Abstract
Background:
Despite its promise as a highly useful therapy for pancreatic cancer (PC), the addition of external beam radiation therapy to PC treatment has shown varying success in clinical trials. Understanding PC radioresistance and discovery of methods to sensitise PC to radiation will increase patient survival and improve quality of life. In this study, we identified PC radioresistance-associated pathways using global, unbiased techniques.
Methods:
Radioresistant cells were generated by sequential irradiation and recovery, and global genome cDNA microarray analysis was performed to identify differentially expressed genes in radiosensitive and radioresistant cells. Ingenuity pathway analysis was performed to discover cellular pathways and functions associated with differential radioresponse and identify potential small-molecule inhibitors for radiosensitisation. The expression of FDPS, one of the most differentially expressed genes, was determined in human PC tissues by IHC and the impact of its pharmacological inhibition with zoledronic acid (ZOL, Zometa) on radiosensitivity was determined by colony-forming assays. The radiosensitising effect of Zol in vivo was determined using allograft transplantation mouse model.
Results:
Microarray analysis indicated that 11 genes (FDPS, ACAT2, AG2, CLDN7, DHCR7, ELFN2, FASN, SC4MOL, SIX6, SLC12A2, and SQLE) were consistently associated with radioresistance in the cell lines, a majority of which are involved in cholesterol biosynthesis. We demonstrated that knockdown of farnesyl diphosphate synthase (FDPS), a branchpoint enzyme of the cholesterol synthesis pathway, radiosensitised PC cells. FDPS was significantly overexpressed in human PC tumour tissues compared with healthy pancreas samples. Also, pharmacologic inhibition of FDPS by ZOL radiosensitised PC cell lines, with a radiation enhancement ratio between 1.26 and 1.5. Further, ZOL treatment resulted in radiosensitisation of PC tumours in an allograft mouse model.
Conclusions:
Unbiased pathway analysis of radioresistance allowed for the discovery of novel pathways associated with resistance to ionising radiation in PC. Specifically, our analysis indicates the importance of the cholesterol synthesis pathway in PC radioresistance. Further, a novel radiosensitiser, ZOL, showed promising results and warrants further study into the universality of these findings in PC, as well as the true potential of this drug as a clinical radiosensitiser.
Keywords: pancreatic cancer, radiation resistance, cholesterol, zoledronic acid, radiosensitiser
Pancreatic cancer (PC) is the fourth leading cause of cancer death in the United States and has a 5-year survival rate of 6% (Siegel et al, 2013). Patients with PC are usually diagnosed late in the cancer's progression, and therefore only 15–20% of patients are eligible for curative-intent surgery. For patients diagnosed with resectable or locally advanced disease, accounting for ∼40% of PC patients, external beam radiation therapy (EBRT) is considered a staple of therapy to either help prevent local failure or shrink the tumour to the point of surgical resectability, respectively (Callery et al, 2009; Hazard, 2009; Ryan and Mamon, 2009). Clinical trials that have tested the ability of radiation to prolong progression-free survival, increase overall survival, or improve quality of life in PC patients, either alone or in combination with chemotherapy, have shown mixed results (reviewed in Hazard (2009); Goodman and Hajj (2013)). Further, only 20% of pancreatic primary tumours show any significant response to radiation (Roldan et al, 1988; Hazard, 2009).
This lack of clinical efficacy of PC irradiation, combined with the substantial benefit that efficacious PC EBRT could provide, mandates exploration of methods to overcome radiation resistance in PC. Successful PC radiosensitisation could provide significant benefit to patients diagnosed with resectable disease, as an estimated 21% suffer solely from local failure post resection, whereas an additional 20% fail both locally and distantly (Oettle et al, 2007; Asiyanbola et al, 2009). To understand how to overcome PC's radioresistance and offer potential methods for therapeutic radiosensitisation, the mechanisms underlying the lack of radiation-induced cytotoxicity in PC must be elucidated.
The molecular pathways contributing to apparent resistance to ionising radiation (IR) in PC remain poorly understood (Brunner et al, 2005; Kimple et al, 2010; Fokas et al, 2012; Engelke et al, 2013). We employed a novel in vitro model of PC radiation resistance to determine the global transcriptional differences between radiosensitive and radioresistant PC cells. Several genes were identified and validated, including many in the cholesterol synthesis pathway, whose differential expressions significantly correlated with in vitro PC radioresponse. Further, through these methods, a putative radiosensitiser for PC was tested, zoledronic acid (ZOL, Zometa, Novartis, East Hanover, NJ, USA), currently used clinically for non-IR-related purposes. Finally, tumour-specific EBRT was performed using a linear accelerator for treatment of a subcutaneous allograft model of PC, testing whether ZOL could radiosensitise in vivo.
Materials and Methods
Cell culture and materials
The human PC cell lines Panc-1 and BxPC3 were obtained from the American Type Culture Collection (Manassas, VA, USA). The L3.6pl human PC cell line was a kind gift from Dr Michel Ouellette (UNMC). The mouse PC cell line UN-KPC-961 was established in our laboratory from a primary tumour generated in a spontaneous mouse model of PC, the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mouse model (Torres et al, 2013). Cell lines were cultured per established lab protocol (Singh et al, 2004). ZOL was purchased from Sigma (St Louis, MO, USA) and was dissolved in sterile water. Clinical grade ZOL (4 mg per 5 ml concentrate) was received from the oncology pharmacy (Nebraska Medical Center, Omaha, NE, USA).
In vitro irradiation
In vitro irradiation was accomplished via a linear accelerator in the Department of Radiation Oncology at UNMC. Briefly, cells in exponential growth phase were plated at 40% confluence 24 h before irradiation. Flasks were placed on 10 cm of solid water (phantom material used for radiation beam calibration) positioned in the centre of the 40 cm × 40 cm radiation field and irradiated with 6 MV X-rays at a rate of 2.73 Gy min−1 from the posterior direction, with the media being 100 cm from the X-ray target. The dose to the media was verified with MOSFET detectors (Best Medical Canada, Ottawa, ON, Canada).
Analysis of radiosensitivity of PC cell lines
Cellular radioresponse was determined by colony survival assay (CSA) using standard protocol (Boothman et al, 1987). On the day of staining, cells were washed, fixed in MeOH, and stained using 0.4% crystal violet in 25% MeOH. Colonies containing >50 cells were counted for each well and the number of colonies corresponding to each radiation dose averaged. Surviving fraction at each dose was determined by the formula (number of surviving colonies in dose X)/(number of cells seeded for dose X × (average colonies arising from the non-irradiated cells (0 Gy)/number of non-irradiated cells seeded)) (Kimple et al, 2010). Log (surviving fraction) was plotted against dose for each condition. The linear quadratic model (Y=e^-[α*X+β*X2]) was fit to the clonogenic survival curve for each condition, where the parameters α and β were calculated according to the method of Fertil (Fertil et al, 1984). The survival curve and linear quadratic fit were plotted using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA) according to a least-squares fit, weighted to minimise the relative distances squared as described previously (Kimple et al, 2010). The mean inactivation dose (equal to the area under the survival curve) was also calculated according to the method of Fertil (Fertil et al, 1984).
The radiation enhancement ratio (ER) was calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose of experimental conditions (that is, another cell line, or siRNA or drug treatment), as described previously (Morgan et al, 2008). A value significantly >1 indicates radiosensitisation.
Generation of radioresistant PC cell lines
Selection for radiation-resistant subpopulations within parental PC cell lines was attempted originally for both Panc-1 and BxPC3, chosen due to their primary tumour origins as well as their poorly and moderately differentiated status, respectively. Radioselection was also performed in the L3.6pl cell line (derived from COLO 357, which was established from a lymph node metastasis and has well-differentiated status) for validation of results. Cells were subjected to 2 Gray (Gy) radiation daily for 2 weeks, taking weekends off (giving a total of 10 fractions). Throughout the irradiation process and recovery time, cells were kept at 40–70% confluency to ensure potential for exponential growth. After completion of the second week of radiation, cells were maintained in media containing 10% FBS, being washed and re-fed daily for the first 7 days and then every third day thereafter. Radioselected radioresistance was verified by comparing the radiosensitivity of the radiation-selected cells (after a recovery period) with their respective parental cell lines by CSA as described above.
RNA extraction and cDNA synthesis from PC cell lines
Total RNA was extracted using the Qiagen RNAeasy RNA isolation kit (Qiagen, Valencia, CA, USA). RNA was converted to cDNA using 2 μg of total RNA, oligo(dT)18 primer, and the SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA).
Global gene expression analysis
Global genome microarray analysis was performed by the UNMC microarray core facility. Phalanx whole-genome cDNA microarray platforms were used containing 30 275 features probing for ∼22 000 unique genes. To ensure valid comparison across cell lines, all samples were normalised against a universal human reference (Stratagene, Cat: 740000, Cedar Creek, TX, USA).
Discovery of radioresistance-associated pathways
Radioresistance-associated pathways were determined by comparative gene expression analysis of radioresistant cell line (Panc-1RR) with that of parental cell line exhibiting relative radiosensitivity (Panc-1). On completion of unbiased discovery of radioresistance-associated gene differential expression, ingenuity pathway analysis was performed to identify pathways and cellular functions most implicated in our radioresistance model, as well as to determine potential targets for radiosensitisation.
Quantitative real-time PCR validation of microarray transcriptional analysis
After analysis of microarray results for radioresistance-associated differential expression, altered transcription of 12 selected genes associated with PC radioresistance was validated using quantitative real-time PCR (qRT-PCR) using SYBR green-based chemistry. qRT-PCR was performed using 1 μl of a 1 : 5 dilution of first-strand cDNA using the SYBR Premix Ex Taq kit (Takara Bio, Madison, WI, USA) and specific primers (sequence in Supplementary Table 4) on a Roche Light Cycler 480 system (Roche Diagnostics, Mannheim, Germany). Fold change in gene expression was determined by the delta-delta CT method as described previously (Baine et al, 2011).
RNA interference and transient transfection
Farnesyl diphosphate synthase (FDPS) siRNA pool of three target-specific 19–25 nt siRNAs was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) (sc-75011). For transient transfection, recipient cells were seeded in a six-well plate 24 h before transfection. Following serum starvation for 4 h, the cells were transfected using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer's protocol. Four hours following transfections, the cells were replenished with serum-containing media and protein was isolated from the cells 48 h post transfection.
Whole-cell lysate preparation
Cells were rinsed with PBS and then lysed on culture dishes using RIPA buffer containing protease and phosphatase inhibitors (Moniaux et al, 2004) and stored at −20°C. Thawed samples were passed through a 27-gauge needle and syringe, and clarified by centrifugation at 14 000 r.p.m. at 4 °C for 20 min. Protein concentration in lysates was determined by the Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
SDS–PAGE and western blot analysis
Equal amounts of protein were separated on 12% SDS–polyacrylamide gels and blotted onto polyvinylidene fluoride membranes (EMB Millipore, Billerica, MA, USA) and developed per lab protocol (Andrianifahanana et al, 2005). Horseradish peroxidase-conjugated appropriate secondary antibodies were diluted at 1 : 3000 in PBS and incubated for 1 h at room temperature. After three washes in PBST, membranes were developed using the Pierce ECL Western Blotting Substrate (Thermo, Rockford, IL, USA) and blots were exposed to ECL-sensitive film (MIDSCI, St Louis, MO, USA). The films were digitally scanned, and figures were assembled using Microsoft PowerPoint.
Immunohistochemistry
Tissue arrays comprised of normal pancreas (from donors for pancreas transplant) and PC patient samples were obtained from the Rapid Autopsy Program of the University of Nebraska Medical Center (PI: Michael Hollingsworth, IRB# 091-01-FB). The tissues were stained with anti-FDPS polyclonal antibody (ab38854, Abcam, Cambridge, MA, USA) using an antibody dilution of 1 : 100. The immunostaining technique was performed according to the standard laboratory procedure (Moniaux et al, 2008). The FDPS staining was scored by a pathologist using the criteria described in Supplementary Methods. Representative photographs were taken under a Nikon light microscope with a Q-capture Micropublisher 5.0 camera (Leeds Precision Instruments, Minneapolis, MN, USA) by utilising the Q-capture suite software package (QImaging, Surrey, BC, Canada).
Evaluation of pathway-directed pharmaceuticals for cell growth inhibition and radiosensitisation
MTT assay was performed with 10 nM–100 μM ZOL to evaluate toxicity. Subsequently, cell lines were exposed to ZOL in conjunction with radiation and assessed by colony forming assay as described above. Concentrations of 10 nM–10 μM of ZOL were used with radiation, as these concentrations have been previously shown to inhibit pathways of interest (Chen et al, 2002; Skerjanec et al, 2003; Almubarak et al, 2011). Cells were treated with ZOL either before and after radiation or only after as indicated. Once cells were switched to media containing 5% FBS, all media given to the cells was drug free.
Tumour implantation
All animal studies were performed under an approved protocol by the University of Nebraska Medical Center Institutional Animal Care and Use Committee (IACUC). Subcutaneous tumour growth was evaluated with the UN-KPC-961 mouse PC cell line. Cells (5 × 106) suspended in 50 μl sterile PBS were injected in two areas (one to be irradiated and one unirradiated) in immune-competent mixed Sv129/C57BL/6 background mice on the right and left posterior lateral thorax caudal to the axilla. Before implantation, mice were shaved at the site of injection. Tumours were allowed to grow for 2 weeks before randomisation. Tumour growth was monitored by measuring with vernier calipers.
Radiation treatment planning
Planning CT scans (Sensation Open; Siemens Healthcare, Eschborn, Germany) at a slice thickness of 0.6 mm were performed in anaesthetised mice that were implanted with pancreatic tumours. Before scanning, mice were anaesthetised with xylazine/ketamine, and restrained in the prone position. Gross tumour volume and the organs at risk such as lung and spinal cord were contoured on axial CT slices of all mice. A planning target volume expansion of 3 mm was used. The iPlan (Brainlab AG, Feldkirchen, Germany) system was used by a radiation oncologist and radiation physicist to generate 3D conformal plans, providing a steep dose fall-off in normal tissue away from the target to be irradiated (Supplementary Figure 1A). The prescribed target dose was 35 Gy in 7 Gy daily fractions. At least 95% of the prescribed dose encompassed 100% of the planning target volume and at least 95% of the planning target volume received 100% of the prescribed dose.
Radiation treatment
Radiation was delivered 2–3 days after planning CT scans. One hour before irradiation, mice were randomised to receive clinical grade ZOL (2 mg kg−1, 50 μl of a 4 mg per 5 ml solution for a 20 mg mouse; equivalent to a 10-mg human dose calculated based on body surface area (Reagan-Shaw et al, 2008)) or vehicle control by intraperitoneal injection.
The Novalis accelerator (Brainlab AG) was used to deliver stereotactic radiation therapy. It incorporates stereotactic X-ray capabilities for verifying target position (ExacTrac; Brainlab AG). For soft-tissue targets, the system is designed to be used with radio-opaque metal markers placed near the target. These markers can be observed within the field of view of the X-ray localisation system at the time of treatment. In detail, each mouse was anaesthetised and restrained. A metal bb was placed on the tumour, and the locating lasers in the x–y–z planes were placed directly on the bb/dot. Each mouse was imaged with ExacTrac, and the mouse spine from the ExacTrac image was superimposed onto the mouse spine from the pre-treatment CT image (Supplementary Figure 1B shows before and after ExacTrac). The couch position was adjusted accordingly. Radiation treatment consisted of 35 Gy in 7 Gy daily fractions delivered in 5 consecutive days with 3–5.5-mm multileaf collimators (Novalis) and 6 MV photons with a dose rate of 480–650 cGy min−1. No animal shielding was used, and no obvious radiation toxicity was observed for subcutaneous tumour-targeted radiation.
Measurement of treatment responses
Tumour volumes were measured with calipers immediately before irradiation, and every 3–5 days after that. Tumour volume was calculated using the formula: volume=[length × (width^2)]/2.
Histological analysis of radiated and ZOL-treated tumours
Pathologic responses were scored by a pathologist. Necrosis was measured in percentages at 10% increments. Fibrosis was measured as described in Supplementary Methods.
Statistical analysis
CSAs were performed no less than on two separate dates for each cell line. Mean surviving colonies and s.e. were calculated for each radiation dose and compared between cell lines. Colony survival was considered a continuous variable and cell lines were compared using two-tailed Student's t-test with P-values <0.05 being considered statistically significant. All CSA data were analysed using the MedCalc version 9.3.0.0 software (MedCalc Software bvba, Ostend, Belgium) and GraphPad Prism v.5.0.
Microarray analysis was accomplished through log2 transformation of all ratios followed by normalisation to ‘centre' each array using lowess smoother through BRB ArrayTools (Simon et al, 2007). Genes in which the percent of spots missing or filtered out exceeded 50% were excluded. Genes whose expression differed by at least 1.5-fold from the median in at least 20% of the samples were retained, genes not meeting this criteria were excluded due to low variability across the samples. Univariate analyses were performed comparing each utilised cell line and cell line derivative using a two-sample t-test using the random variance model. P-values <0.001 were considered significant.
The non-parametric Wilcoxon rank sum test was used to compare protein levels with radiation and chemotherapy status. P-values <0.05 are statistically significant.
Response to treatment of tumour allografts was analysed with a two-sample t-test, with P-value <0.05 considered significant.
Results
Natural radiosensitivity varies across PC cell lines
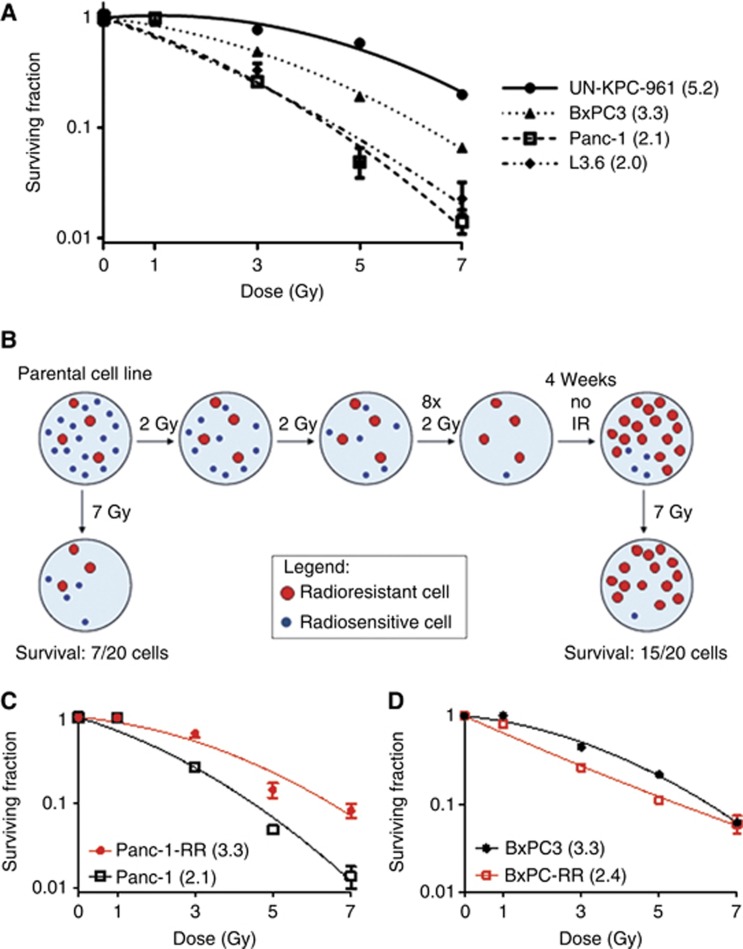
CSAs of PC cell lines revealed variation in their radiosensitivity (Figure 1A). Of note, Panc-1 and BxPC3 revealed a significant difference in natural radiosensitivity, with BxPC3 being more radioresistant than Panc-1 at doses of 3 Gy and above. These results indicate that an average of 1.6-fold radiation dose is required to produce equivalent cell death in BxPC3 as in Panc-1. UN-KPC-961 was the most resistant line used. The UN-KPC-961 cell line was derived and characterised in our laboratory (Torres et al, 2013) from a primary tumour developing in KPC (LSL-KrasG12D/+;Trp53R172H/+;Pdx-1-Cre) genetically engineered mouse model of PC (Hingorani et al, 2005). It was observed that the immortalised human pancreatic ductal epithelial cell line (HPDE) was highly sensitive to radiation, with no colonies observed with a 2 Gy treatment and consequently, could not be included in CSA analysis. Summary of cell line mutations is presented in Supplementary Table 1.
Figure 1.
Response of PC cell lines to radiation and generation of radiation-resistant sublines. (A) Colony formation assays of PC cell lines exposed to the indicated dose of radiation reveal a heterogeneous response. It was observed that the immortalised human pancreatic ductal epithelial cell line (HPDE) was highly sensitive to radiation, with no colonies being observed with 2 Gy treatment (not shown). Cell line names are given with the mean inactivation dose, ( QUOTE ), given in parentheses. Mean inactivation dose has units of Gy. (B) Schematic of the generation of radiation-resistant sublines. Cell lines were exposed to 10 fractions of 2 Gy irradiation over a 2-week period and then allowed to recover. Subsequently, colony formation assays were done following exposure to varying doses of radiation to compare the sensitivity between the parental and radioresistant sublines. (C) Colony formation assay of Panc-1RR vs Panc-1. The Panc-1 subline exposed to fractionated radiation required 1.6-fold higher IR dose to elicit equivalent cell death as the parental Panc-1, and was thus referred to as radioresistant, Panc-1RR. The mean inactivation dose, ( QUOTE ), is given in parentheses. (D) Colony formation assay of BxPC3-RR vs BxPC3. No difference was observed between the naturally radioresistant parental line BxPC3 and the BxPC3 subline exposed to fractionated radiation (BxPC3-RR).
Generation of radioresistant cell lines
For the generation of radioresistant cell lines, cells were exposed to 10 fractions of 2 Gy X-rays and then allowed to recover for 4 weeks (6–10 passages; Figure 1B). After this recovery period, the Panc-1 subline that had been exposed to 10 fractions of 2 Gy had a significant increase in radioresistance as compared with parental Panc-1, with CSAs indicating that Panc-1RR requires a 1.6-fold higher IR dose as compared with parental Panc-1 to elicit equivalent cell death (Figure 1C). This Panc-1 subline with confirmed induced radioresistance was subsequently referred to as Panc-1RR. Importantly, this increased radioresistance was found to be stable, with similar results obtained 6 and 18 passages after completion of the fractionated IR regimen. No difference was observed in BxPC3 after fractionated IR exposure (BxPC3-RR vs BxPC3), potentially indicating that BxPC3 cells are either more homogenous in their radiosensitivity or are incapable of becoming more radioresistant (Figure 1D).
Global expression analysis of PC cell lines
Microarray analysis comparing global expression levels across cell lines revealed notable differential expression profiles. A total of 54 genes were found to be differentially expressed (P⩽0.001), with fold differences ranging from six-fold higher (FDPS) to five-fold lower (LOC146481) in Panc-1RR as compared with Panc-1 parental cell line (Supplementary Table 2). In contrast, only a few genes were differentially expressed between BxPC3-RR and BxPC3, with expression levels of only 8 genes found to be differentially expressed between the two (P⩽0.001), ranging from three-fold higher (RASSF8) to four-fold lower (ASIP) in BxPC3-RR (Supplementary Table 3).
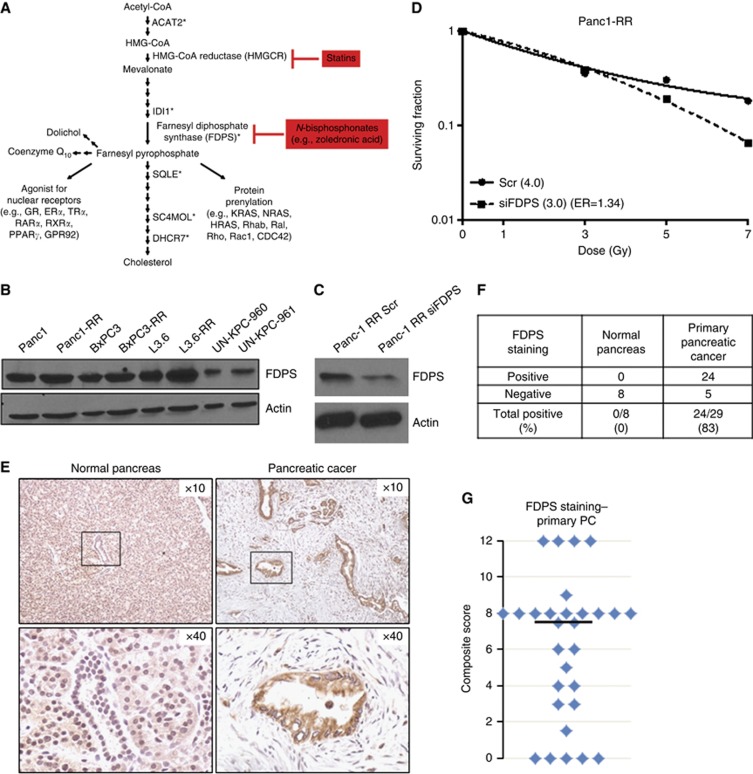
Ingenuity pathway analysis of the differentially expressed genes in Panc-1RR compared with Panc-1 indicates an array of cellular functions that have a role in PC radioresistance, with the two most associated cellular functions being lipid metabolism (P=2.66E−08 to 6.83E−03) and small molecule biochemistry (that is, synthesis of cholesterol and lipids; P=2.66E−08 to 6.83E−03). Further, the canonical pathways exhibiting association with the differentially expressed genes are those of the superpathway of cholesterol biosynthesis (P=4.47E−11) (Figure 2A; the top eight canonical pathways are involved in cholesterol/mevalonate synthesis) and fatty acid biosynthesis (P=4.43E−3).
Figure 2.
Cholesterol (mevalonate) synthesis pathway genes are upregulated in radiation-resistant cells. (A) Simplified cholesterol biosynthesis pathway. Asterisks (*) mark upregulated genes in Panc-1 radiation-resistant (Panc-1RR) cells. FDPS is a branchpoint enzyme in the pathway, producing farnesyl pyrophosphate that is used for cholesterol synthesis, post-translational modification of small GTPases, including KRAS, termed farnesylation or geranylgeranylation (collectively called prenylation), which helps traffic proteins to membranes, and recently discovered to be an agonist for several nuclear receptors. ZOL (Zometa) inhibits FDPS, whereas the cholesterol-lowering statins inhibit HMG-CoA reductase (HMGCR), the rate-limiting enzyme of the cholesterol pathway. (B) Immunoblot of FDPS protein expression in various PC cell lines. (C) Immunoblot of Panc-1RR cells transfected with control scrambled (Scr) siRNA and FDPS siRNA indicating efficient knockdown at 48 h. (D) Colony formation assay comparing Panc-1RR cells transfected with scrambled control (Scr) or FDPS siRNA. Cells were irradiated 48 h after transfection, and then seeded for colony assay. siFDPS cells were sensitised to radiation, with ER being 1.34. Surviving fraction is expressed relative to non-irradiated cells in the respective treatment group. Graph shows the mean±s.e. of triplicate samples of a representative experiment. (E) Immunohistochemical analysis of normal pancreas (left) and PC tissue (right) for FDPS. Normal pancreas samples were from donors for pancreas transplant while PC specimens were from the Rapid Autopsy Program at the University of Nebraska Medical Center. Samples were mounted as tissue arrays and all were stained at the same time. Scoring was done by one pathologist. (F) Table summarising the incidence of FDPS overexpression in pancreatic tumours. (G) Scatter plot indicating the distribution of composite score for FDPS staining in pancreatic tumours. The median composite score (black bar) was 7.5.
qRT-PCR validation of radioresistance-associated genes
To validate the results of the microarray analysis, 11 genes (FDPS, ACAT2, AG2, CLDN7, DHCR7, ELFN2, FASN, SC4MOL, SLC12A2, SQLE, and IDI1) found to be associated with PC radioresistance were validated by qRT-PCR for differential expression between Panc-1RR and Panc-1. From this analysis, 100% validation of microarray results was observed (Table 1).
Table 1. Validated genes differentially expressed in radioselected Panc-1RR vs parental Panc-1 cell lines (fold change ratio shown).
|
Panc-1RR
vs
Panc-1 |
||
|---|---|---|
| Gene name | qRT-PCR | Microarray |
| FDPS | 7.14 | 5.93 |
| ACAT2 | 2.49 | 3.03 |
| AG2 | 1.87 | 2.59 |
| CLDN7 | 0.07 | 0.49 |
| DHCR7 | 1.15 | 2.96 |
| ELFN2 | 0.01 | 0.34 |
| FASN | 1.97 | 2.62 |
| SC4MOL | 2.83 | 3.57 |
| SLC12A2 | 1.49 | 1.99 |
| SQLE | 2.86 | 2.5 |
| IDI1 | 7.23 | 4.15 |
Abbreviation: qRT-PCR=quantitative real-time PCR.
Cell line expression of FDPS and siRNA knockdown for radiosensitisation
As FDPS was the top differentially upregulated gene in our microarray and because FDPS is a major branchpoint enzyme of the cholesterol synthesis pathway, we further investigated its role in radioresistance. Western blotting revealed that FDPS is expressed in all PC cell lines tested. Marginal increases in FDPS protein expression could be seen in the Panc-1RR cells compared with parental Panc-1, and in the recently induced radiation-resistant subline L3.6pl-RR compared with parental L3.6pl (Figure 2B).
To determine whether FDPS contributes to radiation resistance, Panc-1RR cells were transfected with pooled siRNA to specifically knockdown FDPS expression. Panc-1RR cells were successfully transfected with either FDPS-specific siRNA or nonspecific control scrambled siRNA, with knockdown of FDPS seen at 48 h after transfection (Figure 2C). Radiosensitivity was examined at this 48 h time point after transfection. Knockdown of FDPS increased radiosensitivity, with an ER of 1.34 (Figure 2D).
Immunohistochemistry of FDPS in PC patient samples
As knockdown of FDPS resulted in radiosensitisation in PC cells, we wanted to explore the presence of FDPS in human PC samples. PC patient tissue array samples were obtained from the Rapid Autopsy Program at the UNMC. Remarkably, staining with anti-FDPS antibody revealed low expression of FDPS in normal pancreas samples (n=8) from donors for pancreas transplant, but was significantly overexpressed in 83% (24 out of 29) of primary pancreatic adenocarcinoma patient samples (Figure 2E and F). Tumour samples had a median composite score of 7.5 (range 0–12, with 12 being the maximum score possible), while the mean composite score of normal pancreas ranged from 0 to 1 (Figure 2G). No significant association between FDPS composite score and patient exposure to treatment with radiation or chemotherapy was observed, as assessed by Wilcoxon rank sum test (not shown).
ZOL radiosensitises PC cells
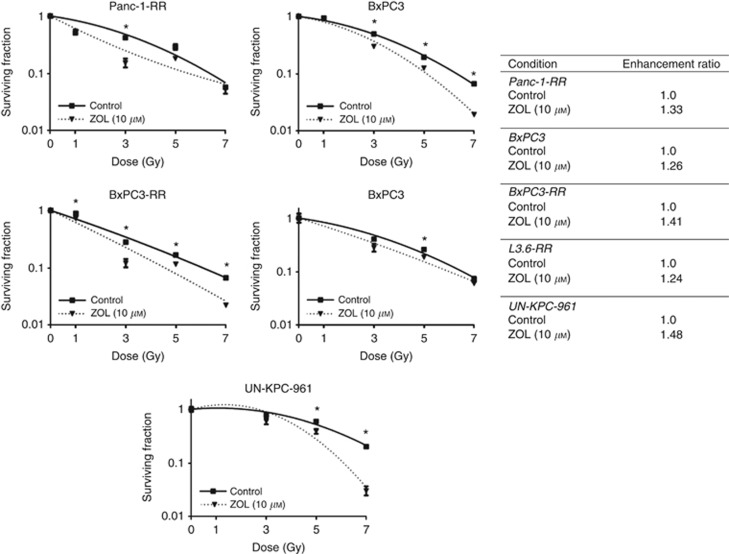
ZOL inhibits FDPS and thus, was used for further study as a potential radiosensitiser. On analysis, it was found that 10 μM ZOL acts as a potent radiosensitiser of Panc-1RR, BxPC3, and BxPC3-RR cell lines, but not the naturally sensitive Panc-1, with radiation ER of 1.26, 1.41, and 1.33 for BxPC3, BxPC3-RR, and Panc-1RR, respectively (Figure 3). In MTT assays, ZOL at 10 μM exhibited mild toxicity (data not shown); however, this toxicity was accounted for in CSAs, as surviving fraction is normalised to unirradiated control for each concentration of ZOL used. ZOL was also found to radiosensitise the radioselected L3.6-RR cells and the naturally radioresistant UN-KPC-961 cell line (Figure 3).
Figure 3.
Inhibition of FDPS radiosensitises PC cells. (Left) CSAs of radioselected Panc-1RR, BxPC3, fractionated BxPC3-RR, UN-KPC-961, and radioselected L3.6-RR cells following radiation treatment in the absence (control) and presence of indicated concentration of ZOL. ZOL treatment was 24 h before and 72 h after radiation treatment for Panc-1RR, BxPC3, fractionated BxPC3-RR, and 72 h after radiation treatment for UN-KPC-961 and radioselected L3.6-RR cells. Surviving fraction is expressed relative to non-irradiated cells in the respective treatment group. Graph shows the mean±s.e. of triplicate samples of a representative experiment. Asterisks indicate P-value <0.05. (Right) ER for each cell line following ZOL treatment. A value significantly >1 indicates radiosensitisation.
In vivo radiation treatment and ZOL radiosensitisation
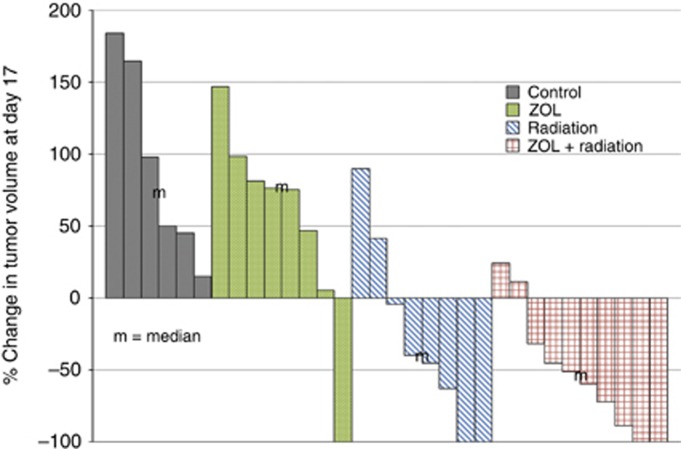
To determine whether ZOL treatment could radiosensitise PC cells in vivo, UN-KPC-961 tumours were established subcutaneously on the right (to be irradiated) and left (unirradiated control) posterior lateral thorax of Sv129/C57BL/6 mixed background mice. Each tumour-bearing mouse received a planning CT scan and 3D conformal radiation treatment plan, generated within 2 days of the start of radiation treatment (Supplementary Figure 1A). Mice bearing tumours of at least 100 mm3 were randomly assigned to receive a single dose of either ZOL (2 mg kg−1) or vehicle by intraperitoneal injection 1 h before radiation. Tumours on the right lateral chest received five fractions of 7 Gy (total 35 Gy) on 5 consecutive days. Even without shielding of the mice, no obvious radiation toxicity could be seen in mice receiving 35 Gy total dose targeted to subcutaneous tumours. Tumour volume was measured with vernier calipers during the course of treatment to determine response compared with the first day of radiation.
Unirradiated tumours in the mice that received a single injection of ZOL were seen to shrink in the first week after treatment, but then grew at the same rate as untreated tumours, and by day 17 were not significantly different in mean percent change in tumour volume. Tumours receiving radiation alone had maximal tumour volume shrinkage by day 10, but in most mice it exhibited increased size by day 17. Tumours receiving a single injection of ZOL plus radiation had maximal tumour volume shrinkage at day 17. Treatment response at day 17 was plotted and compared by waterfall plot (Figure 4). Although 63% (5 out of 8) of animals showed response with radiation alone, ZOL plus radiation produced a response rate of 80% (8 out of 10). The mean percent change in tumour volume for tumours receiving ZOL plus radiation was not seen to be statistically significant (P=0.404) compared with tumours receiving radiation alone. Histological comparison of tumours revealed increased areas of fibrosis with less tumour cell density in tumours receiving radiation, but no significant differences were observed for fibrosis score or tumour cell dropout in radiated tumours receiving ZOL compared with radiation alone (Supplementary Figure 2).
Figure 4.
Effect of ZOL on radiation therapy of subcutaneous mouse allograft tumours. Immune-competent mice were injected s.c. with UN-KPC-961 cells in two areas – one to be irradiated and one as unirradiated control. Half of the mice were given ZOL (2 mg kg−1) 1–2 h before the first radiation dose. Each mouse was aligned to its CT scan by ExacTrac and then stereotactically irradiated on 5 consecutive days with 7 Gy fractions. Waterfall plot showing percentage change in tumour volume at day 17 as observed by caliper measurement of tumours for all mouse tumours examined in the study. Similar results were observed with volume measured by CT imaging. Median of each treatment group is marked with ‘m'.
Discussion
Successful radiosensitisation has significant potential to provide improved prognosis in PC patients. Increasing radiotherapy effectiveness would greatly improve outcome for patients with locally advanced disease, as a greater proportion of patients would be successfully downstaged to resectable disease, thereby improving their 5-year survival rate from 8.6 to 21.5% and providing them with access to the only PC therapy with reasonable curative potential (SEER; National Cancer Institute, 2011). This study determined pathways associated with PC radioresistance through comparison of natural and radioselected radioresistant cell lines with their differentially radiosensitive counterparts. These methods allow for the discovery of genes common to both PC cell lines with inherent radioresistance and the radioresistant subpopulations of PC cell lines with relative radiosensitivity, providing comprehensive insight into genes associated with PC's evasion of IR-induced cell death.
Such methods for isolating radioresistant subpopulations from cell lines have been previously reported using breast (Li et al, 2001), oesophageal (Fukuda et al, 2004), and lung cancer (Xu et al, 2008; Lee et al, 2010) cell lines. Unbiased analysis of radioresistance in PC has been attempted only once before (Ogawa et al, 2006), with some major differences with our study. Ogawa et al used high-dose fractions and high total dose (10 Gy fractions with 14-day recovery period between each fraction for total dose 150–180 Gy), whereas we used 10 fractions of 2 Gy in a 2-week period (total dose 20 Gy). Ogawa et al used a different microarray platform and used only one cell line in common with our study, Panc-1, but were unable to establish a radioresistant subline from it. These differences may explain why none of the differentially expressed genes were common between their study and ours. Ogawa et al found 73 upregulated genes common to radioresistant sublines, including the growth factor amphiregulin, and genes associated with cell proliferation (MAPKAPK2), intracellular calcium signalling (regucalcin) and disruption of vascularisation (angiopoietin 2). Downregulated genes included genes associated with apoptosis (caspase 8) and others. Encouragingly, siRNA screens are now being used to identify radiosensitisation targets, such as PPP2R1A, a scaffolding protein of phosphatase 2A (Wei et al, 2013). Of the 69 confirmed hits from this screen done in MiaPaCa-2 cells, suspected (Chk1, ATR, MCL1) and novel genes (PLA2G4B, GPR2, MTMR3, RAD9A, KLC2L, KIFAP3, KLF3, DVL2, LZTR1, SIAH2, PLCD1) were identified as targets for radiosensitisation. More cell lines require testing with these screens to capture the heterogeneities in PC radioresponse.
Such unbiased studies are necessary as previous attempts at determining the causes of PC radioresistance relied primarily on specific pathways related to apoptosis, cell survival, and cell cycle progression while overlooking other pathways and cellular processes that are considered to likely result in increased radioresistance, such as DNA damage recognition and repair (DiBiase et al, 1999; Satyamoorthy et al, 2000; Macaulay et al, 2001; Munshi et al, 2004; Kim et al, 2007; Hui et al, 2009; Wang et al, 2009; Romano et al, 2010). In addition, despite the few pathways currently implicated in PC radioresistance, none has yet been effectively targeted to elicit therapeutic radiosensitisation clinically.
The results of this study indicate the importance of cholesterol and fatty acid biosynthesis in PC radiation resistance. Such implication has only been indicated once before in a directed study showing that fatty acid synthase (FASN) is associated with PC radioresponse (Yang et al, 2011). Importantly, increased expression of FASN was found to be strongly associated with both natural and radioselected radioresistance in our study as well, validating both our methods as well as the observations of this previous study. We specifically found that FDPS, an important branchpoint enzyme of the cholesterol synthesis pathway, is directly involved in radioresistance (Figure 2C and D). Moreover, FDPS was expressed in all PC cell lines tested (Figure 2B) and is overexpressed in human PC tissues (Figure 2E–G). Low-to-moderate expression in normal pancreas as observed in our study has also been reported earlier (Romanelli et al, 2009). Although FDPS has been seen to be overexpressed in multiple solid cancers by gene expression microarray (Pilarsky et al, 2004), to our knowledge, this is the first report to determine FDPS protein levels in human cancer tissues. Although elucidation of the molecular mechanisms underlying the association of FDPS, FASN, as well as the nine other radioresistance-associated genes, with PC radioresistance has not yet been undertaken in this or previous works, previous studies indicating the necessity for cholesterol biosynthesis in the membrane association and activity of both mutant and wild-type KRAS in PC may explain our observations (Ura et al, 1994). To this point, ZOL and cholesterol-lowering statins (that is, HMG-CoA reductase inhibitors) have been shown to decrease Ras prenylation or Ras protein levels in cells with mutant Ras (Tassone et al, 2003; Marten et al, 2007; Wasko et al, 2011).
Our studies indicate the potential of targeting cholesterol biosynthesis for radiosensitisation of PC through the successful synergy of siRNA knockdown of FDPS and IR in vitro, and ZOL and IR in vitro and in vivo. Currently, ZOL, a potent inhibitor of FDPS, is used in the treatment of patients with breast and prostate cancers that have metastasised to the bone, to prevent treatment-associated bone loss in breast cancer, as well as to slow the progression of osteoporosis (Brufsky et al, 2007; Gnant et al, 2007). ZOL has also undergone clinical trials as an adjuvant to standard of care for breast cancer, showing mixed efficacy results (Brufsky et al, 2007; Gnant et al, 2007; Coleman et al, 2011). ZOL has been seen to radiosensitise fibrosarcoma (Koto et al, 2013), osteosarcoma (Ryu et al, 2010), oesophageal squamous cell carcinoma (You et al, 2014), and breast cancer cells (Ural et al, 2006) in preclinical studies. Importantly, ZOL has recently undergone preclinical study as a chemosensitiser for PC, specifically as an add-on therapy to enhance the efficacy of gemcitabine, with promising results (Zhao et al, 2012). A phase II clinical study of ZOL with gemcitabine in advanced PC patients (n=35 enroled) showed the combination to be well-tolerated, although final results have not been published (ASCO 2006 abstract; Cov et al, 2006). An additional phase I trial (n=15) for neoadjuvant ZOL treatment for patients with resectable PC also was well tolerated (Sanford et al, 2013). To date, however, no study has examined ZOL specifically as a radiosensitiser in PC. Our results indicate that ZOL treatment can significantly radiosensitise PC cell lines in vitro (Figure 3). The ER of 1.3–1.5 is comparable with that reported for known radiosensitisers, such as gemcitabine (Pauwels et al, 2005) and cisplatin (Skov and MacPhail, 1991), and for other reported radiosensitisers, such as dual prenyl transferase inhibitors (Brunner et al, 2005), the HIV protease inhibitor nelfinavir (Kimple et al, 2010), and Chk1 and PARP inhibitors (Vance et al, 2011).
ZOL treatment also provided a trend towards radiosensitisation of allograft tumours of a radioresistant murine cell line derived from a genetically engineered KPC mouse tumour (Figure 4). The dose of ZOL used (2 mg kg−1) is equivalent to a 10 mg human dose calculated based on body surface area (Reagan-Shaw et al, 2008), and it should be noted that the standard clinical dose of ZOL, administered as an infusion every 3–4 weeks in the treatment of cancer-induced bone disease, is 4 mg, which yields a peak plasma concentration (Cmax) of 1–2 μM (Rogers and Holen, 2011). This dose was chosen because it is within the range of ZOL used in clinical trials (4–16 mg) and because it is well below the highest reported non-lethal single dose (10 mg kg−1) in mice (Zometa package insert, 2006). Possible reasons for not reaching significance in the in vivo studies could be single dose administration of ZOL to more accurately reflect clinical dosing, use of a highly radioresistant cell line, or a sample size of mice too small to reach significance. Further study is needed to determine whether multiple doses of ZOL may enhance radiosensitisation.
Using a clinical CT scanner, clinical treatment planning software, and linear accelerator, we were able to accurately and precisely irradiate subcutaneous tumours at high doses without shielding. Although most preclinical studies use small-dose fractions (3–10 fractions of 1–2 Gy; Morgan et al, 2008; Kimple et al, 2010; Wei et al, 2013), we were able to deliver high-dose fractions (five fractions of 7 Gy), which are being investigated in stereotactic hypofractionated regimens for PC at UNMC and other institutions. By using immune-competent mice in our study, we better modeled the effects of radiation on the immune system, clearance of radiated cancer cells by the immune system, and possible immunogenicity of radiated tumours (abscopal effect). This also provides for added anti-tumour effects of ZOL from a competent immune system, such as through activation of gamma delta (γδ) T cells (Marten et al, 2007), repolarisation of macrophages (Coscia et al, 2010; Rogers and Holen, 2011), and impairment of myeloid-derived suppressor cells (Porembka et al, 2012).
Despite the novelty and positive results of this work, several pitfalls remain. Currently, the only in vivo model available for PC radiation research consists of subcutaneous transplantation that has proven a poor indicator of clinical efficacy for both chemo- and radiotherapy. In addition, only two cell lines and two cell line derivatives were utilised in the gene expression microarray profiling in this study (although more were used to check FDPS expression and to test ZOL), potentiating that our results may not remain consistent across all PC cells lines or in the complexities of PC patients. It is further important to note that the radioresisant phenotype was diminished beyond 18 passages in the absence of continued radiation exposure. Also, we are yet to determine whether ZOL can radiosensitise normal organs involved in radiation toxicity, such as small intestine. Biochemical tests are also needed to determine tumour uptake of ZOL, as bisphosphonates accumulate highly in bones. However, this study describes comprehensive pathway analysis from the context of PC radiation resistance and opens avenues for future studies both in PC as well as in other cancers with low radiation efficacy. For example, several interesting upregulated genes in our microarray could be investigated for their role in radioresistance, including the chemokine CCL2 and the splicing factor SFPQ (PSF), which is associated with DNA repair and RNA biogenesis and mediates radioresistance of HeLa cells (Ha et al, 2011). Downregulated genes in our microarray, such as the pro-apoptotic BNIP3 and galectin-3 (LGALS3), could prove to be important radioresponse genes.
On the basis of our results, several future studies are warranted. Microarray analysis and correlation of gene expression with radioresistance, both natural and radioselected, should be undertaken in a greater number of cell lines to determine the universality of the identified genes across all PC. This approach could also be used to compare the gene expression profiles of radiation responder vs non-responder human tumours. For example, unbiased analysis of tumour samples taken pre-irradiation could be correlated with treatment response. In addition, ZOL should be further studied as a radiosensitiser using either spontaneous genetically engineered mouse models or directly in phase I trials. Further, unbiased studies such as this should be undertaken in other cancers such as renal cell carcinoma and melanoma, in which radiotherapy has a significant role in patient treatment but lacks general efficacy, to determine novel pathways and targets for radioresistance.
Acknowledgments
We thank Dr Michel Ouellette and Dr Ying Yan for discussions. We also thank the UNMC Microarray Core Facility for technical assistance; Dr Michael Hollingsworth for providing tissue arrays of PC patient samples and Judy Anderson and Dr Paul Grandgenett for help acquiring patient treatment status; and the patients who donated their tissues for research. This work was in part supported by grants from the National Institutes of Health (TMEN U54 CA163120, EDRN UO1 CA111294, and SPORE P50 CA127297).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Almubarak H, Jones A, Chaisuparat R, Zhang M, Meiller TF, Scheper MA. Zoledronic acid directly suppresses cell proliferation and induces apoptosis in highly tumorigenic prostate and breast cancers. J Carcinog. 2011;10:2. doi: 10.4103/1477-3163.75723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Andrianifahanana M, Agrawal A, Singh AP, Moniaux N, van Seuningen I, Aubert JP, Meza J, Batra SK. Synergistic induction of the MUC4 mucin gene by interferon-gamma and retinoic acid in human pancreatic tumour cells involves a reprogramming of signalling pathways. Oncogene. 2005;24 (40:6143–6154. doi: 10.1038/sj.onc.1208756. [DOI] [PubMed] [Google Scholar]
- Asiyanbola B, Gleisner A, Herman JM, Choti MA, Wolfgang CL, Swartz M, Edil BH, Schulick RD, Cameron JL, Pawlik TM. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg. 2009;13 (4:752–759. doi: 10.1007/s11605-008-0762-x. [DOI] [PubMed] [Google Scholar]
- Baine MJ, Chakraborty S, Smith LM, Mallya K, Sasson AR, Brand RE, Batra SK. Transcriptional profiling of peripheral blood mononuclear cells in pancreatic cancer patients identifies novel genes with potential diagnostic utility. PLoS One. 2011;6 (2:e17014. doi: 10.1371/journal.pone.0017014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman DA, Greer S, Pardee AB. Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by beta-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran- 5,6-dione), a novel DNA repair inhibitor. Cancer Res. 1987;47 (20:5361–5366. [PubMed] [Google Scholar]
- Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, Hohneker J, Lacerna L, Petrone S, Perez EA. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25 (7:829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- Brunner TB, Cengel KA, Hahn SM, Wu J, Fraker DL, McKenna WG, Bernhard EJ. Pancreatic cancer cell radiation survival and prenyltransferase inhibition: the role of K-Ras. Cancer Res. 2005;65 (18:8433–8441. doi: 10.1158/0008-5472.CAN-05-0158. [DOI] [PubMed] [Google Scholar]
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William TL, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16 (7:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42 (11:1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Ritchie D, Pugh J, Gaunt C, Rea U, Peterson J, Davies C, Hiley V, Gregory W, Bell R. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365 (15:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Monkkonen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14 (12:2803–2815. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cov J, Cartwright T, Neubauer M, McCollum D, Sandbach J, Monticelli K, Boehm K, Ilegbodu D, Asmar L. A Phase II study of gemcitabine plus zoledronic acid in subjects with Stage IV pancreatic cancer. J Clin Oncol. 2006;24 (18S:14002. [Google Scholar]
- DiBiase SJ, Guan J, Curran WJ, Jr, Iliakis G. Repair of DNA double-strand breaks and radiosensitivity to killing in an isogenic group of p53 mutant cell lines. Int J Radiat Oncol Biol Phys. 1999;45 (3:743–751. doi: 10.1016/s0360-3016(99)00229-1. [DOI] [PubMed] [Google Scholar]
- Engelke CG, Parsels LA, Qian Y, Zhang Q, Karnak D, Robertson JR, Tanska DM, Wei D, Davis MA, Parsels JD, Zhao L, Greenson JK, Lawrence TS, Maybaum J, Morgan MA. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res. 2013;19 (16:4412–4421. doi: 10.1158/1078-0432.CCR-12-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99 (1:73–84. [PubMed] [Google Scholar]
- Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, Vallis KA, Hammond EM, Olcina MM, Gillies MW, Muschel RJ, Brunner TB. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, Yamagishi H. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer. 2004;91 (8:1543–1550. doi: 10.1038/sj.bjc.6602187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, Menzel C, Piswanger-Soelkner JC, Galid A, Mittlboeck M, Hausmaninger H, Jakesz R. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25 (7:820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- Goodman KA, Hajj C. Role of radiation therapy in the management of pancreatic cancer. J Surg Oncol. 2013;107 (1:86–96. doi: 10.1002/jso.23137. [DOI] [PubMed] [Google Scholar]
- Ha K, Takeda Y, Dynan WS. Sequences in PSF/SFPQ mediate radioresistance and recruitment of PSF/SFPQ-containing complexes to DNA damage sites in human cells. DNA Repair (Amst) 2011;10 (3:252–259. doi: 10.1016/j.dnarep.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazard L. The role of radiation therapy in pancreas cancer. Gastrointest Cancer Res. 2009;3 (1:20–28. [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7 (5:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hui Z, Tretiakova M, Zhang Z, Li Y, Wang X, Zhu JX, Gao Y, Mai W, Furge K, Qian CN, Amato R, Butler EB, Teh BT, Teh BS. Radiosensitization by inhibiting STAT1 in renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2009;73 (1:288–295. doi: 10.1016/j.ijrobp.2008.08.043. [DOI] [PubMed] [Google Scholar]
- Kim DW, Seo SW, Cho SK, Chang SS, Lee HW, Lee SE, Block JA, Hei TK, Lee FY. Targeting of cell survival genes using small interfering RNAs (siRNAs) enhances radiosensitivity of Grade II chondrosarcoma cells. J Orthop Res. 2007;25 (6:820–828. doi: 10.1002/jor.20377. [DOI] [PubMed] [Google Scholar]
- Kimple RJ, Vaseva AV, Cox AD, Baerman KM, Calvo BF, Tepper JE, Shields JM, Sartor CI. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16 (3:912–923. doi: 10.1158/1078-0432.CCR-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koto K, Murata H, Kimura S, Sawai Y, Horie N, Matsui T, Ryu K, Ashihara E, Maekawa T, Kubo T, Fushiki S. Zoledronic acid significantly enhances radiationinduced apoptosis against human fibrosarcoma cells by inhibiting radioadaptive signaling. Int J Oncol. 2013;42 (2:525–534. doi: 10.3892/ijo.2012.1735. [DOI] [PubMed] [Google Scholar]
- Lee YS, Oh JH, Yoon S, Kwon MS, Song CW, Kim KH, Cho MJ, Mollah ML, Je YJ, Kim YD, Kim CD, Lee JH. Differential gene expression profiles of radioresistant non-small-cell lung cancer cell lines established by fractionated irradiation: tumor protein p53-inducible protein 3 confers sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;77 (3:858–866. doi: 10.1016/j.ijrobp.2009.12.076. [DOI] [PubMed] [Google Scholar]
- Li Z, Xia L, Lee LM, Khaletskiy A, Wang J, Wong JY, Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155 (4:543–553. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Macaulay VM, Salisbury AJ, Bohula EA, Playford MP, Smorodinsky NI, Shiloh Y. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene. 2001;20 (30:4029–4040. doi: 10.1038/sj.onc.1204565. [DOI] [PubMed] [Google Scholar]
- Marten A, Lilienfeld-Toal M, Buchler MW, Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J Immunother. 2007;30 (4:370–377. doi: 10.1097/CJI.0b013e31802bff16. [DOI] [PubMed] [Google Scholar]
- Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, Lele SM, Ouellette M, Pour PM, Sasson AR, Brand RE, Hollingsworth MA, Jain M, Batra SK. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98 (9:1540–1547. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52 (2:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14 (16:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A, Kurland JF, Nishikawa T, Chiao PJ, Andreeff M, Meyn RE. Inhibition of constitutively activated nuclear factor-kappaB radiosensitizes human melanoma cells. Mol Cancer Ther. 2004;3 (8:985–992. [PubMed] [Google Scholar]
- National Cancer Institute Surveillance, Epidemiology, and End Results Program, Cancer Statistics Review, 1975–2006 (2011
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297 (3:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Utsunomiya T, Mimori K, Tanaka F, Haraguchi N, Inoue H, Murayama S, Mori M. Differential gene expression profiles of radioresistant pancreatic cancer cell lines established by fractionated irradiation. Int J Oncol. 2006;28 (3:705–713. [PubMed] [Google Scholar]
- Pauwels B, Korst AE, Lardon F, Vermorken JB. Combined modality therapy of gemcitabine and radiation. Oncologist. 2005;10 (1:34–51. doi: 10.1634/theoncologist.10-1-34. [DOI] [PubMed] [Google Scholar]
- Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6 (6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61 (9:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22 (3:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan GE, Gunderson LL, Nagorney DM, Martin JK, Ilstrup DM, Holbrook MA, Kvols LK, McIlrath DC. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61 (6:1110–1116. doi: 10.1002/1097-0142(19880315)61:6<1110::aid-cncr2820610610>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Romanelli MG, Lorenzi P, Sangalli A, Diani E, Mottes M. Characterization and functional analysis of cis-acting elements of the human farnesyl diphosphate synthetase (FDPS) gene 5' flanking region. Genomics. 2009;93 (3:227–234. doi: 10.1016/j.ygeno.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Romano S, D'Angelillo A, Pacelli R, Staibano S, De LE, Bisogni R, Eskelinen EL, Mascolo M, Cali G, Arra C, Romano MF. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 2010;17 (1:145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- Ryan DP, Mamon H.2009. Management of locally advanced and borderline resectable pancreatic exocrine cancer. Up to Date, Wolter Kluwer Health: Alphen aan den Rijn, Netherlands.
- Ryu K, Murata H, Koto K, Horie N, Matsui T, Nishigaki Y, Sakabe T, Takeshita H, Itoi M, Kimura S, Ashihara E, Maekawa T, Fushiki S, Kubo T. Combined effects of bisphosphonate and radiation on osteosarcoma cells. Anticancer Res. 2010;30 (7:2713–2720. [PubMed] [Google Scholar]
- Sanford DE, Porembka MR, Panni RZ, Mitchem JB, Belt BA, Plambeck-Suess SM, Lin G, Denardo DG, Fields RC, Hawkins WG, Strasberg SM, Lockhart AC, Wang-Gillam A, Goedegebuure SP, Linehan DC. A study of zoledronic acid as neo-adjuvant, perioperative therapy in patients with resectable pancreatic ductal adenocarcinoma. J Cancer Ther. 2013;4 (3:797–803. doi: 10.4236/jct.2013.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, Chehab NH, Waterman MJ, Lien MC, El-Deiry WS, Herlyn M, Halazonetis TD. Aberrant regulation and function of wild-type p53 in radioresistant melanoma cells. Cell Growth Differ. 2000;11 (9:467–474. [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63 (1:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64 (2:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- Skerjanec A, Berenson J, Hsu C, Major P, Miller WH, Jr, Ravera C, Schran H, Seaman J, Waldmeier F. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol. 2003;43 (2:154–162. doi: 10.1177/0091270002239824. [DOI] [PubMed] [Google Scholar]
- Skov K, MacPhail S. Interaction of platinum drugs with clinically relevant x-ray doses in mammalian cells: a comparison of cisplatin, carboplatin, iproplatin, and tetraplatin. Int J Radiat Oncol Biol Phys. 1991;20 (2:221–225. doi: 10.1016/0360-3016(91)90094-k. [DOI] [PubMed] [Google Scholar]
- Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D'Alessandro A, Galea E, Goel A, Abbruzzese A, Boland CR, Venuta S. Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer. 2003;88 (12:1971–1978. doi: 10.1038/sj.bjc.6600986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MP, Rachagani S, Souchek JJ, Mallya K, Johansson SL, Batra SK. Novel pancreatic cancer cell lines derived from genetically engineered mouse models of spontaneous pancreatic adenocarcinoma: applications in diagnosis and therapy. PLoS One. 2013;8 (11:e80580. doi: 10.1371/journal.pone.0080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura H, Obara T, Nishino N, Tanno S, Okamura K, Namiki M. Cytotoxicity of simvastatin to pancreatic adenocarcinoma cells containing mutant ras gene. Jpn J Cancer Res. 1994;85 (6:633–638. doi: 10.1111/j.1349-7006.1994.tb02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ural AU, Avcu F, Candir M, Guden M, Ozcan MA. In vitro synergistic cytoreductive effects of zoledronic acid and radiation on breast cancer cells. Breast Cancer Res. 2006;8 (4:R52. doi: 10.1186/bcr1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance S, Liu E, Zhao L, Parsels JD, Parsels LA, Brown JL, Maybaum J, Lawrence TS, Morgan MA. Selective radiosensitization of p53 mutant pancreatic cancer cells by combined inhibition of Chk1 and PARP1. Cell Cycle. 2011;10 (24:4321–4329. doi: 10.4161/cc.10.24.18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Wang ZX, Qiu Y, Xiong J, Chen YX, Miao DS, De W. Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits growth, reduces invasion, and enhances radiosensitivity in human osteosarcoma cells. Mol Cell Biochem. 2009;327 (1-2:257–266. doi: 10.1007/s11010-009-0064-y. [DOI] [PubMed] [Google Scholar]
- Wasko BM, Smits JP, Shull LW, Wiemer DF, Hohl RJ. A novel bisphosphonate inhibitor of squalene synthase combined with a statin or a nitrogenous bisphosphonate in vitro. J Lipid Res. 2011;52 (11:1957–1964. doi: 10.1194/jlr.M016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, Morgan MA. Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res. 2013;19 (16:4422–4432. doi: 10.1158/1078-0432.CCR-13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu QY, Gao Y, Liu Y, Yang WZ, Xu XY. Identification of differential gene expression profiles of radioresistant lung cancer cell line established by fractionated ionizing radiation in vitro. Chin Med J (Engl) 2008;121 (18:1830–1837. [PubMed] [Google Scholar]
- Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L, Chen JH, Zhang JT. Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol. 2011;2 (1:89–98. [PMC free article] [PubMed] [Google Scholar]
- You Y, Liu J, Wang Z, Zhang Y, Ran Y, Guo X, Liu H, Wang H. The enhancement of radiosensitivity in human esophageal squamous cell carcinoma cells by zoledronic acid and its potential mechanism. Cytotechnology. 2014;66 (1:17–25. doi: 10.1007/s10616-012-9532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Tominaga Y, Ohuchida K, Mizumoto K, Cui L, Kozono S, Fujita H, Maeyama R, Toma H, Tanaka M. Significance of combination therapy of zoledronic acid and gemcitabine on pancreatic cancer. Cancer Sci. 2012;103 (1:58–66. doi: 10.1111/j.1349-7006.2011.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.