | Referent | ||
| Previous parity | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | ||
| 1 | 2.85 | 1.39 | 5.83 | 1.60 | 0.68 | 3.73 |
| ≥2 | 1.49 | 0.64 | 3.45 | 1.06 | 0.40 | 2.80 |
| Previous spontaneous abortions | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | ||
| ≥1 | 0.69 | 0.29 | 1.66 | 0.64 | 0.25 | 1.69 |
| Plurality | ||||||
| Single | 1.00 | Referent | 1.00 | Referent | ||
| Twin | 2.51 | 0.83 | 7.57 | 2.95 | 0.92 | 9.45 |
| Maternal age | ||||||
| <20 | 1.00 | Referent | 1.00 | Referent | ||
| 20–24 | 0.91 | 0.40 | 2.06 | 1.24 | 0.53 | 2.90 |
| 25–29 | 0.86 | 0.37 | 2.00 | 1.38 | 0.56 | 3.38 |
| 30–34 | 0.57 | 0.24 | 1.36 | 0.91 | 0.36 | 2.28 |
| ≥35 | 1.83 | 0.50 | 6.66 | 2.89 | 0.74 | 11.21 |
| Parental age difference | ||||||
| Mother same age or older | 0.94 | 0.33 | 2.68 | 0.83 | 0.26 | 2.64 |
| Mother 1–2 years younger | 1.00 | Referent | 1.00 | Referent | ||
| Mother 3–4 years younger | 0.84 | 0.29 | 2.41 | 0.58 | 0.18 | 1.90 |
| Mother >4 years younger | 2.44 | 0.74 | 8.11 | 2.91 | 0.75 | 11.29 |
ETOPFA, elective termination of pregnancy for fetal anomalies; aOR, adjusted odds ratio.
OR computed only for the 16 programs reporting ETOPFA; surveillance programs with more than 20% missing data were excluded from the analysis; seven cases with syndromes were excluded from the analysis.
Adjustments were made for tertiles of percentage of MCA cases in each program.
Table VIII lists the specific defects associated with amelia (excluding other limb reduction defects), and their frequencies by three-digit International Classification of Diseases, Tenth Revision (ICD-10) code among cases with MCA (n = 218). Defects present in more than 10% of amelia cases were: musculoskeletal congenital malformations not elsewhere classified (39.9%); congenital malformations of the spine and bony thorax (22.5%); congenital malformations involving the limbs (excluding limb reduction defects) (21.1%); absence, atresia, or stenosis of the large intestine (18.8%); renal agenesis and other reduction of kidney (16.5%); indeterminate sex and pseudohermaphroditism (14.7%); musculoskeletal deformities of the head, face, spine, and chest (13.8%); congenital deformity of the feet (13.8%); cleft palate with cleft lip (11.0%); congenital malformations of the cardiac septa (11.0%); and anencephaly (10.1%). For other defects that have been associated with amelia in the literature, we found the following percentages (data not shown in Table VIII): gastroschisis was observed in 11.9% of our MCA cases, omphalocele in 9.2%, diaphragmatic defects in 3.2%, and anorectal atresia or stenosis in 16.1% of MCA cases in our series.
TABLE VIII.
Associated Defects Among Nonsyndromic Amelia Cases, Excluding Other Limb Reduction Defects, Reported by 20 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| Associated defects | ICD-10 Code (3 digits) | N | % |
|---|---|---|---|
| Anencephaly | Q00 | 22 | 10.1 |
| Encephalocele | Q01 | 13 | 6.0 |
| Microcephaly | Q02 | 2 | 0.9 |
| Hydrocephalus | Q03 | 18 | 8.3 |
| Other CM of brain | Q04 | 14 | 6.4 |
| Spina bifida | Q05 | 9 | 4.1 |
| Other CM of spinal cord | Q06 | 2 | 0.9 |
| CM of eyelid, lacrimal system and orbit | Q10 | 2 | 0.9 |
| Anophthalmos/microphtalmos and macrophthalmos | Q11 | 15 | 6.9 |
| CM of the lens | Q12 | 1 | 0.5 |
| CM of posterior segment of eye | Q14 | 1 | 0.5 |
| Other CM of eye | Q15 | 8 | 3.7 |
| CM of ear causing impairment of hearing | Q16 | 6 | 2.8 |
| Other CM of ear | Q17 | 20 | 9.2 |
| CM of face and neck | Q18 | 18 | 8.3 |
| CM of cardiac chambers and connections | Q20 | 6 | 2.8 |
| CM of cardiac septa | Q21 | 24 | 11.0 |
| CM of pulmonary and tricuspid valves | Q22 | 5 | 2.3 |
| CM of aortic and mitral valves | Q23 | 3 | 1.4 |
| Other CM of heart | Q24 | 14 | 6.4 |
| CM of great arteries | Q25 | 7 | 3.2 |
| CM of great veins | Q26 | 1 | 0.5 |
| Other CM of peripheral vascular system | Q27 | 15 | 6.9 |
| Other CM of circulatory system | Q28 | 1 | 0.5 |
| CM of nose | Q30 | 8 | 3.7 |
| CM of lung | Q33 | 17 | 7.8 |
| Other CM of respiratory system | Q34 | 7 | 3.2 |
| Cleft palate | Q35 | 6 | 2.8 |
| Cleft lip | Q36 | 6 | 2.8 |
| Cleft palate with cleft lip | Q37 | 24 | 11.0 |
| Other CM of tongue, mouth and pharynx | Q38 | 6 | 2.8 |
| CM of esophagus | Q39 | 8 | 3.7 |
| Absence, atresia, and stenosis of small intestine | Q41 | 4 | 1.8 |
| Absence, atresia, and stenosis of large intestine | Q42 | 41 | 18.8 |
| Other CM of intestine | Q43 | 13 | 6.0 |
| CM of gallbladder, bile ducts, and liver | Q44 | 4 | 1.8 |
| Other CM of digestive system | Q45 | 2 | 0.9 |
| CM of ovaries, fallopian tubes and broad ligament | Q50 | 12 | 5.5 |
| CM of uterus and cervix | Q51 | 8 | 3.7 |
| Other CM of female genitalia | Q52 | 9 | 4.1 |
| Undescended and ectopic testicle | Q53 | 7 | 3.2 |
| Hypospadias | Q54 | 4 | 1.8 |
| Other CM of male genital organs | Q55 | 11 | 5.0 |
| Indeterminate sex and pseudohermaphroditism | Q56 | 32 | 14.7 |
| Renal agenesis and other reduction of kidney | Q60 | 36 | 16.5 |
| Cystic kidney | Q61 | 7 | 3.2 |
| Obstructive defects of renal pelvis and ureter | Q62 | 17 | 7.8 |
| Other CM of kidney | Q63 | 8 | 3.7 |
| Other CM of urinary system | Q64 | 11 | 5.0 |
| Congenital deformity of hips | Q65 | 3 | 1.4 |
| Congenital deformity of feet | Q66 | 30 | 13.8 |
| Musculoskeletal deformities of head, face, spine, and chest | Q67 | 30 | 13.8 |
| Other musculoskeletal deformities | Q68 | 9 | 4.1 |
| Polydactyly | Q69 | 6 | 2.8 |
| Syndactyly | Q70 | 14 | 6.4 |
| Other CM of limb(s) | Q74 | 46 | 21.1 |
| Other CM of skull and face bones | Q75 | 11 | 5.0 |
| CM of spine and bony thorax | Q76 | 49 | 22.5 |
| Non elsewhere classified musculoskeletal CM | Q79 | 87 | 39.9 |
| Other CM of skin | Q82 | 12 | 5.5 |
| CM of breast | Q83 | 3 | 1.4 |
| Other CM of integument | Q84 | 3 | 1.4 |
| Other syndromes affecting multiple systems | Q87 | 17 | 7.8 |
| Other CM, not elsewhere classified | Q89 | 18 | 8.3 |
| Total | 218 | 100.0 |
CM, congenital malformations.
DISCUSSION
This report is based on the largest series of amelia cases known to date. Among more than 23.1 million births from all over the world, the overall total prevalence of amelia was 1.41 per 100,000, and ranged from a minimum of 0.42 to a maximum of 2.44. This overall total prevalence falls within the range described by other authors among total births of 0.95 per 100,000 [Källén et al., 1984] to 1.71 per 100,000 [Castilla et al., 1995] (Table III). However, some of the cases included in these two reports were also included in our study. The high total prevalences observed in China Beijing and Mexico RYVEMCE in our study (although marginally significant in this last program) are even higher than that of Castilla et al. [1995]. The apparently low total prevalence reported by Spain ECEMC (also marginally significant) is probably due to the lack of inclusion of ETOPFA in the prevalence estimate, since prior to passage in 1985 of a law permitting ETOPFA in Spain the total birth prevalence was even higher (1.83 per 100,000; CI: 0.74–3.77) than the overall total prevalence in our study (although not significantly different). Another factor contributing to the lowest end of the prevalence in our study could be under-registration (e.g., Italy North East and Italy Campania—for this last registry only a marginally statistically significant result was obtained for its low total prevalence). However, since amelia is a very obvious defect, it is unlikely that it goes unnoticed. Therefore, under-ascertainment does not seem a plausible explanation and we consider that a more likely contributor to the variation in total prevalence among programs might be differences in classification of amelia cases under other less specific categories of limb defects, such as transverse limb deficiencies. This issue highlights one of the primary problems regarding limb defects: their classification. In many studies in the literature, limb defects are analyzed together as a single group; however, in other studies, different classification systems have been used preventing the comparison of results. In several instances, amelia has been analyzed jointly with phocomelia [Källén et al., 1984], with other transverse limb defects [Calzolari et al., 1990; Lin et al., 1993], or with limb reduction defects considered as a whole in many studies. This lack of standardization or harmonization could reflect the lack of a completely satisfactory classification for limb deficiencies, one that complies with both developmental and causal boundaries, as stated by Botto et al. [1998]. Other factors that could contribute to under-registration of amelia cases may be linked to the methods and organization of the surveillance programs, especially if birth defects reported on notification forms are the main or only source of case identification.
It is important to underline the need for a proper examination of cases, in order to confirm the absence of the proximal segment of the humerus or femur before considering that a case has amelia. For these purposes, a radiological examination is essential to exclude the presence of any bony structure in the limb. Moreover, taking into account that many of the pregnancies in which the fetus presents with amelia are subject to ETOPFA, a complete study of those fetuses is essential to adequately characterize not only amelia, but all the defects to which it is associated (what is also critical to provide a proper counseling to the parents regarding recurrence risks and early detection possibilities in future pregnancies). This could be a limitation of this study, because although the study protocol included a very clear and strict definition of amelia, if in some cases the radiological study was not available for review, or if in cases of ETOPFA the fetus could not be completely studied, some misclassification cannot be completely excluded.
Regarding the outcome, we observed that 25.8% of amelia cases were SB, this estimate being higher than that reported by Martínez-Frías et al. [1997a] (16.7%, n = 18), and lower than that reported in the study of Castilla et al. [1995] (34%, n = 50). Our percentage of cases with other defects associated with amelia (69% when including the seven cases with different syndromes) is higher than that reported by Froster-Iskenius and Baird [1990] among liveborn cases (61%, n = 18), and Evans et al. [1994] (56.3%, n = 16), but slightly lower than the 72% (n = 50) described by Castilla et al. [1995] (72%, n = 50).
The small number of syndromes detected among amelia cases in our study is striking. This finding is consistent with the low frequency of syndromes associated with amelia in the literature (Table II); the hypothesis that amelia has fewer etiologies compared with the broader categories of limb reduction defects [Brent and Holmes, 1988]; and the fact that amelia is not generally considered to be of genetic origin [Lenz, 1980]. Two of the amelia cases included in our study had already being described in two other reports of a possibly new syndrome characterized by the presence of severe limb defects (including amelia), vertebral hypersegmentation and mirror polydactyly, and with suggested autosomal recessive inheritance [Urioste et al., 1996; Martínez-Frías et al., 1997b]. Each of these reports included two cases each, and there have not been any further reports of similar cases in the literature.
Regarding the limbs affected, 65.2% of cases in our study were monomelic, similar to the 58% (n = 24) observed in the study of Evans et al. [1994] among cases with other associated defects, and lower than the 83.3% (n = 18) observed by Martínez-Frías et al. [1997a]. Among our monomelic cases, both sides were equally involved, in contrast to the results of Froster-Iskenius and Baird [1990], who found more left-sided defects, and Martínez-Frías et al. [1997a], who found more right-sided defects. In our study, the upper limbs were more frequently affected (53.9% of monomelic cases); in contrast, Froster-Iskenius and Baird [1990] found that amelia affected equally the upper and lower limbs, and Martínez-Frías et al. [1997a] reported a higher frequency among the lower limbs (72.2%). From our larger series, it seems likely that there is no clear tendency of either side to be more frequently affected, although the upper limbs seem to be more frequently involved. This tendency appears more marked among dimelic cases.
Overall, cases in our study were more often males, and this was more marked among the isolated cases. However, Martínez-Frías et al. [1997a] found a nonsignificant excess of females affected (seven males to nine females), although it could be due to the smaller numbers. Froster-Iskenius and Baird [1990] reported a sex-ratio favoring males (11 males to 7 females), which did not differ from that among LB in the general population from which the cases were ascertained.
Regarding the tendency for amelia to be associated with other congenital anomalies, we found that more cases had MCA than not, and this tendency was more marked among SB and ETOPFA. This was not unexpected, since amelia originates during blastogenesis and blastogenetic defects tend to be associated with other severe and multiorganic defects. The fact that MCA are more frequent among stillborn cases could indicate that the most severe defects with which amelia may be associated, might cause an early death. These deaths of the more severely affected fetuses would result in a group of surviving fetuses capable of progressing to be born at term, and this could also explain the low percentage of cases with a birth weight below 1,500g observed in our data.
One of the most interesting findings in this series is the higher prevalence of amelia among younger mothers. As for other defects showing a similar association with a younger maternal age (such as gastroschisis), this finding might indicate that lifestyle or environmental influences could be contributing factors to at least some amelia cases. Moreover, since this association has already been found also in relation with gastroschisis, it could be hypothesized that a vascular disruption might be in the origin of amelia, so our results could also provide a clue on the pathogenesis of this congenital defect. To this respect, taking this into account and all the knowledge on thalidomide’s action mechanism, it could also be hypothesized that there might be other drugs or other environmental factors with some influence on the vasculature or blood supply to the fetus that should be investigated in relation with amelia.
Finally, the relative lack of information on risk factors and causes of amelia highlighted by a thorough review of the literature underscores the need for good classification and coding systems and more collaborative research on modifiable risk factors and causes for this rare but severe congenital defect. In this sense, according to present times, it could be helpful to indicate that up to now there has not been enough experience with chromosome microarray testing (at least in published reports), and this could open new avenues in the research on the causes of amelia. Therefore, although quite speculative at present, it would be worthwhile to explore whole genome microarray tests in patients with amelia in order to find genomic variants that could be directly associated to it, or other that combined with some enviromental hazards could increase the risk for this severe limb defect.
Acknowledgments
The authors are grateful to each surveillance program’s staff and members for their work in collecting case data and submission to the ICBDSR Centre. We also thank Dr. Adolfo Correa, from the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA (USA), for his helpful comments in the preparation of the manuscript. This work was partly supported by Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) of Spain, and the Fundación 1000 sobre Defectos Congénitos of Spain. CIBERER is an initiative of ISCIII. Components of ECEMC’s Peripheral Group are gratefully acknowledged. The work conducted at the ICBDSR Centre was supported by the Centers for Disease Control and Prevention (1U50DD000524-02). Grant sponsor for South America ECLAMC: MCT/CNPq, Brazil; grant numbers: 573993/2008-4, 476978/2008-4, 554755/2009-2, 306750/2009-0, 402045/2010-6. The Tuscany Registry of Birth Defects is funded by the “Direzione Generale Diritti di cittadinanza e Coesione sociale—Regione Toscana.”
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham G, Clarke NMP. Genetic regulation of embryological limb development with relation to congenital limb deformity in humans. J Child Orthop. 2008;2:1–9. doi: 10.1007/s11832-008-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazet JD, Zeller R. Vertebrate limb development: Moving from classical morphogen gradients to an integrated 4-dimensional patterning system. Cold Spring Harb Perspect Biol. 2009;1:a001339. doi: 10.1101/cshperspect.a001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Sánchez E, Cuevas L, Amar E, Bianca S, Bianchi F, Botto LD, Canfield MA, Castilla EE, Clementi M, Cocchi G, Landau D, Leoncini E, Li Z, Lowry RB, Mastroiacovo P, Mutchinick OM, Rissmann A, Ritvanen A, Scarano G, Siffel C, Szabova E, Martínez-Frías ML. Phocomelia: A worldwide descriptive epidemiologic study in a large series of cases from the International Clearinghouse for Birth Defects Surveillance and Research, and overview of the literature. Am J Med Genet C Semin Med Genet. 2001 doi: 10.1002/ajmg.c.30320. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Jensen A. Opera ex Domo Biol Hered Hum Univ Harniensis 19. Copenhagen: Munksgaard; 1949. Congenital deformities of the upper extremities. Quoted by Froster-Iskenius and Baird, 1990. [Google Scholar]
- Bod M, Czeizel A, Lenz W. Incidence at birth of different types of limb reduction abnormalities in Hungary 1975–1977. Hum Genet. 1983;65:27–33. doi: 10.1007/BF00285024. [DOI] [PubMed] [Google Scholar]
- Botto LD, Olney RS, Moore CA, Khoury MJ, Mastroiacovo P. (Mis)classifying limb deficiencies: A reply to “Academicians are more likely to share each other’s toothbrush than each other’s nomenclature (Cohen, 1982)”. Am J Med Genet. 1998;76:359–360. [PubMed] [Google Scholar]
- Botto LD, Olney RS, Erickson JD. Vitamin supplements and the risk for congenital anomalies other than neural tube defects. Am J Med Genet Part C Semin Med Genet. 2004;125C:12–21. doi: 10.1002/ajmg.c.30004. [DOI] [PubMed] [Google Scholar]
- Brent RL, Holmes LB. Clinical and basic science lessons from the thalidomide tragedy: What have we learned about the causes of limb defects? Teratology. 1988;38:241–251. doi: 10.1002/tera.1420380308. [DOI] [PubMed] [Google Scholar]
- Calzolari E, Manservigi D, Garani GP, Cocchi G, Magnani C, Milan M. Limb reduction defects in Emilia Romagna, Italy: Epidemiological and genetic study in 173,109 consecutive births. J Med Genet. 1990;27:353–357. doi: 10.1136/jmg.27.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla EE, Mastroiacovo P. Very rare defects: What can we learn? Am J Med Genet C Semin Med Genet. 2011 doi: 10.1002/ajmg.c.30315. (in press) [DOI] [PubMed] [Google Scholar]
- Castilla EE, Cavalcanti D, Dutra M, López-Camelo J, Paz J, Gadow E. Limb reduction defects in South America. Br J Obstet Gynaecol. 1995;102:393–400. doi: 10.1111/j.1471-0528.1995.tb11292.x. [DOI] [PubMed] [Google Scholar]
- Charite J, de Graaff W, Shen S, Deschamps J. Ectopic expression of Hoxb-8 causes duplication of the ZPA in the forelimb and homeotic transformation of axial structures. Cell. 1994;78:589–601. doi: 10.1016/0092-8674(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Czeizel AE. The primary prevention of birth defects: Multivitamins or folic acid? Int J Med Sci. 2004;1:50–61. doi: 10.7150/ijms.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Evans JA, Kodaj I, Lenz W. Genetic and teratologic epidemiological studies. Budapest: Akadémiai Kiadó; 1994. Congenital limb deficiencies in Hungary; p. 353. [Google Scholar]
- Evans JA, Vitez M, Czeizel AE. Congenital abnormalities associated with limb deficiency defects: A population study based on cases from the Hungarian Congenital Malformation Registry (1975–1984) Am J Med Genet. 1994;49:52–66. doi: 10.1002/ajmg.1320490111. [DOI] [PubMed] [Google Scholar]
- Eyaid W, Al-Qattan MM, Al Abdulkareem I, Fetaini N, Al Balwi M. A novel homozygous missense mutation (c.610G>A, p.Gly204Ser) in the WNT7A gene causes tetra-amelia in two Saudi families. Am J Med Genet Part A. 2011;155A:599–604. doi: 10.1002/ajmg.a.33717. [DOI] [PubMed] [Google Scholar]
- Foster JW. Mutations in SOX9 cause both autosomal sex reversal and campomelic dysplasia. Acta Paediatr Jpn. 1996;38:405–411. doi: 10.1111/j.1442-200x.1996.tb03515.x. [DOI] [PubMed] [Google Scholar]
- Frantz CH, O’Rahilly R. Congenital skeletal deficiencies. J Bone Joint Surg. 1961;8:1202–1224. [Google Scholar]
- Froster U, Baird P. Congenital defects of the limbs in stillbirths: Data from a population-based study. Am J Med Genet. 1993;46:479–482. doi: 10.1002/ajmg.1320460502. [DOI] [PubMed] [Google Scholar]
- Froster-Iskenius U, Baird P. Amelia: Incidence and associated defects in a large population. Teratology. 1990;41:23–31. doi: 10.1002/tera.1420410104. [DOI] [PubMed] [Google Scholar]
- Ghosh G, Gupta S. Amelia with anorectal and external genitalia atresia. Indian Pediatr. 2004;41:1267. [PubMed] [Google Scholar]
- Glass IA, Walford-Moore J, Chapman S, Farndon PA. Ear anomalies, clefting and limb reduction defects: A new autosomal recessive condition? Clin Dysmorphol. 1994;3:150–156. [PubMed] [Google Scholar]
- Hoyme HE, Jones KL, Van Allen MI, Saunders BS, Bernischke K. Vascular pathogenesis of transverse limb reduction defects. J Pediatr. 1982;101:839–843. doi: 10.1016/s0022-3476(82)80343-0. [DOI] [PubMed] [Google Scholar]
- ICBDSR (International Clearinghouse for Birth Defects Surveillance and Research) [Accessed July 29, 2011];Web page. 2011a World Wide Web http://www.icbdsr.org.
- ICBDSR (International Clearinghouse for Birth Defects Surveillance and Research) Annual Report 2009 with data for 2007. Rome: ICBD; 2011b. [Accessed July 29, 2011]. World Wide Web http://www.icbdsr.org/filebank/documents/ar2005/AR%202009_web.pdf. [Google Scholar]
- Källén B, Rahmani TMZ, Winberg J. Infants with congenital limb reduction registered in the Swedish register of congenital malformations. Teratology. 1984;29:73–85. doi: 10.1002/tera.1420290109. [DOI] [PubMed] [Google Scholar]
- Kariminejad A, Goodarzi P, Asghari-Roodsari A, Kariminejad MH. Amelia, cleft lip, and holoprosencephaly: A distinct entity. Am J Med Genet Part A. 2009;149A:2828–2831. doi: 10.1002/ajmg.a.32933. [DOI] [PubMed] [Google Scholar]
- Lai CY, Hsu WM, Peng SSF. Tubular jejunal duplication, amelia and congenital diaphragmatic eventration in a neonate. Clin Dysmorphol. 2010;19:169–171. doi: 10.1097/MCD.0b013e328339ab0c. [DOI] [PubMed] [Google Scholar]
- Laufer E, Dahn R, Orozco OE, Yeo CY, Pisenti J, Henrique D, Abbott UK, Fallon JF, Tabin C. Expression of radical fringe in limb-bud ectoderm regulates apical ectodermal ridge formation. Nature. 1997;386:366–373. doi: 10.1038/386366a0. Erratum in: Nature 1997. 388(6640):400. [DOI] [PubMed] [Google Scholar]
- Lazjuk GI, Lurie IW, Cherstvoy ED, Ussova YI. A syndrome of multiple congenital malformations including amelia and oligodactyly occurring in half-cousins. Teratology. 1976;13:161–166. [Google Scholar]
- Lenz W. Kindliche Mißildungen nach Medicament-Einnahme während der Gravidität? Dtsch Med Wschr. 1961;86:2555–2556. [Google Scholar]
- Lenz W. Thalidomide and congenital abnormalities. Lancet. 1962;1:271. [Google Scholar]
- Lenz W. Genetics and limb deficiencies. Clin Orthop. 1980;148:9–17. [PubMed] [Google Scholar]
- Lin S, Marshall EG, Davidson GK, Roth GB, Druschel CM. Evaluation of congenital limb reduction defects in upstate New York. Teratology. 1993;47:127–135. doi: 10.1002/tera.1420470205. [DOI] [PubMed] [Google Scholar]
- Linder N, Amarilla M, Hernandez A, Tamiri T, Sirota L, Klinger G, Levy I, Merlob P. Association of high-dose bifonazole administration during early pregnancy and severe limb reduction defects in the newborn. Birth Defects Res. 2010;88:201–204. doi: 10.1002/bdra.20644. [DOI] [PubMed] [Google Scholar]
- Marles SL, Reed M, Evans JA. Humeroradial synostosis, ulnar aplasia and oligodactyly, with contralateral amelia, in a child with prenatal cocaine exposure. Am J Med Genet Part A. 2003;116A:85–89. doi: 10.1002/ajmg.a.10731. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML. Epidemiological analysis of outcomes of pregnancy in diabetic mothers: Identification of the most characteristic and most frequent congenital anomalies. Am J Med Genet. 1994;51:108–113. doi: 10.1002/ajmg.1320510206. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML, Bermejo E, Aparicio P, Blanco M, Burón E, Cuevas L, Espinosa MJ, Fondevilla J, Gallo M, Hernández F, Marco JJ, Martínez S, Morales MC, Mújica I, Paisán L, Valdivia L. Amelia: Analysis of its epidemiological and clinical characteristics. Am J Med Genet. 1997a;73:189–193. [PubMed] [Google Scholar]
- Martínez-Frías ML, Arroyo I, Bermejo E, Espinosa J, García MJ. Severe limb deficiencies, vertebral hypersegmentation, and mirror polydactyly: Two additional cases that expand the phenotype to a more generalized effect on blastogenesis. Am J Med Genet. 1997b;73:205–209. doi: 10.1002/(sici)1096-8628(19971212)73:2<205::aid-ajmg18>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mastroiacovo P, Källén B, Knudsen LB, Lancaster PAL, Castilla EE, Mutchinick O, Robert E. Absence of limbs and gross body wall defects: An epidemiological study of related rare malformation conditions. Teratology. 1992;46:455–464. doi: 10.1002/tera.1420460510. [DOI] [PubMed] [Google Scholar]
- McCredie J, Willert HG. Longitudinal limb deficiencies and the sclerotomes: An analysis of 378 dysmelic malformations induced by thalidomide. J Bone Joint Surg Br. 1999;81:9–23. doi: 10.1302/0301-620x.81b1.8448. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branch-less. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The developing human: Clinically oriented embryology. Philadelphia: Saunders; 2008. p. 536. [Google Scholar]
- Ohro Y, Suzuki Y, Tsutsumi Y, Ogata T. Female external genitalia, absent uterus, and probable agonadism in a 46,XY infant with bilateral upper amelia. Clin Genet. 1998;54:52–55. doi: 10.1111/j.1399-0004.1998.tb03693.x. [DOI] [PubMed] [Google Scholar]
- OMIM database: Online Mendelian Inheritance in Man, OMIM (™) McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University; Baltimore, MD: National Center for Biotechnology Information, National Library of Medicine; Bethesda, MD: 2011. [Accessed July 29, 2011]. World Wide Web http://www.ncbi.nlm.nih.gov/omim/ [Google Scholar]
- Parazzini F, La Vecchia C, Levi F, Franceschi S. Trends in male:female ratio among newborn infants in 29 countries from five continents. Hum Reprod. 1998;13:1394–1396. doi: 10.1093/humrep/13.5.1394. [DOI] [PubMed] [Google Scholar]
- Pauli RM, Feldman PF. Major limb malformations following intrauterine exposure to ethanol: Two additional cases and literature review. Teratology. 1986;33:273–280. doi: 10.1002/tera.1420330304. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Geer JS, Blackburn WR. Vascular anastomoses leading to amelia and cutis aplasia in a dizygotic twin pregnancy. Clin Genet. 1998;53:126–130. doi: 10.1111/j.1399-0004.1998.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Rallis C, Bruneau BG, Del Buono J, Seidman CE, Seidman JG, Nissim S, Tabin CJ, Logan MP. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- Ratan SK, Rattan KN, Ratan J, Sodhi PK, Bhatia V. A neonate with anorectal malformation with rare limb defects: Report of a case. Pediatr Surg Int. 2005;21:825–828. doi: 10.1007/s00383-005-1515-5. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisúa Belmonte JC. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398:814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- Rosano A, Botto LD, Olney RS, Khoury MJ, Ritvanen A, Goujard J, Stoll C, Cocchi G, Merlob P, Mutchinick O, Cornel MC, Castilla EE, Martínez-Frías ML, Zampino G, Erickson JD, Mastroiacovo P. Limb defects associated with major congenital anomalies: Clinical and epidemiological study from the International Clearinghouse for Birth Defects Monitoring Systems. Am J Med Genet. 2000;93:110–116. doi: 10.1002/1096-8628(20000717)93:2<110::aid-ajmg6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Langman’s medical embryology. 11. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- Sawaya D, Goldstein S, Seetharamaiah R, Suson K, Nabaweesi R, Colombani P, Gearhart J. Gastrointestinal ramifications of the cloacal extrophy complex: A 44-year experience. J Pediatr Surg. 2010;45:171–176. doi: 10.1016/j.jpedsurg.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Scadding SR. Citral, an inhibitor of retinoic acid synthesis, modifies pattern formation during limb regeneration in the axolotl Ambystoma mexicanum. Can J Zool. 1999;77:1835–1837. [Google Scholar]
- Sonderegger A. Missgeburten und Wundergestalten in Einblattdrucken und Handzeichungen des 16 Jahrhunderts. Zürich-Leipzing-Berlin: Orell Füssli; 1927. [Google Scholar]
- Summerbell D. A quantitative analysis of effect of excision of the AER from the chick limb bud. J Embryol Exp Morphol. 1974;32:651–660. [PubMed] [Google Scholar]
- Swanson AB. A classification for congenital limb malformations. J Hand Surg. 1976;1:8–22. doi: 10.1016/s0363-5023(76)80021-4. [DOI] [PubMed] [Google Scholar]
- Tadmor OP, Kreisbe GA, Achiront R, Porat S, Yagel S. Limb amputation in amniotic band syndrome: Serial ultrasonographic and Doppler observations. Ultrasound Obstet Gynecol. 1997;10:312–315. doi: 10.1046/j.1469-0705.1997.10050312.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Matsumoto K, Vogel-Hopker A, Naitoh-Matsuo M, Ogura K, Takahashi N, Yasuda K, Ogura T. Tbx5 and Tbx4 genes determine the wing/leg identity of limb buds. Nature. 1999;398:810–814. doi: 10.1038/19762. [DOI] [PubMed] [Google Scholar]
- Tickle C. Patterning systems—From one end of the limb to the other. Dev Cell. 2003;4:449–458. doi: 10.1016/s1534-5807(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Urioste M, Lorda-Sánchez I, Blanco M, Burón E, Aparicio P, Martínez-Frías ML. Severe congenital limb deficiencies, vertebral hypersegmentation, absent thymus and mirror polydactyly: A defect expression of a developmental control gene? Hum Genet. 1996;97:214–217. doi: 10.1007/BF02265268. [DOI] [PubMed] [Google Scholar]
- Vogel A, Rodríguez C, Izpisúa-Belmonte JC. Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development. 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- Weaver DD. Vascular etiology of limb defects: The subclavian artery supply disruption sequence. In: Herring JA, Birch JG, editors. The child with a limb deficiency. Chicago: American Academy of Orthopaedic Surgeons; 1998. pp. 25–37. [Google Scholar]
- Winter R, Baraitser M. Winter-Baraitser Dysmorphology Database. 2010 ( http//www.lmdatabases.com). Version 1.0.22.
- Yang Q, Khoury MJ, Olney RS, Mulinare J. Does periconceptional multivitamin use reduce the risk for limb deficiency in offspring? Epidemiology. 1997;8:157–161. doi: 10.1097/00001648-199703000-00006. [DOI] [PubMed] [Google Scholar]