Abstract
Background:
The impact of combining plasma fibrinogen levels with Epstein–Barr Virus DNA (EBV DNA) levels on the prognosis for patients with nasopharyngeal carcinoma (NPC) was evaluated.
Methods:
In this observational study, 2563 patients with non-metastatic NPC were evaluated for the effects of circulating plasma fibrinogen and EBV DNA levels on disease-free survival (DFS), distant metastasis-free survival (DMFS), and overall survival (OS).
Results:
Compared with the bottom biomarker tertiles, TNM stage-adjusted hazard ratios (HR, 95% confidence intervals (CIs)) for predicting DFS in fibrinogen tertiles 2 to 3 were 1.26 (1.00 to 1.60) and 1.81 (1.45 to 2.26), respectively; HR for EBV DNA tertiles 2 to 3 were 1.49 (1.12 to 1.98) and 4.24 (3.27 to 5.49), respectively. After additional adjustment for established risk factors, both biomarkers were still associated (P for trend <0.001) with reduced DFS (HR: 1.79, 95% CI, 1.43 to 2.25 for top fibrinogen tertiles; HR: 4.04, 95% CI: 3.10 to 5.27 for top EBV DNA tertiles compared with the bottom tertiles). For patients with advanced-stage disease, those with high fibrinogen levels (⩾3.34 g l−1) presented with worse DFS, regardless of EBV DNA ⩾4000 or <4000 copies ml−1 subgroup. Similar findings were observed for DMFS and OS.
Conclusions:
Circulating fibrinogen and EBV DNA significantly correlate with NPC patients survival. Combined fibrinogen and EBV DNA data lead to improved prognostic prediction in advanced-stage disease.
Keywords: nasopharyngeal carcinoma, EBV DNA, fibrinogen, survival
Nasopharyngeal carcinoma (NPC) is endemic in Southern China and Southeast Asia, where a peak incidence of 50 cases per 100 000 has been reported (Wee et al, 2010). Radiotherapy is the primary treatment modality, and concurrent chemoradiotherapy with or without adjuvant chemotherapy is the primary regimen for patients with locoregionally advanced NPC. However, patients with similar stages and histologic classifications have markedly different survival outcomes given the heterogeneity of protein expression profiles (Ludwig and Weinstein, 2005; Wei and Sham, 2005). Various studies have attempted to identify molecular biomarkers to predict NPC progression, and numerous promising biomarkers have been evaluated as potential prognosis predictors of NPC. Recently, pretreatment plasma Epstein–Barr Virus (EBV) DNA levels were clinically employed as a useful tool for NPC diagnosis, risk stratification, monitoring and prognosis (Lo et al, 1999; Chan et al, 2002; Leung et al, 2003a; Lin et al, 2004; Leung et al, 2006). EBV DNA levels are considered the most attractive potential biomarker that complements TNM classification in NPC (Ng et al, 2014). Given the biological heterogeneity of cancer, the present staging system, even in combination with plasma EBV DNA levels, remains inadequate for predicting NPC patient prognosis. Therefore, we hypothesise that additional biomarkers could complement EBV DNA levels. These biomarkers could be used in combination to improve the prognostic stratification of NPC patients.
Fibrinogen, a circulating glycoprotein that is a nonspecific acute-phase reactant and important clotting factor, might serve as a useful biomarker in this context. Fibrinogen converts to insoluble fibrin via thrombin, thereby significantly affecting inflammatory response, fibrinolysis, blood clotting, wound healing and neoplasia (Mosesson, 2005). Increased fibrinogen levels influence cancer cell growth, progression and metastasis. Hyperfibrinogenaemias are associated with various human malignancies, including oesophageal (Takeuchi et al, 2007), colorectal (Yamashita et al, 2009), ovarian (Polterauer et al, 2009a), cervical (Polterauer et al, 2009b), and pancreatic cancer (Guo et al, 2009). Nasopharyngeal carcinoma is associated with EBV infection and hence chronic inflammation. Nevertheless, little information is known regarding the clinical significance of fibrinogen and the potential complementary role of fibrinogen and EBV DNA levels in predicting NPC carcinogenesis and progression. Therefore, this large cohort study compared the efficacy of EBV DNA and fibrinogen alone and in combination for predicting NPC patient survival. In addition, this study provides information regarding personalised therapy.
Materials and Methods
Two thousand seven hundred and sixty-seven patients with primary NPC were consecutively recruited from January 2007 to December 2011 at the Sun Yat-sen University Cancer Center, Guangzhou, China. Patients were excluded from this study if they met the following criteria: (1) previously received any anticancer therapy (n=24); (2) <18 years old (n=12); (3) pregnant or lactating (n=8); (4) unsuitable for chemotherapy as a result of a liver, kidney, lung or heart deficiency (n=15); (5) a history of previous or synchronous malignant tumours (n=17); (6) have primary NPC metastasis (n=90); or (7) lost during follow-up (n=38). In total, 2563 patients with non-metastatic primary NPC were eligible for analysis.
The routine staging work-up included clinical examination of the head and neck region, magnetic resonance imaging scan from the suprasellar cistern to the collarbone, fibreoptic nasopharyngoscopy, chest radiography, abdominal sonography and whole-body bone scan or whole-body FDG PET/CT. All patients were restaged according to the seventh American Joint Committee on Cancer (AJCC) TNM staging manual. In total, 1126 (43.9%) patients were treated with conventional two-dimensional (2D) or three-dimensional (3D) conformal radiotherapy radiotherapy, and 1437 (56.1%) patients were treated with intensity-modulated radiotherapy. In addition, 2183 (87.5%) patients with stage II–IV disease received platinum-based chemotherapy. Concurrent chemoradiotherapy with or without neoadjuvant or adjuvant chemotherapy was administered for advanced-stage disease (stages III and IV). A stratified multitherapeutic protocol was used. Radiation alone was administered for stage I disease, and radiation alone or with concurrent platinum-based chemotherapy was administered for stage II disease (Chen et al, 2011). Concurrent chemoradiotherapy with or without neoadjuvant or adjuvant chemotherapy was administered for advanced-stage disease (stages III and IV). Neoadjuvant or adjuvant chemotherapy consisting of cisplatin plus 5-fluorouracil or cisplatin plus taxane was administered every 3 weeks for two or three cycles (Chen et al, 2012). Concurrent cisplatin chemotherapy was administered on weeks 1, 4 and 7 of RT. All patients were treated according to the principles of treatment for NPC patients at the Sun Yat-sen University Cancer Center, Guangzhou, China.
Collection of data
Before treatment, baseline clinical data were collected regarding sex, age, hereditary NPC, smoking status and PS as assessed by the Eastern Cooperative Oncology Group (ECOG). Information regarding relevant concurrent diseases, such as cardiovascular disease, diabetes and chronic hepatitis, was collected as previous studies have indicated that these factors promote increased plasma fibrinogen levels (Mora et al, 2006; Sinning et al, 2006; Calvaruso et al, 2008; Lowe et al, 2013; Sapkota et al, 2013). These comorbidities and smoking status were defined as follows: chronic hepatitis B: HBsAg-positive >6 months and serum HBV-DNA ⩾2000 IU ml−1 (104 copies ml−1) with or without increased alanine transaminase/aspartate transaminase levels; diabetes: fasting plasma glucose level 7.0 mmol l−1 and/or 2 h plasma glucose level 11.1 mmol l−1 after a 75 g glucose load or a previous diagnosis of diabetes by a healthcare professional; cardiovascular disease: coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, pulmonary embolism, hypertension (systolic blood pressure 140 mm Hg, diastolic blood pressure 90 mm Hg) or a previous diagnosis of any of these diseases made by a healthcare professional; smoking: patients were identified as current, former or never smokers. Patients who smoked or reported smoking cessation within 1 year at the time of the diagnosis were considered current smokers. Patients who had smoked less than 100 cigarettes during their lifetime were considered never smokers.
Plasma fibrinogen evaluation
A 3 ml fasting blood sample was collected from each patient before treatment and processed within 3 h of collection. The plasma was stored at −80 °C until use in assays. Fibrinogen values were measured by the Clauss method using an Automatic Analyser Sysmex CA7000 (Sysmex Corporation, Kobe, Japan) and reagents according to the SIEMENS AG guidelines (Munich, Germany) (Clauss, 1957; Cook and Ubben, 1990). The results were obtained using a standard curve prepared according to the manufacturer's instructions, and the interassay imprecision (coefficient of variation) was <10%. Plasma D-dimer concentrations <3.5 g l−1 were considered normal based on the manufacturer's instructions.
EBV DNA, VCA-IgA and EA-IgA measurement
As previously described (Lo et al, 1999; Shao et al, 2004; An et al, 2011), patient plasma EBV DNA concentrations were routinely measured by q-PCR before treatment. EBV-specific VCA/IgA antibodies and EBV-specific EA/IgA antibodies were assessed using a previously described immunoenzymatic assay (Liu et al, 2012b).
Clinical outcome assessment and patient follow-up
Our primary endpoint was disease-free survival (DFS), and our secondary endpoints were distant metastasis-free survival (DMFS) and overall survival (OS). Disease-free survival was calculated from the date of the first NPC diagnosis to the date of the first relapse at any site, death from any cause or the date of the last follow-up visit. DMFS was determined from the date of the first NPC diagnosis to the date of distant relapse or patient censoring at the date of the last follow-up. Overall survival was calculated from the date of the first NPC diagnosis to the date of death from any cause or patient censoring at the date of the last follow-up. Patients still alive on 31 December 2013 (end of follow-up) were censored at the date of the last contact. After the treatment was complete, the patients were evaluated at 3-month intervals for the first 3 years and every 6 months thereafter.
Statistical analysis
Spearman rank correlation coefficients (rs) were calculated for continuous variables, and fibrinogen and EBV DNA values were divided into tertiles. The Kaplan–Meier method was used to estimate the cumulative survival plot in relation to the variables divided according to their tertiles. The survival among groups was compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for EBV DNA and fibrinogen tertiles were estimated using Cox proportional hazards regression. We first adjusted for TNM stage and then further adjusted for age (years), sex, ECOG performance, pathological type, disease stage, treatment allocation, LDH, VCA-IgA, EA-IgA, smoking (never, former, current), cardiovascular disease (yes, no), diabetes mellitus (yes, no), familial history of NPC (yes, no) and body mass index. To assess potentially confounding variables or effect mediation by other biomarkers, the models assessing the association of fibrinogen with survival were further adjusted for EBV DNA and vice versa. We then evaluated the combined association of EBV DNA and fibrinogen with NPC patient survival by dividing participants into prespecified groups. We analysed the combined association according to high or low fibrinogen (above or below top tertile) and high or low EBV DNA (⩾4000 copies ml−1 or <4000 copies ml−1; Chan et al, 2002; Leung et al, 2006). In addition, we repeated this analysis of the combined association of EBV DNA and fibrinogen using tertiles of the above two biomarkers. Finally, statistical tests for interaction between fibrinogen and EBV DNA tertiles were performed using the TNM stage-adjusted Cox regression models. All reported probability values were two tailed, and P<0.05 was considered significant. Statistical analyses were performed with STATA version 8.2 and SPSS 17.0.
Results
Patient characteristics and association with clinical variables
The characteristics of the 2563 NPC patients are listed in Table 1. The median follow-up time was 37 months (interquartile range (IQR): 28–46).The median fibrinogen level in patients with (n=513) and without (n=2050) relapse were 3.21 (IQR: 2.67–4.06) g l−1 and 2.95 (IQR: 2.49–3.47) g l−1, respectively (P<0.001). The median EBV DNA levels were 22 500 (IQR: 3280–130 000) copies ml−1 and 1940 (IQR: 0–14 225) copies ml−1 in patients with and without relapse, respectively (P<0.001). When examined as continuous variables, fibrinogen was positively correlated with EBV DNA (rs=0.222, P<0.001). In addition, both fibrinogen and EBV DNA concentrations were significantly correlated with TNM staging (rs=0.231, P<0.001; rs=0.369, P<0.001; respectively). In total, 208 patients developed locoregional recurrences, 344 patients had distant metastases and 215 were deceased at the last follow-up.
Table 1. Patient demographics and clinical characteristics.
|
Number of patients (fibrinogen and EBV DNA tertiles,
n=2563) | ||||||
|---|---|---|---|---|---|---|
|
Fibrinogen, g l−1 |
EBV DNA, copies ml−1 |
|||||
| Characteristic | <2.67 | 2.67–3.34 | ⩾3.34 | <326 | 326–11 333 | ⩾11 333 |
|
Age, years | ||||||
| Median | 44 | 47 | 48 | 46 | 46 | 47 |
| Mean | 44.82 | 47.61 | 48.33 | 46.27 | 47.15 | 47.31 |
|
Sex | ||||||
| Male | 635 | 607 | 645 | 608 | 635 | 644 |
| Female | 226 | 256 | 194 | 246 | 220 | 210 |
|
Histology, WHO type | ||||||
| III | 820 | 830 | 812 | 810 | 824 | 828 |
| II | 39 | 28 | 26 | 39 | 30 | 24 |
| I | 2 | 5 | 1 | 5 | 1 | 2 |
|
ECOG | ||||||
| 0–1 | 856 | 861 | 837 | 853 | 851 | 850 |
| 2 | 5 | 2 | 2 | 1 | 4 | 4 |
|
Clinical stage | ||||||
| I | 40 | 23 | 6 | 59 | 8 | 2 |
| II | 158 | 100 | 46 | 210 | 61 | 33 |
| III | 474 | 482 | 446 | 439 | 542 | 421 |
| IV | 189 | 258 | 341 | 146 | 244 | 398 |
|
Tumour stage | ||||||
| T1 | 103 | 62 | 27 | 111 | 50 | 31 |
| T2 | 237 | 164 | 113 | 221 | 150 | 143 |
| T3 | 390 | 435 | 420 | 398 | 448 | 399 |
| T4 | 131 | 202 | 279 | 124 | 207 | 281 |
|
Node stage | ||||||
| N0 | 167 | 167 | 105 | 292 | 97 | 50 |
| N1 | 333 | 310 | 257 | 367 | 322 | 211 |
| N2 | 298 | 309 | 370 | 168 | 384 | 425 |
| N3 | 63 | 77 | 107 | 27 | 57 | 168 |
|
Treatment | ||||||
| Radiotherapy | 168 | 125 | 80 | 229 | 83 | 61 |
| Chemotherapy and radiotherapy | 693 | 738 | 759 | 625 | 772 | 793 |
|
Radiotherapy technique | ||||||
| 2DRT/3DCRT | 414 | 355 | 357 | 355 | 367 | 404 |
| IMRT | 447 | 508 | 482 | 499 | 488 | 450 |
|
VCA-IgA | ||||||
| <1 : 80 | 246 | 249 | 231 | 321 | 223 | 182 |
| ⩾1 : 80 | 615 | 614 | 608 | 533 | 632 | 672 |
|
EA-IgA | ||||||
| <1 : 10 | 391 | 375 | 343 | 465 | 356 | 288 |
| ⩾1 : 10 | 470 | 488 | 496 | 389 | 499 | 566 |
|
LDH, U l−1 | ||||||
| <170 | 489 | 428 | 340 | 494 | 439 | 324 |
| ⩾170 | 372 | 435 | 499 | 360 | 416 | 530 |
|
Body mass index, kg m−2 | ||||||
| <23 | 504 | 449 | 448 | 431 | 470 | 500 |
| ⩾23 | 357 | 414 | 391 | 423 | 385 | 354 |
|
Smoking | ||||||
| Never | 558 | 550 | 467 | 566 | 535 | 474 |
| Ever | 40 | 50 | 46 | 45 | 41 | 50 |
| Current | 263 | 263 | 326 | 243 | 279 | 330 |
|
Chronic HBV
infection | ||||||
| Yes | 69 | 60 | 54 | 58 | 62 | 63 |
| No | 792 | 803 | 785 | 796 | 793 | 791 |
|
Cardiovascular disease | ||||||
| Yes | 35 | 57 | 65 | 51 | 47 | 59 |
| No | 826 | 806 | 774 | 803 | 805 | 795 |
|
Diabetes mellitus | ||||||
| Yes | 15 | 21 | 21 | 22 | 18 | 17 |
| No | 846 | 842 | 818 | 832 | 837 | 837 |
|
Family history of NPC | ||||||
| Yes | 97 | 84 | 83 | 92 | 90 | 82 |
| No | 764 | 779 | 756 | 762 | 765 | 772 |
| Median follow-up (months) | 40 | 35 | 35 | 37 | 36 | 37 |
|
Outcome features | ||||||
| PR | 129 | 159 | 225 | 81 | 122 | 310 |
| Non-PR | 732 | 704 | 614 | 773 | 733 | 544 |
| DM | 80 | 104 | 160 | 40 | 82 | 222 |
| Non-DM | 781 | 759 | 679 | 814 | 773 | 632 |
| LR | 57 | 68 | 83 | 44 | 50 | 114 |
| Non-LR | 804 | 795 | 756 | 810 | 805 | 710 |
| Deaths | 45 | 57 | 113 | 27 | 40 | 148 |
| Non-deaths | 816 | 806 | 726 | 827 | 815 | 706 |
Abbreviations: DM=the number of patients presenting with distant metastasis at the last follow-up; EA=early antigen; ECOG=Eastern Cooperative Oncology Group; HBV=chronic hepatitis B virus; IMRT=intensity-modulated radiotherapy; LDH=serum lactate dehydrogenase levels; LR=the number of patients presenting with local or regional relapse at the last follow-up; Non-DM=the number of patients without distant metastasis at the last follow-up; Non-LR=the number of patients without local or regional relapse at the last follow-up; Non-PR=the number of patients who had not progressed at the last follow-up; NPC=nasopharyngeal carcinoma; PR=the number of patients who progressed at the last follow-up; VCA=viral capsid antigen; WHO, World Health Organization; 2DRT=two-dimensional radiotherapy; 3DCRT=three-dimensional conformal radiotherapy.
Deaths=the number of deceased patients at the last follow-up; Non-deaths=the number of patients alive at the last follow-up.
HRs and 95% CIs comparing fibrinogen and EBV DNA tertiles
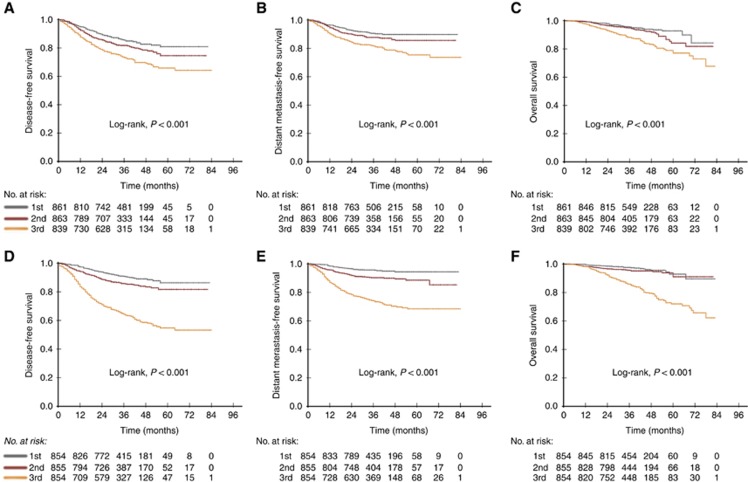
Cumulative DFS, DMFS and OS probabilities for NPC patients indicate that EBV DNA tertiles are superior in survival prediction compared with fibrinogen tertiles (Figure 1), with P<0.001 from the log-rank significance tests across the tertile of either biomarker. As shown in Tables 2 and 3, both fibrinogen and EBV DNA are associated with DFS (TNM stage-adjusted HR: 1.81, 95% CI: 1.45 to 2.26 for top fibrinogen tertiles; TNM stage-adjusted HR: 4.79, 95% CI: 3.33 to 6.89 for top EBV DNA tertiles; both compared with the bottom tertiles). Linear associations were observed for fibrinogen tertiles 2 to 3 and EBV DNA tertiles 2 to 3. After adjusting for age and other risk factors (Tables 2 and 3), the upper tertiles of both biomarkers remained associated with DFS (P for trend <0.001).The HRs were 1.79 (95% CI: 1.43 to 2.25) for the upper fibrinogen tertiles and 4.04 (95% CI, 3.10 to 5.27) for the upper EBV DNA tertiles.
Figure 1.
Upper fibrinogen (A–C) and EBV DNA (D–F) tertiles are associated with DFS, DMFS and OS. P<0.001 for both variables as determined by log-rank significance tests.
Table 2. DFS, DMFS and OS HRs according to fibrinogen tertiles.
|
Fibrinogen tertile, g l−1, n=2563 |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| <2.67 (Bottom) | 2.67–3.34 | ⩾3.34 | P-value(trend) | |
|
DFS | ||||
| TNM stage-adjusteda | 1.00 | 1.26 (1.00–1.60) | 1.81 (1.45–2.26) | <0.001 |
| Plus risk factorsb | 1.00 | 1.30 (1.03–1.65) | 1.79 (1.43–2.25) | <0.001 |
| Plus EBV DNAc | 1.00 | 1.25 (0.98–1.58) | 1.63 (1.30–2.04) | <0.001 |
|
DMFS | ||||
| TNM stage-adjusteda | 1.00 | 1.27 (0.95–1.70) | 1.93 (1.47–2.53) | <0.001 |
| Plus risk factorsb | 1.00 | 1.31 (0.97–1.76) | 1.89 (1.43–2.50) | <0.001 |
| Plus EBV DNAc | 1.00 | 1.24 (0.92–1.67) | 1.68 (1.27–2.23) | <0.001 |
|
OS | ||||
| TNM stage-adjusteda | 1.00 | 1.26 (0.85–1.86) | 2.31 (1.63–3.29) | <0.001 |
| Plus risk factorsb | 1.00 | 1.21 (0.81–1.80) | 2.08 (1.45–2.98) | <0.001 |
| Plus EBV DNAc | 1.00 | 1.16 (0.78–1.73) | 1.85 (1.29–2.65) | <0.001 |
Abbreviations: DFS=disease-free survival; DMFS=distant metastasis-free survival; EBV DNA=Epstein–Barr Virus DNA; HR=hazard ratio; OS=overall survival; TNM stage=clinical stage for NPC based on the seventh American Joint Committee on Cancer (AJCC) TNM staging manual.
The values represent hazard ratios (95% confidence interval).
Obtained from Cox proportional hazard regression models adjusted for TNM stage (IV vs III vs II vs I).
Obtained from Cox proportional hazard regression models adjusted for age (⩾46 years vs <46 years), sex (male vs female), WHO pathological type (undifferentiated non-keratinising vs differentiated non-keratinising vs keratinising squamous cell), ECOG performance status (2 vs 0–1), chemoradiotherapy (yes vs no), radiation technique (intensity-modulated radiotherapy vs 3D-CRT/2D-CRT), lactate dehydrogenase (⩾170 U l−1 vs <170 U l−1), viral capsid antigen (⩾1 : 80 vs<1 : 80), early antigen (⩾1 : 10 vs<1 : 10), body mass index (⩾23 kg m−2 vs <23 kg m−2), smoking status (yes vs no), concurrent cardiovascular disease (yes vs no), diabetes (yes vs no), chronic hepatitis disease (yes vs no) and family history of nasopharyngeal carcinoma (yes vs no). The lowest tertile of each biomarker served as the reference category for the hazard ratios. P-values were obtained from models, which were used to assess linear trends.
Adjusted for all the above variables and EBV DNA.
Table 3. DFS, DMFS and OS HRs according to EBV DNA tertiles.
|
EBV DNA tertile, copies ml−1, n=2563 |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| <326 | 326–11 333 | ⩾11 333 | P (trend) | |
|
DFS | ||||
| TNM stage-adjusteda | 1.00 | 1.49 (1.12–1.98) | 4.24 (3.27–5.49) | <0.001 |
| Plus risk factorsb | 1.00 | 1.46 (1.10–1.95) | 4.04 (3.10–5.27) | <0.001 |
| Plus Fibrinogenc | 1.00 | 1.45 (1.09–1.94) | 3.91 (2.99–5.10) | <0.001 |
|
DMFS | ||||
| TNM stage-adjusteda | 1.00 | 1.94 (1.32–2.85) | 5.51 (3.88–7.84) | <0.001 |
| Plus risk factorsb | 1.00 | 1.95 (1.32–2.87) | 5.34 (3.72–7.66) | <0.001 |
| Plus Fibrinogenc | 1.00 | 1.92 (1.31–2.83) | 5.12 (3.57–7.35) | <0.001 |
|
OS | ||||
| TNM stage-adjusteda | 1.00 | 1.29 (0.78–2.11) | 4.38 (2.86–6.70) | <0.001 |
| Plus risk factorsb | 1.00 | 1.28 (0.78–2.11) | 3.98 (2.57–6.16) | <0.001 |
| Plus Fibrinogenc | 1.00 | 1.25 (0.76–2.06) | 3.76 (2.42–5.82) | <0.001 |
Abbreviations: DFS=disease-free survival; DMFS=distant metastasis-free survival; EBV DNA=Epstein–Barr Virus DNA; HR, hazard ratio, OS=overall survival; TNM stage=clinical stage for NPC based on the seventh American Joint Committee on Cancer (AJCC) TNM staging manual.
The values represent hazard ratios (95% confidence interval).
Obtained from Cox proportional hazard regression models adjusted for TNM stage (IV vs III vs II vs I).
Obtained from Cox proportional hazard regression models adjusted for age (⩾46 years vs <46 years), sex (male vs female), WHO pathological type (undifferentiated non-keratinising vs differentiated non-keratinising vs keratinising squamous cell), ECOG performance status (2 vs 0–1), chemoradiotherapy (yes vs no), radiation technique (intensity-modulated radiotherapy vs 3D-CRT/2D-CRT), lactate dehydrogenase (⩾170 U l−1 vs <170 U l−1), viral capsid antigen (⩾1 : 80 vs<1 : 80); early antigen (⩾1 : 10 vs<1 : 10), body mass index (⩾23 kg m−2 vs <23 kg m−2), smoking status (yes vs no), concurrent cardiovascular disease (yes vs no), diabetes (yes vs no), chronic hepatitis disease (yes vs no) and family history of NPC (yes vs no). The lowest tertile of each biomarker served as the reference category for the hazard ratios. P-values were obtained from models used to assess linear trends.
Adjusted for all the above variables and fibrinogen.
We further adjusted fibrinogen for EBV DNA in a Cox model. The HR comparing the top and bottom fibrinogen tertiles was slightly attenuated to 1.63 (95% CI: 1.30 to 2.04); however, the trend across tertiles remained significant (P for trend<0.001; Table 2). Similarly, in a Cox model that adjusted EBV DNA for fibrinogen, the HR comparing the top and bottom EBV DNA tertiles was also mildly attenuated to 3.91 (95% CI: 2.99 to 5.10), but the trend across tertiles remained significant (P for trend<0.001; Table 3). A similar finding was also observed for DMFS and OS regardless of HR adjusted for TNM stage or other risk factors.
Prognostic value of integrating plasma EBV DNA and fibrinogen levels
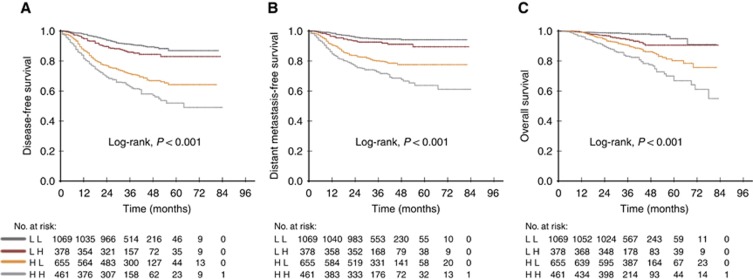
Combinatorial analyses of four prespecified groups of high and low EBV DNA or fibrinogen with DFS, DMFS and OS were performed (Figure 2). Reduced DFS, DMFS and OS were significantly associated with increased levels of both EBV DNA and fibrinogen. Increased DFS, DMFS and OS were significantly associated with low levels of both biomarkers (P log-rank<0.001). Of note, patients with high EBV DNA and low fibrinogen levels displayed increased event rates during follow-up compared with patients with low EBV DNA and high fibrinogen levels (Table 4). In subgroup analysis of advanced-stage disease (stages III and IV), the patients with high fibrinogen levels presented with worse DFS, DMFS and OS regardless of EBV DNA ⩾4000 or <4000 copies ml−1 subgroups. However, a significant difference for predicting distant metastasis in the low DNA group was not achieved. For patients with early stage disease (stages I and II), in the low DNA subgroup, high fibrinogen levels were associated with reduced DFS, DMFS and OS. Statistical significance was achieved for DMFS, but not for DFS and OS. In the high DNA subgroup, patients with high hs-CRP levels maintained reduced DFS, DMFS, and OS. However, statistical significance was only achieved for OS, but not for DFS and DMFS (Table 4).
Figure 2.
Kaplan–Meier curves of DFS, DMFS and OS according to the combination of pretreatment EBV DNA and fibrinogen levels in NPC patients. DFS (A), DMFS (B) and OS (C) values for 2563 patients. LL, <4000 copies ml−1 EBV DNA and <3.34 g l−1 fibrinogen; LH, <4000 copies ml−1 EBV DNA and ⩾3.34 g l−1 fibrinogen; HL, ⩾4000 copies ml−1 EBV DNA and <3.34 g l−1 fibrinogen; HH, ⩾4000 copies ml−1 EBV DNA and ⩾3.34 g l−1 fibrinogen.
Table 4. Log-rank test on DFS, DMFS and OS for TNM stages split by EBV DNA and fibrinogen combination.
|
DFS |
DMFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | No. of patients | Events (No.) | DFS (%) at 3 years | P-value | Events (No.) | DMFS (%) at 3 years | P-value | Events (No.) | OS (%) at 3 years | P-value |
|
All patients | ||||||||||
|
Low DNA | ||||||||||
| Low Fbg | 1069 | 96 | 89 (97.0–90.1) | 54 | 94 (92.0–96.0) | 22 | 98 (96.0–100.0) | |||
| High Fbg | 378 | 51 | 85 (81.1–88.9) | 0.007 | 30 | 91 (87.1–94.9) | 0.031 | 23 | 91 (87.1–94.9) | <0.001 |
|
High DNA | ||||||||||
| Low Fbg | 655 | 192 | 67 (63.1–70.9) | 130 | 78 (74.1–81.9) | 80 | 86 (82.1–89.9) | |||
| High Fbg | 461 | 174 | 58 (52.1–63.9) | 0.001 | 130 | 68 (82.1–73.9) | <0.001 | 90 | 78 (74.1–81.9) | <0.001 |
|
I+II | ||||||||||
|
Low DNA | ||||||||||
| Low Fbg | 277 | 15 | 94 (92.0–96.0) | 5 | 98 (96.0–99.9) | 4 | 99 (97.0–100.0) | |||
| High Fbg | 44 | 5 | 85 (71.3–98.7) | 0.132 | 1 | 95 (85.0–100) | 0.041 | 2 | 93 (83–100.0) | 0.192 |
|
High DNA | ||||||||||
| Low Fbg | 44 | 16 | 59 (41.4–76.6) | 12 | 73 (59.3–86.7) | 2 | 98 (94.1–100.0) | |||
| High Fbg | 8 | 5 | 30 (0.0–67.2) | 0.084 | 3 | 44 (0.00–93.0) | 0.431 | 4 | 63 (29.7–96.3) | 0.001 |
|
III+IV | ||||||||||
|
Low DNA | ||||||||||
| Low Fbg | 792 | 81 | 87 (85.0–89.0) | 49 | 93 (91.0–95.0) | 18 | 97 (95.0–99.0) | |||
| High Fbg | 334 | 34 | 85 (81.1–88.9) | 0.054 | 29 | 90 (86.1–93.9) | 0.108 | 21 | 91 (87.1–94.9) | 0.001 |
|
High DNA | ||||||||||
| Low Fbg | 611 | 176 | 68 (64.1–71.9) | 118 | 78 (74.1–81.9) | 78 | 85 (81.1–88.9) | |||
| High Fbg | 453 | 169 | 58 (52.1–63.9) | 0.001 | 127 | 69 (63.1–74.9) | <0.001 | 86 | 78 (74.1–81.9) | 0.001 |
Abbreviations: DFS=disease-free survival; DMFS=distant metastasis-free survival, EBV DNA=Epstein-Barr Virus DNA; HR=hazard ratio; OS=overall survival; TNM stage=clinical stage for NPC based on the seventh American Joint Committee on Cancer (AJCC) TNM staging manual.
Low DNA defined as <4000 copies ml−1 EBV DNA; high DNA defined as ⩾4000 copies ml−1 EBV DNA; low Fbg defined as <3.34 g l−1 fibrinogen; high Fbg defined as ⩾3.34 g l−1 fibrinogen; P-values compared for overall log-rank trend test. Events (No.)=the total number of events that occurred at the last follow-up. Values in parentheses indicate 95% confidence interval ranges.
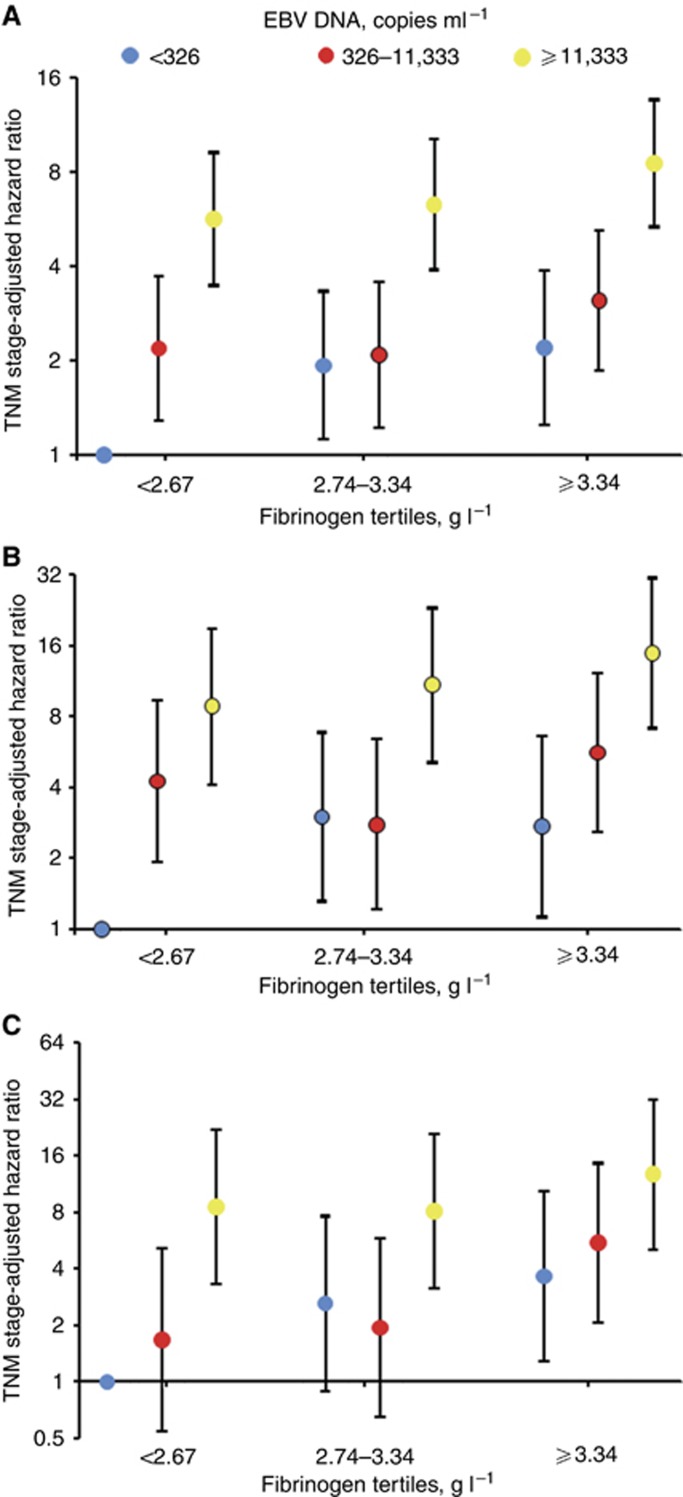
We then divided the participants into nine categories according to EBV DNA and fibrinogen tertiles. Using patients with the lowest EBV DNA and fibrinogen levels (EBV DNA <326 copies ml−1 and fibrinogen <2.67 g l−1) as the reference group, the TNM stage-adjusted HRs associated with DFS, DMFS and OS in patients with EBV DNA ⩾11 133 copies ml−1 and fibrinogen ⩾3.34 g l−1 were 8.51(95% CI, 5.34–13.56), 14.86 (7.13 to 30.95) and 12.77 (5.08 to 32.12), respectively (Figure 3). When examined separately using the above cut-offs of high vs low fibrinogen and EBV DNA levels, the TNM stage-adjusted HRs associated with DFS, DMFS and OS were 1.60 (95% CI: 1.34 to 1.91), 1.69 (95% CI: 1.36 to 2.10) and 2.05 (95% CI: 1.56 to 2.69), respectively, for fibrinogen and 3.35 (95% CI: 2.78 to 4.03), 3.64 (95% CI: 2.89 to 4.59) and 3.77 (95% CI: 2.80 to 5.08), respectively, for EBV DNA (data not shown). Patients with either upper values for EBV DNA but lower or intermediate values for fibrinogen or upper values for fibrinogen but lower or intermediate values for EBV DNA displayed poorer survival compared with patients with lower values for both biomarkers. Therefore, similar results were obtained when we employed tertile cut-offs to define increased EBV DNA levels compared with previously reported cut-offs. Multiplicative interactions between fibrinogen tertiles and EBV DNA categories with regard to relapse, distant metastasis and death were not observed; the P-values for these interactions were 0.554, 0.760 and 0.067, respectively.
Figure 3.
TNM stage-adjusted HRs predicting recurrence (A), distant metastasis (B) and death (C) are presented for EBV DNA and fibrinogen on the y axis. The fibrinogen tertile limits were <2.67 g l−1, 2.67 to 3.34 g l−1 and ⩾3.34 g l−1. The EBV DNA tertile limits were <326 copies ml−1, 326 to 11 333 copies ml−1 and ⩾11 333 copies ml−1.
Discussion
In this large-scale observational study of 2563 patients with non-metastatic primary NPC, we found that increased EBV DNA and fibrinogen levels alone and in combination are associated with reduced DFS, DMFS and OS. Despite the positive correlation between EBV DNA and fibrinogen, increased levels of these biomarkers together were associated with reduced survival. The predictive value of EBV DNA was superior to that of fibrinogen, and the combinatorial effect was greater than the individual effects of either biomarker alone, without evidence of multiplicative interactions. Patients with ⩾11 133 copies ml−1 of EBV DNA and ⩾3.34 g l−1 of fibrinogen display a greater than eightfold increased risk of disease progression compared with patients with <326 copies ml−1 of EBV DNA and <2.67 g l−1 of fibrinogen.
Previous studies (Lo et al, 1999; Chan et al, 2002; Lin et al, 2004; Leung et al, 2006; An et al, 2011) have examined the association between EBV DNA and NPC prognosis. In addition, the prognostic value of fibrinogen has also been demonstrated in other cancers (Takeuchi et al, 2007; Yamashita et al, 2009; Polterauer et al, 2009a; Polterauer et al, 2009b). However, the clinical value of fibrinogen alone or in combination with plasma EBV DNA has not been assessed in NPC. Interestingly, our findings demonstrate that EBV DNA and fibrinogen display an additive effect even upon adjustment for established risk factors. These results suggest a complementary role for these biomarkers in risk prediction that is not provided by standard risk factors or either biomarker alone.
Recent advancements in NPC patient classification and NPC molecular alterations, including microRNA signatures and the NPC-SVM classifier (Wang et al, 2011a; Liu et al, 2012a), have been made. However, these developments required expensive and complicated procedures, and rapid clinical implementation was difficult to achieve. To date, routine prognostic risk assessment of NPC patients still relies on traditional clinico-pathological prognostic variables and EB virus-associated blood tests. Plasma fibrinogen levels are established, routinely measured blood-based parameters that are reproducibly detected without additional laborious efforts. Thus, fibrinogen is an attractive biomarker that is potentially useful for improving the prognostic stratification of NPC patients. Although EBV DNA combined with fibrinogen did not enhance prognostic prediction compared with EBV DNA alone in patients with early stage disease, the combination improved prognostic stratification in patients with advanced NPC (Table 4).These results suggest that these biomarkers may be selectively useful in advanced stage NPC. Although other established roles of plasma/serum EBV DNA were confirmed in monitoring relapse (Leung et al, 2003b) and response to treatment (Chan et al, 2004), in patient follow-up (Wang et al, 2011b) (Hsu et al, 2013) and in population screening (Chan et al, 2013), the value of fibrinogen alone or combined with EBV DNA in these fields will require further exploration.
The mechanism by which these biomarkers combination improves the prognostic stratification of NPC patients is unclear. In addition to its role as an inflammatory biomarker, fibrinogen is the predominant coagulation factor in blood plasma and has an important role in platelet aggregation and fibrin formation (Hackam and Anand, 2003; Kerlin et al, 2004). Increased platelet counts promote distant metastases in NPC patients (Gao et al, 2013). Increased plasma fibrinogen levels may result from the production of tumour-associated cytokines or endothelial cells via the host vs tumour response, the endogenous production of fibrinogen by tumour cells or tumour growth-induced hypercoagulation and hypoxia (Wenger et al, 1995; Palumbo et al, 2000; Wang et al, 2005; Sahni et al, 2008). Tumour growth increases hypoxia and induces tumour cell apoptosis. Previous studies demonstrated that EBV DNA molecules are released into the circulation by apoptosis and represent the tumour load (Mutirangura et al, 1998; Chan et al, 2003). This finding partially explains why EBV DNA levels correlate with fibrinogen levels. In addition, our findings potentially suggest that fibrinogen and EBV DNA may represent different signalling pathways or biological behaviours that contribute to NPC progression.
The major drawback of our study is the single measurement of both EBV DNA and fibrinogen. A single measurement potentially underestimates the magnitude of the association between the biomarkers and survival. The second limitation is that the data were obtained exclusively from one centre. Although our cancer centre treats a large number of NPC patients, these results need to be validated in other data sets. The third limitation is that the median follow-up time was 37 months. Patients remain closely followed, and we will report 5-year follow-up results as available.
The study's strengths include reliable fibrinogen and EBV DNA measurements with high accuracy in a core laboratory and well-characterised risk factor profiles of patient that allowed for the control of potential confounding variables. In addition, the large sample size allowed both biomarkers to be examined individually and in combination for incident events and subgroup analysis.
In conclusion, our results suggest that plasma fibrinogen may serve as a new tool for assessing prognosis of NPC patients. In addition, the baseline assessment of both plasma EBV DNA and serum fibrinogen levels significantly improves DFS, DMFS and OS predictions as well as prognostic stratification of NPC patients according to TNM staging.
If the results from the forthcoming large collaborative studies confirm our results, this simple noninvasive approach may prove beneficial in prognostic stratification of NPC patients in clinical trials. The approach could be used to guide individual treatment, ultimately improving NPC outcome. However, randomised biomarker trials are required to determine whether this molecular staging strategy can improve NPC management compared with the conventional staging approach before implementation.
Acknowledgments
We gratefully recognise the patients who participated in this study. This study was partly supported by the Ministry of Science and Technology of China (No. 2011CB504300), the National Natural Science Foundation of China (81025014, 81230045, 91019015, 81071932, 30600755 and 81072226), the 863 Project (No: 2012AA02A501), the National Key Basic Research Program of China (No: 2013CB910304), the Sci-Tech Project Foundation of Guangdong Province (No: 2011B080701034), the Sci-Tech Project Foundation of Guangzhou City (No: 2011J4300100), the Sun Yat-sen University Clinical Research 5010 Program and the Fundamental Research Funds for the Central Universities.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- An X, Wang FH, Ding PR, Deng L, Jiang WQ, Zhang L, Shao JY, Li YH. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117 (16:3750–3757. doi: 10.1002/cncr.25932. [DOI] [PubMed] [Google Scholar]
- Calvaruso V, Maimone S, Gatt A, Tuddenham E, Thursz M, Pinzani M, Burroughs AK. Coagulation and fibrosis in chronic liver disease. Gut. 2008;57 (12:1722–1727. doi: 10.1136/gut.2008.150748. [DOI] [PubMed] [Google Scholar]
- Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, Mo F, Lai M, Ho S, Huang DP, Johnson PJ. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94 (21:1614–1619. doi: 10.1093/jnci/94.21.1614. [DOI] [PubMed] [Google Scholar]
- Chan AT, Ma BB, Lo YM, Leung SF, Kwan WH, Hui EP, Mok TS, Kam M, Chan LS, Chiu SK, Yu KH, Cheung KY, Lai K, Lai M, Mo F, Yeo W, King A, Johnson PJ, Teo PM, Zee B. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22 (15:3053–3060. doi: 10.1200/JCO.2004.05.178. [DOI] [PubMed] [Google Scholar]
- Chan KC, Hung EC, Woo JK, Chan PK, Leung SF, Lai FP, Cheng AS, Yeung SW, Chan YW, Tsui TK, Kwok JS, King AD, Chan AT, van Hasselt AC, Lo YM. Early detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA analysis in a surveillance program. Cancer. 2013;119 (10:1838–1844. doi: 10.1002/cncr.28001. [DOI] [PubMed] [Google Scholar]
- Chan KC, Zhang J, Chan AT, Lei KI, Leung SF, Chan LY, Chow KC, Lo YM. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res. 2003;63 (9:2028–2032. [PubMed] [Google Scholar]
- Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Mao YP, Tang LL, Chen YM, Liu MZ, Ma J. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13 (2:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, Li NW, Xiang YQ, Luo DH, Qiu F, Sun R, Deng MQ, Chen MY, Hua YJ, Guo X, Cao KJ, Hong MH, Qian CN, Mai HQ. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011;103 (23:1761–1770. doi: 10.1093/jnci/djr432. [DOI] [PubMed] [Google Scholar]
- Clauss A. [Rapid physiological coagulation method in determination of fibrinogen] Acta Haematol. 1957;17 (4:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Cook NS, Ubben D. Fibrinogen as a major risk factor in cardiovascular disease. Trends Pharmacol Sci. 1990;11 (11:444–451. doi: 10.1016/0165-6147(90)90125-r. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhang HY, Xia YF. Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34 (1:39–45. doi: 10.1007/s13277-012-0508-y. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38 (3:e75–e79. doi: 10.1097/MPA.0b013e3181987d86. [DOI] [PubMed] [Google Scholar]
- Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA. 2003;290 (7:932–940. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Chan SC, Chang KP, Lin TL, Lin CY, Hsieh CH, Huang SF, Tsang NM, Lee LY, Ng SH, Wang HM. Clinical scenario of EBV DNA follow-up in patients of treated localized nasopharyngeal carcinoma. Oral Oncol. 2013;49 (6:620–625. doi: 10.1016/j.oraloncology.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, Zogg M, Hendrickson SB, Mosesson MW, Lord S, Weiler H. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood. 2004;103 (5:1728–1734. doi: 10.1182/blood-2003-08-2886. [DOI] [PubMed] [Google Scholar]
- Leung SF, Chan AT, Zee B, Ma B, Chan LY, Johnson PJ, Lo YM. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer. 2003;98 (2:288–291. doi: 10.1002/cncr.11496. [DOI] [PubMed] [Google Scholar]
- Leung SF, Lo YM, Chan AT, To KF, To E, Chan LY, Zee B, Huang DP, Johnson PJ. Disparity of sensitivities in detection of radiation-naive and postirradiation recurrent nasopharyngeal carcinoma of the undifferentiated type by quantitative analysis of circulating Epstein-Barr virus DNA1,2. Clin Cancer Res. 2003;9 (9:3431–3434. [PubMed] [Google Scholar]
- Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai M, Chan KC, Chan LY, Kwan WH, Lo YM, Chan AT. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24 (34:5414–5418. doi: 10.1200/JCO.2006.07.7982. [DOI] [PubMed] [Google Scholar]
- Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, Jiang RS. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350 (24:2461–2470. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei RR, Zhang MY, Sun Y, Huang BJ, Chen M, He QM, Jiang N, Chen L, Cho WC, Yun JP, Zeng J, Liu LZ, Li L, Guo Y, Wang HY, Ma J. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13 (6:633–641. doi: 10.1016/S1470-2045(12)70102-X. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang Q, Liu W, Liu Q, Jia W, Chang E, Chen F, Liu Z, Guo X, Mo H, Chen J, Rao D, Ye W, Cao S, Hong M. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer. 2012;131 (2:406–416. doi: 10.1002/ijc.26380. [DOI] [PubMed] [Google Scholar]
- Lo YM, Chan LY, Lo KW, Leung SF, Zhang J, Chan AT, Lee JC, Hjelm NM, Johnson PJ, Huang DP. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59 (6:1188–1191. [PubMed] [Google Scholar]
- Lowe G, Woodward M, Hillis G, Rumley A, Li Q, Harrap S, Marre M, Hamet P, Patel A, Poulter N, Chalmers J. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes mellitus and cardiovascular disease or risk factors: the advance study. Diabetes. 2013;63 (3:1115–1123. doi: 10.2337/db12-1625. [DOI] [PubMed] [Google Scholar]
- Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5 (11:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- Mora S, Rifai N, Buring JE, Ridker PM. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114 (5:381–387. doi: 10.1161/CIRCULATIONAHA.106.634089. [DOI] [PubMed] [Google Scholar]
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3 (8:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Mutirangura A, Pornthanakasem W, Theamboonlers A, Sriuranpong V, Lertsanguansinchi P, Yenrudi S, Voravud N, Supiyaphun P, Poovorawan Y. Epstein-Barr viral DNA in serum of patients with nasopharyngeal carcinoma. Clin Cancer Res. 1998;4 (3:665–669. [PubMed] [Google Scholar]
- Ng WT, Yuen KT, Au KH, Chan OS, Lee AW. Staging of nasopharyngeal carcinoma - The past, the present and the future. Oral Oncol. 2014;50 (6:549–554. doi: 10.1016/j.oraloncology.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96 (10:3302–3309. [PubMed] [Google Scholar]
- Polterauer S, Grimm C, Seebacher V, Concin N, Marth C, Tomovski C, Husslein H, Leipold H, Hefler-Frischmuth K, Tempfer C, Reinthaller A, Hefler L. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: a multicenter study. Oncologist. 2009;14 (10:979–985. doi: 10.1634/theoncologist.2009-0079. [DOI] [PubMed] [Google Scholar]
- Polterauer S, Seebacher V, Hefler-Frischmuth K, Grimm C, Heinze G, Tempfer C, Reinthaller A, Hefler L. Fibrinogen plasma levels are an independent prognostic parameter in patients with cervical cancer. Am J Obstet Gynecol. 2009;200 (6:647 e1–647 e7. doi: 10.1016/j.ajog.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2) J Thromb Haemost. 2008;6 (1:176–183. doi: 10.1111/j.1538-7836.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- Sapkota B, Shrestha SK, Poudel S. Association of activated partial thromboplastin time and fibrinogen level in patients with type II diabetes mellitus. BMC Res Notes. 2013;6:485. doi: 10.1186/1756-0500-6-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, Cheng G, Hu LF, Ernberg I, Zeng YX. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100 (6:1162–1170. doi: 10.1002/cncr.20099. [DOI] [PubMed] [Google Scholar]
- Sinning JM, Bickel C, Messow CM, Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Lackner KJ, Tiret L, Munzel T, Blankenberg S. Impact of C-reactive protein and fibrinogen on cardiovascular prognosis in patients with stable angina pectoris: the AtheroGene study. Eur Heart J. 2006;27 (24:2962–2968. doi: 10.1093/eurheartj/ehl362. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Ikeuchi S, Kitagawa Y, Shimada A, Oishi T, Isobe Y, Kubochi K, Kitajima M, Matsumoto S. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol. 2007;22 (12:2222–2227. doi: 10.1111/j.1440-1746.2006.04736.x. [DOI] [PubMed] [Google Scholar]
- Wang HY, Sun BY, Zhu ZH, Chang ET, To KF, Hwang JS, Jiang H, Kam MK, Chen G, Cheah SL, Lee M, Liu ZW, Chen J, Zhang JX, Zhang HZ, He JH, Chen FL, Zhu XD, Huang MY, Liao DZ, Fu J, Shao Q, Cai MB, Du ZM, Yan LX, Hu CF, Ng HK, Wee JT, Qian CN, Liu Q, Ernberg I, Ye W, Adami HO, Chan AT, Zeng YX, Shao JY. Eight-signature classifier for prediction of nasopharyngeal [corrected] carcinoma survival. J Clin Oncol. 2011;29 (34:4516–4525. doi: 10.1200/JCO.2010.33.7741. [DOI] [PubMed] [Google Scholar]
- Wang WY, Twu CW, Lin WY, Jiang RS, Liang KL, Chen KW, Wu CT, Shih YT, Lin JC. Plasma Epstein-Barr virus DNA screening followed by (1)(8)F-fluoro-2-deoxy-D-glucose positron emission tomography in detecting posttreatment failures of nasopharyngeal carcinoma. Cancer. 2011;117 (19:4452–4459. doi: 10.1002/cncr.26069. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a ‘Cantonese cancer'. Chin J Cancer. 2010;29 (5:517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365 (9476:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Rolfs A, Marti HH, Bauer C, Gassmann M. Hypoxia, a novel inducer of acute phase gene expression in a human hepatoma cell line. J Biol Chem. 1995;270 (46:27865–27870. doi: 10.1074/jbc.270.46.27865. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Kitayama J, Taguri M, Nagawa H. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J Surg. 2009;33 (6:1298–1305. doi: 10.1007/s00268-009-9992-7. [DOI] [PubMed] [Google Scholar]