Abstract
Objective
Percutaneous cochlear implant surgery consists of a single drill path from the lateral mastoid cortex to the cochlea via the facial recess. We sought to clinically validate this technique in patients undergoing traditional cochlear implant surgery.
Study Design
Prospective clinical trial.
Methods
After institutional regulatory board approved protocols, five ears were studied via the following steps. 1) In the clinic under local anesthesia, bone-implanted anchors were placed surrounding each mastoid. 2) Temporal-bone computed tomography (CT) scans were obtained. 3) On the CT scans, paths were planned from the lateral mastoid cortex, through the facial recess, to the basal turn of the cochlea both “manually” and “automatically” using computer software. 4) Customized microstereotactic frames were rapid-prototyped to serve as drill guides constraining the drill to follow the appropriate path. 5) During cochlear implant surgery, after drilling of the facial recess, drill guides were mounted on the bone-implanted anchors. 6) Accuracy of paths was assessed via intraoperative photodocumentation.
Results
All surgical paths successfully traversed the facial recess and hit the basal turn of the cochlea. Distance in millimeters (average SD) from the midpoint of the drill to the facial nerve was 1.18 ± 0.68 for the “manual” path and 1.24 ± 0.44 mm for the “automatic” path and for the chorda tympani 0.986 ± 0.48 for the “manual” path and 1.22 ± 0.62 for the “automatic” path.
Conclusions
Percutaneous cochlear implant access using customized drill guides based on preoperative CT scans and image-guided surgery technology can be safely accomplished.
INTRODUCTION
Within all fields of surgery there has been a push towards minimizing the invasiveness of procedures. This has occurred to limit co-morbidity (1) as well as to limit cost (2,3). Within the field of otolaryngology, perhaps the most notable shift to minimally-invasive surgery is that of functional endoscopic sinus surgery (4,5,6). Other areas that are undergoing such transition include thyroid surgery (7,8) and laryngeal surgery (9,10,11). In the continued effort to minimize invasiveness, image-guided surgical system (IGS), are being utilized more and more frequently. Using pre or intraoperatively acquired radiographs, these systems allow surgeons to determine the boundaries of the surgical field and positions of vital anatomical structures. Parallel development of IGS took place in America, Japan and Switzerland (12,13,14). Credit for introducing IGS to Otolaryngology goes to Schlöndorff (15,16).
Within the subspecialty of otology/neurotology, an area where minimally-invasive surgery has garnered attention is cochlear implant surgery. Early attempts to minimize cochlear implant surgery included the endomeatal approach where a trough was drilled in the posterior external auditory canal to accommodate the connecting wire from the internal receiver to the electrode array. This approach was complicated by infection, wire extrusion into the external auditory canal, and cholesteatoma (17, 18). More recent efforts have focused on smaller incision sizes (19,20), micromastoids (21) and single drill troughs to the middle ear via the attic (22).
The ultimate, minimally-invasive cochlear implant is one in which a single drill pass goes from the mastoid cortex to the basal turn of the cochlea. This has been termed “percutaneous cochlear implantation.” To achieve this radical approach, image guidance based on a pre-operative CT scan is necessary. Initial reports regarding this technique were made using IGS with freehand drilling on cadaveric specimens (23). The authors of this study noted difficulty in keeping the drill on the correct trajectory. The technique was then modified to use a microstereotactic frame as a drill guide constraining the drill to pass in the pre-determined path (24). Both of these reports were done on cadaveric specimens.
Contained herein is an initial report on clinical translation of the drill guide technique for percutaneous cochlear implantation. This first step consists of validation of a safe drill path. To accomplish this, patients underwent pre-operative imaging after having bone-implanted anchors placed surrounding the ear. A customized drill guide was created which constrained the path of a drill through the facial recess and to the basal turn of the cochlea. Then, after undergoing traditional cochlear implant surgery via mastoidectomy and facial recess but before performing the cochleostomy and implantation, the drill guide was affixed to the anchors to determine whether the percutaneous technique would have been successful. The results for our first four patients (five ears) are reported below.
METHODS
Institutional Regulatory Board (IRB) approval was obtained. Each patient recruited underwent extensive pre-procedural counseling detailing the specifics about the following procedures which were performed.
(1) Anchor/fiducial marker placement
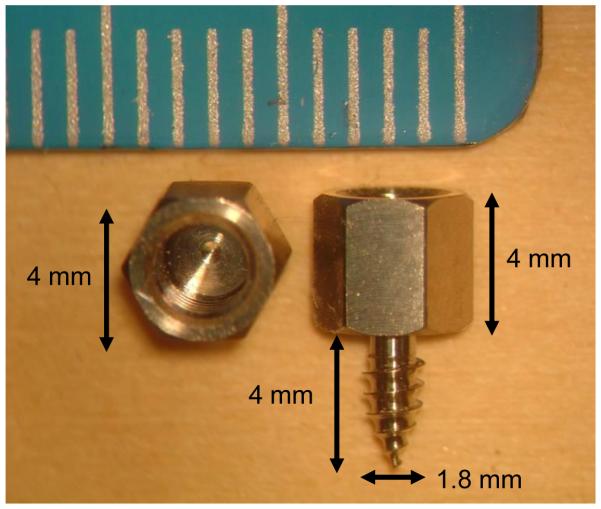
Anchors with self-tapping threads were screwed into the skull surrounding the temporal bone. These anchors serve two purposes. First, they are used to register the patient’s anatomy to the CT scan to allow referencing of the drill path (from lateral skull to cochlea) to the position of the anchors. Second, they have a hollow interior which is tapped to allow mounting of the drill guide at the time of surgery. Each anchor is made of titanium and is 4mm wide and 8 mm long with 4mm of this length screwed into the skull. The shape and size can be seen in Figure 1. Three anchors were placed surrounding the mastoid. Their locations are “mastoid”, “suprahelix”, and “posterior” as shown in Figure 2, which is a view of a patient after anchor insertion and skin closure and the corresponding radiograph.
Figure 1.
Anchor. Left sample shown is from the top. Right sample is shown from the side. The anchor is titanium with a self-tapping screw. The hole at the upper end has female threads to which a bolt can be attached. The bolt connects the drill guide rigidly to a set of these anchors. The smaller markings on the ruler are millimeters.
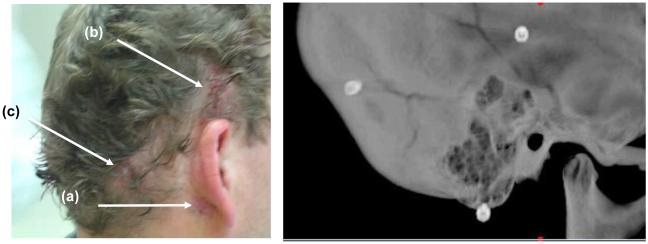
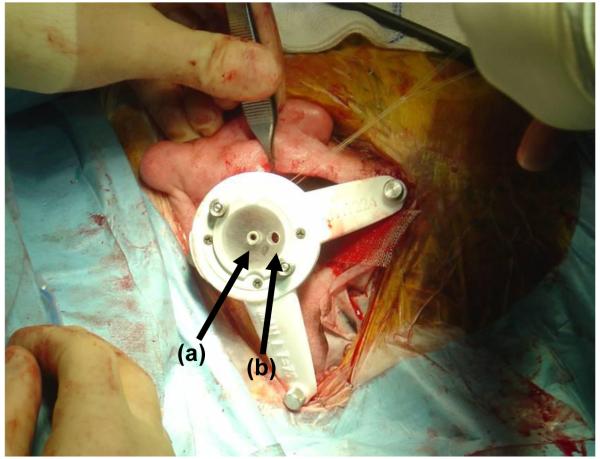
Figure 2.
Anchor positioning. After anchor insertion and skin closed. Locations as named in the text (a) “Mastoid”, (b) “Suprahelix”, (c) “Posterior”.
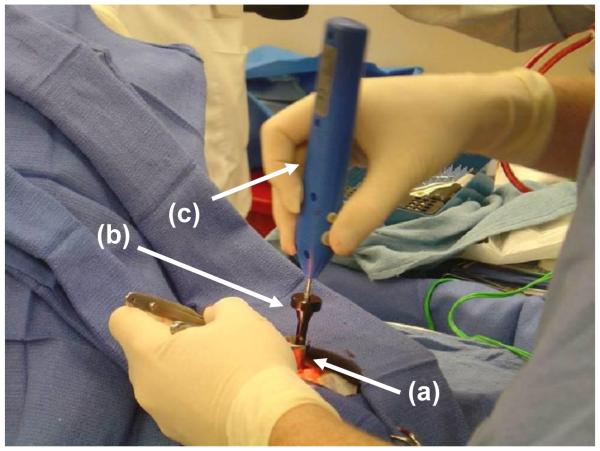
Anchor placement was accomplished using local anesthesia in a procedure room in the clinic. The anchor sites were marked with an indelible pen, cleaned with alcohol, and then injected with 1% lidocaine with epinephrine. The area was prepped and draped without trimming of hair. At each site, a stab incision was made with a scalpel down to and through periosteum. Hemostasis was achieved with direct pressure, and, if necessary, bipolar electrocautery. A nasal speculum was used for exposure. A periosteal elevator was used to expose bone. A modified surgical screwdriver (WayPoint driver; FHC, Inc; Bowdoin, ME) was used in conjunction with an Osteomed electric driver (Model 450-0600; Adison, TX) to place anchors into the cranium to a depth of 4 mm, as shown in Figure 3. Incision sites were closed with subcutaneous 3-0 vicryl, 5-0 plain gut to skin, and dressed with bacitracin zinc ointment.
Figure 3.
Anchor insertion. (a) Nasal speculum maintains exposure. (b) Waypoint™ driver passes through speculum to attach to anchor (not visible). (c) Osteomed electric driver (model 450-0600) inserted into Waypoint driver provides torque.
(2) CT scanning
After anchor placement, patients went directly to a CT scanner where clinically applicable CT scans were obtained (helical scans, 0.8 mm slice thickness with 0.4 mm overlap). Figure 4 shows CT images of the three anchors. These CT scans were loaded into proprietary software which was used to automatically detect the position of the anchors.
Figure 4.
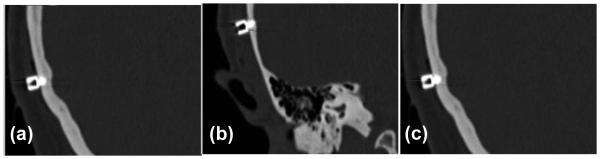
CT images of three anchors surrounding the left ear. The positions are, as described in the text, (a) Mastoid, (b) Suprahelix, and (c) Posterior.
(3) Path planning
Next an appropriate path from the lateral skull base to the cochlea was planned either “manually” or “automatically.” To manually plan, the surgeon picked the target (the basal turn of the cochlea) as well as the mid portion of the facial recess. This trajectory was then traced back to the surface of the lateral skull. Confirmation that this path did not violate any vital structures was performed using a number of views including a “path of flight” view where the CT scans were aligned on the trajectory allowing subjective determination of distance to the facial nerve, chorda tympani, and external auditory canal.
The automated path planning was done by means of a computer program written in Matlab (The Mathworks, Inc., Natick, MA). The program comprises the following three steps: (i) Identify and delimit select anatomy. Select anatomic landmarks (external auditory canal, the short process of the incus, and the basal turn of the cochlea) were automatically localized in the patient’s CT using an atlas-based technique (25). Temporal bone CT scans with normal anatomy (as identified and labeled by an experienced otologist) were defined as atlases and used to create a master atlas. Then, for each patient plan, this atlas is registered to the patient’s CT scan using affine registration, which consists of rotation, translation, skewing, and stretching of the atlas so that it fits the unknown scan (26,27,28,29). As a result of the registration, the pre-mapped anatomic landmarks in the atlas image can be superimposed on the patient image, thus automatically identifying and delimiting those structures. It is important to note that the final location of the basal turn of the cochlea is dependent upon the manually-identified location selected in the original atlas. This location was defined as the intracochlear, fluid-containing space anterior and inferior to the round window membrane. (ii) Refine the position of the facial nerve and chorda tympani. Following registration, specific segmentation of the facial nerve is necessary because atlas-based registration alone was found to be an insufficiently accurate predictor of its course. To segment the facial nerve, its beginning (most inferior 5%) and its ending (most anterior 5%) are identified from the aforementioned atlas based on registration. These two points are then connected by a curved line to segment the track of the facial nerve by means of minimization of a cost function where cost is a function of voxel intensity and shape of the track. This method results in excellent identification of the facial nerve. A similar procedure is performed with the chorda tympani picking a beginning 5mm inferior to the atlas-determined branch point and the ending being the superior medial corner of the annular ring. As with the facial nerve, this method resulted in excellent identification of the chorda tympani. (30) (iii) Identify boundaries of extended facial recess. Next, the extended facial recess is identified using the location of the facial nerve, external auditory canal, and short process of incus. The center of the recess is identified, and a perpendicular line is drawn from the center of the facial recess to the basal turn of the cochlea. This procedure allows a rough estimation of the plane of the facial recess. (iv) Identify all paths that pass through the facial recess plane and hit the target—the basal turn of the cochlea. Within the aforementioned plane of the facial recess, a dense set of possible linear paths to the basal turn of the cochlear are enumerated. (v) Calculation of safety. For each possible drill path, the path is subjected to thousands of random deviations of approximately the level of accuracy of the system. This creates a “cloud of uncertainty” for each possible drill path. The fraction of times the “cloud” hits any vital structure (facial nerve, external auditory canal, chorda tympani, and short process of the incus) are calculated. This fraction represents the probability, given the accuracy of the system, that a given drill path will violate one of these structures. Minimum acceptable safety probabilities were set such that there would be 99.9% certainty of avoiding the facial nerve, 95% certainty of avoiding the external auditory canal, 80% certainty of avoiding the incus, and 67% of avoiding the chorda tympani. The path which achieves the highest probability for avoidance of each structure yet satisfies these minimum probabilities was chosen as the best path. An example of an automatically chosen path is displayed in Figure 5 The entire path planning program takes approximately 35 minutes on a 3 GHz PC running Windows XP.
Figure 5.
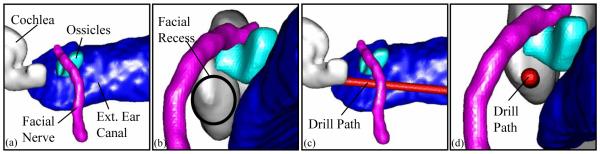
Example of automatically planned drill path for a right ear. (a) View from posterior-to-anterior. (b) View through facial recess. (c) Same view as (a) but with drill path (1mm diameter) shown. (d) Same view as (b) but with drill path shown.
(4) Rapid prototyping of drill guides
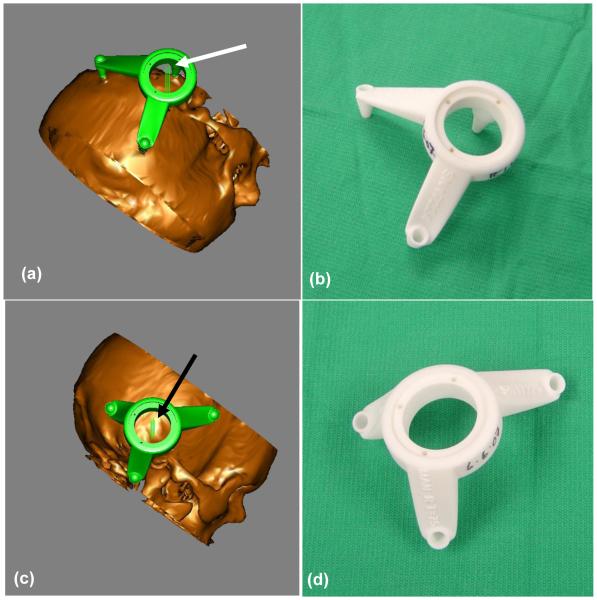
For each patient, a drill guide was created that (a) mounted to the anchors and (b) constrained the passage of a surgical drill to either the “manual” or “automatic” path. Proprietary software was used to create a digital file defining the drill guide which satisfies (a) and (b). This digital file was then sent to a manufacturing facility where the drill guide was built using rapid prototype technology (FHC Inc.; Bowdoin, ME). Manufactured drill guides were sent via overnight shipping and were sterilized prior to use. Figure 6 shows both the graphic depiction of a drill guide as seen during planning, and the resulting guides as fabricated from the plan. Two examples are shown—one for a left ear and a second for a right ear. Figure 7 shows the physical arrangement of a drill guide and anchors. During surgery a bolt is passed through each foot of the drill guide into the corresponding anchor.
Figure 6.
Drill guide as designed and as a fabricated device. (a) Left ear design, (b) Left ear device, (c) Right ear design, (d) Right ear device. The arrows point to pairs of parallel lines that represent the planned drill paths. The distance between the lines of a pair are equal to the planned drill width (1mm).
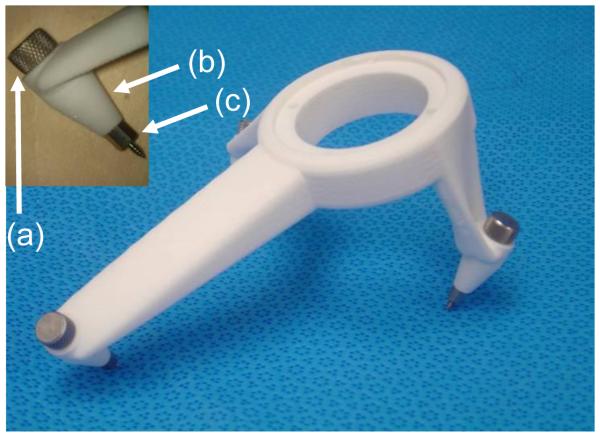
Figure 7.
The drill guide. A bolt (a) passes through a hole in each foot (b) attaching the foot to an anchor (c), as shown in the inset at the upper left.
(5) Intraoperative Validation
Standard cochlear implant surgery was performed. The mastoid and suprahelical anchors were exposed during initial post-auricular incision. After completion of mastoidectomy and extended facial recess, the cochlear promontory was visualized. Next, the posterior anchor was exposed with a stab incision. To determine whether the proposed drill path would safely traverse the facial recess and hit the basal turn of the cochlea, the customized drill guide was attached to the anchors, as shown in Figure 8, and sham drill bits (devoid of cutting flutes) of 1, 2, and 3 mm were passed via the drill guide to the cochlear promontory. The largest size bit which safely traversed the facial recess was noted. The 1mm sham drill bit was coated with ink from an operative marking pen and used to mark where on the promontory the cochleostomy would have occurred. Photo documentation was performed using a zero-degree Hopkins rod which traversed parallel to the drill path. Imaging software was used to measure the distance from the drill to vital anatomy.
Figure 8.
Customized drill guide after attachment to the three anchors in the operating room. (a) Entry for drill bit. (b) Entry for endoscope (Hopkins rod). Images in Figure Figure 10 were acquired with the Hopkins rod.
RESULTS
Four patients agreed to partake in the study with one undergoing bilateral testing for a total of five ears. Anchor placement was tolerated by all patients. The first patient took two hydrocodone 7.5mg-acetaminophen 500mg pain tablets in the 24 hours post-procedure. The second patient rated the pain as 1.5 out of 10 and took two pain tablets that evening. The third patient, who underwent bilateral testing, rated pain for the first five anchors as 2 out of 10 but for the last anchor had intense pain of 9.5 out of 10. Notably, he did not verbalize pain during the procedure. He took four pain tablets in the 48 hours post-procedure. The last patient reported pain of 1 out of 10 during the procedure and 2 out of 10 post-procedural due to jaw discomfort. She took at a total of three pain tablets. All patients reported transient tenderness and pain with mastication. Fourteen of fifteen anchor sites healed nicely. The lone difficulty was delayed healing without infection. Of note, this one site was the same site where the patient experienced discomfort and was the only site where bipolar cautery was necessary to control bleeding.
Results of intraoperative validation testing are shown in Figure 9 and Table I. For each ear, the results for both manual and automatic path planning are summarized in Table I including (i) the largest sham drill bit which traversed the facial recess and (ii) the distance from the midline of the trajectory to the facial recess and (iii) the distance from the midline of the trajectory to the chorda tympani.
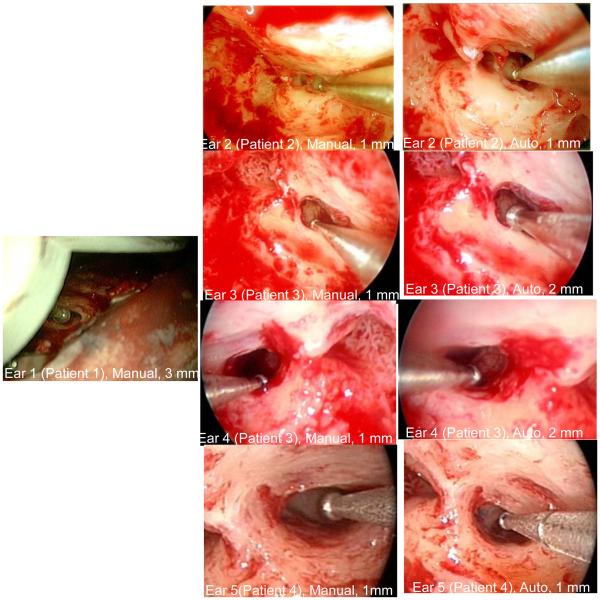
Figure 9.
Intraoperative Validation. Each image shows a sham drill bit (diameter noted under each picture) that safely traversed the facial recess. “Manual” means that a manual plan was used. “Automatic” means that the plan based on the computer algorithm was used. No automatic validation was possible for Ear 1 (see text).
Table I.
| Ear | Patient | Planning Method |
Largest Drill Bit to Pass Recess |
Distance to Facial Nerve |
Distance to Chorda Tympani |
|---|---|---|---|---|---|
| 1 | 1 | Manual | 3 mm | 2.23 mm | 1.13 mm |
| 1 | 1 | Automatic | NA | NA | NA |
| 2 | 2 | Manual | 1 mm | 1.09 mm | 0.87 mm |
| 2 | 2 | Automatic | 2 mm | 1.22 mm | 1.57 mm |
| 3 | 3 | Manual | 1 mm | 0.69 mm | 1.73 mm |
| 3 | 3 | Automatic | 2 mm | 1 mm | 1.59 mm |
| 4 | 3 | Manual | 1 mm | 0.5 mm | 0.5 mm |
| 4 | 3 | Automatic | 1 mm | 0.87 mm | Hit |
| 5 | 4 | Manual | 2 mm | 1.4 mm | 0.7 mm |
| 5 | 4 | Automatic | 1 mm | 1.85 mm | 0.5 mm |
Note that no “automatic” path is included for Ear 1. This first patient had the posterior anchor extrude while changing from the manual to automatic path drill guide. Averaging the remaining four ears, distance to facial nerve (± standard deviation) was 1.24 (± 0.44) mm for the automated path planning and 1.18 (±0.68) mm for the manual path planning. The chorda tympani was sacrificed in one “automatic” path. For the remaining “automatic” paths, the average distance (± standard deviation) from the midpoint of the drill path to the chorda tympani was calculated to be 1.22 (± 0.62) mm. The chorda tympani was preserved in all “manual” paths where the average distance was calculated to be 0.986 (±0.48) mm.
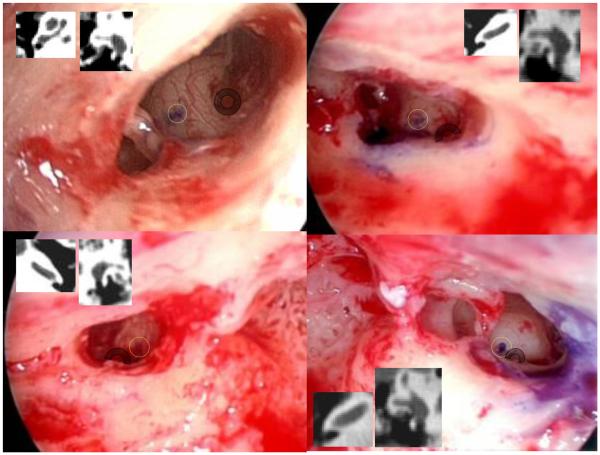
Shown in Figure 10 are the positions of the cochleostomy for ears 2-5. Ear 1 is not included as the technique of photo documentation was not in place for this first case. The raw data—the purple dot where the sham drill bit hit the cochlear promontory—is highlighted by a yellow circle. Also highlighted, by transparent grey annuli, are the round window niches. Inset within each frame are the corresponding axial and coronal CT scan slices which show the boney overhang of the round window. These insets are included as they describe the relationship between the relevant surface and sub-surface anatomy.
Figure 10.
The positions of the cochleostomy for each path are shown as a purple spot on the lateral wall of the cochlea. Transparent yellow circles have been added to highlight this position while transparent grey annuli overlie the round window niche. Inset within each panel are the corresponding axial and coronal scans showing boney overhand of the round window. (Note: No data is presented for the first ear as the technique was developed after the first surgery.)
DISCUSSION
Presented herein is clinical validation of the concept of percutaneous cochlear implantation. This concept, which has undergone extensive pre-clinical testing (23,24), consists of using image-guided surgical technology to create customized drill guides, the path of which traverses the facial recess (without injury to the facial nerve) and hits the cochlea anterior to the round window in the position loco typico for a cochleostomy. This technique has been translated from neurosurgery where such devices are FDA-approved for placement of deep brain stimulators in the subthalamic nucleus.
While this is but a preliminary report, we are encouraged by these initial findings especially (i) patient tolerance of the procedure and (ii) accuracy of the device in bypassing the facial nerve and hitting the basal turn of the cochlea. Regarding (i), the most arduous part of the project for participants—placement of bone-implanted anchors within the clinic using local anesthesia—was tolerated quite well with 14 of 15 anchors occurring with no more than slight discomfort. The single anchor that caused significant discomfort appeared to be a technical error in the placement of the local anesthesia, and the patient voiced no complaints until after the procedure was complete.
Regarding (ii), all trajectories bypassed the facial nerve and hit the cochlea at the intended target location. This result was not unexpected as a prior study established the accuracy of the microstereotactic frame as 0.44 mm at a depth of 125mm from the surface—the depth typically used for deep brain implants (31). The cochlea is more superficial than neurosurgical targets, and for cochlear implant use a depth of 75mm is used. Assuming an approximately linear distribution of error from the surface of the frame, we propose that the error for cochlear implant applications is 0.26 mm (0.44*75/125). This accuracy, afforded by the bone-implanted fiducial markers which also serve as anchors for the frame, is well below the 0.5 mm projected by Schipper et al. (32) as necessary to achieve reliable access to the scala tympani.
The location of the cochleostomy proved difficult to assess. Our original concept of putting dye on the tip of a sham drill bit produced the results shown in Figure 10. While these show cochleostomy locations acceptable for access to the cochlea, it is difficult to interpret where the cochlea would be entered for two reasons. First, the endoscopic pictures are slightly skewed as they are 2-D representation of 3-D objects and therefore dependent upon angle. For example, the image in the top left of Figure 10 is taken from below the proposed trajectory looking up to the cochlear promontory. From this perspective, the round window niche (which can be seen in its entirety) appears much lower than the proposed cochleostomy. Learning from this experience, subsequent images were taken in a much more uniform manner photo documenting along the axis of the trajectory producing the pictures in the remaining three panels. Second, the amount of boney overhang from the round window niche obscures the relationship between the surface anatomy (the round window niche) to the subsurface anatomy (scalas vestibule, media, and tympani). Therefore, we have included the axial and coronal CT views of the round window to allow readers to form their own interpretations about the proposed cochleostomy data. Recognizing the need to document precisely where in the cochlea the proposed trajectory lands, we have modified our future experimental protocol to include drilling away the round window overhang before documentation of the proposed cochleostomy site.
The automated path planning worked as well if not better than manual path planning. We attribute this to the computer’s ability to utilize 3-D relationships better than a surgeon whose path planning is achieved with a collection of 2-D views of a 3-D structure. While we have limited data, the automated path appeared to have more respect for the facial nerve than the manual path planning performed by the surgeon. This may reflect the human tendency to desire a perfect solution (bypassing BOTH the facial nerve and the chorda tympani) where in cases in which the facial recess is extremely narrow this may not be technically achievable or advisable. Ear 4 is just such an instance where the manual path successfully traversed a very narrow facial recess (≈ 1mm) while the automated path resulted in sacrifice of the chorda tympani in keeping an acceptable margin of safety in avoidance of the facial nerve. Intraoperatively, the chorda tympani had to be sacrificed in order to allow visualization and instrumentation within the middle ear. This incidence of injury of chorda tympani sacrifice is in line with the literature which reports an incidence of approximately 20% (33).
Perhaps a more fundamental question is: “Why pursue percutaneous cochlear implantation at all given an existing surgical approach (mastoidectomy with facial recess) which has an excellent track record?” We argue that percutaneous cochlear implantation holds out the prospect of benefit in several areas including (a) standardization of the surgical approach, (b) decreased operative time and overall cost, and (c) affording the possibility of unique implant opportunities. Each of these will be discussed below.
Standardization of surgical approach
In developing countries, where Otolaryngology-Head and Neck Surgery training is highly variable, cochlear implant surgery outcomes and complication rates are likewise variable. In such settings, standardization of the surgical approach with automated path planning would offer advantages including reduced complications and improved outcomes. The technique proposed herein requires access to a CT scanner with the rest of the technology being accessed from afar via transfer of electronic files. For application in developing countries, it is envisioned that a portable CT scanner (approximately $250,000 at the time of this writing) would be centrally located in a facility where CI programming would be performed.
Even in countries where otolaryngological surgical training is highly regimented (e.g. United States, Europe) and complications rates are low (injury to the facial nerve estimated at 1.5% (34,35,36) disagreement about such fundamental questions such as proper position of the cochleostomy persists (37,38,39). Automated path planning holds out the prospect of optimizing electrode placement potentially improving outcome by decreasing power consumption.
Decreased operative time and overall cost
Surgical times for cochlear implantation are frequently discussed but rarely published. Work from a decade ago estimated operative time at three hours for inexperienced (i.e. resident) surgeons (40). Estimates for experienced surgeons range from 50-75 minutes with outliers occurring routinely. Retrospectively reviewing data from two experienced institutions with multiple surgeons showed the following results. Institute A: n = 457; average surgical time 209 minutes; minimum 40 minutes; maximum 310 minutes. Institute B: n = 108: average surgical time 147 minutes; minimum 84 minutes; maximum 280 minutes. (Exclusion criteria - bilateral implantation, revision cases, anomalies, and patients involved in intraoperative research studies. Note that reported operative times include intraoperative audiological testing which take up to 30 minutes for each patient.) Using this information and the extremely limited data regarding time for implantation, a very conservative estimate is that an experienced surgeon can safely perform a routine cochlear implantation in 70 minutes and an inexperienced surgeon can do such in 150 minutes. Conservatively estimating the individual components of a percutaneous cochlear implant, we predict that percutaneous cochlear implantation would take approximately 50 minutes (exposure of anchors 10 minutes, attachment of drill guide 5 minutes, drilling from lateral cortex to middle ear 5 minutes, creation of pocket for internal receiver 10 minutes, cochleostomy and insertion of electrode 5 minutes, removal of drill guide 2.5 minutes, removal of anchors 2.5 minutes, closure 10 minutes). While this time differential is not dramatic for an experienced surgeon, it could have a dramatic impact upon surgical times for inexperienced surgeons.
Regarding actual surgical costs, as with surgical times limited data is available. A report from Germany estimates cost to vary between $46,000 and $57,000 depending on the discount on device (41). In the United Kingdom costs are approximately $44,000 (42). Reimbursement from private insurance companies in the USA is approximately $44,000 (43). In comparing cost of traditional cochlear implant surgery to percutaneous cochlear implant surgery, we propose that a more appropriate comparison is to equate operative time with operative cost. At the home institution of the lead author, the first ½ hour facility fee is $1581 and subsequent ½ hour fees are $1265. Also taking into account the additional cost of the drill guide (≈ $2000), the facility fee cost for experienced versus inexperienced surgeons is estimated below in Table II. As with time, while the cost savings for experienced surgeons may be minimal, the cost savings for inexperienced surgeons potentially could be substantial.
Table II.
Time and Cost Estimates for Cochlear Implant Surgery
| Traditional CI surgery | Percutaneous CI surgery | |
|---|---|---|
| TIME experienced surgeon | 70 minutes | 50 minutes |
| COST experienced surgeon | $4111 | $4046 |
| TIME inexperienced surgeon | 150 minutes | 50 minutes |
| COST inexperienced surgeon | $6641 | $4046 |
Unique implant opportunities
The cost estimate noted above takes into account only the time for procedural intervention. Significant cost savings could also be realized if the intervention could be moved from the operating room to a procedure room under local anesthesia. Local anesthesia has been used in select cases for cochlear implant surgery (44,45) and is the preferred mode of anesthesia for placement of deep brain stimulators using techniques similar to those described in this manuscript. Given that the procedure could be performed under local anesthesia, the concept of same-day service for cochlear implantation may be achievable. This would include audiological testing, CT acquisition, automated path planning, custom drill guide creation, implantation, and activation. Such a radical paradigm shift may allow percutaneous cochlear implantation to become analogous to LASIX surgery.
The preceding four paragraphs provide a glimpse at the possibilities which percutaneous cochlear implant surgery may allow. At present, we have demonstrated the feasibility of the technique on patients undergoing traditional cochlear implantation. This continuing study, funded by the NIDCD, now begins a multi-site component where the findings contained within this initial report will be tested by other surgeons. Pending confirmation of the success of the technique and addressing other technical issues (e.g. electrode insertion tools and drill strategies currently under development in our temporal bone lab), the method will be utilized to perform cochlear implantation in the near future.
CONCLUSIONS
Contained herein are the initial validation studies documenting translation of the concept of “percutaneous cochlear access” from the laboratory to the operating room. To accomplish this, under IRB-approved protocols, the following procedures were performed: (1) Patients had three bone-implanted markers placed surrounding the mastoid. (2) Patients underwent clinically applicable CT scanning. (3) Using proprietary computer software and each patient’s CT scan, trajectories from the lateral cortex of the mastoid, through the facial recess, and to the basal turn of the cochlear were planned. (4) Customized drill-guides to achieve these trajectories were rapid prototyped. (5) Intraoperatively, after traditional cochlear implant surgery, drill-guides were mounted on the markers, and accuracy of the proposed trajectory was assessed. All planned trajectories were accurate with five out of five traversing the facial recess without injury to the facial nerve. Four out of five trajectories also bypassed the chorda tympani, with the lone chorda tympani injury planned a priori on the pre-intervention CT scan. While additional work is necessary before full clinical implementation, these results support the feasibility of this novel surgical approach.
AKNOWLEDGEMENTS
Funded by NIH (NIBIB R01 DC008408-01A1)
REFERENCES
- (1).Attwood SE, Hill AD, Mealy K. A prospective comparison of laparoscopic versus open cholecystectomy. Ann R Coll Surg Engl. 1992;74(6):397–400. [PMC free article] [PubMed] [Google Scholar]
- (2).Soper NJ, Barteau JA, Clayman RV, Ashley SW. Comparison of early postoperative results for laparoscopic versus standard open cholecystectomy. Surg Gynecol Obstet. 1992;174(2):114–118. [PubMed] [Google Scholar]
- (3).Vierra M. Minimally invasive surgery. Annu Rev Med. 1995;46:147–158. doi: 10.1146/annurev.med.46.1.147. [DOI] [PubMed] [Google Scholar]
- (4).Messerklinger W. Endoscopy of the nose. Urban&Schwarzenberg (Baltimore, Munich) 1978 [Google Scholar]
- (5).Draf W. Therapeutic endoscopy of the paranasal sinuses. Endoscopy. 1978;10(4):247–254. doi: 10.1055/s-0028-1098303. [DOI] [PubMed] [Google Scholar]
- (6).Kennedy DW, Zinreich SJ, Rosenbaum AE. Functional endoscopic sinus surgery. Theory and diagnostic evaluation. Arch Otolaryngol. 1985;111(9):576–582. doi: 10.1001/archotol.1985.00800110054002. [DOI] [PubMed] [Google Scholar]
- (7).Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg. 2007;31(3):601–606. doi: 10.1007/s00268-006-0481-y. [DOI] [PubMed] [Google Scholar]
- (8).Terris DJ, Gourin CG. Minimally invasive thyroidectomy: basic and advanced techniques. Laryngoscope. 2006;116(3):350–356. doi: 10.1097/01.mlg.0000191462.58630.e4. [DOI] [PubMed] [Google Scholar]
- (9).Iro H, Waldfahrer F, tendorf-Hofmann A, Weidenbecher M, Sauer R. Transoral laser surgery of supraglottic cancer: follow-up of 141 patients. Arch Otolaryngol Head Neck Surg. 1998;124(11):1245–1250. doi: 10.1001/archotol.124.11.1245. [DOI] [PubMed] [Google Scholar]
- (10).Strong MS. Laser surgery in the larynx. Early clinical experience with continuous CO 2 laser. Ann Otol Rhinol Laryngol. 1972;81(6):791–798. doi: 10.1177/000348947208100606. [DOI] [PubMed] [Google Scholar]
- (11).Vaughan CW. Transoral laryngeal surgery using the CO2 laser: laboratory experiments and clinical experience. Laryngoscope. 1978;88(9):1399–1420. doi: 10.1002/lary.1978.88.9.1399. Pt 1. [DOI] [PubMed] [Google Scholar]
- (12).Watanabe E, Watanabe T, Manaka S, Mayanagi Y. Three-dimensional digitizer (neuronavigator): new equipment for computed tomography-guided stereotaxic surgery. Surg Neurol. 1987;27(6):543–547. doi: 10.1016/0090-3019(87)90152-2. [DOI] [PubMed] [Google Scholar]
- (13).Reinhardt H, Meyer H. A computer-assisted device for the intraoperative CT-correlated localization of brain tumors. Eur Surg Res. 1988;20(1):51–58. doi: 10.1159/000128741. [DOI] [PubMed] [Google Scholar]
- (14).Roberts DW, Strohbehn JW, Hatch JF, Murray W. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65(4):545–549. doi: 10.3171/jns.1986.65.4.0545. [DOI] [PubMed] [Google Scholar]
- (15).Mösges R. A new imaging method for intraoperative therapy control in skull-base surgery. Neurosurg Rev. 1988;11(3-4):245–247. doi: 10.1007/BF01741417. [DOI] [PubMed] [Google Scholar]
- (16).Schlöndorff G, Mösges R, Meyer-Ebrecht D, Krybus W. CAS (computer assisted surgery). Ein neuartiges Verfahren in der Kopf- und Halschirurgie [CAS (computer assisted surgery). A new procedure in head and neck surgery] HNO. 1989;37(5):187–190. [PubMed] [Google Scholar]
- (17).Banfai P, Kubik S. Our extra-scalar operating method of cochlear implantation. Experience with 46 cases. Acta Otolaryngol Suppl. 1984;411:9–12. doi: 10.3109/00016488409099535. [DOI] [PubMed] [Google Scholar]
- (18).Chouard CH. Implantation of multiple intracochlear electrodes for rehabilitation of total deafness: preliminary report. Laryngoscope. 1976;86(11):1743–1751. doi: 10.1288/00005537-197611000-00021. [DOI] [PubMed] [Google Scholar]
- (19).Roberson JB, Stidham KR, Scott KM. Cochlear implantation: minimal hair removal technique. Otolaryngol Head Neck Surg. 2000;122(5):625–629. doi: 10.1016/S0194-5998(00)70186-0. [DOI] [PubMed] [Google Scholar]
- (20).O'Donoghue GM. Minimal access surgery for pediatric cochlear implantation. Otol Neurotol. 2002;23(6):891–894. doi: 10.1097/00129492-200211000-00014. [DOI] [PubMed] [Google Scholar]
- (21).Kiratzidis T. 'Veria operation': cochlear implantation without a mastoidectomy and a posterior tympanotomy. A new surgical technique. Adv Otorhinolaryngol. 2000;57:127–130. doi: 10.1159/000059218. [DOI] [PubMed] [Google Scholar]
- (22).Kronenberg J, Migirov L. Suprameatal approach: new surgical approach for cochlear implantation. J Laryngol Otol. 2001;115(4):283–285. doi: 10.1258/0022215011907451. [DOI] [PubMed] [Google Scholar]
- (23).Labadie RF, Chodhury P, Cetinkaya E, et al. Minimally invasive, image-guided, facial-recess approach to the middle ear: demonstration of the concept of percutaneous cochlear access in vitro. Otol Neurotol. 2005;26(4):557–562. doi: 10.1097/01.mao.0000178117.61537.5b. [DOI] [PubMed] [Google Scholar]
- (24).Warren F, Balachandran R, Fitzpatrick J. Percutaneous Cochlear Access Using Bone-Mounted, Customized Drill Guides: Demonstration of Concept In Vitro. Otol Neurotol. 2007;28(3):325–9. doi: 10.1097/01.mao.0000253287.86737.2e. [DOI] [PubMed] [Google Scholar]
- (25).Al-Marzouqi H, Noble J, Warren F, Labadie R, Fitzpatrick J. Michael, Dawant B. Planning a safe drilling path for cochlear implantation surgery using image registration techniques. SPIE Conf. on Med. Im. 2007 Feb;8 28. [Google Scholar]
- (26).Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997 Apr;16(2):187–98. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- (27).Wells WM, III, Viola P, Atsumi H, Nakajima S, Kikinis R. Multi-modal Volume Registration by Maximization of Mutual Information. Medical Image Analysis. 1996;1(1):35–51. doi: 10.1016/s1361-8415(01)80004-9. [DOI] [PubMed] [Google Scholar]
- (28).Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G. In: Bizais Y, Barillot C, Di Paola R, editors. Automated multimodality medical image registration using information theory; Proc. 14th Int. Conf. Information Processing in Medical Imaging (IPMI’95); Ile de Berder, France. Jun, 1995. pp. 263–274. Computational Imaging and Vision. [Google Scholar]
- (29).Rohde Gustavo K., Aldroubi Akram, Dawant Benoit M. The adaptive bases algorithm for intensity-based nonrigid image registration. IEEE Trans. Med. Imag. 2003 Nov;22:1470–1479. doi: 10.1109/TMI.2003.819299. [DOI] [PubMed] [Google Scholar]
- (30).Noble JH, Warren FH, Labadie RF, Dawant BA. Proc. Medical Imaging. San Diego, CA: Feb, 2008. 2008. Automatic Segmentation of the Facial Nerve and Chorda Tympani Using Image Registration and Statistical Priors. [Google Scholar]
- (31).Balachandran R, Mitchell J, Dawant B, Fitzpatrick JM. Evaluation of targeting frames for deep-brain stimulation using virtual targets. IEEE International Symposium on Biomedical Imaging: From Nanao to Macro. 2007:1184–1187. [Google Scholar]
- (32).Schipper J, Klenzner T, Aschendorff A, Arapakis I, Ridder GJ. Navigiert-kontrollierte Kochleostomie. Ist eine Verbesserung der Ergebnisqualitat in der Kochleaimplantatchirurgie möglich? [Navigation-controlled cochleostomy. Is an improvement in the quality of results for cochlear implant surgery possible?] HNO. 2004;52(4):329–335. doi: 10.1007/s00106-004-1057-5. [DOI] [PubMed] [Google Scholar]
- (33).Bhatia K, Gibbin KP, Nikolopoulos TP. Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otol Neurotol. 2004;25(5):730–739. doi: 10.1097/00129492-200409000-00015. [DOI] [PubMed] [Google Scholar]
- (34).Fayad JN, Wanna GB, Micheletto JN. Facial nerve paralysis following cochlear implant surgery. Laryngoscope. 2003;113(8):1344–1346. doi: 10.1097/00005537-200308000-00014. [DOI] [PubMed] [Google Scholar]
- (35).Cohen NL. Complications of cochlear implant surgery in adults and children. Ann Otol Rhinol Laryngol. 1991;100(9):708–711. doi: 10.1177/000348949110000903. Pt 1. [DOI] [PubMed] [Google Scholar]
- (36).Hoffman RA. Complications of cochlear implant surgery. Ann Otol Rhinol Laryngol Suppl. 1995;166:420–422. [PubMed] [Google Scholar]
- (37).Adunka OF, Radeloff A, Gstoettner WK, Pillsbury HC. Scala tympani cochleostomy II: Topography and histology. Laryngoscope. 2007 doi: 10.1097/MLG.0b013e3181453a53. in press. [DOI] [PubMed] [Google Scholar]
- (38).Buchman CA. Scala tympani cochleostomy I: Results of a Survey. Laryngoscope. 2007 doi: 10.1097/MLG.0b013e3181453a6c. in press. [DOI] [PubMed] [Google Scholar]
- (39).Roland PS, Wright GG, Issacson B. Cochlear implant electrode insertion: the round window revisited. Laryngoscope. 2007;117:1397–1402. doi: 10.1097/MLG.0b013e318064e891. [DOI] [PubMed] [Google Scholar]
- (40).Labadie RF, Carrasco VN, Gilmer CH, Pillsbury HC. Cochlear implant performance in older adults. Otolaryngol Head Neck Surg. 2000;123:419–24. doi: 10.1067/mhn.2000.109759. [DOI] [PubMed] [Google Scholar]
- (41).Schulze-Gattermann H, Illg A, Schoenermark M, Lenarz T&L-SA. Cost-benefit analysis of pediatric cochlear implantation: German experience. Otol Neurotol. 2002;23(5):674–681. doi: 10.1097/00129492-200209000-00013. [DOI] [PubMed] [Google Scholar]
- (42).O'Neill C. Cost-utility analysis of pediatric cochlear implantation. Laryngoscope. 2000;110(7):1239. doi: 10.1097/00005537-200007000-00035. [DOI] [PubMed] [Google Scholar]
- (43).Cheng AK, Rubin HR, Powe NR, Mellon NK, Francis HW. Cost-utility analysis of the cochlear implant in children. JAMA. 2000;284(7):850–856. doi: 10.1001/jama.284.7.850. [DOI] [PubMed] [Google Scholar]
- (44).Toner JG, John G. Cochlear implantation under local anaesthesia, the Belfast experience. J Laryngol Otol. 1998;112(6):533–536. doi: 10.1017/s0022215100141027. [DOI] [PubMed] [Google Scholar]
- (45).Djalilian HR, Roy S, Benson AG, Regala C, McDonald TB. Transcanal cochlear implantation under monitored anesthesia care. Otol Neurotol. 2005;26(4):674–677. doi: 10.1097/01.mao.0000178127.58859.e0. [DOI] [PubMed] [Google Scholar]