Abstract
Background
The World Health Organization (WHO) recommends artemisinin‐based combination therapy (ACT) for treating people with Plasmodium falciparum malaria. Five combinations are currently recommended, all administered over three days. Artemisinin‐naphthoquine is a new combination developed in China, which is being marketed as a one‐day treatment. Although shorter treatment courses may improve adherence, the WHO recommends at least three days of the short‐acting artemisinin component to eliminate 90% P. falciparum parasites in the bloodstream, before leaving the longer‐acting partner drug to clear the remaining parasites.
Objectives
To evaluate the efficacy and safety of the artemisinin‐naphthoquine combination for treating adults and children with uncomplicated P. falciparum malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library; MEDLINE; EMBASE; and LILACS up to January 2015. We also searched the metaRegister of Controlled Trials (mRCT) using 'malaria' and 'arte* OR dihydroarte*' as search terms.
Selection criteria
Randomized controlled trials comparing artemisinin‐naphthoquine combinations with established WHO‐recommended ACTs for the treatment of adults and children with uncomplicated malaria due to P. falciparum.
Data collection and analysis
Two review authors independently assessed trials for eligibility and risk of bias, and extracted data. We analysed primary outcomes in line with the WHO 'Protocol for assessing and monitoring antimalarial drug efficacy’ and compared drugs using risk ratios (RR) and 95% confidence intervals (CI). Secondary outcomes were effects on gametocytes, haemoglobin, and adverse events. We assessed the quality of evidence using the GRADE approach.
Main results
Four trials, enrolling 740 adults and children, met the inclusion criteria. Artemisinin‐naphthoquine was administered as a single dose (two trials), as two doses given eight hours apart (one trial), and once daily for three days (one trial), and compared to three‐day regimens of established ACTs. Three additional small pharmaceutical company trials have been carried out. We have requested the data but have not received a response from the company.
Artemisinin‐naphthoquine versus artemether‐lumefantrine
In three small trials from Benin, Côte d'Ivoire, and Papua New Guinea, both combinations had a very low incidence of treatment failure at Day 28, and there were no differences demonstrated in PCR‐unadjusted, or PCR‐adjusted treatment failure (three trials, 487 participants, low quality evidence). Only the single study from Papua New Guinea followed participants up to Day 42, and the number of treatment failures remained very low with both combinations (one trial, 186 participants, very low quality evidence).
Artemisinin‐naphthoquine versus dihydroartemisinin‐piperaquine
In a single small trial from Indonesia, treatment failure at Day 28 and Day 42 was very low in both groups with no differences demonstrated (one trial, 144 participants, very low quality evidence).
Authors' conclusions
The results of these few trials of artemisinin‐naphthoquine are promising, but further trials from multiple settings are required to reliably demonstrate the relative efficacy and safety compared to established ACTs. Future trials should be adequately powered to demonstrate non‐inferiority, and regimens incorporating three days of the artemisinin component are probably preferable to the one‐day regimens.
15 April 2019
Update pending
Studies awaiting assessment
A search for studies (2 Jul, 2018) has identified potentially relevant studies (see 'Characteristics of studies awaiting classification'). These studies have not yet been incorporated into this Cochrane Review.
Keywords: Adult; Child; Humans; Antimalarials; Antimalarials/therapeutic use; Artemether, Lumefantrine Drug Combination; Artemisinins; Artemisinins/therapeutic use; Drug Combinations; Ethanolamines; Ethanolamines/therapeutic use; Fluorenes; Fluorenes/therapeutic use; Malaria, Falciparum; Malaria, Falciparum/drug therapy; Naphthoquinones; Naphthoquinones/therapeutic use; Quinolines; Quinolines/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Artemisinin‐naphthoquine for treating uncomplicated Plasmodium falciparum malaria
This Cochrane Review summarises trials evaluating the effects of artemisinin‐naphthoquine compared to other artemisinin‐based combination therapies (ACTs) recommended by the World Health Organization (WHO) for treating adults and children with uncomplicated P. falciparum malaria. After searching for relevant trials up to January 2015, we included four randomized controlled trials, enrolling 740 adults and children.
What is uncomplicated malaria and how might artemisinin‐naphthoquine work
Uncomplicated malaria is the mild form of malaria which usually causes a fever, with or without headache, tiredness, muscle pains, abdominal pains, nausea, and vomiting. If left untreated, uncomplicated malaria can develop into severe malaria with kidney failure, breathing difficulties, fitting, unconsciousness, and eventually death.
The WHO recommends ACT for treating people with P. falciparum malaria. Five combinations are currently recommended, all administered over three days. Artemisinin‐naphthoquine is a new combination developed in China, which is being marketed and evaluated as one‐day or three‐day regimens.
What the research says
Artemisinin‐naphthoquine versus artemether‐lumefantrine
In three small trials from Benin, Côte d'Ivoire, and Papua New Guinea, both artemisinin‐naphthoquine and AL had a very low incidence of treatment failure at Day 28 (low quality evidence), and in the trial from Papua New Guinea it remained low in both groups at Day 42 (very low quality evidence).
Artemisinin‐naphthoquine versus dihydroartemisinin‐piperaquine
In a single small study from Indonesia, treatment failure at Day 28 and Day 42 was very low with both artemisinin‐naphthoquine and DHA‐P (very low quality evidence).
Conclusions
The results of these few trials of artemisinin‐naphthoquine are promising, but larger trials from multiple settings are required to be confident that artemisinin‐naphthoquine is as effective and well tolerated as other antimalarials.
Summary of findings
Summary of findings for the main comparison. Artemisinin‐naphthoquine versus AL for treating uncomplicated P. falciparum malaria.
| Artemisinin‐naphthoquine versus AL for treating uncomplicated P. falciparum malaria | |||||
| Patient or population: Adults and children with uncomplicated P. falciparum malaria Settings: Malaria endemic settings Intervention: Artemisinin‐naphthoquine (ART‐NQ) (one or three‐day course) Comparison: Artemether‐lumefantrine (AL) (three‐day course) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| AL | ART‐NQ | ||||
| Treatment failure at day 28 | PCR‐unadjusted | RR 1.02 (0.24 to 4.37) | 487 (3 trials) | ⊕⊕⊝⊝ low1,2,3,4 | |
| 1 per 100 | 1 per 100 (0 to 4) | ||||
| PCR‐adjusted | RR 1.03 (0.15 to 7.07) | 485 (3 trials) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 0 per 100 | 0 per 100 (0 to 0) | ||||
| Treatment failure at day 42 | PCR‐unadjusted |
RR 0.09 (0.00 to 1.59) |
186 (1 trial) |
⊕⊝⊝⊝ very low5,6,7 | |
| 5 per 100 | 0 per 100 (0 to 8) | ||||
| PCR‐adjusted |
RR 0.33 (0.01 to 7.91) |
186 (1 trial) |
⊕⊝⊝⊝ very low5,6,7 | ||
| 1 per 100 | 0 per 100 (0 to 8) | ||||
| The basis for the assumed risk is the mean control group risk across included studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Two studies adequately concealed allocation to be at low risk of selection bias. In the other study the process of randomization and allocation concealment was unclear. 2 No serious inconsistency: Statistical heterogeneity was low. 3 Downgraded by 1 for serious indirectness: Three studies have now evaluated this comparison, but only one used a three‐day regimen as recommended by the WHO. The three studies are from Benin, Côte d'Ivoire, and Papua New Guinea, and the level of treatment failure with both artemisinin‐naphthoquine and AL was very low, lower than seen in many trials of AL. Further studies from additional settings are required before this result can be generalized to elsewhere. 4 Downgraded by 1 for serious imprecision: to demonstrate non‐inferiority at 95% efficacy requires a sample size of 472. These trials are individually significantly underpowered, and the number of events is too low to have full confidence in this result. 5 No serious risk of bias: This study adequately concealed allocation to be at low risk of selection bias. 6 Downgraded by 1 for serious indirectness: This single study is from Papua New Guinea. Further studies from additional settings are required before this result can be generalized to elsewhere. 7 Downgraded by 2 for very serious imprecision: This trial is significantly underpowered to demonstrate non‐inferiority.
Summary of findings 2. Artemisinin‐naphthoquine versus DHA‐P for treating uncomplicated P. falciparum malaria.
| Artemisinin‐naphthoquine versus DHA‐P for treating uncomplicated P. falciparum malaria | |||||
| Patient or population: Adults and children with uncomplicated P. falciparum malaria Settings: Malaria endemic settings Intervention: Artemisinin‐naphthoquine (ART‐NQ) (one‐day course) Comparison: Dihydroartemisinin‐piperaquine (DHA‐P) (three‐day course) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| DHA‐P | ART‐NQ | ||||
| Treatment failure at day 28 | PCR‐unadjusted | Not estimable | 143 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 | |
| 0 per 100 | 0 per 100 | ||||
| PCR‐adjusted | Not estimable | 143 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 0 per 100 | 0 per 100 | ||||
| Treatment failure at day 42 | PCR‐unadjusted | RR 0.91 (0.13 to 6.26) | 143 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 | |
| 3 per 100 | 3 per 100 (0 to 19) | ||||
| PCR‐adjusted | RR 0.19 (0.01 to 3.82) | 141 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 3 per 100 | 0 per 100 (0 to 11) | ||||
| The basis for the assumed risk is the mean control group risk on the included studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Although the description of the randomization procedure is vague, this trial is probably at low risk of selection bias. 2 Downgraded by 1 for serious indirectness: This comparison has only been evaluated in a single setting. Further studies from additional settings are required before this result can be generalized to elsewhere. 3 Downgraded by 2 for very serious imprecision: to demonstrate non‐inferiority at 95% efficacy would require a sample size of 472. This trial is significantly underpowered.
Background
Description of the condition
Malaria is a febrile illness caused by infection with the protozoan parasite Plasmodium, and transmitted from person to person by the bite of infected mosquitoes. Five Plasmodium species are capable of causing malaria in humans, of which Plasmodium falciparum is the most common, responsible for over 90% of cases and almost all of the malaria deaths worldwide (WHO 2012).
Uncomplicated malaria is the mild form of the disease, characterised by fever with or without associated headache, tiredness, muscle pains, abdominal pains, rigors, and nausea and vomiting (WHO 2010a). If left untreated, uncomplicated malaria can rapidly develop into severe, life threatening forms of the disease, particularly in those without acquired immunity. Effective immunity generally requires repeated infections over five to 10 years, and is reduced during pregnancy. Consequently, in highly endemic settings, as seen in many areas of rural sub‐Saharan Africa, young children and pregnant women are most at risk, while in settings with low or seasonal transmission, all age groups can be equally at risk (WHO 2010a).
P. falciparum has now developed resistance in many parts of the world to most antimalarial drugs used as monotherapy (White 2004; WHO 2010b). Consequently, the World Health Organization (WHO) now recommends that P. falciparum malaria is always treated with a combination of two drugs that act at different biochemical sites within the parasite (WHO 2010a). If a parasite mutation producing drug resistance arises spontaneously during treatment, the parasite should then be killed by the partner drug, reducing or delaying the development of resistance, and increasing the useful lifetime of the individual drugs (White 1996; White 1999).
Description of the intervention
Five artemisinin‐based combination therapies (ACTs) are recommended for the first‐line treatment of uncomplicated malaria; artemether‐lumefantrine (AL), artesunate plus amodiaquine (AS+AQ), artesunate plus mefloquine (AS+MQ), artesunate plus sulphadoxine‐pyrimethamine (AS+SP), and dihydroartemisinin‐piperaquine (DHA‐P) (WHO 2010a). The artemisinin components (artemether, artesunate, and dihydroartemisinin) are highly effective schizonticides, and over three days of treatment rapidly eliminate up to 90% of the blood stage asexual forms of P. falciparum. The partner drugs are longer acting and are used to clear any residual infection (WHO 2010a). The combinations with very long half‐lives (AS+MQ and DHA‐P), can provide a period of post‐treatment prophylaxis which may last for up to six weeks (Sinclair 2009).
Artemisinin‐naphthoquine is a new ACT developed by the Academy of Military Medical Sciences Research Institute for Microbial Epidemics in China (Wang 2004). Contrary to the WHO recommendation for three‐days of the artemisinin‐derivative (WHO 2010a), this combination is being promoted for use as either a single dose or two dose regimen administered over 24 hours (Hombhanje 2010). The rationale provided for the shortened regimen is to improve compliance with treatment.
Naphthoquine is a tetra‐aminoquinoline that was developed in China in the late 1980s. Whilst it was used as monotherapy within China, it has never been widely used in other countries (Hombhanje 2010). It is reported to be well absorbed orally, with high bioavailability, and is excreted mainly via the kidneys (Wang 2004). Several pharmacokinetic studies are available but with conflicting estimates of the elimination half‐life (Liu 2012). Early reports were of a half‐life of two to three days (Wang 2004), but subsequent published studies found a mean half‐life of 10.6 days in healthy adult Chinese volunteers (Qu 2010), and 22.8 days in children aged five to 12 years with malaria (Batty 2012). For comparison, the elimination half‐lives of lumefantrine and mefloquine in people with uncomplicated malaria are around three days, and 14 days respectively (Ezzet 2000; Karbwang 1990; WHO 2010a).
Artemisinin is a naturally occurring antimalarial compound which can be extracted from the plant Artemisia annua. Since its discovery in the 1970s, product development has concentrated on its semi‐synthetic derivatives (artesunate, artemether, dihydroartemisinin) due to the poor water solubility of artemisinin (Pawluk 2013; Woodrow 2005). Once absorbed, these derivatives are rapidly converted to the active metabolite dihydroartemisinin. Parasites resistant to the artemisinin derivatives were first reported in Cambodia in 2007 (Noedl 2008), and confirmed in Cambodia in 2008 (Dondorp 2009), and in Thailand in 2012 (Phyo 2012; WHO 2011).
Assessment of antimalarial drug efficacy
The WHO recommends that new antimalarials should have a treatment failure rate of less than 5%, and failure rates with existing first‐line antimalarials higher than 10% should trigger a change in treatment policy (WHO 2010a).
The late reappearance of P. falciparum parasites in the blood can be due to failure of the drug to completely clear the original parasite infection (a recrudescence) or due to a new infection, which is especially common in areas of high transmission. A molecular genotyping technique called polymerase chain reaction (PCR) can be used in clinical trials to distinguish between recrudescence and new infection, giving a clearer picture of the efficacy of the drug and its post‐treatment prophylactic effect (Cattamanchi 2003; White 2002; WHO 2008).
The WHO recommends a minimum follow‐up period of 28 days for antimalarial efficacy trials, but longer periods of follow‐up may be required for antimalarials with long elimination half‐lives (Bloland 2003; White 2002). This is because treatment failure due to true recrudescence of malaria parasites may be delayed until the drug concentration falls below the minimum concentration required to inhibit parasite multiplication, which may be beyond 28 days. The WHO recommends 42 days follow‐up for trials involving lumefantrine and piperaquine and 63 days for trials of mefloquine (WHO 2010a).
Why it is important to do this review
This Cochrane Review aims to systematically evaluate the available studies on the efficacy and safety of the artemisinin‐naphthoquine combination for consideration by global and national policy makers.
Objectives
To evaluate the efficacy and safety of the artemisinin‐naphthoquine combination for treating adults and children with uncomplicated P. falciparum malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs). We excluded quasi‐RCTs.
Types of participants
Adults and children (including pregnant women and infants) with symptomatic, microscopically confirmed, uncomplicated P. falciparum malaria.
Types of interventions
Intervention
A course of artemisinin‐naphthoquine given as a single dose, or multiple doses over one, two, or three days.
Control
A three‐day course of a WHO‐recommended ACT.
The specific ACTs included are: DHA‐P; AS+MQ; AL (six doses); AS+AQ; and AS+SP.
Types of outcome measures
Primary outcomes
Treatment failure at Days 28, 42, and 63; PCR‐adjusted and PCR‐unadjusted.
Secondary outcomes
Fever clearance.
Parasite clearance.
Gametocyte carriage at Day 7 or 14 (preference for Day 14 in data analysis).
Gametocyte development (negative at baseline, and positive at follow‐up).
Change in haemoglobin from baseline (minimum 28 day follow‐up).
Adverse events
Deaths occurring during follow‐up.
Serious adverse events (life threatening, causing admission to hospital, or discontinuation of treatment).
Haematological and biochemical adverse effects (for example, neutropenia, liver toxicity).
Early vomiting.
Other adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 13 January 2015 using the search terms detailed in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library; MEDLINE; EMBASE; and LILACS.
Searching other resources
We contacted the manufacturer of artemisinin‐naphthoquine in October 2013 requesting further unpublished data.
We also checked the reference lists of all trials identified by the database search.
Data collection and analysis
Selection of studies
Rachel Isba (RI) and Babalwa Zani (BZ) independently reviewed the results of the literature search and obtained full‐text copies of all potentially relevant trials. We checked each trial report for evidence of multiple publications from the same data set. RI and BZ then independently assessed each trial for inclusion in this review using an eligibility form based on the inclusion criteria. We resolved any disagreements through discussion or, where necessary, by consultation with DS. If clarification was necessary, we attempted to contact the trial authors for further information.
Data extraction and management
Two review authors (RI and BZ) independently extracted data using a pre‐tested data extraction form. We extracted data on trial characteristics including methods, participants, interventions, and outcomes as well as data on dose and drug ratios of the combinations.
We extracted the number of participants randomized and the number analysed in each treatment group for each outcome. We calculated and report the loss to follow‐up in each group.
For dichotomous outcomes, we recorded the number of participants experiencing the event and the number of participants in each treatment group. For continuous outcomes, we extracted the arithmetic means and standard deviations for each treatment group together with the numbers of participants in each group. If the data were reported using geometric means, we recorded this information and extracted standard deviations on the log scale. If medians were reported we extracted medians and ranges.
Primary outcome
Our primary analysis drew on the WHO protocol for assessing and monitoring antimalarial drug efficacy (Bloland 2003). This protocol has been used to guide most efficacy trials since its publication in 2003, even though it was designed to assess the level of antimalarial resistance in the study area rather than for comparative trials. As a consequence, a high number of randomized participants are excluded from the final efficacy outcome as losses to follow‐up, or voluntary or involuntary withdrawals. For this reason we conducted a sensitivity analysis to restore the integrity of the randomization process and test the robustness of the results to this methodology. (For a summary of the methodology and sensitivity analysis see Table 3).
1. Primary outcome measure (Total failure).
| Analysis | Participants | PCRb‐unadjusted | PCR‐adjusted | ||
| Numerator | Denominator | Numerator | Denominator | ||
| Primary analysisa | Exclusions after enrolment | Excludedc | Excluded | Excluded | Excluded |
| Missing or indeterminate PCR | Included as failures | Included | Excluded | Excluded | |
| New infections | Included as failures | Included | Excluded | Excluded | |
| Sensitivity analysis 1d | As 'Primary analysis' except: missing or indeterminate PCR | — | — | Included as failures | Included |
| Sensitivity analysis 2e | As 'Sensitivity analysis 1' except: new infections | — | — | Included as successes | Included |
| Sensitivity analysis 3f | As 'Sensitivity analysis 2' except: exclusions after enrolment | Included as failures | Included | Included as failures | Included |
| Sensitivity analysis 4g | As 'Sensitivity analysis 2' except: exclusions after enrolment | Included as successes | Included | Included as successes | Included |
a Note: participants who were found to not satisfy the inclusion criteria after randomization are removed from all calculations. b PCR: polymerase chain reaction. c 'Excluded' means removed from the calculation. d To re‐classify all indeterminate or missing PCR results as treatment failures in the PCR‐adjusted analysis. e To re‐classify all PCR‐confirmed new infections as treatment successes in the PCR‐adjusted analysis. (This analysis may overestimate efficacy as PCR is not wholly reliable and some recrudescences may be falsely classified as new infections. Also some participants may have gone on to develop a recrudescence after the new infection.) f To re‐classify all exclusions after enrolment (losses to follow‐up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment failures. For PCR‐unadjusted total failure this represents a true worse‐case scenario. g To re‐classify all exclusions after enrolment (losses to follow‐up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment successes.
PCR‐unadjusted total failure
We calculated PCR‐unadjusted total failure (P. falciparum) as the sum of early treatment failures and late treatment failures (without PCR adjustment). The denominator excluded participants for whom an outcome was not available (for example, those who were lost to follow‐up, withdrew consent, took other antimalarials, or failed to complete treatment) and those participants who were found not to fulfil the inclusion criteria after randomization.
PCR‐adjusted total failure
PCR‐adjusted total failure (P. falciparum) was calculated as the sum of early treatment failures plus late treatment failures due to PCR‐confirmed recrudescence. We treated participants with indeterminate PCR results, missing PCR results, or PCR‐confirmed new infections as involuntary withdrawals and excluded them from the calculation. The denominator excludes participants for whom an outcome was not available (for example, those who were lost to follow‐up, withdrew consent, took other antimalarials, or failed to complete treatment) and those participants who were found not to fulfil the inclusion criteria after randomization.
These primary outcomes relate solely to failure due to P. falciparum. For both PCR‐unadjusted and PCR‐adjusted total failure, participants infected with P. vivax during follow‐up were retained in the calculation if they were treated with chloroquine and continued in follow‐up. As long as they did not go on to develop P. falciparum parasitaemia they were classified as treatment successes. We excluded from the calculation those participants who were infected with P. vivax and were removed from the trial's follow‐up at the time of P. vivax parasitaemia.
Assessment of risk of bias in included studies
Two review authors (RI and BZ) independently assessed the risk of bias for each trial using The Cochrane Collaboration's tool for assessing the 'Risk of bias' (Higgins 2011). We resolved any differences of opinion through discussion with a third review author. We followed the guidance to assess whether adequate steps were taken to reduce the risk of bias across six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias.
For sequence generation and allocation concealment, we report the methods used. For blinding, we describe who was blinded and the blinding method. For incomplete outcome data, we report the percentage and proportion lost to follow‐up. For selective outcome reporting, we state any discrepancies between the methods used and the results, in terms of the outcomes measured or the outcomes reported. For other biases, we describe any other trial features that we think could affect the trial result (for example, if the trial was stopped early).
We then categorized our judgements as 'low', 'high', or 'unclear' risk of bias, and used this information to guide our interpretation of the presented data. Where our judgement was unclear, we attempted to contact the trial authors for clarification and resolved any differences of opinion through discussion.
Measures of treatment effect
We analysed the data using Review Manager (RevMan). Dichotomous data were combined and presented using risk ratios. For continuous data summarized by arithmetic means and standard deviations, we combined data using mean differences. Risk ratios and mean differences are accompanied by 95% CIs.
Unit of analysis issues
We did not encounter any unit of analysis issues.
Dealing with missing data
When trial reports were insufficient, unclear, or missing, we attempted to contact the trial authors for additional information.
Assessment of heterogeneity
We assessed heterogeneity amongst trials by inspecting the forest plots, applying the Chi² test with a 10% level of statistical significance, and also using the I² statistic with a value of 50% used to denote moderate levels of heterogeneity.
Assessment of reporting biases
We planned to assess the possibility of publication bias by examining funnel plots for asymmetry, but there were too few trials to make this meaningful.
Data synthesis
We gave the included trials identity codes which include the three‐letter international country code, and listed the trials in forest plots in chronological order (by the final date of enrolment).
Treatments were compared directly using pair‐wise comparisons. For outcomes that were measured at different time points, we stratified the analysis by the time point.
We performed meta‐analysis where appropriate after assessment and investigation of heterogeneity. In the first instance we used a fixed‐effect model, and used a random‐effects model when the Chi² test P value was less than 0.1 or the I² statistic greater than 50%.
Quality of evidence
We assessed the quality of evidence for each outcome measure using the GRADE approach. The quality rating across studies has four levels: high, moderate, low, or very low. Randomized trials are initially categorized as high quality but downgraded after assessment of five criteria: risk of bias, consistency, directness, imprecision, and publication bias (Guyatt 2008).
Subgroup analysis and investigation of heterogeneity
We planned to investigate potential sources of heterogeneity through a series of analyses subgrouping the trials by: geographical region, intensity of malaria transmission (low to moderate versus high malaria transmission), known parasite resistance, allocation concealment, participant age, and drug dose (comparing regimens where there are significant variations in drug dose). However, there were too few trials to make this meaningful.
Sensitivity analysis
We conducted a series of sensitivity analyses to investigate the robustness of the methodology used in the primary analysis. We aimed to restore the integrity of the randomization process by adding excluded groups back into the analysis in a stepwise fashion (see Table 3 for details).
Results
Description of studies
Results of the search
We identified 15 articles as potentially relevant to this Cochrane Review. Four RCTs met our inclusion criteria and we excluded 11 articles (see Figure 1).
1.

Study flow diagram.
Included studies
The four trials randomized 740 participants with uncomplicated P. falciparum malaria. Two trials (Laman 2014 PNG; Tjitra 2012 IDN) also included participants with P. vivax malaria or mixed infections but we excluded these participants from this review.
The trials were conducted in Benin, Côte d'Ivoire, Papua New Guinea, and Indonesia. The trial sites in Benin and Côte d'Ivoire are described as having high transmission intensity and high levels of resistance to chloroquine and SP (Kinde‐Gazard 2012 BEN; Toure 2009 CIV). In Indonesia, there were multiple trial sites which are likely to have covered variable levels of transmission (although this was not explicitly stated), and Indonesia has reported resistance to chloroquine, quinine, and SP (Tjitra 2012 IDN). Endemicity and resistance are not described in the study from Papua New Guinea (Laman 2014 PNG).
Kinde‐Gazard 2012 BEN recruited participants older than six months, Toure 2009 CIV recruited children aged six months to 15 years, Laman 2014 PNG recruited children aged 6 months to five years, and Tjitra 2012 IDN only recruited adults.
Three trials compared artemisinin‐naphthoquine with a three‐day course of AL, but each trial used a different regimen of artemisinin‐naphthoquine: Kinde‐Gazard 2012 BEN gave a single dose, Toure 2009 CIV gave two doses eight hours apart, and Laman 2014 PNG gave a daily dose for three days. It is unclear whether the total dose is comparable across these three trials. Tjitra 2012 IDN compared a single dose of artemisinin‐naphthoquine with a three day course of DHA‐P.
Kinde‐Gazard 2012 BEN and Toure 2009 CIV only report the primary outcome at Day 28 post‐treatment, while Laman 2014 PNG and Tjitra 2012 IDN report both Day 28 and Day 42.
Excluded studies
The excluded studies and reasons for their exclusion are given in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We summarised the 'Risk of bias' assessments in Figure 2 and the reasons for these judgements in the 'Characteristics of included studies' table.
2.
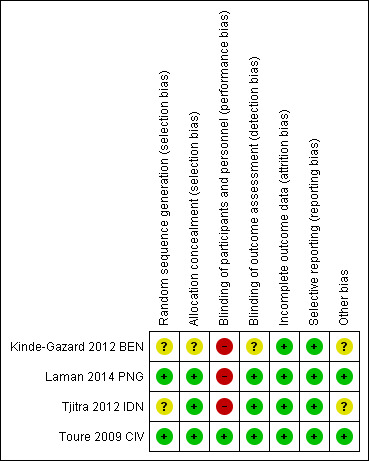
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Two trials adequately described random sequence generation and allocation concealment to be considered at low risk of selection bias (Laman 2014 PNG; Toure 2009 CIV). In the other two trials the description was unclear (Kinde‐Gazard 2012 BEN; Tjitra 2012 IDN).
Blinding
Three trials adequately blinded the outcome assessors (laboratory staff and study physicians) to be at low risk of detection bias. In the remaining trial it was unclear whether outcome assessments had been adequately blinded (Kinde‐Gazard 2012 BEN).
Incomplete outcome data
All four trials were judged to be of low risk for attrition bias.
Selective reporting
We found no evidence of selective reporting.
Other potential sources of bias
The drug manufacturer was involved in three trials (Kinde‐Gazard 2012 BEN; Tjitra 2012 IDN; Toure 2009 CIV); however, it is clearly stated in one of these that they had no involvement in the design or analysis of the study (Toure 2009 CIV).
Effects of interventions
Comparison 1. Artemisinin‐naphthoquine versus AL
Three trials compared artemisinin‐naphthoquine with AL. These trials recruited adults and children and administered artemisinin‐naphthoquine as a single dose (Kinde‐Gazard 2012 BEN), as two doses eight hours apart (Toure 2009 CIV), or once daily for three days (Laman 2014 PNG). It is unclear whether the total dose is comparable across these three trials.
Early clinical response to treatment
Two trials reported on fever clearance, with no significant differences between groups in the risk of remaining febrile after 24, 48, or 72 hours (two trials, 321 participants, Analysis 1.1).
1.1. Analysis.

Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 1 Fever clearance.
All three trials reported parasite clearance, with no significant differences between groups at 24, 48 or 72 hours (three trials, 494 participants, Analysis 1.2).
1.2. Analysis.

Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 2 Parasite clearance.
Treatment failure
Across all three trials, only four participants had recurrent parasitaemia before Day 28, and only two were deemed to have a recrudescence after PCR‐adjustment. Consequently, there were no statistically significant differences between groups (three trials, 487 participants, Analysis 1.3; Analysis 1.4). The trial from Papua New Guinea continued follow‐up until Day 42, by which time there were five treatment failures with AL (one recrudescence and four new infections) compared to none with artemisinin‐naphthoquine (one trial, 186 participants, Analysis 1.5; Analysis 1.6).
1.3. Analysis.

Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 3 PCR‐unadjusted treatment failure at day 28.
1.4. Analysis.

Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 4 PCR‐adjusted treatment failure at day 28.
1.5. Analysis.
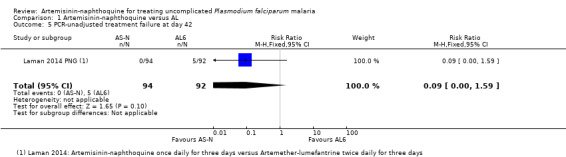
Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 5 PCR‐unadjusted treatment failure at day 42.
1.6. Analysis.
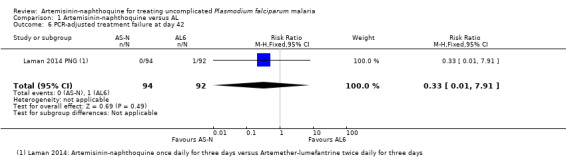
Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 6 PCR‐adjusted treatment failure at day 42.
Gametocytemia
Two trials reported on gametocyte carriage (Toure 2009 CIV and Laman 2014 PNG). Gametocyte carriage was very low at baseline in both groups in the trial from Côte d'Ivoire. Gametocyte carriage was higher at baseline in the trial from Papua New Guinea and AL appeared to clear gametocytes quicker than artemisinin‐naphthoquine (Day 7 gametocyte carriage: RR 2.56, 95% CI 1.42 to 4.60, one trial, 197 participants, Analysis 1.7).
1.7. Analysis.

Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 7 Gametocyte carriage.
Anaemia
Toure 2009 CIV reported the number of participants who were anaemic on Day 7 and found no significant difference between the two groups (one trial, 120 participants, Analysis 1.8). Laman 2014 PNG presented mean haemoglobin for both groups graphically over 42 days follow‐up. There was a small reduction in mean haemoglobin in both groups during the first week which recovered over the following five weeks. There was no difference between groups at any time point other than Day 42 when mean haemoglobin was slightly lower with AL (P < 0.001, authors' own figures).
1.8. Analysis.
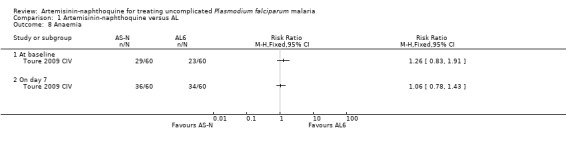
Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 8 Anaemia.
Adverse events
Across the three trials only one severe adverse event is described, and this was considered non‐drug‐related; one child given artemisinin‐naphthoquine was admitted and treated for lobar pneumonia (Laman 2014 PNG; see Table 4).
2. Serious adverse events.
| Comparison | Trial ID | Description of severe adverse events provided by publication |
| ART‐NQ versus AL | Kinde‐Gazard 2012 BEN | Not mentioned. |
| Toure 2009 CIV | "No severe alterations in renal, haematologic or hepatic function were observed with any of the drug combinations under study." Other adverse events are described as mild. |
|
| Laman 2014 PNG | "The only severe adverse event was considered non‐drug related. A 48 month old child allocated to artemisinin‐naphthoquine was hospitalized and treated successfully for lobar pneumonia." | |
| ART‐NQ versus DHA‐P | Tjitra 2012 IDN | "There were no serious adverse events reported in malaria subjects treated with ART‐NQ and DHA‐P during the study". |
All three trials conducted some form of clinical adverse event monitoring and no differences were reported in clinical symptoms after treatment (three trials, 554 participants, Analysis 1.9).
1.9. Analysis.

Comparison 1 Artemisinin‐naphthoquine versus AL, Outcome 9 Adverse events.
One trial also conducted biochemical monitoring for adverse events on Days 0, 3 and 7. No clinically important differences were seen in tests of renal or liver function (Laman 2014 PNG). The same trial also conducted ECG monitoring on Day 0, 2, 3 and 7 in a non‐random sample of participants. After the second dose on day two there were statistically significant differences in the QT interval with 33.3% of those treated with artemisinin‐naphthoquine having a QTc > 460 msec compared with 3.7% with AL (P value not reported). Differences were not statistically significant at Day 3 or 7.
Comparison 2. Artemesinin‐naphthoquine versus DHA‐P
One multi‐centre trial in Indonesia compared artemisinin‐naphthoquine with DHA‐P (Tjitra 2012 IDN). This trial recruited only adults and administered artemisinin‐naphthoquine as a single dose.
Early response to treatment
There was no significant differences in fever clearance, or parasite clearance between the two groups (one trial, 149 participants, Analysis 2.1; Analysis 2.2). All blood slides were clear of parasites by Day 3.
2.1. Analysis.

Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 1 Fever clearance.
2.2. Analysis.
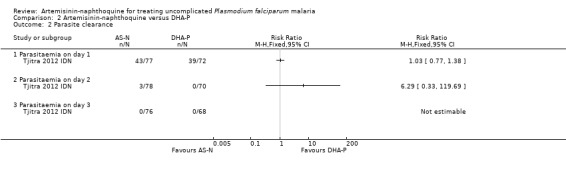
Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 2 Parasite clearance.
Treatment failure
There were no PCR‐unadjusted or PCR‐adjusted treatment failures before Day 28 in either group (one trial, 143 participants, Analysis 2.3; Analysis 2.4). By Day 42, two participants in each group had recurrent parasitaemia, and after PCR‐adjustment the participants given artemisinin‐naphthoquine were deemed to have new infections, and those given DHA‐P were deemed to have recrudescences (one trial, 143 participants; Analysis 2.5; Analysis 2.6).
2.3. Analysis.

Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 3 PCR‐unadjusted treatment failure at day 28.
2.4. Analysis.
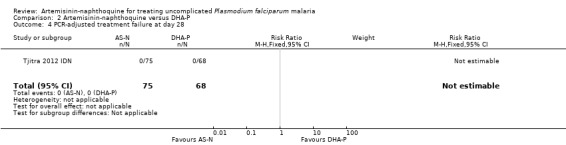
Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 4 PCR‐adjusted treatment failure at day 28.
2.5. Analysis.
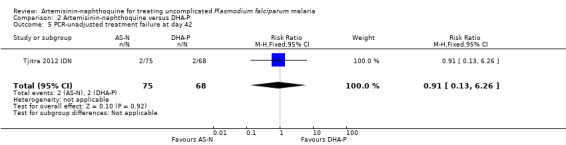
Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 5 PCR‐unadjusted treatment failure at day 42.
2.6. Analysis.

Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 6 PCR‐adjusted treatment failure at day 42.
Gametocytemia
On Day 7, there was no significant differences in gametocytaemia between the two trial arms (one trial, 150 participants, Analysis 2.7).
2.7. Analysis.
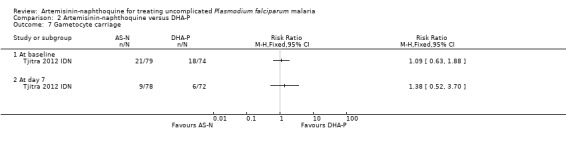
Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 7 Gametocyte carriage.
Anaemia
Not reported.
Adverse events
No serious adverse events were reported, and adverse events were rare with no differences detected between the two treatments (one trial, 152 participants, Analysis 2.8; see Table 4).
2.8. Analysis.
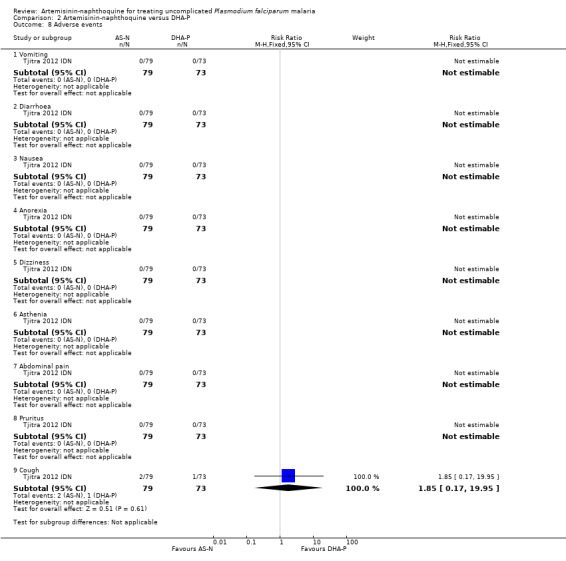
Comparison 2 Artemisinin‐naphthoquine versus DHA‐P, Outcome 8 Adverse events.
Discussion
Summary of main results
In three small trials from Benin, Côte d'Ivoire, and Papua New Guinea, both artemisinin‐naphthoquine and AL had a very low incidence of treatment failure at day 28 (low quality evidence).
In a single small study from Indonesia, treatment failure at day 28 and day 42 was very low with both artemisinin‐naphthoquine and DHA‐P (very low quality evidence).
These trials were underpowered to detect clinically important differences.
Overall completeness and applicability of evidence
To date, there are only very limited data available on either the efficacy or safety of artemisinin‐naphthoquine and much larger trials, from a wider variety of epidemiological settings will be required before this combination could be recommended. It is perhaps helpful to note that DHA‐P had been evaluated in 22 RCTs enrolling almost 15,000 adults and children before it was formally recommended by the WHO (Sinclair 2009), and artesunate plus pyronaridine has been evaluated in three large multicentre trials enrolling over 3000 participants but still requires further evidence of efficacy and safety to have confidence in its effects (Bukirwa 2014).
Trials of around 500 participants are required to demonstrate equivalent efficacy in a single setting (Table 5), and trials from multiple settings are required to demonstrate that the findings can be generalised to regions or continents, particularly for infectious diseases such as malaria where infection patterns and drug resistance vary widely. To rule out serious side‐effects, particularly rare ones, much larger patient numbers are required and this is usually done through observational cohorts.
3. Optimal information size calculations.
| Outcome | Hypothesis | Example | Power | α error | Proportion in control group | Proportion in intervention group | Maximum risk difference | Total sample size |
| PCR‐adjusted treatment failure | Superiority | Assuming a 10% failure rate with the old drug and that a new drug should be at least 95% effective | 80% | 5% | 0.10 | 0.05 | — | 864 |
| Non‐inferiority | Assuming that both drugs are 95% effective and that there is no more than a 5% difference in efficacy | 80% | 5% | 0.05 | 0.05 | 0.05 | 472 |
We performed calculations with http://www.sealedenvelope.com
The trials included in this review suggest that this combination has potential, but it is unclear whether the rationale of a shortened 24 hour regimen is justified. The current WHO recommendation for three‐day regimens is based on a trade‐off between compliance (enhanced by shorter regimens), efficacy (enhanced by longer regimens), and the desire to reduce the risk of drug resistance developing (enhanced by combinations of two drugs acting via different mechanisms until parasitaemia is reduced to very low levels). While compliance with the three‐day regimens of established ACTs has been poor in some studies, it is hard to understand why shortening the regimen to 24 hours with artemisinin‐naphthoquine would be any different to ensuring very poor compliance with any other ACT. Consequently, it would probably be preferable if future studies evaluated a three‐day regimen.
Quality of the evidence
We assessed the quality of the evidence in this review using the GRADE approach and presented the evidence in two 'Summary of findings' tables for efficacy (Table 1; Table 2). We judge the evidence to be of low or very low quality meaning that we have little confidence in the findings of no statistically significant difference between the tested ACTs. We downgraded the evidence by one level for serious indirectness as artemisinin‐naphthoquine has only been evaluated in a limited number of settings and the findings are not easily generalized, and by one or two levels for serious imprecision as the trials are severely underpowered to detect differences.
Potential biases in the review process
None identified.
Agreements and disagreements with other studies or reviews
We found two review articles authored by representatives of the pharmaceutical developers (Hombhanje 2010; Wang 2004). Both are narrative overviews rather than systematic reviews. Hombhanje 2010 includes the data from the three trials included here plus some additional data from three unpublished trials. We have contacted the pharmaceutical company requesting access to these data but have not yet received it. Should these become available, we will include them in the future updates of this review. Hombhanje 2010 concludes that "ARCO® demonstrated high level of efficaciousness and safety" but notes that further research is still necessary. We are more conservative in our conclusions, and feel that neither the efficacy nor the safety has yet been reliably established.
Authors' conclusions
Implications for practice.
The results of these few trials of artemisinin‐naphthoquine are promising, but further trials from multiple settings are required to reliably demonstrate the relative efficacy and safety compared to established ACTs.
Implications for research.
Future trials should be adequately powered to demonstrate non‐inferiority, and regimens incorporating three days of the artemisinin component are probably preferable to the one‐day regimens.
Acknowledgements
We thank Vittoria Lutje for conducting the searches. We also acknowledge the contributions of Sarah Donegan and Piero Olliaro to developing the protocol for Sinclair 2009 which was also used for this Cochrane Review.
This document is an output from a project funded by UKaid from the UK Government for the benefit of developing countries. The views expressed are not necessarily those of the Department for International Development (DFID).
Appendices
Appendix 1. Detailed search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACS |
| 1 | malaria | Malaria ti, ab, Mesh | Malaria ti, ab, Mesh | Malaria ti, ab, Emtree | malaria |
| 2 | arte* | arte* ti, ab | arte* ti, ab | arte* | Arte$ |
| 3 | dihydroarte* | dihydroarte* ti, ab | dihydroarte* ti, ab | dihydroarte* | Dihydroarte$ |
| 4 | Coartem* | Coartem* ti, ab | Coartem* ti, ab | Coartem$ | Coartem$ |
| 5 | lumefantrine | Lumefantrine ti, ab | Lumefantrine ti, ab | lumefantrine | lumefantrine |
| 6 | 2 or 3 or 4 or 5 | 2 or 3 or 4 or 5 | 2 or 3 or 4 or 5 | 2 or 3 or 4 or 5 | 2 or 3 or 4 or 5 |
| 7 | Naphthoquin* | Naphthoquin* ti, ab | Naphthoquin* ti, ab | Naphthoquin* ti, ab | Naphthoquin$ |
| 8 | 1 and 6 and 7 | Naphthoquinones[Mesh] | Naphthoquinones[Mesh] | Naphthoquinone [Emtree] | 1 and 6 and 7 |
| 9 | 7 or 8 | 7 or 8 | 7 or 8 | ||
| 10 | 1 and 6 and 9 | 1 and 6 and 9 | 1 and 6 and 9 |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2011).
Appendix 2. Adverse event monitoring
| Trial ID | Sample Size | Blinding | Clinical symptoms monitoring | Biochemistry | Haematological | Electrocardiogram |
| Laman 2014 PNG | 198 | Open label | Standardized assessment on days 1, 2, 3, 7, 14, 28 and 42. | Hepatorenal function on days 0, 3 and 7. | Full blood count, on days 0, 3 and 7. | Electrocardiogram on days 0, 3 and 7. An additional electrocardiogram was performed 4 hours after day 2 dose in those treated with ART‐NQ and in a convenience sample of 30 people treated with artemether‐lumefantrine. |
| Tjitra 2012 IDN | 243 | Open label | Limited physical exam on days 1 to 2, 3, 7, 14, 12, 28, 35, and 42 and if clinically indicated. | Blood chemistry at days 3, 7, and 28. | Haematology at days 3, 7, and 28. | ECG 2 to 4 hours after drug administration and on follow‐up days 7, 28, and 42. |
| Kinde‐Gazard 2012 BEN | 174 | Single | Patients were hospitalized for the first three days, and monitored clinically; following discharge patients were seen on days 7, 14, 21, 28 and a symptom questionnaire was conducted at each visit. | Biochemistry (U and E, LFT) whilst patients were hospitalized for the first three days; then following discharge on days 7, 14, 21, 28. | Haematology whilst patients were hospitalized for the first three days; then following discharge on days 7, 14, 21, 28. | — |
| Toure 2009 CIV | 125 | Single | Follow‐up: on days 1, 2, 3, 7, 14, 21, and 28 (or any other day if they felt ill). Follow‐up evaluation was history and examination. "All observed adverse events were monitored actively and passively from the time the participant has taken one dose of study treatment through last visit, and were recorded on the Case Report Form (CRF) according to Good Clinical Practice (GCP) and ICH guidelines." | Day 7 follow‐up included liver profile. | Day 7 follow‐up included liver profile. | — |
Data and analyses
Comparison 1. Artemisinin‐naphthoquine versus AL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever clearance | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fever on day 1 | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.29] |
| 1.2 Fever on day 2 | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.89, 8.43] |
| 1.3 Fever on day 3 | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.39, 3.52] |
| 2 Parasite clearance | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Parasitaemia on day 1 | 3 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.19] |
| 2.2 Parasitaemia on day 2 | 3 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 2.3 Parasitaemia on day 3 | 3 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.70] |
| 3 PCR‐unadjusted treatment failure at day 28 | 3 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.24, 4.37] |
| 4 PCR‐adjusted treatment failure at day 28 | 3 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 7.07] |
| 5 PCR‐unadjusted treatment failure at day 42 | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.59] |
| 6 PCR‐adjusted treatment failure at day 42 | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 7 Gametocyte carriage | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 At baseline | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.87, 1.80] |
| 7.2 At day 7 | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.42, 4.60] |
| 7.3 At day 14 | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.13 [1.75, 98.47] |
| 8 Anaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 At baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 On day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Vomiting | 2 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.39, 2.64] |
| 9.2 Diarrhoea | 2 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.30, 2.54] |
| 9.3 Nausea | 2 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.40, 2.25] |
| 9.4 Abdominal pain | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.73, 2.45] |
| 9.5 Anorexia | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.32, 27.60] |
| 9.6 Dizziness | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.14] |
| 9.7 Headaches | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.61, 2.92] |
| 9.8 Asthenia | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.38] |
| 9.9 Trouble sleeping | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.30] |
| 9.10 Cough | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.81, 1.84] |
| 9.11 Difficulty breathing | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.19] |
| 9.12 Pruritus | 2 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.29, 7.34] |
| 9.13 Skin rash | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.26, 4.00] |
Comparison 2. Artemisinin‐naphthoquine versus DHA‐P.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever clearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fever on day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Fever on day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Parasite clearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Parasitaemia on day 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Parasitaemia on day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Parasitaemia on day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PCR‐unadjusted treatment failure at day 28 | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PCR‐adjusted treatment failure at day 28 | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 PCR‐unadjusted treatment failure at day 42 | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.26] |
| 6 PCR‐adjusted treatment failure at day 42 | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.82] |
| 7 Gametocyte carriage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 At baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Vomiting | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Diarrhoea | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Nausea | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Anorexia | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Dizziness | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Asthenia | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 Abdominal pain | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.8 Pruritus | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Cough | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.17, 19.95] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kinde‐Gazard 2012 BEN.
| Methods | Trial design: RCT Follow‐up: Patients were hospitalized for the first three days, and monitored clinically and biochemically. Following discharge patients were seen on Day 7, 14, 21, and 28 with a malaria blood film at each visit. Adverse event monitoring: A symptom questionnaire, biochemistry (U and E, LFT), and haematology were conducted at each visit. |
|
| Participants | Number: 174 participants randomized Inclusion criteria: Age 6 months to 15 years, clinical signs of uncomplicated malaria, temp > 37.5°C or history of fever in the last 24 hours, asexual P. falciparum density > 2000/μL, able to take oral medication, informed consent. Exclusion criteria: Signs of severe malaria, known hypersensitivity to study medications, treatment with antimalarials within the past 7 days, positive pregnancy test. In addition the trial authors state that they planned to exclude the following groups from the study: severe toxicity, abnormal biochemical tests, unsatisfactory therapeutic response. However, no participants appear to have been excluded for these reasons. |
|
| Interventions | 1. Artemesinin‐naphthoquine 125 mg/50 mg, fixed‐dose combination (Arco, Kunming Pharmaceutical Corporation, China):
2. AL 20 mg/120 mg, fixed‐dose combination (Coartem, Novartis SA, Switzerland):
|
|
| Outcomes |
|
|
| Notes | Country: Benin Setting: Hospital Transmission: High Resistance: Chloroquine and SP Dates: July to Oct 2008 and May to Sept 2009 Funding: None stated, however the randomization procedure was done by the manufacturer of artemisinin‐naphthoquine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "According to a randomization method". |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as "single blind", however as the drug regimens differed significantly blinding of patients and personnel is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Funding is unclear, but the pharmaceutical company appear to be involved. |
Laman 2014 PNG.
| Methods | Trial design: open‐label, randomized controlled trial Follow‐up: Standardized assessment including axillary temp and blood film on Days 1, 2, 3, 7, 14, 28 and 42. Adverse event monitoring: Standardized assessment at each visit plus blood tests for full blood count, hepatorenal function, and an electrocardiogram on Days 0, 3 and 7. An additional electrocardiogram was performed 4 hours after Day 2 dose in those treated with ART‐NQ and in a convenience sample of 30 people treated with artemether‐lumefantrine. |
|
| Participants | Number: 198 participants with P. falciparum randomized. Inclusion criteria: Age 6 months to 5 years, axillary temp > 37.5°C or history of fever in the last 24 hours, asexual P. falciparum density > 1000/μL, or P. vivax > 250/μL. Exclusion criteria: Signs of severe malaria, taken study drug in the previous 14 days, known allergy to study medications, evidence of other infection or co‐morbidity. |
|
| Interventions | 1. Artemesinin‐naphthoquine; fixed‐dose combination (Kunming Pharmaceutical Corporation, China):
2. AL: fixed‐dose combination (Novartis Pharma, Switzerland):
Direct observation of morning AL dose only |
|
| Outcomes |
|
|
| Notes | Country: Papua New Guinea Setting: Health centres Transmission: Not described Resistance: Not described Dates: March 2011 to April 2013 Funding: National Health and Medical Research Council, Australian Award PhD Scholarship, Esso‐Highlands PNGIMR scholarship |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated block randomization". |
| Allocation concealment (selection bias) | Low risk | "Allocated treatments were concealed in sealed numbered envelopes that were opened in sequence by study medical or nursing staff, and the specified treatment was administered." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Treatments were not blinded, primarily because the endpoints were based on objective clinical and parasitologic criteria". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All blood films were reexamined independently by two skilled microscopists who were blind to allocated treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At day 28: 2/98 (2%) were lost to follow‐up with artemisinin‐naphthoquine versus 6/100 (6%) with artemether‐lumefantrine. At day 42: 4/98 (4%) were lost to follow‐up with artemisinin‐naphthoquine versus 8/100 (8%) with artemether‐lumefantrine. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript." |
Tjitra 2012 IDN.
| Methods | Trial design: A phase III, randomized, open label, multi‐centre, comparative study. Follow‐up: Limited physical exam on Days 1‐2, 3, 7, 14, 12, 28, 35, and 42 and if clinically indicated. ECG 2 to 4 hours after drug administration and on follow‐up Days 7, 28, and 42. Thick and thin smears eight hourly in Days 0 to 2, then Days 3, 7, 14, 21, 28, 35, and 42. Haematology and blood chemistry at Days 3, 7, and 28. Blood spot for PCR at Day 42 or failure. Urinalysis on Day 3. HCG for women on Days 0 and 28. Adverse event monitoring: "adverse events collected each time" and "Safety was assessed through direct questioning, physical examinations, ECG abnormalities (prolongation QT‐interval), and significant change from baseline clinical laboratory parameters [17]. Adverse events were followed up until the event had resolved." |
|
| Participants | Number: 401 randomized (153 P. falciparum only, 90 mixed, 158 P. vivax only). Inclusion criteria: adult, absence of severe malnutrition, axillary temperature > 37.5°C or a history of fever within the preceding 24 hours, asexual P. falciparum density 1000 to 200,000/μL, P. vivax and other malaria density ≥ 250/μL, able to take oral treatment, informed consent, uncomplicated P. falciparum or P. vivax mono‐infection, or mixed infection. Exclusion criteria: severe vomiting, history or evidence of 'clinically systematic significant disorders', other febrile conditions, hypersensitivity or adverse reactions to antimalarials, history of use of any other antimalarial agent within four weeks of the start of the trial and confirmed by urine test, and pregnancy or lactating. |
|
| Interventions | 1. Artemesinin‐naphthoquine, fixed‐dose combination, 250 mg/100 mg tablets (Arco, Kunming Pharmaceutical Corporation, China):
2. DHA‐P, fixed‐dose combination, 40 mg/320 mg tablets (Duo‐Cotecxin: Holey‐Cotec Pharmaceutical Co. Ltd, China):
All doses supervised. |
|
| Outcomes |
|
|
| Notes | Country: Indonesia Setting: Three Armed Forces hospitals in Jayapura (Marthen Indeys/Army, Soedibjo Sardadi/Navy, and Bhayangkara/Police Hospitals) and one public hospital in Maumere (St. Gabriel Hospital) Transmission: Not reported Resistance: Widely reported resistance of P. falciparum to chloroquine, sulphadoxine‐pyrimethamine, and quinine Dates: 2007 to 2008 Funding: Kunming Pharmaceutical Corporation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible subjects were blindly, randomly assigned equally to one of the two treatment groups using sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | "Eligible subjects were blindly, randomly assigned equally to one of the two treatment groups using sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial described as "open label". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Microscopy results were blind cross‐checked by certified microscopists". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low losses to follow‐up in both groups (3.5% AS‐N versus 5% DHA‐P). |
| Selective reporting (reporting bias) | Low risk | All listed outcomes reported. |
| Other bias | Unclear risk | "We also thank to Kunming Pharmaceutical Corporation for funding the artemisinin‐naphthoquine trial." The role of the pharmaceutical company in the design, conduct, and interpretation of the trial is unclear. |
Toure 2009 CIV.
| Methods | Trial design: randomized single‐blinded clinical trial Follow‐up: on Days 1, 2, 3, 7, 14, 21, and 28 (or any other day if they felt ill). Follow‐up evaluation was history and examination. Day 7 follow‐up included full blood count and liver profile. Blood spots collected for PCR on day of failure. Adverse event monitoring: "All observed adverse events were monitored actively and passively from the time the participant has taken one dose of study treatment through last visit, and were recorded on the Case Report Form (CRF) according to Good Clinical Practice (GCP) and ICH guidelines." |
|
| Participants | Number: 125 randomized Inclusion criteria: ≥ six months old, P. falciparum monoinfection with parasitaemia level of 2000 to 200,000 asexual parasites/μL, axillary temperature > 37.5°C or a history of fever within the preceding 24 hours, no history of serious side effects to study medications, no evidence of concomitant febrile illness, provision of written informed consent by the participant or parent/guardian. Exclusion criteria: symptoms or signs of severe malaria, or both, any "danger sign" (persistent vomiting; inability to sit, stand, drink or breast feed), recent history of convulsions or lethargy, or both, or otherwise impaired consciousness, haemoglobin concentration ≤ 6 mg/dL, serious underlying disease, or known allergy to the study drugs. "Participants were also excluded after randomization, if they repeatedly vomited their first dose of study medications." "Participants were excluded after enrolment if any of the following occurred: (1) use of antimalarial drugs outside of the study protocol; (2) parasitaemia in the presence of a concomitant febrile illness; (3) withdrawal of consent; (4) loss to follow‐up, (5) protocol violation, or (6) death due to a non‐malaria illness." |
|
| Interventions | 1. Artemesinin‐naphthoquine, 125 mg/50 mg fixed‐dose combination at 0, 8 hours (Arco, Kunming Pharmaceutical Corporation, China):
2. AL fixed‐dose combination (Coartem, Novartis SA, Switzerland):
Morning doses supervised and evening doses taken at home. "The empty sachets were returned to study site as evidence of taking the drug". |
|
| Outcomes |
|
|
| Notes | Country: Côte d'Ivoire Setting: Primary care centre in Anonkoua‐kouté (crowded sub‐urban area) Transmission: "extremely high transmission intensity" and "holoendemic". Resistance: "Plasmodium falciparum resistance to affordable anti‐malarial drugs (chloroquine and sulphadoxine‐pyrimethamine) has reached high levels". Dates: November 2006 to January 2007 Funding: Institut Pasteur, Kunming Pharmaecutical Corporation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants recruited into the study were allocated to two treatment groups using a computer generated random list based on a simple random selection procedure without the use of blocking or stratification by an off‐site investigator." |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, sealed envelopes containing the treatment group assignments were prepared from the randomization list. The study clinical investigators assigned treatment numbers sequentially and a third party investigator who is an appropriately qualified member of the study site, allocated treatment by opening the envelope corresponding to the treatment number. The randomization codes were secured in a locked cabinet accessible only by the third party. Participants were enrolled by the study physicians, and treatments were assigned and administered by the third party." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants were not informed of their treatment regimen." Comment: As the regimens are different and no placebos were used participants were essentially unblinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Only the third party was aware of treatment assignments. All other study personnel, including the study physicians and laboratory personnel involved in assessing outcomes, were blinded to the treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from each group didn't complete the study: 1 from AN lost to follow‐up and 1 from AL excluded. |
| Selective reporting (reporting bias) | Low risk | All listed outcomes reported. |
| Other bias | Low risk | No other sources of bias identified. "Artemisinin/naphthoquine and Artemether/lumefantrine were provided free of charge respectively by Kunming Pharmaceutical Corp. and Novartis S A. The funders had no involvement in the study design, data collection and its analysis and interpretation, in the writing of paper or in decision to submit it for publication". |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batty 2012 | Study 1 ‐ no control group. Study 2 ‐ not a relevant comparison. This study is a pharmacokinetic study comparing a single dose of AS‐N with two doses of AS‐N given 24 hours apart. |
| Benjamin 2012 | Not a relevant comparison: this study compares a single dose of AS‐N given with water versus a single dose of AS‐N given with milk versus two doses of AS‐N. |
| Guo 2003 | Not a relevant comparison: this study compares naphthoquine monotherapy with artesunate monotherapy or mefloquine monotherapy. |
| Hombhanje 2009 | Not a relevant comparison: this study compares a single dose of ART‐NQ with a three day course of chloroquine plus sulphadoxine‐pyrimethamine. |
| Liu 2012 | A review article: discusses a pharmacokinetic study (Batty 2012). |
| Lui 2013 | Not a relevant comparison: this study compares a three day course of ART‐NQ with CQ plus primaquine for treating P. vivax. |
| Meremikwu 2012 | Not a relevant comparison: this study compares a single dose of ART‐NQ versus a high single dose of ART‐NQ versus two dose of ART‐NQ. |
| Tun 2009 | No control group: a single arm trial of a single dose ART‐NQ in adults with uncomplicated P. falciparum malaria. |
| Wang 2002 | No control group: single‐arm trial of dihydroartemisinin combined with naphthoquine. |
| Wang 2003 | Not randomized. |
| Wang 2004 | A review article. |
Contributions of authors
DS and BZ contributed to the development of the standard protocol as used in Sinclair 2009. BZ drafted the background. RI and MG reviewed the reference list, extracted data, and entered it into Review Manager (RevMan). RI, MG, and DS conducted the analyses, constructed 'Summary of findings' tables, and evaluated the quality of evidence using the GRADE approach. RI wrote the first draft and all authors reviewed and contributed to the final draft.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development (DFID), UK.
Declarations of interest
The review authors have no conflicting interests.
Unchanged
References
References to studies included in this review
Kinde‐Gazard 2012 BEN {published data only}
- Kinde‐Gazard D, Ogouyèmi‐Hounto A, Capo‐Chichi L, Gbaguidi J, Massougbodji A. [A randomized clinical trial comparing the effectiveness and tolerability of artemisinine‐naphthoquine (Arco®) and artemether‐lumefantrine (Coartem®) in the treatment of uncomplicated malaria in Benin] [Essai clinique randomisé comparant l'efficacité et la tolérance de la combinaison artémisinine‐naphthoquine (Arco®) et artéméther‐luméfantrine (Coartem®) dans le traitement du paludisme simple au Bénin]. Bulletin de la Société de Pathologie Exotique 2012;105(3):208‐14. [DOI] [PubMed] [Google Scholar]
Laman 2014 PNG {published data only}
- Laman M, Moore BR, Benjamin JM, Yadi G, Bona C, Warrel J, et al. Artemisinin‐Naphthoquine versus Artemether‐Lumefantrine for Uncomplicated Malaria in Papua New Guinean Children: An Open‐Label Randomized Trial. PLoS Medicine 2014;11(12):e1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tjitra 2012 IDN {published data only}
- Tjitra E, Hasugian AR, Siswantoro H, Prasetyorini B, Ekowatiningsih R, Yusnita EA, et al. Efficacy and safety of artemisinin‐naphthoquine versus dihydroartemisinin‐piperaquine in adult patients with uncomplicated malaria: a multi‐centre study in Indonesia. Malaria Journal 2012;11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toure 2009 CIV {published data only}
- Toure OA, Penali LK, Yapi JD, Ako BA, Toure W, Djerea K, et al. A comparative, randomized clinical trial of artemisinin/naphthoquine twice daily one day versus artemether/lumefantrine six doses regimen in children and adults with uncomplicated falciparum malaria in Côte d'Ivoire. Malaria Journal 2009;8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Batty 2012 {published data only}
- Batty KT, Salman S, Moore BR, Benjamin J, Lee ST, Page‐Sharp M, et al. Artemisinin‐naphthoquine combination therapy for uncomplicated pediatric malaria: a pharmacokinetic study. Antimicrobial Agents and Chemotherapy 2012;56(5):2472‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2012 {published data only}
- Benjamin J, Moore B, Lee ST, Senn M, Griffin S, Lautu D, et al. Artemisinin‐naphthoquine combination therapy for uncomplicated pediatric malaria: a tolerability, safety, and preliminary efficacy study. Antimicrobial Agents and Chemotherapy 2012;56(5):2465‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2003 {published data only}
- Guo WZ, Guo XB, Zheng QJ, Tan B, Chen RJ, Ou FZ, et al. [A randomized comparative study of naphtoquine, mefloquine and artsunate in the treatment of falciparum malaria]. Zhonghua Yi Xue Za Zhi 2003;83(16):1406‐8. [PubMed] [Google Scholar]
Hombhanje 2009 {published data only}
- Hombhanje FW, Linge D, Saweri A, Kuanch C, Jones R, Toraso S, et al. Artemisinin‐naphthoquine combination (ARCO) therapy for uncomplicated falciparum malaria in adults of Papua New Guinea: a preliminary report on safety and efficacy. Malaria Journal 2009;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu R, Dong HF, Jiang MS. A pharmacokinetic approach to assess artemisinin‐naphthoquine combination therapy for uncomplicated pediatric malaria. Expert Review of Clinical Pharmacology 2012;5(5):521‐4. [DOI] [PubMed] [Google Scholar]
Lui 2013 {published data only}
- Liu H, Yang HL, Xu JW, Wang JZ, Nie RH, Li CF. Artemisinin‐naphthoquine combination versus chloroquine‐primaquine to treat vivax malaria: an open‐label randomized and non‐inferiority trial in Yunnan Province, China. Malaria Journal 2013;12:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meremikwu 2012 {published data only}
- Meremikwu MM, Odey F, Oringanje C, Oyo‐Ita A, Effa E, Esu EB, et al. Open‐label trial of three dosage regimens of fixed‐dose combination of artemisinin and naphthoquine for treating uncomplicated falciparum malaria in Calabar, Nigeria. Malaria Journal 2012;11:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tun 2009 {published data only}
- Tun T, Tint HS, Lin K, Kyaw TT, Myint MK, Khaing W, et al. Efficacy of oral single dose therapy with artemisinin‐naphthoquine phosphate in uncomplicated falciparum malaria. Acta Tropica 2009;111(3):275‐8. [DOI] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang SQ, Meng F, Shen H, Wen Y, Zhuo KR, Zhu QX, et al. [Therapeutic effect of dihydroartemisinin combined with naphthoquine phosphate in patients with falciparum malaria]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2002;20(3):180‐2. [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang JY, Shan CQ, Fu DD, Sun ZW, Ding DB. [Efficacy of naphthoquine, artemisinine and a combination of the two drugs in the treatment of falciparum malaria]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2003;21(3):131‐3. [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang JY, Cao WC, Shan CQ, Zhang M, Li GF, Ding DB, et al. Naphthoquine phosphate and its combination with artemisinine. Acta Tropica 2004;89(3):375‐81. [DOI] [PubMed] [Google Scholar]
Additional references
Bloland 2003
- Bloland PB. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria [WHO/HTM/RBM/2003.50]. Geneva: World Health Organization, 2003. [Google Scholar]
Bukirwa 2014
- Bukirwa H, Unnikrishnan B, Kramer CV, Sinclair D, Nair S, Tharyan P. Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD006404.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cattamanchi 2003
- Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp‐1, msp‐2, and glurp. American Journal of Tropical Medicine and Hygiene 2003;68(2):133‐9. [PubMed] [Google Scholar]
Dondorp 2009
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine 2009;361(5):455‐67. [DOI: 10.1056/NEJMoa0808859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ezzet 2000
- Ezzet F, Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrobial Agents and Chemotherapy 2000;44(3):697‐704. [DOI: 10.1128/AAC.44.3.697-704.2000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hombhanje 2010
- Hombhanje FW, Huang Q. Artemisinin‐naphthoquine combination (ARCO®): an overview of the progress. Pharmaceuticals 2010;3:3581‐93. [Google Scholar]
Karbwang 1990
- Karbwang J, White NJ. Clinical pharmacokinetics of mefloquine. Clinical Pharmacokinetics 1990;19(4):264‐79. [PUBMED: 2208897] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.0.1. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Noedl 2008
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. Evidence of artemisinin‐resistant malaria in western Cambodia. New England Journal of Medicine 2008;359(24):2619‐20. [DOI: 10.1056/NEJMc0805011] [DOI] [PubMed] [Google Scholar]
Pawluk 2013
- Pawluk SA, Wilby KJ, Ensom MH. Pharmacokinetic profile of artemisinin derivatives and companion drugs used in artemisinin‐based combination therapies for the treatment of Plasmodium falciparum malaria in children. Clinical Pharmacokinetics 2013;52(3):153–67. [DOI] [PubMed] [Google Scholar]
Phyo 2012
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, et al. Emergence of artemisinin‐resistant malaria on the western border of Thailand: a longitudinal study. Lancet 2012;379(9830):1960‐6. [DOI: 10.1016/S0140-6736(12)60484-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qu 2010
- Qu HY, Gao HZ, Hao GT, Li YY, Li HY, Hu JC, et al. Single‐dose safety, pharmacokinetics, and food effects studies of compound naphthoquine phosphate tablets in healthy volunteers. Journal of Clinical Pharmacology 2010;50(11):1310‐8. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sinclair 2009
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1996
- White NJ, Olliaro PL. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitology Today 1996;12(10):399‐401. [DOI] [PubMed] [Google Scholar]
White 1999
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
White 2002
- White NJ. The assessment of antimalarial drug efficacy. Trends in Parasitology 2002;18(10):458‐64. [DOI] [PubMed] [Google Scholar]
White 2004
- White NJ. Antimalarial drug resistance. Journal of Clinical Investigation 2004;113(8):1084‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2008
- WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. http://whqlibdoc.who.int/publications/2008/9789241596305_eng.pdf (accessed 28 December 2010).
WHO 2010a
- WHO. Guidelines for the treatment of malaria. Second edition March 2010. www.who.int/malaria/publications/atoz/9789241547925/en/index.html (accessed 20 November 2010).
WHO 2010b
- WHO. Global report on antimalarial drug efficacy and drug resistance: 2000‐2010. www.who.int/malaria/publications/atoz/9789241500470/en/index.html (accessed 28 December 2010).
WHO 2011
- WHO. Global plan for artemesinin resistance containment (GPARC). www.who.int/malaria/publications/atoz/9789241500838/en/index.html (accessed 04 February 2011).
WHO 2012
- WHO Global Malaria Programme. World Malaria Report 2012. Geneva: WHO, 2012. [Google Scholar]
Woodrow 2005
- Woodrow CJ, Haynes RK, Krishna S. Artemisinins. Postgraduate Medical Journal 2005;81(952):71‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


