Abstract
Background:
To determine the contribution of cytochrome P4502C9 (CYP2C9), vitamin K epoxide reductase (VKORC1) and factor VII genotypes, age, body mass index (BMI), international normalized ratio (INR) and other individual patient characteristics on warfarin dose requirements in an adult Turkish population.
Methods:
Blood samples were collected from 101 Turkish patients. Genetic analyses for CYP2C9*2 and *3, VKORC1 -1639 G>A and factor VII -401 G>T polymorphisms were performed. Age, INR, BMI values and other individual patient characteristics were also recorded.
Results:
The mean daily warfarin dosage was significantly higher in patients with the CYP2C9*1/*1 genotype than in the CYP2C9*2/*2 and CYP2C9*1/*3 groups (p ≤ 0.05). With respect to the VKORC1 -1639 G>A polymorphism, the mean warfarin daily dose requirement was higher in the wild type group compared to the heterozygous group (p≤0.001). The mean daily dose requirement for patients with the GG form of factor VII was significantly higher than that of patients with the TT genotype (p ≤ 0.05). Age, gender, BMI, INR had no statistically significant correlation with warfarin dose (p ≥ 0.05).
Conclusions:
Polymorphisms in CYP2C9, VKORC1 and factor VII did partially affect daily warfarin dose requirements, while age, gender, BMI and INR do not. However, further case-control studies with a larger study size and different genetic loci are needed to confirm our study.
Keywords: Warfarin, CYP2C9, VKORC1, factor VII, polymorphism
Introduction
Vitamin K antagonists are effective at preventing cardioembolic stroke, myocardial infarction and venous thrombosis, but they double the incidence of hemorrhage. The hemorrhage risk is greatest during the first weeks to months of therapy1.
Oral administration of anticoagulants in the prevention and treatment of thromboembolic disorders is the most commonly used method of treatment in clinical practice. Population differences in drug-metabolizing enzymes are important for modifying therapeutic doses and safety profiles of drugs among populations. Pharmacogenetic differences are regarded as an important factor to consider in the treatment of diseases and conditions with personalized medicine. Warfarin is the most widely prescribed oral anticoagulant in the world1-7. Clinically available warfarin consists of a racemic mixture of two active optical isomers, the (R)- and (S)- isoforms. Their pharmacokinetic properties differ considerably because they are cleared via different pathways. (S)- warfarin has about a 1.5-fold greater systemic clearance and is 5-fold more potent than the (R)- isoform1,4,8,9.
However, warfarin has a narrow therapeutic range and a given dose may result in a large inter-individual variation. An insufficient dose may fail to prevent thromboembolism, while an overdose increases the risk of bleeding. The degree of anticoagulation achieved in each patient is measured by obtaining the prothrombin time (PT) and is expressed as the international normalized ratio (INR)10.
The relationship between the dose prescribed and the individual response is regulated by genetic and environmental factors. Warfarin dose requirements are affected by many factors, such as gender, age, diet, race, concomitant medication and genetic factors. Pharmacogenomics, the study of the interaction of an individual's genotype and drug response, can help to optimize drug efficacy while minimizing adverse drug reactions4,5,10.
In particular, molecular analysis of two genes that encode the enzyme responsible for the warfarin (S)-isoform catabolism (CYP2C9) and the target enzyme vitamin K epoxide reductase complex 1 (VKORC1) strongly suggests that their genetic variations greatly affect the individual response to oral anticoagulants. Genotype-based modeling explained a large portion of the dose variation3,4,6,10. Possession of the CYP2C9*2 or CYP2C9*3 allele variants, which result in decreased enzyme activity, is associated with a significant decrease in the mean warfarin dose. Several single nucleotide polymorphisms (SNPs) in VKORC1 are associated with warfarin dose across the normal dose range. Haplotypes based on these SNPs explain a large fraction of the inter-individual variation in the warfarin dose and VKORC1 variation has an approximately three-fold greater effect than CYP2C9. Additionally, polymorphisms within the genes encoding transporters, receptors and vitamin K-dependent clotting factors (II, VII, IX, X) and the anticoagulant proteins C and S have been suggested to predict sensitivity to warfarin therapy2,5,6,10,11. Factor VII (FVII) is a vitamin K-dependent inactive protease, which is converted to its active form on contact with the tissue factor and then activates factor X and factor IX. Functional genetic polymorphisms in the gene encoding FVII could modify the response to warfarin during the first days of treatment6,12.
Algorithms incorporating genetic (CYP2C9 and VKORC1), demographic and clinical factors to estimate the warfarin dosage, could potentially minimize the risk of over dose during warfarin induction10. Until now, there was no accurate way to estimate the dose to reduce the risk of hemorrhage1.
It appears that it would be useful to increase the number of candidate genes involved in the metabolism of oral anticoagulants; pharmacogenomic profiling according to these genes could be a powerful, easily adoptable tool to improve oral anticoagulant therapy management4.
In this study, we evaluated the effects of CYP2C9, VKORC1 and factor VII polymorphisms and other patient characteristics on the warfarin dose required to maintain a therapeutic INR (2.0-3.0) in Turkish patients.
Material and Methods
Participants
The Human Research Committee at Eskisehir Osmangazi University approved this study (28.07.2009/309) and all participants provided written informed consent. Participants requiring warfarin therapy were recruited from the outpatient clinic of the Cardiology Department of Eskisehir Osmangazi University Faculty of Medicine. In total, 101 consecutive patients fulfilled the inclusion criteria for this study, which included 1) use of warfarin for at least one month and 2) a stable INR of 2.0-3.0 since joining the clinic. Patients were still eligible for this study if there were transient alterations in warfarin requirements attributable to acute illness or medication changes, provided that they returned to their usual warfarin dose when the illness had resolved or the medication was discontinued. Patients with higher warfarin target INRs were included if a stable dose of warfarin maintained their INR between 2.0 and 3.0 for at least one month. The results of standard biochemical tests, age, height, body weight, gender, body mass index (BMI), indication for warfarin use, daily prescribed warfarin dose, comorbidity and concomitant medications were recorded on the study form. All subjects participating in this study were Turkish.
The exclusion criteria included non-genetic explanations for altered warfarin requirements, such as hepatic dysfunction, cancer, liver disease, renal disease, advanced heart failure, hypothyroidism, hyperthyroidism, diseases with bleeding tendency, alcohol consumption (>7 drinks/week), underweight (BMI < 18 kg/m2), concurrent rifampin or herbal use, a diet rich in green leafy vegetables including broccoli, spinach, cauliflower (> 2 servings/day), age > 80 years and use of > 13 acetaminophen (325 mg) tablets/week.
Genotyping
Genotyping was performed by experimenters blinded to the associated clinical information. We collected 10 mL blood in EDTA from each participant. Genomic DNA was isolated from blood samples. Genotyping of CYP2C9*2 (rs1799853), (Arg144Cys), CYP2C9*3 (rs1057910) (Ile359Leu), VKORC1 -1639 G>A (rs9923231) and factor VII -401 G>T (rs3093229) alleles were performed according to previously described methods2,13,14.
Subjects with either of the two non-functional alleles (*2 and *3) of CYP2C9 were defined as poor metabolizers (PMs). Homozygotes for the *1 allele were defined as homozygous extensive metabolizers (hmEms) and heterozygotes for the mutant allele were defined as heterozygous extensive metabolizers (htEms). For VKORC1 -1639 G>A, subjects with GG, GA and AA alleles were defined as wild type, heterozygous and homozygous, respectively. For factor VII -401 G>T, subjects with GG, GT and TT alleles were defined as wild type, heterozygous and homozygous, respectively.
Statistical analysis
All data analyses were performed in IBM Statistical Package for the Social Sciences (SPSS) version 20 (SPSS Inc., Chicago, IL, USA). The properties of our data distribution were investigated using the Kolmogorov-Smirnov and Shapiro-Wilk tests. In each group, all continuous variables (age, BMI, dose and INR) were compared across genotypes using a one-way ANOVA for normally distributed variables and a Kruskal-Wallis test for non-normally distributed variables. A Kolmogorov-Smirnov normality test was used to determine the normally distributed variables. Chi-square analyses were used for nominal variables. The effects of the tested parameters on warfarin dose were assessed using simple and multiple linear regression analysis. The results were evaluated in 95 % confidence intervals and p ≤ 0.05 was accepted as statistically significant.
Results
A total of 101 warfarin-treated patients, including 60 females (59.41%) and 41 males (40.59%), were accepted for the study. The age range was 26-80 years, with a mean age of 63.74 ± 11.43 years. The median maintenance daily dose of warfarin was 4.07 mg/day. Average warfarin dose requirements ranged from 1.13 to 7.86 mg/day. Characteristics of the study group are shown in Table 1a. Concomitant interacting medications and comorbidity are shown in Table 1b.
Table 1a. Characteristics of the study group consisting of 101 Turkish patients that underwent genetic analyses for CYP2C9*2 and *3, VKORC1 -1639 G>A and factor VII-401 G>T polymorphisms.
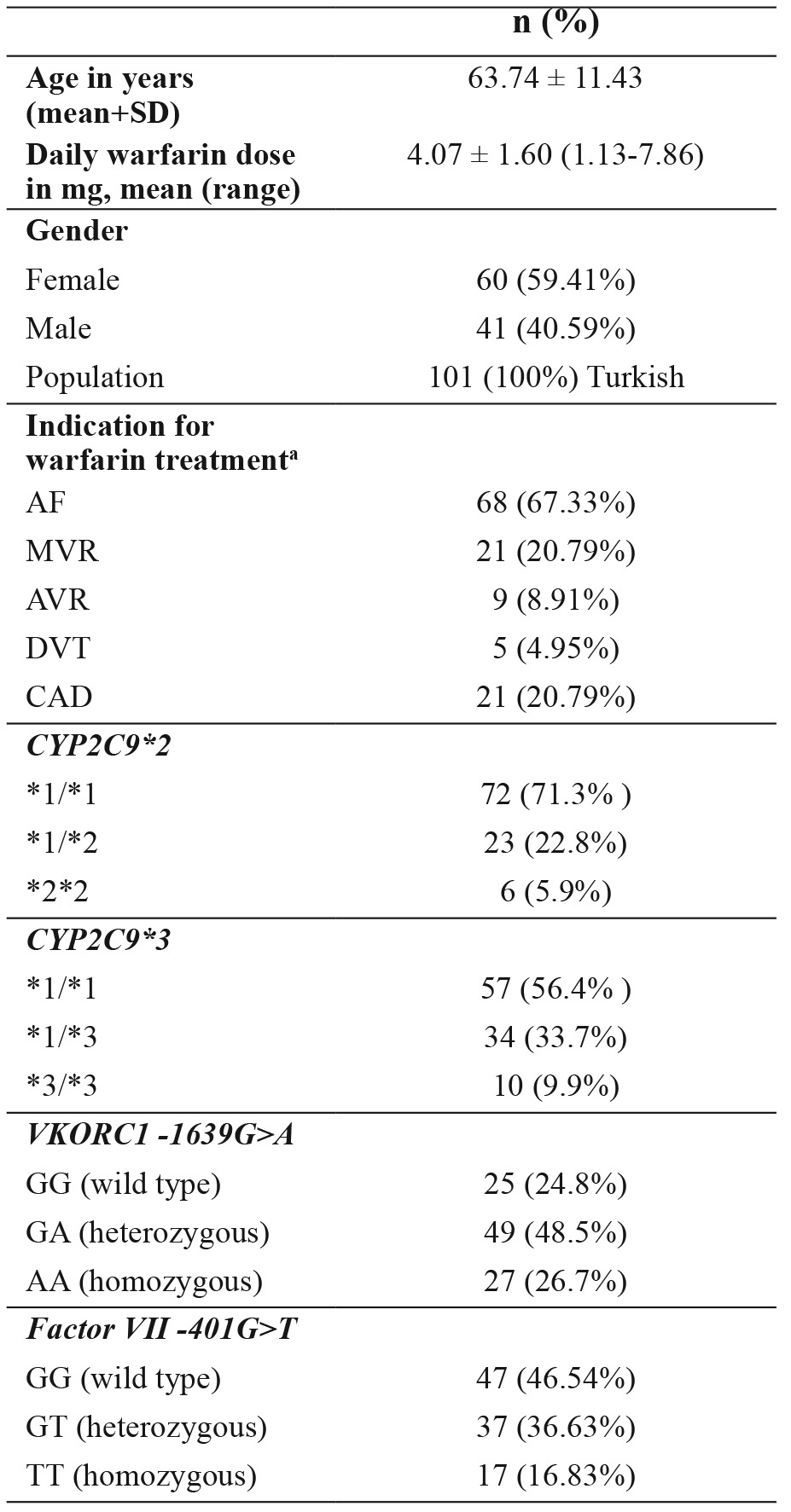
Data are presented as mean ± standard deviation or number of patients (%), a: patients may have had more than one indication for warfarin therapy, DVT: deep vein thrombosis, MVR: mitral valve replacement, AVR: aortic valve replacement, AF: atrial fibrillation, CAD: coronary artery disease, CYP2C9: cytochrome P450 2C9 genotype, VKORC1: vitamin K epoxide reductase genotype, factor VII: factor VII genotype.
Table 1b. Concomitant interacting medications and comorbidity of the 101 warfarin-treated patients included in the study.
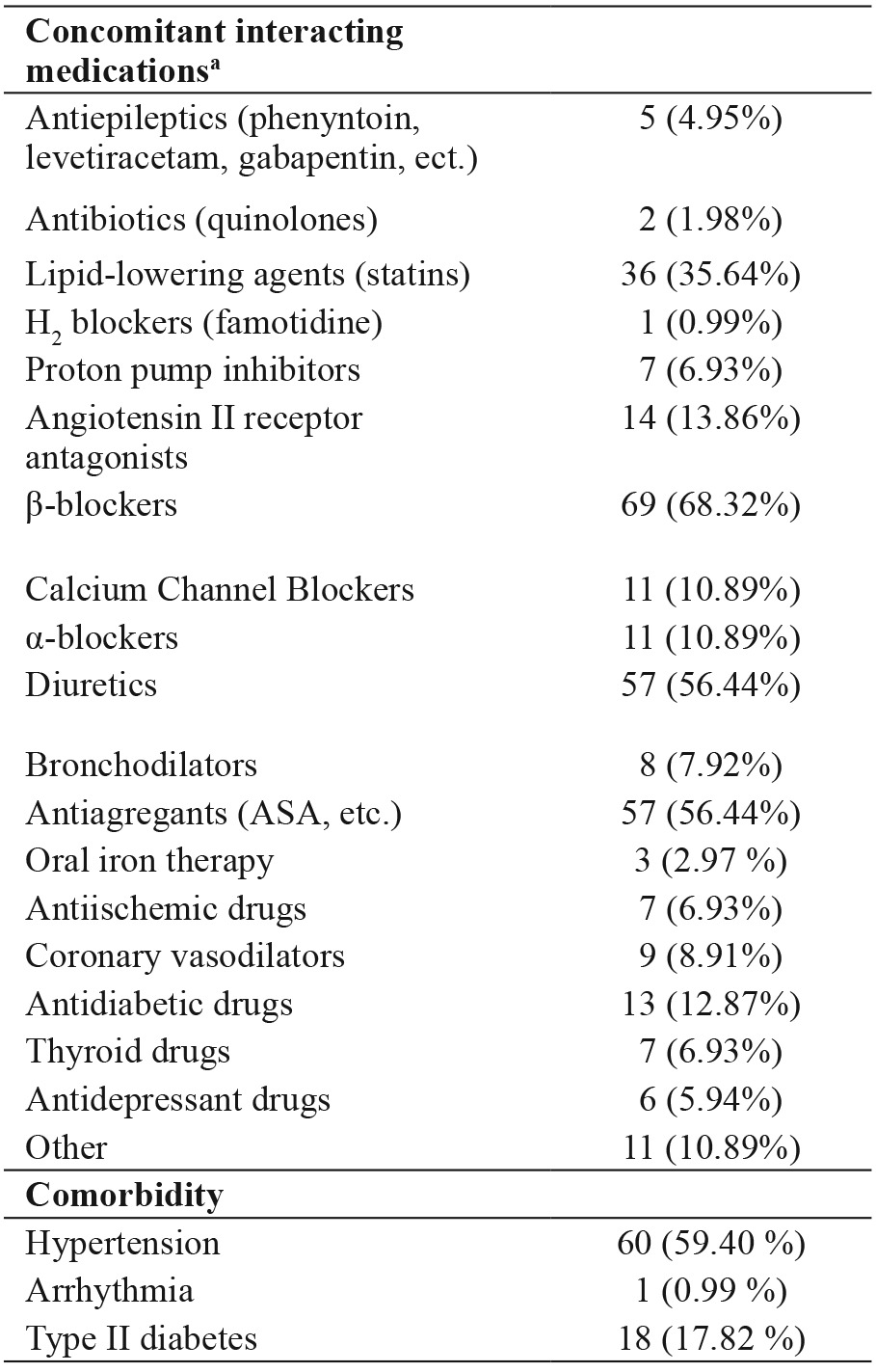
Data are presented as number of patients (%), a: patients might have used more than one concomitant interacting medication.
1. The mean daily warfarin dosage was significantly higher in patients with the CYP2C9*1/*1 genotype [median: 3.93 (2.5-5.36) mg] than in patients with the CYP2C9*2*2 genotype [median: 2.5 (1.88-3.12) mg] (p<0.05). There was no statistically significant difference between the CYP2C9*1/*1 group and the CYP2C9*1/*2 group [3.93 (3.21-5) mg]. Additionally, there was no statistically significant difference between the CYP2C9*1/*2 group and the CYP2C9*2*2 group (p > 0.05) (Table 2).
Table 2. Daily warfarin dose and clinical characteristics in patients with various CYP2C9*2 genotypes.
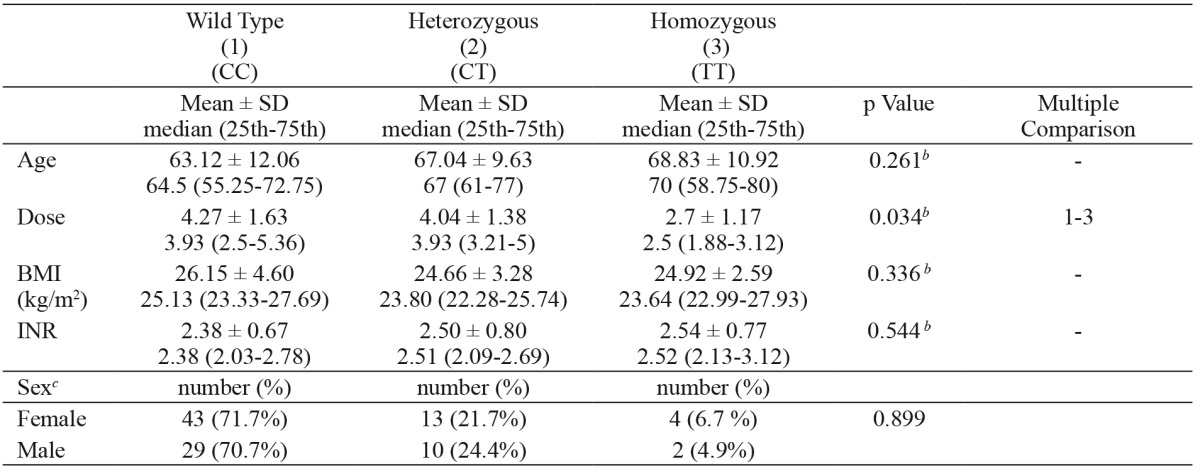
SD: standard deviation, CYP2C9*2: cytochrome P450 2C9*2 genotype, a: one-way analysis of variance, b: Kruskal-Wallis Test, c: Chi-square test for sex, *: p≤0.05.
2. The mean daily warfarin dosage was significantly higher in patients with the CYP2C9*1/*1 genotype [5 (3.93-5.71) mg] than in patients with the CYP2C9*1/*3 genotype [3.21 (2.5-5) mg] (p<0.05). There was no statistically significant difference between the CYP2C9*1/*1 group and the CYP2C9*3/*3 group [3.93 (2.85-5) mg]. Additionally, there was no statistically significant difference between the CYP2C9*1/*3 group and the CYP2C9*3/*3 group (p > 0.05) (Table 3).
Table 3. Daily warfarin dose and clinical characteristics in patients with various CYP2C9*3 genotypes.
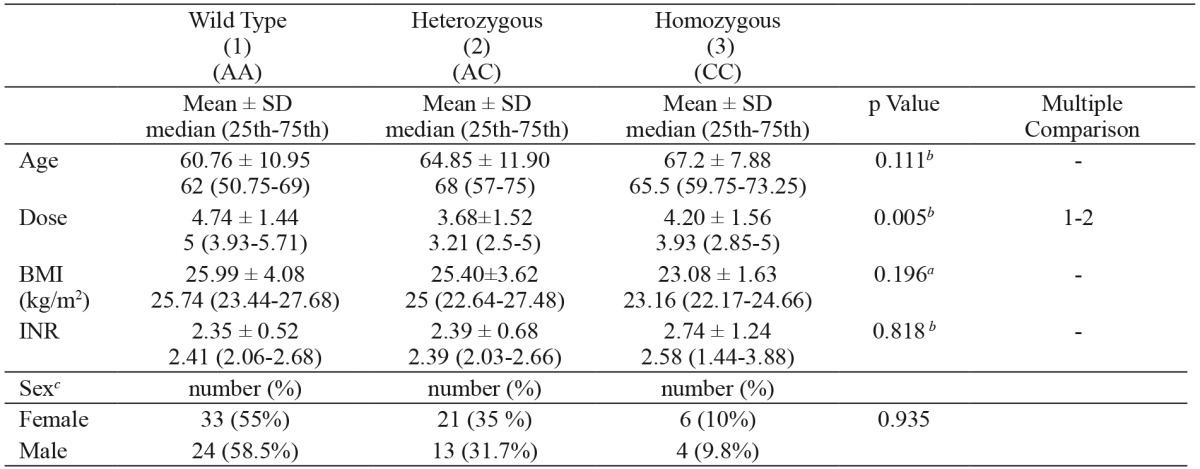
SD: standard deviation, CYP2C9*3: cytochrome P450 2C9*3 genotype, a: one-way analysis of variance, b: Kruskal-Wallis Test, c: Chi-square test for sex, *: p≤0.05.
3. The mean daily dose of the patients with the VKORC1 -1639 GG genotype [5 (3.93-6.07) mg] was significantly higher than that of the patients with the VKORC1 -1639 GA [3.21 (2.5-4.82) mg] genotype (p<0.001). There was no statistically significant difference between the mean daily doses in patients with the VKORC1 -1639 GA genotype and patients with the VKORC1 -1639 AA genotype (p > 0.05) (Table 4).
Table 4. Daily warfarin dose and clinical characteristics in patients with various VKORC1 genotypes.
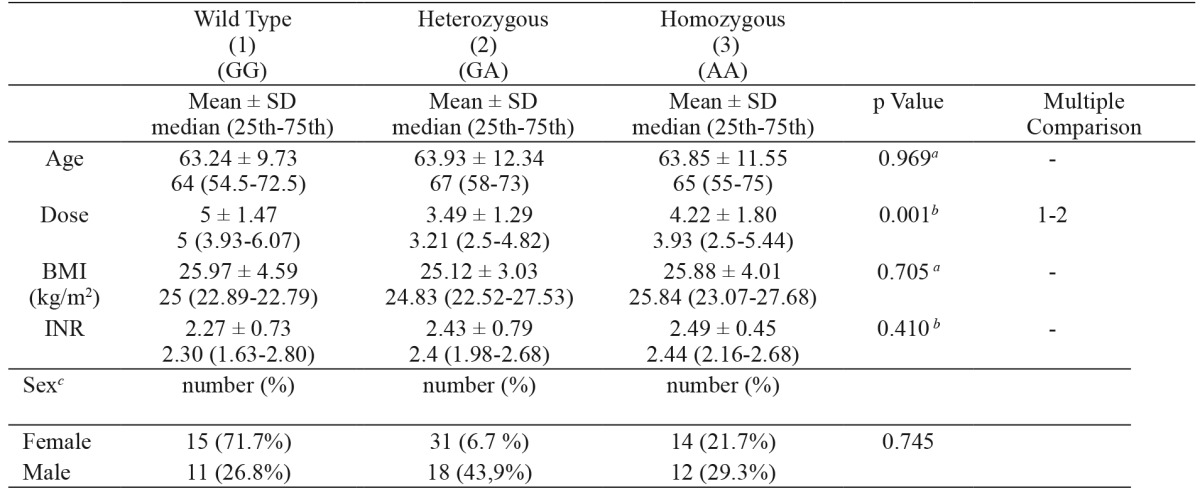
SD: standard deviation, VKORC1: Vitamin K epoxide reductase genotype, a: one-way analysis of variance, b: Kruskal-Wallis Test, c: Chisquare test for sex, ** p≤0.001.
4. The mean daily dose of the patients with the factor VII -401 GG genotype [5 (3.93-6.96) mg] was significantly higher than that of the patients with the factor VII -401 TT genotype [3.57 (2.5-5) mg] (p<0.05). There was no statistically significant difference between the mean daily doses in patients with the factor VII -401 GG genotype and patients with the factor VII -401 GT genotype (p > 0.05) (Table 5).
Table 5. Daily warfarin dose and clinical characteristics in patients with various factor VII genotypes.
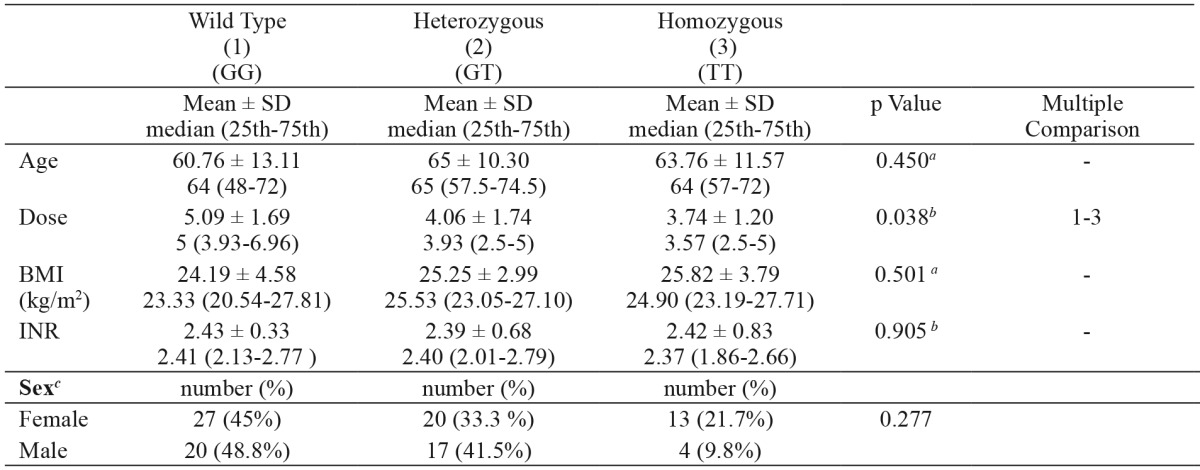
SD: standard deviation, factor VII: coagulation factor VII genotype, a: one-way analysis of variance, b: Kruskal-Wallis Test, c: Chi-square test for sex, ** p≤0.001.
Age, gender, BMI and INR parameters showed no statistically significant difference (p>0.05) across the CYP2C9*2, CYP2C9*3, VKORC1 -1639 G>A and factor VII -401 G>T genotype groups (Table 2 - Table 6). A multivariate regression model including age (R2= 1.4 %), BMI (R2= 1 %), INR (R2= 2 %), CYP2C9*2 (R2= 0.2 %), CYP2C9*3 (R2= 1.1 %), VKORC1 (R2= 14.9 %) and factor VII (R2= 2.2 %) and used to estimate warfarin dose (R2= 18.2 %) is shown in Table 7.
Table 7. Regression equations for modeling warfarin daily dose requirements based on age, BMI, INR and VKORC1, CYP2C9 and factor VII genotypes.
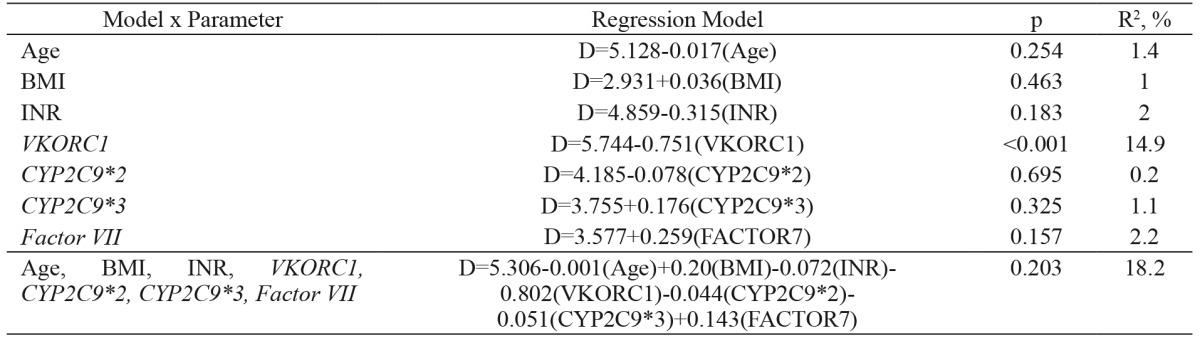
Age: input age in years, BMI: body mass index, INR: international normalized ratio; VKORC1 genotype: input 1 for GG, 2 for GA, 3 for AA; CYP2C9*2 genotype: input 1 for CC, 2 for GT, 3 for TT; CYP2C9*3 genotype: input 1 for AA, 2 for AC, 3 for CC; factor VII genotype: input 1 for GG, 2 for GT, 3 for TT. The y variable is dose (D) in all models.
Discussion
In the present study, we investigated genetic [CYP2C9*2, CYP2C9*3, VKORC1 -1639G>A and factor VII -401G>T polymorphisms] and non-genetic predictors of warfarin dose requirements in 101 patients from Turkey.
Warfarin is a commonly used drug, but it is difficult to manage clinically. Warfarin therapy, especially at initiation, is particularly problematic. There is greater than 10-fold inter-individual variability in the dose required to attain a therapeutic response. The inter-individual variability in response to this drug requires patients to routinely visit coagulation clinics and undergo repeated INR measurements4,7,10,15.
Genetic polymorphisms in the genes encoding for VKORC1 and CYP2C9 have been shown to correlate with the response to warfarin during both induction and maintenance6,16-19. Many large and retrospective clinical studies of various populations have found that the CYP2C9 (*2,*3), VKORC1 -1639 G>A and factor VII -401 G> T polymorphisms and their allelic variants are important in determining the warfarin dose requirement5,10,20-28. Recent genome-wide association studies have confirmed known polymorphisms in CYP2C9 and VKORC1 as the primary genetic determinants of the stabilized warfarin dose29.
On the other hand, numerous clinical factors, including age, race, weight, height, smoking status and medication use, have been associated with variability in maintenance warfarin dose requirements30-32.
Our study assessed the contributions of the CYP2C9, VKORC1 and factor VII genotypes to inter-individual variability in the Turkish population. Determinable demographic factors such as age, gender and BMI, which have been considered as contributing covariates, along with INR parameters, were found not to be associated with warfarin dose requirements in our study. Based on these results, we conclude that patient age, gender, BMI, INR and genetic polymorphism of CYP2C9, VKORC1 and factor VII account for approximately 18.2% of the variability in the daily warfarin dose requirement, though the contributions of age, gender, BMI and INR were not statistically significant.
Thus, our analysis revealed that despite the results of other studies20,22,27,28, genetic polymorphisms in the genes encoding CYP2C9, VKORC1 and factor VII were partially associated with warfarin dose requirement.
We confirmed that the presence of the CYP2C9*2 polymorphism can explain only approximately 0.2% of the inter-individual variability in the warfarin dose requirement in our model, while CYP2C9*3 explains only 1.1%. Furthermore, we found that the daily warfarin doses of patients with the CYP2C9*1/*1 genotype were only significantly higher than those of patients with the CYP2C9*2/*2 or CYP2C9*1/*3 genotypes (p < 0.05).
Thus far, more than 50 variant alleles of CYP2C9 have been identified. R- and S-enantiomers of warfarin are metabolized via different pathways. While the S-isoform is mainly metabolized by CYP2C9, the R-isoform is metabolized by the CYP3A4, 1A2 and 1A1 enzymes. Genetic variations in these regions may lead to inter-individual differences in the effective warfarin dose4,10,21,28,33,34.
Our results are similar to those of Miao et al, who concluded that the CYP2C9*3 allele can only explain approximately 1.7% of the inter-individual variability and those of Suriapranata et al, who revealed that CYP2C9*3 was not significantly associated with warfarin dose requirement. Additionally, our study is similar to previous studies demonstrating that CYP2C9 allelic variants fail to account for changes in the dose-anticoagulant effect of warfarin and that other factors may be involved29,35-37.
We confirmed that the VKORC1-1639 G>A polymorphism can only explain approximately 14.9% of the inter-individual variability in the warfarin dose requirement; as such, it made the largest contribution to estimations of warfarin dose in our study. The daily doses for patients with the VKORC1 -1639 GG genotype were only significantly higher than those of patients with the VKORC1 -1639 GA genotype.
VKORC1 is the target of coumarin anticoagulants. Various mutations in the VKORC1 gene have been found in warfarin-resistant patients22,38. Reieder et al, previously reported that VKORC1 haplotypes can be used to classify patients into low, medium and high warfarin dose groups and that differences in VKORC1 may explain the variation in warfarin dose requirements between the patients39. Although VKORC1 -1639 G>A explains the highest percentage of variation in the warfarin dose, other SNPs may have independent effects and may explain additional variation that is not attributed to -1639G>A. It is necessary to note that different VKORC1 genotypes in various populations result in different warfarin-dosing patterns in these populations. It has been previously reported that ethnic differences in the VKORC1 gene may play an important role in warfarin sensitivity 4,23,26,38. On the other hand, most polymorphisms in the VKORC1 gene have been found to be associated with a normal warfarin dose range10.
We confirmed that the factor VII -401 G>T polymorphism only explains approximately 2.2% of the inter-individual variability in the warfarin dose requirement. The daily warfarin doses of the patients with the factor VII -401 GG genotype were only significantly higher than those of the patients with the factor VII -401 TT genotype. The factor VII gene is located on chromosome 13 and five polymorphic sites have been identified to date12. It has been proposed that the circulating FVII plasma levels are genetically determined to 30 %. The promoter variant at nt -402 has been associated with elevated plasma FVII levels, whereas the R353Q (rs6046) and -323Ins10 (rs36208070) polymorphisms have been associated with a 20% to 25% reduction in FVII plasma levels. There are a number of other factors that influence FVIIc (coagulant activity) levels. Age, body mass index (BMI) and, in women, the use of oral contraceptives and onset of menopause are associated with higher levels. In the general population, some 47% of the total variance in FVIIc levels is attributable to the variability between individuals12,21,40-42. As reported previously, the relationship between INR and the plasma concentrations of the vitamin K-dependent factors II and VII may significantly differ during warfarin induction and maintenance. Thus, even when identical INR values are measured, the plasma concentration of these coagulation factors may greatly vary between induction and stable anticoagulation within the same patient. Thus, it is possible that variability in the FVII concentration, similar to what may be expected among different R353Q genotype carriers, is not a powerful determinant of INR variability during stable anticoagulation6. Our data confirm these earlier findings. In this study, we used patients with stable INR values.
Many other factors are important for determining a safe and effective dose of warfarin at the beginning of therapy10. Drugs, diet, various diseases, ethnicity and environmental factors can change the pharmacokinetics of warfarin. Certain drugs (e.g. phenylbutazone, sulfinpyrazone, metronidazole, trimethoprim sulfamethoxazole, and amiodarone) may affect the oxidative metabolism of warfarin4,31,32,43. Others decrease the plasma levels of warfarin by reducing its absorption (e.g., cholestyramine) or increasing its hepatic clearance (e.g. barbiturates, rifampicin and carbamazepine). As a rule, if a patient receives a large number of medications, it is difficult to control anticoagulation. Long-term alcohol intake may increase the clearance of warfarin. Dietary vitamin K decreases the anticoagulant effects of warfarin, as does consumption of a large amount of green vegetables. Often, various foods and herbal medications, such as avocado and gingko, affect the stability of warfarin and may reduce the need for the dose of warfarin4,10,43.
Increased patient age may lead to a higher sensitivity to warfarin because there is a negative correlation between age and warfarin clearance. At the same time, as a result of age-related decreases in liver mass, the liver content of vitamin K epoxide reductase is reduced, causing increased sensitivity to warfarin4,24,26. To minimize these problems, which are often observed in elderly patients with age-related liver and kidney dysfunction, we tried to enroll patients with normal liver and renal function.
According to Suriapranata et al (2011), age, body weight, height and genetic variants of CYP2C9 and VKORC1 can explain approximately 15.4% of the variability in the daily warfarin dose requirement28. Other studies have demonstrated that genetic polymorphisms lead to inter-individual differences in the effective warfarin dose; the data obtained to date show that CYP2C9 polymorphisms explain 5-22% and VKORC1 polymorphisms 6-37% of the warfarin dose variability4,42,44. When taken together with age, gender, body weight, height and clinical factors such as other drug treatments and therapeutic indications, 33-57% of the dose of warfarin can be estimated in advance4,10,31.
In addition, the contribution of ethnicity is an important factor in determining the effective maintenance dose of warfarin. For example, previous studies have demonstrated that in Asian patients, the maintenance dose of warfarin was approximately 30-40% less than in Caucasians; this difference has been found to be connected to genetic variants of CYP2C9 and VKORC19,32,35. Also previous Clarification of Optimal Anticoagulation through Genetics (COAG) trial study showed that among black patients, the mean percentage of time in the therapeutic range was less in the genotype-guided group than in the clinically guided group32. The comparison of the allelic frequencies of CYP2C9*2,*3 and VKORC1 -1639 G>A across four different populations (Turkish, Asian, African-American and Caucasian) revealed that the allelic frequencies of these two genes in Turkish populations are very different from those in African-Americans and Asians but relatively close to those reported for Caucasians11. Turkish society is composed of people of many different ethnic backgrounds. In our study, due to a lack of sufficient and satisfactory information, the genetic origins of our patients could not be determined.
Based on the currently available clinical and pharmacogenetic information concerning warfarin, the source(s) of 40% of the variation in the warfarin dose requirement is still unknown10. It is likely that the inter-individual variability in warfarin-dose requirements is due at least in part to additional genetic factors, including polymorphisms in genes encoding apolipoprotein E, multidrug resistance 1 (MDR1), vitamin K-dependent clotting factors and possibly additional components of the vitamin K epoxide reductase complex, such as gamma glutamyl carboxylase (GGCX), microsomal epoxide hydrolase1 (mEH1), and calumenin (CALU)4,10,24,38. Cytochrome P4504F2 (CYP4F2) activity was recently found to be associated with warfarin dose23,24. Thus, the inter-individual variability in warfarin dose requirements is due at least in part to additional genetic and non-genetic factors that need further investigation. For a more complete survey of the genetic background for enzymes affecting individual warfarin pharmacokinetics and/or pharmacodynamics, full genotyping of the current list of SNPs is required.
Conclusions
In summary, the results of the present study show that a warfarin dosing regimen formulated using clinical data (age, dose, BMI, and INR) and pharmacogenetic information concerning the CYP2C9, VKORC1 and factor VII genotypes could benefit patients treated with warfarin. It is important that prospective clinical studies assess dosing algorithms that incorporate the contributions of age, genotype and weight, to allow for the individualization of warfarin dose during both the initiation and maintenance stages of therapy. On the other hand, polymorphisms in other genes that may help determine the dose of warfarin should be investigated. Ethnic differences should also be considered. Treatment algorithms that incorporate pharmacogenomic data must be evaluated prospectively in a randomized controlled clinical trial before being integrated into routine clinical practice. These new algorithms could help physicians to determine more accurate dosages for the patients and reduce the chances of bleeding during warfarin treatment.
Conflict of interest
The authors report no conflict of interest.
Acknowledgement
This work was funded by the Eskisehir Osmangazi University Scientific Research Projects Commission (Project number: 200911013).
Table 6. Daily warfarin dose requirements based on gender in patients with various CYP2C9, VKORC1 and factor VII genotypes.
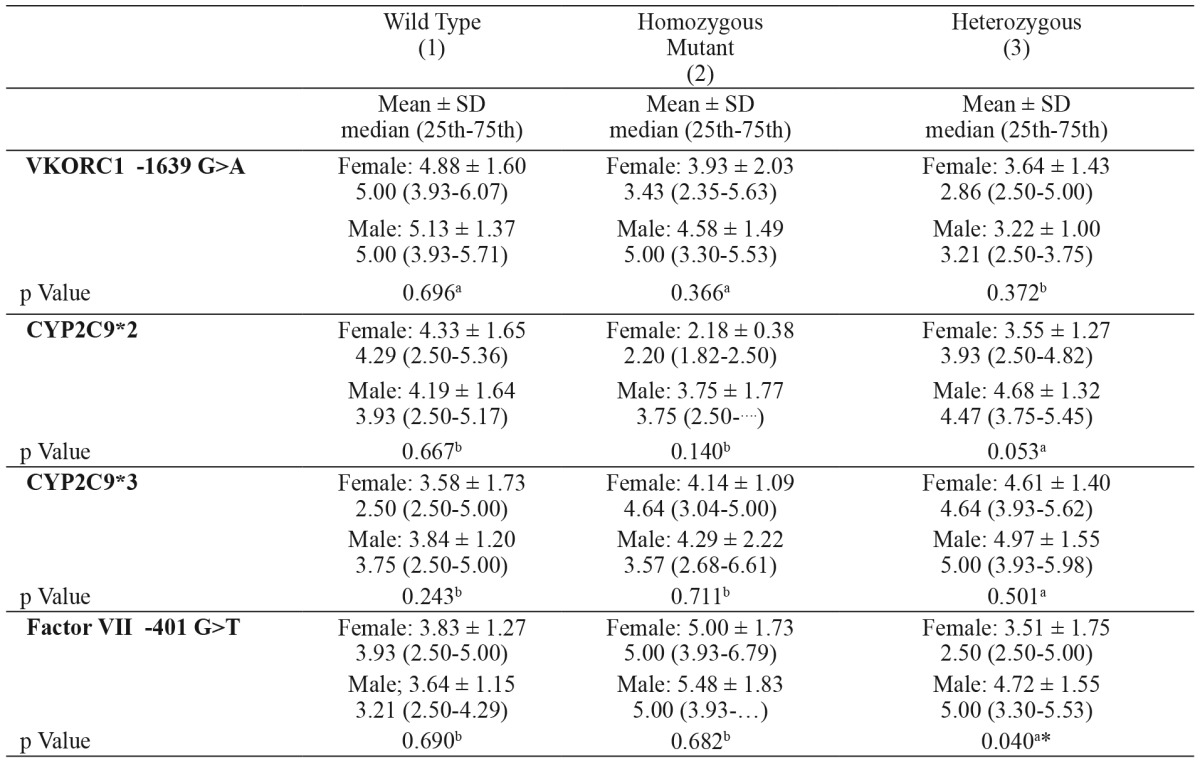
VKORC1: Vitamin K epoxide reductase genotype, CYP2C9*2: cytochrome P450 2C9*2 genotype, CYP2C9*3: cytochrome P450 2C9*3 genotype, factor VII: coagulation factor VII genotype, a: t-test, b: Mann-Whitney Test.
References
- 1.Gage BF. Pharmacogenetics-based coumarin therapy. Hematology Am Soc Hematol Educ Program. 2006:67–473. doi: 10.1182/asheducation-2006.1.467. [DOI] [PubMed] [Google Scholar]
- 2.Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 3.Wiwanitkit V. Pharmacogenomic effect of cytochrome P450 2C9 polymorphisms in different populations. Clin Appl Thromb Hemost. 2006;12:219–222. doi: 10.1177/107602960601200211. [DOI] [PubMed] [Google Scholar]
- 4.D'Andrea G, D'Ambrosio R, Margaglione M. Oral anticoagulants: Pharmacogenetics. Relationship between genetic and non-genetic factors. Blood Rev. 2008;22:127–140. doi: 10.1016/j.blre.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Ozer N, Cam N, Tangurek B, Ozer S, Uyarel H, Oz D, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements in an adult Turkish population. Heart Vessels. 2010;25:155–162. doi: 10.1007/s00380-009-1177-7. [DOI] [PubMed] [Google Scholar]
- 6.Mlynarsky L, Bejarano-Achache I, Muszkat M, Caraco Y. Factor VII R353Q genetic polymorphism is associated with altered warfarin sensitivity among CYP2C9 *1/*1 carriers. Eur J Clin Pharmacol. 2012;68:617–627. doi: 10.1007/s00228-011-1143-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Cheong HS, Kim LH, Kim JO, Seo DW, Kim YH, et al. Screening of Genetic Polymorphisms of CYP3A4 and CYP3A5 Genes. Korean J Physiol Pharmacol. 2013;17:479–484. doi: 10.4196/kjpp.2013.17.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7:97–104. doi: 10.1097/01.gim.0000153664.65759.cf. [DOI] [PubMed] [Google Scholar]
- 9.Wilke RA, Berg RL, Vidaillet HJ, Caldwell MD, Burmester JK, Hillman MA. Impact of age, CYP2C9 genotype and concomitant medication on the rate of rise for prothrombin time during the first 30 days of warfarin therapy. Clin Med Res. 2005;3:207–213. doi: 10.3121/cmr.3.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin T, Miyata T. Warfarin dose and the pharmacogenomics of CYP2C9 and VKORC1 - rationale and perspectives. Thromb Res. 2007;120:1–10. doi: 10.1016/j.thromres.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Oner Ozgon, Langaee TY, Feng H, Buyru N, Ulutin T, Hatemi AC, et al. VKORC1 and CYP2C9 polymorphisms are associated with warfarin dose requirements in Turkish patients. Eur J Clin Pharmacol. 2008;64:889–894. doi: 10.1007/s00228-008-0507-5. [DOI] [PubMed] [Google Scholar]
- 12.Greisenegger S, Weber M, Funk M, Endler G, Lang W, Ferrari J, et al. Polymorphisms in the coagulation factor VII gene and risk of primary intracerebral hemorrhage. Eur J Neurol. 2007;14:1098–1101. doi: 10.1111/j.1468-1331.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- 13.van 't Hooft FM, Silveira A, Tornvall P, Iliadou A, Ehrenborg E, Eriksson P, et al. Two common functional polymorphisms in the promoter region of the coagulation factor VII gene determining plasma factor VII activity and mass concentration. Blood. 1999;93:3432–3441. [PubMed] [Google Scholar]
- 14.Joffe HV, Xu R, Johnson FB, Longtine J, Kucher N, Goldhaber SZ. Warfarin dosing and cytochrome P450 2C9 polymorphisms. Thromb Haemost. 2004;91:1123–1128. doi: 10.1160/TH04-02-0083. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell MD, Berg RL, Zhang KQ, Glurich I, Schmelzer JR, Yale SH, et al. Evaluation of genetic factors for warfarin dose prediction. Clin Med Res. 2007;5:8–16. doi: 10.3121/cmr.2007.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, Lange LA, Li X, Susswein L, Bryant B, Malone R, et al. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J Med Genet. 2006;43:740–744. doi: 10.1136/jmg.2005.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schelleman H, Chen J, Chen Z, Christie J, Newcomb CW, Brensinger CM, et al. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin Pharmacol Ther. 2008;84:332–339. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. EU-PACT Group A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 20.D'Ambrosio RL, D'Andrea G, Cappucci F, Chetta M Di Perna P, Brancaccio V, et al. Polymorphisms in factor II and factor VII genes modulate oral anticoagulation with warfarin. Haematologica. 2004;89:1510–1516. [PubMed] [Google Scholar]
- 21.Shikata E, Ieiri I, Ishiguro S, Aono H, Inoue K, Koide T, et al. Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gama-glutamyl carboxylase) gene variants with warfarin sensitivity. Blood. 2004;103:2630–2635. doi: 10.1182/blood-2003-09-3043. [DOI] [PubMed] [Google Scholar]
- 22.Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–1751. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 23.Daly AK. Pharmacogenomics of anticoagulants: steps toward personal dosage. Genome Med. 2009;1:10. doi: 10.1186/gm10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonas DE, McLeod HL. Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol Sci. 2009;30:375–386. doi: 10.1016/j.tips.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.International Warfarin Pharmacogenetics Consortium, Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Q, Kong Y, Schneede J, Xiao YB, Chen L, Zhong QJ, et al. VKORC1-1639G>A, CYP2C9, EPHX1691A>G genotype, body weight, and age are important predictors for warfarin maintenance doses in patients with mechanical heart valve prostheses in southwest China. Eur J Clin Pharmacol. 2010;66:1217–1227. doi: 10.1007/s00228-010-0863-9. [DOI] [PubMed] [Google Scholar]
- 27.Eroğlu A, Oztürk A, Akar N. Asssociation between the -402GA, -401GT, and -323ins10-bp polymorphisms of factor VII gene and breast cancer. Breast Cancer. 2011;18:282–285. doi: 10.1007/s12282-009-0189-6. [DOI] [PubMed] [Google Scholar]
- 28.Tatarūnas V, Lesauskaitė V, Veikutienė A, Jakuška P, Benetis R. The influence of CYP2C9 and VKORC1 gene polymorphisms on optimal warfarin doses after heart valve replacement. Medicina (Kaunas) 2011;47:25–30. [PubMed] [Google Scholar]
- 29.Suriapranata IM, Tjong WY, Wang T, Utama A, Raharjo SB, Yuniadi Y, et al. Genetic factors associated with patient-specific warfarin dose in ethnic Indonesians. BMC Med Genet. 2011;12:80. doi: 10.1186/1471-2350-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84:326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinxadi P, Blockman M. Warfarin resistance. Cardiovasc J Afr. 2008;19:215–217. [PubMed] [Google Scholar]
- 32.Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, et al. A Pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefferts JA, Schwab MC, Dandamudi UB, Lee HK, Lewis LD, Tsongalis GJ. Warfarin genotyping using three different platforms. Am J Transl Res. 2010;2:441–446. [PMC free article] [PubMed] [Google Scholar]
- 34.Lenzini P, Wadelius M, Kimmel S, Anderson JL, Jorgensen AL, Pirmohamed M, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther. 2010;87:572–578. doi: 10.1038/clpt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao L, Yang J, Huang C, Shen Z. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol. 2007;63:1135–1141. doi: 10.1007/s00228-007-0381-6. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Ge W, Yu F, Zhu H. Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement--a systematic review and meta analysis. Thromb Res. 2010;125:e159–e166. doi: 10.1016/j.thromres.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Shahin MH, Khalifae SI, Gong Y, Hammad LN, Sallam MT, El Shafey M, et al. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics. 2011;21:130–135. doi: 10.1097/FPC.0b013e3283436b86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Eng J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 40.Green F, Kelleher C, Wilkes H, Temple A, Meade T, Humphries S. A common genetic polymorphism associated with lower coagulation factor VII levels in healthy individuals. Arterioscler Thromb. 1991;11:540–546. doi: 10.1161/01.atv.11.3.540. [DOI] [PubMed] [Google Scholar]
- 41.Fuchshuber-Moraes M, Perini JA, Rosskopf D, Suarez-Kurtz G. Exploring warfarin pharmacogenomics with the extreme-discordant-phenotype methodology: impact of FVII polymorphisms on stable anticoagulation with warfarin. Eur J Clin Pharmacol. 2009;65:789–793. doi: 10.1007/s00228-009-0651-6. [DOI] [PubMed] [Google Scholar]
- 42.Mo X, Hao Y, Yang X, Chen S, Lu X, Gu D. Association between polymorphisms in the coagulation factor VII gene and coronary heart disease risk in different ethnicities: a meta-analysis. BMC Med Genet. 2011;12:107. doi: 10.1186/1471-2350-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31:326–343. doi: 10.1007/s11239-011-0561-1. [DOI] [PubMed] [Google Scholar]
- 44.Lubitz SA, Scott SA, Rothlauf EB, Agarwal A, Peter I, Doheny D, et al. Comparative performance of gene-based warfarin dosing algorithms in a multiethnic population. J Thromb Haemost. 2010;8:1018–1026. doi: 10.1111/j.1538-7836.2010.03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


