A combination of histological and molecular studies uncovers mechanisms of root hair development in monocots.
Abstract
A priority in many crop improvement programs for a long time has been to enhance the tolerance level of plants to both abiotic and biotic stress. Recognition that the root system is the prime determinant of a plant’s ability to extract both water and minerals from the soil implies that its architecture is an important variable underlying a cultivar’s adaptation. The density and/or length of the root hairs (RHs) that are formed are thought to have a major bearing on the plant’s performance under stressful conditions. Any attempt to improve a crop’s root system will require a detailed understanding of the processes of RH differentiation. Recent progress in uncovering the molecular basis of root epidermis specialization has been recorded in the grasses. This review seeks to present the current view of RH differentiation in grass species. It combines what has been learned from molecular-based analyses, histological studies, and observation of the phenotypes of both laboratory- and field-grown plants.
WHY ARE INVESTIGATIONS OF ROOT HAIR DEVELOPMENT IMPORTANT?
The root epidermis houses two distinct cell types: trichoblasts, which produce tubular outgrowths that are called root hairs (RHs), and atrichoblasts, which do not. It has been postulated for about a century that RHs are important for plant growth and development, since they are intimately involved in the uptake of both soil water and mineral nutrients, in the anchoring of the root as it penetrates the soil, and in interactions with the soil microfauna (Comber, 1922). The root system of an individual cereal rye (Secale cereale) plant develops up to 14 billion RHs, thereby increasing its root surface area by some 400 m2 (Dittmer, 1937). This large increase in the extent of the contact between the root system and the soil is postulated to have a major impact on the plant’s capacity to take up both water and minerals (Richardson et al., 2011; Brown et al., 2012; Haling et al., 2014). A number of indications have emerged that the RHs are important for the ability of grass species to maintain a sufficient level of water and nutrient acquisition when challenged by drought and/or a nutrient deficiency. The participation of the RHs in the uptake of phosphorus (P) has been identified in barley (Hordeum vulgare), wheat (Triticum aestivum), maize (Zea mays), and bermudagrass (Cynodon dactylon; Green et al., 1991; Gahoonia et al., 1997; Brown et al., 2013b). In barley (Brown et al., 2012) and maize (Zhu et al., 2010), low P availability induces an increase in RH length, which is assumed to boost the efficiency of P uptake (Haling et al., 2013). Under P-limiting conditions, up to 90% of the mineral appears to be taken up by the RHs in different plant species (Fohse et al., 1991), a proportion that readily explains the major difference in the P acquisition capacity between barley accessions that form short as opposed to long RHs (Gahoonia and Nielsen, 2004; Brown et al., 2012). The importance of RHs for both cadmium and zinc acquisition has been illustrated by comparing the performance of the barley root-hairless mutant bald root barley (brb) with that of its parent cv Pallas. When wild-type and mutant plants are provided with sufficient zinc, both shoot dry matter and shoot zinc content are indistinguishable, but significant differences in performance set in once the level of zinc becomes limited (Genc et al., 2007). Similarly, the brb mutant is less able to take up cadmium, which leads to the conclusion that up to 45% of the uptake occurs through the RHs (Zheng et al., 2011).
Since RHs significantly increase root surface area, their role in water uptake is postulated by many authors; however, there is little experimental evidence to confirm this hypothesis. Segal et al. (2008) showed that only the tip domain of the RH is directly involved in water uptake in barley. The water potential of the soil lodged between adjacent RHs of cv Pallas has been shown to become equilibrated with that inside the root tissues within 1 min, after which time water uptake ceases (Segal et al., 2008). The same authors further showed that the efficiency of water uptake is 55% higher in wild-type roots than in those of the hairless mutant brb, thus reflecting the large root surface available for water uptake. On the other hand, no water-deficient features were observed for the root-hairless mutants rhl1.a to rhl1.c when plants were grown under optimal conditions in a growth chamber (Chmielewska et al., 2014), giving the assumption that RHs are not essential for water uptake in well-watered soil. RH elongation is more pronounced in plants that are grown in dry soil than in those that do not experience any moisture stress. However, under combined drought and P-deficiency stresses, barley mutants impaired in RH growth showed a limitation in agronomy-important features, such as grain weight, number of grains, number of tillers, and aboveground biomass, in comparison with wild-type plants (Brown et al., 2012). It has been demonstrated that water availability may influence the length of the RHs. In the case of mutants with short RHs grown under drought conditions, the length of the RH was increased slightly, although it never reached the value observed in the wild type (Haling et al., 2014). The overall conclusion is that the increase of RH length may be an adaptive trait to drought stress, similar to nutrient deficiency.
In the dicotyledonous species soybean (Glycine max), proteomic analysis has identified that many transporters are involved in the uptake of water and mineral nutrients (Brechenmacher et al., 2012), and a similar scenario applies to Arabidopsis (Arabidopsis thaliana), where a root-hairless transgenic line, NR23, overexpressing the 23-residue N-terminal domain of the plasma membrane-associated Ca2+-BINDING PROTEIN2 showed 47% reduction in water uptake under normal conditions and exhibited less drought tolerance in comparison with wild-type plants (Tanaka et al., 2014). The indications are that any enhancement in RH function may offer a potential route to improving the productivity and stress tolerance of crops that are grown in poor soils (Meister et al., 2014). Last, but not least, the root epidermis of grass species represents a useful model for cell differentiation (Marzec and Kurczynska, 2014).
“TO BE OR NOT TO BE, THAT IS THE QUESTION”: ROOT EPIDERMIS PATTERNING
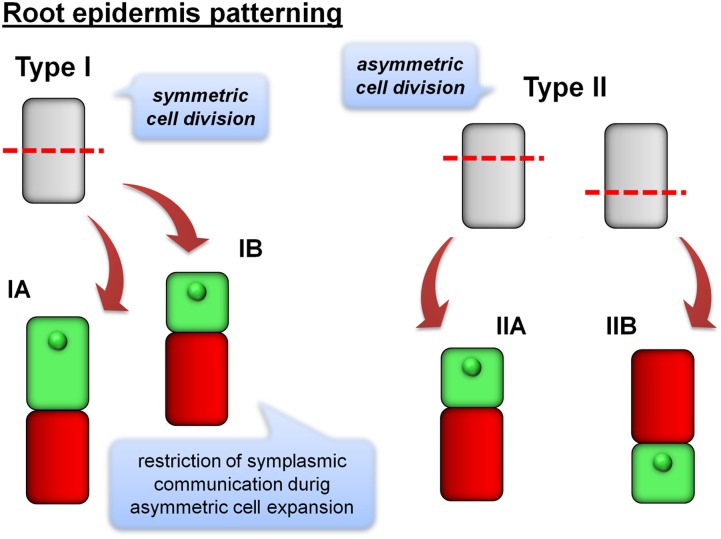
RH development is conventionally regarded as a three-phase process: the first stage sees the establishment of root epidermis patterning, which involves a population of trichoblasts and atrichoblasts; the second comprises the formation of the bulge on the root surface, presaging the formation of an RH tube; and finally, the RH tip begins to grow. The current understanding of root epidermis patterning in dicotyledonous plant species is largely based on the behavior of Arabidopsis, in which trichoblasts and atrichoblasts are arranged in rows. The key regulators of cell fate have been identified (Bruex et al., 2012; Grebe, 2012). There are three types of rhizodermis patterning in plants: (1) all epidermal cells may produce RHs; (2) trichoblasts and atrichoblasts are present in each rhizodermal cell file; and (3) cell files composed only of trichoblasts and atrichoblasts are present (Dolan, 1996). At least two modes of patterning have been documented in the grasses: in the first, every epidermal cell is capable of differentiating into an RH, while in the other, it is exclusively the shorter cells that are endowed with the dense cytoplasm that later emerge as trichoblasts (Clowes, 2000; Kim and Dolan, 2011). Marzec et al. (2014a) have proposed a more nuanced classification of cell epidermis patterning, which is based on observations of the final division of the epidermal cell. In this scheme, type I cells describe those in which the last division is symmetrical. Two type I subtypes can be distinguished: in subtype IA cells, trichoblasts can only be distinguished from atrichoblasts by the presence/absence of an RH tube, while subtype IB cells first divide symmetrically but then expand asymmetrically, with the larger daughter cells differentiating into trichoblasts and the smaller ones into atrichoblasts. In type II cells, the last division is asymmetrical: subtype IIA trichoblasts end up on the upper (shoot-ward) side, while subtype IIB trichoblasts finish on the lower (root-ward) side (Fig. 1).
Figure 1.
Two types of the establishment of root epidermis patterning in monocots. Type I is characterized by an initial symmetric division of a mother cell. In subtype IA, the two identical daughter cells do not show any differences, except for the presence or absence of an RH tube. In subtype IB, the two daughter cells develop into trichoblasts and atrichoblasts through asymmetric expansion, which correlates with a restriction of symplasmic communication between neighboring cells. In type II, the last division is asymmetric. A shorter cell may be located in the shoot-ward (subtype IIA) or root-ward (subtype IIB) position.
The question of which genes are responsible for root epidermis patterning in grass species still remains open. Histological analyses of the root epidermis in barley, Brachypodium distachyon, and rice (Oryza sativa) have demonstrated some distinct species differences, thus indicating that the process is not rigidly conservative. In barley and rice, following the symmetric cell division, an as yet unidentified signal directs daughter cell differentiation (Kim and Dolan, 2011; Marzec et al., 2013), while in B. distachyon, certain extrinsic factors, which appear to be activated prior to the last cell division, exert control over daughter cell identity (Kim and Dolan, 2011). The implication is that any attempt to elucidate the mechanism based on the early stages of RH development should focus on the factors that are related to asymmetric cell division and expansion. A restriction in symplasmic communication is observed between neighboring cells during plant cell differentiation (Marzec and Kurczynska, 2014). In wild-type barley, symplasmic communication is limited to the root zone in which cells start to develop, whereas in the root-hairless mutant rhl1.b, all epidermal cells, even those in the mature zone of the root, remain interconnected; the absence of callose deposits in the plasmodesmata has been suggested as the basis for this difference (Marzec et al., 2014b). Apart from the allelic mutants that have been isolated in barley (Gahoonia et al., 2001; Chmielewska et al., 2014), hairless mutants have not as yet been obtained in the grasses. In rice and maize, some of the mutants that were classified previously as root hairless in fact develop very short RHs, while others exhibit bulges on the root that fail to develop into a recognizable RH (Hochholdinger et al., 2008; Yuo et al., 2009, 2011).
“RETURNING WERE AS TEDIOUS AS GO O’ER”: RH INITIATION
Although root epidermis patterning differs between species, the staging of RH differentiation, beginning with bulge formation and ending with the elongation of the RHs, is universal (Fig. 2). The formation of the RH bulge has been well researched in Arabidopsis (Galway et al., 2011; Pei et al., 2012), but among the grasses, only a handful of mutants in which RH development has been arrested at this stage have been described. Even in these, the RH bulge is still present, as the mutant phenotype results from the inhibition of RH elongation (Szarejko et al., 2005). Only one mutant (rth3 in maize) represents a genuine disturbance of bulge formation (Wen and Schnable, 1994). The target of this mutation encodes a protein that belongs to the grass clade of COBRA proteins (Hochholdinger et al., 2008). They harbor a glycosylphosphatidylinositol anchor that is attached to the C terminus and are thought to be involved in posttranslational modification and intracellular trafficking (Fujita and Kinoshita, 2012). The up-regulation of RTH3 in young primary roots and the localization of its transcription to the trichoblasts are consistent with the participation of these COBRA-like proteins in RH differentiation. Their mechanism of action remains unclear, since RTH3 transcription can be detected almost throughout the maize plant, whereas at the phenotypic level, the mutant and the wild type only differ from one another with respect to RH development. The proposition is that the RTH3 protein contributes to cell wall synthesis and cell expansion and acts as a regulator of a small group of RH-specific genes (Hochholdinger et al., 2008).
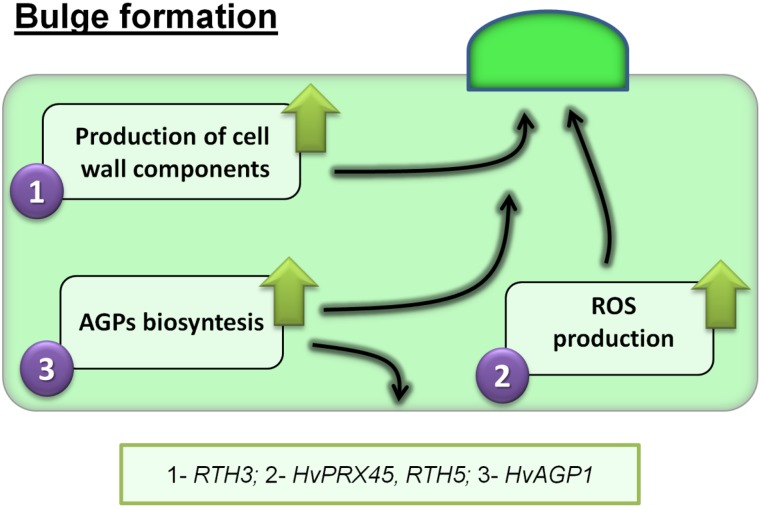
Figure 2.
Mechanisms and genes that are involved in the formation of the RH bulge in monocots. This stage of development is related to three processes: (1) increased biosynthesis of cell wall components and their transport to the forming bulge; (2) higher activity of the enzymes that produce reactive oxygen species (ROS); and (3) production and subcellular transport of arabinogalactan proteins (AGPs).
Ten other genes that are potentially involved in RH development in barley were identified via a transcriptomic comparison between cv Karat and the rhl1.a mutant. Three of these encode peroxidases, two xyloglucan endotransglycosylases, one an arabinogalactan protein, one an extensin, one a Leu-rich repeat protein, one a phosphatidylinositol phosphatidylcholine transfer protein, and the last a RhoGTPase GDP dissociation inhibitor (Kwasniewski et al., 2010). The transcript abundance of all 10 genes is lower in the mutant than in the wild-type plant, while there is no transcriptional difference between the wild type and the rhp1.b mutant that produces an RH bulge. The products of all of these genes are related to either the cell wall or the plasma membrane, modifications to which may be key during bulge formation. The involvement of peroxidases in bulge formation could be confirmed by the detection of unusually high levels of ROS in the trichoblasts, especially in the cell wall of a bulge and at the tip of a growing RH (Kwasniewski et al., 2013a). Furthermore, roots harvested from seedlings that are grown in a medium containing an inhibitor of peroxidase develop a much lower density of RHs. Finally, in situ mRNA hybridization experiments have shown that the transcription of HvPEROXIDASE45 is restricted to individual root epidermal cells (Kwasniewski et al., 2013a). In maize, the gene RTH5 encodes a grass species-specific NADPH oxidase; its loss-of-function mutation resulted in a marked drop in both RH density and RH length (Nestler et al., 2014). The phenotype can be related to a reduced accumulation of ROS in the trichoblasts. Thus, it would appear that ROS, along with the enzymes that are required for their production, are necessary for RH differentiation to occur in the grasses, as is also the case in Arabidopsis (Huang et al., 2013a; Sundaravelpandian et al., 2013).
The possible involvement of AGPs in an early stage of RH development in barley, which is based on their nonhomogenous distribution in root epidermal cells at an early stage of specialization, was recently posited by Marzec et al. (2015). Treatment with a reagent that binds to all classes of AGP, and thereby strips the root surface of functional AGPs, has been shown to suppress RH expansion. The AGPs that are present in the extracellular matrix are believed to affect the organization of the cortical microtubules that are involved in epidermal cell elongation (Nguema-Ona et al., 2007), whereas periplasmic AGPs may function as calcium capacitors (Lamport and Várnai, 2013). Considering the established role of AGPs in pollen tube elongation (Nguema-Ona et al., 2012), the proposition is that the AGPs are important for normal cell tip growth in barley and, hence, for all stages of RH development (Marzec et al., 2015).
“TIS WITHIN OURSELVES THAT WE ARE THUS OR THUS”: ELONGATION OF THE RH TUBE
Studies of tip growth have featured strongly in analyses of RH development in the grasses (Fig. 3). Tip growth follows the elongation of the RHs or the pollen tubes (Rounds and Bezanilla, 2013). The establishment of a gradient in both ROS and Ca2+ is required to determine the direction of cell expansion, the reorganization of the cytoskeleton, and the vesicular transport of the cell wall components to the tip (Ketelaar, 2014; Zhou et al., 2014). Since rapid cell elongation requires a sufficient supply of cell wall building blocks, specifically cellulose, hemicellulose, and pectin, any distortion in their synthesis or transport will inevitably result in growth inhibition (Chebli et al., 2013). The earliest reported involvement of cellulose synthase in RH elongation in the grasses relates to the product of OsCSLD1, a rice gene that encodes a cellulose synthase-like D1 protein (Kim et al., 2007). This gene is expressed throughout the plant as well as in trichoblasts, atrichoblasts, and root cortex cells (Yuo et al., 2011). A Dissociation element (Ds) insertion mutant suffered a 65% to 75% reduction in RH length but no difference in RH density or distribution, which was taken to imply a role for OsCSLD1 in RH elongation. Confirmation was obtained from the behavior of an OsCSLD1 overexpressor, which produced RHs that were double the length of those developed by wild-type plants (Kim et al., 2007). A second rice mutant, termed rth2, was shown to be a loss-of-function allele of OsCSLD1 (Yuo et al., 2011). Unlike those of the Ds insertion mutant, rth2 roots fail to develop bulges on the root surface. The gene copy in the former line is disrupted by the presence of a transposon close to the 3′ end of the first exon, which results in the production of a nonstandard transcript; in the latter, a premature stop codon is created close to the 5′ end of same exon. A similar situation occurs in Arabidopsis, where a mutated form of the ubiquitously expressed gene CSLD3 severely inhibits RH elongation, although it does not compromise the formation of the bulge (Wang et al., 2001). Defects in OsCLDS1 or AtCSLD3 are generally complemented by various paralogs, although this is not the case for the development of RHs (Yuo et al., 2011). Moreover, a mutation in the OsXXT1 gene, encoding xyloglucan 6-xylosytransferase, results in abnormal RH elongation. The product of OsXXT1 is involved in establishing the cellulose-xyloglucan network, and a mutant phenotype indicates that this network is crucial in the cell wall formation during RH elongation (Wang et al., 2014).
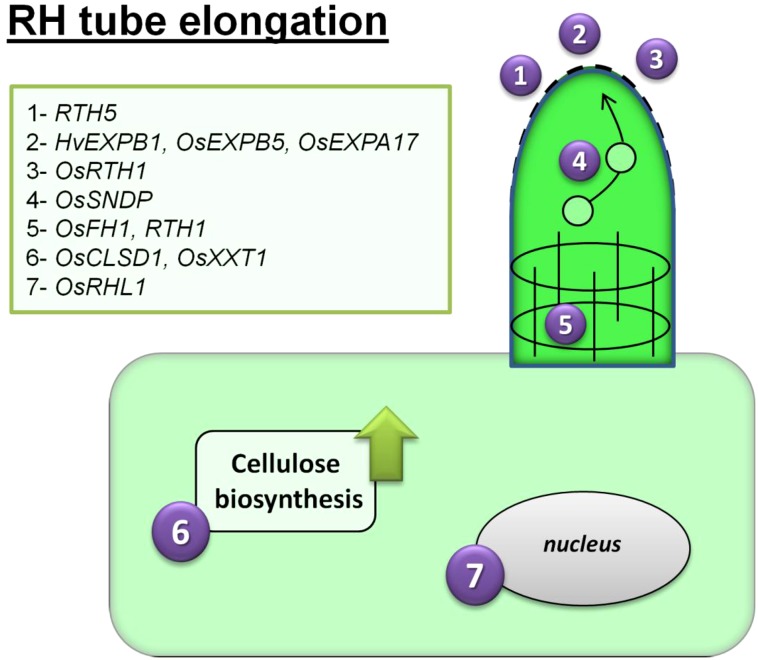
Figure 3.
Scheme of RH tube elongation in monocots. The following seven processes are involved in tip growth: (1) loosening of the cell wall by expansins; (2) vesicle transport of cell wall components such as cellulose to the tube tip; (3) extracellular ATP hydrolysis by apyrases, which leads to their accumulation on the RH tip; (4) and (5) reorganization of the cytoskeleton is related to vesicle transport and tube elongation; (6) induction of cellulose synthesis, its transport to the growing RH tube, and formation of the cellulose-xyloglucan network; and (7) a transcription factor with a basic helix-loop-helix (bHLH) domain is active in the nucleus of the RH cell.
The cell wall components that are required for RH elongation are transported to the growing tip via vesicle transport and exocytosis to the extracellular matrix (Lombardo and Lamattina, 2012). To date, the only RH gene that is related to exocytosis that has been identified in the grasses is RTH1, a maize gene that encodes a homolog of the subunit of exocyst3 (SEC3). While a mutation in this gene induces a major shortening of RH length, it has no effect on pollen tube elongation, thus suggesting an RH-specific function for RTH1 (Wen et al., 2005). In Arabidopsis, a mutation in the homolog EXO70A1 results in a negative pleiotropic effect on both RH length and plant organ size (Synek et al., 2006).
A second major group of proteins that have been determined to be related to tip growth are the expansins, which help to loosen the cell wall during tube elongation (Gu and Nielsen, 2013). Three expansins, which are encoded by HvEXPB1 (Kwasniewski and Szarejko, 2006), OsEXPB5, and OsEXPA17 (Won et al., 2010; ZhiMing et al., 2011), have been proven to be involved in RH initiation and elongation. Mutant analysis showed that the HvEXPB1 transcript is present in the root but not in the shoot (Kwasniewski and Szarejko, 2006). The RH-specific cis-element features in the promoters of both HvEXPB1 and OsEXPB5. Promoter experiments have demonstrated that both are active in an RH-specific manner when they are expressed in either Arabidopsis or rice and, more specifically, both at an early stage of bulge formation and during RH elongation (Won et al., 2010). A rice line that produced RHs that were 70% shorter than those of the wild type has been shown to harbor a single nucleotide variant for OsEXPA17. This gene is specifically transcribed in the RH, and its promoter also includes an RH-specific cis-element (ZhiMing et al., 2011). The mutant phenotype proved to be partially reversible by constitutively expressing OsEXPA30, which is a gene encoding an RH-specific expansin. A recent report showed that the overexpression of OsEXPA8 increases the length of rice RHs, primary roots, and the number of lateral roots that are formed (Ma et al., 2013).
In addition to the contribution of cellulose synthases and expansins to RH tip growth, some regulatory genes have also been identified. Among these is OsRHL1, a rice gene encoding a bHLH transcription factor, the overexpression of which increases RH length (Ding et al., 2009). Apart from producing foreshortened RHs, no other discernible phenotype could be associated with its loss of function. The gene is transcribed strictly in the trichoblasts, but surprisingly, its product localizes to the nucleus, thereby indicating a regulatory function. The participation of the bHLH transcription factors in RH elongation was also noted in both Lotus japonicus (LjRHL1) and Arabidopsis (AtLRL1 to AtLRL3; Karas et al., 2009). Mutants for each of the three Arabidopsis genes are associated with an RH phenotype that is similar to that of Osrhl1, which is involved in the inhibition of RH development at the bulge stage. LjRHL1 deposition is restricted to the root epidermis nucleus, which has been taken to indicate a universal bHLH-mediated mechanism for the regulation of RH elongation (Karas et al., 2009).
Analysis of another rice mutant that develops greatly foreshortened RHs led to the identification of OsRTH1 (encoding an apyrase), the product of which exerts a pleiotropic effect over plant stature, seminal root length, and the outgrowth of root bulges. The ATP content of the mutant’s roots is double that present in the wild-type root (Yuo et al., 2009), a finding that may explain the disruption to RH tip growth, since some of the ATP that is hydrolyzed by apyrase activity is used for cell growth (Roux and Steinebrunner, 2007; Wu et al., 2007). In Arabidopsis, barrel medic (Medicago truncatula), and wheat, ATP was localized within the extracellular matrix of the tip of a growing tube (Kim et al., 2006). The proposition is that an unequal distribution over the RH surface of this ATP is essential for the establishment of the ROS and Ca2+ gradients that are required for RH elongation (Choi et al., 2014).
Phosphoinositide is a particularly important signaling molecule in the context of RH elongation. Disorders in its metabolism can lead to defective RH development, including either their foreshortening and/or branching (Heilmann, 2009; Yoo et al., 2012). Although the involvement of phosphoinositide in RH development has been well studied in Arabidopsis (Kusano et al., 2008; Stenzel et al., 2008), to date, only one gene, rice Sec14-Nodulin Domain-Containing Protein1 (OsSNDP1), has been implicated in this process in the grasses. This gene encodes a phosphatidylinositol transfer protein (Huang et al., 2013b). As in the Arabidopsis Sec fourteen homologs1 mutant (Vincent et al., 2005), the RHs that are formed by the Ossndp1 mutant are shorter than those of the wild type and frequently form branches. This phenotype suggests a severe disorder with respect to polar growth and probably reflects cytoskeleton disorganization and/or a disturbed vesicle transport. Since the overexpression of OsSNDP1 has no impact on RH length, the likelihood is that its product is not directly involved in RH elongation but, rather, in the determination of the direction of tube expansion (Huang et al., 2013b).
A final group of proteins that are implicated in RH development in the grasses is the formins. These proteins are involved in cell division and organ expansion as well as in tip growth (Yang et al., 2011; Wang et al., 2012). They are also important for actin polymerization and, thus, in the organization of the cytoskeleton (van Gisbergen and Bezanilla, 2013). An analysis of a short RH rice mutant revealed a point mutation in the gene rice Formin Homology1 (OsFH1), which encodes a formin-like protein (Huang et al., 2013c). The expression of a mutant phenotype is environmentally dependent, however. In plants that are raised in a liquid medium, the RH of the mutant is somewhat shorter than that of the wild type, but when the seedlings are raised on a solidified medium, this difference disappears. Treatment with either auxin or ethylene, or the exposure of the plants to either P or iron starvation, has no effect on Osfh1 RH length. It has been suggested that the roots of the mutants may be more sensitive to oxygen depletion than the wild-type roots, a condition that tends to prevail in liquid cultures (Huang et al., 2013c). Nevertheless, this is, to our knowledge, the first report of a mutation in a gene that is associated with formin affecting RH elongation; in doing so, it confirms the importance of cytoskeleton organization in this process.
WHAT DO WE KNOW?
A focus on root traits has been a feature of research that is associated with the breeding of wheat (Wasson et al., 2012), barley (Brown et al., 2013a; Haling et al., 2013), and maize (Bayuelo-Jiménez et al., 2011). Simultaneously, attempts have been made to uncover the molecular basis of RH development in the grasses at both the transcriptomic (Kwasniewski et al., 2010) and proteomic (Nestler et al., 2011; Janiak et al., 2012) levels. Mutants have provided an invaluable means for identifying the key genes that are involved in the various stages of RH formation as well as to validate conclusions that are based on large-scale analyses (Chmielewska et al., 2014). Meanwhile, histological analyses of cell modifications during RH development have led to a better understanding of the differentiation process (Kim and Dolan, 2011; Marzec et al., 2013, 2014b). The use of confocal laser scanning, transmission, and scanning electron microscopy has enabled a number of insights to be gained into how the RHs behave under controlled experimental conditions. A more novel development is the deployment of synchrotron radiation x-ray tomographic microscopy to investigate RH function under natural conditions (Keyes et al., 2013). Gradually, a fuller picture of the RH differentiation process is being assembled (Table I).
Table I. Genes that are involved in RH development in monocots.
| Gene | Encoded Protein | Protein Function | Species | Mutant | References |
|---|---|---|---|---|---|
| Stage of RH development: bulge formation | |||||
| RTH3 | COBRA-like protein | Cell expansion, cell wall components, biosynthesis | Maize | + | Hochholdinger et al. (2008) |
| HvPRX45 | Peroxidase | ROS production | Barley | − | Kwasniewski et al. (2013a) |
| Stage of RH development: tip growth | |||||
| RTH5 | NAPDH oxidase | Establishment of a high level of ROS in RH tips | Maize | + | Nestler et al. (2014) |
| HvEXPB1 | Expansin | Cell wall loosening | Barley | − | Kwasniewski and Szarejko (2006); Won et al. (2010) |
| OsEXPB5 | Expansin | Cell wall loosening | Rice | − | Won et al. (2010) |
| OsEXPA17 | Expansin | Cell wall loosening | Rice | + | ZhiMing et al. (2011) |
| OsRTH1 | Apyrase | Hydrolyzation of extracellular ATP | Rice | + | Yuo et al. (2009) |
| OsSNDP | Sec14-nodulin domain-containing protein | Vesicle transport and organization of the cytoskeleton | Rice | + | Huang et al. (2013c) |
| RTH1 | Exocyst subunit SEC3 | Polar exocytosis | Maize | + | Wen et al. (2005) |
| OsFH1 | Formin-like protein | Polymerization of the actin cytoskeleton | Rice | + | Huang et al. (2013b) |
| OsCLSD1 | Cellulose synthase-like D1 protein | Synthesis of cellulose | Rice | + | Kim et al. (2007); Yuo et al. (2011) |
| OsXXT1 | Xyloglucan 6-xylosytransferase | Formation of the cellulose-xyloglucan network | Rice | + | Wang et al. (2014) |
| OsRHL1 | bHLH transcription factor | Function unknown | Rice | + | Ding et al. (2009) |
WHAT WE STILL NEED TO KNOW
Although the last few years have seen significant progress being made in uncovering the mechanisms that are involved in RH differentiation in the grasses, major knowledge gaps still persist, especially as compared with the much fuller information that is available for Arabidopsis, in which 138 genes related to various stages of RH development have already been identified (www.iroothair.org; Kwasniewski et al., 2013b). Some commonalities have been established between monocotyledonous and dicotyledonous species, but major differences in the initial rhizodermis specialization step remain. While 21 genes that are involved in the production of trichoblasts and atrichoblasts have been defined in Arabidopsis, the number of grass species genes that are related to the early stage of rhizodermis differentiation is still zero. Additionally, there appear to be significant differences between the various grasses with respect to trichoblast/atrichoblast formation. At present, recognition of the molecular mechanisms that underlie rhizodermis patterning in grasses is the most pressing research priority. Achieving this should be possible through a combination of molecular, cytological, and histochemical studies that are targeted at RH mutants.
CONCLUSION
Continued progress in crop plant improvement implies the development of cultivars that are better adapted to drought stress and low soil fertility. Given that the roots represent the front line for both water and nutrient uptake, it is logical to focus resources on improving the root system (Lynch, 2011; White et al., 2013). While root traits are not easy to quantify, both the density and length of RHs are readily measurable and are likely to be highly correlated with a plant’s ability to take up water and minerals from the soil (Brown et al., 2013a; George et al., 2014). Thus, a greater knowledge of the molecular basis of RH differentiation offers the potential to make significant advances that will be relevant to growing the crops of the future. At the same time, the importance of a plant’s performance in a field situation should not be underestimated. Recent data indicate that, in some cases, the perceived genetic differences in RH length, which were concluded from laboratory-based experiments, can be modified when the plants are exposed to drought stress or nutrient deficiency (Haling et al., 2013, 2014). The take-home message is that the development of improved cultivars cannot be based on the performance of single mutants or on the modification of single genes or proteins, unless perhaps the gene/protein that is concerned plays a regulatory role over root epidermis differentiation.
Glossary
- RH
root hair
- P
phosphorus
- ROS
reactive oxygen species
- AGP
arabinogalactan protein
- bHLH
basic helix-loop-helix
Footnotes
This work was supported by the Polish National Science Center (grant nos. 2011/01/M/NZ2/02979 and 2013/08/T/NZ3/00811), the European Union (FP7 project EUROOT grant no. 289300), and the Foundation for Polish Science (grant no. START 071.2014).
References
- Bayuelo-Jiménez JS, Gallardo-Valdéz M, Pérez-Decelis VA, Magdaleno-Armas L, Ochoa I, Lynch JP (2011) Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res 121: 350–362 [Google Scholar]
- Brechenmacher L, Nguyen TH, Hixson K, Libault M, Aldrich J, Pasa-Tolic L, Stacey G (2012) Identification of soybean proteins from a single cell type: the root hair. Proteomics 12: 3365–3373 [DOI] [PubMed] [Google Scholar]
- Brown LK, George TS, Barrett GE, Hubbard SF, White PJ (2013a) Interactions between root hair length and arbuscular mycorrhizal colonisation in phosphorus deficient barley (Hordeum vulgare). Plant Soil 372: 195–205 [Google Scholar]
- Brown LK, George TS, Dupuy LX, White PJ (2013b) A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Ann Bot (Lond) 112: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, George TS, Thompson JA, Wright G, Lyon J, Dupuy L, Hubbard SF, White PJ (2012) What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann Bot (Lond) 110: 319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruex A, Kainkaryam RM, Wieckowski Y, Kang YH, Bernhardt C, Xia Y, Zheng X, Wang JY, Lee MM, Benfey P, et al. (2012) A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet 8: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Kroeger J, Geitmann A (2013) Transport logistics in pollen tubes. Mol Plant 6: 1037–1052 [DOI] [PubMed] [Google Scholar]
- Chmielewska B, Janiak A, Karcz J, Guzy-Wrobelska J, Forster BP, Nawrot M, Rusek A, Smyda P, Kedziorski P, Maluszynski M, et al. (2014) Morphological, genetic and molecular characteristics of barley root hair mutants. J Appl Genet 55: 433–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G (2014) Identification of a plant receptor for extracellular ATP. Science 343: 290–294 [DOI] [PubMed] [Google Scholar]
- Clowes FAL. (2000) Pattern in root meristem development in angiosperms. New Phytol 146: 83–94 [Google Scholar]
- Comber NM. (1922) The availability of mineral plant food. J Agric Sci 12: 363–369 [Google Scholar]
- Ding W, Yu Z, Tong Y, Huang W, Chen H, Wu P (2009) A transcription factor with a bHLH domain regulates root hair development in rice. Cell Res 19: 1309–1311 [DOI] [PubMed] [Google Scholar]
- Dittmer HJ. (1937) A quantitative study of the roots and root hairs of a winter rye plant (Secale cereale). Am J Bot 24: 417–420 [Google Scholar]
- Dolan L. (1996) Pattern in the root epidermis: an interplay of diffusible signals and cellular geometry. Ann Bot (Lond) 77: 547–553 [Google Scholar]
- Fohse D, Claassen N, Jungk A (1991) Phosphorus efficiency of plants. Plant Soil 132: 261–272 [Google Scholar]
- Fujita M, Kinoshita T (2012) GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta 1821: 1050–1058 [DOI] [PubMed] [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE (1997) Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 191: 181–188 [Google Scholar]
- Gahoonia TS, Nielsen NE (2004) Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 262: 55–62 [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A (2001) A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant Soil 235: 211–219 [Google Scholar]
- Galway ME, Eng RC, Schiefelbein JW, Wasteneys GO (2011) Root hair-specific disruption of cellulose and xyloglucan in AtCSLD3 mutants, and factors affecting the post-rupture resumption of mutant root hair growth. Planta 233: 985–999 [DOI] [PubMed] [Google Scholar]
- Genc Y, Huang CY, Langridge P (2007) A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J Exp Bot 58: 2775–2784 [DOI] [PubMed] [Google Scholar]
- George TS, Brown LK, Ramsay L, White PJ, Newton AC, Bengough AG, Russell J, Thomas WT (2014) Understanding the genetic control and physiological traits associated with rhizosheath production by barley (Hordeum vulgare). New Phytol 203: 195–205 [DOI] [PubMed] [Google Scholar]
- Grebe M. (2012) The patterning of epidermal hairs in Arabidopsis: updated. Curr Opin Plant Biol 15: 31–37 [DOI] [PubMed] [Google Scholar]
- Green RL, Beard JB, Oprisko MJ (1991) Root hairs and root lengths in nine warm-season turfgrass genotypes. J Am Soc Hortic Sci 116: 965–969 [Google Scholar]
- Gu F, Nielsen E (2013) Targeting and regulation of cell wall synthesis during tip growth in plants. J Integr Plant Biol 55: 835–846 [DOI] [PubMed] [Google Scholar]
- Haling RE, Brown LK, Bengough AG, Valentine TA, White PJ, Young IM, George TS (2014) Root hair length and rhizosheath mass depend on soil porosity, strength and water content in barley genotypes. Planta 239: 643–651 [DOI] [PubMed] [Google Scholar]
- Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS (2013) Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. J Exp Bot 64: 3711–3721 [DOI] [PubMed] [Google Scholar]
- Heilmann I. (2009) Using genetic tools to understand plant phosphoinositide signalling. Trends Plant Sci 14: 171–179 [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Wen TJ, Zimmermann R, Chimot-Marolle P, da Costa e Silva O, Bruce W, Lamkey KR, Wienand U, Schnable PS (2008) The maize (Zea mays L.) roothairless 3 gene encodes a putative GPI-anchored, monocot-specific, COBRA-like protein that significantly affects grain yield. Plant J 54: 888–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GQ, Li E, Ge FR, Li S, Wang Q, Zhang CQ, Zhang Y (2013a) Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol 200: 1089–1101 [DOI] [PubMed] [Google Scholar]
- Huang J, Kim CM, Xuan YH, Liu J, Kim TH, Kim BK, Han CD (2013b) Formin homology 1 (OsFH1) regulates root-hair elongation in rice (Oryza sativa). Planta 237: 1227–1239 [DOI] [PubMed] [Google Scholar]
- Huang J, Kim CM, Xuan YH, Park SJ, Piao HL, Je BI, Liu J, Kim TH, Kim BK, Han CD (2013c) OsSNDP1, a Sec14-nodulin domain-containing protein, plays a critical role in root hair elongation in rice. Plant Mol Biol 82: 39–50 [DOI] [PubMed] [Google Scholar]
- Janiak A, Piórko S, Matros A, Mock HP, Kwaśniewski M, Chwiałkowska K, Chmielewska B, Szarejko I (2012) A comparative analysis of proteins that accumulate during the initial stage of root hair development in barley root hair mutants and their parent varieties. J Appl Genet 53: 363–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas B, Amyot L, Johansen C, Sato S, Tabata S, Kawaguchi M, Szczyglowski K (2009) Conservation of lotus and Arabidopsis basic helix-loop-helix proteins reveals new players in root hair development. Plant Physiol 151: 1175–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar T. (2014) Live cell imaging of Arabidopsis root hairs. Methods Mol Biol 1080: 195–199 [DOI] [PubMed] [Google Scholar]
- Keyes SD, Daly KR, Gostling NJ, Jones DL, Talboys P, Pinzer BR, Boardman R, Sinclair I, Marchant A, Roose T (2013) High resolution synchrotron imaging of wheat root hairs growing in soil and image based modelling of phosphate uptake. New Phytol 198: 1023–1029 [DOI] [PubMed] [Google Scholar]
- Kim CM, Dolan L (2011) Root hair development involves asymmetric cell division in Brachypodium distachyon and symmetric division in Oryza sativa. New Phytol 192: 601–610 [DOI] [PubMed] [Google Scholar]
- Kim CM, Park SH, Je BI, Park SH, Park SJ, Piao HL, Eun MY, Dolan L, Han CD (2007) OsCSLD1, a Cellulose Synthase-Like D1 gene, is required for root hair morphogenesis in rice. Plant Physiol 143: 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Sivaguru M, Stacey G (2006) Extracellular ATP in plants: visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol 142: 984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H, Testerink C, Vermeer JE, Tsuge T, Shimada H, Oka A, Munnik T, Aoyama T (2008) The Arabidopsis phosphatidylinositol phosphate 5-kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 20: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasniewski M, Chwialkowska K, Kwasniewska J, Kusak J, Siwinski K, Szarejko I (2013a) Accumulation of peroxidase-related reactive oxygen species in trichoblasts correlates with root hair initiation in barley. J Plant Physiol 170: 185–195 [DOI] [PubMed] [Google Scholar]
- Kwasniewski M, Janiak A, Mueller-Roeber B, Szarejko I (2010) Global analysis of the root hair morphogenesis transcriptome reveals new candidate genes involved in root hair formation in barley. J Plant Physiol 167: 1076–1083 [DOI] [PubMed] [Google Scholar]
- Kwasniewski M, Nowakowska U, Szumera J, Chwialkowska K, Szarejko I (2013b) iRootHair: a comprehensive root hair genomics database. Plant Physiol 161: 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasniewski M, Szarejko I (2006) Molecular cloning and characterization of β-expansin gene related to root hair formation in barley. Plant Physiol 141: 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport DT, Várnai P (2013) Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol 197: 58–64 [DOI] [PubMed] [Google Scholar]
- Lombardo MC, Lamattina L (2012) Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J Exp Bot 63: 4875–4885 [DOI] [PubMed] [Google Scholar]
- Lynch JP. (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Wang Y, Qiu S, Kang Z, Che S, Wang G, Huang J (2013) Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS ONE 8: e75997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Kurczynska E (2014) Importance of symplasmic communication in cell differentiation. Plant Signal Behav 9: e27931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Melzer M, Szarejko I (2013) Asymmetric growth of root epidermal cells is related to the differentiation of root hair cells in Hordeum vulgare (L.). J Exp Bot 64: 5145–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Melzer M, Szarejko I (2014a) The evolutionary context of root epidermis cell patterning in grasses (Poaceae). Plant Signal Behav 9: e27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Muszynska A, Melzer M, Sas-Nowosielska H, Kurczynska EU (2014b) Increased symplasmic permeability in barley root epidermal cells correlates with defects in root hair development. Plant Biol (Stuttg) 16: 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Szarejko I, Melzer M (2015) Arabinogalactan proteins are involved in root hair development in barley. J Exp Bot 66: 1245–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister R, Rajani MS, Ruzicka D, Schachtman DP (2014) Challenges of modifying root traits in crops for agriculture. Trends Plant Sci 19: 779–788 [DOI] [PubMed] [Google Scholar]
- Nestler J, Liu S, Wen TJ, Paschold A, Marcon C, Tang HM, Li D, Li L, Meeley RB, Sakai H, et al. (2014) Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J 79: 729–740 [DOI] [PubMed] [Google Scholar]
- Nestler J, Schütz W, Hochholdinger F (2011) Conserved and unique features of the maize (Zea mays L.) root hair proteome. J Proteome Res 10: 2525–2537 [DOI] [PubMed] [Google Scholar]
- Nguema-Ona E, Bannigan A, Chevalier L, Baskin TI, Driouich A (2007) Disruption of arabinogalactan proteins disorganizes cortical microtubules in the root of Arabidopsis thaliana. Plant J 52: 240–251 [DOI] [PubMed] [Google Scholar]
- Nguema-Ona E, Coimbra S, Vicré-Gibouin M, Mollet JC, Driouich A (2012) Arabinogalactan proteins in root and pollen-tube cells: distribution and functional aspects. Ann Bot (Lond) 110: 383–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W, Du F, Zhang Y, He T, Ren H (2012) Control of the actin cytoskeleton in root hair development. Plant Sci 187: 10–18 [DOI] [PubMed] [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, et al. (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349: 121–156 [Google Scholar]
- Rounds CM, Bezanilla M (2013) Growth mechanisms in tip-growing plant cells. Annu Rev Plant Biol 64: 243–265 [DOI] [PubMed] [Google Scholar]
- Roux SJ, Steinebrunner I (2007) Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci 12: 522–527 [DOI] [PubMed] [Google Scholar]
- Segal E, Kushnir T, Mualem Y, Shani U (2008) Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone J 7: 1027–1034 [Google Scholar]
- Stenzel I, Ischebeck T, König S, Hołubowska A, Sporysz M, Hause B, Heilmann I (2008) The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell 20: 124–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaravelpandian K, Chandrika NN, Schmidt W (2013) PFT1, a transcriptional Mediator complex subunit, controls root hair differentiation through reactive oxygen species (ROS) distribution in Arabidopsis. New Phytol 197: 151–161 [DOI] [PubMed] [Google Scholar]
- Synek L, Schlager N, Eliás M, Quentin M, Hauser MT, Zárský V (2006) AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J 48: 54–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarejko I, Janiak A, Chmielewska B, Nawrot M (2005) Genetic analysis of several root hair mutants of barley. Barley Genet Newsl 35: 36–38 [Google Scholar]
- Tanaka N, Kato M, Tomioka R, Kurata R, Fukao Y, Aoyama T, Maeshima M (2014) Characteristics of a root hair-less line of Arabidopsis thaliana under physiological stresses. J Exp Bot 65: 1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen PA, Bezanilla M (2013) Plant formins: membrane anchors for actin polymerization. Trends Cell Biol 23: 227–233 [DOI] [PubMed] [Google Scholar]
- Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, Allen N, Bibikova TN, Gilroy S, Bankaitis VA (2005) A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol 168: 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li S, Ng S, Zhang B, Zhou Y, Whelan J, Wu P, Shou H (2014) Mutation in xyloglucan 6-xylosytransferase results in abnormal root hair development in Oryza sativa. J Exp Bot 65: 4149–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xue X, Ren H (2012) New insights into the role of plant formins: regulating the organization of the actin and microtubule cytoskeleton. Protoplasma (Suppl 2) 249: S101–S107 [DOI] [PubMed] [Google Scholar]
- Wang X, Cnops G, Vanderhaeghen R, De Block S, Van Montagu M, Van Lijsebettens M (2001) AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol 126: 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63: 3485–3498 [DOI] [PubMed] [Google Scholar]
- Wen TJ, Hochholdinger F, Sauer M, Bruce W, Schnable PS (2005) The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol 138: 1637–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TJ, Schnable PS (1994) Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. Am J Bot 81: 833–842 [Google Scholar]
- White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot (Lond) 112: 207–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SK, Choi SB, Kumari S, Cho M, Lee SH, Cho HT (2010) Root hair-specific EXPANSIN B genes have been selected for Graminaceae root hairs. Mol Cells 30: 369–376 [DOI] [PubMed] [Google Scholar]
- Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ (2007) Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol 144: 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Ren S, Zhang X, Gao M, Ye S, Qi Y, Zheng Y, Wang J, Zeng L, Li Q, et al. (2011) BENT UPPERMOST INTERNODE1 encodes the class II formin FH5 crucial for actin organization and rice development. Plant Cell 23: 661–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CM, Quan L, Cannon AE, Wen J, Blancaflor EB (2012) AGD1, a class 1 ARF-GAP, acts in common signaling pathways with phosphoinositide metabolism and the actin cytoskeleton in controlling Arabidopsis root hair polarity. Plant J 69: 1064–1076 [DOI] [PubMed] [Google Scholar]
- Yuo T, Shiotani K, Shitsukawa N, Miyao A, Hirochika H, Ichii M, Taketa S (2011) Root hairless 2 (rth2) mutant represents a loss-of-function allele of the cellulose synthase-like gene OsCSLD1 in rice (Oryza sativa L.). Breed Sci 61: 225–233 [Google Scholar]
- Yuo T, Toyota M, Ichii M, Taketa S (2009) Molecular cloning of a root hairless gene rth1 in rice. Breed Sci 59: 13–20 [Google Scholar]
- Zheng R, Li H, Jiang R, Römheld V, Zhang F, Zhao FJ (2011) The role of root hairs in cadmium acquisition by barley. Environ Pollut 159: 408–415 [DOI] [PubMed] [Google Scholar]
- ZhiMing Y, Bo K, XiaoWei H, ShaoLei L, YouHuang B, WoNa D, Ming C, Hyung-Taeg C, Ping W (2011) Root hair-specific expansins modulate root hair elongation in rice. Plant J 66: 725–734 [DOI] [PubMed] [Google Scholar]
- Zhou L, Lan W, Jiang Y, Fang W, Luan S (2014) A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant 7: 369–376 [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang C, Lynch JP (2010) The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct Plant Biol 37: 313–322 [Google Scholar]