Abstract
OBJECTIVE
Minimally-invasive image-guided approach to cochlear implantation (CI) involves drilling a narrow, linear tunnel to the cochlea. Reported herein is the first clinical implementation of this approach.
STUDY DESIGN
Prospective, cohort study.
METHODS
On preoperative CT, a safe linear trajectory through the facial recess targeting the scala tympani was planned. Intraoperatively, fiducial markers were bone-implanted, a second CT was acquired, and the trajectory was transferred from preoperative to intraoperative CT. A customized microstereotactic frame was rapidly designed and constructed to constrain a surgical drill along the desired trajectory. Following sterilization, the frame was employed to drill the tunnel to the middle ear. After lifting a tympanomeatal flap and performing a cochleostomy, the electrode array was threaded through the drilled tunnel and into the cochlea.
RESULTS
Eight of nine patients were successfully implanted using the proposed approach with six insertions completely within scala tympani. Traditional mastoidectomy was performed on one patient following difficulty threading the electrode array via the narrow tunnel. Other difficulties encountered included use of the back-up implant when an electrode was dislodged during threading via the tunnel, tip fold-over, and facial nerve paresis (House-Brackmann II/VII at 12 months) secondary to heat during drilling. Average time of intervention was 182±36 minutes.
CONCLUSION
Minimally-invasive, image-guided CI is clinically achievable. Further clinical study is necessary to address technological difficulties during drilling and insertion and to assess potential benefits including decreased time of intervention, standardization of surgical intervention, and decreased tissue dissection potentially leading to shorter recovery and earlier implant activation.
Keywords: Minimally-invasive, cochlear implantation, image guided, stereotactic frame
INTRODUCTION
To date, over 320,000 hearing-impaired individuals have undergone cochlear implantation (CI) to restore the ability to hear.1 Surgical treatment typically involves a mastoidectomy with facial recess approach following which the cochlea is entered via either the round window or a separate cochleostomy. The surgeon aims to place the electrode array within the scala tympani (ST) as it is larger than scala vestibuli (SV). More recently, several studies have suggested that implantation fully within ST portends better audiological outcomes.2,3,4 Other studies support that less traumatic electrode insertion, as assessed by preservation of residual hearing, is associated with better audiological outcomes.5
To achieve accurate and precise implantation, it might be expected that computer-guided systems would be utilized as employed in other fields including neurosurgery, orthopedics, and rhinology. However, to date, the vast majority of surgeries are performed manually as described by CI pioneers including William House.6 Success rates are high and complications are low with permanent injury to the facial nerve less than 0.1%.7
To facilitate ease of CI surgery for surgeons uncomfortable with the facial recess approach, several alternative approaches have been developed. The most widely cited of these is the suprameatal approach,8,9 in which, based upon anatomical measurements, a hole is drilled blindly from the mastoid cortex to the attic. After lifting a tympanomeatal flap and making a cochleostomy, the CI is passed via the attic to the middle ear and subsequently into the cochlea. While this approach requires highly flexible CI electrode arrays and offers a suboptimal insertion vector, over 500 suprameatal approaches have been performed to date with an impressive safety profile.
Capitalizing on image-guidance technology, we present a minimally invasive approach that can provide the advantages of both the traditional CI and the suprameatal approaches but without the disadvantages—i.e. it allows a minimally invasive approach via an optimal insertion vector tangential to the basal turn of ST. Previously referred to as “percutaneous cochlear implantation,”10,11,12 herein and henceforth we will refer to this technique as “minimally invasive, image-guided cochlear implantation,” and herein we present the first report of clinical implementation.
MATERIALS AND METHODS
Informed consent
Approval was obtained from our Institutional Review Board, following which informed consent was obtained. Owing to the success of the traditional technique and the potential risks of the image-guided approach, the informed-consent process was thorough and involved watching a video about the procedure, discussing the procedure with both a surgeon and a research nurse, and providing a detailed written consent form that was sent home with the patient for further consideration of their voluntary involvement. A second discussion was held on the day of surgery, after which written consent was obtained.
Preoperative Path Planning
On each patient’s preoperative CT scan, anatomy of interest was automatically segmented and a safe linear trajectory avoiding vital anatomy and targeting the ST was identified. Segmentation was accomplished via atlas-based segmentation for the external ear canal and ossicles,13 active shape modeling for the labyrinth and its subcomponents,14 and optimal path finding for the tube-shaped facial nerve and chorda tympani.15 Trajectory generation specified avoidance of the facial nerve and external ear canal but did allow violation of the chorda tympani if no other solutions were possible.16
Automatic segmentation and path planning required approximately three minutes on an Intel Xeon 2.4 GHz dual quad-core 64-bit machine with 16 GB RAM. Results were graphically displayed to the surgeon (Figure 1), who was required to verify the accuracy of segmentation and safety of the trajectory.
Figure 1.
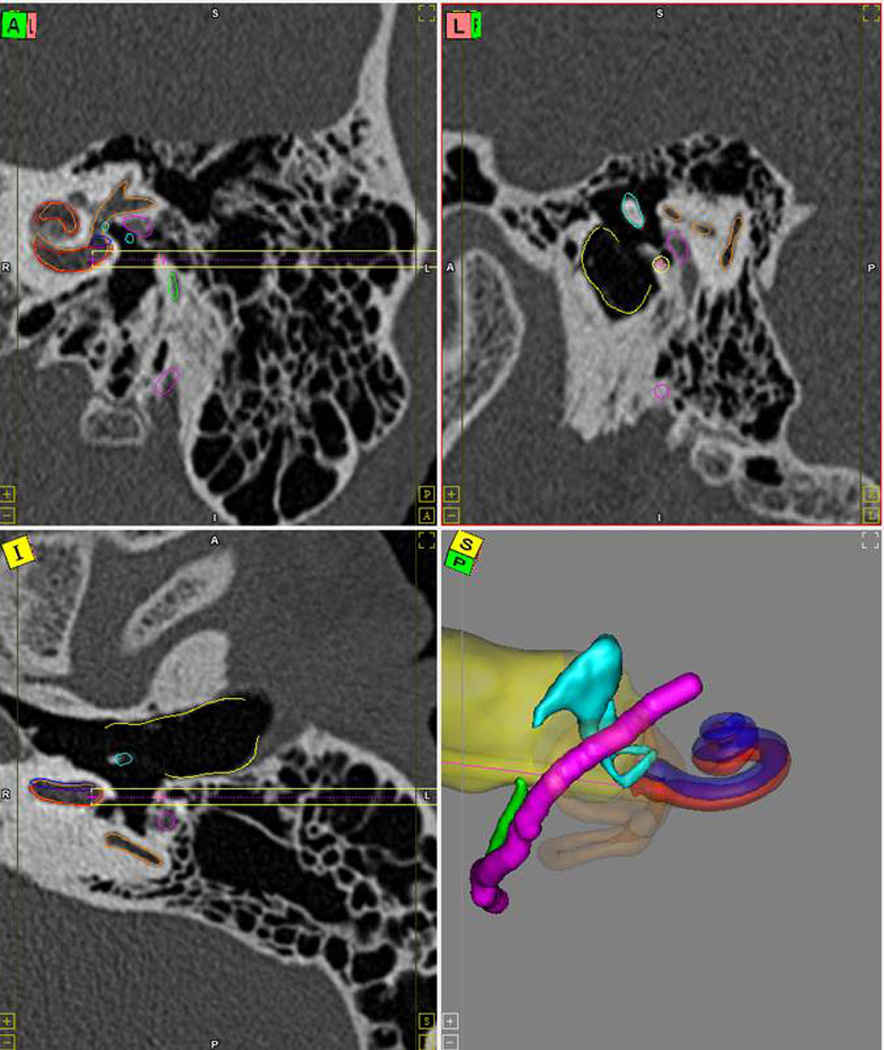
Preoperative path planning. The results of the automatic segmentation and path planning are displayed to the surgeon for verification. Segmented structures include the scala tympani (red), scala vestibuli (blue), facial nerve (magenta), chorda tympani (green), ossicles (blue), and external auditory canal (yellow). The drill path is shown as a golden cylinder. Red line in the three-dimensional rendering shows the trajectory line.
Implantation of fiducial markers
Once the patient was prepped, three titanium self-tapping anchors were screwed into the skull—one at the mastoid tip, one posterior to the midpoint of the sigmoid sinus, and one superior to the helix (Figure 2). Onto each of these, a titanium extender with spherical tip was attached. The spherical tips, whose centers can be found automatically in the CT image, act both as (a) fiducials for registering the patient’s anatomy to the CT scan and (b) attachment points for a miniature stereotactic frame. This step takes approximately eight minutes.
Figure 2.
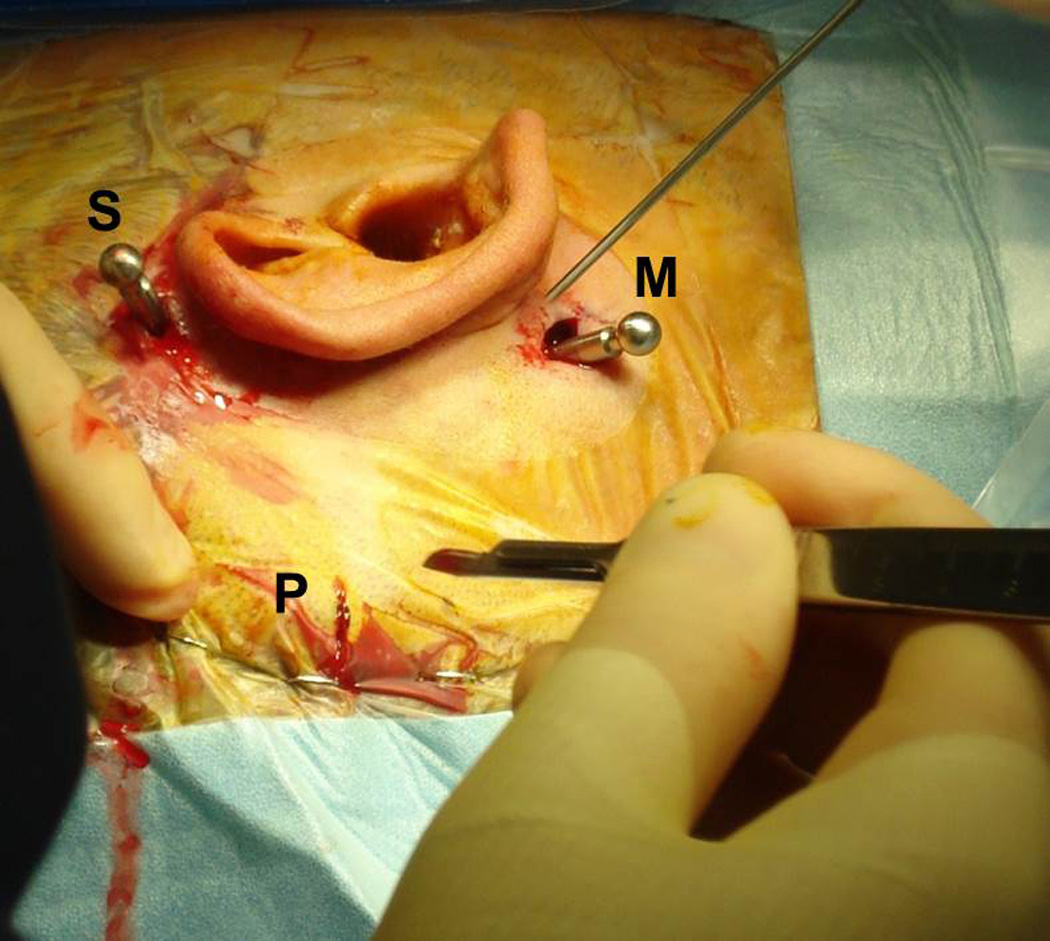
Implantation of fiducial markers for a right ear. Three anchors are screwed into the mastoid bone via stab incisions—one at the mastoid tip (M), one posterior to the midpoint of the sigmoid sinus (P), and one superior to the helix (S). An extender with a spherical tip is attached to each anchor.
Acquisition of an intraoperative, temporal bone CT scan
One of two different intraoperative flat-panel volumetric CT scanners—the Allura Xpert FD20 (Philips Medical Systems, Eindhoven, The Netherlands) and the xCAT ENT scanner (Xoran Technologies Inc., Ann Arbor, MI, USA) (Figure 3) was used. Time for scanning and reconstruction was 5–10 minutes.
Figure 3.
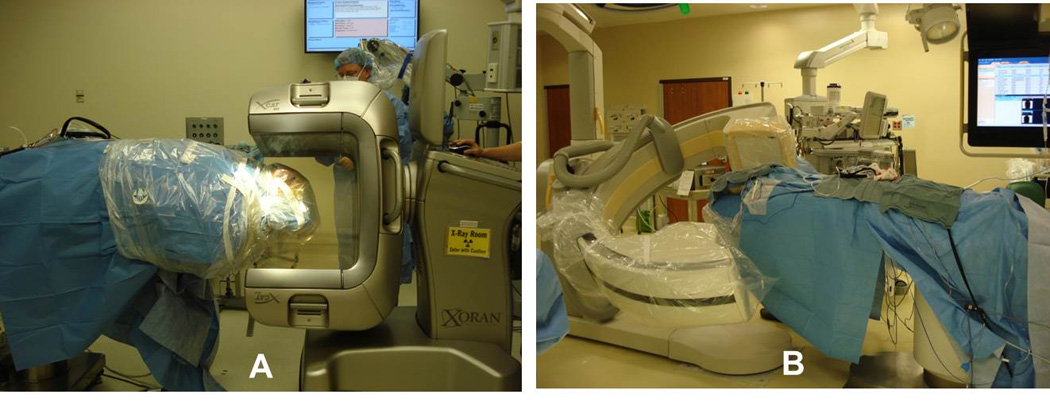
Intraoperative scanners. Two intraoperative scanners were used in the study. (A) Portable xCAT ENT scanner from Xoran Technologies Inc. and (B) Allura Xpert FD20 from Philips Medical Systems.
Intraoperative planning
The segmented anatomy and drill trajectory from the preoperative CT scan were mapped to the intraoperative CT scan by rigidly registering the two volumes using a feature based method17 followed by a standard mutual-information intensity-based method.18 Additionally, the center of each fiducial was localized in the intraoperative CT by means of an algorithm that fits a model of the fiducial shape to the image.19 This step takes approximately four minutes.
Design and construction of custom microstereotactic frame
This process, detailed in a prior publication,20 involves fabricating “on-the-fly” using a computer-numeric-control (CNC) machine (a.k.a. milling machine) a miniature tabletop that mounts onto the spherical fiducial markers via legs of specified lengths. A virtual model of the customized frame is created automatically by means of planning software written in Matlab (The Mathworks, Natick, MA, USA). The input to the software is the location of the spherical fiducial markers and the desired trajectory. The output is a set of commands in G-code (CNC programming language) for milling four holes in the tabletop (3 holes for the legs and a central hole for the drill) as well as three leg lengths selected from three standard lengths. Legs couple to corresponding spherical markers via a ball-and-socket assembly held in place by pivoting fingers that, when tightened, grasp the spherical end of the fiducial markers (Figure 4). Design and fabrication takes approximately eight minutes, and sterilization approximately ten minutes.
Figure 4.
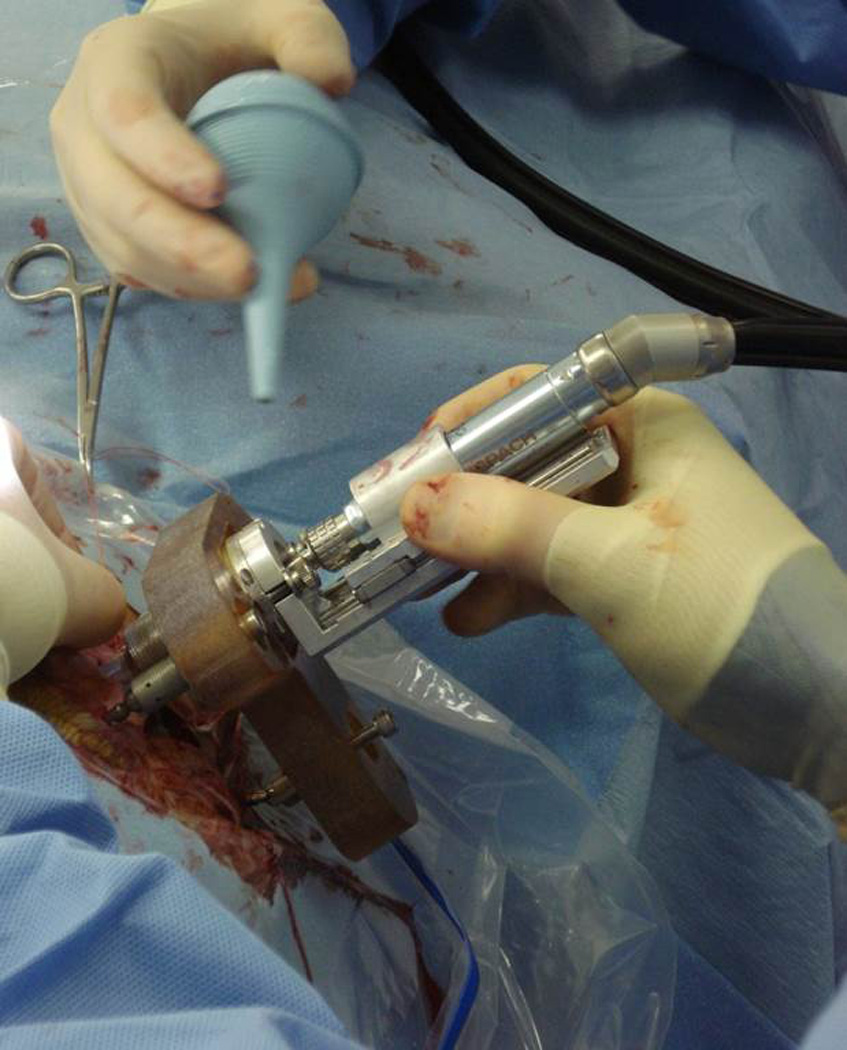
Drilling using custom microstereotactic frame. Custom microstereotactic frames are designed and rapidly manufactured to mount on the fiducial markers and constrain the drill along the desired trajectory. The frame is rigidly attached to the head by tightening the grippers to hold on to the spherical markers. While drilling saline is irrigated onto the drill bit.
Drilling
A drill press, designed specifically for linear drilling using the microstereotactic frame,21 was set to a pre-planned distance based on patient anatomy. It was attached to the sterilized microstereotactic frame, and the entire assembly was mounted on the fiducial markers (Figure 4). A stab incision was made at the site where the drill hit the skin and bone was exposed. Drilling was done at ≤ 50,000 RPM with twist drill bits. Lateral to the facial nerve a large bore (3.797 mm diameter) bit was used to allow irrigation during deeper drilling and serve as a receptacle for a bushing that further constrained the deeper drilling. As an additional safety measure, after lateral drilling a second intraoperative CT scan was obtained to confirm that drilling was on-course. The drill bit was then replaced with a smaller bore (1.59 mm diameter) bit for drilling through the facial recess. Bushings of corresponding sizes were used during the lateral and medial drilling to restrict lateral movement. Facial nerve monitoring was continuously performed. Confirmation of correct location was made using an endoscope passed via the drilled tunnel to the middle ear (Figure 5). Time for drilling was approximately two minutes.
Figure 5.
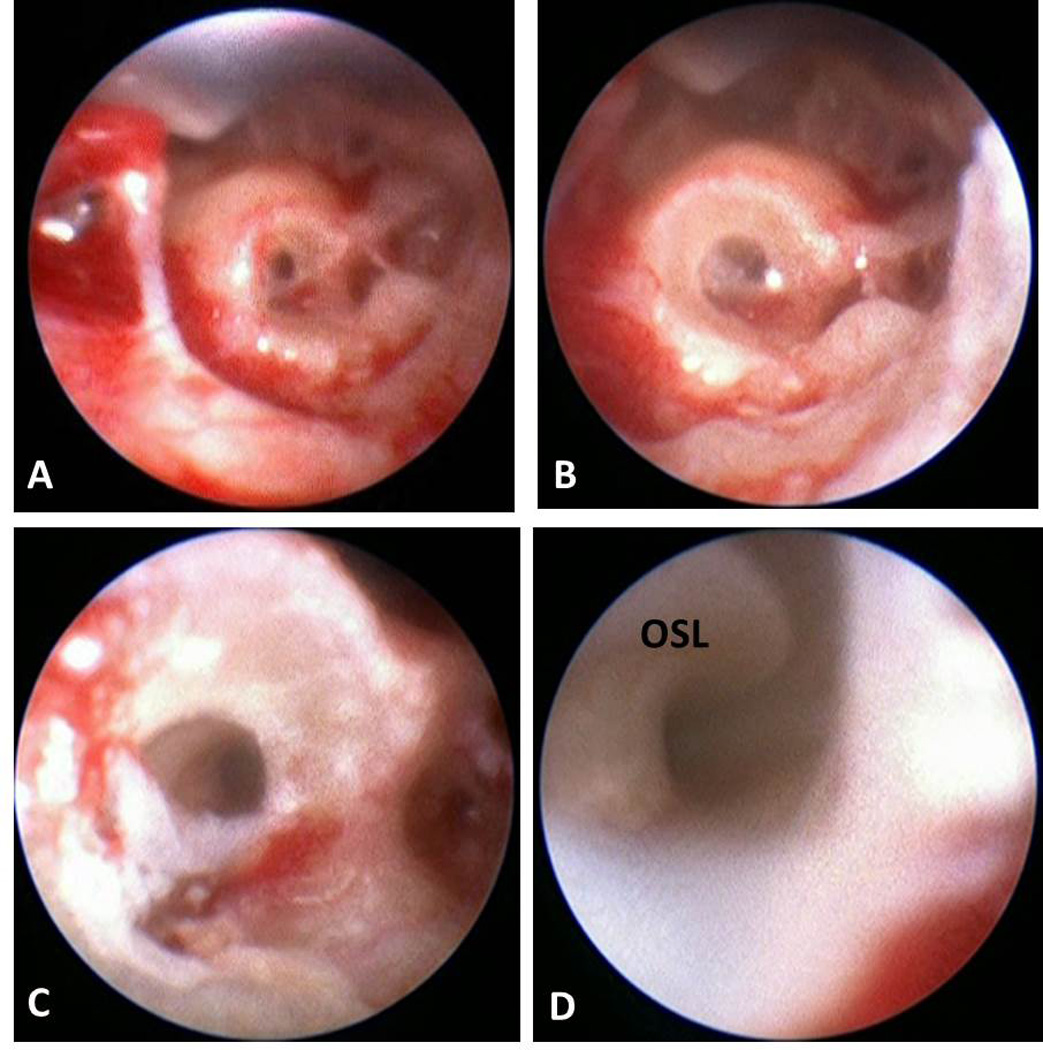
Endoscopic confirmation of trajectory in sequential order from (a) just past the facial recess, (b) in the middle ear, (c) prior to entering the cochlea, and (d) inside the cochlea with osseous spiral lamina (OSL) visible.
Insertion of electrode array into the cochlea
While awaiting construction of the customized frame, a subperiosteal receiving well and path to the drill hole was made via a 2–3 cm incision. For these first clinical implementations, a tympanomeatal flap was raised to allow access to the middle ear for anterior, inferior round window cochleostomy. After securing the internal receiver, the electrode array was passed through the subperiosteal path, into the drilled tunnel, and into the cochlea under direct visualization provided by the tympanomeatal flap. Confirmation of insertion was made with a final intraoperative CT scan, which was also used for assessment of post-operative location within the cochlea (i.e. ST versus SV).22
Closure
Hardware was removed and incisions closed using standard technique.
Results
Nine patients consented to the minimally-invasive, image-guided approach. In seven, a single drill tunnel was made from the surface of the mastoid to the middle ear. In the remaining two, image-guided drilling was restricted to the facial recess as one patient had undergone a cortical mastoidectomy in the distant past and the other had a soft tissue mass just lateral to the facial recess that was remotely suspicious for a facial nerve neuroma (intraoperatively, the mass was found to be mucous).
For the first six patients, styleted CI electrode arrays were used. The stylet was left in place for Patient 2 as it was felt that removal would dislodge the electrode array. In Patient 5, a banded electrode became dislodged from the electrode array during withdrawal and repositioning as it caught on a boney edge. The back-up array was inserted without further incident. For Patient 6, successful image-guided drilling was performed, but the styleted implant could not be inserted via the drilled path because the tip of the electrode array was repeatedly diverted into large air cells along the drilled path. The surgeon converted to a traditional open technique using the back-up array.
For the last three patients, lateral wall electrodes were used. Post-op CT scanning showed tip fold-over of the distal two electrode arrays for Patient 7 (Figure 6).
Figure 6.
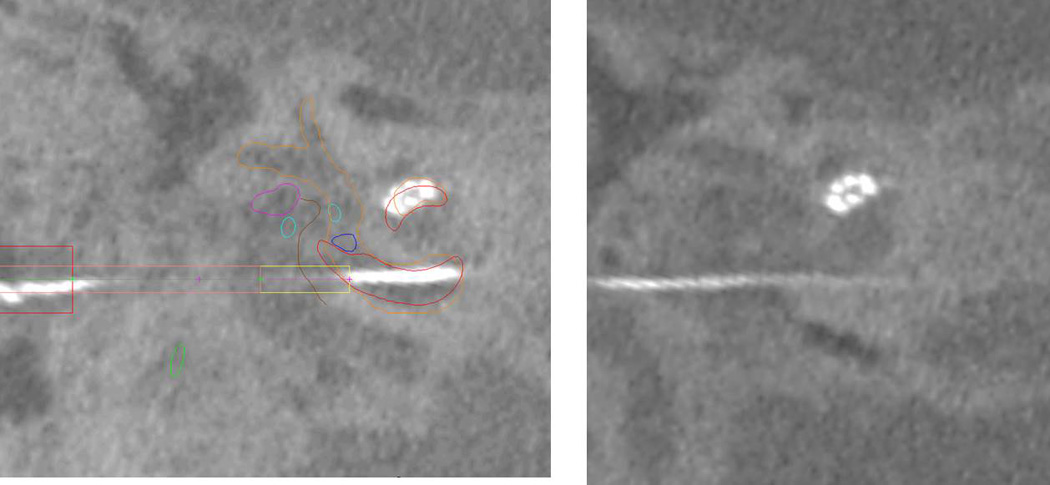
Post-operative screenshot of Patient 7 showing the tip fold-over.
During medial drilling for Patient 8, the facial nerve monitor beeped once for a very short duration. Since the duration and intensity of the alarm were minimal and the planned trajectory was close to the chorda tympani, the alarm was interpreted as secondary to chorda tympani stimulation, and drilling proceeded. The patient experienced immediate post-operative facial nerve weakness that did not resolve after 24 hours. He subsequently underwent exploratory surgery the next day at which time the chorda tympani and facial nerve were found to be structurally intact with bone covering each (Figure 7). This was confirmed by a second surgeon who decompressed the structurally intact nerve, and the patient was placed on steroids for two weeks. The patient has experienced steady recovery and, at twelve months post-op, is a House-Brackmann II/VI (Figure 8).
Figure 7.
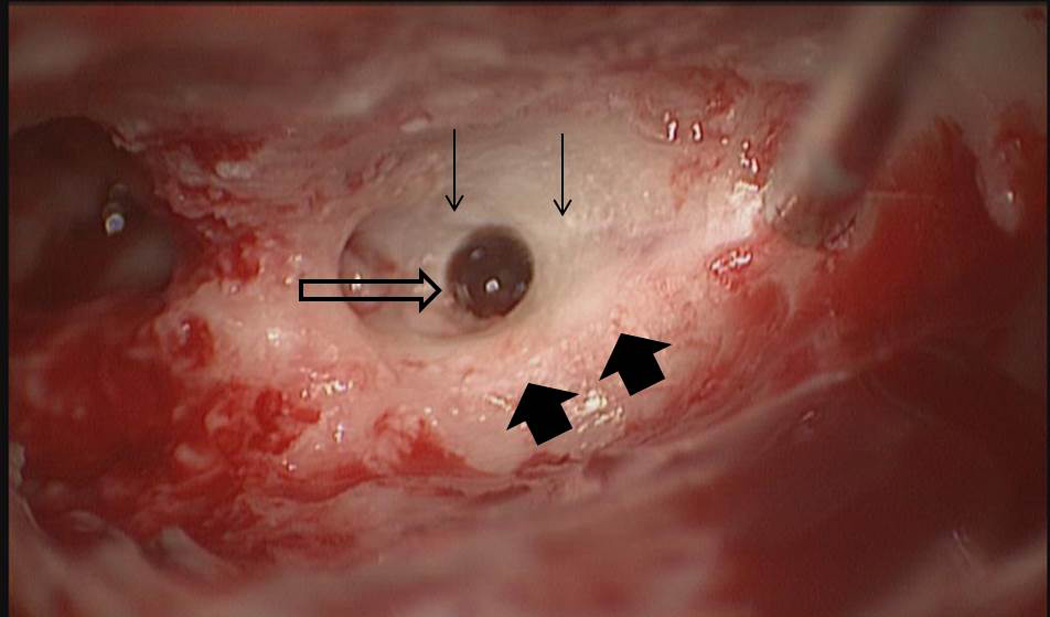
Intraoperative exploration of patient 8 who had post-operative facial nerve paresis. The drilled trajectory (open arrow) bypassed the facial nerve (wide arrows) and chorda tympani (thin arrows). This, in combination with bench-top testing, led to the conclusion that heat led to the temporarily severe paresis.
Figure 8.
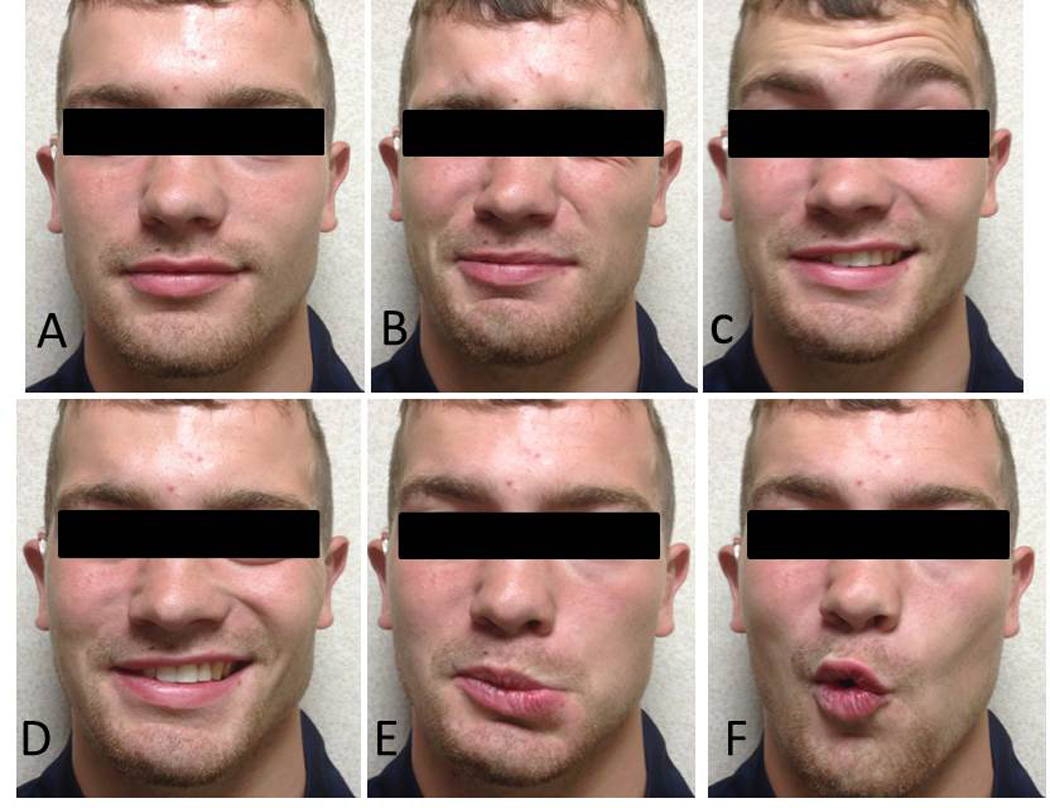
Facial movement at 12 months post-op for patient 8 who had immediate, complete facial nerve paralysis following surgery. The panels show the patient (A) at rest, (B) closing eyes (complete closure is achieved), (C) wrinkling of forehead, (D) smiling, (E) frowning, and (F) puckering. At 12 months, the patient was graded a House-Brackmann II/VI.
For Patient 9 (Figure 9), CI activation was initially performed in the recovery room and further mapped on post-operative day #1. The patient returned to work on post-operative day #5 wearing CI.
Figure 9.
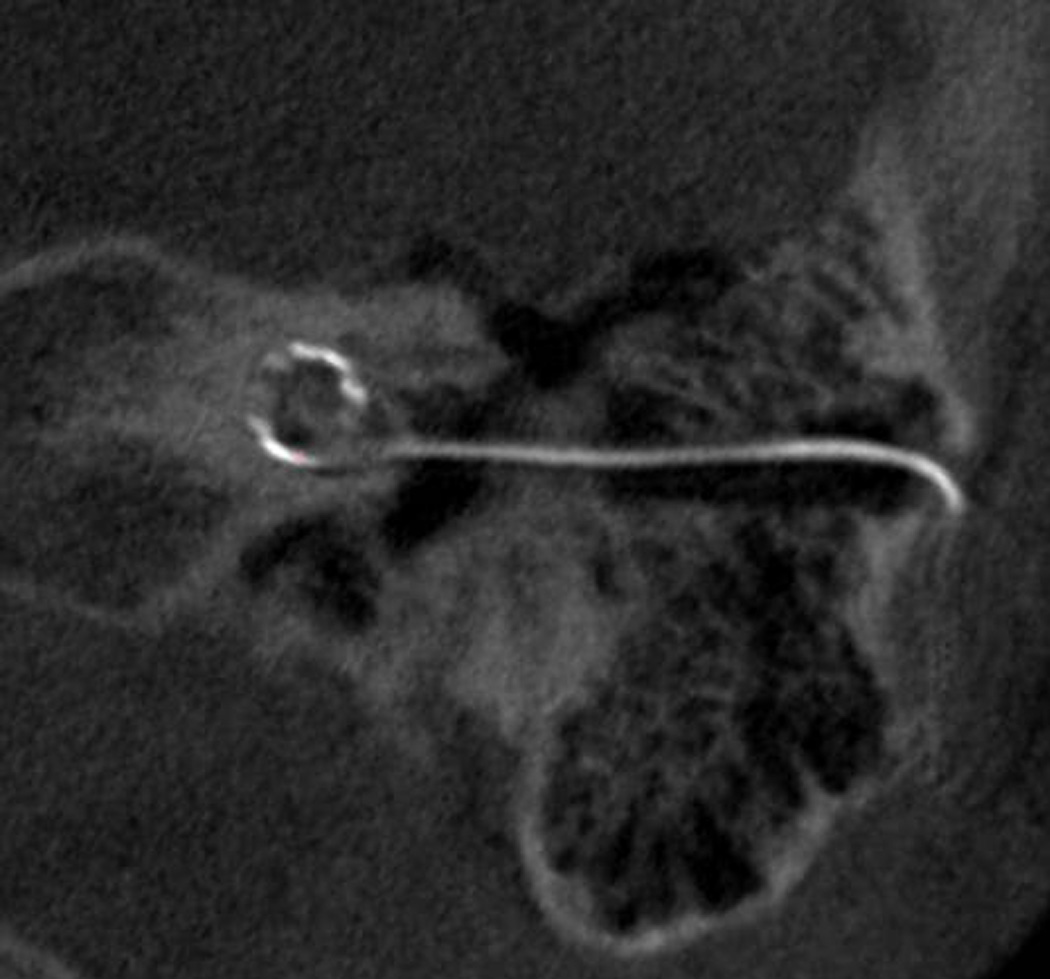
Post-op CT scan of Medel electrode inserted via minimally invasive technique.
Post-operative CTs were analyzed to determine the location of the electrode arrays within the cochlea. Of the eight patients for whom insertion was made via the drilled path, six were completely within ST, one crossed from ST to SV at approximately 180° (Figure 10), and one could not be determined due to an unresolvable CT artifact.
Figure 10.
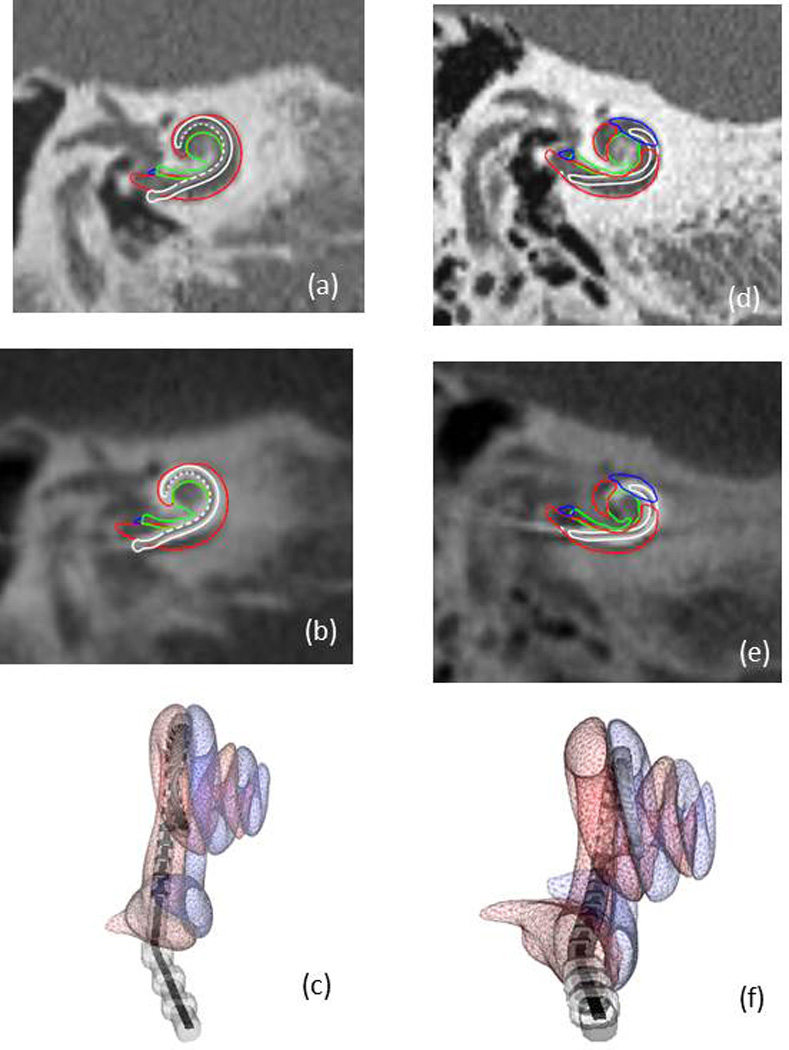
Shown in (a)-(c) are a coronal slice of a pre-operative CT, the same slice in post-operative CT, and a 3D rendering, respectively, of the scala tympani (red), scala vestibuli (blue), modiolus (green), and the implanted electrode array (white and gray) for a subject where the electrode array is completely within the scala tympani. Panels (d)-(f) show similar views for another subject where the electrode array crosses from scala tympani to scala vestibuli.
Average surgery time from incision to closure over all nine patients was recorded as 182±36 minutes. The surgery time ranged from 141 to 248 minutes, with 248 minutes required when the minimally invasive approach was aborted and a traditional approach undertaken.
Discussion
This paper is the first report of clinical implementation of the minimally-invasive, image-guided approach for routine cochlear implantation. We have previously reported on the use of this technique for implantation in an ossified cochlea.23 Clinical translation of this concept occurred only after extensive in vitro10,21 and in vivo11,12 research. As described above, we have performed this procedure in seven patients, for six of whom we were able to insert the electrode array via the drilled tunnel. Additionally, we performed stereotactic drilling through the facial recess in two patients who had undergone cortical mastoidectomies. During this period of clinical translation, five of the nine procedures were without incident. Difficulties encountered include the inability to place the implant necessitating conversion to traditional CI approach, damage to the CI electrode necessitating use of a back-up device, tip fold-over, and facial nerve paresis. At this juncture, as we pursue an Investigational Device Exemption from the United States Food and Drug Administration for continued study of this technique, it is prudent that we critically analyze our progress to date.
What have we learned?
To date, we have shown that the technique is feasible. However the complication rate and time of intervention are greater than for traditional CI surgery. While this is typical with the introduction of new technology (i.e. laparoscopic cholecystectomy24), each complication must be thoroughly addressed to understand why it occurred and mitigate possible future occurrences.
Three issues – inability to place the electrode array necessitating conversion to a traditional approach, damage to the electrode array necessitating use of the back-up array, and tip fold-over – were related to CI electrode design. Our first six surgeries utilized the Cochlear Freedom Advance, a styleted array which has a slight curvature that makes threading the array through the drilled tunnel difficult. As a result, we feel that flexible, lateral wall electrodes are preferred for our approach. We also noted tip fold-over with a lateral wall electrode, which has been reported to occur in 1.4%25 to 5.5% of cases.26
The complication of greatest concern was the facial nerve paresis. Extensive work was done to minimize the risk of this occurrence. Based on findings from our clinical validation testing,11,12 raw statistical risk of violating the facial nerve is < 3.5%. With additional procedures, including review/modification of the preoperative plan and confirmatory CT scan between the lateral and medial drilling, we feel confident that the risk of permanent facial nerve injury is well below the current clinical standard of 0.1%.7 Consistent with this, the facial nerve paresis which occurred did not result from violation of the nerve, which was found to be structurally intact at the time of exploratory surgery and confirmed by a second surgeon. The most likely mechanism of injury, despite copious irrigation with saline, was heat injury. Benchtop experimentation showed that the heat of the drill bit may transiently exceed 200°F. As a result, we have spent considerable effort towards improving our drilling protocols as detailed below.
What additional work will be necessary to bring this concept to clinical use?
First and foremost, otologic surgeons will need to be open to and comfortable with image-guidance and stereotactic-frame technology. This technology is a shift from the open-field approaches currently in use. All necessary equipment is typically available in tertiary-care medical centers including intraoperative CT scanners, which are now routinely available in neurovascular, vascular, and interventional cardiology suites. At our institution we have used both portable CT scanners and biplane fluoroscopy units within a neurovascular operating room. Alternatively, as we did in our initial validation trials11 and as is currently done with placement of deep-brain stimulators by neurosurgeons, the bone-implanted markers can be placed outside the operating room and CT scans obtained in the radiographic suite. Exposure to additional radiation accompanies the second CT scan. While flat panel scanners, such as the Xoran XCAT, deliver a considerably lower radiation dose, approximating that of a chest x-ray, some patients may feel uncomfortable with the additional radiation exposure.
Improvements in drilling technology, which we are currently exploring, will be necessary to minimize risk of injury to the facial nerve and allow atraumatic cochleostomy. To drill the access tunnel, drills bits should have a cutting head that is slightly larger in diameter than the shaft so that after the head passes the depth of the facial nerve the shaft will have little contact with tissue, thereby minimizing damage and heat conduction. Additionally, we are currently implementing “peck” drilling, in which small forward movements of the drill (e.g. 0.5 mm in < 1 second) are interspaced with short breaks (e.g. 2–3 seconds) to allow the drill bit to cool. To facilitate minimally-traumatic cochleostomy, we envision use of a torque-controlled drill such as that developed at Aston University.27 With these modifications and use of an endoscope28 to confirm accurate location of the cochleostomy both before and after drilling, the tympanomeatal flap may not be necessary. Despite promising work on these and other issues, we recognize that it may be years before routine clinical use may ever be realized.
What realistic future benefits will the technology hold?
When we originally embarked on the research and development of this technology, we hypothesized that it would dramatically reduce time of intervention. With recent reports citing time of CI surgery within academic centers of 80–90 minutes,29 we think that it will be difficult to dramatically improve upon this time for experienced surgeons. At present our strategy requires the same level of extensive otologic surgical experience as that required by the conventional technique. However, we still hypothesize that consistently lower times of intervention will be achievable for less experienced surgeons, especially those who have limited familiarity with the facial recess approach.
Another potential benefit is the ability to standardize the surgical intervention, potentially leading to optimal placement within ST. Our early results show complete ST placement in six of eight procedures. Without question, the minimally-invasive approach leads to less tissue dissection, but the clinical implications of this are not clear.
Conclusion
We have shown that minimally-invasive image-guided CI is clinically achievable. As with the introduction of other technology, this has involved a learning curve with more early complications than encountered in traditional approaches. We recognize areas that need further refinement including development of drilling technology both to limit heat production and to allow atraumatic entry into the cochlea. We conclude that further clinical study is necessary to address the significance of potential benefits including decreased time of surgical intervention, standardization of surgical intervention, and reduced tissue resection potentially leading to quicker activation and shorter patient recovery.
Acknowledgements
We are grateful to Lueder Kahrs, PhD who assisted with collection of endoscopic images such as those seen in Figure 5 and Wendy Lipscomb, RN who was instrumental in consenting patients and ensuring compliance with our Institutional Review Board.
Financial Disclosures: The project described was supported by Award Number R01DC008408 from the National Institute on Deafness and other Communication Disorders (NIDCD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
Footnotes
Conflict of Interests: Drs. Dawant, Fitzpatrick, Noble, and Labadie hold intellectual property rights on aspects of the technology described herein some of which may lead to commercialization with the potential for financial benefit to them.
References
- 1. http://www.nytimes.com/2013/09/10/health/lasker-awards-winners-2013.html?_r=0
- 2.Aschendorff A, Kubalek R, Turowski B, Zanella F, Hochmuth A, Schumacher M, Klenzner T, Laszig R. Quality control after cochlear implant surgery by means of rotational tomography. Otology & Neurotology. 2005;26:34–37. doi: 10.1097/00129492-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JH, Saxon EA, Hullar TE, Finley CG. In-vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Annals of Otology, Rhinology, & Laryngology. 2007;116(4) Suppl 197:1–24. [PubMed] [Google Scholar]
- 4.Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, Skinner MW. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008 Oct;29(7):920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson ML, Driscoll CL, Gifford RH, Service GJ, Tombers NM, Hughes-Borst BJ, Neff BA, Beatty CW. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011 Aug;32(6):962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.House W. Surgical considerations in cochlear implantation. Ann Otol Rhinol Laryngol Supp. 1982;91(2 Pt 3):15–20. [PubMed] [Google Scholar]
- 7.Thom JJ, Carlson ML, Olson MD, Neff BA, Beatty CW, Facer GW, Driscoll CL. The prevalence and clinical course of facial nerve paresis following cochlear implant surgery. Laryngoscope. 2013 Apr;123(4):1000–1004. doi: 10.1002/lary.23316. [DOI] [PubMed] [Google Scholar]
- 8.Kronenberg J, Migirov L, Dagan T. Suprameatal approach: new surgical approach for cochlear implantation. The Journal of laryngology and otology. 2001;115(4):283–285. doi: 10.1258/0022215011907451. Epub 2001/03/29. PubMed PMID: 11276329. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg J, Baumgartner W, Migirov L, Dagan T, Hildesheimer M. The suprameatal approach: an alternative surgical approach to cochlear implantation. Otol Neurotol. 2004;25(1):41–44. doi: 10.1097/00129492-200401000-00008. discussion 4–5. Epub 2004/01/16. PubMed PMID: 14724490. [DOI] [PubMed] [Google Scholar]
- 10.Warren FM, Balachandran R, Fitzpatrick JM, Labadie RF. Percutaneous Cochlear Access Using Bone-Mounted, Customized Drill Guides: Demonstration of Concept In-Vitro. Otology & Neurotology. 2007;28(3):325–329. doi: 10.1097/01.mao.0000253287.86737.2e. PMID: 17414037. [DOI] [PubMed] [Google Scholar]
- 11.Labadie RF, Noble JH, Dawant BM, Majdani O, Balachandran R, Fitzpatrick JM. Clinical validation of percutaneous cochlear implant surgery: initial report. Laryngoscope. 2008 Jun;118(6):1031–1039. doi: 10.1097/MLG.0b013e31816b309e. PMID: 18401279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labadie RF, Balachandran R, Mitchell J, Noble JH, Majdani O, Haynes DS, Bennett M, Dawant BM, Fitzpatrick JM. Clinical Validation Study of Percutaneous Cochlear Access Using Patient Customized Microstereotactic Frames. Otology & Neurotology. 2010 Jan;31(1):94–99. doi: 10.1097/MAO.0b013e3181c2f81a. PMID: 20019561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble JH, Dawant BM, Warren FM, Labadie RF. Automatic identification and 3D rendering of temporal bone anatomy. Otol Neurotol. 2009;30(4):436–442. doi: 10.1097/MAO.0b013e31819e61ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble JH, Labadie RF, Majdani O, Dawant BM. Automatic Segmentation of Intracochlear Anatomy in Conventional CT. IEEE Transactions on Biomedical Engineering. 2011;58(9):2625–2632. doi: 10.1109/TBME.2011.2160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble JH, Warren FM, Labadie RF, Dawant BM. Automatic segmentation of the facial nerve and chorda tympani in CT images using spatially dependent feature values. Medical Physics. 2008;35(12):5375–5384. doi: 10.1118/1.3005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble JH, Majdani O, Labadie RF, Dawant B, Fitzpatrick JM. Automatic determination of optimal linear drilling trajectories for cochlear access accounting for drill-positioning error. Int J Med Robot. 2010;6(3):281–290. doi: 10.1002/rcs.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reda FA, Noble JH, Labadie RF, Dawant BM. Automatic Pre- to Intra-Operative CT Registration for Image-Guided Cochlear Implant Surgery. IEEE Transactions on Biomedical Engineering. 2012;59(11):3070–3077. doi: 10.1109/TBME.2012.2214775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging. 1997 Apr;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Cevikalp H, Fitzpatrick JM. Marker orientation in fiducial registration. Proc. SPIE Medical Imaging 2003; Feb 2003; San Diego, CA. pp. 1176–1185. [Google Scholar]
- 20.Labadie RF, Mitchell J, Balachandran R, Fitzpatrick JM. Customized, rapid-production microstereotactic table for surgical targeting: description of concept and in vitro validation. Int J CARS. 2009;4:273–280. doi: 10.1007/s11548-009-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balachandran R, Mitchell JE, Blachon G, Noble JH, Dawant BM, Fitzpatrick JM, Labadie RF. Percutaneous Cochlear Implant Drilling via Customized Frames: an in vitro study. Otolaryngology-Head & Neck Surgery. 2010;142(3):421–426. doi: 10.1016/j.otohns.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF. Anatomic Verification of a Novel, Non-rigid Registration Method for Precise Intrascalar Localization of Cochlear Implant Electrodes in Adult Human Temporal Bones Using Clinically-available Computerized Tomography. Laryngoscope. 2010;120(11):2277–2283. doi: 10.1002/lary.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanna GA, Carlson ML, Blachon, Noble JH, Reda F, Dawant BM, Labadie RF, Balachandran R. Implantation of the completely ossified cochlea: An image-guided approach. Otology and Neurotology. 2013;34(3):522–525. doi: 10.1097/MAO.0b013e31827d8aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddick EJ, Olsen DO. Larparoscopic laser cholecystectomy. Surg Endosc. 1989;3:131–133. doi: 10.1007/BF00591357. [DOI] [PubMed] [Google Scholar]
- 25.Cosetti MK, Troob SH, Latzman JM, Shaprio WH, Roland JY, Waltzman SB. An evidence-based algorithm for intraoperative monitoring during cochlear implantation. Otology & Neurotology. 2012;33:169–176. doi: 10.1097/MAO.0b013e3182423175. [DOI] [PubMed] [Google Scholar]
- 26.Grolman W, Maat A, Verdam F, Simis Y, Carelsen B, Freling N, Tange RA. Spread of excitation measurements for the detection of electrode array foldovers: a prospective study comparing 3-dimenstional rotational x-ray and intraoperative spread of excitation measurement. Otology & Neurotology. 2008;30:27–33. doi: 10.1097/mao.0b013e31818f57ab. [DOI] [PubMed] [Google Scholar]
- 27.Brett PN, Taylor RP, Proops D, Coulson C, Reid A, Griffiths MV. A surgical robot for cochleostomy. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1229–1232. doi: 10.1109/IEMBS.2007.4352519. [DOI] [PubMed] [Google Scholar]
- 28.Kahrs LA, McRackan TR, Labadie RF. Intracochlear visualization: comparing established and novel endoscopy techniques. Otol Neurotol. 2011;32(9):1590–1595. doi: 10.1097/MAO.0b013e3182390248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A Semaan MT, Fredman ET, Shah JR, Fares SA, Murray GS, Megerian CA. Surgical duration of cochlear implantation in an academic university-based practice. Am J Otolaryngol. 2013 Feb 12; doi: 10.1016/j.amjoto.2013.01.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


