Abstract
Background
In settings where both Plasmodium vivax and Plasmodium falciparum infection cause malaria, rapid diagnostic tests (RDTs) need to distinguish which species is causing the patients' symptoms, as different treatments are required. Older RDTs incorporated two test lines to distinguish malaria due to P. falciparum, from malaria due to any other Plasmodium species (non‐falciparum). These RDTs can be classified according to which antibodies they use: Type 2 RDTs use HRP‐2 (for P. falciparum) and aldolase (all species); Type 3 RDTs use HRP‐2 (for P. falciparum) and pLDH (all species); Type 4 use pLDH (fromP. falciparum) and pLDH (all species).
More recently, RDTs have been developed to distinguish P. vivax parasitaemia by utilizing a pLDH antibody specific to P. vivax.
Objectives
To assess the diagnostic accuracy of RDTs for detecting non‐falciparum or P. vivax parasitaemia in people living in malaria‐endemic areas who present to ambulatory healthcare facilities with symptoms suggestive of malaria, and to identify which types and brands of commercial test best detect non‐falciparum and P. vivax malaria.
Search methods
We undertook a comprehensive search of the following databases up to 31 December 2013: Cochrane Infectious Diseases Group Specialized Register; MEDLINE; EMBASE; MEDION; Science Citation Index; Web of Knowledge; African Index Medicus; LILACS; and IndMED.
Selection criteria
Studies comparing RDTs with a reference standard (microscopy or polymerase chain reaction) in blood samples from a random or consecutive series of patients attending ambulatory health facilities with symptoms suggestive of malaria in non‐falciparum endemic areas.
Data collection and analysis
For each study, two review authors independently extracted a standard set of data using a tailored data extraction form. We grouped comparisons by type of RDT (defined by the combinations of antibodies used), and combined in meta‐analysis where appropriate. Average sensitivities and specificities are presented alongside 95% confidence intervals (95% CI).
Main results
We included 47 studies enrolling 22,862 participants. Patient characteristics, sampling methods and reference standard methods were poorly reported in most studies.
RDTs detecting 'non‐falciparum' parasitaemia
Eleven studies evaluated Type 2 tests compared with microscopy, 25 evaluated Type 3 tests, and 11 evaluated Type 4 tests. In meta‐analyses, average sensitivities and specificities were 78% (95% CI 73% to 82%) and 99% (95% CI 97% to 99%) for Type 2 tests, 78% (95% CI 69% to 84%) and 99% (95% CI 98% to 99%) for Type 3 tests, and 89% (95% CI 79% to 95%) and 98% (95% CI 97% to 99%) for Type 4 tests, respectively. Type 4 tests were more sensitive than both Type 2 (P = 0.01) and Type 3 tests (P = 0.03).
Five studies compared Type 3 tests with PCR; in meta‐analysis, the average sensitivity and specificity were 81% (95% CI 72% to 88%) and 99% (95% CI 97% to 99%) respectively.
RDTs detecting P.vivax parasitaemia
Eight studies compared pLDH tests to microscopy; the average sensitivity and specificity were 95% (95% CI 86% to 99%) and 99% (95% CI 99% to 100%), respectively.
Authors' conclusions
RDTs designed to detect P. vivax specifically, whether alone or as part of a mixed infection, appear to be more accurate than older tests designed to distinguish P. falciparum malaria from non‐falciparum malaria. Compared to microscopy, these tests fail to detect around 5% ofP. vivax cases. This Cochrane Review, in combination with other published information about in vitro test performance and stability in the field, can assist policy‐makers to choose between the available RDTs.
12 April 2019
No update planned
Review superseded
This Cochrane Review has been superseded by Choi 2019 https://doi.org/10.1002/14651858.CD013218
Keywords: Humans; Antigens, Protozoan; Antigens, Protozoan/analysis; Cohort Studies; Malaria; Malaria/diagnosis; Malaria/immunology; Malaria/parasitology; Malaria, Vivax; Malaria, Vivax/diagnosis; Malaria, Vivax/immunology; Microscopy; Parasitemia; Parasitemia/diagnosis; Plasmodium; Plasmodium/immunology; Plasmodium vivax; Plasmodium vivax/immunology; Polymerase Chain Reaction; Reagent Kits, Diagnostic; Reagent Kits, Diagnostic/parasitology; Sensitivity and Specificity; Species Specificity
Plain language summary
Rapid tests for diagnosing malaria caused by Plasmodium vivax or other less common parasites
This review summarises trials evaluating the accuracy of rapid diagnostic tests (RDTs) for diagnosing malaria due to Plasmodium vivax or other non‐falciparum species. After searching for relevant studies up to December 2013, we included 47 studies, enrolling 22,862 adults and children.
What are rapid tests and why do they need to be able to distinguish Plasmodium vivax malaria
RDTs are simple to use, point of care tests, suitable for use in rural settings by primary healthcare workers. RDTs work by using antibodies to detect malaria antigens in the patient's blood. A drop of blood is placed on the test strip where the antibodies and antigen combine to create a distinct line indicating a positive test.
Malaria can be caused any one of five species of Plasmodium parasite, but P. falciparum and P. vivax are the most common. In some areas, RDTs need to be able to distinguish which species is causing the malaria symptoms as different species may require different treatments. Unlike P. falciparum, P. vivax has a liver stage which can cause repeated illness every few months unless it is treated with primaquine. The most common types of RDTs for P. vivax use two test lines in combination; one line specific to P. falciparum, and one line which can detect any species of Plasmodium. If the P. falciparum line is negative and the 'any species' line is positive, the illness is presumed to be due to P. vivax (but could also be caused by P. malariae, or P. ovale). More recently, RDTs have been developed which specifically test for P. vivax.
What does the research say
RDTs testing for non‐falciparum malaria were very specific (range 98% to 100%) meaning that only 1% to 2% of patients who test positive would actually not have the disease. However, they were less sensitive (range 78% to 89%), meaning between 11% and 22% of people with non‐falciparum malaria would actually get a negative test result.
RDTs which specifically tested for P. vivax were more accurate with a specificity of 99% and a sensitivity of 95%, meaning that only 5% of people with P. vivax malaria would have a negative test result.
Summary of findings
Summary of findings'. 'Performance of RDTs for diagnosis of non‐falciparum or P. vivax malaria.
| Patients/populations | People presenting with symptoms suggestive of uncomplicated malaria | |||||
| Prior testing | None | |||||
| Settings | Ambulatory healthcare settings in P. vivax,P. malariae or P. ovale malaria endemic areas in Asia, Africa and South America | |||||
| Index tests | Immunochromatography‐based rapid diagnostic tests (RDTs) for non‐falciparum malaria in the absence of P. falciparum co‐infection, or P. vivax malaria with or without other malaria species | |||||
| Reference standard | Conventional microscopy, polymerase chain reaction (PCR) | |||||
| Importance | Accurate and fast diagnosis allows appropriate and quick treatment for malaria to be provided | |||||
| Studies | 37 unique publications reporting 47 studies (22,862 participants) | |||||
| Quality concerns | Poor reporting of patient characteristics, sampling method and reference standard methods were common concerns | |||||
| Test type |
Quantity of evidence Number of evaluations (malaria cases/participants) |
Average sensitivity (95% CI) | Average specificity (95% CI) | Prevalence (%) | Consequences in a cohort of 1000 | |
| Missed cases | False positives | |||||
| Target condition (reference standard): non‐falciparum malaria (microscopy) | ||||||
|
Type 2 HRP‐2 (P. falciparum specific) and aldolase (pan‐specific) |
11 (958/6879) | 78% (73% to 82%) | 99% (97% to 99%) | 5 | 11 | 10 |
| 15 | 33 | 9 | ||||
| 30 | 66 | 7 | ||||
|
Type 3 HRP‐2 (P. falciparum specific) and pLDH (pan‐specific) |
23 (1537/11,234) | 78% (69% to 84%) | 99% (98% to 99%) | 5 | 11 | 10 |
| 15 | 33 | 9 | ||||
| 30 | 66 | 7 | ||||
|
Type 4 pLDH (P. falciparum specific) and pLDH (pan‐specific) |
10 (986/3831) | 89% (79% to 95%) | 98% (97% to 99%) | 5 | 6 | 19 |
| 15 | 17 | 17 | ||||
| 30 | 33 | 14 | ||||
| Target condition (reference standard): non‐falciparum malaria (PCR) | ||||||
|
Type 3 HRP‐2 (P. falciparum specific) and pLDH (pan‐specific) |
5 (300/1639) | 81% (72% to 88%) | 99% (97% to 99%) | 5 | 10 | 10 |
| 15 | 29 | 9 | ||||
| 30 | 57 | 7 | ||||
| Target condition (reference standard): P.vivax with or without other malaria species (microscopy) | ||||||
| HRP‐2 (P. falciparum specific) and pLDH (P. vivax‐specific) | 8 (580/3682) | 95% (86% to 99%) | 99% (99% to 100%) | 5 | 3 | 10 |
| 15 | 8 | 9 | ||||
| 30 | 15 | 7 | ||||
| Conclusions: The majority of studies evaluated RDTs which are designed to differentiate falciparum malaria from non‐falciparum malaria, but cannot differentiate between different non‐falciparum species or identify non‐falciparum malaria species within a mixed infection. In these types of tests, specificity for non‐falciparum malaria in the absence ofP. falciparum infection was high, but sensitivity was low, tests missing between 11% and 22% of non‐falciparum cases. RDTs which are designed to detect P. vivax specifically, whether alone or part of a mixed infection, were more accurate with tests missing less than 5% of P. vivax cases. This review can help decision‐making about which RDT to use, in combination with other published information about in vitro test performance and stability in the field. | ||||||
Background
Target condition being diagnosed
Malaria is a life‐threatening illness caused by protozoan Plasmodium parasites, which are transmitted by many species of Anopheles mosquitoes. In 2008, there were between 190 and 311 million cases of malaria worldwide (WHO 2009b). The two most common species of parasites that cause malaria are Plasmodium falciparum andPlasmodium vivax. Falciparum malaria is the most common cause of severe malaria and malaria deaths and can also cause other complications, such as anaemia and, in pregnancy, low birthweight babies. Vivax malaria is a relapsing form, which is rarely fatal but can cause serious anaemia in children. Other, less common, Plasmodium species that cause malaria in people include P. malariae and P. ovale. Malaria is a curable disease, and therefore malaria‐related morbidity and mortality can be reduced. Early, prompt and accurate diagnosis followed by appropriate treatment is the key to effective disease management (WHO 2003) and is a basic tenet of current malaria control policy (WHO 2005; Bell 2006).
People who are exposed repeatedly to Plasmodium infection develop a partial and incomplete immunity. This means that in highly endemic areas those most at risk are children under the age of five, who have not yet had the chance to develop immunity. In less endemic areas, or areas of seasonal or epidemic transmission, older children and adults are also at risk due to less developed immunity. Travellers from non‐endemic to endemic countries are at highest risk because they have no immunity at all.
Index test(s)
Rapid diagnostic tests (RDTs) (WHO 2003) detect parasite‐specific antigens in a drop of fresh blood through lateral flow immunochromatography (WHO 2006). The World Health Organization (WHO) currently lists 96 commercially available test kits meeting ISO131485 manufacturing standards (WHO 2009). RDTs do not require a laboratory or any special equipment (WHO 2006), are simple to use and can give results as a simple positive or negative result, at thresholds pre‐set by the manufacturers, within 15 minutes (Talman 2007). Therefore, RDTs are, in general, suitable for remote areas with limited facilities and relatively untrained staff. However, they have a limited shelf life and need to be kept dry and away from temperature extremes. They may also fail to detect malaria where there are low levels of Plasmodium parasites in the blood, for example in young children with low immunity, and false positives are possible due to cross reactions or gametocytaemia (Kakkilaya 2003).
Different types of RDT use different types of antibody or combination of antibodies to detect Plasmodium antigens. Some antibodies aim to detect a particular species while others are pan‐malarial, aiming to detect all types of Plasmodium.Table 2 lists the main types of RDT that were available in 2010. Since this classification was developed, the following test types have also become available:
1. Types of malaria RDTs by antigen combination and parasite species detected.
| Type of test | Antigen combinations | Possible results |
| Type 1 | HRP‐2 (P. falciparum specific) | No Pf; Pf; invalid |
| Type 2 | HRP‐2 (P. falciparum specific) and aldolase (pan‐specific) | No malaria; Pf or mixed; Pv, Pf, or Pm; invalid |
| Type 3 | HRP‐2 (P. falciparum specific) and pLDH (pan‐specific) | No malaria; Pf or mixed; Pv, Pf, or Pm; invalid |
| Type 4 | pLDH (P. falciparum specific) and pLHD (pan‐specific) | No malaria; Pf or mixed; Pv, Pf, or Pm; invalid |
| Type 5 | pLDH (P. falciparum specific) and pLHD (P. vivax‐specific) | No malaria; Pf; Pv; Pf and Pv; invalid |
| Type 6 | HRP‐2 (P. falciparum specific), pLHD (pan‐specific) and pLDH (P. vivax specific) | No malaria; Pf and Pv ± Po and/or Pm; Pf ± Po and/or Pm; Pv ± Po or Pm; Po or Pm; invalid |
| Type 7 | Aldolase (pan‐specific) | No malaria; Pf, Pv, Po,or Pm; invalid |
| Other | HRP‐2 (P. falciparum specific) and pLDH (P. vivax specific) | No malaria; Pf; Pv; Pf and Pv; invalid |
Pan pLDH only, with possible results of: no malaria; P. falciparum (Pf), P. vivax (Pv), P. ovale (Po), or P. malariae (Pm); invalid
P. vivax specific pLDH only, with possible results of: no malaria; Pv; invalid;
P. falciparum specific HRP‐2 and P. vivax specific pLDH, with possible results of: no malaria; Pf, Pv, Pf + Pv; invalid.
HRP‐2 can stay in the blood for 28 days after initiating the antimalarial therapy (Kakkilaya 2003). Because of this 'persistent antigenaemia', it is not possible to use these tests in assessing parasite clearance following treatment, and false positive results may be found in patients who have recently been treated for malaria. In contrast, pLDH is rapidly cleared from the blood following parasite death; in fact it may clear more rapidly than the dead parasites (WHO 2009).
Alternative test(s)
Microscopic examination of Giemsa‐stained thick and thin blood films remains the conventional laboratory method and is still regarded as the 'gold standard'. Microscopic examination provides a good sensitivity and specificity, and it allows species and stage differentiations and quantification of parasites, all of which are important in assessing the disease severity and prescribing appropriate therapy. Intensive examination is more likely to reveal parasitaemia so the test is carried out with a fixed number of fields examined. Infections may be missed if slides are not examined carefully (Wongsrichanalai 2007). Very low parasitaemia may be missed even by good quality microscopy; the limit of detection of thick smear microscopy has been estimated at approximately four to 20 asexual parasites per µL, although under field conditions a threshold of 50 to 100 asexual parasites per µL is more realistic (Wongsrichanalai 2007). False positive results are also possible; if blood slides are not prepared carefully, artefacts may be formed which can be mistaken for Plasmodium parasites (Wongsrichanalai 2007).
The polymerase chain reaction (PCR), which is a molecular method based on DNA amplification, is the most accurate method of detecting parasites in the blood. Compared to microscopy, PCR is less prone to observer error and more sensitive at low levels of parasitaemia (Snounou 1993). For PCR, the limit of detection may be as low as 0.004 asexual parasites per µL (Hänscheid 2002). However, whether this increased ability to detect low level parasitaemia makes it a better diagnostic test is uncertain, as sub‐microscopic parasitaemia are of unknown clinical significance and the prevalence of asymptomatic sub‐microscopic infection is high in some areas (May 1999). PCR is currently not widely available due to logistical constraints and the need for specially trained technicians and a well‐equipped laboratory. It is usually used only for research purposes.
Rationale
A diagnostic test which is simple to perform, rapid and accurate is important in many situations to ensure prompt specific treatment, reduce misdiagnosis of non‐malarial illness as malaria, limit the development of drug resistance (Talman 2007) and reduce drug wastage. The WHO lists some of the situations where RDTs can be particularly useful in remote areas without access to expert microscopy, complex emergencies and severe malaria, where rapid diagnosis is essential to save lives (WHO 2000).
The WHO 2010 guidelines recommend chloroquine for P. vivax malaria in areas in which parasites remain sensitive to this drug, although they are currently considering recommending artemisinin‐based combination therapies (ACTs) for all P. vivax infections as they are effective (Gogtay 2013). Primaquine may be added to immediate treatment of P. vivax (and P. ovale) to effect a radical cure and prevent relapse (WHO 2010). Therefore, in areas where both P. falciparum and P. vivax are endemic, it is often useful to be able to distinguish between the two species.
The relative costs of microscopy and RDTs vary according to context. Where there is a relatively high prevalence of malaria and an established microscopy service, microscopy would usually be less expensive than RDTs because most of the costs associated with microscopy are fixed costs, and microscopy can also be used to diagnose other diseases. In areas where malaria is less prevalent, or very rural areas where access to good quality microscopy services is limited, RDTs may be less expensive than microscopy (WHO 2008). The cost of RDTs also depends on the type of test used, which will depend on the types of malaria parasite endemic in the area; the WHO describes three zones (WHO 2005a) as shown in Table 3.
2. Malaria 'zones' by endemic parasite species and type of test appropriate for each.
| Zone | Endemic malaria parasites | Geographic area | Appropriate test type |
| 1 | P. falciparum only or other species almost always as a mixed infection | Most of sub‐Saharan Africa; lowland Papua New Guinea | Tests using HRP‐2 to detect P. falciparum only (Type 1) |
| 2 | Both P. falciparum and P. vivax, most commonly as a single species |
Asia and the Americas; Ethiopian highlands |
Combination RDTs which detect all species and distinguish between P. falciparum and P. vivax (Types 2 to 6) |
| 3 | Non‐falciparum only |
Vivax‐only areas of East Asia and Central Asia; some highland areas elsewhere | Pan‐specific or vivax‐specific RDTs (Type 7; Pan‐pLDH only; vivax‐pLDH only) |
RDTs may be used to confirm diagnosis before commencing treatment in people with symptoms of malaria where confirmation by microscopy is currently unavailable or unused, thereby increasing the specificity of diagnosis, which would otherwise be made on symptoms only. Alternatively, RDTs may replace microscopy for confirmatory diagnosis, where logistical factors and relative costs indicate that this may be beneficial. The usefulness of RDTs in these roles will depend to a large extent on their accuracy. The sensitivity and specificity thresholds that decide whether a test is useful in practice will depend upon the situation; as malaria endemicity varies enormously by geographic area, and positive and negative predictive values will vary considerably with endemicity, relating to the proportion of patients with fever who have malaria. In addition, microscopy is not a perfect reference standard in itself, and the relative accuracy of RDTs and microscopy will depend to a large extent on the performance of the laboratory facilities and personnel available for microscopy.
Previously published systematic reviews have focused on the accuracy of RDTs for diagnosing malaria in travellers returning to non‐endemic countries from endemic countries (Marx 2005). As far as we know this is the first systematic review to assess the accuracy of the full range of RDTs for diagnosing non‐falciparum orP. vivax malaria in people with symptoms in malaria‐endemic areas.
This review is the second of two Cochrane Reviews assessing the accuracy of RDTs for diagnosing symptomatic uncomplicated malaria in endemic countries. It covers two slightly different target conditions; non‐falciparum malaria in the absence of P. falciparum infection and P. vivax malaria, corresponding to the results obtainable with different RDT test types. The first review reported separately on RDTs for diagnosing P. falciparum malaria (Abba 2011). The summaries in this review are to assist decision making, in conjunction with other relevant information about these tests, including in vitro assessment and tests of stability and costs (WHO 2012).
Objectives
To assess the diagnostic accuracy of RDTs for detecting non‐falciparum or P. vivax malaria parasitaemia in people living in malaria‐endemic areas who present to ambulatory healthcare facilities with symptoms suggestive of malaria and to identify which types and brands of commercial test best detect non‐falciparum and P. vivax malaria.
Investigation of sources of heterogeneity
We planned to investigate heterogeneity in relation to age group, continent where the study took place, and adequacy of reference standard.
Methods
Criteria for considering studies for this review
Types of studies
Studies sampling a consecutive series of patients, or a randomly selected series of patients were eligible. Where the report did not explicitly state that sampling was consecutive, but we judged that consecutive sampling was most probable, we included the report. We excluded studies if they did not present sufficient data to allow us to extract absolute numbers of true positives, false positives, false negatives and true negatives. Due to resource constraints, we also excluded studies if the report did not present enough information to allow full assessment of eligibility or if the study was reported only in a non‐English language.
Participants
Studies recruiting people living in P. vivax,P. ovale or P. malariae endemic areas attending ambulatory healthcare settings with symptoms of uncomplicated malaria were eligible.
We excluded studies if participants:
were non‐immune people returning from endemic countries or were mainly recent migrant or displaced populations from non‐endemic or very low endemicity areas;
had been treated for malaria and the test was performed to assess treatment outcome;
had symptoms of severe malaria;
did not have symptoms of malaria;
were recruited through active case finding (for example, door to door surveys).
In studies where only a subgroup of participants was eligible for inclusion in the review, we included the study provided that we could extract relevant data specific to that subgroup. If studies included some patients with severe malaria, and we could not extract data specific to a subgroup of participants with uncomplicated malaria, we included the study if 90% or more of the participants had uncomplicated malaria.
Index tests
Studies evaluating any immunochromatography‐based RDTs specifically designed to detect non‐falciparum or P. vivax malaria. We included commercial tests that are no longer available because they may use the same antibodies and very similar technology to tests that are currently available or may become available in the future. Older and more recently available versions of the same test, for example, OptiMAL and OptiMAL‐IT were included separately. We also included prototype tests which are not longer available but which correspond to one of the commercial tests.
Comparator tests
We included studies regardless of whether they made comparisons with other RDT tests or not.
Target conditions
Studies aimed to detect non‐falciparum or P. vivax malaria. Where no distinction was made by species, but over 98% of malaria infections were identified by the reference standard as non‐falciparum or P. vivax, the study was eligible for inclusion.
Reference standards
Studies were required to diagnose non‐falciparum or P. vivax malaria using at least one of the following two reference standards:
Conventional microscopy of thick blood smears, thin blood smears or both. Presence of asexual parasites of any density was regarded as a positive smear;
PCR.
The reference standard was required to be performed using blood samples drawn at the same time as those for the index tests. Where studies used more than one reference standard, we presented data relating to comparisons with each reference standard.
Search methods for identification of studies
We used a single search strategy for both Cochrane Reviews in this series (see Abba 2011).
Electronic searches
To identify all relevant studies, we used the search terms and strategy outlined in Appendix 1 to search the following databases: Cochrane Infectious Diseases Group Specialized Register; MEDLINE; EMBASE; MEDION; Science Citation Index; Web of Knowledge; African Index Medicus; LILACS; and IndMED. We based the search on the following MeSH, full text and keyword terms: Malaria, Plasmodium, reagent kits, diagnosis, diagnostics, RDT, dipstick, MRDD, OptiMal, Binax Now, Parasight, Immumochromatography, antigen detection, antigen test, Combo card. We did not limit the search by language or publication status (although we later excluded non‐English language studies due to resource constraints). We restricted the searches to human studies. We updated the search on 31 December 2013.
Searching other resources
We searched the reference lists of included studies for relevant publications. Due to resource constraints, we did not search any other resources.
Data collection and analysis
Selection of studies
We initially used a single selection procedure to identify studies for inclusion in either of the two Cochrane Reviews in this series. The inclusion criteria differed between the reviews only in the target condition and parasite species. Therefore, of the study characteristics examined, we assessed parasite species last , for example a study listed as excluded due to not presenting sufficient data may also have not been a study of non‐falciparum or P. vivax malaria. One author (KA) initially assessed the titles identified by the search, excluding those obviously irrelevant to the diagnosis of malaria using RDTs. We retained titles where we had any doubt regarding inclusion.
Based on abstract examination, we excluded irrelevant letters, review articles and articles and then excluded other irrelevant notes. Using a pro forma, two review authors (KA and NM) independently assessed the eligibility of the remaining potentially relevant articles based on full text publications. We have listed the excluded studies in the Characteristics of excluded studies table. We resolved any discrepancies by discussion. Where we could not reach agreement, we consulted a third author (PG or PO). Where it remained unclear whether a study was eligible for inclusion because of a lack of detail or poor reporting, we excluded it. Similiarly, we excluded non‐English language reports for logistical reasons.
We named studies according to the surname of the first study author and the year of publication. The study naming used in this review uniquely identifies multiple study cohorts within each study report (for example as 'Bell 2001a' and 'Bell 2001b'), each of which use different reference standards or present data separately for more than one population with different characteristics. More than one RDT may be evaluated in each study cohort, thus the number of test evaluations exceeds the number of study cohorts, which exceeds the number of study reports.
Data extraction and management
Two review authors (KA and NM) independently extracted data and resolved any discrepancies by discussion. In cases of studies where only a subgroup of participants met the review inclusion criteria, we extracted and presented data only for that particular subgroup. Where two versions of one reference standard were used, for example local clinic and expert standard microscopy, or field versus laboratory RDT testing, we only included the one most likely to yield the highest quality results.
For each study, we systematically extracted data on the characteristics of the study, as shown in Appendix 2. We also extracted data relating to the sensitivity of the RDT at different levels of parasitaemia (asexual parasites per µL of blood) as presented by the study authors. For each comparison of index test with reference test, we extracted data on the number of true positives, true negatives, false positives and false negatives in the form of a two by two table. RDT results are dichotomous; microscopy results were deemed positive at any level of asexual parasitaemia; and PCR results used the cut‐off points presented by the study authors. Gametocyte‐only parasitaemia was considered negative; where a study was unclear on how they had classed gametocyte‐only parasitaemia, they were assumed to have used the same classification as ourselves and we included the data in the study.
We extracted data for each study (Smidt 2008), using current manufacturers' instructions in interpreting the RDT results. P. falciparum malaria only was considered as negative for parasitaemia. The target condition was defined slightly differently depending on the type of the test, as follows:
Types 2, 3 and 4 ‐ Non‐falciparum malaria in the absence of falciparum malaria
RDT Types 2, 3 and 4 are designed to detect non‐falciparum species (mainly P. vivax in most situations) when they occur without concurrent P. falciparum infection. They have two test lines, one specific for P. falciparum and one pan‐malarial line to detect all malaria species. Non‐falciparum malaria is identified by a positive pan‐malarial line and negative P. falciparum line; mixed infections will produce positive results for both the P. falciparum and pan‐malaria lines and are indistinguishable from P. falciparum alone.
Mixed infections detected by microscopy were considered true negative if RDT indicated P. falciparum; true positive if RDT indicated non‐falciparum in the absence of P. falciparum; and false negative if RDT indicated no malaria. This method corresponded to the method most often described by the authors of the included studies, first described by Tjitra 1999.
Tests using Pf HRP2 and Pv pLDH ‐ P. vivax (whether alone or part of mixed infection)
These types of tests are designed to identify P. vivax parasitaemia specifically, as they have a test line specific to P. vivax. Some also include other test lines, specific to other types of malaria parasite. Test results were considered positive for P. vivax whether or not they also indicated the presence of P. falciparum.
Where study authors interpreted test results or presented data differently, we used all the information presented in the paper to extract data consistent with our own methods; if we were unable to do this, we did not include the data in the analyses.
Assessment of methodological quality
Three researchers (KA, NM and SJ) assessed the quality of each individual study using the checklist adapted from the QUADAS tool (Whiting 2003). We answered each question on the checklist with a yes or no response, or noted unclear if study authors reported insufficient information to enable a judgement, and we documented the reasons for the judgement made. We have summarized the criteria we used in Appendix 3.
Statistical analysis and data synthesis
The comparisons made in this review can be considered in a hierarchy. We classified the data on each test type in the primary studies according to commercial brands. In order to provide a coherent description of the studies contributing to each analysis, we structured the results first by grouping studies according to their commercial brand, then grouping brands to form test types. The analytical strategy thus compared the test accuracy of commercial brands within each test type before making comparisons between test types. Comparative analyses first included all studies with relevant data, and were then restricted to studies that made direct comparisons between tests with the same participants, where such studies existed.
For each test type, we plotted estimates of the observed sensitivities and specificities in forest plots and in receiver‐operating characteristic (ROC) space. These plots illustrate variation in accuracy between studies. Where adequate data were available, we performed meta‐analyses using the bivariate model (Reitsma 2005) to produce summary sensitivities and specificities. Using a random‐effects approach, the model jointly synthesises sensitivity and specificity by allowing for correlation between them across studies. We made comparisons between tests by adding a covariate for brand or test type to the bivariate model to investigate association with sensitivity or specificity, or both. Also, we investigated the effect of test type on the variances of the random effects of logit sensitivity and logit specificity and we included separate variance terms where required. We assessed the significance of the difference in test performance by a likelihood ratio test comparing models with and without covariate terms for sensitivity and specificity. Where inadequate studies were available to estimate all parameters, we simplified the bivariate model to two univariate random‐effects logistic regression models by assuming no correlation between sensitivity and specificity. We fitted the models using the xtmelogit command in StataCorp 2011.
Where more than one commercial brand of the same test type was evaluated on the same patients against the same reference standard, we selected one brand at random from the analysis by test type in order to avoid bias due to inclusion of the same participants more than once in the analysis. We included both brands in any analyses comparing commercial brands.
Investigations of heterogeneity
We inspected forest plots and summary ROC plots to visually assess heterogeneity between study specific estimates of sensitivity and specificity. We planned to investigate the effect of age group, continent where the study took place, and adequacy of the reference standard on summary estimates of sensitivity and specificity by adding each factor as a covariate to the bivariate model.
We did not attempt to assess reporting bias because little is known about how this should be done for diagnostic test accuracy (DTA) reviews.
Results
Results of the search
In the initial search we identified 4837 titles, of which we excluded 4325 based on their title or abstract alone. We were unable to obtain one article in full text form. We retrieved full text articles for 511 titles; of which we excluded 474 articles; 316 because they were initially assessed as ineligible; 22 because the reports did not present enough information for us to assess their eligibility; 21 because they were available only in non‐English languages; 21 because we were unable to extract absolute numbers of true positives, false positives, false negatives and true negatives; and 94 because they did not present data on non‐falciparum or P. vivax malaria, although they were eligible for other reviews in this series. See Figure 1 for a flow diagram of search and eligibility results.
1.
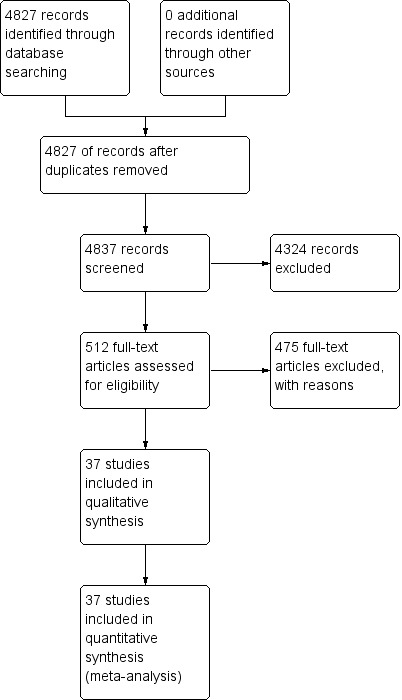
Study flow diagram.
We therefore included a total of 37 study publications. One of the included publications described two related studies, and another publication reported data separately for 10 different sites, making a total of 47 study cohorts. Seven of the 47 cohorts evaluated more than one test; one compared four tests, three compared three tests and three compared two tests. There were a total of 67 test evaluations reporting on a total of 32,466 tests in 22,862 participants. We have given a summary of the number of studies by test type and reference standard (microscopy or PCR) in Table 4.
3. Number of studies by RDT type and reference standard.
| Type of RDT | Number of study cohorts (test evaluations) by reference standard | |
| Microscopy | PCR | |
| Non‐falciparum species in the absence of P. falciparum | ||
| Type 2 | 11 (11) | 0 (0) |
| Type 3 | 23 (25) | 5 (5) |
| Type 4 | 10 (11) | 1 (1) |
| Other type | 1 (1) | 0 (0) |
| P. vivax | ||
| Pf HRP2 and Pv pLDH | 8 (9) | 2 (3) |
| Type 6 | 0 (0) | 1 (1) |
Methodological quality of included studies
We summarised the overall methodological quality of the included studies in Figure 2. Twenty‐seven study cohorts (57%) clearly included a representative spectrum of patients attending ambulatory healthcare setting with symptoms of malaria; the remaining 20 were unclear, most often because they had not adequately described their sampling methods. Twenty study cohorts (43%) reported an adequate reference standard, 19 (40%) did not provide enough information on the reference standard, and eight (17%) had an inadequate reference standard, in all cases because a second microscopist did not verify the results. Thirty‐five study cohorts (75%) reported blinding of the reference standard results, 11 (23%) did not describe whether the reference standard was blinded and one (2%) did not blind the reference standard. Thirty‐seven (79%) study cohorts blinded the RDT results to the results of the reference tests, nine (19%) were unclear and one (2%) did not blind the RDTs. All 47 cohorts reported avoidance of partial verification, differential verification and incorporation.
2.
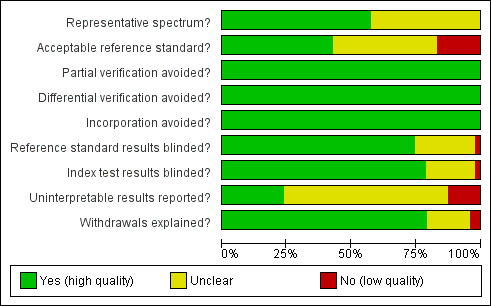
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Eleven study cohorts (23%) reported on uninterpretable test results; of these, three excluded uninterpretable results from the analysis, four reported that there were no uninterpretable results, three repeated any uninterpretable tests and one presented the results for uninterpretable tests. The proportion of uninterpretable tests was low in every study that reported this information (maximum 6%). Thirty study cohorts (64%) did not report on uninterpretable results, but appeared to have no uninterpretable results, because they had an exact correlation between the number of participants enrolled and the number presented in the analysis. Six study cohorts (13%) did not report on uninterpretable results and also either did not clearly state the number of participants initially enrolled or showed a discrepancy between the number of participants enrolled and the number presented in the analysis.
Thirty‐seven study cohorts (79%) reported either no withdrawals from the study or recorded the reasons for any withdrawals; eight (17%) were unclear as to whether there were any withdrawals; one (2%) had one participant missing from the analysis, with no explanation, and another (2%) reported that samples with mixed infection or where microscopists disagreed were excluded, while the number of samples excluded, and the original number of participants enrolled, was not presented.
Findings
Target condition: non‐falciparum malaria only
In this section we present the results for RDTs which identify 'non‐falciparum malaria' by the presence of a positive pan‐malaria antibody line in the absence of a positive P. falciparum specific antibody line.
Verified by microscopy
Type 2 tests
There were 11 evaluations of Type 2 RDTs verified with microscopy (Figure 3); eight were undertaken in Asia, two in Africa and one in South America. The median sample size was 372 (range 113 to 2383), the median prevalence of non‐falciparum only malaria was 14% (range 7% to 32%) and the median percentage of malaria that was non‐falciparum was 46% (range 13% to 80%). None of the evaluations were undertaken only in children under the age of five years. Five different test brands were evaluated: ICT Malaria Pf/Pv (seven); ICT Malaria combo cassette (one), Malascan (one), NOW Malaria ICT (one) and VIKIA Malaria Ag Pf/Pan (one). Sensitivities of the tests ranged from 67% to 90%; specificities ranged from 89% to 100%. In meta‐analysis (11 evaluations, 6879 participants) the pooled sensitivity was 78% (95% confidence interval (CI) 73% to 82%) and the specificity was 99% (95% CI 97% to 99%) (Figure 4). Of the false negative RDT results (where microscopy identified non‐falciparum malaria only, but RDT gave a different result) 65% (95% CI 43% to 81%) of RDT results indicated 'no malaria'; the remaining false negative RDT results indicated P. falciparum or mixed infection (Table 5).
3.
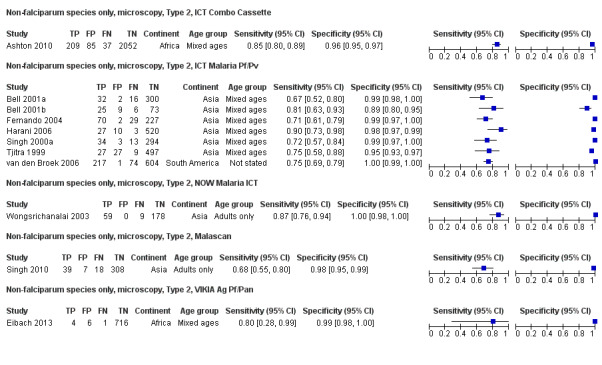
Forest plot of commercial brands of Type 2 tests for detection of non‐falciparum species (verified with microscopy). We ordered studies by continent, age group and study identifier.
4.
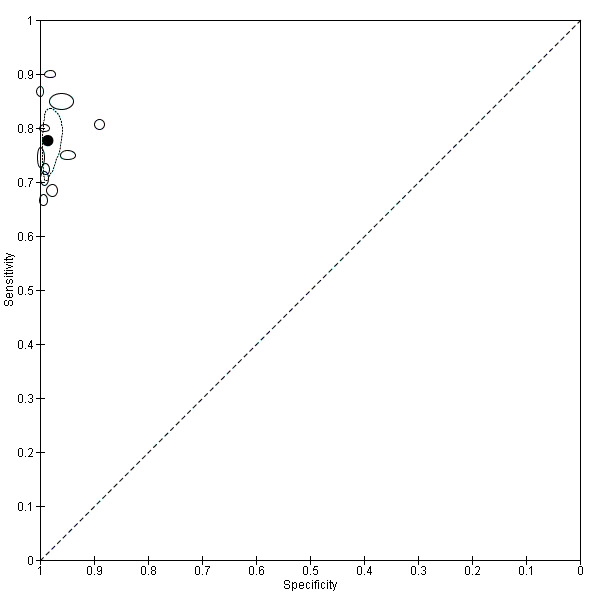
Summary ROC plot of Type 2 tests for detection of non‐falciparum species (verified with microscopy). The black solid circle corresponds to the summary estimate of sensitivity and specificity, and is shown with a 95% confidence region.
4. False negatives for non‐falciparum and P. vivax by RDT type.
| Study | Test | Number of false negatives | % false negatives indicating 'no malaria' | % false negatives indicating 'P. falciparum' |
| Type 2 tests | ||||
| Ashton 2010 | ICT Combo | 37 | 22 | 78 |
| Bell 2001a | ICT Malaria trial 1 | 16 | 13 | 88 |
| Bell 2001b | ICT Malaria trial 2 | 6 | 67 | 33 |
| Fernando 2004 | ICT Malaria Pf/Pv | 29 | 100 | 0 |
| Harani 2006 | ICT Malaria Pf/Pv | 3 | 67 | 33 |
| Singh 2000a | ICT Malaria Pf/Pv | 13 | 62 | 38 |
| Singh 2010 | Malascan | 18 | 67 | 33 |
| Tjitra 1999 | ICT Malaria Pf/Pv | 8 | 75 | 25 |
| van den Broek 2006 | NOW malaria ICT | 72 | 67 | 33 |
| Wongsrichanalai 2003 | ICT Malaria Pf/Pv | 9 | 67 | 33 |
| van den Broek 2006 | OptiMAL‐IT | 34 | 74 | 26 |
| Median (range) | 67 (13 to 100) | 33 (0 to 88) | ||
| Pooled estimate (95% CI)* | 65 (43 to 81) | 35 (19 to 57) | ||
| Type 3 tests | ||||
| Ashton 2010 | Carestart | 37 | 22 | 78 |
| Ashton 2010 | Parascreen | 43 | 14 | 86 |
| Bendezu 2010 | Parascreen | 19 | 84 | 16 |
| Bharti 2008 | First response | 7 | 100 | 0 |
| Dev 2004 | Diamed OptiMAL | 3 | 100 | 0 |
| Eibach 2013 | CareStart | 3 | 100 | 0 |
| Elahi 2013 | Parascreen | 5 | 60 | 40 |
| Kosack 2013 | SD Bioline | 133 | 89 | 11 |
| Moges 2012 | Carestart | 38 | 89 | 11 |
| Ratsimbasoa 2007 | SD Malaria Antigen Bioline | 4 | 100 | 0 |
| Singh 2010 | Parascreen | 13 | 54 | 46 |
| Singh 2010 | First response | 9 | 33 | 67 |
| Singh 2010 | ParaHIT Total | 48 | 92 | 8 |
| Trouvay 2013 | SD Malaria Ag Pf/Pan | 18 | 78 | 22 |
| Yan 2013 | Pf/Pan Device | 24 | 25 | 75 |
| Median (range) | 84 (14 to 100) | 16 (0 to 86) | ||
| Pooled estimate (95% CI) | 74 (52 to 88) | 26 (12 to 48) | ||
| Type 4 tests | ||||
| Andrade 2010 | OptiMAL‐IT | 0 | 0 | 0 |
| Chayani 2004 | OptiMAL | 3 | 100 | 0 |
| Dev 2004 | SD Malaria | 2 | 100 | 0 |
| Kolaczinski 2004 | OptiMAL | 23 | 100 | 0 |
| Metzger 2011 | OptiMAL‐IT | 30 | 100 | 0 |
| Pattanasin 2003 | OptiMAL‐IT | 26 | 65 | 35 |
| Ratsimbasoa 2007 | OptiMAL‐IT | 2 | 100 | 0 |
| Ratsimbasoa 2007 | Carestart Malaria | 3 | 33 | 67 |
| Singh 2003 | OptiMAL (field) | 0 | 0 | 0 |
| Soto Tarazona 2004 | OptiMAL | 3 | 100 | 0 |
| Valecha 2003 | OptiMAL | 13 | 77 | 23 |
| Median (range) | 100 (0 to 100) | 0 (0 to 67) | ||
| Pooled estimate (95% CI) | 87 (79 to 92) | 13 (8 to 21) | ||
*The pooled estimates of the percentage of false negatives indicating 'no malaria' and the percentage of false negatives indicating 'P. falciparum' were computed by using a random effects logistic regression model for Type 2 and Type 3. A fixed effects logistic regression model was used for Type 4.
This table shows participants with non‐falciparum malaria monoinfection identified by microscopy who were negative by non‐falciparum monoinfection by RDT, by whether the RDT incorrectly identified the participant as not having malaria, or as having P. falciparum malaria.
Type 3 tests
There were 25 evaluations of Type 3 RDTs verified with microscopy (Figure 5); eight were undertaken in Asia, 15 in Africa and two in South America. The median sample size was 200 (range 30 to 2585), the median prevalence of non‐falciparum only malaria was 10% (range 7% to 36%) and the median percentage of malaria that was non‐falciparum was 36% (range 17% to 85%). None of the evaluations were undertaken only in children under the age of five years. Five different test brands were evaluated: Parascreen (14), SD Malaria Antigen Bioline (four), Carestart Pf/Pan (four), First Response Malaria Combo (two) and One Step Malaria Pf/Pan (one). Sensitivities of the tests ranged from 25% to 100%; specificities ranged from 94% to 100%. Two studies evaluated two brands and so one brand was selected at random for inclusion in the meta‐analysis. Therefore, based on 23 evaluations (11,234 participants), the pooled sensitivity was 78% (95% CI 69% to 84%) and the specificity was 99% (95% CI 98% to 99%) (Figure 6). Of the false negative RDT results (where microscopy identified non‐falciparum malaria only, but RDT gave a different result), 74% (52% to 88%) of RDT results indicated 'no malaria'; the remaining false negative RDT results indicated P. falciparum or mixed infection (Table 5).
5.
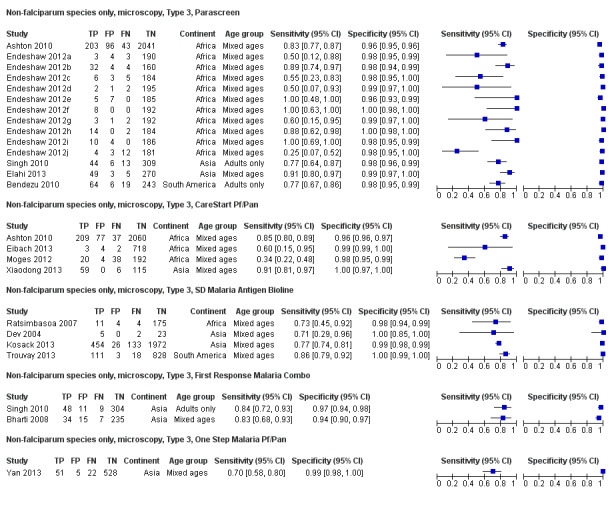
Forest plot of commercial brands of Type 3 tests for detection of non‐falciparum species (verified with microscopy). We ordered studies by continent, age group and study identifier.
6.
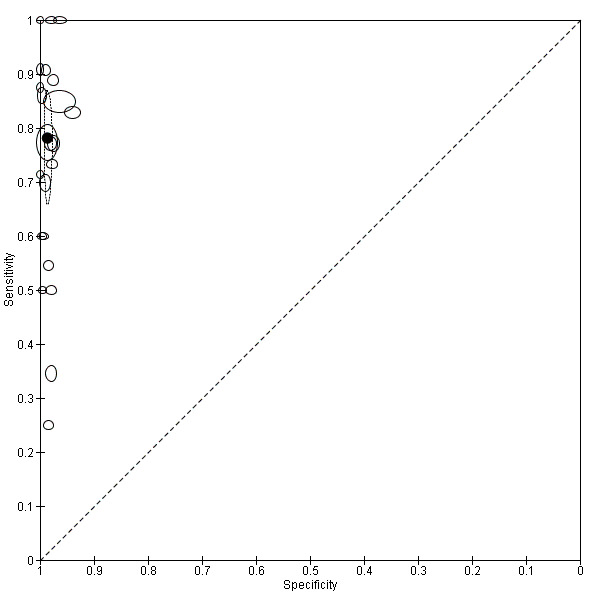
Summary ROC plot of Type 3 tests for detection of non‐falciparum species (verified with microscopy). The black solid circle corresponds to the summary estimate of sensitivity and specificity, and is shown with a 95% confidence region.
Type 4 tests
There were 11 evaluations of Type 4 RDTs compared microscopy (Figure 7); six were undertaken in Asia, two in Africa and three in South America. The median sample size was 289 (range 80 to 896), the median prevalence of non‐falciparum only malaria was 27% (range 8% to 33%) and the median percentage of malaria that was non‐falciparum was 51% (range 21% to 100%). None of the evaluations were undertaken only in children under the age of five years. Three different test brands were evaluated: OptiMAL (six), OptiMAL‐IT (four) and Carestart Malaria Pf/Pan (one). Sensitivities of the tests ranged from 63% to 100%; specificities ranged from 94% to 100%. One study evaluated two brands and so one brand was selected at random for inclusion in the meta‐analysis. Based on 10 evaluations (3831 participants), the pooled sensitivity was 89% (95% CI 79% to 95%) and the specificity was 98% (95% CI 97% to 99%) (Figure 8). Of the false negative RDT results (where microscopy identified non‐falciparum malaria only, but RDT gave a different result), 87% (79% to 92%) of RDT results indicated 'no malaria'; the remaining false negative RDT results indicated P. falciparum or mixed infection (Table 5).
7.
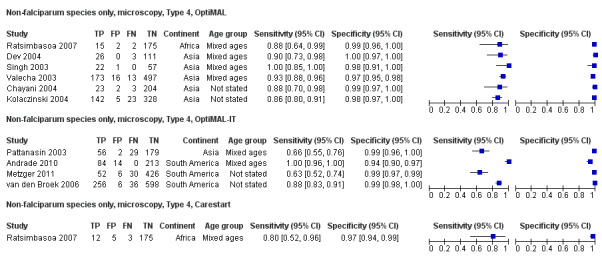
Forest plot of commercial brands of Type 4 tests for detection of non‐falciparum species (verified with microscopy). We ordered studies by continent, age group and study identifier.
8.
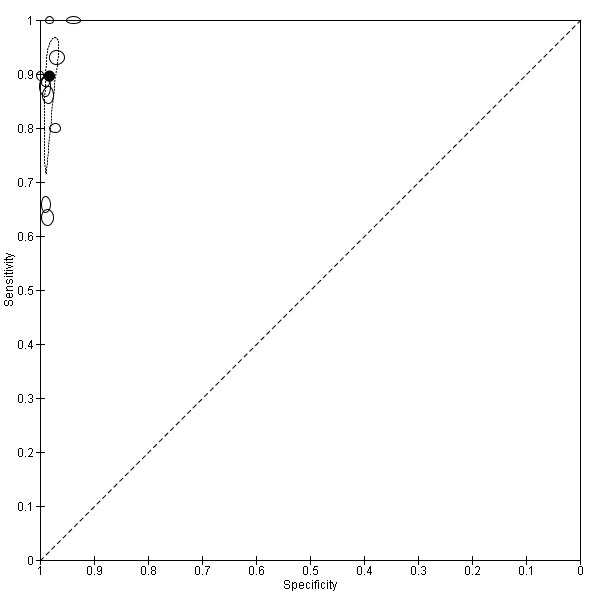
Summary ROC plot of Type 4 tests for detection of non‐falciparum species (verified with microscopy). The black circle corresponds to the summary estimate of sensitivity and specificity, and is shown with a 95% confidence region.
Other test types
There was one evaluation of Malariagen Malaria, a type of test that does not fit into the classification presented in Table 2. Malariagen Malaria uses antibodies to the HRP‐2 antigen of P. falciparum and unspecified monoclonal antibodies for detection of pan‐malarial antigens. The study (Selimuzzaman 2010) was undertaken on a sample of 262 adults in Asia and the study prevalence of non‐falciparum malaria was 5%. The sensitivity of this test verified against microscopy was 92% (95% CI 62% to 100%) and the specificity was 95% (95% CI 92% to 97%).
Comparisons between RDT types
We summarised the comparison of different RDT types in Figure 9, Figure 10, Table 6 and Table 7. There was a statistically significant (P = 0.008) difference in accuracy between test types (Table 6) with Type 4 tests being significantly more sensitive than Type 2 (P = 0.01) and Type 3 (P = 0.03) (Table 7) based on indirect comparisons using all available data. Specificities were similarly high across the three test types. Few studies directly compared tests and so meta‐analyses restricted to direct comparisons were not possible. The results from the only study (van den Broek 2006) that directly compared a Type 2 test and a Type 4 test were consistent with the meta‐analytic finding and also demonstrated a statistically significant difference (P < 0.001) in sensitivity (Appendix 4).
9.
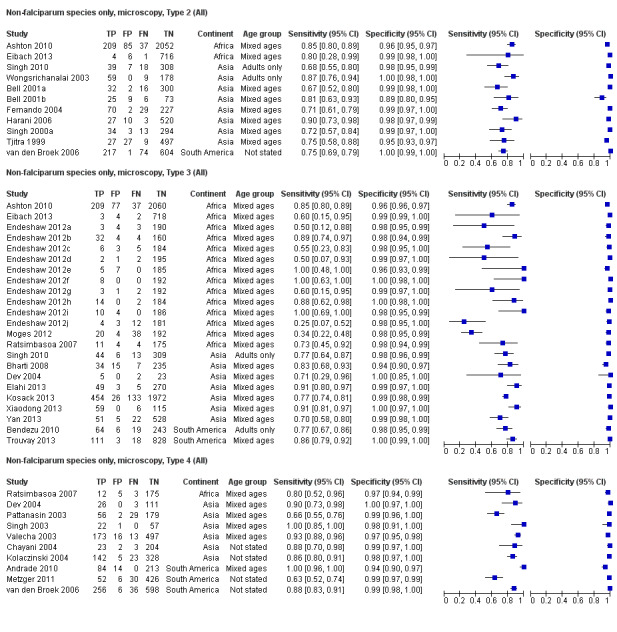
Forest plot of Type 2, Type 3 and Type 4 tests for detection of non‐falciparum species (verified with microscopy). We ordered studies by continent, age group and study identifier.
10.
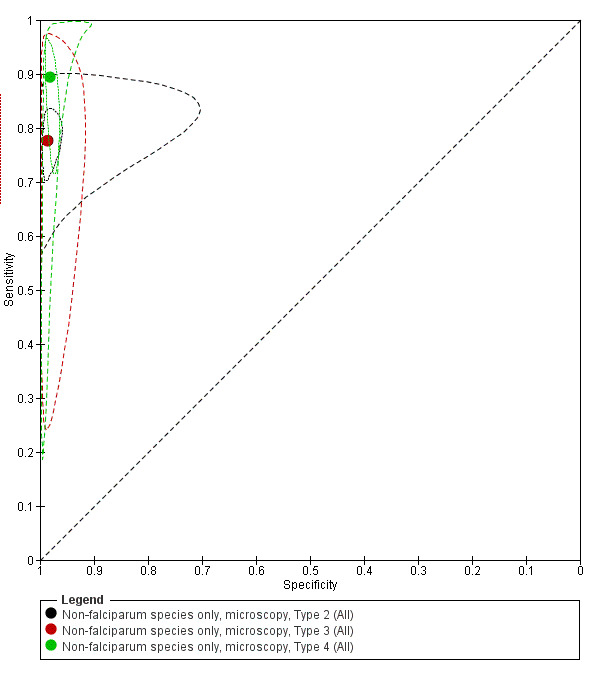
Summary ROC plot comparing Type 2, Type 3 and Type 4 tests for detection of non‐falciparum species (verified with microscopy). The solid circles correspond to the summary estimates of sensitivity and specificity for each test type, and are shown with 95% confidence regions (dotted lines) and 95% prediction regions (dashed lines). The summary points for Type 2 and Type 3 and their 95% confidence regions are identical but the 95% prediction regions differ. The 95% prediction regions illustrate the extent of between study heterogeneity.
5. Non‐falciparum infections by RDT types verified by microscopy.
| RDT Type | Study cohort | Participants | Malaria cases |
Pooled sensitivity (95% CI) (%) |
Pooled specificity (95% CI) (%) |
Test1 |
| Type 2 | 11 | 6879 | 958 | 78 (73 to 82) | 99 (97 to 99) | P = 0.008 |
| Type 3 | 23 | 11,234 | 1537 | 78 (69 to 85) | 99 (98 to 99) | |
| Type 4 | 10 | 3831 | 986 | 90 (79 to 95) | 98 (97 to 99) | |
| Other type | 1 | 262 | 12 | 92 (62 to 100) | 95 (92 to 98) |
1Likelihood ratio test for evidence of a difference in sensitivity or specificity, or both, between Types 2, 3, and 4.
*Only one test brand (randomly selected) from each cohort is included in the analysis of each type.
6. Comparisons of RDT types for non‐falciparum infections verified by microscopy.
|
Ratio of sensitivity (95% CI), P value for comparison Ratio of specificity (95% CI), P value for comparison |
Type 2 | Type 3 | ||
| Studies (participants) | 11 (6879) | 23 (11,234) | ||
| Studies (participants) |
Sensitivity (95% CI) Specificity (95% CI) |
78 (73 to 82) 99 (97 to 99) |
78 (69 to 84) 99 (98 to 99) |
|
| Type 2 | 11 (6879) | 78 (73 to 82) 99 (97 to 99) |
‐ | ‐ |
| Type 3 | 23 (11,234) | 78 (69 to 84) 99 (98 to 99) |
1.00 (0.89 to 1.12), P = 1.00 1.00 (0.99 to 1.01), P = 0.87 |
‐ |
| Type 4 | 10 (3831) | 90 (79 to 95) 98 (97 to 99) |
0.87 (0.78 to 0.96), P = 0.01 1.00 (0.99 to 1.02), P = 0.52 |
0.87 (0.76 to 0.99), P = 0.03 1.01 (1.00 to 1.02), P = 0.29 |
We computed the ratio of sensitivities and specificities by division of the sensitivity and specificity for the column by the sensitivity and specificity for the row. If the ratio of sensitivities is greater than one, the sensitivity of the test for the column is higher than that for the row; if less than one, the sensitivity of the test in the row is higher than in the column. The same applies to the ratio of specificities.
Comparison of brands
We compared the test performance of three Type 3 test brands—Parascreen (14 studies, 547 participants), Carestart Pf/Pan (four studies, 3544 participants) and SD Malaria Antigen Bioline (four studies, 3769 participants). We excluded the other two brands—First Response Malaria Combo (two studies, 663 participants) and One Step Malaria Pf/Pan (one study, 606 participants)—from the analysis due to limited data. There was no evidence (P = 0.88) to suggest that the sensitivity or specificity, or both, of type 3 tests was associated with brand. The summary sensitivity (95% CI) was 79% (67% to 88%) for Parascreen, 74% (45% to 91%) for Carestart Pf/Pan, and 80% (73% to 85%) for SD Malaria Antigen Bioline. The summary specificity (95% CI) was 98% (98% to 99%) for Parascreen, 99% (96% to 100%) for Carestart Pf/Pan, and 99% (98% to 100%) for SD Malaria Antigen Bioline.
For Type 4 tests, we compared the diagnostic accuracy of the OptiMAL (six studies, 1843 participants) and OptiMAL‐IT (four studies, 1987 participants) brands. We excluded a third brand, Carestart Pf/Pan (one study, 195 participants), because of limited data. There was no evidence (P = 0.79) to suggest a difference in the sensitivity or specificity, or both, of the two brands. The summary sensitivity of OptiMAL was 90% (85% to 93%) and that of OptiMAL‐IT was 91% (49% to 99%). The summary specificities were 98% (97% to 99%) and 98% (96% to 99%) for OptiMAL and OptiMAL‐IT respectively.
Investigations of heterogeneity
Due to the limited number of studies available for each test type, we were only able to investigate the effect of continent and adequacy of the reference standard on the sensitivity and specificity of Type 3 tests for detecting non‐falciparum species with microscopy as reference standard. There were three continents—Africa (14 studies, 5551 participants), Asia (eight studies, 4997 participants) and South America (two studies, 704 participants)—but we excluded South America from the analysis due to the limited data available. There was no evidence (P = 0.55) to suggest a difference in sensitivity or specificity, or both, between studies conducted in Africa and those in Asia. The summary sensitivity (95% CI) was 74% (57% to 86%) for Africa and 80% (73% to 85%) for Asia. The summary specificity (95% CI) was 99% (98% to 99%) for Africa and 99% (97% to 99%) for Asia. For adequacy of the reference standard, six studies were scored 'Yes', 12 studies were scored 'No' and five studies were scored 'Unclear'; there was no evidence (P = 0.54) to suggest a difference in sensitivity or specificity, or both. The summary sensitivity and specificity were 77% (67% to 85%) and 99% (98% to 99%) for studies with an acceptable reference standard; 78% (65% to 88%) and 99% (98% to 99%) for studies without an acceptable reference standard; and 86% (78% to 91%) and 98% (97% to 99%) for studies where the assessment was judged to be unclear.
Verified by PCR
Type 2 tests
No study verified a Type 2 test with PCR.
Type 3 tests
There were five evaluations of a Type 3 test verified with PCR (Figure 11); three were undertaken in Asia and two were undertaken in South America. The median sample size was 327 (range 178 to 606), and the median prevalence of non‐falciparum malaria was 15% (range 7% to 33%). None of the evaluations were undertaken only in children under the age of five years. Four different test brands were evaluated; Parascreen (two studies); SD Malaria Antigen Bioline (one study), CareStart Pf/Pan (one study) and One Step Malaria Pf/Pan (one study). Sensitivities of the tests ranged from 64% to 91% and specificities ranged from 97% to 100%. In meta‐analysis, the pooled sensitivity was 81% (95% CI 72% to 88%) and the pooled specificity was 99% (95% CI 97% to 99%).
11.
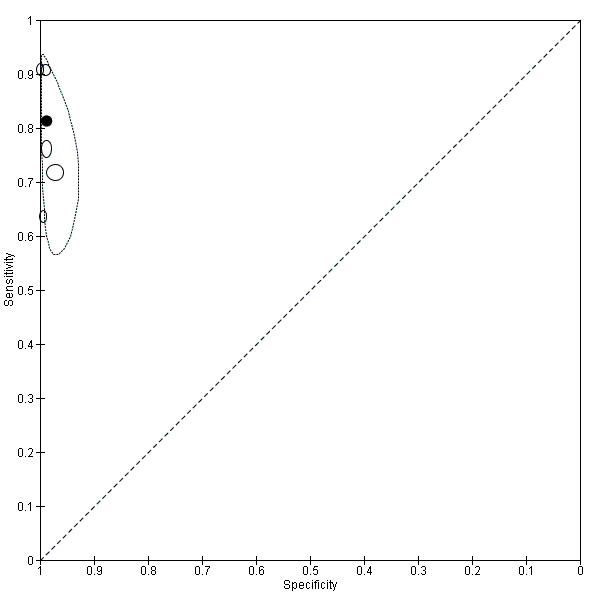
Summary ROC plot of Type 3 tests for detection of non‐falciparum species (verified with PCR). The solid circles correspond to the summary estimate of sensitivity and specificity, and is shown with a 95% confidence region.
Type 4 tests
One study (Rakotonirina 2008) verified a Type 4 test, OptiMAL, against PCR and gave results consistent with the summary results of the six studies that used microscopy as the reference standard (Appendix 5).
Comparison of results verified by microscopy or PCR
Four studies used both microscopy and PCR as reference standards to verify parasitaemia. Elahi 2013 estimated a sensitivity of 91% and specificity of 99% for both PCR and microscopy; Bendezu 2010 estimated a sensitivity of 76% and specificity of 98% with PCR, and sensitivity of 77% and specificity of 99% with microscopy. The accuracy of CareStart Pf/Pan reported by Xiaodong 2013 was similar for both reference standards with sensitivity of 91% and specificity of 100%. Yan 2013 evaluated One Step Malaria Pf/Pan with an estimated sensitivity of 72% when verified against PCR and 70% against microscopy, and a specificity of 97% with PCR and 99% with microscopy. Ratsimbasoa 2008 verified the Malaria Antigen Bioline test against PCR and gave results within the 95% CI of the pooled results of the two studies that used microscopy as the reference standard (Appendix 5).
Target condition: P. vivax
In this section we present the results for RDTs which identify P. vivax by the presence of a positive P. vivax specific antibody line. The majority of the tests had two test lines, an HRP‐2 line to detect P. falciparum and an pLDH line to detect P. vivax. One study, which verified results using PCR, evaluated a Type 6 tests, with additional test with an additional pan line to detect all species of malaria. In each case, only the Pv PLDH line is considered in the analysis.
Verified by microscopy
Eight studies evaluated the performance of Pf HRP‐2 and Pv pLDH antibody tests verified with microscopy—four were undertaken in Africa and four in Asia (Figure 12). The median sample size was 361 (range 240 to 1092), with a median prevalence of P. vivax malaria of 19% (range 2% to 45%). Evaluations were conducted in mixed age groups or adults only. Five different test brands were assessed: CareStart Pf/Pv (three), Falcivax (two), Biotech Malaria Pf/Pv (one), OnSite Pf/Pv (one), and Pf/Pv Malaria Device (one). The sensitivities of the tests ranged from 66% to 100%, and specificities ranged from 98% to 100%. In meta‐analysis (eight evaluations, 3682 participants) the summary sensitivity and specificity (95% CI) were 95% (86% to 99%) and 99% (99% to 100%) respectively (Figure 13).
12.
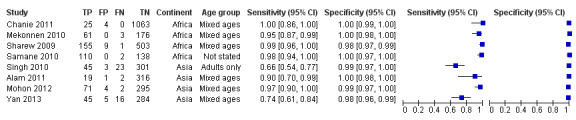
Forest plot of Pf HRP‐2 and Pv pLDH for detection of P. vivax (verified with microscopy). Studies are ordered by continent, age group and study identifier.
13.
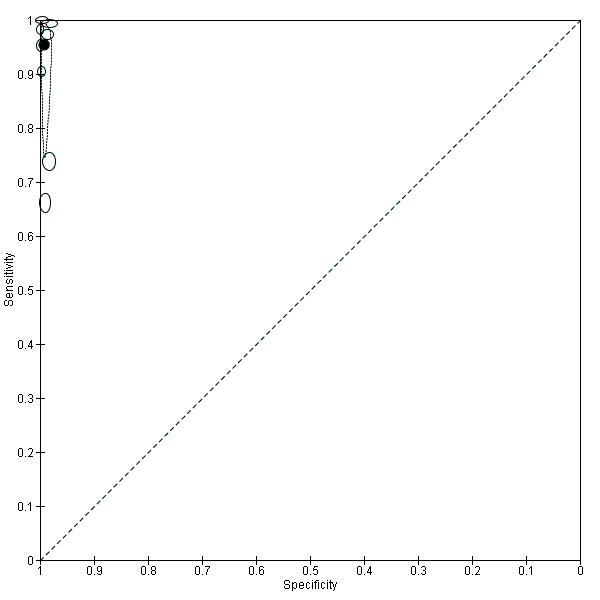
Summary ROC plot Pf HRP‐2 and Pv pLDH for detection of P. vivax (verified with microscopy). The black circle corresponds to the summary estimate of sensitivity and specificity, and is shown with a 95% confidence region.
Verified by PCR
Two studies evaluated the performance of three different brands of Pf HRP‐2 and Pv pLDH antibody tests against PCR. One study was undertaken in Bangladesh and the other in China. Sensitivities ranged from 59% (47% to 70%) to 77% (56% to 91%) and specificities ranged from 97% (95% to 99%) to 100% (99% to 100%). One study evaluated a Type 6 RDT and reported a sensitivity of 90% (70% to 99%) and specificity of 100% (99% to 100%).
Additional analyses
Sensitivities of tests at different levels of P. vivax parasitaemia
Type 2 tests
Four studies presented additional data relating to the sensitivity of Type 2 RDTs against microscopy at different levels of parasitaemia (Fernando 2004; Tjitra 1999; van den Broek 2006; Wongsrichanalai 2003). The findings varied; all found very low sensitivities below 100 parasites per µL, rising with level of parasitaemia, but the level at which a high sensitivity (over 90%) was achieved varied between 500 parasites per µL and 5,000 parasites per µL.
Type 3 tests
Four studies presented additional data relating to the sensitivity of a Type 3 RDT against microscopy at different levels of parasitaemia. In Ratsimbasoa 2008 sensitivity was 93% at levels of 501 to 5000 asexual parasites per µL; and 100% at levels above 5000 asexual parasites per µL. In Mohon 2012, sensitivity ranged from 80% at 1 to 100 asexual parasites per µL, to 90% at 101 to 500 asexual parasites per µL to 100% at 501 or more asexual parasites per µL. In Yan 2013, sensitivity was 73.3% at under 500 asexual parasites per µL to 100%, and 69% at over 500 asexual parasites per µL to 100%. In Kosack 2013 sensitivity was 14% at one to nine asexual parasites per 100 fields, 70% at one to 10 asexual parasites in 10 fields, 96% at one to 10 asexual parasites per field, and 98% at more than 10 asexual parasites per field.
Type 4 tests
Three studies presented additional data relating to the sensitivity of Type 4 RDTs against microscopy at different levels of parasitaemia, although two had only small numbers. One study presented useful data (Valecha 2003), reporting a sensitivity of 30% at under 500 asexual parasites per µL; 48% at 500 to 999 asexual parasites per µL; 91% at 1000 to 5000 asexual parasites per µL; and 100% at over 5000 asexual parasites per µL.
Discussion
Summary of main results
Test Types 2, 3 and 4, and other tests that identified non‐falciparum malaria through deduction of a positive result for pan‐malarial antigens along with a negative result for P. falciparum specific antigens, had sensitivities that ranged in pooled analyses from 78% (Type 2) to 89% (Type 4). Further analysis of the false negative results showed that the majority of non‐falciparum only cases that were missed by the RDTs were indicated as 'no malaria' although some were indicated as P. falciparum or mixed infection. Type 4 tests were significantly more sensitive than Type 2 tests. Specificities were consistently high, ranging from 98% (Type 4) to 99% (Type 2 and Type 3). There were no apparent differences between microscopy and PCR as the reference standard.
In studies that verified RDTs with microscopy, tests that used a P. vivax specific antibody line to identify P. vivax had a pooled sensitivity of 95% (95% CI 86% to 99%) and a pooled specificity of 99% (95% CI 99% to 100%). In contrast, the two studies that verified these types of RDTs with PCR demonstrated much lower sensitivity of 59% (47% to 70%) and 77% (56% to 91%).
In Table 1, assuming prevalences of 5%, 15% and 30%, the number of missed non‐falciparum or P. vivax malaria cases and the number of false positives in a hypothetical cohort of 1000 patients are presented by test type. In the case of tests for non‐falciparum only, the performance may in reality be affected by the prevalence of P. falciparum parasitaemia; this effect is not possible to estimate with any accuracy, however it is likely to be small, and has therefore been ignored.
Strengths and weaknesses of the review
Completeness of evidence
It is probable that some studies eligible for inclusion in the review were missed by our search strategy. DTA studies are known to be poorly indexed, and hence liable to be missed, even when searches are designed to be very sensitive (Whiting 2009). However, our search was comprehensive.
Accuracy of the reference standards used
Microscopy is an imperfect diagnostic test in itself, raising the possibility that in some cases of discordant results between microscopy and RDT, the RDT result may in fact have been correct, and the microscopy results incorrect. However, with the exception of P. vivax specific tests, where only two studies verified by PCR were available, results for studies which verified RDT results against PCR gave similar results to those which used microscopy as a reference standard.
In studies reporting on sensitivity by parasitaemia level, RDTs tended to reach high levels of sensitivity (above 90%) at levels of parasitaemia above 500 to 1000 asexual parasites for P. vivax, but were less reliable at lower levels. This finding corresponds closely with a similar analysis within a DTA review of RDTs for travellers with fever returning from malaria endemic to non‐endemic areas(Marx 2005).
Quality of reporting of the included studies
The quality of reporting of the included studies, as assessed by the number of 'unclear' evaluations of study quality was variable. Nineteen study cohorts (40%) did not provide enough information for us to adequately assess the adequacy of the reference standard, which we judged to be the most important quality indicator for this review.
Quality of the included studies
Where sufficient information was provided to assess the quality of included studies, the quality was variable. Twenty (43%) study cohorts reported an adequate reference standard, while 37 (79%) reported that readers of the reference standard were blinded to the results of the RDTs. For Type 3 tests, we were able to investigate the effect of adequacy of the reference standard on test performance. There was no evidence of a difference in test performance between studies with an adequate, inadequate or unclear reference standard.
Completeness and relevance of the review
This review focused specifically on the use of RDTs for diagnosing non‐falciparum malaria in people living in malaria endemic areas and attending ambulatory health care setting with symptoms of malaria; therefore evaluating the tests in the context in which they are intended to be most often used. Previously published reviews have evaluated the accuracy of RDTs under laboratory conditions (WHO 2012) and for use by travellers returning from malaria endemic to non‐endemic areas (Marx 2005). By classifying asexual parasitaemia as positive and gametocytes only as negative we focused on malarial illness requiring curative treatment, in line with current treatment recommendations (WHO 2010). In the future, as malaria comes closer to elimination, it may become important to cure gametocytaemia to prevent transmission, and diagnostic priorities may change.
Applicability of findings to the review question
Qualities of RDTs
RDT types 2, 3 and 4, which aim to identify 'non‐falciparum malaria only' as a proxy for P. vivax may miss between 11% (Type 4) and 22% (Type 2 and Type 3) of cases, with the majority of missed cases being incorrectly identified as free of malaria. In addition, the design of these tests does not allow the identification of non‐falciparum malaria as part of a mixed infection, or the differentiation of P. vivax from P. ovale and P. malariae. These tests therefore do not appear adequately sensitive for the identification of P. vivax, although they may play a role in areas where both P. vivax and P. falciparum occur and are initially treated with the same drugs. In contrast, RDT types using pLDH designed to detect P. vivax specifically, whether alone or part of a mixed infection appear to be both highly sensitive (missing 5% of cases) and highly specific for P. vivax. However, two studies included in this review, which verified the test with PCR, found much lower sensitivities. Consideration also needs to be made for variation in sensitivity by brand (WHO 2012).
Application to clinical decision‐making in practice
The evaluations presented in this review were conducted in patients with symptoms of clinical malaria and inferences about the results relate to this context, and not to mass surveys of well populations. The evaluations should also be read in conjunction with other published information regarding the in vitro performance, stability and costs of the tests, including the WHO FIND report (WHO 2012). Results between this review and the FIND analysis for some RDTs differ slightly. The FIND report tested individual products under laboratory conditions using standardised blood samples at low and high parasite densities (200 and 2000 parasites per µL) and reported the 'panel detection score'; which is defined as the percentage of times that two tests within a batch detected parasites at low density, and percentage of times that one test detected parasites at high density. This measure is slightly different to sensitivity, as it includes an aspect of consistency, whereas the studies in this review were conducted in field conditions with patients, and this is likely to account for variations between the datasets. The results in our review more closely mimic the conditions in which the tests would be used in practice; where parasite density is generally unknown, and may be affected by storage of the test, quality of a specific batch, local parasite densities, local parasite antigen patterns, quality of local microscopy and accuracy of reading the tests. Equally, these factors bring in more variation than tests from a laboratory using standardised samples.
RDTs can only influence clinical practice if the results are believed and acted upon. There may be reluctance on the part of both health providers and patients to believe negative RDT results, leading to unnecessary repeat testing and prescription of antimalarials for negative cases (Tavrow 2000). Various studies have shown that patients with fever and negative malaria test results, whether by microscopy or RDT, often still receive antimalarials (Hamer 2007), thus reducing their potential usefulness and cost‐effectiveness. However, some educational interventions have been shown to be effective in reducing prescriptions for antimalarials in negative cases (Ngasala 2008).
Authors' conclusions
Implications for practice.
RDT types 2, 3 and 4, which aim to identify 'non‐falciparum malaria only' as a proxy for P. vivax are limited by their design as they are unable to identify P. vivax specifically or to identify any species of non‐falciparum malaria as part of mixed infection. In addition, they have a relatively low sensitivity for 'non‐falciparum malaria only'. They may be useful in areas where the majority of malaria is caused by P. falciparum or mixed infection and where good quality microscopy is not available; our related review (Abba 2011) has shown that these test types are sensitive for the detection of P. falciparum. RDT types which are designed to detect P. vivax specifically, whether alone or part of a mixed infection appear to be both more directly applicable to practice in P. vivax endemic areas and in the majority of published studies have been shown to be more accurate. Data were insufficient to determine test accuracy by parasite density, which will affect the sensitivity and specificity thresholds that decide whether a test is useful in practice.
Implications for research.
More studies are needed to assess the accuracy of the newer RDT types designed to detect P. vivax specifically, particularly in areas with low prevalence.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2015 | Amended | Errors in the number of malaria cases were corrected in the Summary of Findings table. |
Acknowledgements
The academic editor for this review was Dr Karen Steingart.
We are grateful to our affiliated institutions and organizations, and to the Department of International Development (DFID), UK for research grants. We acknowledge the referees for their comments. Also, we thank Nicola Mayan and Sally Jackson for helping with data extraction.
The editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of developing countries.
Appendices
Appendix 1. Search strategy
| Search set | MEDLINE | EMBASE |
| 1 | Exp Malaria[MeSH] | Exp Malaria [Emtree] |
| 2 | Exp Plasmodium [MeSH] | Exp Plasmodium [Emtree] |
| 3 | Malaria ti, ab | Malaria ti, ab |
| 4 | 1 or 2 or 3 | 1 or 2 or 3 |
| 5 | Exp Reagent kits, diagnostics [MeSH] | Exp Diagnostic procedures [Emtree] |
| 6 | rapid diagnos* test* ti, ab | rapid diagnos$ test$ ti, ab |
| 7 | RDT ti, ab | RDT ti, ab |
| 8 | Dipstick* ti, ab | Dipstick$ ti, ab |
| 9 | Rapid diagnos* device* ti, ab | Rapid diagnos$ device$ ti, ab |
| 10 | MRDD ti, ab | MRDD ti, ab |
| 11 | OptiMal ti, ab | OptiMal ti, ab |
| 12 | Binax NOW ti, ab | Binax NOW ti, ab |
| 13 | ParaSight ti, ab | ParaSight ti, ab |
| 14 | Immunochromatograph* ti, ab | Immunochromatography [Emtree] |
| 15 | Antigen detection method* | Antigen detection method$ |
| 16 | Rapid malaria antigen test* | Rapid malaria antigen test$ |
| 17 | Combo card test* ti, ab | Combo card test$ ti, ab |
| 18 | Immunoassay [MeSH] | Immunoassay [Emtree] |
| 19 | Chromatography [MeSH] | Chromatography [Emtree] |
| 20 | Enzyme‐linked immunosorbent assay [MeSH] | Enzyme‐linked immunosorbent assay [Emtree] |
| 21 | Rapid test* ti, ab | Rapid test$ ti, ab |
| 22 | Card test* ti, ab | Card test$ ti, ab |
| 23 | Rapid AND (detection* or diagnos*) ti, ab | Rapid AND (detection$ or diagnos$) ti, ab |
| 24 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 |
| 25 | 4 and 19 | 4 and 19 |
| 26 | Limit 20 to Humans | Limit 20 to Human |
Appendix 2. Data extraction: characteristics of included studies
| Study ID | First author, year of publication. |
| Clinical features and settings | Presenting signs and symptoms, previous treatments for malaria, clinical setting. |
| Participants | Sample size, age, sex, comorbidities or pregnancy, country and locality, P. falciparum malaria endemicity, endemic malaria species, average parasite density in microscopy positive cases. |
| Study design | Were consecutive patients enrolled retrospectively or prospectively? |
| Whether the sampling method was consecutive or random, or whether the method was not described but consecutive sampling was most probable. | |
| If the study evaluated more than one RDT, how were tests allocated to individuals, or did each individual receive all the tests? | |
| Target condition | Malaria parasitaemia. |
| Reference standard | The reference standard test(s) used. |
| If microscopy was used, who performed it, and where? | |
| If microscopy was used, how many high power fields were looked at? | |
| If microscopy was used, how many observers or repeats were used? | |
| If microscopy was used, how were discrepancies between observers resolved? | |
| Index tests | The parasite species the test was designed to detect, the commercial name, and the type of test. Batch numbers if provided. Transport and storage conditions. Details of the test operators, including any special training provided. |
| Notes | Source of funding. |
Appendix 3. Data extraction and criteria for judgement: methodological quality
| Quality indicator | Notes |
| Was the spectrum of patients representative of the spectrum of patients who will receive the test in practice? |
|
| Is the reference standard likely to correctly identify the target condition? |
|
| Is partial verification avoided? |
If not all participants received the reference test, we reported how many did not. |
| Is differential verification avoided? |
If any participants received a different reference test, we reported the reasons stated for this, and how many participants were involved. |
| Is incorporation avoided? (the index test does not form part of the reference standard) | This should be 'Yes' for all studies, as the reference standard is defined in the inclusion criteria as microscopy or PCR. |
| Are the reference standard test results blinded? |
|
| Are the index test results blinded? |
|
| Were uninterpretable results reported? |
We reported how many results were uninterpretable (of the total) and how these were handled in the analysis. |
| Were any withdrawals explained? |
We reported how many participants were excluded from the analysis. |
Appendix 4. Direct comparisons between test types
| Study | Sensitivity (true positives/malaria cases) (%) | Difference (95% CI) (%) | P value | Specificity (true negatives/non‐cases) (%) | Difference (95% CI) (%) | P value | ||
| Type 2 versus Type 3 | ||||||||
| Type 2 | Type 3 | Type 2 | Type 3 | |||||
| Ashton 2010 | 85 (209/246) | 85 (209/246) | 0 (‐6.3 to 6.3) | P = 1.00 | 96 (2052/2137) | 96 (2060/2137) | 0 (‐1.5 to 0.8 ) | P = 0.58 |
| Eibach 2013 | 80 (4/5) | 60 (3/5) | 20.0 (‐35.4 to 75.4) | P = 1.00 | 99 (716/722) | 99 (718/722) | 0 (‐1.1 to 0.6) | P = 0.75 |
| Singh 2010 | 68 (39/57) | 77 (44/57) | ‐8.8 (‐25.0 to 7.5) | P = 0.40 | 98 (308/315) | 98 (309/315) | 0 (‐2.5 to 1.9) | P = 1.00 |
| Type 2 versus Type 4 | ||||||||
| Type 2 | Type 4 | Type 2 | Type 4 | |||||
| van den Broek 2006 | 75 (217/291) | 88 (256/292) | ‐13.1 (‐19.4 to ‐6.8) | P < 0.001 | 100 (604/605) | 99 (598/604) | 0.8 (0 to 1.7) | P = 0.07 |
| Type 3 versus Type 4 | ||||||||
| Type 3 | Type 4 | Type 3 | Type 4 | |||||
| Dev 2004 | 71 (5/7) | 90 26/29 | ‐18.2 (‐53.5 to 17.0) | P = 0.24 | 100 (23/23) | 100 (111/111) | 0 (Not estimable) | Not estimable |
| Ratsimbasoa 2007 | 73 (11/15) | 80 (12/15) | ‐6.7 (‐36.8 to 23.5) | P = 1.0 | 98 (175/179) | 97 (175/180) | 0.50 (‐2.7 to 3.8) | P = 1.0 |
We presented the difference in sensitivities and specificities between test types compared within each study as percentages. If a study evaluated more than one commercial brand of a test type on the same patients against the same reference standard, we randomly selected one brand for the comparison of test types.
Appendix 5. Comparison of microscopy and PCR reference standards for non‐falciparum infections
| Test type, RDT brand | Microscopy | PCR | ||||||
| Number of studies | Number of participants | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | Number of studies | Number of participants | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | |
| Type 3, CareStart Pf/Pan | 4 | 3544 | 74 (45 to 91) | 99 (96 to 100) | 1 | 179 | 91 (81 to 97) | 100 (97 to 100) |
| Type 3, Parascreen | 14 | 5407 | 79 (67 to 88) | 98 (98 to 99) | 2 | 659 | 84 (70 to 92) | 99 (97 to 100) |
| Type 3, One Step Malaria Pf/Pan | 1 | 606 | 70 (58 to 81) | 99 (98 to 100) | 1 | 606 | 72 (60 to 82) | 97 (95 to 98) |
| Type 3, SD Malaria Antigen Bioline | 4 | 3769 | 80 (73 to 85) | 99 (98 to 100) | 1 | 196 | 64 (41 to 83) | 99 (97 to 100) |
| Type 4, OptiMAL | 6 | 1843 | 90 (85 to 93) | 98 (97 to 99) | 1 | 313 | 88 (64 to 99) | 98 (96 to 99) |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

Non‐falciparum species only, microscopy, Type 2, ICT Combo Cassette.
2. Test.
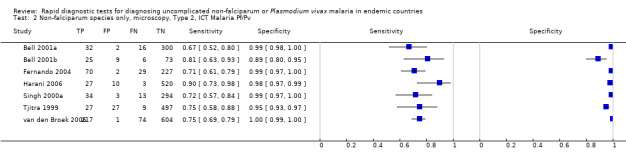
Non‐falciparum species only, microscopy, Type 2, ICT Malaria Pf/Pv.
3. Test.

Non‐falciparum species only, microscopy, Type 2, NOW Malaria ICT.
4. Test.

Non‐falciparum species only, microscopy, Type 2, Malascan.
5. Test.

Non‐falciparum species only, microscopy, Type 2, VIKIA Ag Pf/Pan.
6. Test.
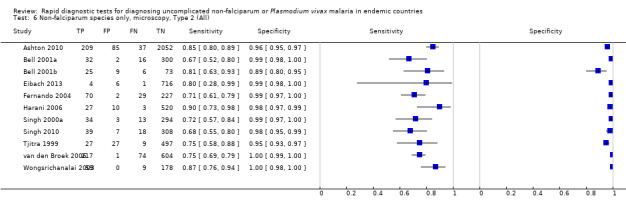
Non‐falciparum species only, microscopy, Type 2 (All).
7. Test.
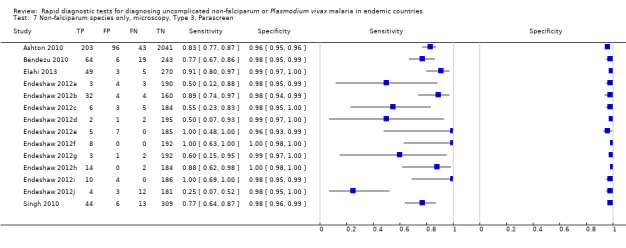
Non‐falciparum species only, microscopy, Type 3, Parascreen.
8. Test.
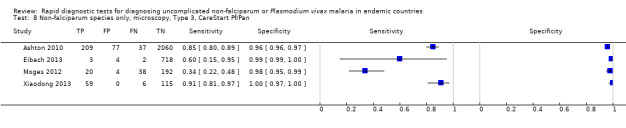
Non‐falciparum species only, microscopy, Type 3, CareStart Pf/Pan.
9. Test.
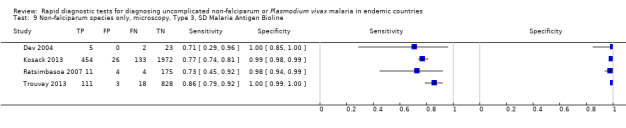
Non‐falciparum species only, microscopy, Type 3, SD Malaria Antigen Bioline.
10. Test.
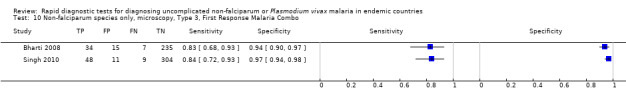
Non‐falciparum species only, microscopy, Type 3, First Response Malaria Combo.
11. Test.

Non‐falciparum species only, microscopy, Type 3, One Step Malaria Pf/Pan.
12. Test.
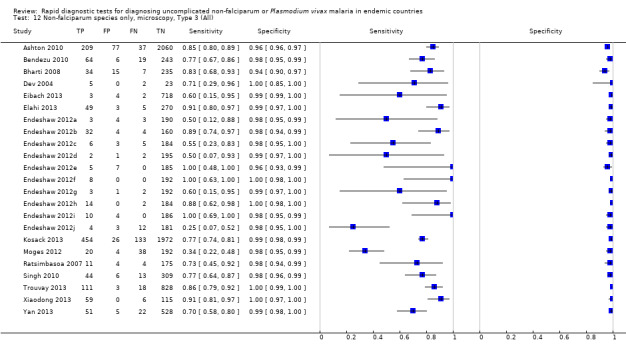
Non‐falciparum species only, microscopy, Type 3 (All).
13. Test.
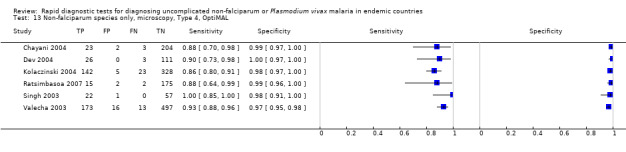
Non‐falciparum species only, microscopy, Type 4, OptiMAL.
14. Test.
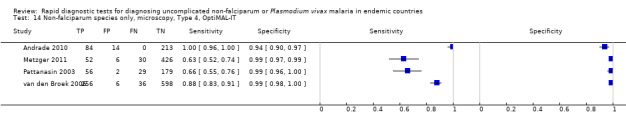
Non‐falciparum species only, microscopy, Type 4, OptiMAL‐IT.
15. Test.

Non‐falciparum species only, microscopy, Type 4, Carestart.
16. Test.
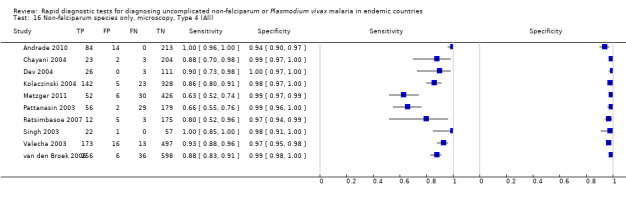
Non‐falciparum species only, microscopy, Type 4 (All).
17. Test.

Non‐falciparum species only, microscopy, Other Type, Malariagen Malaria.
18. Test.

Non‐falciparum species only, PCR, Type 3, CareStart Pf/Pan.
19. Test.
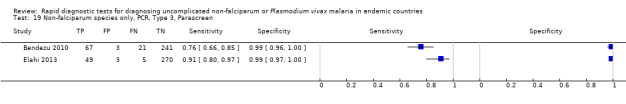
Non‐falciparum species only, PCR, Type 3, Parascreen.
20. Test.

Non‐falciparum species only, PCR, Type 3, One Step Malaria Pf/Pan.
21. Test.

Non‐falciparum species only, PCR, Type 3, SD Malaria Antigen Bioline.
22. Test.
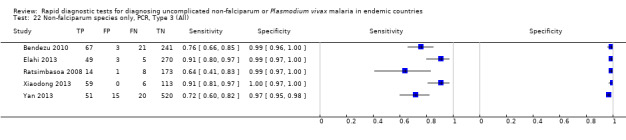
Non‐falciparum species only, PCR, Type 3 (All).
23. Test.

Non‐falciparum species only, PCR, Type 4, OptiMAL (All).
24. Test.

P. vivax, microscopy, Pf HRP‐2 and Pv pLDH, Carestart Pf/Pv (All).
25. Test.

P. vivax, microscopy, Pf HRP‐2 and Pv pLDH, Biotech Malaria Pf/Pv.
26. Test.
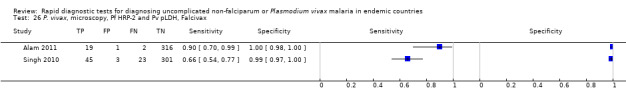
P. vivax, microscopy, Pf HRP‐2 and Pv pLDH, Falcivax.
27. Test.
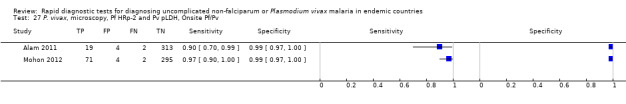
P. vivax, microscopy, Pf HRp‐2 and Pv pLDH, Onsite Pf/Pv.
28. Test.

P. vivax, microscopy, Pf HRP‐2 and Pv pLDH, Pf/Pv Malaria Device.
29. Test.
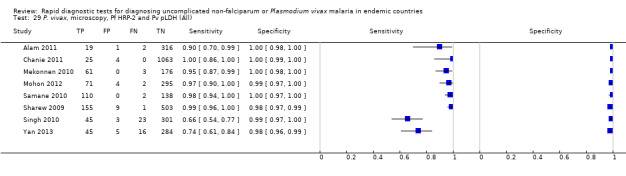
P. vivax, microscopy, Pf HRP‐2 and Pv pLDH (All).
30. Test.

P. vivax, PCR, Pf HRP‐2 and Pv pLDH, Falcivax.
31. Test.

P. vivax, PCR, Pf HRP‐2 and Pv pLDH, OnSite Pf/Pv.
32. Test.

P. vivax, PCR, Pf HRP‐2 and Pv pLDH, Pf/Pv Malaria Device.
33. Test.
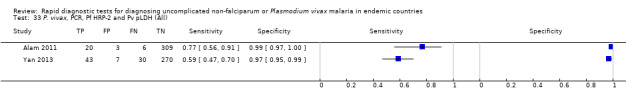
P. vivax, PCR, Pf HRP‐2 and Pv pLDH (All).
34. Test.

P. vivax, PCR, Type 6, PALUTOP (All).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alam 2011.
| Clinical features and settings |
Presenting signs and symptoms: Fever Previous treatments for malaria: No explicit exclusions based on previous treatment and no information presented on previous treatment Clinical setting: Matiranga Upazila Health Complex (UHC) Country: Bangladesh Malaria endemicity: Perennial transmission of malaria with 2 peaks in pre‐monsoon (MarchMay) and post‐monsoon (September to November) periods Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 338 Age: Median age was 14 years and the range was 18 months to 82 years Sex: Both males and females eligible. 50.3% of participants were female Co‐morbidities or pregnancy: Not mentioned, either as an inclusion criteria or characteristic of included participants |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 4 RDTs: Paracheck test was performed at concurrently with the microscopy. The remaining 3 RDTs were performed using stored samples. Samples from each individual were tested by all tests. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum andP. vivax Reference standard test(s) used: Microscopy, thick and thin smear slides and PCR Who performed the reference standard tests, and where? 2 independent microscopists: 1 employed by the study and the other at Matiranga UHC; not reported for PCR If microscopy was used, how many high power fields were looked at? 200 fields in the Giemsa‐stained thick film How many observers or repeats were used? 2 observers How were discrepancies between observers resolved? By a third microscopist posted at the Khagrachari Civil Surgeon's office situated 20 km away from Matiranga UHC |
|
| Index and comparator tests |
Commercial name of the test: Paracheck (Orchid Biomedical System, India), FalciVax Pf (Zephyr Biomedicals, India), Onsite Pf (CTK Biotech Inc, USA) and Onsite Pf/Pv (CTK Biotech Inc, USA) Parasite species the test is designed to detect:
Designated type:
Batch numbers: Not provided Transport and storage conditions: Not provided Who performed the index test, and where? Not reported. All the RDTs were used following the manufacturer's instructions. |
|
| Follow‐up | ||
| Notes | Source of funding: funded by icddr,b and its donors. Paracheck provided by NMCP; Onsite Pf and Onsite Pf/Pv provided by CTK Biotech Inc, USA as a donation. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Febrile patients referred to microscopy for malaria diagnosis. Other characteristics, inclusion and exclusion criteria not described. |
| Acceptable reference standard? All tests | Yes | Microscopy: 2 experienced, independent microscopists assessed each slide. There was provision for a third microscopist to resolve any disagreement between them. 200 fields were viewed, another 200 fields were viewed if malaria was identified, to identify mixed infections. PCR is also a reference standard. |
| Partial verification avoided? All tests | Yes | All participants receiving index tests had their diagnosis verified by reference test. |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The reference standard was microscopy and PCR. |
| Reference standard results blinded? All tests | Unclear | Blinding not described. |
| Index test results blinded? All tests | Unclear | Blinding not described. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | The number recruited into the study was clearly stated and corresponded with the number included in the analysis, therefore there were no withdrawals. |
Andrade 2010.
| Clinical features and settings |
Presenting signs and symptoms: Malaria‐related symptoms Previous treatments for malaria: Not mentioned, either as an inclusion criteria or characteristic of included participants Clinical setting: Diagnostic centres of the Brazilian National Foundation of Health (FUNASA) Country: Brazil Malaria endemicity: Not stated Malaria endemic species: P. falciparum and P. vivax |
|
| Participants |
Sample size: 311 Age: Median age was 33.5 years and the range was 4 to 65 years Sex: Both males and females eligible. 60.5% of participants were male Co‐morbidities or pregnancy: Not mentioned, either as an inclusion criteria or characteristic of included participants |
|
| Study design | Participants were recruited consecutively. The sampling method was not described. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax malaria Reference standard test(s) used: Microscopy thick and thin blood films and nested PCR Who performed the reference standard tests, and where? Experienced malaria field microscopists from the FUNASA performed microscopy; not stated who performed the nested PCR. All tests were repeated and confirmed at the main laboratory at the Centro de Pesquisas Goncalo Moniz, Bahia, Brazil. If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? 2 repeats with 2 different observers How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: Optimal‐IT RDT (DiaMed China Ltd, Hong Kong, China) Parasite species the test is designed to detect:P. falciparum and P. vivax malaria Designated type: Antigens test detects stated Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? Not stated |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: FINEP (010409605)/FNDCT‐CT Amazônia. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Patients were attending a clinic with symptoms of malaria. Study authors excluded people who had lived in the area for less than 6 months or had received antimalarials in the last 2 weeks. |
| Acceptable reference standard? All tests | Unclear | 2 independent microscopists performed microscopy, 1 in the field and 1 in the central laboratory. The number of fields viewed is not stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference tests. |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | Not reported whether the tests were read blindly. |
| Index test results blinded? All tests | Unclear | Not reported whether the tests were read blindly. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals. |
Ashton 2010.
| Clinical features and settings |
Presenting signs and symptoms: Symptoms of uncomplicated malaria (axillary temperature > 37.5°C or report of fever in the previous 48 hours) Previous treatments for malaria: Not mentioned, either as an inclusion criteria or characteristic of included participants Clinical setting: 1 health centre and 3 health posts per woreda Country: Ethiopia Malaria endemicity: Not stated Malaria endemic species: P. falciparum and P. vivax |
|
| Participants |
Sample size: 2400 Age: All ages over 6 months eligible. Actual age profile of participant population not presented. Sex: Both males and females eligible. Actual proportions of males and females in the participant population not stated. Co‐morbidities or pregnancy: Patients with any life‐threatening diseases were excluded. No exclusion criteria based on pregnancy. No details about the frequency of pregnancy in the patient population are presented. |
|
| Study design | Enrolment was consecutive and prospective. 3 RDTs were evaluated (CareStart®, ParaScreen® and ICT Combo®). Each individual received all the tests. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax malaria Reference standard test(s) used: Microscopy thick and thin blood films Who performed the reference standard tests, and where? Staff at the health centre and experienced microscopists at a regional malaria reference laboratory who was blinded to the initial results. And a third, blinded, reading was conducted to address discrepancies If microscopy was used, how many high power fields were looked at? 200 fields at 1000× magnification How many observers or repeats were used? 2 How were discrepancies between observers resolved? They were corrected according to a third, blinded, readings: presence or absence of asexual parasites, difference in species, or > 50% difference in parasite count. Microscopy results and parasite counts were corrected according to the third reading. |
|
| Index and comparator tests |
Commercial name of the test:
Parasite species the test is designed to detect: Multi‐species Designated type:
Batch numbers: Not stated Transport and storage conditions: "RDTs were transported unrefrigerated by air to Addis Ababa, where they were stored at ambient conditions until transfer to field sites. Temperature was monitored (Tinytag, Gemini Data Loggers, UK) but not controlled while RDTs were transported by road to health centres and during storage at the health centres. Temperatures during transport reached a maximum 36°C, but at health facilities temperatures did not exceed 30°C." Who performed the index test, and where? Health extension workers or nurses at the health centres |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: "United States Agency for International Development (Cooperative Agreement 663‐A‐00‐09‐00404‐00). BC is funded by the ACT Consortium which is supported by a grant from the Bill & Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine. Manufacturers supplied CareStart and ParaScreen RDTs free of charge for this evaluation, and ICT Combo was provided at a reduced price." | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were visiting an outpatient department of a health centre with symptoms suggestive of malaria. |
| Acceptable reference standard? All tests | Yes | 2 independent microscopists viewed the slides, a third viewed any discrepancies; 200 fields were looked at. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standards. |
| Reference standard results blinded? All tests | Yes | "RDT and microscopy results were read by different staff at the health centre, each blinded to the results of the other diagnostic technique". |
| Index test results blinded? All tests | Yes | "RDT and microscopy results were read by different staff at the health centre, each blinded to the results of the other diagnostic technique". |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals. |
Bell 2001a.
| Clinical features and settings |
Presenting signs and symptoms: History of fever, headache, chills or rigors occurring within the preceding 3 days; or more distant history of fever or non‐specific signs suggestive of malaria Previous treatment for malaria: Participants who had recently taken antimalarials were not excluded; 5% of participants reported prior antimalarial use Clinical setting: Village health workers in 5 barangaya (districts) Country: Philippines (Agusan del Sur Province in the northeast of the island of Maindano) Malaria endemicity: Generally low perennial transmission, with pockets of high transmission Malaria endemic species:P. falciparum andP. vivax |
|
| Participants |
Sample size: 350 Age: Eligible age range not stated. Mean age of the participants was 19.5 years Sex: Both males and females eligible. There were 171 male and 179 female participants. Co‐morbidities and pregnancy: Not mentioned, either an exclusion criteria or characteristic of included participants Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy of thick and thin blood smears Who performed the reference standard tests, and where? An experienced local microscopist for all slides; selected slides were also read by an experienced parasitologist. Microscopy was performed in a local laboratory and hospital laboratory in Australia If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 1, except in discordant cases where RDT and microscopy results differed, all cases RDT‐positive for P. vivax and 20% of cases negative by slide and RDT, in which case a second reader was used. How were discrepancies between observers resolved? The second, off‐site reading was taken as the correct 1 |
|
| Index and comparator tests |
Commerical name of RDT: ICT Malaria Pf/Pv (Amrad‐ICT, Sydney, Australia) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 4 Batch numbers: Not stated Transport and storage conditions: Refrigerated until 2 weeks before use Person(s) performing RDT: Researchers RDT setting: Study villages |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: The Australian National Health and Medical Research Council. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants had approached village health workers with symptoms suggestive of malaria, but the sampling method was not described. |
| Acceptable reference standard? All tests | Yes | An experienced microscopist viewed at least 100 high powered fields and discordant results were re‐examined. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "slides were read by a local microscopist who was not aware of the results of the ICT tests". |
| Index test results blinded? All tests | Yes | RDTs were performed 2 to 4 weeks before microscopy. |
| Uninterpretable results reported? All tests | Yes | The paper reported that there was 1 uninterpretable microscopy result. |
| Withdrawals explained? All tests | Unclear | The number of participants originally enrolled in the study was not stated; therefore it is unclear whether there were any withdrawals. |
Bell 2001b.
| Clinical features and settings |
Presenting signs and symptoms: History of fever, headache, child or rigors occurring within the preceding 3 days; or more distant history of fever or non‐specific signs suggestive of malaria Previous treatment for malaria: Patients treated with antimalarials during the 4 weeks preceding the test were excluded from the analysis Clinical setting: Health centre in Visaya Country: Philippines (Agusan del Sur Province in the northeast of the island of Maindano) Malaria endemicity: Generally low perennial transmission, with pockets of high transmission Malaria endemic species:P. falciparum andP. vivax |
|
| Participants |
Sample size: 113 Age: Eligible age range not stated. Mean age of the participants was 19.8 years Sex: Both males and females eligible. There were 73 male and 40 female participants. Co‐morbidities and pregnancy: Not mentioned, either as an exclusion criteria or characteristic of included participants Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy of thick and thin blood smears Who performed the reference standard tests, and where? Not stated. Setting: Regional Health Units If microscopy was used, how many high power fields were looked at? Not stated, but probably 100 as in the other trial reported together in the same paper How many observers or repeats were used? Not stated How were discrepancies between observers resolved? Not applicable |
|
| Index and comparator tests |
Commerical name of RDT: ICT Malaria Pf/Pv (Amrad‐ICT, Sydney, Australia) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 4 Batch numbers: Not stated Transport and storage conditions: Stored by barangay health workers at room temperature, averaging about 25°C for up to 6 months Person(s) performing RDT: Barangay health workers RDT setting: Health centre |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: The Australian National Health and Medical Research Council. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were attending a health centre with history of fever, headache, child or rigors within the preceding 3 days; more distant history of fever or non‐specific signs suggestive of malaria; but the sampling method was not described. |
| Acceptable reference standard? All tests | Unclear | No details given of the microscopy process. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Clear that blinding had taken place, as it was not possible to match up all the RDT and microscopy results by name and date. |
| Index test results blinded? All tests | Yes | Clear that blinding had taken place, as it was not possible to match up all the RDT and microscopy results by name and date. |
| Uninterpretable results reported? All tests | Yes | 25 of 393 tests done were considered invalid because of an indistinct control band. Invalid results were excluded from the analysis. |
| Withdrawals explained? All tests | Yes | Only 113 microscopy results could be matched with RDT results by name and date; the others were lost from the analysis. |
Bendezu 2010.
| Clinical features and settings |
Presenting signs and symptoms: History of fever with or without chills, sweating and headache Previous treatments for malaria: No history of anti‐malarial treatment during the last 2 weeks Clinical setting: Health facilities Country: Peru Malaria endemicity: "Despite a reduction of the incidence by up to 40% during the last 4 years in Peru, malaria due to P. falciparum and P. vivax remains an important public health problem, especially in the Amazon region where more than 70% of the cases of the country are reported." Malaria endemic species: P. falciparum and P. vivax |
|
| Participants |
Sample size: 332 Age: Eligible age range not stated. Mean age was 32 ± 16 years Sex: Not mentioned either as an inclusion criteria or a characteristic of participants Co‐morbidities or pregnancy: Not mentioned either as an inclusion criteria or a characteristic of participants |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax malaria Reference standard test(s) used: Microscopy thick and thin blood films and PCR Who performed the reference standard tests, and where? Reference standards were carried out by different staff blinded to each other result. Expert microscopy was carried out by experts in the 6 health centres; not described for PCR If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? 10% of the slides were examined by a second expert microscopist at the reference laboratory How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen (Zephyr Biomedical Systems) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: ParaScreen – Type 3 Batch numbers: Lot 101051 Transport and storage conditions: Not described Who performed the index test, and where? Not stated |
|
| Follow‐up | ||
| Notes | Source of funding: by The Global Fund to fight AIDS, Tuberculosis and Malaria through the ‐ Organismo Andino de Salud ‐ Convenio Hipolito Unanue' (Principal Recipient of the Multi‐Country Malaria Project "Malaria control on the cross border areas of the Andean Region: A community based approach"‐PAMAFRO), Grant Number MAA‐305‐G01‐M; and by the Directorate General for Development Cooperation (DGCD) of the Belgian Government (framework agreement 02, 2003‐2007), project 95501. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were attending health centres with fever and no history of malaria treatment within the last 2 weeks. |
| Acceptable reference standard? All tests | No | Only 10% of slides were viewed twice. The number of high power fields viewed before declaring a sample negative was not stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Reported that the tests were read blindly. |
| Index test results blinded? All tests | Yes | Reported that the tests were read blindly. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals. |
Bharti 2008.
| Clinical features and settings |
Presenting signs and symptoms: Fever or history of fever, and suspicion of malaria Previous treatment for malaria: No exclusions based on previous treatment; it was undertaken in a remote area with no medical facilities Clinical setting: Mobile field clinics in ten villages Country: India (remote forested region of Jabalpur during the peak monsoon season) Malaria endemicity: Low endemic areas with higher transmission during the monsoon Malaria endemic species:P. falciparum andP. vivax |
|
| Participants |
Sample size: 291 Age: All age groups eligible. Actual age range of participants 1 to 60 years Sex: Both males and females eligible. Male: female ratio 1:1.15 Co‐morbidities and pregnancy: No criteria based on co‐morbidities or pregnancy. No details of the frequency of these conditions in the participant population presented. Parasite density of microscopy positive cases: Range 80 to 111,920 parasites per µL, mean 8011, standard deviation 21,595 |
|
| Study design | Enrolment was consecutive and prospective. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick blood films Who performed the reference standard tests, and where? Experienced microscopist in the laboratory of NIMR If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 1 for all samples, 2 independent readers for samples discordant between microscopy and RDT How were discrepancies between observers resolved? Where the second reading gave a different result from the first, the results of the second reading were confirmed by a third examination by another technician |
|
| Index and comparator tests |
Commerical name of RDT: First Response Combo Malaria Ag card test (Premier Medical Corporation Ltd, Mumbai, India) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: 61F0107 Transport and storage conditions: RDTs were stored properly, at temperature of 4°C to 30°C, and used within their shelf life Person(s) performing RDT: Field laboratory assistants. Independent staff re‐read the saved tests after 2 months and matched them with the originally recorded results RDT setting: Field laboratory |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Indian Council of Medical Research, Delhi. Test kits provided by Premier Medical Corporation Ltd. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive sample of people attending mobile field clinics with fever or history of fever, and suspicion of malaria. |
| Acceptable reference standard? All tests | Yes | An experienced microscopist viewed at least 100 high power fields before declaring a slide negative, and results discordant with RDT were independently re‐examined by a second microscopist, and a third if necessary. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Microscopy was undertaken "without reference to the RDT". |
| Index test results blinded? All tests | Yes | RDTs were undertaken on site, and the results recorded before the microscopy results became available. |
| Uninterpretable results reported? All tests | Yes | The paper reported that there were no invalid results. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study was clearly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals. |
Chanie 2011.
| Clinical features and settings |
Presenting signs and symptoms: Suspected malaria: clinical symptoms of malaria, fever Previous treatments for malaria: 12.5% of the subjects had anti‐malaria treatment in the preceding month Clinical setting: Outpatient departments of 3 health facilities Country: Ethiopia Malaria endemicity: High endemicity Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 1092 Age: Mean 22.3 (SD 12.8); range 3 months to 78 years of age Sex: 48.57% female, 51.43% male Co‐morbidities or pregnancy: Co‐morbidities and pregnancy not stated |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy thick and thin smears Who performed the reference standard tests, and where? Experienced malaria technicians performed the microscopic test. Location not reported (presumably at each of the participating health centres) If microscopy was used, how many high power fields were looked at? A minimum of 100 high power fields examined on a thick smear How many observers or repeats were used? Not stated How were discrepancies between observers resolved? 20% of the positive and 10% of the negative slides and discordant results between RDT and microscopic tests were examined by another well experienced technician |
|
| Index and comparator tests |
Commercial name of the test: CareStart Malaria Pf/Pv Combo (Access Bio Inc, New Jersey, USA) Parasite species the test is designed to detect:P. falciparum and P. vivax Designated type: Type Other (HRP‐2 antigen for P. falciparum and pLDH antigen for P. vivax) Batch numbers: Lot No H38 IV and Lot No H28 IV Transport and storage conditions: Lot No H38 IV and Lot No H28 IV Who performed the index test, and where? Experienced malaria technicians performed the index test |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Addis Ababa University, The Federal Ministry of Health of Ethiopia. RDT kits were donated by Acces Bio Inc. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | All participants were attending primary health centres with fever and symptoms of malaria, sampling was consecutive. |
| Acceptable reference standard? All tests | Yes | "...discordant results between RDT and microscopic tests were examined by another well experienced technician". Experienced malaria technicians viewed 200 white blood cells or 100 fields. |
| Partial verification avoided? All tests | Yes | Characteristics of participants are well described and the only exclusion criterion was refusal to participate in the study. |
| Differential verification avoided? All tests | Yes | The same reference standard was used. |
| Incorporation avoided? All tests | Yes | The reference standard was microscopy. |
| Reference standard results blinded? All tests | Unclear | Blinding procedures not stated. However, microscopic evaluation and RDT were performed independently. Results were recorded in separate sheets. |
| Index test results blinded? All tests | Unclear | Blinding procedures not stated. However, microscopic evaluation and RDT were performed independently. Results were recorded in separate sheets. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | The number recruited into the study was clearly stated, and corresponded with the number included in the analysis, therefore there were no withdrawals. |
Chayani 2004.
| Clinical features and settings |
Presenting signs and symptoms: Specific symptoms: rigor, chills, rise of high temperature and profuse sweating; or irregular fever, joint pain and jaundice Previous treatment for malaria: No explicit exclusions based on previous treatment and no information presented on previous treatment Clinical setting: Diagnostic and research centre (takes referrals from physicians for the diagnosis of malaria) Country: Orissa, India Malaria endemicity: Not stated Malaria endemic species: In sample, 78.6% P. falciparum, 21.4% P. vivax |
|
| Participants |
Sample size: 232 Age: Not mentioned, either as inclusion criteria or characteristic of participants Sex: Not mentioned, either as inclusion criteria or characteristic of participants Co‐morbidities and pregnancy: Not mentioned, either as inclusion criteria or characteristic of participants Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was unclear. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and think blood smear Who performed the reference standard tests, and where? Microscopists in a diagnostic and research centre If microscopy was used, how many high power fields were looked at? 200 How many observers or repeats were used? 2 independent observers How were discrepancies between observers resolved? A third microscopist's opinion was taken into account |
|
| Index and comparator tests |
Commerical name of RDT: OptiMAL (DiaMed, AG, Cressier, Switzerland) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 4 Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: Not stated RDT setting: Not stated |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were attending an ambulatory clinic with rigor, chills, rise of high temperature and profuse sweating; or irregular fever, joint pain and jaundice. However the sampling method was not described. |
| Acceptable reference standard? All tests | Yes | 2 independent microscopists viewed 200 high powered fields before declaring a slide negative. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | Blinding not described. |
| Index test results blinded? All tests | Unclear | Blinding not described. |
| Uninterpretable results reported? All tests | No | The number of participants originally enrolled in the study was not explicitly stated; therefore it is not possible to judge whether any were excluded from the analysis due to invalid test results. |
| Withdrawals explained? All tests | Unclear | The number of participants originally enrolled in the study was not explicitly stated; therefore it is not possible to judge whether there were any withdrawals. |
Dev 2004.
| Clinical features and settings |
Presenting signs and symptoms: Fever Previous treatment for malaria: No information presented on previous treatment; no suggestion of any exclusions based on previous treatment Clinical setting: Malaria clinics Country: India (Assam) Malaria endemicity: Mesoendemic Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 336; but varied by RDT evaluated (10 to 139) Age: Infants under 12 months excluded; actual age range 1 to 60 years Sex: Both males and females eligible. Actual proportions of males and females in the participant population not stated Co‐morbidities and pregnancy: No exclusions criteria based on co‐morbidities or pregnancy were stated, and no details of the frequency of these conditions in the participant population is presented. Parasite density of microscopy positive cases: Range 300 to 350,000 parasites per µL, mean 59,842, standard deviation (SD) 78,780 |
|
| Study design | Enrolment was prospective. The sampling method was not described. 7 RDTs were evaluated; it is unclear how each RDT was allocated, as no participant received all the tests. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood smears Who performed the reference standard tests, and where? Technician; all positive slides and 20% of negative slides were also examined by the senior technician for confirmation of result. Setting was the laboratory at the malaria clinics If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 1 in the case of most smears judged negative by the technician. 2 in the case of 20% of those initially judged negative, and all those judged positive. How were discrepancies between observers resolved? The judgement of the senior technician was used |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:
Designated type:
Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: The laboratory attendant performed the test and recorded his or her interpretation. The test kit result was then re‐read for verification by the senior technician. RDT setting: Malaria clinic laboratory |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Mian source of funding not stated. Test kits supplied by the Government of Assam. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were attending malaria clinics with fever; however, during the study period, 6663 blood smears were examined but only 336 were evaluated with RDT kits, and the sampling method for RDT evaluation was unclear. |
| Acceptable reference standard? All tests | Unclear | 2 observers were used in the vast majority of cases; however, it is unclear whether the observers worked independently. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Microscopy and RDT results were compared by an independent observer. |
| Index test results blinded? All tests | Yes | Microscopy and RDT results were compared by an independent observer. |
| Uninterpretable results reported? All tests | No | No information presented on numbers initially allocated each RDT, so not possible to judge this. |
| Withdrawals explained? All tests | Unclear | No information presented on numbers initially allocated each RDT, so not possible to judge this. |
Eibach 2013.
| Clinical features and settings |
Presenting signs and symptoms: Suspected malaria with a temperature > 37.5°C Previous treatments for malaria: More than 90% of the patients reported receiving traditional or registered drugs, including antipyretics, antimalarials and antibiotics previously. However, the quality of drugs, the dosage and the duration of treatment remained unknown. Clinical setting: General health centre Country: Mali Malaria endemicity: Hyperendemic in the peripheral villages, mesoendemic in the periurban area and hypoendemic in the city. Malaria endemic species: 95% P. falciparum |
|
| Participants |
Sample size: 727 Age: Mean 23.5 (SD 14.9) median = 21, range 1 to 60) Sex: Not reported Co‐morbidities or pregnancy: Not reported |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 2 RDTs. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum, PAN malaria Reference standard test(s) used: Microscopy thick and thin smears Who performed the reference standard tests, and where? Local investigators If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? Thick and thin smears were assessed by 2 local investigators, and by an expert at the Parasitology Department of the Lyon University Hospital, as a quality control How were discrepancies between observers resolved? Local investigators resolved all discrepancies between themselves by consensus. All discordant results between microscopy and the 2 RDTs were resolved by PCR and test characteristics were recalculated according to the PCR‐corrected results. |
|
| Index and comparator tests |
Commercial name of the test: VIKIA Malaria Ag Pf/Pan (IMAccess, Lyon, France), CareStart Malaria (AccessBio, USA) Parasite species the test is designed to detect: VIKIA Malaria Ag Pf/Pan: P. falciparum or mixed infection, non‐falciparum malaria species only CareStart Malaria: P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: VIKIA Malaria Ag Pf/Pan: Type 2 CareStart Malaria: Type 3 Batch numbers: VIKIA Malaria Ag Pf/Pan: RD_MA2_110527 CareStart Malaria: G21MR Transport and storage conditions: Not reported Who performed the index test, and where? Local community health workers trained to use both tests . |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: The study was supported by IMACCESS. Data from the Lyon part of the study was not included as it did not match inclusion criteria. The VIKIA Malaria Ag Pf/Pan™ test was read at different time points (15, 20, 30, 60 minutes), while the CareStart Malaria™ test was read after 20 minutes as recommended. 2 drops of blood were spotted onto filter paper, individually stored in a plastic bag and sent to the Parasitology Department of the Lyon University Hospital for PCR correction. |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Consecutive sample of people attending a clinic with symptoms of malaria. |
| Acceptable reference standard? All tests | Yes | Microscopy was undertaken by 2 trained local health workers and corrected by PCR at the Parasitology Department of the Lyon University Hospital. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference tests. |
| Differential verification avoided? All tests | Yes | The same reference test was used. |
| Incorporation avoided? All tests | Yes | Standard microscopy and PCR. |
| Reference standard results blinded? All tests | Yes | All microscopists were blinded to the results of the RDTs. |
| Index test results blinded? All tests | Yes | RDTs were performed immediately after sampling. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled was clearly stated, and the number included in the analysis corresponds to this number, indicating no withdrawals. |
Elahi 2013.
| Clinical features and settings |
Presenting signs and symptoms: Febrile patients with clinical symptoms Previous treatments for malaria: Not described Clinical setting: Health posts in remote border areas Country: Bangladesh Malaria endemicity: Endemic Malaria endemic species: 74% P. falciparum, 26% P. vivax,P. malariae and P. ovale were also present in the area, but were not found within the study sample |
|
| Participants |
Sample size: 327 Age: Not reported Sex: Not reported Co‐morbidities or pregnancy: Not reported |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum, PAN malaria Reference standard test(s) used: Microscopy thick and thin smears; quantitative PCR Who performed the reference standard tests, and where? Experienced microscopists at the field sites If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 2 independent microscopists, blinded to the findings of the other How were discrepancies between observers resolved? Where there was a discrepancy between the 2 microscopists, the sample was excluded from the study. The number excluded for this reason was not stated |
|
| Index and comparator tests |
Commercial name of the test: Parascreen (Zephyr Biomedical Systems, India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: 101159 Transport and storage conditions: Not described Who performed the index test, and where? Laboratory personnel at the Parisitology Laboratory, icddr,b |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: icddr,b and its donors, which provide unrestricted support to icddr,b for its operations and support. Parascreen was donated by the manufacturer. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Recruitment was prospective, but the sampling procedure was not stated. Samples with mixed infections or where the 2 microscopists' findings did not agree were excluded, and the number excluded was not stated. |
| Acceptable reference standard? All tests | Unclear | Microscopy was undertaken by 2 experienced microscopists, but the number of fields viewed before declaring a slide negative was not stated. PCR was also used as a separate, additional reference standard. |
| Partial verification avoided? All tests | Yes | All participants received both the reference test and the index test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as the reference standard. |
| Reference standard results blinded? All tests | Yes | Microscopists were blinded to prior results. |
| Index test results blinded? All tests | Unclear | Blinding not described, however, index and reference tests were undertaken at different locations. |
| Uninterpretable results reported? All tests | No | Uninterpretable results were not reported on. |
| Withdrawals explained? All tests | No | It was stated that samples with mixed infection or where microscopists disagreed were excluded; however, the number of samples excluded and the original number of participants enrolled was not presented. |
Endeshaw 2012a.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: Patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa, India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field was not stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012b.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: Patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa,India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic fields was not stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as a reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012c.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa, India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre, the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012d.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: Patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa, India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre, the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, the number of microscopic fields was not stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012e.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: Patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa,India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012f.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa,India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012g.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa,India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The 10 experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy". "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012h.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa,India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012i.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa,India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Endeshaw 2012j.
| Clinical features and settings |
Presenting signs and symptoms: Clinically presumptive malaria: an axillary temperature greater than or equal to 37.5°C or history of fever in the previous 48 hours Previous treatments for malaria: Not stated Clinical setting: Ten health centres Country: Ethiopia Malaria endemicity: The study was conducted in an area with range of transmission intensities, during the peak transmission period of malaria infection. Malaria endemic species: Not stated |
|
| Participants |
Sample size: 1997. 4 RDTs were not done (reason not reported) Age: Range: 8 months to 85 years. Mean 20.7 (SD not stated) Sex: 56.2 male, 43.8 female Co‐morbidities or pregnancy: patients with other known causes of non malarial febrile illnesses or serious illness were excluded. Pregnancy status not stated. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy, thick and thin smears Who performed the reference standard tests, and where? Microscopy assessment was performed by experienced medical laboratory technicians If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? Slides were also sent for expert microscopy at The Carter Center in Addis Ababa How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: ParaScreen Pan/Pf (Zephyr Biomedical systems, Verna, Goa, India) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 3 Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? The ten experienced laboratory technicians involved in this study were trained on the RDT sampling and evaluation procedures |
|
| Follow‐up | Not applicable | |
| Notes |
Source of funding: Not stated. "Out of 2000 recruited patients, 1997 febrile cases were examined for malaria parasites by blood slide microscopy." "Of the 1997 persons tested by slide, 1993 samples were also examined by ParaScreen RDT at the health centers." |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | In each health centre the first 200 self‐presenting patients of any age and either sex who qualified as clinically presumptive malaria were recruited. |
| Acceptable reference standard? All tests | Unclear | Slides were evaluated by trained technicians at each site and were also sent to a central lab for expert microscopy. However, number of microscopic field have not been stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | Microscopy was used as reference standard. |
| Reference standard results blinded? All tests | Yes | Although blind procedures for local technicians are not reported, slides were also sent for expert microscopy at a central lab where they were examined in blinded fashion. |
| Index test results blinded? All tests | Yes | Microscopy and ParaScreen Pan/PfH RDT were done immediately by health centre technicians. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated as 2000, 1993 were presented in the analysis; therefore there were 7 withdrawals. These were explained as 3 who declined to participate and 4 who did not have RDT because the technicians had too much work. |
Fernando 2004.
| Clinical features and settings |
Presenting signs and symptoms: Fever or history of fever Previous treatment for malaria: No exclusions because of prior antimalarial use, and no data presented on the frequency of recent antimalarial use in the participants Clinical setting: A malaria research station and a malaria clinic Country: Sri Lanka Malaria endemicity: Not stated Malaria endemic species:P. vivax (70%) and P. falciparum |
|
| Participants |
Sample size: 328 Age: All ages above 5 years eligible; mean age 28.3 years (range 5 to 72 years) Sex: Both males and females eligible; 64% of the participants were males Co‐morbidities and pregnancy: No exclusion criteria based on co‐morbidities or pregnancy. No details of the frequency of these conditions in the participant population is presented. Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was consecutive and prospective. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and think blood films Who performed the reference standard tests, and where? Trained microscopists at the clinics and in a laboratory If microscopy was used, how many high power fields were looked at? 400 How many observers or repeats were used? 2 independent readers; 1 at the clinics and another in a laboratory How were discrepancies between observers resolved? There were no discrepancies between the 2 microscopists |
|
| Index and comparator tests |
Commerical name of RDT: ICT Malaria Pf/Pv (Amrad‐ICT, Sydney, Australia) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 2 Batch numbers: Not stated Transport and storage conditions: Stored and used at room temperature which often exceeds 30°C Person(s) performing RDT: The researchers RDT setting: At the clinics |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: National Science Foundation, Sri Lanka | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive sample of people attending clinics with fever or history of fever. |
| Acceptable reference standard? All tests | Yes | 2 independent trained microscopists viewed 400 high power fields before declaring a slide negative. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | Blinding not described. |
| Index test results blinded? All tests | Unclear | Blinding not described. |
| Uninterpretable results reported? All tests | Unclear | The number of participants enrolled was clearly stated, and the number included in the analysis corresponds to this number, indicating no withdrawals. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled was clearly stated, and the number included in the analysis corresponds to this number, indicating no withdrawals. |
Harani 2006.
| Clinical features and settings |
Presenting signs and symptoms: Clinical symptoms of malaria and history of fever over 37.5°C. People with known causes of fever other than malaria were excluded. Previous treatment for malaria: Patients who had been treated for malaria in the previous 4 weeks were excluded from the study Clinical setting: Outpatient department of a reference hospital Country: Pakistan Malaria endemicity: Not stated Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 560 Age: All age groups eligible; actual age range of included participants 2 to 73 years Sex: Both males and females eligibles. Participants included 339 males and 221 females Co‐morbidities and pregnancy: Not mentioned, either as an inclusion criteria or characteristic of included participants Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was tested | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood films Who performed the reference standard tests, and where? Senior technologist and principle author in the Department of Pathology and Microbiology, Aga Khan University If microscopy was used, how many high power fields were looked at? 200 How many observers or repeats were used? Unclear, 2 microscopists were used but how they divided the work between them was not described How were discrepancies between observers resolved? Not applicable |
|
| Index and comparator tests |
Commerical name of RDT: ICT Malaria Pf/Pv (Binax Inc. Portland, Maine, USA) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 2 Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: The second author RDT setting: Microbiology section of Aga Khan University |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were presenting at an outpatients department with symptoms of malaria and history of fever, but the sampling method was not described. |
| Acceptable reference standard? All tests | Unclear | 2 microscopists at a University laboratory viewed 200 high power fields before declaring a slide negative; however, it is unclear how the 2 microscopists worked together. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "The microscopists were unaware of the microscopy results". |
| Index test results blinded? All tests | Yes | "These results were read by the second author who was blind to the microscopy results". |
| Uninterpretable results reported? All tests | Unclear | The number of participants originally enrolled in the study was clearly stated, and corresponds with the number presented in the analysis; therefore there were no exclusions due to invalid results. |
| Withdrawals explained? All tests | Yes | The number of participants originally enrolled in the study was clearly stated, and corresponds with the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
Kolaczinski 2004.
| Clinical features and settings |
Presenting signs and symptoms: Suspected malaria or febrile illness Previous treatment for malaria: No exclusion criteria based on previous use of antimalarials, and no data on previous antimalarial use of the participants was presented Clinical setting: Basic health units within an Afghan refugee camp Country: Pakistan (North West Frontier Province) Malaria endemicity: Not stated Malaria endemic species: 80% P. vivax, 20%P. falciparum |
|
| Participants |
Sample size: 499 Age: All age groups eligible for inclusion; actual age range of the participants not stated Sex: Both males and females eligible for inclusion; actual age range of the participants not stated Co‐morbidities and pregnancy: No exclusions based on co morbidities or pregnancy, and no data presented on the frequency of these conditions in the study population Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was consecutive and prospective. 1 RDT was tested. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and think blood films Who performed the reference standard tests, and where? Microscopists in the basic health units within an Afghan refugee camp and HNI's reference laboratory in Peshawar If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 2, 1 at the BHU and 1 at the reference laboratory How were discrepancies between observers resolved? Unclear "all of the smears checked by the microscopist at each BHU were cross checked at HNI's reference laboratory at Pashawar". |
|
| Index and comparator tests |
Commerical name of RDT: OptiMAL (DiaMed, AG, Cressier, Switzerland) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 4 Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: Microscopists RDT setting: Basic health units |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive series of patients attending a basic health units with suspected malaria. |
| Acceptable reference standard? All tests | Yes | 2 microscopists, 1 working in a central laboratory, viewed at least 100 high power fields before declaring a slide negative. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | No | The index test and reference test were undertaken by the same person. |
| Index test results blinded? All tests | No | The index test and reference test were undertaken by the same person. |
| Uninterpretable results reported? All tests | Unclear | The number of participants originally enrolled in the study was clearly stated, and corresponded to the number presented in the analysis: therefore there were no exclusions due to invalid test results. |
| Withdrawals explained? All tests | Yes | The number of participants originally enrolled in the study was clearly stated, and corresponded to the number presented in the analysis: therefore there were no withdrawals. |
Kosack 2013.
| Clinical features and settings |
Presenting signs and symptoms: Fever or a history of fever in the prior 24 hours Previous treatments for malaria: Not reported Clinical setting: 2 primary care clinics Country: Myanmar Malaria endemicity: High endemicity Malaria endemic species:P. falciparum, P. vivax |
|
| Participants |
Sample size: 2585 Age: Mean 10.9 years (SD not reported), range 0.1 to 94 years Sex: 51.3% male, 48.7% female Co‐morbidities or pregnancy: Pregnant women were not included |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for: Multi‐species malaria Reference standard test(s) used: Microscopy thick and thin smears. Who performed the reference standard tests, and where? A laboratory technician performed the reference test. Location not reported, presumably on site If microscopy was used, how many high power fields were looked at? At least 200 fields How many observers or repeats were used? Slides were sent to a malaria research centre in Thailand, for external quality control How were discrepancies between observers resolved? Not reported |
|
| Index and comparator tests |
Commercial name of the test: SD Bioline Malaria Ag P.f/Pan 05FK60 (Standard Diagnostics, Kyonggi, Republic of Korea), Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum species Designated type: Type 3 Batch numbers: Not reported Transport and storage conditions: Not reported Who performed the index test, and where? Not reported. As patients with fever or history of fever in the past 24 hours were immediately tested, presumable the RDTs were performed at the clinics. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Source of funding not reported | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | All non‐pregnant patients visiting primary care clinics with fever or a history of fever in the prior 24 hours were included. |
| Acceptable reference standard? All tests | No | It is reported that the program follows the WHO Malaria Microscopy Quality Assurance recommendation (reference provided). 1 technician read the slide on site. 900 (450 negative and 450 positive) of 2585 slides were sent to a malaria research unit in Thailand for external control. |
| Partial verification avoided? All tests | Yes | All participants had their RDT results verified by microscopic reference test. |
| Differential verification avoided? All tests | Yes | Microscopy was used for all samples. |
| Incorporation avoided? All tests | Yes | Microscopy was used for all samples. |
| Reference standard results blinded? All tests | Yes | The laboratory technician was not aware of the RDT result when examining the smear. |
| Index test results blinded? All tests | Yes | RDT was performed immediately. The slide for microscopic evaluation was prepared after the RDT was performed. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals. |
Mekonnen 2010.
| Clinical features and settings |
Presenting signs and symptoms: Febrile, clinically suspected for malaria Previous treatment for malaria: No exclusions based on previous treatment, and no relevant data presented Clinical setting: Outpatient department of a health centre Country: Ethiopia (Jimma, South‐West) ‐ 300 km south‐west of Addis Ababa, 1760 m above sea level Malaria endemicity: Not stated: transmission takes place throughout the year Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 240 Age: Eligible age range not stated. Actual age range of participants was 1 to 60 years, with a mean age of 25 years Sex: Both males and females eligible. 57.5% of the study participants were male, 42.5% female Co‐morbidities and pregnancy: Not mentioned, either as an exclusion criteria or characteristic of the included participants. Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood films Person(s) performing microscopy: Experienced malaria technicians Microscopy setting: Not stated Number of high power fields examined before declaring negative: 300 Number of observer or repeats: Discordant results between RDTs and slides were repeated. Resolution of discrepancies between observers: Not described. |
|
| Index and comparator tests |
Commerical name of RDT: CareStart Malaria Pf/Pv Combo (Access Bio Inc, Monmouth Junction, New Jersey, USA) Parasite(s) designed to detect:P. falciparum and P. vivax Designated type: Type 5 Batch numbers: Not stated Transport and storage conditions: Stored according to the guidelines of the manufacturer and quality of package desiccant was checked before use Person(s) performing RDT: Experienced malaria technicians RDT setting: Not stated |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Recieved financial support from the School of Laboratory Studies of the Jimma Univeristy and the VLIR‐IUC program between Flanders and Jimma Univeristy. Access Bio Ltd donated the CareStart Malaria Pf/Pv Combo test kit | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were attending a clinic with fever and suspected malaria, but the sampling method was not described. |
| Acceptable reference standard? All tests | Yes | Experienced technicians independently viewed 300 high power fields before declaring a slide negative. Discordant results was repeated independently. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Blinding not described. |
| Index test results blinded? All tests | Yes | "Results of the CareStart tests were determined prior to microscopic results with strict blinding to the microscopic examination of the blood film". |
| Uninterpretable results reported? All tests | Unclear | The number of participants originally enrolled in the study was clearly stated, and corresponded to the number presented in the analysis: therefore there were no exclusions due to invalid test results. |
| Withdrawals explained? All tests | Yes | The number of participants originally enrolled in the study was clearly stated, and corresponded to the number presented in the analysis: therefore there were no withdrawals. |
Metzger 2011.
| Clinical features and settings |
Presenting signs and symptoms: Not stated Previous treatments for malaria: Not mentioned, either as an exclusion criteria or characteristic of included participants Clinical setting: Health posts Country: Venezuela Malaria endemicity: "In 2007, the annual parasite index (API) was 68.4 cases/1000 inhabitants, but hot spots of higher malaria risk were seen in some indigenous ethnic groups." Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 550 Age: Not mentioned either as an inclusion criteria or a characteristic of participants Sex: Not mentioned either as an inclusion criteria or a characteristic of participants Co‐morbidities or pregnancy: Not mentioned either as an inclusion criteria or a characteristic of participants |
|
| Study design | No details of the enrolment and sampling method were reported. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: microscopy Who performed the reference standard tests, and where? Slides were examined by microscopists in health posts, then all positive and 10% of negative slides were re‐examined by microscopists at the Regional Central Laboratory. Slides were then sent to the National Amazon Centre for Research and Control of Tropical Diseases in Puerto Ayacucho for re‐examination by expert microscopists. If microscopy was used, how many high power fields were looked at? In the health posts and Regional Central Laboratory 200 fields of thick blood smears, and in the National Amazon Centre for Research and Control of Tropical Diseases the complete blood smear was read before being declared negative. How many observers or repeats were used? 3 How were discrepancies between observers resolved? Not reported |
|
| Index and comparator tests |
Commercial name of the test: OptiMAL‐IT (Diamed AG, Cressier sur Morat, Switzerland) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum malaria species only Designated type: Type 4 Batch numbers: Not stated Transport and storage conditions: Samples were transported by messengers using boat, aeroplane, motorbike, bicycle and foot transportation, sometimes taking up to 4 weeks. Due to lack of refrigerators or due to electrical power cuts, or both, samples were often exposed to local ambient conditions (study average temperature of 26.9C, with frequent peaks up to 40°C). Who performed the index test, and where? Microscopists at health posts and expert microscopists at the Amazon Centre for Research and Control of Tropical Diseases in Puerto Ayacucho. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: UNICEF | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | No details of the characteristics of the participants were reported. |
| Acceptable reference standard? All tests | Yes | 3 microscopists read the slides at either 200 fields or the complete smear before declaring a test as negative. |
| Partial verification avoided? All tests | Yes | "550 RDTs (OptiMAL‐IT) ans concomitant slides originating from the HPs of Atures municipality were received in the order of their arrival at the RCL in Puerto Ayacucho". |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Reported blinding. |
| Index test results blinded? All tests | Yes | Reported blinding. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | "36 tests had to be excluded because coding was lost during transport and/or because they could not be clearly allocated". |
Moges 2012.
| Clinical features and settings |
Presenting signs and symptoms: Suspected malaria: fever, headache, fatigue, sweating/chills/rigors, vomiting, splenomegaly, myalgia and arthralgia, anaemia, hypoglycaemia. Previous treatments for malaria: Patients who had received anti‐malarial drugs during the past 4 weeks were excluded. Clinical setting: Medical and paediatric out‐patient departments of a health centre. Country: Ethiopia Malaria endemicity: High endemicity Malaria endemic species:P. vivax and P. falciparum |
|
| Participants |
Sample size: 254 Age: Mean 21.4 (SD14.76), range 0.4 to 75 years Sex: 61% male, 39% female Co‐morbidities or pregnancy: Co‐morbities are not reported. However, critically ill patients who were unable to give blood were excluded from the study. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy thick and thin smears Who performed the reference standard tests, and where? The tests were performed by an experienced laboratory technician at the health centre and an experienced microscopist at a university hospital laboratory If microscopy was used, how many high power fields were looked at? 200 fields How many observers or repeats were used? 2 observers How were discrepancies between observers resolved? A third of discordant results, a third expert reader was used. This third reader's results were considered final. |
|
| Index and comparator tests |
Commercial name of the test: CareStart™ Malaria HRP2/pLDH COMBO (Access Bio Inc., USA) Parasite species the test is designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 3 Batch numbers: Not reported Transport and storage conditions: Not reported Who performed the index test, and where? The index test was performed at the health centre. Information on the person who performed the test is not reported. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Source of funding not reported. RDT kits were supplied by the North Gondar Zonal Health Bureau | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Subjects with suspected malaria symptoms were recruited (all symptoms reported). |
| Acceptable reference standard? All tests | Yes | Microscopy evaluations were performed by 2 independent observers (200 fields). Discordant results were referred to a third observer. |
| Partial verification avoided? All tests | Yes | All participants receiving the index test had their diagnosis verified my microscopy. |
| Differential verification avoided? All tests | Yes | Microscopy was used as reference test for all samples. |
| Incorporation avoided? All tests | Yes | The reference standard for all samples was microscopy. |
| Reference standard results blinded? All tests | Yes | The microscopists were blinded to the RDT results. |
| Index test results blinded? All tests | Unclear | The same finger‐prick blood sample used for microscopy was used to perform the index in parallel. |
| Uninterpretable results reported? All tests | Unclear | The number of participants enrolled in the study was clearly stated and corresponded to the number presented in the analysis; therefore there were no exclusions due to uninterpretable test results. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study was clearly stated and corresponded to the number included in the analysis; therefore there were no withdrawals. |
Mohon 2012.
| Clinical features and settings |
Presenting signs and symptoms: Fever Previous treatments for malaria: Not reported Clinical setting: Upazila Health Complexes Country: Bangladesh Malaria endemicity: Hypo‐endemicity Malaria endemic species: 95% P. falciparum |
|
| Participants |
Sample size: 372 Age: Median 19.4, range 1.5 to 82 years Sex: 52.8 male, 47.2% female Co‐morbidities or pregnancy: Co‐morbidities and pregnancy not reported. |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum, P. vivax Reference standard test(s) used: Microscopy thick and thin smears, nested PCR. Who performed the reference standard tests, and where? Microscopy was performed by experienced microscopists on site and the icddr,b laboratory If microscopy was used, how many high power fields were looked at? Microscopy was performed following the standard procedure. Details, not reported(reference provided). How many observers or repeats were used? 2 How were discrepancies between observers resolved? Not reported |
|
| Index and comparator tests |
Commercial name of the test: OnSite (Pf /Pan) (CTK Biotech Inc, USA) Parasite species the test is designed to detect:P. falciparum, non‐falciparum, P. vivax Designated type: Type 3 Batch numbers: Not reported Transport and storage conditions: Not reported Who performed the index test, and where? The index test was performed at the icddr,b Parasitology Laboratory |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: International Centre for Diarrheal Research Bangladesh (icddr,b) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Febrile patients were recruited. However, patients with mixed infections and those with discordant microscopy/PCR results were excluded and the numbers excluded for these reasons are not stated. |
| Acceptable reference standard? All tests | Yes | Microscopy was verified with PCR. |
| Partial verification avoided? All tests | Yes | All participants that received the index test had their diagnosis verified by reference test. |
| Differential verification avoided? All tests | Yes | PCR adjusted microscopy was used as the reference test. |
| Incorporation avoided? All tests | Yes | PCR adjusted microscopy was used as the reference test. |
| Reference standard results blinded? All tests | Unclear | Blinding not reported. |
| Index test results blinded? All tests | Unclear | Blinding not reported. |
| Uninterpretable results reported? All tests | No | The number of participants originally enrolled in the study was not explicitly stated; therefore it is unclear whether there were any exclusions due to uninterpretable test results. |
| Withdrawals explained? All tests | Unclear | The number of participants originally enrolled in the study was not explicitly stated; therefore it is unclear whether there were any withdrawals. |
Pattanasin 2003.
| Clinical features and settings |
Presenting signs and symptoms: Fever or history of fever and suspected diagnosis of uncomplicated malaria Previous treatment for malaria: No mention of previous treatment for malaria, either as an exclusion criteria or a characteristic of included participants Clinical setting: Not stated Country: Thailand (Mae Sod) Malaria endemicity: Not stated, peak transmission season Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 271 Age: Children aged under 2 years were excluded. The study included participants aged 2 to 81 years; 71% were aged under 15 years Sex: Male: female ratio was 1.7:1 Co‐morbidities and pregnancy: Pregnant women were excluded Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was not described. 2 RDTs were evaluated, the vast majority of participants received both RDTs. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood film Person(s) performing microscopy: Not stated Microscopy setting: Not stated Number of high power fields examined before declaring negative: Not stated Number of observer or repeats: Not stated Resolution of discrepancies between observers: Not applicable |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:
Designated type:
Batch numbers: Not stated Transport and storage conditions: Kept at room temperature and opened just before performing the test to avoid humidity Person(s) performing RDT: Not stated RDT setting: Not stated |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants had a fever and suspected malaria, but the exact clinical setting and the sampling method were not described. |
| Acceptable reference standard? All tests | Unclear | No details of the microscopy process were given. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | Blinding not described. |
| Index test results blinded? All tests | Yes | Test results were recorded without reference to the microscopy results. |
| Uninterpretable results reported? All tests | Yes | Doubtful and invalid results were reported (5 of 271). |
| Withdrawals explained? All tests | Unclear | Almost all participants were reported to receive the same index and reference tests (271 participants in total, 266 received OptMAL, 269 received Paracheck‐Pf); the numbers presented in the analysis correspond. |
Rakotonirina 2008.
| Clinical features and settings |
Presenting signs and symptoms: Fever over 37.5°C or history of fever in the previous 24 hours Previous treatment for malaria: Participants with recent antimalarial use were not excluded from the study; 34% of participants declared antimalarial use Clinical setting: 2 primary health centres Country: Madagascar (Tsiroanomandidy on the west foothill areas of the Highlands) Malaria endemicity: Low and predominantly seasonal Malaria endemic species:P. falciparum (80%) andP. vivax |
|
| Participants |
Sample size: 313 Age: All age groups were eligible for inclusion; the actual age range of the included participants was 6 months to 79 years (median age 10 years) Sex: Male: female ratio was 1.2:1 Co‐morbidities and pregnancy: Pregnant women were excluded, as were people with signs of severe or complicated malaria Parasite density of microscopy positive cases: Range 32 to 52,750 parasites per µL, mean 4104, SD 7894 |
|
| Study design | Enrolment was consecutive and prospective. 2 RDTs were evaluated, all participants received both RDTs. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: PCR |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:
Designated type:
Batch numbers:
Transport and storage conditions: Transported and maintained at the study sites (primary health centres) at room temperature and opened just before use to avoid humidity damage Person(s) performing RDT: Trained technician RDT setting: Primary health centres |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Global Fund Project for Madagascar, Round 3 | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive sample of patients attending primary health centres with fever or history of fever in the previous 24 hours. |
| Acceptable reference standard? All tests | Yes | Reference standard was PCR. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Stated that the PCR operator was blind to the results of the other tests performed. |
| Index test results blinded? All tests | Yes | Stated that the test readers were blind to the results of the other tests performed. |
| Uninterpretable results reported? All tests | Yes | There were no test failures with either RDT. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study is clearly stated and corresponds to the number presented in the analysis. |
Ratsimbasoa 2007.
| Clinical features and settings |
Presenting signs and symptoms: Fever over 37.5°C or history of fever in the previous 24 hours, with typical malaria symptoms. Patients with signs of severe or complicated malaria were excluded. Previous treatment for malaria: Participants with recent antimalarial use were not excluded from the study; 17% of participants reported antimalarial use Clinical setting: Primary health centres Country: Madagascar. Rural areas of Mahasolo (western foothills areas of the highlands) and Saharevo (eastern foothills areas of the highlands) Malaria endemicity: Low and predominantly seasonal in both areas Malaria endemic species: Predominantly P. falciparum; someP. vivax |
|
| Participants |
Sample size: 194 Age: All groups eligible for inclusion not stated; actual age range of the included participants was 1 to 79 years (mean age 15.2 years). 12.9% were under 5 years of age Sex: Male: female ratio was 0.98:1 Co‐morbidities and pregnancy: Pregnant women were excluded, as were people with signs of severe or complicated malaria Parasite density of microscopy positive cases: Range 16 to 233,600 parasites per µL, mean 6564, SD 26,553 |
|
| Study design | Enrolment was prospective. The sampling method was not described. 2 RDTs were evaluated, all participants received both RDTs. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood films Person(s) performing microscopy: An experienced technician Microscopy setting: Not stated Number of high power fields examined before declaring negative: 200 Number of observer or repeats: 1 Resolution of discrepancies between observers: Not applicable |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 4 Batch numbers:
Transport and storage conditions: Transported and maintained at the study sites (primary health centres) at room temperature and opened just before use to avoid humidity damage Person(s) performing RDT: A technician RDT setting: Not stated |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Global Fund Project for Madagascar, Round 3. The manufacturers supplied the test kits. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were attending primary health centres with fever and symptoms of malaria, but the sampling method was not described. |
| Acceptable reference standard? All tests | No | An expert technician viewed 200 high power fields before declaring a slide negative; however their findings were not verified by a second independent reader. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "Analyzed without reference to the RDT results". |
| Index test results blinded? All tests | Yes | The RDTs were undertaken before the microscopy. |
| Uninterpretable results reported? All tests | Unclear | The number recruited into the study was clearly stated, and corresponded with the number included in the analysis. |
| Withdrawals explained? All tests | Yes | The number recruited into the study was clearly stated, and corresponded with the number included in the analysis. |
Ratsimbasoa 2008.
| Clinical features and settings |
Presenting signs and symptoms: Fever or fever in the previous 24 hours with typical malaria symptoms Previous treatment for malaria: Participants with recent antimalarial use were not excluded from the study; 13% of participants declared antimalarial use Clinical setting: Primary Health Centre Country: Madagascar (Ampasimpotsy, Central Highlands) Malaria endemicity: Transmission is low and predominantly seasonal. This study was carried out in the low season Malaria endemic species:P. falciparum (approximately 75%) andP. vivax |
|
| Participants |
Sample size: 200 Age: Eligible age range not stated; actual age range of the included participants was 6 months to 73 years (40% under 5 years, 26.5% 5 to 15 years) Sex: Male: female ratio was 1.2:1 Co‐morbidities and pregnancy: Pregnant women were excluded, as were people with signs of severe or complicated malaria Parasite density of microscopy positive cases: Range 16 to 285,000 parasites per µL, mean 16,757, SD 42,631 |
|
| Study design | Enrolment was prospective. The sampling method was not described. 2 RDTs were evaluated, all participants received both RDTs. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: PCR |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:
Designated type:
Batch numbers:
Transport and storage conditions: All tests were kept at room temperature and opened just before use to avoid humidity damage. Person(s) performing RDT: Not stated RDT setting: Not stated |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Kozone, representing Standard Diagnostics Inc in Madagascar | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Participants were all attending a health centre with fever and typical symptoms of malaria, but the sampling method was not described. |
| Acceptable reference standard? All tests | Yes | The reference standard was PCR. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | PCR was carried out by technicians blind to the results of RDT testing. |
| Index test results blinded? All tests | Yes | RDTs were undertaken before the results of PCR were known |
| Uninterpretable results reported? All tests | Yes | Uninterpretable results are reported and excluded from the analysis. There were 2 invalid results for Bioline Pf and 1 for Bioline Pf/Pan. |
| Withdrawals explained? All tests | No | There was 1 participant missing from the analysis from Bioline Pf/Pan, with no explanation. |
Samane 2010.
| Clinical features and settings |
Presenting signs and symptoms: Suspected malaria with symptoms including fever or chills of several days, or both Previous treatments for malaria: Not mentioned, either as an exclusion criteria or characteristic of included participants Clinical setting: Health centres Country: Iran Malaria endemicity: Not stated Malaria endemic species: Not stated |
|
| Participants |
Sample size: 250 Age: Not mentioned either as an inclusion criteria or a characteristic of participants Sex: Not mentioned either as an inclusion criteria or a characteristic of participants Co‐morbidities or pregnancy: Not mentioned either as an inclusion criteria or a characteristic of participants |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum andP. vivax Reference standard test(s) used: Microscopy Who performed the reference standard tests, and where? Experienced microscopists performed the test, it is not stated where this was done. If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? 2 How were discrepancies between observers resolved? Not stated |
|
| Index and comparator tests |
Commercial name of the test: BIOTEC Malaria Pv/Pf Rapid Device Parasite species the test is designed to detect:P. falciparum andP. vivax Designated type: Other type. HRP‐2 for P. falciparum and pLDH for P. vivax. Batch numbers: Not stated Transport and storage conditions: Not reported Who performed the index test, and where? Not reported |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Characteristics of participants not adequately described, although all had symptoms of malaria. |
| Acceptable reference standard? All tests | Unclear | 2 independent microscopists viewed the slides, but the number of fields viewed was not reported. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference tests. |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference test. |
| Reference standard results blinded? All tests | Yes | Reported that the tests were read blindly. |
| Index test results blinded? All tests | Yes | Reported that the tests were read blindly. |
| Uninterpretable results reported? All tests | No | Number enrolled in the study was explicitly stated but did not correspond to the number presented in the analysis ‐ 250 patients were enrolled but 276 were included in the analysis. |
| Withdrawals explained? All tests | Unclear | Number enrolled in the study was explicitly stated but did not correspond to the number presented in the analysis ‐ 250 patients were enrolled but 276 were included in the analysis. |
Selimuzzaman 2010.
| Clinical features and settings |
Presenting signs and symptoms: Fever with oral temperature 100 °F or more or with convincing history of fever Previous treatments for malaria: "Patients taking anti‐malarial drugs for current illness or providing history of anti‐malarial therapy within previous four weeks or taking anti‐malarial prophylaxis were excluded from the study" Clinical setting: Sick bay of 37 Rifle Battalion Headquarters Country: Bangladesh Malaria endemicity: "Malaria endemic zone" Malaria endemic species: Not stated |
|
| Participants |
Sample size: 271 Age: Ranged from 18 years to 57 years Sex: Male Co‐morbidities or pregnancy: Not mentioned either as an inclusion criteria or a characteristic of participants |
|
| Study design |
Were participants consecutively enrolled in the study?: Yes
Were they enrolled prospectively?: Yes If the study evaluated more than one RDT, how were tests allocated to individuals, or did each individual receive all the tests? 1 RDT was evaluated |
|
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax malaria Reference standard test(s) used: Microscopy thick and thin blood films Who performed the reference standard tests, and where? Experienced microscopists at Armed Forces Medical College in Dhaka examined the slides. If microscopy was used, how many high power fields were looked at? 200 How many observers or repeats were used? 1 How were discrepancies between observers resolved? Only 1 microscopist read each slide |
|
| Index and comparator tests |
Commercial name of the test: MALARIGEN MALARIA Pf/Pv Antigen Rapid Test (Biotest Diagnostic Corp., Denville, NJ, USA) Parasite species the test is designed to detect:P. falciparum, P. vivax malaria Designated type: Unclear, HRP‐2 antigen of P. falciparum and unspecified monoclonal antibodies for detection of non‐falciparum malarial parasites. Batch numbers: Not stated Transport and storage conditions: Not stated Who performed the index test, and where? Experienced microscopists at Armed Forces Medical College in Dhaka examined the RDT kits. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive series of patients with clinical signs and symptoms of malaria. |
| Acceptable reference standard? All tests | No | One microscopist read each slide. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference tests. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standards. |
| Reference standard results blinded? All tests | Unclear | Described as a single blinded study, but no further details reported. |
| Index test results blinded? All tests | Unclear | Described as a single blinded study, but no further details reported. |
| Uninterpretable results reported? All tests | Yes | "Three out of 271 (1.11%) cases did not demonstrate control band or become positive only after a long time lag and were excluded from the study. Thin blood films of 6 patients (2.21%) were marked by the microscopist as poor quality and were excluded from the study." |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study is clearly stated and corresponds to the number presented in the analysis minus the number reported to have invalid test results or incomplete data. |
Sharew 2009.
| Clinical features and settings |
Presenting signs and symptoms: Febrile patients, clinically suspected for malaria Previous treatment for malaria: No exclusions based on previous treatment. Information on previous treatment collected, but actual data not provided. Clinical setting: Outpatient departments of 2 health centres Country: Ethiopia (Southern ‐ Wondo Genet) Malaria endemicity: Takes place throughout the year Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 668 Age: All age groups eligible. Actual age range 6 months to 75 years. Sex: 361 (54%) males, 307 (46%) females Co‐morbidities and pregnancy: No exclusion criteria based on co‐morbidities or pregnancy. No details of the frequency of these conditions in the participant population is presented. Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was consecutive and prospective. 2 different RDTs were evaluated, and each participant received both tests. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood films Who performed the reference standard tests, and where? Experienced malaria technicians. The microscopy setting was not stated, but in the Wondo Genet area If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 2 independent technicians, also checked by the team leader How were discrepancies between observers resolved? All discordant results between microscopy and RDTs were repeated. |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:P. falciparum
Designated type:
Batch numbers: Not stated Transport and storage conditions: As per the instructions of the manufacturer Person(s) performing RDT: Not stated RDT setting: 2 health centres |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: School of Graduate Studies of the Addis Adaba University through the Graduate Programme in Tropical and Infectious Diseases, Aklilu Lemma Institute of Pathobiology and from the Federal Ministry of Health of Ethiopia. Federal Ministry of Health of Ethiopia and Access Bio Inc donated the test kits. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive sample of febrile patients attending health centres with suspected malaria. |
| Acceptable reference standard? All tests | Yes | 2 experienced microscopists independently viewed 100 high power fields before declaring a slide negative. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | Not described. |
| Index test results blinded? All tests | Yes | Strict blinding with the results available before microscopy reported. |
| Uninterpretable results reported? All tests | Yes | If a test was un‐interpretable then it was repeated. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study was clearly stated and corresponds to the number included in the analysis; therefore there were no withdrawals. |
Singh 2000a.
| Clinical features and settings |
Presenting signs and symptoms: Fever suspected to be malaria Previous treatment for malaria: There were no exclusions based on previous treatment, and no information presented; this was an outbreak in a rural area Clinical setting: Mobile field laboratory Country: India (forest villages in Chhindwara, central India) Malaria endemicity: Outbreak situation Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 344 Age: All age groups eligible. Actual age range 6 months to 65 years Sex: Both males and females eligible. Actual proportions of males and females in the participant population not stated Co‐morbidities and pregnancy: No exclusion criteria based on co‐morbidities or pregnancy. No details of the frequency of these conditions in the participant population is presented. Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was consecutive and prospective. 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick blood film Who performed the reference standard tests, and where? Experienced microscopist for all slides; expert microscopist for re‐examined slides. Setting was a mobile field laboratory for all slides; Malaria Research Centre at Jabalur for re‐examined slides If microscopy was used, how many high power fields were looked at? Not stated. However, 200 white blood cells were counted as an alternative indicator; or 500 WBCs for slides that were re‐examined How many observers or repeats were used? 1, but negative blood smears were re‐examined if the patient was having severe symptoms, the corresponding RDT result was positive or if P. vivax was diagnosed How were discrepancies between observers resolved? Not described, most likely accepted the findings of second microscopist |
|
| Index and comparator tests |
Commerical name of RDT: ICT Malaria Pf/Pv (AMRAD, Australia) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 2 Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: Field laboratory assistants RDT setting: Mobile field laboratory |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Becton Dickinson provided financial support and supplied the RDTs free of charge | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | All participants were attending an ambulatory setting with fever suspected to be malaria, and enrolment was consecutive. |
| Acceptable reference standard? All tests | No | Microscopy was undertaken by 1 microscopist only; and the number of high power fields viewed was unclear (200 white blood cells). |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "Blood films were examined...without reference to the results of ICT". |
| Index test results blinded? All tests | Yes | "All specimens were tested...who were blinded to the results of the blood smear tests". |
| Uninterpretable results reported? All tests | Unclear | The numbers of participants originally enrolled in the study was clearly stated and the numbers presented in the analysis correspond; therefore there were no exclusions due to uninterpretable test results. |
| Withdrawals explained? All tests | Yes | The numbers of participants originally enrolled in the study was clearly stated and the numbers presented in the analysis correspond; therefore there were no withdrawals. |
Singh 2003.
| Clinical features and settings |
Presenting signs and symptoms: Fever or history of fever Previous treatment for malaria: No explicit exclusions based on previous treatment, and no data reported Clinical setting: Hospital malaria clinic Country: India, Jabalpur Malaria endemicity: Not stated Malaria endemic species:P. falciparum and P. vivax in roughly equal proportions |
|
| Participants |
Sample size: 80 Age: All age groups eligible. Adults and children included; mean age 27.7 (SD 16.42) for males and 29 (SD 12.8) for females Sex: Both males and females eligible; included 28 males and 18 females Co‐morbidities and pregnancy: No explicit exclusion criteria based on co‐morbidities or pregnancy. No details of the frequency of these conditions in the participant population is presented. Parasite density of microscopy positive cases: Range 40 to 370,574 parasites per µL for P. falciparum and 318 to 9970 for P. vivax |
|
| Study design | Enrolment was prospective. The sampling method was not described. Only 1 RDT was evaluated. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick blood films Who performed the reference standard tests, and where? Not stated. Setting was a hospital laboratory If microscopy was used, how many high power fields were looked at? Not stated How many observers or repeats were used? If the results of the OptiMAL conflicted with that of microscopy for any sample, the blood smear was re‐examined by a different technician How were discrepancies between observers resolved? If the re‐examination of discordant results gave a different result to the first examination, the second results was confirmed by yet another technician |
|
| Index and comparator tests |
Commerical name of RDT: OptiMAL Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 4 Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: A technician RDT setting: Hospital clinic or laboratory |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Participants were all attending a clinic with fever or history of fever, but the sampling method was not described. |
| Acceptable reference standard? All tests | Unclear | Discordant results between RDT and microscopy were re‐examined; however the number of high power fields viewed before declaring a sample negative was not stated. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | Blinding not described. |
| Index test results blinded? All tests | Yes | Technican were blinded to the results of the blood smear examination. |
| Uninterpretable results reported? All tests | Unclear | The numbers of participants originally enrolled in the study was clearly stated and the numbers presented in the analysis correspond; therefore there were no exclusions due to uninterpretable test results. |
| Withdrawals explained? All tests | Yes | The numbers of participants originally enrolled in the study was clearly stated and the numbers presented in the analysis correspond; therefore there were no withdrawals. |
Singh 2010.
| Clinical features and settings |
Presenting signs and symptoms: Clinical suspicion of malaria Previous treatments for malaria: Patients were excluded due to recent anti‐malarial intake Clinical setting: Field clinic Country: India Malaria endemicity: Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 409 Age: All ages were included. Mean age was 15 (SD 14). Sex: Both sexes were included, ratio was not reported. Co‐morbidities or pregnancy: Pregnant women were excluded from participating. Co‐morbidities not mentioned either as an inclusion criteria or a characteristic of participants. |
|
| Study design | Enrolment was prospective and consecutive. 5 RDTs were evaluated; each participant received all the tests. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy and PCR Who performed the reference standard tests, and where? Microscopy was conducted by an experienced microscopist in the laboratory. PCR was also performed in the laboratory, by an independent research assistant. If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 1 How were discrepancies between observers resolved? "All negative slides that test positive on the RDT/PCR or all positive slides that test negative on the RDT/PCR were re‐examined by another expert technician blinded to the results of microscopy, RDT/PCR and clinical status of the patients." |
|
| Index and comparator tests |
Commercial name of the test:
Parasite species the test is designed to detect:
Designated type:
Batch numbers: Not stated Transport and storage conditions: "RDTs were stored at 25°C on receipt in the study sites, then allocated to separate groups for storage at 35°C & 45°C for 90 days, at 60°C for 48 hours, and at ‐10°C for 60 minutes before testing. At the start of the study, the incubators were stabilized at the required temperature for three days before the RDTs to be tested were placed inside. RDTs were removed from storage to reach room temperature for 2 hours before testing and comparisons were made with control RDTs kept at 25°C until use and with microscopy." Who performed the index test, and where? 2 research assistants tested in the RCTs in field in 10 villages of Satanwada Primary Health Centre. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: WHO Country Office, New Delhi, India | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants were a consecutive series of patients attending clinics with clinical signs and symptoms of malaria. |
| Acceptable reference standard? All tests | No | Only 1 microscopist used, except in cases of discordant results between microscopy and RDT. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference tests were used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standards. |
| Reference standard results blinded? All tests | Yes | Reported that the tests were read blindly. |
| Index test results blinded? All tests | Yes | Reported that the tests were read blindly. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | 37 patients (9%) were excluded as not fulfilling the study enrolment criteria due to recent anti‐malarial intake. |
Tjitra 1999.
| Clinical features and settings |
Presenting signs and symptoms: Symptomatic with a presumptive clinical diagnosis of malaria: fever or history of fever in the last 24 hours and no other obvious cause of fever Previous treatment for malaria: Prior use of antimalarials was not an exclusion criteria. Approximately half of the participants reported use of antimalarials within the previous 4 weeks Clinical setting: Primary health centre Country: Indonesia (Laratama sub district, West Sumba, East Nusa Tenggara Province, Eastern Indonesia) Malaria endemicity: Infection rate in children 0 to 9 years of 5.1% Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 560 Age: All ages eligible. Actual age range of the participants 0 to 80 years Sex: Males and females eligible; 289 males and 271 females included Co‐morbidities: Not mentioned either as an exclusion criteria or a characteristic of the included participants Parasite density of microscopy positive cases:P. vivax mean 7157 parasites per µL |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was tested. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood smears Who performed the reference standard tests, and where? Expert microscopists with over 20 years experience each. The setting was one local (exact setting not stated); cross‐checking was done in Darwin, Australia If microscopy was used, how many high power fields were looked at? at least 100 for all slides, at least 200 for those cross‐checked How many observers or repeats were used? 1 observer for the majority of slides; discordant results between microscopy and RDT and 20% of slides with concordant results were cross‐checked by a 2nd microscopist, blind to the results of 1st microscopy and RDT How were discrepancies between observers resolved? Not described |
|
| Index and comparator tests |
Commerical name of RDT: ICT Malaria Pf/Pv Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 2 Batch numbers: 100088 for the first 393 tests, and 041388 for the remaining 167 tests Transport and storage conditions: Not described Person(s) performing RDT: Performed by trained health workers and read by a study physician blinded to the microscopy results RDT setting: Primary health centre |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Financial assistance received from the Northern Territory Government 50th Anniversary of Indonesian Independence Malaria‐Tuberculosis Research Fellowships. ICT Pf/Pv kits and some logistical costs were supported by AMRAD‐ICT Sydney, New South Wales, Australia | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Participants were all attending a primary health care centre with fever and symptoms of malaria, but the sampling method was not described. |
| Acceptable reference standard? All tests | Yes | All slides were read by an experienced microscopist viewing at least 100 high power fields, and results discordant with RDT were re‐examined by another, independent microscopist. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "The microscopist was unaware of the immunochromatographic test result". |
| Index test results blinded? All tests | Yes | "The results were read by a study physician who was blinded to the microscopy results". |
| Uninterpretable results reported? All tests | Unclear | The number of participants enrolled in the study was clearly stated and correspond to the number presented in the analysis; therefore there were no exclusions due to uninterpretable test results. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study was clearly stated and corresponded to the number included in the analysis; therefore there were no withdrawals. |
Trouvay 2013.
| Clinical features and settings |
Presenting signs and symptoms: Febrile patients who consulted for suspected malaria Previous treatment for malaria: Not reported on, but there were no exclusion criteria based on antimalarial use Clinical setting: Not clear Country: French Guiana Malaria endemicity: At a low number of focal points on the coast, associated with gold mining Malaria endemic species: 31% P. falciparum and 68.5% P. vivax. P. malariae cases are occasional. |
|
| Participants |
Sample size: 960 Age: All ages eligible. Actual age range of the participants 1 to 92 years (median age 25.8 years) Sex: Males and females eligible; ratio of male to female was 1.2:1 Co‐morbidities: Not mentioned either as an exclusion criteria or a characteristic of the included participants Parasite density of microscopy positive cases:P. vivax mean 0.11% |
|
| Study design | Enrolment was prospective, with all eligible participants were included. 1 RDT was tested. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood smears Who performed the reference standard tests, and where? An expert microscopist at Cayenne Hospital laboratory If microscopy was used, how many high power fields were looked at? 200 fields in the thin film How many observers or repeats were used? 1 observer. How were discrepancies between observers resolved? Not applicable. However, PCR was conducted on samples where microscopy and RDT gave different results |
|
| Index and comparator tests |
Commerical name of RDT: SD malaria Ag Pf/Pan Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 3 Batch numbers: not stated Transport and storage conditions: Tests were guaranteed to have been stored at the correct temperature (24 to 28C) and were used within their recommended shelf life. Person(s) performing RDT: Technician RDT setting: Cayenne Hospital |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: French Ministry of Health | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | All febrile patients who consulted with suspected malaria during a prospective study were initially included. P. malariae cases were subsequently excluded, however, only 3 of 960 enrolled participants were excluded for this reason. |
| Acceptable reference standard? All tests | No | Only 1 microscopist was used. In case of discordant results between RDT and microscopy, PCR was used to determine infections and species. However, the PCR results were not used to adjust the microscopy results. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | The microscopic examination was carried out simultaneously. |
| Index test results blinded? All tests | Yes | Interpretation of the test was carried out independently of the microscopic examination. |
| Uninterpretable results reported? All tests | Yes | No invalid RDTs were observed. |
| Withdrawals explained? All tests | Yes | There were 3 exclusions post‐enrolment, due to P. malariae infection. |
Valecha 2003.
| Clinical features and settings |
Presenting signs and symptoms: Fever or history of fever Previous treatment for malaria: Not mentioned, either as an exclusion criteria or a characteristic of included participants Clinical setting: Malaria clinics and village health workers Country: India (Delhi, Nadiad, Jabalpur and Sonapur) Malaria endemicity: 4 sites of different endemicities Malaria endemic species:P. falciparum and P. vivax |
|
| Participants |
Sample size: 699 Age: All ages eligible; age range of included participants 1 to 75 years (mean 22.8) Sex: Included 395 males and 304 females Co‐morbidities: Not mentioned, either as an exclusion criteria or a characteristic of included participants Parasite density of microscopy positive cases:P. vivax range 40 to 44,000 parasites/µL, median 1020, P. falciparum range 120 to 68,480 parasites/µL, median 2000 |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was tested. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy Who performed the reference standard tests, and where? Microscopist. Setting was not stated If microscopy was used, how many high power fields were looked at? 100 How many observers or repeats were used? 1 for most slides. All results discordant with RDT results and 20% of concordant results were cross‐checked. Negative slides which tested positive by kit were re‐examined by counting up to 2000 WBCs. How were discrepancies between observers resolved? In the case of initially negative slides looked at in more detail because of discordant results, the second reading was taken as true. |
|
| Index and comparator tests |
Commerical name of RDT: OptiMAL (DiaMed, AG, Cressier, Switzerland) Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 4 Batch numbers: 46050.24.05 Transport and storage conditions: Stored below 30°C Person(s) performing RDT: Not stated RDT setting: At the study sites (clinic and villages) |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Not stated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were all attending clinics or approaching village health workers with fever or history of fever, but the sampling method was not described. |
| Acceptable reference standard? All tests | Unclear | Microscopists viewed 100 high power fields before declaring a slide negative, and results discordant with RDTs were cross‐checked. However, it is not clear whether the person doing the cross‐checking was a different microscopist working independently. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "Microscopists were blinded to the rapid test results". |
| Index test results blinded? All tests | Yes | The RDT was done before the microscopy. |
| Uninterpretable results reported? All tests | No | The number of participants originally enrolled in the study was not explicitly stated; therefore it is unclear whether there were any exclusions due to uninterpretable test results. |
| Withdrawals explained? All tests | Unclear | The number of participants originally enrolled in the study was not explicitly stated; therefore it is unclear whether there were any withdrawals. |
van den Broek 2006.
| Clinical features and settings |
Presenting signs and symptoms: New episode of suspected malaria, which could include fever, history or other complaints indicating possible malaria infection Previous treatment for malaria: Excluded if malaria confirmed (treated or untreated) within the previous 4 weeks Clinical setting: Malaria outpatient centre Country: Colombia Malaria endemicity: Hypoendemic, annual parasite rate 2 to 5% Malaria endemic species:P. vivax (54%) P. falciparum (46%) |
|
| Participants |
Sample size: 896 Age: All ages eligible. Actual numbers of children and adults not stated, although the report mentions that many workers were included, Sex: Both males and females eligible. Most of the participants were male (646, 79%) Co‐morbidities and pregnancy: No exclusions criteria based on co‐morbidities. No details of the frequency of these conditions in the participant population is presented. Parasite density of microscopy positive cases: Geometric mean approximately 2300 parasites per µL for both P. falciparum and P. vivax |
|
| Study design | Enrolment was prospective. The sampling method was not described. 3 RDTs were tested. All individuals received all 3 tests. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood smears Person(s) performing microscopy: Well trained, experienced microscopists Microscopy setting: Not stated Number of high power fields examined before declaring negative: At least 200 Number of observer or repeats: 1, except for about one third of the slides (especially low density parasitaemias and mixed infections). In this case, another microscopist viewed the slide and discordant results between microscopists or between slides and RDTs were sent to the University of Antioquia for external cross‐checking. Resolution of discrepancies between observers: Disagreements between the internal and external results were sent to a third laboratory, of the National Health Institute in Bogota. In cases where both external laboratories disagreed with the internal laboratory, results were corrected accordingly. |
|
| Index and comparator tests |
Commerical name of RDT:
Parasite(s) designed to detect:
Designated type:
Batch numbers: Not stated Transport and storage conditions: Not described Person(s) performing RDT: A bacteriologist. Where the result was ambiguous, 2 bacteriologists read the test results. RDT setting: At the malaria centre |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Medicins Sans Frontières, Holland, and its donors. The American Society of Tropical Medicine and Hygiene assisted with publication expenses. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were patients presenting with suspected malaria, but the sampling method was not described. |
| Acceptable reference standard? All tests | No | Microscopists viewed at least 200 high power fields before declaring a slide negative; however the findings were only verified by a second independent reader for a third of slides. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Report states that microscopists were blinded to the results of RDTs. |
| Index test results blinded? All tests | Yes | Report states that RDTs were blinded to the results of microscopy. |
| Uninterpretable results reported? All tests | Yes | There were no uninterpretable results; and weak lines were scored as positive. |
| Withdrawals explained? All tests | Unclear | The number of participants originally enrolled in the study was not explicitly stated; therefore it was not possible to assess whether there were any withdrawals. |
Wongsrichanalai 2003.
| Clinical features and settings |
Presenting signs and symptoms: Oral temperature over 38°C, headache or a history of fever in the previous 72 hours Previous treatment for malaria: No exclusions based on previous episodes or treatment for malaria; no data presented on recent antimalarial use in the children Clinical setting: Malaria clinics Country: Thailand (Maesod) Malaria endemicity: Not stated Malaria endemic species:P. falciparum andP. vivax. |
|
| Participants |
Sample size: 246 Age: Inclusion criteria stipulated over 20 years old Sex: Both males and females were eligible Co‐morbidities and pregnancy: Not mentioned, either as an exclusion criteria or characteristic of the included participants Parasite density of microscopy positive cases: Not presented |
|
| Study design | Enrolment was prospective. The sampling method was not described. 1 RDT was tested. | |
| Target condition and reference standard(s) |
Target condition: Malaria parasitaemia Reference standard: Microscopy thick and thin blood smears Who performed the reference standard tests, and where? Experienced microscopists at the Armed Forces Research Institute of Medical Sciences If microscopy was used, how many high power fields were looked at? 200 How many observers or repeats were used? 2 independent observers, blinded to each others findings How were discrepancies between observers resolved? Resolved by a third expert microscopist, whose reading was accepted as final. Where there was species discrepancy between microscopy and NOW ICT, PCR was done. |
|
| Index and comparator tests |
Commerical name of RDT: NOW ICT Malaria Pf/Pv Parasite(s) designed to detect:P. falciparum or mixed infection, non‐falciparum species only Designated type: Type 2 Batch numbers: 030611 Transport and storage conditions: Not described Person(s) performing RDT: Technician RDT setting: Armed Forces Research Institute of Medical Sciences |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: US Army Medical Material Development Activity | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | All participants were attending malaria clinics with temperature over 38°C, headache or a history of fever in the previous 72 hours, but the sampling method was not adequately described. |
| Acceptable reference standard? All tests | Yes | 2 independent microscopists at a research laboratory viewed at least 200 high power fields before declaring a slide negative. |
| Partial verification avoided? All tests | Yes | All participants who received the index test also received the reference test. |
| Differential verification avoided? All tests | Yes | The same reference test was used regardless of the index test results. |
| Incorporation avoided? All tests | Yes | The index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | "read by two microscopists blinded to...the NOW ICT results". |
| Index test results blinded? All tests | Yes | The RDT was carried out before microscopy. |
| Uninterpretable results reported? All tests | Yes | The RDTs had to be repeated in 39 of 285 assays. A successful test was eventually completed for each sample. |
| Withdrawals explained? All tests | Yes | The number of participants enrolled in the study was clearly stated and corresponded with the number included in the analysis, indicating no withdrawals. |
Xiaodong 2013.
| Clinical features and settings |
Presenting signs and symptoms: Suspected malaria Previous treatments for malaria: Not reported. Clinical setting: TengChong CDC, China and Health Unlimited clinic in Myanmar (China‐Myanmar border). Country: China, Myanmar Malaria endemicity: Endemic Malaria endemic species:P. falciparum,P. vivax |
|
| Participants |
Sample size: 241 Age: Mean 29.62 (11.21), range 3 to 58 years Sex: 78.01% male, 21.99% female Co‐morbidities or pregnancy: Not reported |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. The study evaluated 1 RDT. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for:P. falciparum and P. vivax Reference standard test(s) used: Microscopy (thick and thin blood smears) corrected by PCR assays. Who performed the reference standard tests, and where? The microscopic evaluation was done by experienced microscopists. Place not reported. Not reported for PCR. If microscopy was used, how many high power fields were looked at? 100 fields How many observers or repeats were used? 2 independent microscopists. Also, a double‐blind cross reading of a random 50 blood slides was performed by a senior microscopist. How were discrepancies between observers resolved? In the case of discordant results between microscopy and PCR, the results of PCR were used as the standard method. |
|
| Index and comparator tests |
Commercial name of the test: CareStart malaria HRP2/pLDH (Pf/pan) combo test Parasite species the test is designed to detect: Multi species Designated type: Type 3 Batch numbers: C201R Transport and storage conditions: Not reported. Who performed the index test, and where? 3 health worker‐observers. Place not reported. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: Source of funding not reported. CDC (Chinese Center for Disease Control and Prevention). It is stated that individual biodata and malaria history in the previous 1 year were documented from each suspected case. However, co‐morbidities and treatment history have not been reported. Index test was performed by 3 health worker‐observers: the first observer performed readings at 20 minutes (recommended by the manufacturer) and the other 2 observers, within the next 10 minutes. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Consecutive patients with suspected malaria were enrolled. Then all patients who were positive for malaria by microscopy and a random sample of negative samples were included in the analysis. |
| Acceptable reference standard? All tests | Yes | Microscopy was undertaken by 2 independent experienced microscopists (100 fields) and species identifications was conformed PCR assays. |
| Partial verification avoided? All tests | Yes | All participants receiving the index tests had their diagnosis verified by reference standard. |
| Differential verification avoided? All tests | Yes | Microscopy was used as a reference standard for all samples, regardless of index test. |
| Incorporation avoided? All tests | Yes | Microscopy and PCR was used. |
| Reference standard results blinded? All tests | Unclear | Blinding of microscopists not reported. |
| Index test results blinded? All tests | Yes | The 3 observers were blinded to each other's readings and to the results of microscopy and PCR assay. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. In case the index test result was considered invalid, the test was repeated. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals |
Yan 2013.
| Clinical features and settings |
Presenting signs and symptoms: Suspected uncomplicated malaria, fever with axillary temperature above 37.5°C at the time of examination. Previous treatments for malaria: Not reported Clinical setting: Local malaria clinics and hospitals at the Laiza township. Country: Myanmar (China‐Myanmar border) Malaria endemicity: Endemic. Seasonal; mostly in the rainy season from April to November. Malaria endemic species: Predominantly P. falciparum and P. vivax |
|
| Participants |
Sample size: 606 Age: Median 20.3 years, range 6 months to 88 years. Sex: ˜ 50% male 50% female Co‐morbidities or pregnancy: Not reported |
|
| Study design | Participants prospectively enrolled, not reported whether participants consecutively enrolled. All 606 samples were evaluated microscopically and by One Step Malaria Pf/Pan test. A subset of 350 were also evaluated by Malaria Pv/Pf test device. | |
| Target condition and reference standard(s) |
Type(s) of malaria parasite tested for: Multiple species; falciparum and non‐falciparum. Reference standard test(s) used: Microscopy thick and thin blood smears and PCR Who performed the reference standard tests, and where? The reference standard was performed by experienced microscopists. Location not reported. Not reported for PCR. If microscopy was used, how many high power fields were looked at? 100 fields How many observers or repeats were used? 2 independent microscopists How were discrepancies between observers resolved? The results were combined |
|
| Index and comparator tests |
Commercial name of the test:
Parasite species the test is designed to detect:
Designated type:
Batch numbers: Not reported Transport and storage conditions: Not reported Who performed the index test and where? Not reported. |
|
| Follow‐up | Not applicable | |
| Notes | Source of funding: The National Institute of Allergy and Infectious Diseases, National Institutes of Health (U19 AI089672). | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Patients with suspected malaria, having fever with axillary temperature above 37.5°C were included in the study. Sampling method was not reported, only a subsample received Pf/Pv test and sampling method for this not described. |
| Acceptable reference standard? All tests | Yes | Microscopy was performed by 2 independent microscopists. 100 fields. PCR was also done. |
| Partial verification avoided? All tests | Yes | All participants receiving the index tests had their diagnosis verified by the reference test |
| Differential verification avoided? All tests | Yes | The same reference test was used. |
| Incorporation avoided? All tests | Yes | The reference test was microscopy and PCR. |
| Reference standard results blinded? All tests | Yes | The microscopists were blinded to the results of additional diagnostic tests. |
| Index test results blinded? All tests | Yes | The readers of RDTs were blinded to the results of microscopy and PCR. |
| Uninterpretable results reported? All tests | Unclear | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals due to invalid results. |
| Withdrawals explained? All tests | Yes | Number enrolled in the study was explicitly stated and corresponded to the number presented in the analysis; therefore there were no withdrawals. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| A‐Elgayoum 2009 | Not a study of RDTs (compared usual with expert microscopy). |
| Abeku 2008 | No data presented on non‐falciparum malaria. |
| Abul Faiz 2000 | Participants had cerebral malaria. |
| Ademowo 2012 | P. falciparum malaria only. |
| Adesanmi 2011 | P. falciparum malaria only. |
| Afzaal 2001 | Review or narrative. |
| Ahmad 2003 | Report does not contain enough information to assess eligibility. |
| Ahmed 2010 | Case‐control study. |
| Albertini 2012 | Not a DTA study. |
| Allen 2011 | P. falciparum malaria only. |
| Anonymous 2005 | Review or narrative. |
| Ansah 2008 | Report does not contain enough information to assess eligibility. |
| Ansah 2010 | P. falciparum malaria only. |
| Araz 2000 | Some participants did not have symptoms of malaria. |
| Arcanjo 2007 | Non‐English language. |
| Ardic 2012 | Non‐English language. |
| Arora 2003 | Participants have severe or complicated malaria. |
| Arróspide 2004a | Most participants had no symptoms of malaria. |
| Arróspide 2004b | Non‐English language. |
| Arróspide 2006 | |
| Ashley 2009 | Not able to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Aslan 2001 | Participants were hospital inpatients. |
| Assal 1999 | Not immunochromatographic RDTs. |
| Avila 2002 | Participants were travellers returning from an endemic to a non‐endemic region. |
| Ayeh‐Kumi 2011 | P. falciparum malaria only. |
| Azazy 2004 | Only participants with malaria positive blood films by microscopy received the RDT. |
| Azikiwe 2012 | P. falciparum malaria only. |
| Babacar 2008 | Not a DTA study. |
| Baiden 2012 | P. falciparum malaria only. |
| Baltzell 2013 | Not a DTA study. |
| Banchongaksorn 1996 | No data presented on non‐falciparum malaria. |
| Banchongaksorn 1997 | No data presented on non‐falciparum malaria. |
| Barber 2013 | Only participants with malaria positive blood films by microscopy were included. |
| Bartoloni 1998 | Single case study. |
| Bassene 2009 | Not a DTA study. |
| Bassett 1991 | Not a DTA study. |
| Batwala 2011 | P. falciparum malaria only. |
| Beadle 1994 | Most participants did not have symptoms of malaria. |
| Bechem 1999 | Did not present sufficient data to enable extraction of the numbers of true positives, false positive, true negatives and false positives. |
| Beg 2005 | All participants were positive for malaria by microscopy. |
| Belizario 2005 | Participants were recruited by active case finding. |
| Bell 2005 | Not a consecutive sample: excluded a random sample of participants who were negative for malaria by microscopy. |
| Bell 2006 | Review or narrative. |
| Bellagra 1998 | Participants were travellers returning from an endemic to a non‐endemic area. |
| Bendezu 2008 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Berens‐Riha 2009 | Subjects were dead. |
| Bhandari 2008 | All participants were positive for malaria by microscopy. |
| Bhat 2012 | Recruited from a tertiary care hospital. |
| Bhatt 1994 | Review or narrative. |
| Birku 1999 | Participants had severe or complicated malaria. |
| Bisoffi 2009a | Not a DTA study. |
| Bisoffi 2009b | Review or narrative. |
| Bisoffi 2011 | Not a DTA study. |
| Biswas 2004 | Not a DTA study. |
| Biswas 2006 | Not an immunochromatographic test. |
| Bjorkman 2011 | Report does not contain enough information to assess eligibility. |
| Bojang 1999 | No data presented on non‐falciparum malaria. |
| Bouchaud 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Bouyou Akotet 2013 | Unable to extract raw data. |
| Brenier‐Pinchart 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Bruxvoort 2008 | Participants were recruited by active case finding. |
| Bualombai 2003 | No data presented on non‐falciparum malaria. |
| Bualombai 2008 | Report does not contain enough information to assess eligibility. |
| Buchachart 2004 | Participants are hospital in‐patients. |
| Buhalata 2011 | P. falciparum malaria only. |
| Bujanover 2002 | Not a DTA study. |
| Cabezas 2004 | Not a DTA study (comparing 'field' and laboratory RDT results). |
| Caraballo 1996 | No data presented on non‐falciparum malaria. |
| Carmona Fonseca 2010 | Non‐English language. |
| Cavallo 1997 | Participants were travellers returning from endemic to non‐endemic countries. |
| Chaijaroenkul 2011 | Included participants without symptoms of malaria. |
| Chatterjee 2008 | Report did not contain enough information to assess eligibility. |
| Cheng 2006 | Review or narrative. |
| Chilton 2006 | Not a DTA study. |
| Chinkhumba 2010 | P. falciparum malaria only. |
| Chinkhumba 2012 | P. falciparum malaria only. |
| Chiodini 1998 | Review or narrative. |
| Chiodini 2005 | Not a DTA study. |
| Chitkara 2004 | No data presented on non‐falciparum malaria. |
| Cho 2001 | Not undertaken in a malaria endemic area. |
| Cho 2011 | Case‐control study in travellers returning from an endemic to a non‐endemic area. |
| Cnops 2011 | Samples not collected in a malaria endemic area. |
| Coleman 2002a | Most participants did not have symptoms of malaria. |
| Coleman 2002b | Most participants did not have symptoms of malaria. |
| Cong le 2002 | Non‐English language. |
| Cooke 1999 | No data presented on non‐falciparum malaria. |
| Craig 1997 | Tested blood films with artificially cultured and diluted malaria parasites. |
| Craig 2002 | The participants were positive for malaria by microscopy. |
| Cropley 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Cuadros 2007 | Participants were travellers returning from endemic to non‐endemic areas. |
| Davoodian 2011 | Not enough information presented to judge eligibility. |
| Dawoud 2008 | No data presented on non‐falciparum malaria. |
| de Carsalade 2009 | Non‐English language. |
| de Dominguez 1996 | Not a DTA study. |
| De Monbrison 2004 | Participants were travellers returning from endemic to non‐endemic areas. |
| de Oliveira 2007 | No data presented on non‐falciparum malaria. |
| Delaunay 2008 | Review or narrative. |
| Deletoille 1987 | Not commercially available RDTs. |
| Devi 2002 | No data presented on non‐falciparum malaria. |
| Di Perry 1997 | All participants were positive for malaria by microscopy. |
| Di Santi 2011 | Positive and negative blood samples selected for the study. |
| Diarra 2012 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives for individual malaria species. |
| Dietze 1995 | Some participants did not have symptoms of malaria. |
| Drakeley 2009 | Review or narrative. |
| Dubarry 1990 | Not evaluating an immunochromatographic RDT. |
| Durand 2005 | Review or narrative. |
| Durand 2007 | Participants were travellers returning from endemic to non‐endemic areas. |
| Durrheim 1998 | No data presented on non‐falciparum malaria. |
| Dyer 2000 | All participants were positive for malaria by microscopy. |
| Dzakah 2013 | Positive and negative blood samples selected for the study. |
| Eisen 2000 | Not undertaken in a malaria endemic area. |
| El‐Moamly 2007 | Participants were travellers returning from a malaria endemic to a non endemic area. |
| Elmardi 2009 | Not a DTA study. |
| Endeshaw 2008 | Most participants did not have symptoms of malaria. |
| Endeshaw 2010 | Unable to extract raw data for 2 x 2 table. |
| Existe 2010 | Not enough information presented to judge eligibility. |
| Falade 2013 | P. falciparum malaria only. |
| Fan 2000 | Non‐English language. |
| Fancony 2013 | Included assymptomatic individuals (population survey). |
| Farcas 2003 | Participants were travellers returning from endemic to non‐endemic areas. |
| Farcas 2004 | Not an immunochromatographic test. |
| Ferro 2002 | Participants were travellers returning from an endemic area to an non‐endemic area. |
| Figueiredo Filho 2003 | All participants were positive for malaria by microscopy. |
| Fogg 2008 | No data presented on non‐falciparum malaria. |
| Forney 2001 | No data presented on non‐falciparum malaria. |
| Forney 2003 | No data presented on non‐falciparum malaria. |
| Fryauff 1997 | Report did not contain enough information to assess eligibility. |
| Fryauff 2000 | Participants did not have symptoms of malaria. |
| Funk 1999 | Participants were travellers returning from endemic to non‐endemic areas. |
| Garavelli 2002 | Participants were travellers returning from endemic to non‐endemic areas. |
| Garcia 1996 | Report did not contain enough information to assess eligibility. |
| Gatti 2002 | Participants were travellers returning from endemic to non‐endemic areas. |
| Gatti 2007 | Participants were travellers returning from endemic to non‐endemic areas. |
| Gaye 1998 | No data presented on non‐falciparum malaria. |
| Gaye 1999 | No data presented on non‐falciparum malaria. |
| Gelaglie 2010 | Not enough information presented to judge eligibility. |
| Gerstl 2009 | No data presented on non‐falciparum malaria. |
| Ghanchi 2009 | Not a DTA study. |
| Ghosh 2000 | No data presented on non‐falciparum malaria. |
| Ghouth 2012 | P. falciparum malaria only. |
| Gillet 2009a | Participants were travellers returning from endemic to non‐endemic areas. |
| Gillet 2009b | Not a DTA study. |
| Gillet 2009c | Participants were travellers returning from an endemic to a non‐endemic area. |
| Gillet 2011 | Not a DTA study: patients negative by reference standard standard were excluded. |
| Gogtay 1999 | Participants had severe or complicated malaria. |
| Gogtay 2003 | Participants were all positive for malaria by blood smear. |
| Goh 2013 | Does not evaluate an RDT. |
| Gomes 2013 | Selected positve and negative samples by reference test. |
| Gonzáles‐Cerón 2005 | Non‐English language. |
| Grobusch 1999 | Not undertaken in a malaria endemic area. |
| Grobusch 2002 | Not undertaken in a malaria endemic area. |
| Grobusch 2003a | Participants were travellers returning from endemic to non‐endemic areas. |
| Grobusch 2003b | Participants were travellers returning from endemic to non‐endemic areas. |
| Gupta 2001 | Some participants had severe or complicated malaria. |
| Guthmann 2002 | No data presented on non‐falciparum malaria. |
| Gutierrez 2005 | Not a DTA study. |
| Hada 2011 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives for individual malaria species. |
| Haditsch 2004 | Review or narrative. |
| Hance 2005 | Review or narrative. |
| Happi 2004 | All participants were positive for malaria by microscopy. |
| Harchut 2013 | P. falciparum malaria only. |
| Hashizume 2006 | Participants were displaced people from mainly very low endemicity areas. |
| Hawkes 2009 | Not a DTA study. |
| Hernandes 2001 | Participants were travellers returning from endemic to non‐endemic areas. |
| Holmberg 1992 | Not a DTA study. |
| Hopkins 2007 | No data presented on non‐falciparum malaria. |
| Hopkins 2008 | No data presented on non‐falciparum malaria. |
| Hossain 2008 | Participants had severe or complicated malaria. |
| Houmsou 2011 | Report does not contain enough information to assess eligibility. |
| Houzé 2009 | All participants were positive for malaria by microscopy. |
| Houzé 2011 | Participants were travellers returning from endemic to non‐endemic areas. |
| Humar 1997 | Participants were travellers returning from endemic to non‐endemic areas. |
| Huong 2002 | Not based on a consecutive sample; included a group malaria positive by microscopy and an asymptomatic malaria negative control group. |
| Hänscheid 1999 | Review or narrative. |
| Iqbal 2000 | Not a consecutive sample: participants were selected to have a high risk of rheumatoid factor. |
| Iqbal 2001 | Participants were travellers returning from endemic to non‐endemic areas. |
| Iqbal 2002 | Participants were travellers returning from endemic to non‐endemic areas. |
| Iqbal 2003 | No data presented on non‐falciparum malaria. |
| Iqbal 2004 | All participants were positive for malaria by microscopy. |
| Ishengoma 2011 | P. falciparum malaria only. |
| Jang 2013 | Participants were travellers returning from endemic to non‐endemic areas. |
| Jelinek 1996 | Does not evaluate an immunochromatographic RDT for malaria. |
| Jelinek 1999 | Participants were travellers returning from endemic to non‐endemic areas. |
| Jelinek 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Jelinek 2001 | Participants were travellers returning from endemic to non‐endemic areas. |
| Jeurissen 1999 | Review or narrative. |
| John 1998 | All participants were positive for malaria by microscopy. |
| Joshi 2004 | Not evaluating an immunochromatographic RDT. |
| Kaewsonthi 1996 | Not a DTA study. |
| Kahama‐Maro 2008 | Report does not contain enough information to assess eligibility. |
| Kahama‐Maro 2011 | No data presented on non‐falciparum malaria. |
| Kakkilaya 2003 | Review or narrative. |
| Kamugisha 2008 | Most participants did not have symptoms of malaria. |
| Kar 1998 | No data presented on non‐falciparum malaria. |
| Karbwang 1996 | All participants were positive for malaria by microscopy. |
| Karimov 2011 | Non‐English language. |
| Kashif 2013 | P. falciparum malaria only. |
| Katakai 2011 | Positive and negative blood samples selected for the study. |
| Kattenberg 2011 | Not enough information presented to judge eligibility. |
| Kaur 2000 | All participants had cerebral malaria. |
| Kaushal 1995 | Tested for P. knowlesi infection in monkeys. |
| Kaushal 1997 | Review or narrative. |
| Kawai 2009 | Tested for P. knowlesi infection in monkeys. |
| Keating 2009 | Most participants did not have symptoms of malaria. |
| Khairnar 2009 | Participants were travellers returning from an endemic to a non‐endemic area. |
| Khan 2004 | Participants were hospital inpatients. |
| Kilian 1997 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Kilian 1999 | No data presented on non‐falciparum malaria. |
| Kim 2008 | Includes a symptomatic group with malaria infection identified by microscopy, and an asymptomatic group with no malaria infection by microscopy. |
| Kim 2011 | Only participants with malaria positive blood films by microscopy received the RDT. |
| Kim 2013 | Includes a symptomatic group with malaria infection identified by microscopy, and an asymptomatic group with no malaria infection by microscopy. |
| Knappik 2002 | Participants were travellers returning from endemic to non‐endemic areas. |
| Kodisinghe 1997 | Some participants did not have symptoms of malaria. |
| Koita 2012 | P. falciparum malaria only. |
| Kumar 1996 | No data presented on non‐falciparum malaria. |
| Kumar 2000 | Participants were migrants from a very low endemicity area. |
| Kumar 2004 | No data presented on non‐falciparum malaria. |
| Kumar 2012 | Not a DTA study. |
| Kumar 2013 | Not a diganostic test accuracy study. |
| Kweka 2011 | P. falciparum malaria only. |
| Kyabayinze 2008 | No data presented on non‐falciparum malaria. |
| Labbé 2001 | No data presented on non‐falciparum malaria. |
| Lee 1999 | Some participants did not have symptoms of malaria. |
| Lee 2008 | Participants were soldiers usually residing in non‐endemic areas. |
| Lee 2011 | Positive and negative blood samples selected for the study. |
| Lema 1999 | Some participants were attending for follow‐up of a previously diagnosed and treated case of malaria. |
| Lepère 2004 | Not a DTA study. |
| Lim 2001 | Half the participants had malaria confirmed by microscopy before enrolment. |
| Llanos Zavalaga 2000 | Not a DTA study. |
| Llanos‐Zavalaga 2002 | Non‐English language. |
| Mahajan 2000 | Participants were hospital inpatients. |
| Makler 1998 | Review or narrative. |
| Makler 2009 | Review or narrative. |
| Malik 2004 | Study was based at a tertiary referral centre with a high percentage of patients with complicated malaria. |
| Mankhambo 2002 | Most participants did not have symptoms of malaria. |
| Mason 2002 | Some participants did not have symptoms of malaria. |
| Mawili‐Mboumba 2010 | P. falciparum malaria only. |
| Mayxay 2004 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Mboera 2006a | No data presented on non‐falciparum malaria. |
| McCutchan 2008 | Review or narrative. |
| McMorrow 2010 | P. falciparum malaria only. |
| Meena 2009 | Participants were all hospital inpatients. |
| Menan 1996 | Not a study of malaria RDTs. |
| Mendiratta 2006 | No data presented on non‐falciparum malaria. |
| Mendoza 2007 | Report does not contain enough information to assess eligibility. |
| Mendoza 2013 | Report does not contain enough information to assess eligibility. |
| Mengesha 1999 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Mens 2007 | No data presented on non‐falciparum malaria. |
| Mens 2010 | RDT evaluated is not an immunochromatographic test. |
| Metzger 2008 | Participants were recruited by active case finding. |
| Metzger 2009 | Not a DTA study. |
| Mharakurwa 1997a | Participants had all been recently treated for malaria. |
| Mharakurwa 1997b | No data presented on non‐falciparum malaria. |
| Miantuasila 2012 | P. falciparum malaria only. |
| Mikhail 2011 | Positive and negative blood samples selected for the study. |
| Miller 2001 | Letter. |
| Miller 2008 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Mills 1999 | Participants were travellers returning from endemic to non‐endemic areas. |
| Mills 2007 | Report does not contain enough information to assess eligibility. |
| Mills 2010 | P. falciparum malaria only. |
| Mills 2010a | P. falciparum malaria only. |
| Minja 2012 | Not all participants had symptoms of malaria: prospective cohort of pregnant women. |
| Minodier 2005 | Review or narrative. |
| Mishra 1999 | Not a consecutive sample; comprised a malaria positive group by microscopy and negative control groups. |
| Mishra 2007 | Report did not contain enough information to assess eligibility. |
| Mohanty 1999 | Report did not contain enough information to assess eligibility. |
| Mohapatra 1996 | No data presented on non‐falciparum malaria. |
| Montoya 2008 | Non‐English language. |
| Moody 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Moody 2002a | Review or narrative. |
| Moody 2002b | RDTs tested on artificially cultured blood samples. |
| Moonasar 2007 | Not a DTA study. |
| Moonasar 2009 | No data presented on non‐falciparum malaria. |
| Morankar 2011 | P. falciparum malaria only. |
| Moulin 2009 | Review or narrative. |
| Msellem 2009 | No data presented on non‐falciparum malaria. |
| Mtove 2011 | P. falciparum malaria only. |
| Mueller 2007 | Participants not representative of people presenting to ambulatory care setting with symptoms of malaria. |
| Muhindo 2012 | No data presented on non‐falciparum malaria. |
| Munier 2009 | Report does not contain enough information to assess eligibility. |
| Murahwa 1999 | No data presented on non‐falciparum malaria. |
| Murray 2003 | Review or narrative. |
| Murray 2008 | Review or narrative. |
| Mwanza 2005 | No data presented on non‐falciparum malaria. |
| Myjak 2004 | Participants were travellers returning from endemic to non‐endemic areas. |
| Naing 2002a | No data presented on non‐falciparum malaria. |
| Nema 2005 | All participants were positive for malaria by microscopy. |
| Neumann 2008 | Most participants did not have symptoms of malaria. |
| Nicastri 2009a | No data presented on non‐falciparum malaria. |
| Nigussie 2008 | No data presented on non‐falciparum malaria. |
| Nkrumah 2010 | RDT evaluated is not an immunochromatographic test. |
| Nkrumah 2011 | P. falciparum malaria only: Only 2 cases of non‐falciparum malaria (263 study participants). |
| Nour 2011 | Not enough information presented to extract numbers of true postives, false positives, true negatives and false negatives. |
| Nwuba 2001 | No data presented on non‐falciparum malaria. |
| Nyunt 2013 | Not a DTA study: all participants had positive blood slide for P. falciparum |
| Ochola 2006 | Review or narrative. |
| Omar 1999 | No data presented on non‐falciparum malaria. |
| OMS 1999 | Not a DTA study. |
| Onile 2005 | Review or narrative. |
| Osman 2010 | Less than one percent of the malaria detected was P. vivax. |
| Ouattara 2011 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives for individual malaria species (mainly P. falciparum but numbers not provided). |
| Ozbilge 2006 | Not an immunochromatographic test. |
| Pabon 2007 | Non‐English language. |
| Pakalapati 2013 | Unable to extract data on numbers of true positives, false positive, true negatives and false negatives. |
| Palmer 1998 | Report did not contain enough information to assess eligibility. |
| Palmer 1999 | All participants were positive for malaria by microscopy. |
| Palmer 2003 | Participants were travellers returning from endemic to non‐endemic areas. |
| Pammenter 1988 | Review or narrative. |
| Pandey 1995 | Review or narrative. |
| Pandya 2001 | No data presented on non‐falciparum malaria. |
| Park 2003 | Not a consecutive sample; included a known malaria group and negative control group by microscopy. |
| Park 2006 | Written in Korean only. |
| Parra 1991 | Not a DTA study. |
| Peng 2012 | P. falciparum malaria only. |
| Penhalbel 2005 | Not a consecutive sample; included a known malaria group and negative control group by microscopy. |
| Peyron 1999 | Review or narrative. |
| Phommanivong 2010 | Not a DTA study. |
| Pica 2005 | Review or narrative. |
| Pieroni 1998 | Participants were travellers returning from endemic to non‐endemic areas. |
| Pinto 1999 | All participants had previous tested negative for malaria and had symptoms that meant complicated malaria could not be ruled out. |
| Piper 1999 | Half the participants lived in non‐endemic areas. |
| Pividal 1994 | Not a DTA study. |
| Planche 2001 | Review or narrative. |
| Playford 2002 | Participants were travellers returning from endemic to non‐endemic areas. |
| Popov 2000 | Non‐English language. |
| Popov 2004 | Non‐English language. |
| Premji 1994 | Participants did not have symptoms of malaria. |
| Prou 1988 | Not an immunochromatographic test. |
| Proux 2001 | Majority of participants did not have symptoms of malaria. |
| Pérez 2007 | Review or narrative. |
| Quintana 1998 | Report did not contain enough information to assess eligibility. |
| Rabinovich 2006 | Non‐English language. |
| Radrianasolo 2007 | Non‐English language. |
| Rahim 2002 | All participants were positive for malaria by microscopy. |
| Rajendran 2006 | Report does not contain enough information to assess eligibility. |
| Ramutton 2012 | Participants had severe malaria. |
| Ratnawati 2008 | Many participants were recruited by active case finding. |
| Ratsimbasoa 2012 | P. falciparum malaria only. |
| Rehlis 2004 | Non‐English language. |
| Reyburn 2007 | Not a DTA study. |
| Ricci 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Richardson 2002 | Participants were travellers returning from endemic to non‐endemic areas. |
| Richter 2004a | Review or narrative. |
| Richter 2004b | Participants were travellers returning from endemic to non‐endemic areas. |
| Rimón 2003 | No data presented on non‐falciparum malaria. |
| Roche 1995 | Not an immunochromatographic test. |
| Rodríguez‐Iglesias 2005 | Review or narrative. |
| Rodulfo 2007 | Some of the participants did not have symptoms of malaria. |
| Rolland 2006 | Not a DTA study. |
| Rosenthal 2012 | Not a DTA study: editorial. |
| Rubio 2001 | Participants were travellers returning from endemic to non‐endemic areas. |
| Runsewe‐Abiodun 2012 | Not enough information presented to absolute numbers of true positives, true negatives, false positives and false negatives. |
| Ryan 2002 | Not a DTA study. |
| Samal 1998 | Not an immunochromatographic test. |
| Saranya 2003 | Review or narrative. |
| Sayang 2009 | No data presented on non‐falciparum malaria. |
| Schachterle 2011 | P. falciparum only. |
| Schmidt 2003 | Review or narrative. |
| Schmidt 2011 | Only participants with positive P. falciparum malaria slides were included. |
| Seidahmed 2008 | Not a DTA study. |
| Senn 2012 | Not a diagnostic test accuracy study: blood slide was performed to assess treatment outcome. |
| Sezibera 2009 | Not a DTA study. |
| Shah 2004 | All participants were positive for malaria by microscopy. |
| Shaikh 2013 | Does not differentiate malaria parasitiaemia by species. |
| Shakya 2012 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Shamsi 1999 | Report did not contain enough information to assess eligibility. |
| Sharma 1999 | No data presented on non‐falciparum malaria. |
| Sharma 2008 | Some participants did not have symptoms of malaria. |
| She 2007 | Not undertaken in a malaria endemic area. |
| Shenoi 1996 | Report did not contain enough information to assess eligibility. |
| Shiff 1993 | Some participants did not have symptoms of malaria. |
| Shillcutt 2008 | Not a DTA study. |
| Shirayama 2008 | Not a DTA study. |
| Shujatullah 2006 | Participants had severe or complicated malaria. |
| Shujatullah 2009 | Participants were hospital inpatients. |
| Singer 2004 | Most participants did not have symptoms of malaria. |
| Singh 1997a | No data presented on non‐falciparum malaria. |
| Singh 1997b | No data presented on non‐falciparum malaria. |
| Singh 2000b | Some participants did not have symptoms of malaria. |
| Singh 2000c | No data presented on non‐falciparum malaria. |
| Singh 2001 | Participants were recruited by active case finding. |
| Singh 2002a | Most participants did not have symptoms of malaria. |
| Singh 2002b | All participants were positive for malaria by microscopy. |
| Singh 2004a | Participants had severe or complicated malaria. |
| Singh 2005a | Most participants did not have symptoms of malaria. |
| Singh 2005b | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Singh 2005c | Some participants did not have symptoms of malaria. |
| Singh 2007 | Most participants did not have symptoms of malaria. |
| Singh 2013 | Participants selected through active case detection. |
| Skarbinski 2009 | Not a DTA study. |
| Smego 2000 | Review or narrative. |
| Sotimehin 2007 | Most participants did not have symptoms of malaria. |
| Soto Tarazona 2004 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Srinivasan 2000 | Participants were travellers returning from endemic to non‐endemic areas. |
| Stauffer 2005 | Participants were refugees from an endemic to a non‐endemic country. |
| Stauffer 2006 | Participants were travellers returning from endemic to non‐endemic areas. |
| Stauffer 2009 | Participants were all travellers returning from an endemic to a non‐endemic area. |
| Stephens 1999 | No data presented on non‐falciparum malaria. |
| Stow 1999 | No data presented on non‐falciparum malaria. |
| Strøm 2013 | No cases of non‐falciparum malaria. |
| Stürenburg 2009 | Review or narrative. |
| Surpur 2010 | Data not presented for P. falciparum and P. vivax separately. |
| Susi 2005 | Participants were all travellers returning from an endemic to a non‐endemic area. |
| Swarthout 2007 | All participants were positive for malaria by microscopy. |
| Tagbo 2007 | No data presented on non‐falciparum malaria. |
| Tagbor 2008 | Most participants did not have symptoms of malaria. |
| Tahar 2013 | P. falciparum malaria only: only 4 cases of non‐falciparum malaria (179 participants). |
| Tarimo 2001 | Unable to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Taylor 2002 | All participants were positive for malaria by microscopy. |
| Tekeste 2012 | P. falciparum malaria only. |
| Tham 1999 | Participants were all travellers returning from an endemic to a non‐endemic area. |
| Thepsamarn 1997 | All participants were positive for malaria by microscopy. |
| Tietche 1996 | Not a DTA study. |
| Tjitra 2001a | All participants were positive for malaria by microscopy. |
| Tjitra 2001b | All participants were positive for malaria by microscopy. |
| Trachsler 1999 | Not a DTA study. |
| Uguen 1995 | Participants were travellers returning from endemic to non‐endemic areas. |
| Uneke 2008a | Review or narrative. |
| Uneke 2008b | Not a DTA study. |
| Uzochukwu 2009 | No data presented on non‐falciparum malaria. |
| Valecha 1998 | Report does not contain enough information to assess eligibility. |
| Valecha 2002 | Participants were recruited by active case finding. |
| Valéa 2009 | No data presented on non‐falciparum malaria. |
| Van den Ende 1998 | Participants were travellers returning from endemic to non‐endemic areas. |
| Van der Palen 2009 | Participants were travellers returning from endemic to non‐endemic areas. |
| Van Dijk 2009 | Participants were travellers returning from an endemic to a non‐endemic area. |
| van Hellemond 2009 | Not a DTA study. |
| VanderJagt 2005 | Most participants had no symptoms of malaria. |
| Venkatesh 2007 | Participants had severe or complicated malaria. |
| Verlé 1996 | No data presented on non‐falciparum malaria. |
| Voller 1993 | Review or narrative. |
| Waltz 2007 | Review or narrative. |
| Wang J‐Y 2007 | Not a commercial test kit. |
| Wanji 2008 | Participants did not have symptoms of malaria. |
| WHO 1996 | Review or narrative. |
| Wiese 2006 | Participants were travellers returning from endemic to non‐endemic areas. |
| Willcox 2009 | No data presented on non‐falciparum malaria. |
| Williams 2008 | Not a DTA study. |
| Wilson 2013 | Review or narrative. |
| Win 2001 | Review or narrative. |
| Wolday 2001 | No data presented on non‐falciparum malaria. |
| Wongsrichanalai 1999 | No data presented on non‐falciparum malaria. |
| Wongsrichanalai 2001 | Review or narrative. |
| Wongsrichanalai 2007 | Review or narrative. |
| Woyessa 2013 | Participants recruited through active case detection (population survey). |
| Wu 2005 | Not an immunochromatographic RDT kit. |
| Yadav 1997 | No data presented on non‐falciparum malaria. |
| Yadav 2012 | Not enough information presented to assess eligibility (not clear where participants presented with symptoms). |
| Yavo 2002 | No data presented on non‐falciparum malaria. |
| Zakai 2003 | Review or narrative. |
| Zerpa 2007 | Not able to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives. |
| Zheng 1999 | Non‐English language. |
| Zhu 1998 | Non‐English language. |
| Zikusooka 2008 | Not a DTA study. |
| Zurovac 2008 | Not a DTA study. |
Differences between protocol and review
In the protocol, we considered RDTs for the detection of P. falciparum and non‐falciparum malaria within one Cochrane Review. However, it became apparent during production of the review that such a publication would be very large. For this reason we decided to split results for the different target conditions into two separate Cochrane Reviews.
In the protocol, we stated that in the search for eligible studies we would contact test manufacturers to identify any unpublished studies, handsearch conference proceedings and contact study authors and other experts for information on ongoing and unpublished studies. However, due to the number of citations returned by our search (over 4000) and the large size of the reviews, we did not have the resources to undertake any of these additional search methods, and the methods stated in the review reflect this.
Since the publication of the protocol, we added three additional exclusion criteria relating to study eligibility. We excluded studies if the study authors used active case detection to recruit participants, as we felt the threshold of symptoms leading to testing may be lower than for a self‐selecting sample attending healthcare facilities and that this may influence the findings. We also excluded studies if they did not present sufficient data to allow us to extract or calculate absolute numbers of true positives, false positives, false negatives and true negatives, as we considered it would be distracting to the reader to present data on studies that did not contribute to the analyses. Due to resource constraints, we excluded studies if they were written in non‐English languages, or if they did not provide enough information to enable a full assessment of their eligibility for the review.
Contributions of authors
The review authors jointly developed the protocol. Katharine Abba applied inclusion criteria, oversaw the data extractions and entered the data. Yemisi Takwoingi, Sarah Donegan, Amanda Kirkham and Jon Deeks performed statistical analyses. All review authors contributed to the final manuscript.
Sources of support
Internal sources
-
International Medical University, Malaysia.
Research grant ID 134/2007
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Research Programme Grant
Declarations of interest
PG is Director of Evidence Building and Synthesis Research Consortium that receives money to increase the number of evidence‐informed decisions by intermediary organizations, including WHO and national decision‐makers that benefit the poor in middle‐ and low‐income countries. PG is the coordinator of a WHO Collaborating Centre for Evidence Synthesis for Infectious and Tropical Diseases; one of the Centre's aims is to help WHO in its role as an infomediary in communicating reliable summaries of research evidence to policy makers, clinicians, teachers, and the public in developing countries.
Unchanged
References
References to studies included in this review
Alam 2011 {published data only}
- Alam MS, Mohon AN, Mustafa S, Khan WA, Islam N, Karim MJ, et al. Real‐time PCR assay and rapid diagnostic tests for the diagnosis of clinically suspected malaria patients in Bangladesh. Malaria Journal 2011;10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrade 2010 {published data only}
- Andrade BB, Reis‐Filho A, Barros AM, Souza‐Neto SM, Nogueira LL, Fukatano KF, et al. Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malaria Journal 2010;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashton 2010 {published data only}
- Ashton RA, Kefyalew T, Tesfaye G, Counihan H, Yadeta D, Cundill B, et al. Performance of three multi‐species rapid diagnostic tests for diagnosis of Plasmodium falciparum and Plasmodium vivax malaria in Oromia Regional State, Ethiopia. Malaria Journal 2010;9:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2001a {published data only}
- Bell D, Go R, Miguel C, Walker J, Cacal L, Saul A. Diagnosis of malaria in a remote area of the Philippines: comparison of techniques and their acceptance by health workers and the community. Bulletin of the World Health Organization 2001;79(10):933‐41. [PMC free article] [PubMed] [Google Scholar]
Bell 2001b {published data only}
- Bell D, Go R, Miguel C, Walker J, Cacal L, Saul A. Diagnosis of malaria in a remote area of the Philippines: comparison of techniques and their acceptance by health workers and the community. Bulletin of the World Health Organization 2001;79(10):933‐41. [PMC free article] [PubMed] [Google Scholar]
Bendezu 2010 {published data only}
- Bendezu J, Rosas A, Grande T, Rodriguez H, Llanos‐Cuentas A, Escobedo J, et al. Field evaluation of a rapid diagnostic test (Parascreen) for malaria diagnosis in the Peruvian Amazon. Malaria Journal 2010;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bharti 2008 {published data only}
- Bharti PK, Silawat N, Singh PP, Singh MP, Shukla M, Chand G, et al. The usefulness of a new rapid diagnostic test, the First Response Malaria Combo (pLDH/HRP2) card test, for malaria diagnosis in the forested belt of central India. Malaria Journal 2008;7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanie 2011 {published data only}
- Chanie M, Erko B, Animut A, Legesse M. Performance of CareStartTM Malaria Pf/Pv Combo test for the diagnosis of Plasmodium falciparum and Plasmodium vivax infections in the Afar Region, North East Ethiopia. Ethiopian Journal of Health Development 2011;25(3):206‐11. [Google Scholar]
Chayani 2004 {published data only}
- Chayani N, Das B, Sur M, Bajoria S. Comparison of parasite lactate dehydrogenase based immunochromatographic antigen detection assay (OptiMAL) with microscopy for detection of malaria parasites. Indian Journal of Medical Microbiology 2004;22(2):104‐6. [PubMed] [Google Scholar]
Dev 2004 {published data only}
- Dev V. Relative utility of dipsticks for diagnosis of malaria in mesoendemic area for Plasmodium falciparum and P. vivax in Northeastern India. Vector‐Borne and Zoonotic Diseases 2004;4(2):123‐30. [DOI] [PubMed] [Google Scholar]
Eibach 2013 {published data only}
- Eibach D, Traore B, Bouchrik M, Coulibaly B, Coulibaly N, Siby F, et al. Evaluation of the malaria rapid diagnostic test VIKIA malaria Ag Pf/PanTM in endemic and non‐endemic settings. Malaria Journal 2013;12:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elahi 2013 {published data only}
- Elahi, R, Mohon, A.N, Khan, W.W, Haque, R, Alam, M.S. Performance of a HRP‐2/pLDH based rapid diagnostic test at the Bangladesh‐India‐Myanmar border area for diagnosis of clinical malaria. Malaria Journal 2013;12:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012a {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centers in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012b {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012c {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012d {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012e {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012f {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012g {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012h {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012i {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2012j {published data only}
- Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centres in Northwest Ethiopia. PLoS One 2012;7(4):e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernando 2004 {published data only}
- Fernando SD, Karunaweera ND, Fernando WP. Evaluation of a rapid whole blood immunochromatographic assay for the diagnosis of Plasmodium falciparum and Plasmodium vivax malaria. Ceylon Medical Journal 2004;49(1):7‐11. [DOI] [PubMed] [Google Scholar]
- Fernando SD, Karunaweera ND, Fernando WP, Attanayake N, Wickremasinghe AR. A cost analysis of the use of the rapid, whole‐blood, immunochromatographic Pf/Pv assay for the diagnosis of Plasmodium vivax malaria in rural areas of Sri Lanka. Annals of Tropical Medicine and Parasitology 2004;98(1):5‐13. [DOI] [PubMed] [Google Scholar]
Harani 2006 {published data only}
- Harani MS, Beg MA, Khaleeq L, Adil SN, Kakepoto GN, Khurshid M. Role of ICT Malaria immunochromatographic test for rapid diagnosis of malaria. Journal of the Pakistan Medical Association 2006;56(4):167‐71. [PubMed] [Google Scholar]
Kolaczinski 2004 {published data only}
- Klolaczinski J, Mohammed N, Ali A, Ali M, Khan N, Ezard N, Rowland M. Comparison of the OptiMAL rapid antigen test with field microscopy for the detection of Plasmodium vivax and P. falciparum: considerations for the application of the rapid test in Afghanistan. Annals of Tropical Medicine and Parasitology 2004;98(1):15‐20. [DOI] [PubMed] [Google Scholar]
Kosack 2013 {published data only}
- Kosack CS, Naing WT, Piriou E, Shanks L. Routine parallel diagnosis of malaria using microscopy and the malaria rapid diagnostic test SD 05FK60: the experience of Médecins Sans Frontières in Myanmar. Malaria Journal 2013;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mekonnen 2010 {published data only}
- Mekonnen Z, Ali S, Belay G, Suleman S, Chatterjee S. Evaluation of the performance of Carestart Malaria Pf/Pf Combo rapid diagnostic test for the diagnosis of malaria in Jimma, southwestern Ethiopia. Acta Tropica 2010;113(3):285‐8. [DOI] [PubMed] [Google Scholar]
Metzger 2011 {published data only}
- Metzger WG, Vivas‐Martínez S, Giron A, Vaccari E, Campos E, Rodríguez I, et al. Assessment of routine malaria diagnosis in the Venezuelan Amazon. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(5):262‐8. [DOI] [PubMed] [Google Scholar]
Moges 2012 {published data only}
- Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, et al. Comparison of CareStart HRP2/pLDH COMBO rapid malaria test with light microscopy in north‐west Ethiopia. Malaria journal 2012;11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohon 2012 {published data only}
- Mohon AN, Elahi R, Podder MP, Mohiuddin K, Hossain MS, Khan WA, et al. Evaluation of the OnSite (Pf/Pan) rapid diagnostic test for diagnosis of clinical malaria. Malaria Journal 2012;11:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pattanasin 2003 {published data only}
- Pattanasin S, Proux S, Chompasuk D, Luwiradaj K, Jacquier P, Looareesuwan S, et al. Evaluation of a new plasmodium lactate dehydrogenase assay (OptiMAL‐IT) for the detection of malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(6):672‐4. [DOI] [PubMed] [Google Scholar]
Rakotonirina 2008 {published data only}
- Rakotonirina H, Barnadas C, Raherijafy R, Andrianantenaina H, Ratsimbasoa A, Randrianasolo L, et al. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria‐endemic countries. American Journal of Tropical Medicine and Hygiene 2008;78(2):217‐21. [PubMed] [Google Scholar]
Ratsimbasoa 2007 {published data only}
- Ratsimbasoa A, Randriamanantena A, Raherinjafy R, Rasoarilalao N, Ménard D. Which malaria rapid test for Madagascar? Field and laboratory evaluation of three test and expert microscopy of samples from suspected malaria patients in Madagascar. American Journal Of Tropical Medicine and Hygiene 2007;76(3):481‐5. [PubMed] [Google Scholar]
Ratsimbasoa 2008 {published data only}
- Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D. Short report: Evaluation of two new immunochromatographic assays for diagnosis of malaria. American Journal of Tropical Medicine and Hygiene 2008;79(5):670‐2. [PubMed] [Google Scholar]
Samane 2010 {published data only}
- Samane AK, Nahid HZ, Saaed S, Khazen H, Ali H, Ahmad R, et al. Comparison of microscopy and RDTs techniques for laboratory detection of malaria. African Journal of Biotechnology 2010;9(10):1514‐6. [Google Scholar]
Selimuzzaman 2010 {published data only}
- Selimuzzaman SM, Islam SJ, Nahar Z, Das R, Rahman MA, Rahman MA. Malariagen malaria Pf/Pv antigen rapid test: a simple and effective tool for diagnosis of malaria in the far‐flung hilly areas of Bangladesh. Mymensingh Medical Journal 2010;19(1):94‐9. [PubMed] [Google Scholar]
Sharew 2009 {published data only}
- Sharew B, Legesse M, Animut A, Jima D, Medhim G, Erkko B. Evaluation of the performance of CareStart Malaria Pf/Pv Combo and Paracheck Pf tests for the diagnosis of malaria in Wondo Genet, southern Ethiopia. Acta Tropica 2009;111(3):321‐4. [DOI] [PubMed] [Google Scholar]
Singh 2000a {published data only}
- Singh N, Saxena A, Valecha N. Field evaluation of the ICT Malaria Pf/Pv immunochromatographic test for diagnosis of Plasmodium falciparum and P. vivax infection in forest villages in Chhindwara, central India. Tropical Medicine and International Health 2000;5(11):765‐70. [DOI] [PubMed] [Google Scholar]
Singh 2003 {published data only}
- Singh N, Valecha N, Nagpal AC, Mishra SS, Varma HS, Subbaro SK. The hospital‐ and field‐based performances of the OptiMAL tests, for malaria diagnosis and treatment monitoring in central India. Annals of Tropical Medicine and Parasitology 2003;97(1):5‐13. [DOI] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh N, Shukla MM, Shukla MK, Mehra RK, Sharma S, Bharti PK, et al. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malaria Journal 2010;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tjitra 1999 {published data only}
- Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM. Field evaluation of the ICT malaria Pf/Pv immunochromatographic test in detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern Indonesia. Journal of Clinical Microbiology 1999;37(8):2412‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trouvay 2013 {published data only}
- Trouvay M, Palazon G, Berger F, Volney B, Blanchet D, Faway E, et al. High performance of histidine‐rich protein 2 based rapid diagnostic tests in French Guiana are explained by the absence of pfhrp2 gene deletion in P. falciparum. PLoS One 2013;8(9):e74269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valecha 2003 {published data only}
- Valecha N, Singh N, Yadav RS, Dev V, Aggarwal A, Subbarao SK. Field evaluation of OptiMAL48 rapid malaria diagnostic test in India. Acta Parasitologica 2003;48(3):229‐32. [Google Scholar]
van den Broek 2006 {published data only}
- Broek I, Hill O, Gordillo F, Angarita B, Hamade P, Counihan H, et al. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. American Journal of Tropical Medicine and Hygiene 2006;75(6):1209‐15. [PubMed] [Google Scholar]
Wongsrichanalai 2003 {published data only}
- Wongsrichanalai G, Arevalo I, Laoboonchai A, Yingyuen K, Miller RS, Magill AJ, et al. Rapid diagnostic devices for malaria: field evaluation of a new prototype immunochromatographic assay for the detection of Plasmodium falciparum and non‐falciparumPlasmodium. American Journal of Tropical Medicine and Hygiene 2003;69(1):26‐30. [PubMed] [Google Scholar]
Xiaodong 2013 {published data only}
- Xiaodong S, Tambo E, Chun W, Zhibin C, Yan D, Jian W, et al. Diagnostic performance of CareStartTM malaria HRP2/pLDH (Pf/pan) combo test versus standard microscopy on falciparum and vivax malaria between China‐Myanmar endemic borders. Malaria Journal 2013;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2013 {published data only}
- Yan J, Li N, Wei X, Li P, Zhao Z, Wang L, et al. Performance of two rapid diagnostic tests for malaria diagnosis at the China‐Myanmar border area. Malaria Journal 2013;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abeku 2008 {published data only}
- Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, et al. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malaria Journal 2008;7:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abul Faiz 2000 {published data only}
- Abul Faiz M, Rashid R, Palit R, Rahman MR, BinYunus E, Hussain A, et al. ParaSight‐F test results in cerebral malaria patients before and after treatment in Chittagong Medical College Hospital, Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(1):56‐7. [DOI] [PubMed] [Google Scholar]
Ademowo 2012 {published data only}
- Ademowo G. Evaluation of the relative performance of rapid diagnostic test products (Partec and Paracheck‐Pf) in the diagnosis of malaria in febrile children. 6th EDCTP Forum: Strengthening Research Partnerships for Better Health and Sustainable Development Addis Ababa Ethiopia; 2011 09‐12 Oct; Addis Ababa. Tropical Medicine and International Health. 2012:58‐9.
Adesanmi 2011 {published data only}
- Adesanmi TA, Okafor HU, Okoro AB, Mafe AG. Diagnosis of malaria parasitemia in children using a rapid diagnostic test. Nigerian Journal of Clinical Practice 2011;14(2):195‐200. [DOI] [PubMed] [Google Scholar]
A‐Elgayoum 2009 {published data only}
- A‐Elgayoum SME, El‐Feki AE‐KA, Mahgoub BA, El‐Rayah el‐A, Giha HA. Malaria overdiagnosis and burden of malaria misdiagnosis in the suburbs of central Sudan: special emphasis on artemisinin‐based combination therapy era. Diagnostic Microbiology and Infectious Disease 2009;64(1):28‐34. [DOI] [PubMed] [Google Scholar]
Afzaal 2001 {published data only}
- Afzaal S, Singh M, Fatima S, Koshy AA. Rapid diagnostic tests for malaria. Journal of the Association of Physicians of India 2001;49:261‐5. [PubMed] [Google Scholar]
Ahmad 2003 {published data only}
- Ahmad SQ, Abbasi SA, Tariq MA, Mirza SA, Salamat A. Evaluation of Plasmodium‐lactate dehydrogenase based immunochromatographic kit for the diagnosis of malaria. Journal of the College of Physicians and Surgeons of Pakistan 2003;13(3):176‐7. [PubMed] [Google Scholar]
Ahmed 2010 {published data only}
- Ahmed MU, Mahmud MC, Shamsuzzaman AK, Musa AK, Ahmed SU, Alam M, et al. Role of immunochromatographic test for rapid diagnosis of malaria. Mymensingh Medical Journal 2010;19(1):106‐9. [PubMed] [Google Scholar]
Albertini 2012 {published data only}
- Albertini A, Djalle D, Faye B, Gamboa D, Luchavez J, Mationg ML, et al. Preliminary enquiry into the availability, price and quality of malaria rapid diagnostic tests in the private health sector of six malaria‐endemic countries. Tropical Medicine and International Health 2012;17(2):147‐52. [DOI] [PubMed] [Google Scholar]
Allen 2011 {published data only}
- Allen LK, Hatfield JM, DeVetten G, Ho JC, Manyama M. Reducing malaria misdiagnosis: the importance of correctly interpreting Paracheck Pf "faint test bands" in a low transmission area of Tanzania. BMC Infectious Diseases 2011;11:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Micro moves against malaria. New Scientist 2005;187(2517):53. [Google Scholar]
Ansah 2008 {published data only}
- Ansah EK. A comparison of microscopy with rapid diagnostic tests for malaria in rural Ghana. American Journal of Tropical Medicine and Hygiene 2008;79(6):91. [Google Scholar]
Ansah 2010 {published data only}
- Ansah EK, Narh‐Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, et al. Rapid testing for malaria in settings where microscopy is available and peripheral clinical where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ 2010;340:c930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Araz 2000 {published data only}
- Araz E, Tanyuksel M, Ardic N, Tabuk C. Performance of a commercial immunochromatographic test for the diagnosis of vivax malaria in Turkey. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(1):55‐6. [DOI] [PubMed] [Google Scholar]
Arcanjo 2007 {published data only}
- Arcanjo AL, Lacerda MVG, Alecrim WD, Alecrim MDC. Evaluation of the Optimal‐IT® and ICT P.f./P.v® rapid dipstick tests for diagnosing malaria within primary healthcare in the municipality of Manaus, Amazonas [Avaliação dos testes rápidos Optimal‐IT® e ICTP.f./P.v.® para o diagnóstico da malária, na Atenção Básica de Saúde, no município de Manaus, Amazonas]. Revista Da Sociedade Brasileira de Medicina Tropical 2007;40(1):88‐90. [DOI] [PubMed] [Google Scholar]
Ardic 2012 {published data only}
- Ardic N, Koru O. Rapid diagnostic tests in malaria. Nobel Medicus 2012;8(2):10‐5. [Google Scholar]
Arora 2003 {published data only}
- Arora S, Gaiha M, Arora A. Role of the Parasight‐F test in the diagnosis of complicated Plasmodium falciparum malarial infection. Brazilian Journal of Infectious Diseases 2003;7(5):332‐8. [DOI] [PubMed] [Google Scholar]
Arróspide 2004a {published data only}
- Arróspide N, Puray C, Guzmán E, Verano M, Medina S, Mendizábal S, et al. Use of rapid tests for detecting Plasmodium falciparum in blood donors in Peru [Uso de pruebas rápidas immunocromatográficas para la detección de Plasmodium falciparum en donantes de sangre en Perú]. Revista Peruana de Medicina Experimental y Salud Pública 2004;21(2):76‐82. [Google Scholar]
Arróspide 2004b {published data only}
- Arróspide NV, Marquiño WQ, Gutiérrez SG. Evaluation of an immunoassay ICT P.f/P.v for the diagnosis of Plasmodium falciparum and Plasmodium vivax in the local macroregion of northern Peru [Evaluación de una prueba inmunocromatográfica ICT P.f/P.v para el diagnóstico de malaria por Plasmodium falciparum y Plasmodium vivax en establecimientos de la macroregión norte del Perú]. Revista Peruana de Medicina Experimental y Salud Pública 2004;21(3):134‐8. [Google Scholar]
Arróspide 2006 {published data only}
- Arróspide V, Flores RP, Ruiz JC. Evaluation of a rapid test based on pLDH detection for the diagnosis of malaria in endemic areas of Peru [Evaluación de una prueba rápida basada para el diagnóstico de malaria en áreas endémicas del Perú]. Revista Peruana de Medicina Experimental y Salud Pública 2006;23(2):81‐6. [Google Scholar]
Ashley 2009 {published data only}
- Ashley EA, Touabi M, Ahrer M, Hutagalung R, Htun K, Luchavez J, et al. Evaluation of three parasite lactate dehydrogenase‐based rapid diagnostic tests for the diagnosis of falciparum and vivax malaria. Malaria Journal 2009;8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley EA, Touabi M, Ahrer M, Hutagalung R, Htun K, Lwin M, et al. Evaluation of 3 rapid diagnostic tests: CareStartTM Malaria 3 line pLDH (pan, Pf), Optimal‐IT® PLDH (pan, Pf) and CarestartTM 2 line pLDH (pan) for the diagnosis of malaria in Myanmar. American Journal of Tropical Medicine and Hygiene 2008;79(6):966. [Google Scholar]
Aslan 2001 {published data only}
- Aslan G, Ulukanligil M, Seyrek A, Erel O. Diagnostic performance characteristics of rapid dipstick test for Plasmodium vivax malaria. Memórias do Instituto Oswaldo Cruz 2001;96(5):683‐6. [DOI] [PubMed] [Google Scholar]
Assal 1999 {published data only}
- Assal A, Kauffmann‐Lacroix C, Rodier MH, Dardé ML, Houssay D, Jacquemin JL. Comparison of two techniques for detection of anti‐Plasmodium falciparum antibodies: Falciparum‐spot IF (Biomerieux) and Malaria IgG Celisa (BMD). Transfusion Clinique et Biologique 1999;6(2):119‐23. [DOI] [PubMed] [Google Scholar]
Avila 2002 {published data only}
- Avila PE, Kirchgatteri K, Brunialti KCS, Oliveira AM, Siciliano RF, Santi SM. Evaluation of a rapid dipstick test, Malar‐check, for the diagnosis of Plasmodium falciparum malaria in Brazil. Revista do Instituto de Medicina Tropical de São Paulo 2002;44(5):293‐6. [DOI] [PubMed] [Google Scholar]
Ayeh‐Kumi 2011 {published data only}
- Ayeh‐Kumi PF, Akalifa BG, Obeng Nkrumah N, Asmah RH, Dayie NTKD. Performance of rapid DiaMed OptiMal‐IT® malaria test in an endemic Ghanaian setting. Journal of Parasitic Diseases 2011;35(2):129‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azazy 2004 {published data only}
- Azazy AA. Performance and accuracy of an immunodiagnostic antigen detection test in diagnosing Plasmodium falciparum among Yemeni patients. Annals of Saudi Medicine 2004;24(1):50‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azikiwe 2012 {published data only}
- Azikiwe CCA, Ifezulike CC, Siminialayi IM, Amazu LU, Enye JC, Nwakwunite OE. A comparative laboratory diagnosis of malaria: Microscopy versus rapid diagnostic test kits. Asian Pacific Journal of Tropical Biomedicine 2012;2(4):307‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babacar 2008 {published data only}
- Babacar F, Ndiaye JL, Diallo I, Tine RC, Seck I, Ba‐Fall F, et al. Feasibility of the rapid diagnostic tests (RDTs) field use for malaria case management in Senegal. American Journal of Tropical Medicine and Hygiene 2008;79(6):967. [Google Scholar]
Baiden 2012 {published data only}
- Baiden F, Webster J, Tivura M, Delimini R, Berko Y, Amenga‐Etego S, et al. Accuracy of rapid tests for malaria and treatment outcomes for malaria and non‐malaria cases among under‐five children in rural Ghana. PLoS One 2012;7(4):e34073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baltzell 2013 {published data only}
- Baltzell KA, Shakely D, Hsiang M, Kemere J, Ali AS, Björkman A, et al. Prevalence of PCR detectable malaria infection among febrile patients with a negative Plasmodium falciparum specific rapid diagnostic test in Zanzibar. American Journal of Tropical Medicine and Hygiene 2013;88(2):289‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banchongaksorn 1996 {published data only}
- Banchongaksorn T, Yomokgul P, Panyim S, Rooney W, Vickers P. A field trial of the ParaSight‐F test for the diagnosis of Plasmodium falciparum infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(3):244‐5. [DOI] [PubMed] [Google Scholar]
Banchongaksorn 1997 {published data only}
- Banchongaksorn T, Prajakwong S, Rooney W, Vickers P. Operational trial of ParaSight‐F (dipstick) in the diagnosis of falciparum malaria at the primary health care level. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(2):243‐6. [PubMed] [Google Scholar]
Barber 2013 {published data only}
- Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. Evaluation of the sensitivity of a pLDH‐based and an aldolase‐based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR‐confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. Journal of Clinical Microbiology 2013;51(4):1118‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartoloni 1998 {published data only}
- Bartoloni A, Strohmeyer M, Sabatinelli G, Benucci M, Serni U, Paradisi F. False positive ParaSight‐F test for malaria in patients with rheumatoid factor. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(1):33‐4. [DOI] [PubMed] [Google Scholar]
Bassene 2009 {published data only}
- Bassene H, Kenge P, Ndiath MO, Sokhna C, Dupressoir T, Fontenille D, et al. Comparison of PCR, ELISA‐CSP and direct microscopic observation methods for the detection of Plasmodium falciparum sporozoites in Anopheles gambiae M in Sengal. Bulletin de la Société de Pathologie Exotique 2009;102(4):233‐7. [PubMed] [Google Scholar]
Bassett 1991 {published data only}
- Bassett MT, Taylor P, Bvirakare J, Chiteka F, Govera E. Clinical diagnosis of malaria: can we improve?. Journal of Tropical Medicine and Hygiene 1991;94(1):65‐9. [PubMed] [Google Scholar]
Batwala 2011 {published data only}
- Batwala V, Magnussen P, Hansen KS, Nuwaha F. Cost‐effectiveness of malaria microscopy and rapid diagnostic tests versus presumptive diagnosis: implications for malaria control in Uganda. Malaria Journal 2011;10:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beadle 1994 {published data only}
- Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, et al. Diagnosis of malaria by detection of Plasmodium falciparum HRP‐2 antigen with a rapid dipstick antigen‐capture assay. The Lancet 1994;343(8897):564‐8. [DOI] [PubMed] [Google Scholar]
Bechem 1999 {published data only}
- Bechem NN, Leke RFG, Tietche F, Taylor DW. Evaluation of rapid test for histidine rich protein 2 for diagnosis of Plasmodium falciparum infection in Cameroonian children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(1):46. [DOI] [PubMed] [Google Scholar]
Beg 2005 {published data only}
- Beg MA, Ali SS, Haqqee R, Khan MA, Qasim Z, Hussain R, et al. Rapid immunochromatography‐based detection of mixed‐species malaria infection in Pakistan. Southeast Asian Journal of Tropical Medicine and Public Health 2005;36(3):562‐4. [PubMed] [Google Scholar]
Belizario 2005 {published data only}
- Belizario VY, Pasay CJ, Bersabe MJ, Leon WU, Guerrero DM, Bugaoisan VM. Field evaluation of malaria rapid diagnostic tests for the diagnosis of P. falciparum and non‐P. falciparum infections. Southeast Asian Journal of Tropical Medicine and Public Health 2005;36(3):552‐61. [PubMed] [Google Scholar]
Bell 2005 {published data only}
- Bell DR, Wilson DW, Martin LB. False‐positive results of a Plasmodium falciparum histidine‐rich protein 2‐detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. American Journal of Tropical Medicine and Hygiene 2005;73(1):199‐203. [PubMed] [Google Scholar]
Bell 2006 {published data only}
- Bell D, Peeling RW, WHO‐Regional Office for the Western Pacific/TDR. Evaluation of rapid diagnostic tests: malaria. Nature 2006;4(9 Suppl):S34‐8. [DOI] [PubMed] [Google Scholar]
Bellagra 1998 {published data only}
- Bellagra N, Ajana F, Caillaux M. ParaSight F in the diagnosis of Plasmodium falciparum malaria. Pathologie Biologie 1998;46(5):301‐6. [PubMed] [Google Scholar]
Bendezu 2008 {published data only}
- Bendezu J. Field evaluation of a rapid malaria diagnostic test (Parascreen) for malaria diagnosis in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene 2008;79(6):960. [Google Scholar]
Berens‐Riha 2009 {published data only}
- Berens‐Riha N, Sinicina E, Fleischmann E, Löscher T. Comparison of different methods for delayed post‐mortem diagnosis of falciparum malaria. Malaria Journal 2009;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 2008 {published data only}
- Bhandari TS, Rai S, Naik R, Raghuveer CV. Specificity and sensitivity of rapid diagnostic test in the detection of falciparum malaria. Indian Journal of Medical Research 2008;127:638. [Google Scholar]
Bhat 2012 {published data only}
- Bhat Sandhya K, Sastry Apurba S, Nagaraj ER, Mannur S, Sastry Anand S. Laboratory diagnosis of malaria by conventional peripheral blood smear examination with Quantitative Buffy Coat (QBC) and Rapid Diagnostic Tests (RDT) ‐ A comparative study. International Journal of Collaborative Research on Internal Medicine and Public Health 2012;4(10):1746‐55. [Google Scholar]
Bhatt 1994 {published data only}
- Bhatt KM. Laboratory diagnosis of malaria: an overview. African Journal of General Practice 1994;1(1):12. [PubMed] [Google Scholar]
Birku 1999 {published data only}
- Birku Y, Welday D, Ayele D, Shepherd A. Rapid diagnosis of severe malaria based on the detection of Pf‐HRP‐2 antigen. Ethiopian Medical Journal 1999;37(3):173‐9. [PubMed] [Google Scholar]
Bisoffi 2009a {published data only}
- Bisoffi Z, Sirima BS, Angheben A, Lodesani C, Gobbi F, Tinto H, et al. Rapid malaria diagnostic tests vs. clinical management of malaria in rural Burkina Faso: safety and effect on clinical decisions. A randomized trial. Tropical Medicine and International Health 2009;14(5):491‐8. [DOI] [PubMed] [Google Scholar]
Bisoffi 2009b {published data only}
- Bisoffi Z, Gobbi F, Angheben A, Ende J. The role of rapid diagnostic tests in managing malaria. PLoS Medicine 2009;6(4):e1000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisoffi 2011 {published data only}
- Bisoffi Z, Sodiomon Sirima B, Meheus F, Lodesani C, Gobbi F, Angheben F, et al. A cost benefit analysis of malaria rapid diagnostic tests for adults and children in Burkina Faso. 7th European Congress on Tropical Medicine and International Health; 2011 03‐06 Oct; Barcelona. Tropical Medicine and International Health. 2011:107.
Biswas 2004 {published data only}
- Biswas S. Inter‐test comparison between filter paper absorbed blood eluate and serum for malaria serology by enzyme immunoassay: an operational feasibility. Journal of Immunoassay and Immunochemistry 2004;25(4):399‐410. [DOI] [PubMed] [Google Scholar]
Biswas 2006 {published data only}
- Biswas S. Assessment of immunometric parameters in malaria: role of enzyme immunoassay. Journal of Immunoassay and Immunochemistry 2006;27(4):341‐50. [DOI] [PubMed] [Google Scholar]
Bjorkman 2011 {published data only}
- Bjorkman A. The use of rapid diagnostic tests for measuring malaria transmission. 7th European Congress on Tropical Medicine and International Health; 2011 03‐06 Oct; Barcelona. Tropical Medicine and International Health. 2011:12.
Bojang 1999 {published data only}
- Bojang KA. The diagnosis of Plasmodium falciparum infection in Gambian children, by field staff using the rapid, manual, ParaSight‐F test. Annals of Tropical Medicine and Parasitology 1999;93(7):685‐7. [DOI] [PubMed] [Google Scholar]
Bouchaud 2000 {published data only}
- Bouchaud O, Houzé S, Longuet C, Piazza JP, Ruggieri C, Sécardin Y, et al. Use of the Parasight‐F diagnostic test for imported malaria in a travel clinic. American Journal of Tropical Medicine and Hygiene 2000;63(1‐2):76‐9. [DOI] [PubMed] [Google Scholar]
Bouyou Akotet 2013 {published data only}
- Bouyou Akotet MK, Mawili‐Mboumba DP, Madoungou B, Kombila M. Performances of malaria P.f/Pan rapid test device Acon® (Pf HRP2/pan aldolase) and malaria Pf rapid test device Acon® (Pf HRP2) for the diagnosis of malaria in adults and children living in Gabon, Central Africa. Diagnostic Microbiology and Infectious Disease 2013;77(1):58‐63. [DOI] [PubMed] [Google Scholar]
Brenier‐Pinchart 2000 {published data only}
- Brenier‐Pinchart MP, Pinel C, Croisonnier A, Brion JP, Faure O, Ponard D, et al. Diagnosis of malaria in non‐endemic countries by the ParaSight‐F test. American Journal of Tropical Medicine and Hygiene 2000;63(3‐4):150‐2. [DOI] [PubMed] [Google Scholar]
Bruxvoort 2008 {published data only}
- Bruxvoort K, Khatib RA, Abdulah SM, Kahigwa E, Kachur SP, McMorrow ML. Variable sensitivity of malaria rapid diagnostic tests in household surveys ‐ Tanzania 2006. American Journal of Tropical Medicine and Hygiene 2008;79(6):957. [Google Scholar]
Bualombai 2003 {published data only}
- Bualombai P, Prajakwong S, Aussawatheerakul H, Congpuong K, Sudathip S, Thimasarn K, et al. Determining cost‐effectiveness and cost‐component of three malaria diagnostic models being used in remote non‐microscope areas. Southeast Asian Journal of Tropical Medicine and Public Health 2003;34(2):322‐33. [PubMed] [Google Scholar]
Bualombai 2008 {published data only}
- Bualombai P, Balachandra K, Dhepaksorn P, Congpuong K, Satimai W. The validation of DMSC Malaria Pf/Pv rapid diagnostic device for the detection of non‐falciparum malaria in Thailand in 2006. American Journal of Tropical Medicine and Hygiene 2008;79(6):958. [Google Scholar]
Buchachart 2004 {published data only}
- Buchachart K, Krudsood S, Nacher M, Chindanond D, Rungmatcha P, Kano S, et al. Evaluation of the KAT‐Quick Malaria Rapid Test for rapid diagnosis of falciparum malaria in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2004;35(1):35‐7. [PubMed] [Google Scholar]
Buhalata 2011 {published data only}
- Buhalata SN, Massaga JJ. Performance of ParaHIT and OptiMAL tests in the diagnosis of malaria in Mwanza, north‐western Tanzania. Tanzania Journal of Health Research 2011;13(1):48‐53. [DOI] [PubMed] [Google Scholar]
Bujanover 2002 {published data only}
- Bujanover S, Shwartz E. Quick detection of malaria. Israel Medical Association Journal 2002;4(12):1167. [PubMed] [Google Scholar]
Cabezas 2004 {published data only}
- Cabezas CS, Arróspide NV, Marquiño WQ, Gutiérrez SS, Álvarez EM, Chuquipiondo JR, et al. Evaluation of the use of a rapid immunochromatographic test for health workers to diagnose malaria in rural areas of the Peruvian Amazon [Evaluación del uso de una prueba rápida inmunocromatográfica en promotores de salud para el diagnóstico de la malaria en áreas rurales de la Amazonia peruana]. Revista Peruana de Medicina Experimental y Salud Publica 2004;20(1):4‐11. [Google Scholar]
Caraballo 1996 {published data only}
- Caraballo A, Ache A. The evaluation of a dipstick test for Plasmodium falciparum in mining areas of Venezuela. American Journal of Tropical Medicine and Hygiene 1996;55(5):482‐4. [DOI] [PubMed] [Google Scholar]
Carmona Fonseca 2010 {published data only}
- Carmona Fonseca J, Gallego AF, Flórez EA, Agudelo García OM, Maestre Buitrago A. Now ICT malaria Pf/Pv® frente a microscopía (gota gruesa‐extendido) para diagnóstico de malaria en Urabá (Colombia). Iatreia 2010;23(2):137‐45. [Google Scholar]
Cavallo 1997 {published data only}
- Cavallo JD, Hernandez E, Gerome P, Plotton N, Debord T, Vagueresse R. Serum HRP‐2 antigens and imported Plasmodium falciparum malaria: comparison of ParaSight‐F and ICT malaria P.f. Médecine Tropicale 1997;57(4):353‐6. [PubMed] [Google Scholar]
Chaijaroenkul 2011 {published data only}
- Chaijaroenkul W, Wongchai T, Ruangweerayut R, Na‐Bangchang K. Evaluation of rapid diagnostics for Plasmodium falciparum and P. vivax in Mae Sot malaria endemic area, Thailand. Korean Journal of Parasitology 2011;49(1):33‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatterjee 2008 {published data only}
- Chatterjee K, Chand P. Evaluation of the Rapid in Bios malaria kit for the detection of malaria LDH antigen in human blood. Vox Sanguinis 2008;95:31. [Google Scholar]
Cheng 2006 {published data only}
- Cheng A, Bell D. Evidence behind the WHO guidelines: hospital care for children: what is the precision of rapid diagnostic tests for malaria?. Journal of Tropical Pediatrics 2006;52(6):386‐9. [DOI] [PubMed] [Google Scholar]
Chilton 2006 {published data only}
- Chilton D, Malik ANJ, Armstrong M, Kettelhut M, Parker‐Williams J, Chiodini PL. Use of rapid diagnostic tests for diagnosis of malaria in the UK. Journal of Clinical Pathology 2006;59(8):862‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chinkhumba 2010 {published data only}
- Chinkhumba J, Skarbinksi J, Chilima B, Campbell C, Ewing V, San Joaquin M, et al. Comparative field performance and adherence to test results of four rapid diagnostic tests among febrile patients more than five years of age in Blantyre, Malawi. Malaria Journal 2010;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chinkhumba 2012 {published data only}
- Chinkhumba J, Nyanda M, Skarbinski J, Mathanga DP. Performance of two malaria rapid diagnostic tests in febrile adult patients with and without human immunodeficiency virus‐1 infection in Blantyre, Malawi. American Journal of Tropical Medicine and Hygiene 2012;86(2):199‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiodini 1998 {published data only}
- Chiodini PL. Non‐microscopic methods for diagnosis of malaria. Lancet 1998;351(9096):80‐1. [DOI] [PubMed] [Google Scholar]
Chiodini 2005 {published data only}
- Chiodini PL. New diagnostics in parasitology. Infectious Disease Clinics of North America 2005;19(1):267‐70. [DOI] [PubMed] [Google Scholar]
Chitkara 2004 {published data only}
- Chitkara A, Ahmed FU. Test for rapid diagnosis of Plasmodium falciparum infection. Indian Journal of Community Medicine 2004;23:173‐4. [Google Scholar]
Cho 2001 {published data only}
- Cho D, Kim KH, Park SC, Kim YK, Lee KN, Lim CS. Evaluation of rapid immunocapture assays for diagnosis of Plasmodium vivax in Korea. Parasitology Research 2001;87(6):445‐8. [DOI] [PubMed] [Google Scholar]
Cho 2011 {published data only}
- Cho C‐H, Nam MH, Kim JS, Han ET, Lee WJ, Oh JS, et al. Genetic variability in Plasmodium vivax aldolase gene in Korean isolates and the sensitivity of the Binax Now malaria test. Tropical Medicine and International Health 2011;16(2):223‐6. [DOI] [PubMed] [Google Scholar]
Cnops 2011 {published data only}
- Cnops L, Boderie M, Gillet P, Esbroeck M, Jacobs J. Rapid diagnostic tests as a source of DNA for Plasmodium species‐specific real‐time PCR. Malaria Journal 2011;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2002a {published data only}
- Coleman RE, Maneechai N, Ponlawat A, Kumpitak C, Rachapaew N, Miller RS, et al. Short report: Failure of the OptiMAL rapid malaria test as a tool for the detection of asymptomatic malaria in an area of Thailand endemic for Plasmodium falciparum and P. vivax. American Journal of Tropical Medicine and Hygiene 2002;67(6):563‐5. [DOI] [PubMed] [Google Scholar]
Coleman 2002b {published data only}
- Coleman RE, Maneechai N, Rachapaew N, Kumpitak C, Soyseng V, Miller RS, et al. Field evaluation of the ICT Malaria Pf/Pv immunochromatographic test for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. American Journal of Tropical Medicine and Hygiene 2002;66(4):379‐83. [DOI] [PubMed] [Google Scholar]
Cong le 2002 {published data only}
- Cong le D, Sergiev VP, Rabinovich SA, Nhah DH, Huong NV, Morozov EN, et al. Efficiency and specificity of the KAT‐test for rapid diagnosis of falciparum malaria. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2002;2:17‐20. [PubMed] [Google Scholar]
Cooke 1999 {published data only}
- Cooke AH, Chiodini PL, Doherty T, Moody AH, Ries J, Pinder M. Comparison of a parasite lactate dehydrogenase‐based immunochromatographic antigen detection assay (OptiMAL) with microscopy for the detection of malaria parasites in human blood samples. American Journal of Tropcial Medicine and and Hygiene 1999;60(2):173‐6. [DOI] [PubMed] [Google Scholar]
Craig 1997 {published data only}
- Craig MH, Sharp BL. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(3):279‐82. [DOI] [PubMed] [Google Scholar]
Craig 2002 {published data only}
- Craig MH, Bredenkamp BL, Williams CHV, Rossouw EJ, Kelly VJ, Kleinschmidt I, et al. Field and laboratory comparative evaluation of ten rapid malaria diagnostic tests. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(3):258‐65. [DOI] [PubMed] [Google Scholar]
Cropley 2000 {published data only}
- Cropley IM, Lockwood DN, Mack D, Pasvol G, Davidson RN. Rapid diagnosis of Falciparum malaria by using the ParaSight F test in travellers returning to the United Kingdom: prospective study. British Medical Journal 2000;321(7259):484‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuadros 2007 {published data only}
- Cuadros J, Martín‐Rabadán P, Merino FJ, Delgado‐Irribarren A, Garcia‐Bujalance S, Rubio JM. Malaria diagnosis by NOW ICT and expert microscopy in comparison with multiplex polymerase chain reaction in febrile returned travellers. European Journal of Clinical Microbiology and Infectious Diseases 2007;26(9):671‐3. [DOI] [PubMed] [Google Scholar]
Davoodian 2011 {published data only}
- Davoodian P, Daryanavard A, Safari R, Eqbal Eftekhaari T, Khalilzadeh M, Fekri S, et al. Rapid diagnosing test in malaria screening. 21st ECCMID/27th ICC; 2011 07‐10 May; Milan. Clinical Microbiology and Infection. 2011:S396.
Dawoud 2008 {published data only}
- Dawoud HA, Ageely HM, Heiba AA. Comparison of two commercial assays and microscopy with PCR for diagnosis of malaria. Journal of the Egyptian Society of Parasitology 2008;38(2):329‐38. [PubMed] [Google Scholar]
de Carsalade 2009 {published data only}
- Carsalade GY, Lam Kam R, Lepere JF, Brettes A, Peyramond D. Can the thick drop/smear examination for malaria be replaced by a rapid diagnostic test in first intention? The Mayotte experience. Médecine et Maladies Infectieuses 2009;39(1):36‐40. [DOI] [PubMed] [Google Scholar]
de Dominguez 1996 {published data only}
- Dominguez N, Rodriguez‐Acosta A. Glutamate dehydrogenase antigen detection in Plasmodium falciparum infections. Korean Journal of Parasitology 1996;34(4):239‐46. [DOI] [PubMed] [Google Scholar]
Delaunay 2008 {published data only}
- Delaunay P, Estran‐Pomares C, Marty P. Malaria diagnosis: thickdrop and bloodsmear examination, and rapid test. Médecine et Maladies Infectieuses 2008;38(Suppl 2):S121‐3. [DOI] [PubMed] [Google Scholar]
Deletoille 1987 {published data only}
- Deletoille P, Prou O. Value of rapid diagnosis of Plasmodium falciparum using indirect monoclonal immunofluorescence. Bulletin de la Société de Pathologie Exotique et de Ses Filiales 1987;80(3 Pt 2):569‐80. [PubMed] [Google Scholar]
De Monbrison 2004 {published data only}
- Monbrison F, Gérome P, Chaulet JF, Wallon M, Picot S, Peyron F. Comparative diagnostic performance of two commercial rapid tests for malaria in a non‐endemic area. European Journal of Clinical Microbiology and Infectious Diseases 2004;23(10):784‐6. [DOI] [PubMed] [Google Scholar]
de Oliveira 2007 {published data only}
- Oliveira AM, Skarbinski J, Ouma PO, Kariuki S, Barnwell JW, Otieno K, et al. Malaria rapid diagnostic test use in performance by facility‐based health workers in western Kenya. American Journal of Tropical Medicine and Hygiene 2007;77:338 (abstract number). [Google Scholar]
- Oliveira AM, Skarbinski J, Ouma PO, Kariuki S, Barnwell JW, Otieno K, et al. Peformance of malaria rapid diagnostic tests as part of routine malaria case management in Kenya. American Journal of Tropical Medicine and Hygiene 2009;80(3):470‐4. [PubMed] [Google Scholar]
Devi 2002 {published data only}
- Devi G, Indumathi VA, Sridharan D, Srinivas BPR, Sandhya BMR. Evaluation of paraHIT strip test for diagnosis of malaria infection. Indian Journal of Medical Sciences 2002;56(10):489‐94. [PubMed] [Google Scholar]
Diarra 2012 {published data only}
- Diarra A, Nébié I, Tiono A, Sanon S, Soulama I, Ouédraogo A, et al. Seasonal performance of a malaria rapid diagnosis test at community health clinics in a malaria‐hyperendemic region of Burkina Faso. Parasites and Vectors 2012;5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietze 1995 {published data only}
- Dietze R, Perkins M, Boulos M, Luz F, Reller B, Corey G R. The diagnosis of Plasmodium falciparum infection using a new antigen detection system. American Journal of Tropical Medicine and Hygiene 1995;52(1):45‐9. [DOI] [PubMed] [Google Scholar]
Di Perry 1997 {published data only}
- Perry G, Olliaro P, Nardi S, Allegranzi B, Deganello R, Vento S, et al. The Parasight‐F rapid dipstick antigen capture assay for monitoring parasite clearance after drug treatment for Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):403‐5. [DOI] [PubMed] [Google Scholar]
Di Santi 2011 {published data only}
- Santi SM, Lima G, Levi JE, Geraldi M, Arroyo Sanchez MC, Segurado AA, et al. Real‐time PCR, nested PCR and immunoassay in blood samples processed in pool, as a platform for molecular andserological diagnosis of malaria on large‐scale. 7th European Congress on Tropical Medicine and International Health; 2011 03‐06 October; Barcelona. Tropical Medicine and International Health. 2011:129.
Drakeley 2009 {published data only}
- Drakeley C, Reyburn H. Out with the old, in with the new: the utility of rapid diagnostic tests for malaria diagnosis in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(4):333‐7. [DOI] [PubMed] [Google Scholar]
Dubarry 1990 {published data only}
- Dubarry M, Luilier M, Malot N, Bayard P, Lambin P, Prou O, et al. Enzyme immunoassays for detection of malarial antigens in human plasma by Plasmodium falciparum monoclonal antibodies. American Journal of Tropical Medicine and Hygiene 1990;43(2):116‐23. [DOI] [PubMed] [Google Scholar]
Durand 2005 {published data only}
- Durand F, Faure O, Brion JP, Pelloux H. Invalid result of Plasmodium falciparum malaria detection with the BinaxNOW Malaria rapid diagnostic test. Journal of Medical Microbiology 2005;54(Pt 11):1115. [DOI] [PubMed] [Google Scholar]
Durand 2007 {published data only}
- Durand F, Crassous B, Fricker‐Hidalgo H, Carpentier F, Brion JP, Grillot R, et al. Performance of the Now Malaria rapid diagnostic test with returned travellers: a 2‐year retrospective study in a French teaching hospital. Clinical Microbiology and Infection 2007;11(11):903‐7. [DOI] [PubMed] [Google Scholar]
Durrheim 1998 {published data only}
- Durrheim DN, Govere J, Grange JJP, Mabuza A. Rapid immunochromatographic diagnosis and Rolling Back Malaria ‐ experiences from an African control programme. African Journal of Medicine and Medical Sciences 2001;30:Suppl: 21‐4. [PubMed] [Google Scholar]
- Durrheim DN, Grange JJ, Govere J, Mngomezulu NM. Accuracy of a rapid immunochromatographic card test for Plasmodium falciparum in a malaria control programme in South Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(1):32‐3. [DOI] [PubMed] [Google Scholar]
Dyer 2000 {published data only}
- Dyer ME, Tjitra E, Currie BJ, Anstey NM. Failure of the 'pan‐malarial' antibody of the ICT Malaria P.f/P.v immunochromatographic test to detect symptomatic Plasmodium malariae infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(5):518. [DOI] [PubMed] [Google Scholar]
Dzakah 2013 {published data only}
- Dzakah EE, Kang K, Ni C, Wang H, Wu P, Tang S, et al. Plasmodium vivax aldolase‐specific monoclonal antibodies and its application in clinical diagnosis of malaria infections in China. Malaria Journal 2013;12:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisen 2000 {published data only}
- Eisen DP, Saul A. Disappearance of pan‐malarial antigen reactivity using the ICT Malaria P.f/P.v (TM) kit parallels decline of patent parasitaemia as shown by microscopy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(2):169‐70. [DOI] [PubMed] [Google Scholar]
Elmardi 2009 {published data only}
- Elmardi KA, Malik EM, Abdelgadir T, Ali SH, Elsyed AH, Mudather MA, et al. Feasibility and acceptability of home‐based management of malaria strategy adapted to Sudan's conditions using artemisinin‐based combination therapy and rapid diagnostic test. Malaria Journal 2009;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Moamly 2007 {published data only}
- Moamly AMAR. Antigen capture immuno‐chromatographic strip format in detecting parasite‐specific lactate dehydrogenase to diagnose malaria in non‐immune patients. Journal of the Egyptian Society of Parasitology 2007;37(3):1017‐30. [PubMed] [Google Scholar]
Endeshaw 2008 {published data only}
- Endeshaw TG, Teshome NJ, Graves PM, Shargie EB, Ejigsemahu Y, Ayele B, et al. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malaria Journal 2008;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endeshaw 2010 {published data only}
- Endeshaw T, Graves PM, Shargie EB, Gebre T, Ayele B, Yohannes G, et al. Comparison of Parascreen Pan/Pf, Paracheck Pf and light microscopy for detection of malaria among febrile patients, Northwest Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010;104(7):467‐74. [DOI] [PubMed] [Google Scholar]
Existe 2010 {published data only}
- Existe A, Freeman N, Boncy J, Magloire R, Vely JF, Chang M, et al. Rapid tests for malaria‐Haiti 2010. Journal of the American Medical Association 2010;304(23):2585‐6. [Google Scholar]
Falade 2013 {published data only}
- Falade CO, Adesina‐Adewole B, Dada‐Adegbola HO, Ajayi IO, Akinyemi JO, Ademowo OG, et al. Evaluation of Paracheck‐PfTM rapid malaria diagnostic test for the diagnosis of malaria among HIV‐positive patients in Ibadan, south‐western Nigeria. Pathogens and Global Health 2013;107(2):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2000 {published data only}
- Fan B, Zhang ZX, Wen RS. Diagnosis of falciparum malaria using ICT. Chinese Journal of Parasitology and Parasitic Diseases 2000;18(5):281. [PubMed] [Google Scholar]
Fancony 2013 {published data only}
- Fancony C, Sebastião YV, Pires JE, Gamboa D, Nery SV. Performance of microscopy and RDTs in the context of malaria prevalence survey in Angola: a comparison using PCR as the gold standard. Malaria Journal 2013;12:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farcas 2003 {published data only}
- Farcas GA, Zhong KJY, Lovegrove FE, Graham CM, Kain KC. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travellers. American Journal of Tropical Medicine and Hygiene 2003;69(6):589‐92. [PubMed] [Google Scholar]
Farcas 2004 {published data only}
- Farcas GA, Zhong KJY, Mazzulli T, Kain KC. Evaluation of the RealArt Malaria LC real‐time PCR assay for malaria diagnosis. Journal of Clinical Microbiology 2004;42(2):636‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferro 2002 {published data only}
- Ferro BE, Gonzalez IJ, Carvajal Fd, Palma GI, Saravia NG. Performance of OptiMAL® in the diagnosis of Plasmodium vivax and Plasmodium falciparum infections in a malaria referral center in Colombia. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 2002;97(5):731‐5. [DOI] [PubMed] [Google Scholar]
Figueiredo Filho 2003 {published data only}
- Figueiredo Filho AF, Figueredo MC, Nascimento JM, Calvosa VSP, Póvoa MM, Machado RLD. Performance of an immunochromatography test for vivax malaria in the Amazon region, Brazil. Revista de Saúde Pública 2003;37(3):390‐2. [DOI] [PubMed] [Google Scholar]
Fogg 2008 {published data only}
- Fogg C, Twesigye R, Batwala V, Piola P, Nabasumba C, Kiguli J, et al. Assessment of three new parasite lactate dehydrogenase (pan‐pLDH) tests for diagnosis of uncomplicated malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):25‐31. [DOI] [PubMed] [Google Scholar]
Forney 2001 {published data only}
- Forney JR, Magill AJ, Wongsrichanalai C, Sirichaisinthop J, Bautista CT, Heppner DG, et al. Malaria rapid diagnostic devices: performance characteristics of the ParaSight F device determined in a multisite field study. Journal of Clinical Microbiology 2001;39(8):2884‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill AJ, Wongrichalanai C, Forney JR, Bautista C, Sirichasinthop A, Andersen EM, et al. Performance characteristics of a prototype malaria rapid diagnostic device (MRDD) for the detection of Plasmodium falciparum and Plasmodium vivax. Clinical Infectious Diseases 2000;31(1):472. [Google Scholar]
Forney 2003 {published data only}
- Forney JR, Wongsrichanalai C, Magill AJ, Craig LG, Sirichaisinthop J, Bautista CT, et al. Devices for rapid diagnosis of malaria: evaluation of prototype assays that detect Plasmodium falciparum histidine‐rich protein 2 and a Plasmodium vivax‐specific antigen. Journal of Clinical Microbiology 2003;41(6):2358‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fryauff 1997 {published data only}
- Fryauff DJ, Gomez‐Saladin E, Purnomo, Sumawinata I, Sutamihardja MA, Tuti S, et al. Comparative performance of the ParaSight F test for detection of Plasmodium falciparum in malaria‐immune and nonimmune populations in Irian Jaya, Indonesia. Bulletin of the World Health Organization 1997;75(6):547‐52. [PMC free article] [PubMed] [Google Scholar]
Fryauff 2000 {published data only}
- Fryauff DJ, Purnomo, Sutamihardja MA, Elyazar IR, Susanti I, Krisin, et al. Performance of the OptiMAL assay for detection and identification of malaria infections in asymptomatic residents of Irian Jaya, Indonesia. American Journal of Tropical Medicine and Hygiene 2000;63(3‐4):139‐45. [DOI] [PubMed] [Google Scholar]
Funk 1999 {published data only}
- Funk M, Schlagenhauf P, Tschopp A, Steffen R. MalaQuick versus ParaSight F as a diagnostic aid in travellers' malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(3):268‐72. [DOI] [PubMed] [Google Scholar]
Garavelli 2002 {published data only}
- Garavelli PL. Diagnosis of malaria with immunochromatographic test: The Novara experience. Recenti Progressi in Medicina 2002;93(12):682. [PubMed] [Google Scholar]
Garcia 1996 {published data only}
- Garcia M, Marlborough D. A rapid immunochromatographic tests (ICT) for the diagnosis of Plasmodium falciparum malaria. Journal of Parasitic Diseases 1996;20(1):64. [Google Scholar]
Gatti 2002 {published data only}
- Gatti S, Bernuzzi AM, Bisoffi Z, Raglio A, Gulletta M, Scaglia M, et al. Multicentre study in patients with imported malaria, on the sensitivity and specificity of a dipstick test (ICT Malaria P.f./P.v.) compared with expert microscopy. Annals of Tropical Medicine and Parasitology 2002;96(1):15‐8. [DOI] [PubMed] [Google Scholar]
Gatti 2007 {published data only}
- Gatti S, Gramegna M, Bisoffi Z, Raglio A, Gulletta M, Klersy C, et al. A comparison of three diagnostic techniques for malaria: a rapid diagnostic test (NOW Malaria), PCR and microscopy. Annals of Tropical Medicine and Parasitology 2007;101(3):195‐204. [DOI] [PubMed] [Google Scholar]
Gaye 1998 {published data only}
- Gaye O, Diouf M, Dansokho EF, McLaughlin G, Diallo S. Diagnosis of Plasmodium falciparum malaria using ParaSight F, ICT malaria Pf and malaria IgG CELISA assays. Parasite 1998;5(2):189‐92. [DOI] [PubMed] [Google Scholar]
Gaye 1999 {published data only}
- Gaye O, Diouf M, Diallo S. A comparison of thick smears, QBC malaria, PCR and PATH falciparum malaria test trip in Plasmodium falciparum diagnosis. Parasite 1999;6(3):273‐5. [DOI] [PubMed] [Google Scholar]
Gelaglie 2010 {published data only}
- Gelaglie AK. Performance of four rapid diagnostic tests for diagnosis of falciparum and non‐falciparum malaria in endemic areas of Gondar region, Northern Ethopia. 14th International Congress on Infectious Diseases (ICID); 2010 09‐12 Mar; Miami. 14th International Congress on Infectious Diseases (ICID) Abstracts. 2010.
Gerstl 2009 {published data only}
- Gerstl S, Dunkley S, Mukhtar A, Smet M, Baker A, Maikere J. Assessment of two malaria rapid diagnostic tests, with follow‐up of positive pLDH test results, in a hyperendemic falciparum malaria area. Tropical Medicine and International Health 2009;14(Suppl 2):92. [Google Scholar]
Ghanchi 2009 {published data only}
- Ghanchi NK, Beg MA, Hussain R. Estimation of parasite load using rapid diagnostic test ICT (R) Now Malaria P.f/P.v in Plasmodium falciparum malaria. Scandinavian Journal of Infectious Diseases 2009;41(8):597‐601. [DOI] [PubMed] [Google Scholar]
Ghosh 2000 {published data only}
- Ghosh SK, Titus Burk E, Valecha N, Murugendrappa MV, Sharma VP. Evaluation of a rapid immunochromatographic test (ICT) for detection of Plasmodium falciparum malaria in Karnataka, India. Journal of Parasitic Diseases 2000;24:39‐42. [Google Scholar]
Ghouth 2012 {published data only}
- Ghouth AS, Nasseb FM, Al‐Kaldy KH. The accuracy of the first response histidine‐rich protein2 rapid diagnostic test compared with malaria microscopy for guiding field treatment in an outbreak of falciparum malaria. Tropical Parasitology 2012;2(1):35‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillet 2009a {published data only}
- Gillet P, Bosselaers K, Cnops L, Bottieau E, Esbroeck M, Jacobs J. Evaluation of the SD FK70 malaria Ag Plasmodium vivax rapid diagnostic test in a non‐endemic setting. Malaria Journal 2009;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillet 2009b {published data only}
- Gillet P, Mori M, Esbroeck M, Ende J, Jacobs J. Assessment of the prozone effect in malaria rapid diagnostic tests. Malaria Journal 2009;8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillet 2009c {published data only}
- Gillet P, Dijk DP, Bottieau E, Cnops L, Esbroeck M, Jacobs J. Test characteristics of the SD FK80 Plasmodium falciparum/ Plasmodium vivax malaria rapid diagnostic test in a non‐endemic setting. Malaria Journal 2009;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillet 2011 {published data only}
- Gillet P, Scheirlinck A, Stokx J, Weggheleire A, Chaúque HS, Canhanga OD, et al. Prozone in malaria rapid diagnostics tests: how many cases are missed?. Malaria Journal 2011;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gogtay 1999 {published data only}
- Gogtay NJ, Kotwani RN, Rajgor D, Kanbur A, Karnad DR, Kshirsagar NA. Serial ParaSight‐F test in patients with severe malaria. Indian Journal of Malariology 1999;36(3‐4):94‐5. [PubMed] [Google Scholar]
Gogtay 2003 {published data only}
- Gogtay NJ, Dalvi SS, Rajgor D, Chogle AR, Karnad DR, Ramdas M, et al. Diagnostic and prognostic utility of rapid strip (OptiMAL and Paracheck) versus conventional smear microscopy in adult patients of acute, uncomplicated P. falciparum malaria in Mumbai, India. Journal of the Association of Physicians of India 2003;51:762‐5. [PubMed] [Google Scholar]
Goh 2013 {published data only}
- Goh XT, Lim YA, Vythilingam I, Chew CH, Lee PC, Ngui R, et al. Increased detection of Plasmodium knowlesi in Sandakhan division, Sabah as revealed by PlasmoNexTM. Malaria Journal 2013;12:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomes 2013 {published data only}
- Gomes LT, Tada MS, Katsuragawa TH, Povoa MM, Viana GMR, Alecrim MdGC, et al. Low sensitivity of malaria rapid diagnostic tests stored at room temperature in the Brazilian Amazon region. Journal of Infection in Developing Countries 2013;7(3):243‐52. [DOI] [PubMed] [Google Scholar]
Gonzáles‐Cerón 2005 {published data only}
- González‐Cerón L, Rodríguez MH, Betanzos AF, Abadía A. Efficacy of a rapid test to diagnose Plasmodium vivax in symptomatic patients of Chiapas, Mexico. Salud Pública de México 2005;47(4):282‐7. [DOI] [PubMed] [Google Scholar]
Grobusch 1999 {published data only}
- Grobusch MP, Alpermann U, Schwenke S, Jelinek T, Warhurst DC. False‐positive rapid tests for malaria in patients with rheumatoid factor. Lancet 1999;353(9149):297. [DOI] [PubMed] [Google Scholar]
Grobusch 2002 {published data only}
- Grobusch MP, Hänscheid T, Zoller T, Jelinek T, Burchard GD. Rapid immunochromatographic malarial antigen detection unreliable for detecting Plasmodium malariae and Plasmodium ovale. European Journal of Clinical Microbiology and Infectious Diseases 2002;21(11):818‐20. [DOI] [PubMed] [Google Scholar]
Grobusch 2003a {published data only}
- Grobusch MP, Hänscheid T, Göbels K, Slevogt H, Zoller T, Rögler G, et al. Sensitivity of P. vivax rapid antigen detection tests and possible implications for self‐diagnostic use. Travel Medicine and Infectious Disease 2003;1(2):119‐22. [DOI] [PubMed] [Google Scholar]
Grobusch 2003b {published data only}
- Grobusch MP, Hänscheid T, Göbels K, Slevogt H, Zoller T, Rögler G, et al. Comparison of three antigen detection tests for diagnosis and follow‐up of falciparum malaria in travellers returning to Berlin, Germany. Parasitology Research 2003;89(5):354‐7. [DOI] [PubMed] [Google Scholar]
Gupta 2001 {published data only}
- Gupta MK, Misra RN, Chawla N, Mani H, Chowdhry CN, Singh SP. Immunochromatographic test: a new dimensions in diagnosis of Plasmodium falciparum malaria. Medical Journal of the Armed Forces of India 2001;57(3):188‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guthmann 2002 {published data only}
- Guthmann JP, Ruiz A, Priotto G, Kiguli J, Bonte L, Legros D. Validity, reliability and ease of use in the field of five rapid tests for the diagnosis of Plasmodium falciparum malaria in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(3):254‐7. [DOI] [PubMed] [Google Scholar]
Gutierrez 2005 {published data only}
- Gutierrez Y, Paco G, Romero L, Gonzáles J‚ Peñar LM, Gimenez T. Fluorometers; a rapid and simple method for evaluating the Antimalarial Activity [Fluorometría; un método rápido y sencillo para evaluar la Actividad Antipalúdica]. Biofarbo 2005;13(13):3‐10. [Google Scholar]
Hada 2011 {published data only}
- Hada S, Das ML, Singh YI. Diagnostic methods of malaria in Eastern Nepal: a comparative study of traditional and two rapid diagnostic tests. Nepal Medical College Journal: NMCJ 2011;13(4):261‐6. [PubMed] [Google Scholar]
Haditsch 2004 {published data only}
- Haditsch M. Quality and reliability of current malaria diagnostic methods. Travel Medicine and Infectious Disease 2004;2(3‐4):149‐60. [DOI] [PubMed] [Google Scholar]
Hance 2005 {published data only}
- Hance P, Garnotel E, Pina JJ, Vedy S, Ragot C, Chadli M, et al. Rapid immunochromatographic tests for detection of malaria: principles and strategies for use. Médecine Tropicale 2005;65(4):389‐93. [PubMed] [Google Scholar]
Hänscheid 1999 {published data only}
- Hänscheid T. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clinical and Laboratory Haematology 1999;21(4):235‐45. [DOI] [PubMed] [Google Scholar]
Happi 2004 {published data only}
- Happi CT, Gbotosho GO, Sowunmi A, Falade CO, Akinboye DO, Oladepo O, et al. Malaria diagnosis: false negative ParaSight‐F tests in falciparum malaria patients in Nigeria. African Journal of Medicine and Medical Sciences 2004;33(1):15‐8. [PubMed] [Google Scholar]
Harchut 2013 {published data only}
- Harchut K, Standley C, Dobson A, Klaassen B, Rambaud‐Althaus C, Althaus F, et al. Over‐diagnosis of malaria by microscopy in the Kilombero Valley, Southern Tanzania: an evaluation of the utility and cost‐effectiveness of rapid diagnostic tests. Malaria Journal 2013;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashizume 2006 {published data only}
- Hashizume M, Kondo H, Murakami T, Kodama M, Nakahara S, Lucas MES, et al. Use of rapid diagnostic tests for malaria in an emergency situation after the flood disaster in Mozambique. Public Health 2006;120(5):444‐7. [DOI] [PubMed] [Google Scholar]
Hawkes 2009 {published data only}
- Hawkes M, Katsuva JP, Masumbuko CK. Use and limitations of malaria rapid diagnostic testing by community health workers in war‐torn Democratic Republic of Congo. Malaria Journal 2009;8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandes 2001 {published data only}
- Hernandez E, Pina JJ, Fabre R, Garrabe E, Raphenon G, Cavallo JD. Evaluation of the OptiMal test in the diagnosis of imported malarial outbreak. Médecine Tropicale 2001;61(2):153‐7. [PubMed] [Google Scholar]
Holmberg 1992 {published data only}
- Holmberg M, Wahlberg J, Lundeberg J, Pettersson U, Uhlén M. Colorimetric detection of Plasmodium falciparum and direct sequencing of amplified gene fragments using a solid phase method. Molecular and Cellular Probes 1992;6(3):201‐8. [DOI] [PubMed] [Google Scholar]
Hopkins 2007 {published data only}
- Hopkins H, Kambale W, Kamya MR, Staedke SG, Dorsey G, Rosenthal PJ. Comparison of HRP2 and pLDH‐based rapid diagnostic tests for malaria with longitudinal follow‐up in Kampala, Uganda. American Journal of Tropical Medicine and Hygeine 2007;76(6):1092‐7. [PubMed] [Google Scholar]
Hopkins 2008 {published data only}
- Hopkins H, Bebell L, Kambales W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. Journal of Infectious Diseases 2008;197(4):510‐8. [DOI] [PubMed] [Google Scholar]
Hossain 2008 {published data only}
- Hossain MA, Afroj S, Rahman MR, Yunus EB, Samad R, Asna ZH, et al. Evaluation of alternative diagnostic techniques for diagnosis of cerebral malaria in a tertiary referral hospital in Bangladesh. Mymensingh Medical Journal 2008;17(2):180‐5. [PubMed] [Google Scholar]
Houmsou 2011 {published data only}
- Houmsou RS, Amuta EU, Sar TT, Adagba AH. Malarial infection among patients attending a Nigerian semi‐urban based hospital and performance of HRP‐2 pf Rapid diagnostic Test (RDT) in screening clinical cases of Plasmodium falciparum malaria. Translational Biomedicine 2011;2:1. [Google Scholar]
Houzé 2009 {published data only}
- Houzé S, Boly MD, Bras J, Deloron P, Faucher JF. PfHRP‐2 and PfLDH antigen detection for monitoring the efficacy of artemisinin‐based combination therapy (ACT) in the treatment of uncomplicated falciparum malaria. Malaria Journal 2009;8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houzé 2011 {published data only}
- Houzé S, Hubert V, Cohen DP, Rivetz B, Bras J. Evaluation of the Clearview® Malaria pLDH Malaria Rapid Diagnostic Test in a non‐endemic setting. Malaria Journal 2011;10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humar 1997 {published data only}
- Humar A, Ohrt C, Harrington MA, Pillai D, Kain KC. Parasight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. American Journal of Tropical Medicine and Hygiene 1997;56(1):44‐8. [DOI] [PubMed] [Google Scholar]
Huong 2002 {published data only}
- Huong NM, Davis TME, Hewitt S, Huong N, Uyen TT, Nhan DH, et al. Comparison of three antigen detection methods for diagnosis and therapeutic monitoring of malaria: a field study from southern Vietnam. Tropical Medicine and International Health 2002;7(4):304‐8. [DOI] [PubMed] [Google Scholar]
Iqbal 2000 {published data only}
- Iqbal J, Sher A, Rab A. Plasmodium falciparum histidine‐rich protein 2‐based immunocapture diagnostic assay for malaria: cross‐reactivity with rheumatoid factors. Journal of Clinical Microbiology 2000;38(3):1184‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 2001 {published data only}
- Iqbal J, Hira PR, Sher A, Al‐Enezi AA. Diagnosis of imported malaria by Plasmodium lactate dehydrogenase (pLDH) and histidine‐rich protein 2 (PfHRP‐2)‐based immunocapture assays. American Journal of Tropical Medicine and Hygiene 2001;64(1‐2):20‐3. [DOI] [PubMed] [Google Scholar]
Iqbal 2002 {published data only}
- Iqbal J, Khalid N, Hira PR. Comparison of two commercial assays with expert microscopy for confirmation of symptomatically diagnosed malaria. Journal of Clinical Microbiology 2002;40(12):4675‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 2003 {published data only}
- Iqbal J, Muneer A, Khalid N, Ahmed MA. Performance of the OptiMAL test for malaria diagnosis among suspected malaria patients at the rural health centres. American Journal of Tropical Medicine and Hygiene 2003;68(5):624‐8. [DOI] [PubMed] [Google Scholar]
Iqbal 2004 {published data only}
- Iqbal J, Siddique A, Jameel M, Hira PR. Persistent histidine‐rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. Journal of Clinical Microbiology 2004;42(9):4237‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishengoma 2011 {published data only}
- Ishengoma DS, Francis F, Mmbando BP, Lusingu JP, Magistrado P, Alifrangis M, et al. Accuracy of malaria rapid diagnostic tests in community studies and their impact on treatment of malaria in an area with declining malaria burden in north‐eastern Tanzania. Malaria Journal 2011;10:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2013 {published data only}
- Jang JW, Cho CH, Han ET, An SS, Lim CS. pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test. Malaria Journal 2013;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jelinek 1996 {published data only}
- Jelinek T, Kilian AH, Henk M, Mughusu EB, Nothdurft HD, Löscher T, et al. Parasite‐specific lactate dehydrogenase for the diagnosis of Plasmodium falciparum infection in an endemic area in west Uganda. Tropical Medicine and International Health 1996;1(2):227‐30. [DOI] [PubMed] [Google Scholar]
Jelinek 1999 {published data only}
- Jelinek T, Grobusch MP, Schwenke S, Steidl S, Sonnenburg F, Nothdurft HD, et al. Sensitivity and specificity of dipstick tests for rapid diagnosis of malaria in nonimmune travelers. Journal of Clinical Microbiology 1999;37(3):721‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jelinek 2000 {published data only}
- Jelinek T, Grobusch MP, Nothdurft HD. Use of dipstick tests for the rapid diagnosis of malaria in nonimmune travelers. Journal of Travel Medicine 2000;7(4):175‐9. [DOI] [PubMed] [Google Scholar]
Jelinek 2001 {published data only}
- Jelinek T, Grobusch MP, Harms G. Evaluation of a dipstick test for the rapid diagnosis of imported malaria among patients presenting within the network TropNetEurop. Scandinavian Journal of Infectious Diseases 2001;33(10):752‐4. [DOI] [PubMed] [Google Scholar]
Jeurissen 1999 {published data only}
- Jeurissen A, Beert J. Two rapid tests for the detection of Plasmodium falciparum. Tijdschrift voor Geneeskunde 1999;55:1088‐92. [Google Scholar]
John 1998 {published data only}
- John SM, Sudarsanam A, Sitaram U, Moody AH. Evaluation of OptiMAL, a dipstick test for the diagnosis of malaria. Annals of Tropical Medicine and Parasitology 1998;92(5):621‐2. [DOI] [PubMed] [Google Scholar]
Joshi 2004 {published data only}
- Joshi HH, Mahakunkijcharoen Y, Tantivanich S, Sharma AP, Khusmith S. Detection of P. vivax antigens in malaria endemic populations of Nepal by ELISA using monoclonal antibodies raised against Thai isolates. Southeast Asian Journal of Tropical Medicine and Public Health 2004;35(4):828‐33. [PubMed] [Google Scholar]
Kaewsonthi 1996 {published data only}
- Kaewsonthi S, Harding AG, Kidson C, Indaratna K. Assessing the economic impact of a rapid on‐site malaria diagnostic test. Southeast Asian Journal of Tropical Medicine and Public Health 1996;27(2):210‐5. [PubMed] [Google Scholar]
Kahama‐Maro 2008 {published data only}
- Kahama‐Maro J, D'Acremont V, Mtasiwa D, Genton B, Lengeler C. Low quality of routine microscopy for malaria at different health systems levels in Dar es Salaam: rapid diagnostic tests should also be implemented in hospitals and urban settings. American Journal of Tropical Medicine and Hygiene 2008;79(6):394. [Google Scholar]
Kahama‐Maro 2011 {published data only}
- Kahama‐Maro J, D'Acremont V, Mtasiwa D, Genton B, Lengeler C. Low quality of routine microscopy for malaria at different levels of the health system in Dar es Salaam. Malaria Journal 2011;10:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakkilaya 2003 {published data only}
- Kakkilaya BS. Rapid diagnosis of malaria. Laboratory Medicine 2003;34(8):602‐8. [Google Scholar]
Kamugisha 2008 {published data only}
- Kamugisha ML, Msangeni H, Beale E, Malecela EK, Akida JI, Ishengoma DR, et al. Paracheck Pf compared with microscopy for diagnosis of Plasmodium falciparum malaria among children in Tanga City, north‐eastern Tanzania. Tanzania Journal of Health Research 2008;10(1):14‐9. [DOI] [PubMed] [Google Scholar]
Kar 1998 {published data only}
- Kar I, Eapen A, Adak T, Sharma VP. Trial with ParaSight‐F in the detection of Plasmodium falciparum infection in Chennai (Tamil Nadu) India. Indian Journal of Malariology 1998;35(3):160‐2. [PubMed] [Google Scholar]
Karbwang 1996 {published data only}
- Karbwang J, Tasanor O, Kanda T, Wattanagoon Y, Ibrahim M, Na‐Bangchang K, et al. ParaSight‐F test for the detection of treatment failure in multidrug resistant Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(5):513‐5. [DOI] [PubMed] [Google Scholar]
Karimov 2011 {published data only}
- Karimov SS, Baranova AM, Saĭburkhonov DS. [Evaluation of the efficiency of rapid tests to detect malaria patients and parasite carriers in Tajikistan]. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2011;3:46‐7. [PubMed] [Google Scholar]
Kashif 2013 {published data only}
- Kashif AH, Adam GK, Mohmmed AA, Elzaki SE, AbdelHalim AM, Adam I. Reliability of rapid diagnostic test for diagnosing peripheral and placental malaria in an area of unstable malaria transmission in Eastern Sudan. Diagnostic Pathology 2013;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katakai 2011 {published data only}
- Katakai Y, Komaki‐Yasuda K, Tangpukdee N, Wilairatana P, Krudsood S, Kano S. Evaluation of the NOW malaria immunochromatographic test for quantitative diagnosis of falciparum and vivax malaria parasite density. Tropical Medicine and Health 2011;39(4):105‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kattenberg 2011 {published data only}
- Kattenberg J, Tahita M, Taal A, Zoeten J, Versteeg I, Tinto H, et al. Antigen persistence of rapid diagnostic tests and its implications for the diagnosis of malaria in pregnancy. An evaluation in Nanoro, Burkina Faso. 7th European Congress on Tropical Medicine and International Health; 2011 03‐06 Oct; Barcelona. Tropical Medicine and International Health. 2011:138‐9. [DOI] [PubMed]
Kaur 2000 {published data only}
- Kaur H, Mani A. Evaluation & usefulness of a immunochromatographic test for rapid detection of Plasmodium falciparum infection. Indian Journal of Medical Sciences 2000;54(10):421‐4. [PubMed] [Google Scholar]
Kaushal 1995 {published data only}
- Kaushal DC, Kaushal N, Chandra D, Palni R. Immunodiagnosis of malaria based on detection of parasite enzyme. Journal of Parasitic Diseases 1995;19:21‐4. [Google Scholar]
Kaushal 1997 {published data only}
- Kaushal DC, Kaushal NA. Immunodiagnosis of malaria. Journal of Parasitic Diseases 1997;21(1):31‐40. [Google Scholar]
Kawai 2009 {published data only}
- Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. Cross‐reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitology International 2009;58(3):300‐2. [DOI] [PubMed] [Google Scholar]
Keating 2009 {published data only}
- Keating J, Miller JM, Bennett A, Moonga HB, Eisele TP. Plasmodium falciparum parasite infection prevalence from a household survey in Zambia using microscopy and a rapid diagnostic test: implications for monitoring and evaluation. Acta Tropica 2009;112(3):277‐82. [DOI] [PubMed] [Google Scholar]
Khairnar 2009 {published data only}
- Khairnar K, Martin D, Lau R, Ralevski F, Pillai DR. Multiplex real‐time quantitative PCR, microscopy and rapid diagnostic immuno‐chromatographic tests for the detection of Plasmodium spp: performance, limit of detection analysis and quality assurance. Malaria Journal 2009;8:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2004 {published data only}
- Khan SA, Anwar M, Hussain S, Qureshi AH, Ahmad A, Afzal S. Comparison of OptiMAL malarial test with light microscopy for the diagnosis of malaria. Journal of the Pakistan Medical Association 2004;54(8):404‐7. [PubMed] [Google Scholar]
Kilian 1997 {published data only}
- Kilian AHD, Mughusu EB, Kabagambe G, Sonnenburg F. Comparison of two rapid, HRP‐2‐based diagnostic tests for Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(6):666‐7. [DOI] [PubMed] [Google Scholar]
Kilian 1999 {published data only}
- Kilian AHD, Kabagambe G, Byamukama W, Langi P, Weis P, Sonnenburg F. Application of the ParaSight‐F dipstick test for malaria diagnosis in a district control programme. Acta Tropica 1999;72(3):281‐93. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim SH, Nam MH, Roh KH, Park HC, Nam DH, Park GH, et al. Evaluation of a rapid diagnostic test specific for Plasmodium vivax. Tropical Medicine and International Health 2008;13(12):1495‐500. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim KH, Jang JW, Woo MK, Oh JS, Han ET, Lee WJ, et al. Evaluation of four rapid diagnostic tests for the diagnosis of Plasmodium vivax in Korea. Tropical Medicine and International Health 2011;16(11):1427‐31. [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim JY, Ji SY, Goo YK, Na BK, Pyo HJ, Lee HN, et al. Comparison of rapid diagnostic tests for the detection of Plasmodium vivax malaria in South Korea. PLoS One 2013;8(5):e64353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knappik 2002 {published data only}
- Knappik M, Peyerl‐Hoffmann G, Jelinek T. Plasmodium falciparum: use of a NANP19 antibody‐test for the detection of infection in non‐immune travellers. Tropical Medicine and International Health 7;8:652‐6. [DOI] [PubMed] [Google Scholar]
Kodisinghe 1997 {published data only}
- Kodisinghe HM, Perera KL, Premawansa S, Naotunne T, Wickramasinghe AR, Mendis KN. The ParaSight‐F dipstick test as a routine diagnostic tool for malaria in Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):398‐402. [DOI] [PubMed] [Google Scholar]
Koita 2012 {published data only}
- Koita OA, Doumbo OK, Ouattara A, Tall LK, Konaré A, Diakité M, et al. False‐negative rapid diagnostic tests for malaria and deletion of the histidine‐rich repeat region of the hrp2 gene. American Journal of Tropical Medicine and Hygiene 2012;86(2):194‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 1996 {published data only}
- Kumar A, Sharma VP, Thavaselvam D, Sumodan PK. Clinical trials of a new immunochromatographic test for diagnosis of Plasmodium falciparum malaria in Goa. Indian Journal of Malariology 1996;33(4):166‐72. [PubMed] [Google Scholar]
Kumar 2000 {published data only}
- Kumar A, Sumodan PK, Sharma VP. Clinical trials of an indigenous diagnostic kit Paracheck‐F for the diagnosis of Plasmodium falciparum malaria in Goa. Journal of Parasitic Diseases 2000;24:43‐5. [Google Scholar]
Kumar 2004 {published data only}
- Kumar KR, Sudarshan KS. Clinical evaluation of a rapid diagnostic kit (Paracheck‐Pf) for diagnosis of Plasmodium falciparum in Karnataka state of India. Indian Journal of Preventive and Social Medicine 2004;35(1):10‐4. [Google Scholar]
Kumar 2012 {published data only}
- Kumar N, Singh JPN, Pande V, Mishra N, Srivastava B, Kapoor R, et al. Genetic variation in histidine rich proteins among Indian Plasmodium falciparum population: possible cause of variable sensitivity of malaria rapid diagnostic tests. Malaria Journal 2012;11:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2013 {published data only}
- Kumar N, Pande V, Bhatt RM, Shah NK, Mishra N, Srivastava B, et al. Genetic deletion of HRP2 and HRP3 in Indian Plasmodium falciparum population and false negative malaria rapid diagnostic test. Acta Tropica 2013;125(1):119‐21. [DOI] [PubMed] [Google Scholar]
Kweka 2011 {published data only}
- Kweka EJ, Lowassa A, Msangi S, Kimaro EE, Lyatuu EE, Mwang'onde BJ, et al. Low sensitivity of paraHIT‐F rapid malaria test among patients with fever in rural health centers, Northern Tanzania. Journal of Infection in Developing Countries 2011;5(3):204‐8. [DOI] [PubMed] [Google Scholar]
Kyabayinze 2008 {published data only}
- Kyabayinze DJ. Field validity and comparative persistent antigenicity of HRP‐2 rapid diagnostic tests for malaria in a hyperendemic region of Uganda. American Journal of Tropical Medicine and Hygiene 2008;79(6):884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malaria Journal 2008;7:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labbé 2001 {published data only}
- Labbé AC, Pillai DR, Hongvangthong B, Vanisaveth V, Pomphida S, Inkathone S, et al. The performance and utility of rapid diagnostic assays for Plasmodium falciparum malaria in a field setting in the Lao People's Democratic Republic. Annals of Tropical Medicine and Parasitology 95;7:671‐7. [DOI] [PubMed] [Google Scholar]
Lee 1999 {published data only}
- Lee MA, Aw LT, Singh M. A comparison of antigen dipstick assays with polymerase chain reaction (PCR) technique and blood film examination in the rapid diagnosis of malaria. Annal of the Academy of Medicine of Singapore 1999;28(4):498‐501. [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee SW, Jeon K, Jeon BR, Park I. Rapid diagnosis of vivax malaria by the SD Bioline Malaria Antigen test when thrombocytopenia is present. Journal of Clinical Microbiology 2008;46(3):939‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee GC, Jeon ES, Le DT, Kim TS, Yoo JH, Kim HY, et al. Development and evaluation of a rapid diagnostic test for Plasmodium falciparum, P. vivax, and mixed‐species malaria antigens. American Journal of Tropical Medicine and Hygiene 2011;85(6):989‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lema 1999 {published data only}
- Lema OE, Carter JY, Nagelkerke N, Wangai MW, Kitenge P, Gikunda SM, et al. Comparison of five methods of malaria detection in the outpatient setting. American Journal of Tropical Medicine and Hygiene 1999;60(2):177‐82. [DOI] [PubMed] [Google Scholar]
Lepère 2004 {published data only}
- Lepère JF, Macarry A. Malaria diagnosis and treatment in a rural Health Centre in Mayotte (Comoro archipelago, 2002). Santé 2004;14(1):5‐10. [PubMed] [Google Scholar]
Lim 2001 {published data only}
- Lim HS, Kim HS. Evaluation of diagnostic methods of re‐emerging malaria in Korean patients. Yonsei Medical Journal 2001;42(1):84‐90. [DOI] [PubMed] [Google Scholar]
Llanos Zavalaga 2000 {published data only}
- Llanos Zavalaga LF, Huayta Zacarías E, Mendoza Requena D, Rosas Aguirre A, Contreras Ríos C, Peinada Rodríguez J. Knowledge and perceptions of health workers in malaria endemic areas in Peru on the rapid test Parasight‐F [Conocimientos y percepciones de los trabajadores de salud de zona endémica de malaria en el Perú sobre la prueba de diagnóstico rápido ParaSight‐F]. Revista Medica Herediana 2000;11(4):115‐21. [Google Scholar]
Llanos‐Zavalaga 2002 {published data only}
- Llanos‐Zavalaga LF, Villacorta JV, Reyes RL, Lecca LG, Mendoza DR, Mayca JP, et al. Evaluation of the ICT Test Malaria P.f/P.v (AMRAD®) for the detection of P. falciparum and P. vivax malaria in an endemic area of the Peruvian Amazon [Evaluación de la prueba ICT Malaria P.f/P.v (AMRAD®) para la detección de P. falciparum y P. vivax en una zona endémica de la Amazonía peruana]. Revista Peruana de Medicina Experimental y Salud Publica 2002;19(1):39‐42. [Google Scholar]
Mahajan 2000 {published data only}
- Mahajan SK, Siwach SR, Kishore K, Chaudhry D, Sen R, Aggarwal HK, et al. Evaluation of a rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. Indian Practitioner 2009;53(5):325‐9. [Google Scholar]
Makler 1998 {published data only}
- Makler MT, Piper RC, Milhous WK. Lactate dehydrogenase and the diagnosis of malaria. Parasitology Today 1998;14(9):376‐7. [DOI] [PubMed] [Google Scholar]
Makler 2009 {published data only}
- Makler MT, Piper RC. Rapid malaria tests: where do were go after 20 years?. American Journal of Tropical Medicine and Hygiene 2009;81(6):921‐6. [DOI] [PubMed] [Google Scholar]
Malik 2004 {published data only}
- Malik S, Khan S, Das A, Samantaray JC. Plasmodium lactate dehydrogenase assay to detect malarial parasites. National Medical Journal of India 2004;17(5):237‐9. [PubMed] [Google Scholar]
Mankhambo 2002 {published data only}
- Mankhambo L, Kanjala M, Rudman S, Lema VM, Rogerson SJ. Evaluation of the OptiMAL rapid antigen test and species‐specific PCR to detect placental Plasmodium falciparum infection at delivery. Journal of Clinical Microbiology 2002;40(1):155‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2002 {published data only}
- Mason DP, Kawamoto F, Lin K, Laoboonchai A, Wongsrichanalai C. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Tropica 2002;82(1):51‐9. [DOI] [PubMed] [Google Scholar]
Mawili‐Mboumba 2010 {published data only}
- Mawili‐Mboumba DP, Bouyou Akotet MK, Ngoungou EB, Kombila M. Evaluation of rapid diagnostic tests for malaria case management in Gabon. Diagnostic Microbiology and Infectious Disease 2010;66(2):162‐8. [DOI] [PubMed] [Google Scholar]
Mayxay 2004 {published data only}
- Mayxay M, Newton PN, Yeung S, Pongvongsa T, Phompida S, Phetsouvanh T, et al. Short communication: An assessment of the use of malaria rapid tests by village health volunteers in rural Laos. Tropical Medicine and International Health 2004;9(3):325‐9. [DOI] [PubMed] [Google Scholar]
Mboera 2006a {published data only}
- Mboera LEG, Fanello CI, Malima RC, Talbert A, Fogliati P, Bobbio F, et al. Comparison of the Paracheck‐Pf test with microscopy, for the confirmation of Plasmodium falciparum malaria in Tanzania. Annals of Tropical Medicine and Parasitology 2006;100(2):115‐22. [DOI] [PubMed] [Google Scholar]
McCutchan 2008 {published data only}
- McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerging Infectious Diseases 2008;14(11):1750‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMorrow 2010 {published data only}
- McMorrow ML, Masanja MI, Kahigwa E, Abdullah SMK, Kachur SP. Quality assurance of rapid diagnostic tests for malaria in routine patient care in rural Tanzania. American Journal of Tropical Medicine and Hygiene 2010;82(1):151‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meena 2009 {published data only}
- Meena M, Joshi D, Joshi R, Sridhar S, Waghdhare S, Gangane N, et al. Accuracy of a multispecies rapid diagnostic test kit for detection of malarial parasite at the point of care in a low endemicity region. Transactions of the Royal Society of Tropical Medicine and Hygeine 2009;103(12):1237‐44. [DOI] [PubMed] [Google Scholar]
Menan 1996 {published data only}
- Menan EIH, Adou‐Bryn KD, Mobio SP, Cisse M, Penali K, Kone M. Assessment of parasitological examinations of blood for malaria at the Pasteur Institute Côte d'Ivoire (IPCI) in 1992: the impact of chemotherapy on the laboratory results [Bilan des examens parasitologiques du sang pour la recherche du paludisme a l'Institut Pasteur de Côte d'Ivoire (I.P.C.I) en 1992: impact de la chimiotherapie sur les resultats de laboratoire]. Medecine d'Afrique Noire 1996;43(3):129‐33. [Google Scholar]
Mendiratta 2006 {published data only}
- Mendiratta DK, Bhutada K, Narang R, Narang P. Evaluation of different methods for diagnosis of P. falciparum malaria. Indian Journal of Medical Microbiology 2006;24(1):49‐51. [DOI] [PubMed] [Google Scholar]
Mendoza 2007 {published data only}
- Mendoza NM, García M, Cortés LJ, Vela C, Erazo R, Pérez P, et al. Evaluation of two rapid diagnostic tests, NOW ICT Malaria Pf/Pv and OptiMAL, for diagnosis of malaria. Biomedica 2007;27(4):571‐80. [PubMed] [Google Scholar]
Mendoza 2013 {published data only}
- Mendoza NM, Cucunuba ZM, Aponte S, Gonzalez NE, Bernal SD. [Field evaluation for diagnostic accuracy of the rapid test SD Bioline Malaria Antigen Pf/Pv(R) in Colombia]. Biomedica 2013;33(4):587‐597. [DOI] [PubMed] [Google Scholar]
Mengesha 1999 {published data only}
- Mengesha T, Gebreselassie H, Mohammed T, Assefa T, Woldemichael T. ParaSight‐F dipstick antigen tests in the diagnosis of falciparum malaria in Ethiopia. East African Medical Journal 1999;76(11):626‐9. [PubMed] [Google Scholar]
Mens 2007 {published data only}
- Mens P, Spieker N, Omar S, Heijnen M, Schallig H, Kager PA. Is molecular biology the best alternative for diagnosis of malaria to microscopy? A comparison between microscopy, antigen detection and molecular tests in rural Kenya and urban Tanzania. Tropical Medicine and International Health 2007;12(2):238‐44. [DOI] [PubMed] [Google Scholar]
Mens 2010 {published data only}
- Mens PF, Matelon RJ, Nour BYM, Newman DM, Schallig HDFH. Laboratory evaluation on the sensitivity and specificity of a novel rapid detection method for malaria diagnosis based on magneto‐optical technology (MOT). Malaria Journal 2010;9:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger 2008 {published data only}
- Metzger WG, Vivas‐Martínez S, Rodriguez I, Gonçalves J, Bongard E, Fanello CI, et al. Malaria diagnosis under field conditions in the Venezuelan Amazon. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):20‐4. [DOI] [PubMed] [Google Scholar]
Metzger 2009 {published data only}
- Metzger WG, Giron AM, Vivas‐Martínez S, González J, Charrasco AJ, Mordmüller BG, et al. A rapid malaria appraisal in the Venezuelan Amazon. Malaria Journal 2009;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mharakurwa 1997a {published data only}
- Mharakurwa S, Shiff CJ. Post treatment sensitivity studies with the ParaSight‐F test for malaria diagnosis in Zimbabwe. Tropical Medicine and International Health 1997;66(2):61‐7. [DOI] [PubMed] [Google Scholar]
Mharakurwa 1997b {published data only}
- Mharakurwa S, Manyame B, Shiff CJ. Trial of ParaSight‐F test for malaria diagnosis in the primary health care system, Zimbabwe. Tropical Medicine & International Health 1997;2(6):544‐60. [DOI] [PubMed] [Google Scholar]
Miantuasila 2012 {published data only}
- Miantuasila O. Comparison between molecular and conventional diagnostic tools for the diagnosis of malaria and sickle cell gene carriage. 6th EDCTP Forum: Strengthening Research Partnerships for Better Health and Sustainable Development; 2011 09‐12 October; Addis Ababa. Tropical Medicine and International Health. 2012:57‐8.
Mikhail 2011 {published data only}
- Mikhail AF, Leslie TJ, Mayan MI, Zekria R, Mohammad N, Hasanzai MA, et al. Field trial of three different Plasmodium vivax‐detecting rapid diagnostic tests with and without evaporative cool box storage in Afghanistan. Malaria Journal 2011;10:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2001 {published data only}
- Miller RS, McDaniel P, Wongsrichanalai C. Following the course of malaria treatment by detecting parasite lactate dehydrogenase enzyme. British Journal of Haematology 2001;113(2):558‐62. [DOI] [PubMed] [Google Scholar]
Miller 2008 {published data only}
- Miller RS. Comparison of performance characteristics of the Binax NOW Malaria test using venous and fingerstick samples. American Journal of Tropical Medicine and Hygiene 2008;79(6):533. [Google Scholar]
Mills 1999 {published data only}
- Mills CD, Burgess DC, Taylor HJ, Kain KC. Evaluation of a rapid and inexpensive dipstick assay for the diagnosis of Plasmodium falciparum malaria. Bulletin of the World Health Organization 1999;77(7):553‐9. [PMC free article] [PubMed] [Google Scholar]
Mills 2007 {published data only}
- Mills LA, Blank LR, Kagaayi J, Aluma S, Shott J, Bwanika JB, et al. Performance of malaria rapid diagnostic test versus traditional microscopy among rural Ugandan outpatients. American Journal of Tropical Medicine and Hygiene 2007;75(5):96. [Google Scholar]
Mills 2010 {published data only}
- Mills LA, Kagaayi J, Nakigozi G, Galiwango RM, Ouma J, Shott JP, et al. Short report: Utility of a point‐of‐care malaria rapid diagnostic for excluding malaria as the cause of fever among HIV‐positive adults in rural Rakai, Uganda. American Journal of Tropical Medicine and Hygiene 2010;82(1):145‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2010a {published data only}
- Mills LA, Kagaayi J, Shott JP, Newell K, Bwanika JB, Ssempijja V, et al. Performance of prototype malaria rapid diagnostic test versus thick film microscopy among HIV‐postive subjects in rural Rakai, Uganda. Transactions of the Royal Society of Tropical Medicine in Hygiene 2010;104(3):237‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minja 2012 {published data only}
- Minja DT, Schmiegelow C, Oesterholt M, Magistrado PA, Boström S, John D, et al. Reliability of rapid diagnostic tests in diagnosing pregnancy‐associated malaria in north‐eastern Tanzania. Malaria Journal 2012;11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minodier 2005 {published data only}
- Minodier P. Malaria diagnosis: rapid detection tests. Clinical Microbiology Reviews 2005;18(8):386‐8. [Google Scholar]
- Minodier P, Noël G, Blanc P, Retornaz K, Garnier JM. Tests for rapid diagnosis of malaria. Archives de Pediatrie 2005;12(6):697‐9. [DOI] [PubMed] [Google Scholar]
Mishra 1999 {published data only}
- Mishra B, Samantaray J C, Mirdha BR. Evaluation of a rapid antigen capture assay for the diagnosis of falciparum malaria. Indian Journal of Medical Research 1999;109:16‐9. [PubMed] [Google Scholar]
Mishra 2007 {published data only}
- Mishra MN, Misra RN. Immunochromatographic methods in malaria diagnosis. Medical Journal Armed Forces India 2007;63(2):127‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohanty 1999 {published data only}
- Mohanty S, Mishra SK, Mohanty A, Das BS. Immunochromatographic test for the diagnosis of falciparum malaria. Journal of the Association of Physicians of India 1999;47(2):201‐2. [PubMed] [Google Scholar]
Mohapatra 1996 {published data only}
- Mohapatra PK, Prakash A, Khan AM, Bhattacharyya DR, Goswami BK, Mahanta J. Evaluation of a manual immunochromatographic test for detection of Plasmodium falciparum HRP‐2 antigen. Indian Journal of Medical Microbiology 1996;14(4):193‐5. [Google Scholar]
Montoya 2008 {published data only}
- Montoya AE, Menco J, Osorio N, Zuluaga MA, Duque J, Torres G, et al. Concordance between thick blood smear, immunochromatography and polymerase chain reaction for malaria diagnosis. Biomedica 2008;28(2):252‐61. [PubMed] [Google Scholar]
Moody 2000 {published data only}
- Moody A, Hunt‐Cooke A, Gabbett E, Chiodini P. Performance of the OptiMAL malaria antigen capture dipstick for malaria diagnosis and treatment monitoring at the Hospital for Tropical Diseases, London. British Journal of Haematology 2000;109(4):891‐4. [DOI] [PubMed] [Google Scholar]
Moody 2002a {published data only}
- Moody A. Rapid diagnostic tests for malaria parasites. Clinical Microbiology Reviews 2002;15(1):66‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moody 2002b {published data only}
- Moody AH, Chiodini PL. Non‐microscopic method for malaria diagnosis using OptiMAL IT, a second‐generation dipstick for malaria pLDH antigen detection. British Journal of Biomedical Science 2002;59(4):228‐31. [DOI] [PubMed] [Google Scholar]
Moonasar 2007 {published data only}
- Moonasar D, Goga AE, Frean J, Kruger P, Chandramohan D. An exploratory study of factors that affect the performance and usage of rapid diagnostic tests for malaria in the Limpopo Province, South Africa. Malaria Journal 2007;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moonasar 2009 {published data only}
- Moonasar D, Goga AE, Kruger PS, Cock C, Maharaj R, Frean J, et al. Field evaluation of a malaria rapid diagnostic test (ICT Pf). South African Medical Journal 2009;99(11):810‐3. [PubMed] [Google Scholar]
Morankar 2011 {published data only}
- Morankar S, Tegene A, Kassahun W, Sulueiman S, Negatu YA, Yazachew M, et al. Validity and reliability of RDT for diagnosis of malaria among febrile children in Jimma Town: southwest Ethiopia. Ethiopian Medical Journal 2011;49(2):131‐8. [PubMed] [Google Scholar]
Moulin 2009 {published data only}
- Moulin F, Gendrel D. Imported malaria: diagnostic traps and rapid tests. Archives de Pédiatrie 2009;16(Suppl 2):S89‐S92. [DOI] [PubMed] [Google Scholar]
Msellem 2009 {published data only}
- Msellem MI, Mårtensson A, Rotllant G, Bhattarai A, Strömberg J, Kahigwa E, et al. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar: a crossover validation study. PLoS Medicine 2009;6(4):e1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mtove 2011 {published data only}
- Mtove G, Hendriksen ICE, Amos B, Mrema H, Mandia V, Manjurano A, et al. Treatment guided by rapid diagnostic tests for malaria in Tanzanian children: safety and alternative bacterial diagnoses. Malaria Journal 2011;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueller 2007 {published data only}
- Mueller I, Betuela I, Ginny M, Reeder JC, Genton B. The sensitivity of the OptiMAL rapid diagnostic test to the presence of Plasmodium falciparum gametocytes compromises its ability to monitor treatment outcomes in an area of Papua New Guinea in which malaria is endemic. Journal of Clinical Microbiology 2007;45(2):627‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muhindo 2012 {published data only}
- Muhindo HM, Ilombe G, Meya R, Mitashi PM, Kutekemeni A, Gasigwa D, et al. Accuracy of malaria rapid diagnosis test Optimal‐IT(®) in Kinshasa, the Democratic Republic of Congo. Malaria Journal 2012;11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munier 2009 {published data only}
- Munier A, Diallo A, Sokhna C, Chippaux JP. Assessment of a rapid diagnostic test for malaria in rural health care facilities in Senegal. Medicine Tropicale 2009;69(5):496‐500. [PubMed] [Google Scholar]
Murahwa 1999 {published data only}
- Murahwa FC, Mharakurwa S, Mutambu SL, Rangarira R, Musana BJ. Diagnostic performance of two antigen capture tests for the diagnosis of Plasmodium falciparum malaria in Zimbabwe. Central African Journal of Medicine 1999;45(4):97‐100. [DOI] [PubMed] [Google Scholar]
Murray 2003 {published data only}
- Murray CK, Bell D, Gasser RA, Wongsrichanalai C. Rapid diagnostic testing for malaria. Tropical Medicine and International Health 2003;8(10):876‐83. [DOI] [PubMed] [Google Scholar]
Murray 2008 {published data only}
- Murray CK, Gasser RA Jr, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clinical Microbiology Reviews 2008;21(1):97‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwanza 2005 {published data only}
- Mwanza S, Njunju E, Mbewe B, Chileshe N, Mataa N, Kalungwana N. Evaluation of the hexagon malaria rapid diagnostic test kit in five communities on the copperbelt province of Zambia. Acta Tropica 2005;95S:S303‐4. [Google Scholar]
Myjak 2004 {published data only}
- Myjak P, Nahorski W, Zarnowska‐Prymek H, Pietkiewicz H. Usefulness of the "OptiMAL Rapid Malaria test" for rapid detection of malaria imported to Poland. Wiadomości Parazytologiczne 2004;50(2):193‐9. [PubMed] [Google Scholar]
Naing 2002a {published data only}
- Cho‐Min‐Naing, Gatton ML. Performance appraisal of rapid on‐site malaria diagnosis (ICT Malaria Pf/Pv test) in relation to human resources at village level Myanmar. Acta Tropica 2002;81(1):13‐9. [DOI] [PubMed] [Google Scholar]
Nema 2005 {published data only}
- Nema SK, Chopra GS, Gupta RM, Rai R, Diwan RN. Diagnosis of malaria infection using non‐radioactive malaria diagnostic system (NOMADS). Medical Journal Armed Forces India 2005;61(4):336‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2008 {published data only}
- Neumann CG, Bwibo NO, Siekmann JH, McLean ED, Browdy B, Drorbaugh N. Comparison of blood smear microscopy to a rapid diagnostic test for in‐vitro testing for P. falciparum malaria in Kenyan school children. East African Medical Journal 2008;85(11):544‐9. [DOI] [PubMed] [Google Scholar]
Nicastri 2009a {published data only}
- Nicastri E, Bevilacqua N, Sañé Schepisi SM, Paglia MG, Meschi S, Ame SM, et al. Accuracy of malaria diagnosis by microscopy, rapid diagnostic test, and PCR methods and evidence of antimalarial overprescription in non‐severe febrile patients in two Tanzanian hospitals. American Journal of Tropical Medicine and Hygiene 2009;80(5):712‐7. [PubMed] [Google Scholar]
Nigussie 2008 {published data only}
- Nigussie D, Legesse M, Animut A, Mariam AH, Mulu A. Evaluation of Paracheck Pf and Parascreen Pan/Pf tests for the diagnosis of malaria in an endemic area, South Ethiopia. Ethiopian Medical Journal 2008;46(4):375‐81. [PubMed] [Google Scholar]
Nkrumah 2010 {published data only}
- Nkrumah B, Agyekum A, Acquah SEK, May J, Tannich E, Brattig N, et al. Comparison of the novel Partec rapid diagnostic test to the conventional giemsa stain and the gold standard real‐time PCR. Journal of Clinical Microbiology 2010;48(8):2925‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nkrumah 2011 {published data only}
- Nkrumah B, Acquah SE, Ibrahim L, May J, Brattig N, Tannich E, et al. Comparative evaluation of two rapid field tests for malaria diagnosis: Partec Rapid Malaria Test® and Binax Now® Malaria Rapid Diagnostic Test. BMC Infectious Diseases 2011;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nour 2011 {published data only}
- Nour B. Using RDTS as a malaria diagnostic tool in wad Medani different hospitals ‐ Central Sudan. 7th European Congress on Tropical Medicine and International Health; 2011 03‐06 Oct; Barcelona. Tropical Medicine and International Health. 2011:149.
Nwuba 2001 {published data only}
- Nwuba RI, Anumuda CI, Omosun YO, Sodeinde O, Nwagwu M. Evaluation of a rapid immunochromatographic card test for Plasmodium falciparum in Ibadan, Nigeria. African Journal of Medical Science 2001;30(1‐2):123‐4. [PubMed] [Google Scholar]
Nyunt 2013 {published data only}
- Nyunt MH, Kyaw MP, Win KK, Myint KM, Nyunt KM. Field evaluation of HRP2 and pan pLDH‐based immunochromatographic assay in therapeutic monitoring of uncomplicated falciparum malaria in Myanmar. Malaria Journal 2013;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochola 2006 {published data only}
- Ochola LB, Vounatsou P, Smith T, Mabaso MLH, Newton CRJC. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infectious Diseases 2006;6(9):582‐8. [DOI] [PubMed] [Google Scholar]
Omar 1999 {published data only}
- Omar MS, Malik GM, Al‐Amari OM, Abdalla SE, Moosa RA. The rapid manual ParaSight‐F test for diagnosing Plasmodium falciparum malaria in Saudi Arabia. Annals of Saudi Medicine 1999;19(2):159‐62. [DOI] [PubMed] [Google Scholar]
OMS 1999 {published data only}
- Organisation Mondiale de la Sante USAID. Directive pour l'evaluation rapide: reconnaissance des symptomes de maladies pour le paludisme grave et complique. OMS/TDR/USAID, 1999. [Google Scholar]
Onile 2005 {published data only}
- Onile B, Taiwo S. Recent advances in the laboratory diagnosis of malaria. African Journal of Clinical and Experimental Microbiology 2005;6(2):113‐23. [Google Scholar]
Osman 2010 {published data only}
- Osman MMM, Nour BYM, Sedig MF, Bes L, Babikir AM, Mohamedani AA, et al. Informed decision‐making before changing to RDT: a comparison of microscopy, rapid diagnostic test and molecular techniques for the diagnosis and identification of malaria parasites in Kassala, eastern Sudan. Tropical Medicine and International Health 2010;15(12):1442‐8. [DOI] [PubMed] [Google Scholar]
Ouattara 2011 {published data only}
- Ouattara A, Doumbo S, Saye R, Beavogui AH, Traoré B, Djimdé A, et al. Use of a pLDH‐based dipstick in the diagnostic and therapeutic follow‐up of malaria patients in Mali. Malaria Journal 2011;10:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozbilge 2006 {published data only}
- Ozbilge H, Kurcer MA, Dogan N, Zeyrek F. Comparison with Pan Malaria IgG assays for malaria diagnosis and direct microscopy among suspected malaria patients in Sanliurfa. Tropical Doctor 2006;36(1):25‐6. [DOI] [PubMed] [Google Scholar]
Pabon 2007 {published data only}
- Pabón A, Alvarez G, Yánez J, Céspedes C, Rodríguez Y, Restrepo A, et al. Evaluation of ICT malaria immunochromatographic Binax NOW® ICT P.f/P.v test for rapid diagnosis of malaria in a Colombian endemic area. Biomedica 2007;27(2):225‐35. [PubMed] [Google Scholar]
Pakalapati 2013 {published data only}
- Pakalapati D, Garg S, Middha S, Kochar A, Subudhi AK, Arunachalam BP, et al. Comparative evaluation of microscopy, OptiMAL® and 18S rRNA gene based multiplex PCR for detection of Plasmodium falciparum & Plasmodium vivax from field isolates of Bikaner, India. Asian Pacific Journal of Tropical Medicine 2013;6(5):346‐51. [DOI] [PubMed] [Google Scholar]
Palmer 1998 {published data only}
- Palmer CJ, Lindon JF, Klaskala WI, Quesada JA, Kaminsky R, Baum MK, et al. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. Journal of Clinical Microbiology 1998;36(1):203‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 1999 {published data only}
- Palmer CJ, Validum L, Lindo J, Campa A, Validum C, Makler M, et al. Field evaluation of OptiMAL rapid malaria diagnostic test during anti‐malarial therapy in Guyana. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(5):517‐8. [DOI] [PubMed] [Google Scholar]
Palmer 2003 {published data only}
- Palmer CJ, Bonilla JA, Bruckner DA, Barnett ED, Miller NS, Haseeb MA, et al. Multicenter study to evaluate the OptiMAL test for rapid diagnosis of malaria in U.S hospitals. Journal of Clinical Microbiology 2003;41(11):5178‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pammenter 1988 {published data only}
- Pammenter MD. Techniques for the diagnosis of malaria. South African Medical Journal 1988;74(2):55‐7. [PubMed] [Google Scholar]
Pandey 1995 {published data only}
- Pandey J, Talib VH, Ranga S, Gulati IRA, Pandey J, Ranga S. Diagnosis of malaria: an overview. Journal of Parasitic Diseases 1995;19(1):21‐4. [Google Scholar]
Pandya 2001 {published data only}
- Pandya AP, Sahu GC, Anjan JK. The Para Check‐PC Test: ‐ a simple rapid dip stick test to detect Plasmodium falciparum infection. Journal of Communicable Diseases 2001;33(3):224‐5. [PubMed] [Google Scholar]
Park 2003 {published data only}
- Park SK, Lee KW, Hong SH, Kim DS, Lee JH, Jeon BH, et al. Development and evaluation of an immunochromatographic kit for the detection of antibody to Plasmodium vivax infection in South Korea. Yonsei Medical Journal 2003;44(4):747‐50. [DOI] [PubMed] [Google Scholar]
Park 2006 {published data only}
- Park TS, Kim JH, Kang CI, Lee BH, Jeon BR, Lee SM, at al. Diagnostic usefulness of SD malaria antigen and antibody kits for differential diagnosis of vivax malaria in patients with fever of unknown origin. Korean Journal of Laboratory Medicine 2006;26(4):241‐5. [DOI] [PubMed] [Google Scholar]
Parra 1991 {published data only}
- Parra ME, Evans CB, Taylor DW. Identification of Plasmodium falciparum histidine‐rich protein 2 in the plasma of humans with malaria. Journal of Clinical Microbiology 1991;29(8):1629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2012 {published data only}
- Peng YP, Wu JL, Wang JH, Li WM, Yu SJ. Study and evaluation of Wondfo rapid diagnostic kit based on nano‐gold immunochromatography assay for diagnosis of Plasmodium falciparum. Parasitology Research 2012;110(4):1421‐5. [DOI] [PubMed] [Google Scholar]
Penhalbel 2005 {published data only}
- Penhalbel Rde S, Fugikaha E, Lorenzetti A, Alves RT, Cavasini CE, Rossit ARB, et al. Evaluation of an immunochromatography test for malaria diagnosis under different storage conditions. Revista de Sociedad Brasileira de Medicina Tropical 2005;38(2):194‐5. [DOI] [PubMed] [Google Scholar]
Pérez 2007 {published data only}
- Pérez H, Bracho C, Rosa M. Malaria and rapid diagnostic tests [El paludismo y las pruebas rápidas de diagnóstico]. Boletín de Malariología y Salud Ambiental 2007;47(1):3‐13. [Google Scholar]
Peyron 1999 {published data only}
- Peyron F. Parasitological diagnosis of malaria: Routine and new laboratory techniques. Médecine et Maladies Infectieuses 1999;29(Suppl 3):295‐301. [Google Scholar]
Phommanivong 2010 {published data only}
- Phommanivong V, Thongkham K, Deyer G, Rene JP, Barennes H. An assessment of early diagnosis and treatment of malaria by village health volunteers in the Lao PDR. Malaria Journal 2010;9:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pica 2005 {published data only}
- Pica R, Castellano C. Looking for parasitic infection and disease: the Plasmodium falciparum malaria model. Clinica Terapeutica 2005;156(3):131‐4. [PubMed] [Google Scholar]
Pieroni 1998 {published data only}
- Pieroni P, Mills CD, Ohrt C, Harrington MA, Kain KC. Comparison of the ParaSight‐F test and the ICT Malaria Pf test with the polymerase chain reaction for the diagnosis of Plasmodium falciparum malaria in travellers. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(2):166‐9. [DOI] [PubMed] [Google Scholar]
Pinto 1999 {published data only}
- Pinto MJW, Pereira NF, Rodrigues S, Kharangate NV, Verenkar MP. Rapid diagnosis of falciparum malaria by detection of Plasmodium falciparum HRP‐2 antigen. Journal of the Association of Physicians of India 1999;47(11):1076‐8. [PubMed] [Google Scholar]
Piper 1999 {published data only}
- Piper R, Lebras J, Wentworth L, Hunt‐Cooke A, Houzé S, Chiodini P, et al. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). American Journal of Tropical Medicine and Hygiene 1999;60(1):109‐18. [DOI] [PubMed] [Google Scholar]
Pividal 1994 {published data only}
- Pividal J, Monjane AL, Gomes A, Street E, Barreto A. Evaluation and selection of diagnostic techniques in direct malaria [Avaliacao e seleccao de tecnicas de diagnostico directo na malaria]. Revista Medica de Mocambique 1994;5(3):27‐32. [Google Scholar]
Planche 2001 {published data only}
- Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou‐Milama E, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. American Journal of Tropical Medicine and Hygiene 2001;65(5):599‐602. [DOI] [PubMed] [Google Scholar]
Playford 2002 {published data only}
- Playford EG, Walker J. Evaluation of the ICT malaria P.f/P.v and the OptiMal rapid diagnostic tests for malaria in febrile returned travellers. Journal of Clinical Microbiology 2002;40(11):4166‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Popov 2000 {published data only}
- Popov AF, Popova NI. Rapid methods for the diagnosis of tropical malaria. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2000;2:38‐9. [PubMed] [Google Scholar]
Popov 2004 {published data only}
- Popov AF, Nikiforov ND, Ivanis VA, Barkun SP, Sanin BI, Fed'kina LI. Diagnosis of malaria by express methods. Klinicheskaia Laboratornaia Diagnosis 2004;1:46‐8. [PubMed] [Google Scholar]
Premji 1994 {published data only}
- Premji Z, Minjas J N, Shiff CJ. Laboratory diagnosis of malaria by village health workers using the rapid manual ParaSight‐F test. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(4):418. [DOI] [PubMed] [Google Scholar]
Prou 1988 {published data only}
- Prou O, Deletoille P. Rapid detection of Plasmodium falciparum antigens by monofluo Kit Plasmodium falciparum. Médecine et Maladies Infectieuses 1988;18(2):75‐9. [Google Scholar]
Proux 2001 {published data only}
- Proux S, Hkirijareon L, Ngamngonkiri C, McConnell S, Nosten F. Short communication: Paracheck‐Pf®: A new, inexpensive and reliable rapid test for P. falciparum malaria. Tropical Medicine and International Health 2001;6(2):99‐101. [DOI] [PubMed] [Google Scholar]
Quintana 1998 {published data only}
- Quintana M, Piper R, Boling HL, Makler M, Sherman C, Gill E, et al. Malaria diagnosis by dipstick assay in a Honduran population with coendemic Plasmodium falciparum and Plasmodium vivax. American Journal of Tropical Medicine and Hygiene 1998;59(6):868‐71. [DOI] [PubMed] [Google Scholar]
Rabinovich 2006 {published data only}
- Rabinovich SA, Le DK, Nguen VH, Morozov EN, Toropov DE, Kukina IV, et al. Efficiency of KAT‐quick P.f. test (KAT medical, SAR) among the populations of drug‐resistant parasites. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2006;2:10‐2. [PubMed] [Google Scholar]
Radrianasolo 2007 {published data only}
- Radrianasolo L, Tafangy PB, Raharimalala LA, Ratsimbasoa AC, Randriamanantena A, Randrianarivelojosia M. Rapid diagnostic test for malaria: preliminary study in Madagascar in 2003. Santé 2007;17(2):69‐73. [PubMed] [Google Scholar]
Rahim 2002 {published data only}
- Rahim F, Haq HA, Jamal S. Comparison of amradict test with microscopic examinations for rapid diagnosis of malaria. Journal of the College of Physicians and Surgeons Pakistan 2002;12(9):530‐3. [Google Scholar]
Rajendran 2006 {published data only}
- Rajendran C, Dube S. Field evaluation of rapid immunochromatographic test kit for the diagnosis of Plasmodium falciparum and non‐falciparum malaria parasites for Sontipur Distric, Assam. Journal of Parasitic Diseases 2006;30(1):94‐7. [Google Scholar]
Ramutton 2012 {published data only}
- Ramutton T, Hendriksen IC, Mwanga‐Amumpaire J, Mtove G, Olaosebikan R, Tshefu AK, et al. Sequence variation does not confound the measurement of plasma PfHRP2 concentration in African children presenting with severe malaria. Malaria Journal 2012;11:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratnawati 2008 {published data only}
- Ratnawati MH, Hatta M, Smits HL. Point‐of‐care testing for malaria outbreak management. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(7):699‐704. [DOI] [PubMed] [Google Scholar]
Ratsimbasoa 2012 {published data only}
- Ratsimbasoa A, Ravony H, Vonimpaisomihanta JA, Raherinjafy R, Jahevitra M, Rapelanoro R, et al. Management of uncomplicated malaria in febrile under five‐year‐old children by community health workers in Madagascar: reliability of malaria rapid diagnostic tests. Malaria Journal 2012;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rehlis 2004 {published data only}
- Rehlis N, Javor P. Interpretation of immunochromatographic tests with HRP‐2 antigen in children under 5 years in an area of high risk of malaria transmission in Papua New Guinea [Interpretacja testow immunochromatographiczynch z antygenem HRP‐2 dzieci do lat 5 w rejonie o wysokim ryzyku transmisji zimnicy w Papua Nowej Gwinei]. Wiadomości Parazytologiczne 2004;50(2):201‐8. [PubMed] [Google Scholar]
Reyburn 2007 {published data only}
- Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, et al. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ 2007;334(7590):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ricci 2000 {published data only}
- Ricci L, Viani I, Piccolo G, Fabio A, Calderaro A, Galati L, et al. Evaluation of OptiMAL Assay test to detect imported malaria in Italy. New Microbiologica 2000;23(4):391‐8. [PubMed] [Google Scholar]
Richardson 2002 {published data only}
- Richardson DC, Ciach M, Zhong KJY, Crandall I, Kain KC. Evaluation of the Makromed dipstick assay versus PCR for diagnosis of Plasmodium falciparum malaria in returned travelers. Journal of Clinical Microbiology 2002;40(12):4528‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richter 2004a {published data only}
- Richter J, Göbels K, Müller‐Stöver I, Hoppenheit B, Häussinger D. Co‐reactivity of plasmodial histidine‐rich protein 2 and aldolase on a combined immuno‐chromographic‐malaria dipstick (ICT) as a potential semi‐quantitative marker of high Plasmodium falciparum parasitaemia. Parasitology Research 2004;94(5):384‐5. [DOI] [PubMed] [Google Scholar]
Richter 2004b {published data only}
- Richter J, Harms G, Müller‐Stöver I, Göbels K, Häussinger D. Performance of an immunochromatographic test for the rapid diagnosis of malaria. Parasitology Research 2004;92(6):518‐9. [DOI] [PubMed] [Google Scholar]
Rimón 2003 {published data only}
- Rimón MM, Kheng S, Hoyer S, Thach V, Ly S, Permin AE, et al. Malaria dipsticks beneficial for IMCI in Cambodia. Tropical Medicine and International Health 2003;8(6):536‐43. [DOI] [PubMed] [Google Scholar]
Roche 1995 {published data only}
- Roche J, Benito A, Ayecaba S, Amela C, Molina R, Alvar J. Field evaluation of fluorescence microcopy (QBC) for malaria diagnosis. Bulletin de Liaison et de Documentation de L'OCEAC 1995;28(1):26‐9. [Google Scholar]
Rodríguez‐Iglesias 2005 {published data only}
- Rodríguez‐Iglesias M. Rapid serological techniques. Enfermedades Infecciosas y Microbiologia Clinica Monografias 2005;4(2):69‐71. [Google Scholar]
Rodulfo 2007 {published data only}
- Rodulfo H, Donato M, Mora R, González L, Contreras CE. Comparison of the diagnosis of malaria by microscopy, immunochromatography and PCR in endemic areas of Venezuela. Brazilian Journal of Medical and Biological Research 2007;40(4):535‐43. [DOI] [PubMed] [Google Scholar]
Rolland 2006 {published data only}
- Rolland E, Checchi F, Pinoges L, Balkan S, Guthmann JP, Guerin PJ. Operational response to malaria epidemics: are rapid diagnostic tests cost‐effective?. Tropical Medicine and International Health 2006;11(4):398‐408. [DOI] [PubMed] [Google Scholar]
Rosenthal 2012 {published data only}
- Rosenthal PJ. How do we best diagnose malaria in Africa?. American Journal of Tropical Medicine and Hygiene 2012;86(2):192‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubio 2001 {published data only}
- Rubio JM, Buhigas I, Subirats M, Baquero M, Puente S, Benito A. Limited level of accuracy provided by available rapid diagnosis tests for malaria enhances the need for PCR‐based reference laboratories. Journal of Clinical Microbiology 2001;39(7):2736‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Runsewe‐Abiodun 2012 {published data only}
- Runsewe‐Abiodun IT, Efunsile M, Ghebremedhin B, Sotimehin AS, Ajewole J, Akinleye J, et al. Malaria diagnostics: a comparative study of blood microscopy, a rapid diagnostic test and polymerase chain reaction in the diagnosis of malaria. Journal of Tropical Pediatrics 2012;58(2):163‐4. [DOI] [PubMed] [Google Scholar]
Ryan 2002 {published data only}
- Ryan JR, Davé K, Collins KM, Hochberg L, Sattabongkot J, Coleman RE, et al. Extensive multiple test centre evaluation of the VecTest malaria antigen panel assay. Medical and Veterinary Entomology 2002;16(3):321‐7. [DOI] [PubMed] [Google Scholar]
Samal 1998 {published data only}
- Samal KK, Agarwalla A. Intradermal smear vs peripheral blood smear in diagnosis of malaria. Indian Practitioner 1998;51(1):27‐8. [Google Scholar]
Saranya 2003 {published data only}
- Saranya N. Rapid diagnostic tests, benefits and pitfalls. Indian Journal of Practical Pediatrics 2003;5(2):111‐7. [Google Scholar]
Sayang 2009 {published data only}
- Sayang C, Soula G, Tahar R, Basco LK, Gazin P, Moyou‐Somo R, et al. Use of a histidine‐rich protein 2‐based rapid diagnostic test for malaria by health personnel during routine consultation of febrile outpatients in a peripheral health facility in Yaounde, Cameroon. American Journal of Tropical Medicine and Hygiene 2009;81(2):343‐7. [PubMed] [Google Scholar]
Schachterle 2011 {published data only}
- Schachterle SE, Mtove G, Levens JP, Clemens EG, Shi L, Raj A, et al. Prevalence and density‐related concordance of three diagnostic tests for malaria in a region of Tanzania with hypoendemic malaria. Journal of Clinical Microbiology 2011;49(11):3885‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2003 {published data only}
- Schmidt WP. Malaria rapid diagnostic tests ‐ perspectives for malaria endemic and non‐endemic regions. Laboratoriums Medizin 2003;27(7‐8):296‐301. [Google Scholar]
Schmidt 2011 {published data only}
- Schmidt BA, Mubi M, Premji Z, Ngasala BE, Martensson A, Bjorkman A. Clearance of Plasmodium falciparum as assessed withmicroscopy, RDT and PCR after anti‐malarial treatment in Tanzanian children. 7th European Congress on Tropical Medicine and International Health; 2011 03‐06 October; Barcelona. Tropical Medicine and International Health 2011:111. [Google Scholar]
Seidahmed 2008 {published data only}
- Seidahmed OME, Mohamedein MMN, Elsir AA, Ali FT, Malik el F, Ahmed ES. End‐user errors in applying two malaria rapid diagnostic tests in a remote area of Sudan. Tropical Medicine and International Health 2008;13(3):406‐9. [DOI] [PubMed] [Google Scholar]
Senn 2012 {published data only}
- Senn N, Rarau P, Manong D, Salib M, Siba P, Robinson LJ, et al. Rapid diagnostic test‐based management of malaria: an effectiveness study in Papua New Guinean infants with Plasmodium falciparum and Plasmodium vivax malaria. Clinical Infectious Diseases 2012;54(5):644‐51. [DOI] [PubMed] [Google Scholar]
Sezibera 2009 {published data only}
- Sezibera C. Fever and treatment of malaria: importance of a strategy of diagnostic‐treatment of first class level health services [Fièvre et traitement du paludisme: importance d'une stratégie de diagnostic‐traitement au niveau des services de santé de premier echelon]. Thesis 2009.
Shah 2004 {published data only}
- Shah I, Deshmukh CT. A bedside dipstick method to detect Plasmodium falciparum. Indian Pediatrics 2004;41(11):1148‐51. [PubMed] [Google Scholar]
Shaikh 2013 {published data only}
- Shaikh S, Memon S, Memon H, Ahmed I. Role of rapid daignostic tests for guiding outpatient treatment of febrile illness in Liaquat University Hospital. Pakistan Journal of Medical Science 2013;29(5):1167‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakya 2012 {published data only}
- Shakya G, Gupta R, Pant SD, Poudel P, Upadhaya B, Sapkota A, et al. Comparative study of sensitivity of rapid diagnostic (hexagon) test with calculated malarial parasitic density in peripheral blood. Journal of Nepal Health Research Council 2012;10(1):16‐9. [PubMed] [Google Scholar]
Shamsi 1999 {published data only}
- Shamsi TS, Ahmed A, Farooqui AI, Waraich S. Rapid diagnosis of malaria: a new approach. Journal of the Pakistan Medical Association 1999;49(1):16‐7. [PubMed] [Google Scholar]
Sharma 1999 {published data only}
- Sharma SK, Tyagi PK, Haque MA, Padhan K. Field studies on the sensitivity and specificity of an immunochromatographic test for the detection of Plasmodium falciparum malaria in tribal areas of Orissa. Indian Journal of Malariology 1999;36(3‐4):65‐9. [PubMed] [Google Scholar]
Sharma 2008 {published data only}
- Sharma MK, Rao VK, Agarwal GS, Rai GP, Gopalan N, Prakash S, et al. Highly sensitive amperometric immunosensor for detection of Plasmodium falciparum histidine‐rich protein 2 in serum of humans with malaria: comparison with a commercial kit. Journal of Clinical Microbiology 2008;46(11):3759‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
She 2007 {published data only}
- She RC, Rawlins ML, Mohl R, Perkins SL, Hill HR, Litwin CM. Comparison of immunofluorescence antibody testing and two enzyme immunoassays in the serologic diagnosis of malaria. Journal of Travel Medicine 2007;14(2):105‐11. [DOI] [PubMed] [Google Scholar]
Shenoi 1996 {published data only}
- Shenoi UD. Laboratory diagnosis of malaria. Indian Journal of Pathology and Microbiology 1996;39(5):443‐5. [PubMed] [Google Scholar]
Shiff 1993 {published data only}
- Shiff CJ, Minjas J, Premji Z. The ParaSight‐F test: a simple rapid manual dipstick test to detect Plasmodium falciparum infection. Parasitology Today 1994;10(12):494‐5. [DOI] [PubMed] [Google Scholar]
- Shiff CJ, Premji Z, Minjas JN. The rapid manual ParaSight‐F test. A new diagnostic tool for Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(6):646‐8. [DOI] [PubMed] [Google Scholar]
Shillcutt 2008 {published data only}
- Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJM, et al. Cost‐effectiveness of malaria diagnostic methods in sub‐Saharan Africa in an era of combination therapy. Bulletin of the World Health Organization 2008;86(2):101‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirayama 2008 {published data only}
- Shirayama Y, Phompida S, Kuroiwa C. Monitoring malaria control in Khammouane province, Laos: an active case detection survey of Plasmodium falciparum malaria using the Paracheck rapid diagnostic test. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(8):743‐50. [DOI] [PubMed] [Google Scholar]
Shujatullah 2006 {published data only}
- Shujatullah F, Malik A, Khan HM, Malik A. Comparison of different diagnostic techniques in Plasmodium falciparum cerebral malaria. Journal of Vector Borne Diseases 2006;43(4):186‐90. [PubMed] [Google Scholar]
Shujatullah 2009 {published data only}
- Shujatullah F, Khan HM, Malik A, Malik A. Evaluation of ParaSight‐F test in diagnosis of Plasmodium falciparum infection. JK Science 2009;11(1):16‐9. [Google Scholar]
Singer 2004 {published data only}
- Singer LM, Newman RD, Diarra A, Moran AlC, Huber CS, Stennies G, et al. Evaluation of a malaria rapid diagnostic test for assessing the burden of malaria during pregnancy. American Journal of Tropical Medicine and Hygiene 2004;70(5):481‐5. [PubMed] [Google Scholar]
Singh 1997a {published data only}
- Singh N, Valecha N, Sharma VP. Malaria diagnosis by field workers using an immunochromatographic test. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):396‐7. [DOI] [PubMed] [Google Scholar]
Singh 1997b {published data only}
- Singh N, Singh MP, Sharma VP. The use of a dipstick antigen‐capture assay for the diagnosis of Plasmodium falciparum infection in a remote forested area of Central India. American Journal of Tropical Medicine and Hygiene 1997;56(2):188‐91. [DOI] [PubMed] [Google Scholar]
Singh 2000b {published data only}
- Singh N. Usefulness of a dipstick test (ParaSight‐F) in high‐risk groups for Plasmodium falciparum in Central India. Current Science 2000;79(4):406‐7. [Google Scholar]
Singh 2000c {published data only}
- Singh N, Valecha N. Evaluation of a rapid diagnostic test, 'Determine malaria pf', in epidemic‐prone, forest villages of central India (Madhya Pradesh). Annals of Tropical Medicine and Parasitology 2000;94(5):421‐7. [DOI] [PubMed] [Google Scholar]
Singh 2001 {published data only}
- Singh N, Shukla M. An assessment of the usefulness of a rapid immuno‐chromatographic test 'Determine malaria Pf' in evaluation of intervention measures in forest villages of central India. BMC Infectious Diseases 2001;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2002a {published data only}
- Singh N, Saxena A, Sharma VP. Usefulness of an inexpensive, Paracheck test in detecting asymptomatic infectious reservoir of Plasmodium falciparum during dry season in an inaccessible terrain in central India. Journal of Infection 2002;45(3):165‐8. [DOI] [PubMed] [Google Scholar]
Singh 2002b {published data only}
- Singh N, Shukla MM. Short report: Field evaluation of posttreatment sensitivity for monitoring parasite clearance of Plasmodium falciparum malaria by use of the Determine Malaria Pf test in Central India. American Journal of Tropical Medicine and Hygiene 2002;66(3):314‐6. [DOI] [PubMed] [Google Scholar]
Singh 2004a {published data only}
- Singh N, Nagpal AC. Performance of the OptiMAL dipstick test for management of severe and complicated malaria cases in a tertiary hospital, central India. Journal of Infection 2004;48(4):364‐5. [DOI] [PubMed] [Google Scholar]
Singh 2005a {published data only}
- Singh N, Saxena A, Awadhia SB, Shrivastava R, Singh MP. Evaluation of a rapid diagnostic test for assessing the burden of malaria at delivery in India. American Journal of Tropical Medicine and Hygiene 2005;73(5):855‐8. [PubMed] [Google Scholar]
Singh 2005b {published data only}
- Singh N, Mishra AK, Shukla MM, Chand SK, Bharti PK. Diagnostic and prognostic utility of an inexpensive rapid on site malaria diagnostic test (ParaHIT f) among ethnic tribal population in areas of high, low and no transmission in central India. BMC Infectious Diseases 2005;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2005c {published data only}
- Singh N, Saxena A. Usefulness of a rapid on‐site Plasmodium falciparum diagnosis (Paracheck PF) in forest migrants and among the indigenous population at the site of their occupational activities in central India. American Journal of Tropical Medicine and Hygiene 2005;72(1):26‐9. [PubMed] [Google Scholar]
Singh 2007 {published data only}
- Singh PP, Ahmed R, Singh MP, Terlouw DJ, Ter Kuile FO, Desai MR, et al. Evaluation of the new malaria rapid diagnostic test First Response® Pf/Pv, when used as a screening tool for malaria during pregnancy in central India. American Journal of Tropical Medicine and Hygiene 2007;77(5):341. [Google Scholar]
Singh 2013 {published data only}
- Singh N, Bharti PK, Singh MP, Mishra S, Shukla MM, Sharma RK, et al. Comparative evaluation of bivalent malaria rapid diagnostic tests versus traditional methods in field with special reference to heat stability testing in Central India. PLoS One 2013;8(3):e58080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skarbinski 2009 {published data only}
- Skarbinski J, Ouma PO, Causer LM, Kariuki SK, Barnwell JW, Alaii JA, et al. Effect of malaria rapid diagnostic tests on the management of uncomplicated malaria with artemether‐lumefantrine in Kenya: a cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2009;80(6):919‐26. [PubMed] [Google Scholar]
Smego 2000 {published data only}
- Smego RA Jr, Beg A. Rapid diagnostic modalities for malaria. Journal of the Pakistan Medical Association 2000;50(12):398‐9. [PubMed] [Google Scholar]
Sotimehin 2007 {published data only}
- Sotimehin SA, Runsewe‐Abiodun TI, Oladapo OT, Njokanma OF, Olanrewaju DM. Performance of a rapid antigen test for the diagnosis of congenital malaria. Annals of Tropical Paediatrics 2007;27(4):297‐301. [DOI] [PubMed] [Google Scholar]
Soto Tarazona 2004 {published data only}
- Soto Tarazona A, Solari Zerpa L, Mendoza Requena D, Llano‐Cuentas A, Magill A. Evaluation of the rapid diagnostic test OptiMAL for diagnosis of malaria due to Plasmodium vivax. Brazilian Journal of Infectious Diseases 2004;8(2):151‐5. [DOI] [PubMed] [Google Scholar]
Srinivasan 2000 {published data only}
- Srinivasan S, Moody AH, Chiodini PL. Comparison of blood‐film microscopy, the OptiMAL dipstick, Rhodamine‐123 fluorescence staining and PCR, for monitoring antimalarial treatment. Annals of Tropical Medicine and Parasitology 2000;94(3):227‐32. [DOI] [PubMed] [Google Scholar]
Stauffer 2005 {published data only}
- Stauffer WM, Newberry A, Cartwright C, Rosenblatt J, Hanson K, Sloan L, et al. Evaluation of malaria screening in Liberian refugees by blood smear and rapid antigen capture assay (Binax (TM)). Preliminary results. American Journal of Tropical Medicine and Hygiene 2005;73:603. [Google Scholar]
Stauffer 2006 {published data only}
- Stauffer WM, Newberry AM, Cartwright CP, Rosenblatt JE, Hanson KL, Sloan L, et al. Evaluation of malaria screening in newly arrived refugees to the United States by microscopy and rapid antigen capture enzyme assay. Pediatric Infectious Disease Journal 2006;25(10):948‐50. [DOI] [PubMed] [Google Scholar]
Stauffer 2009 {published data only}
- Stauffer WM, Cartwright CP, Olson DA, Juni BA, Taylor CM, Bowers SH, et al. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clinical Infectious Diseases 2009;49(6):908‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephens 1999 {published data only}
- Stephens JK, Phanart K, Rooney W, Barnish G. A comparison of three malaria diagnostic tests, under field conditions in North‐West Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1999;30(4):625‐30. [PubMed] [Google Scholar]
Stow 1999 {published data only}
- Stow NW, Torrens JK, Walker J. An assessment of the accuracy of clinical diagnosis, local microscopy and a rapid immunochromatographic card test in comparison with expert microscopy in the diagnosis of malaria in rural Kenya. Transactions of the Royal Society of Tropical Medicine an Hygiene 1999;93(5):519‐20. [DOI] [PubMed] [Google Scholar]
Strøm 2013 {published data only}
- Strøm GE, Haanshuus CG, Fataki M, Langeland N, Blomberg B. Challenges in diagnosing paediatric malaria in Dar es Salaam, Tanzania. Malaria Journal 2013;12:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stürenburg 2009 {published data only}
- Stürenburg E, Junker R. Point‐of‐care testing in microbiology: the advantages and disadvantages of immunochromatographic test strips. Deutsches Ärzteblatt International 2009;106(4):48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Surpur 2010 {published data only}
- Surpur RR, Basvarajjappa KG, Patil VM, Anitha MR, Vijayanth V. Comparative study of peripheral blood smear and rapid diagnostic test kits for antigen detection for diagnosis of malaria. Journal of Pure and Applied Microbiology 2010;4(2):867‐70. [Google Scholar]
Susi 2005 {published data only}
- Susi B, Whitman T, Blazes DL, Burgess TH, Martin GJ, Freilich D. Rapid diagnostic test for Plasmodium falciparum in 32 Marines medically evacuated from Liberia with a febrile illness. Annals of Internal Medicine 2005;142(6):476‐7. [DOI] [PubMed] [Google Scholar]
Swarthout 2007 {published data only}
- Swarthout TD, Counihan H, Senga RK, Broek I. Paracheck‐Pf accuracy and recently treated Plasmodium falciparum infections: is there a risk of over‐diagnosis?. Malaria Journal 2007;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tagbo 2007 {published data only}
- Tagbo O, Henrietta UO. Comparisons of clinical, microscopic and rapid diagnostic test methods in the diagnosis of Plasmodium falciparum malaria in Enugu, Nigeria. Nigerian Postgraduate Medical Journal 2007;14(4):285‐9. [PubMed] [Google Scholar]
Tagbor 2008 {published data only}
- Tagbor H, Bruce J, Browne E, Greenwood B, Chandramohan D. Performance of the OptiMAL dipstick in the diagnosis of malaria infection in pregnancy. Therapeutics and Clinical Risk Management 2008;4(3):631‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tahar 2013 {published data only}
- Tahar R, Sayang C, Ngane Foumane V, Soula G, Moyou‐Somo R, Delmont J, et al. Field evaluation of rapid diagnostic tests for malaria in Yaounde, Cameroon. Acta Tropica 2013;125(2):214‐9. [DOI] [PubMed] [Google Scholar]
Tarimo 2001 {published data only}
- Tarimo DS, Minjas JN, Bygbjerg IC. Malaria diagnosis and treatment under the strategy of the integrated management of childhood illness (IMCI): relevance of laboratory support from the rapid immunochromatographic tests of ICT Malaria P.f/P.v and OptiMAL. Annals of Tropical Medicine and Parasitology 2001;95(5):437‐44. [DOI] [PubMed] [Google Scholar]
Taylor 2002 {published data only}
- Taylor WRJ, Widjaja H, Basri H, Fryauff DJ, Ohrt CT, Taufik, et al. Assessing the ParaSight®‐F test in Northeastern Papua, Indonesia, an area of mixed Plasmodium falciparum and Plasmodium vivax transmission. American Journal of Tropical Medicine and Hygiene 2002;66(6):649‐52. [DOI] [PubMed] [Google Scholar]
Tekeste 2012 {published data only}
- Tekeste Z, Workineh M, Petros B. Comparison of Paracheck Pf test with conventional light microscopy for the diagnosis of malaria in Ethiopia. Asian Pacific Journal of Tropical Disease 2012;2(1):1‐3. [Google Scholar]
Tham 1999 {published data only}
- Tham JM, Lee SH, Tan TM, Ting RC, Kara UA. Detection and species determination of malaria parasites by PCR: comparison with microscopy and with ParaSight‐F and ICT malaria Pf tests in a clinical environment. Journal of Clinical Microbiology 1999;37(5):1269‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thepsamarn 1997 {published data only}
- Thepsamarn P, Prayoollawongsa N, Puksupa P, Puttoom P, Thaidumrong P, Wongchai S, et al. The ICT Malaria Pf: a simple, rapid dipstick test for the diagnosis of Plasmodium falciparum malaria at the Thai‐Myanmar border. South East Asian Journal of Tropical Medicine and Public Health 1997;28(4):723‐6. [PubMed] [Google Scholar]
Tietche 1996 {published data only}
- Tietche F, Teguia S, Tetanye E, Louis FJ, Mbonda E, Epee MF. Presumptive diagnosis of malaria and access thick drop of positivity in children 0 to 5 years in Yaounde (Cameroon) [Diagnostic presomptif d'acces palustre et positivite de la goutte epaisse chez l'enfant de 0 a 5 ans a Yaounde (Cameroun)]. Medecine d'Afrique Noire 1996;43(6):318‐21. [Google Scholar]
Tjitra 2001a {published data only}
- Tjitra E, Suprianto S, Dyer ME, Currie BJ, Anstey NM. Detection of histidine rich protein 2 and panmalarial ICT Malaria Pf/Pv test antigens after chloroquine treatment of uncomplicated falciparum malaria does not reliably predict treatment outcome in eastern Indonesia. American Journal of Tropical Medicine and Hygiene 2001;65(5):593‐8. [DOI] [PubMed] [Google Scholar]
Tjitra 2001b {published data only}
- Tjitra A, Suprianto S, McBroom J, Currie BJ, Anstey NM. Persistent ICT malaria P.f/P.v. panmalarial and HRP2 antigen reactivity after treatment of Plasmodium falciparum malaria is associated with gametocytemia and results in false‐positive diagnoses of Plasmodium vivax in convalescence. Journal of Clinical Microbiology 2001;39(3):1025‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trachsler 1999 {published data only}
- Trachsler M, Schlagenhauf P, Steffen R. Feasibility of a rapid dipstick antigen‐capture assay for self‐testing of travellers' malaria. Tropical Medicine and International Health 1999;4(6):442‐7. [DOI] [PubMed] [Google Scholar]
Uguen 1995 {published data only}
- Uguen C, Rabodonirina M, Pina JJ, Vigier JP, Martet G, Maret M, et al. ParaSight‐F rapid manual diagnostic test of Plasmodium falciparum infection. Bulletin of the World Health Organization 1995;73(5):643‐9. [PMC free article] [PubMed] [Google Scholar]
Uneke 2008a {published data only}
- Uneke CJ. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub‐Saharan Africa: the challenges and public health implications. Parasitology Research 2008;102(3):333‐42. [DOI] [PubMed] [Google Scholar]
Uneke 2008b {published data only}
- Uneke CJ, Iyare FE, Oke P, Duhlinska DD. Assessment of malaria in pregnancy using rapid diagnostic tests and its association with HIV infection and hematologic parameters in South‐Eastern Nigeria. Haematologica 2008;93(1):143‐4. [DOI] [PubMed] [Google Scholar]
Uzochukwu 2009 {published data only}
- Uzochukwu BSC, Obikeze EN, Onwujekwe OE, Onoka CA, Griffiths UK. Cost‐effectiveness analysis of rapid diagnostic test, microscopy and syndromic approach in the diagnosis of malaria in Nigeria: implications for scaling‐up deployment of ACT. Malaria Journal 2009;8:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valéa 2009 {published data only}
- Valéa I, Tinto H, Nikiema M, Yamuah L, Rouamba N, Drabo M, et al. Performance of OptiMAL compared to microscopy, for malaria detection in Burkina Faso. Tropical Medicine and International Health 2009;14(3):338‐40. [DOI] [PubMed] [Google Scholar]
Valecha 1998 {published data only}
- Valecha N, Sharma VP, Devi CU. A rapid immunochromatographic test (ICT) for diagnosis of Plasmodium falciparum. Diagnostic Microbiology and Infectious DIseases 1998;30:257‐60. [DOI] [PubMed] [Google Scholar]
Valecha 2002 {published data only}
- Valecha N, Eapen A, Usha Devi C, Ravindran J, Aggarwal J, Subbarao S. Field evaluation of the ICT Malaria P.f./P.v. immunochromatographic test in India. Annals of Tropical Medicine and Parasitology 2002;96(3):333‐6. [DOI] [PubMed] [Google Scholar]
Van den Ende 1998 {published data only}
- Ende J, Vervoort T, Gompel A, Lynen L. Evaluation of two tests based on the detection of histidine rich protein 2 for the diagnosis of imported Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(3):285‐8. [DOI] [PubMed] [Google Scholar]
VanderJagt 2005 {published data only}
- VanderJagt TA, Ikeh EI, Ujah IOA, Belmonte J, Glew RH, VanderJagt DJ. Short communication: Comparison of the OptiMAL rapid test and microscopy for detection of malaria in pregnant women in Nigeria. Tropical Medicine and International Health 2005;10(1):39‐41. [DOI] [PubMed] [Google Scholar]
Van der Palen 2009 {published data only}
- Palen M, Gillet P, Bottieau E, Cnops L, Esbroeck M, Jacobs J. Test characteristics of two rapid antigen detection tests (SD FK50 and SD FK60) for the diagnosis of malaria in returned travellers. Malaria Journal 2009;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dijk 2009 {published data only}
- Dijk DP, Gillet P, Vlieghe E, Cnops L, Esbroeck M, Jacobs J. Evaluation of the Palutop+4 malaria rapid diagnostic test in a non‐endemic setting. Malaria Journal 2009;8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hellemond 2009 {published data only}
- Hellemond JJ, Rutten M, Koelewijn R, Zeeman AM, Verweij JJ, Wismans PJ, et al. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerging Infectious Diseases Journal 2009;15(9):1478‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkatesh 2007 {published data only}
- Venkatesh V, Patibandla PK, Agarwal GG, Awasthi S, Ahuja RC, Nag VL, et al. Performance characteristics of a rapid diagnostic test for malaria, when used to confirm cerebral malaria in children and young adults. Annals of Tropical Medicine and Parasitology 2007;101(1):85‐7. [DOI] [PubMed] [Google Scholar]
Verlé 1996 {published data only}
- Verlé P, Binh LN, Lieu TT, Yen PT, Coosemans M. ParaSight‐F test to diagnose malaria in hypo‐endemic and epidemic prone regions of Vietnam. Tropical Medicine and International Health 1996;1(6):794‐6. [DOI] [PubMed] [Google Scholar]
Voller 1993 {published data only}
- Voller A. Immunoassays for tropical parasitic infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(5):497‐8. [DOI] [PubMed] [Google Scholar]
Waltz 2007 {published data only}
- Waltz E. Practical malaria tests promise results in remote regions. Nature Medicine 2007;13(1):6. [DOI] [PubMed] [Google Scholar]
Wang J‐Y 2007 {published data only}
- Wang JY, Shi F, Yang YT, Gao CH, Bao YF, Tang LH. Establishment and evaluation of colloid gold labelled immunochromatographic strip tests for rapid diagnosis of malaria. Chinese Journal of Parasitic Diseases 2007;25(5):415‐8. [PubMed] [Google Scholar]
Wanji 2008 {published data only}
- Wanji S, Kimbi HK, Eyong JE, Tendongfor N, Ndamukong JL. Performance and usefulness of the Hexagon rapid diagnostic test in children with asymptomatic malaria living in the Mount Cameroon region. Malaria Journal 2008;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1996 {published data only}
- World Health Organization. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. WHO Informal Consultation on Recent Advances in Diagnostic Techniques and Vaccines for Malaria. Bulletin of the World Health Organization 1996;74(1):47‐54. [PMC free article] [PubMed] [Google Scholar]
Wiese 2006 {published data only}
- Wiese L, Bruun B, Baek L, Friis‐Møller A, Gahrn‐Hansen B, Hansen J, et al. Bedside diagnosis of imported malaria using the Binax Now malaria antigen detection test. Scandinavian Journal of Infectious Diseases 2006;38(11‐12):1063‐8. [DOI] [PubMed] [Google Scholar]
Willcox 2009 {published data only}
- Willcox ML, Sanogo F, Graz B, Forster M, Dakouo F, Sidibe O, et al. Rapid diagnostic tests for the home‐based management of malaria, in a high‐transmission area. Annals of Tropical Medicine and Parasitology 2009;103(1):3‐16. [DOI] [PubMed] [Google Scholar]
Williams 2008 {published data only}
- Williams HA, Causer L, Metta E, Malila A, O'Reilly T, Abdulla S, et al. Dispensary level pilot implementation of rapid diagnostic tests: an evaluation of RDT acceptance and usage by providers and patients; Tanzania, 2005. Malaria Journal 2008;7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2013 {published data only}
- Wilson ML. Laboratory diagnosis of malaria: conventional and rapid diagnostic methods. Archives of Pathology and Laboratory Medicine 2013;137(6):805‐11. [DOI] [PubMed] [Google Scholar]
Win 2001 {published data only}
- Win TT, Tantular IS, Pusarawati S, Kerong H, Lin K, Matsuoka H, et al. Detection of Plasmodium ovale by the ICT malaria P.f./P.v. immunochromatographic test. Acta Tropica 2001;80(3):283‐4. [DOI] [PubMed] [Google Scholar]
Wolday 2001 {published data only}
- Wolday D, Balcha F, Fessehaye G, Birku Y, Shepherd A. Field trial of the RTM dipstick method for the rapid diagnosis of malaria based on the detection of Plasmodium falciparum HRP‐2 antigen in whole blood. Tropical Doctor 2001;31(1):19‐21. [PubMed] [Google Scholar]
Wongsrichanalai 1999 {published data only}
- Wongsrichanalai C, Chuanak N, Tulyayon S, Thanoosingha N, Laoboonchai A, Thimasarn K, et al. Comparison of a rapid field immunochromatographic test to expert microscopy for the detection of Plasmodium falciparum asexual parasitemia in Thailand. Acta Tropica 1999;73(3):263‐73. [DOI] [PubMed] [Google Scholar]
Wongsrichanalai 2001 {published data only}
- Wongsrichanalai C. Rapid diagnostic techniques for malaria control. Trends in Parasitology 2001;17(7):307‐9. [DOI] [PubMed] [Google Scholar]
Wongsrichanalai 2007 {published data only}
- Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):119‐27. [PubMed] [Google Scholar]
Woyessa 2013 {published data only}
- Woyessa A, Deressa W, Ali A, Lindtjørn B. Evaluation of CareStartTM malaria Pf/Pv combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, south‐central Ethiopia. Malaria Journal 2013;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu YS, Lei LM, Li M. Evaluation of a parasite lactate dehydrogenase‐based colloid gold‐immunochromatography assay for diagnosis of Plasmodium falciparum. Academic Journal of the First Medical College of PLA 2005;25(7):761‐5. [PubMed] [Google Scholar]
Yadav 1997 {published data only}
- Yadav RS, Sharma VP, Srivastava HC. Field evaluation of an antigen detection immunochromatographic test for diagnosis of Plasmodium falciparum malaria in India. Tropical Medicine 1997;39(2):45‐9. [Google Scholar]
Yadav 2012 {published data only}
- Yadav S, Sharma M, Aparna, Chaudhary U. Comparative evaluation of pan‐malaria antigen card test and blood smear for diagnosing malaria. International Journal of Life Sciences Biotechnology and Pharma Research 2012;1(3):56‐8. [Google Scholar]
Yavo 2002 {published data only}
- Yavo W, Ackra KN, Menan EIH, Barro‐Kiki PC, Kassi RR, Adjetey TAK, et al. Comparative study of four techniques used in Cote d'Ivoire for malaria's biological diagnosis. Bulletin de la Société de Pathologie Exotique 2002;95(4):238‐40. [PubMed] [Google Scholar]
Zakai 2003 {published data only}
- Zakai HA. Methods used in the diagnosis of malaria: where do we stand?. Journal of the Egyptian Society of Parasitology 2003;33(3):979‐90. [PubMed] [Google Scholar]
Zerpa 2007 {published data only}
- Zerpa N, Pabón R, Wide A, Gavidia M, Medina M, Cáceres JL. Evaluation of the OptiMAL test for diagnosis of malaria in Venezuela. Investigación Clínica 2007;49(1):93‐101. [PubMed] [Google Scholar]
Zheng 1999 {published data only}
- Zheng X, Tang L, Xu Y, Meng F, Zhu W, Gu Z, et al. Evaluation of immunochromatographic test in the diagnosis of Plasmodium falciparum and Plasmodium vivax. Chinese Journal of Parasitology and Parasitic Diseases 1999;17(4):235‐6. [PubMed] [Google Scholar]
Zhu 1998 {published data only}
- Zhu W, Tang L, Zheng X, Luo M, Gu Z, Qian H, et al. Diagnosis of falciparum malaria by immunochromatographic test. Chinese Journal of Parasitology and Parasitic Diseases 1998;16(2):94‐6. [PubMed] [Google Scholar]
Zikusooka 2008 {published data only}
- Zikusooka CM, McIntyre D, Barnes KI. Should countries implementing an artemisinin‐based combination malaria treatment policy also introduce rapid diagnostic tests?. Malaria Journal 2008;7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zurovac 2008 {published data only}
- Zurovac D, Larson BA, Skarbinski J, Slutsker L, Snow RW, Hamel MJ. Modeling the financial and clinical implications of malaria rapid diagnostic tests in the case‐management of older children and adults in Kenya. American Journal of Tropical Medicine and Hygiene 2008;78(6):884‐91. [PMC free article] [PubMed] [Google Scholar]
Additional references
Abba 2011
- Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008122.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gogtay 2013
- Gogtay N, Kannan S, Thatte UM, Olliaro PL, Sinclair D. Artemisinin‐based combination therapy for treating uncomplicated Plasmodium vivax malaria. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008492.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamer 2007
- Hamer DH, Ndhlovu M, Zurovac D, Fox M, Yeboah‐Antwi K, Chanda P, et al. Improved diagnostic testing and malaria treatment practices in Zambia. Journal of the American Medical Association 2007;297(20):2227‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hänscheid 2002
- Hänscheid T, Grobusch MP. How useful is PCR in the diagnosis of malaria?. Trends in Parasitology 2002;18(9):395‐8. [DOI] [PubMed] [Google Scholar]
Marx 2005
- Marx A, Pewsner D, Egger M, Nüesch R, Bucher HC, Genton B, et al. Meta‐analysis: Accuracy of rapid tests for malaria in travelers returning from endemic areas. Annals of Internal Medicine 2005;142(10):836‐46. [DOI] [PubMed] [Google Scholar]
May 1999
- May J, Mockenhaupt FP, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, et al. High rate of mixed and subpatent malarial infections in Southwest Nigeria. American Journal of Tropical Medicine and Hygiene 1999;61(2):339‐43. [DOI] [PubMed] [Google Scholar]
Ngasala 2008
- Ngasala B, Mubi M, Warsame M, Petzold MG, Massele AY, Gustafsson LL, et al. Impact of training in clinical and microscopy diagnosis of childhood malaria on antimalarial drug prescription and health outcome at primary health care level in Tanzania: a randomized controlled trial. Malaria Journal 2008;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Smidt 2008
- Smidt N, Deeks J, Moore T. Chapter 4: Guide to the contents of a Cochrane review and protocol. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.4 [updated September 2008]. The Cochrane Collaboration, 2008. [Google Scholar]
Snounou 1993
- Snounou G, Viriyakasol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Molecular Biochemistry and Parasitology 1993;61(2):315‐20. [DOI] [PubMed] [Google Scholar]
StataCorp 2011 [Computer program]
- StataCorp. Stata Statistical Software. Version 12. College Station, Texas: StataCorp LP, 2011.
Talman 2007
- Talman AM, Duval L, Legrand E, Hubert V, Yen S, Bell D, et al. Evaluation of the intra and inter‐specific genetic variability of Plasmodium lactate dehydrogenase. Malaria Journal 2007;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tavrow 2000
- Tavrow P, Knebel E, Cogswell L. Using quality design to improve malaria rapid diagnostic tests in Malawi. Published for the United States Agency for International Development (USAID) by the Quality Assurance Project (QAP); Bethesda, Maryland 2000; Vol. Operations Research Results 1(4).
Whiting 2003
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleinjen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic test accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2009
- Whiting P, Westwood M, Burke M, Sterne J, Glanville J. Systematic reviews of test accuracy should search a range of databases to identify primary studies. Journal of Clinical Epidemiology 2009;61(4):357‐64. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. New perspectives: malaria diagnosis: report of a joint WHO/USAID informal consultation 25‐27 October 1999. Geneva: World Health Organization, 2000. [Google Scholar]
WHO 2003
- World Health Organization. Malaria rapid diagnosis:making it work: World Health Organization meeting report 20‐23 January, Manila. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2005
- World Health Organization. Prevention and control of malaria. Strategic orientation paper. Geneva: World Health Organization, 2005. [Google Scholar]
WHO 2005a
- Roll Back Malaria Department, World Health Organization. Interim notes on selection of type of malaria rapid diagnostic test in relation to the occurrence of different parasite species: Guidance for national malaria control programmes. Geneva: World Health Organization, 2005. [Google Scholar]
WHO 2006
- World Health Organization. Towards quality testing of malaria rapid diagnostic tests: evidence and methods. Proceedings of the WHO Informal Consultation on development and methods for testing malaria rapid diagnostic tests 28 February ‐ 2 March 2006. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2008
- World Health Organization Western Pacific Region. Determining cost effectiveness of malaria rapid diagnostic tests in rural areas with high prevalence. www.wpro.who.int/sites/rdt (accessed 17/10/2008).
WHO 2009
- World Health Organization. List of known commercially‐available antigen‐detecting malaria RDTs: Information for national public health services and UN Agencies wishing to procure RDTs. http://www.wpro.who.int/NR/rdonlyres/990245C0‐F157‐417A‐90C7‐B08A7E1A50BA/0/TotallistofISO131485criteria_Rev_24MAR09.pdf accessed 12 09 2012.
WHO 2009b
- World Health Organization. World Malaria Report 2009. Geneva: World Health Organization, 2009. [Google Scholar]
WHO 2010
- World Health Organization. Guidelines for the treatment of malaria. Guidelines for the treatment of malaria. Geneva: World Health Organization, 2010. [Google Scholar]
WHO 2012
- World Health Organization. Malaria rapid diagnostic test performance: results of the WHO product testing of malaria RDTs: Round 1 (2008). Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: Round 4 (2012). Geneva: World Health Organization, 2012. [Google Scholar]


