Abstract
Associations between cells and the basement membrane are critical for a variety of biological events including cell proliferation, cell migration, cell differentiation and the maintenance of tissue integrity. Dystroglycan is a highly glycosylated basement membrane receptor, and is involved in physiological processes that maintain integrity of the skeletal muscle, as well as development and function of the central nervous system. Aberrant O-glycosylation of the α subunit of this protein, and a concomitant loss of dystroglycan's ability to function as a receptor for extracellular matrix (ECM) ligands that bear laminin globular (LG) domains, occurs in several congenital/limb-girdle muscular dystrophies (also referred to as dystroglycanopathies). Recent genetic studies revealed that mutations in DAG1 (which encodes dystroglycan) and at least 17 other genes disrupt the ECM receptor function of dystroglycan and cause disease. Here, we summarize recent advances in our understanding of the enzymatic functions of two of these disease genes: the like-glycosyltransferase (LARGE) and protein O-mannose kinase (POMK, previously referred to as SGK196). In addition, we discuss the structure of the glycan that directly binds the ECM ligands and the mechanisms by which this functional motif is linked to dystroglycan. In light of the fact that dystroglycan functions as a matrix receptor and the polysaccharide synthesized by LARGE is the binding motif for matrix proteins, we propose to name this novel polysaccharide structure matriglycan.
Keywords: dystroglycan, LARGE, muscular dystrophy, O-mannosyl glycan, POMK
Introduction
Cells are associated with the surrounding basement membrane through various cell-surface receptors including dystroglycan, integrins and sulfatides (Roberts et al. 1985; Sonnenberg et al. 1988; Ibraghimov-Beskrovnaya et al. 1992). In muscle cells, such associations maintain the integrity of the cell membrane (sarcolemma); their disruption renders the sarcolemma sensitive to the mechanical stress imposed by cycles of contraction, thus leading to muscle degeneration (Hayashi et al. 1998; Michele et al. 2002). Phenotypes caused by defects in the receptor function of dystroglycan, in both patients with muscular dystrophy and animal models, suggest that this protein is required not only for muscle maintenance but also peripheral-nerve myelination, neuromuscular-junction formation, neuronal migration in the brain, axon guidance and development of eye, brain and other tissues (Moore et al. 2002; Saito et al. 2003; Wright et al. 2012; Meilleur et al. 2014). Although these findings underscore the importance of dystroglycan in various biological processes, the molecular mechanism that renders this receptor capable of ligand binding was elusive until recently. Soon after dystroglycan was identified in skeletal muscle as 156 K dystrophin-associated glycoprotein (156-DAG), treatment with trifluoromethanesulfonic acid revealed that its glycans confer the ability to bind ligands within the extracellular matrix (ECM; Ervasti and Campbell 1993). However, gaining a structural understanding of this “ligand-binding” glycan moiety was a challenge because dystroglycan, which to date is the only protein known to receive this particular modification in native tissues, is heavily O-glycosylated and the O-glycan population that decorates it is heterogeneous across tissues and cell types.
Recent genetic studies on muscular dystrophies identified genes whose products are required for the maturation of dystroglycan into a functional receptor. Molecular studies on these gene products led to significant advances in our understanding of the chemical structure of the ECM ligand-binding moiety and its biosynthetic pathway. In this review, we summarize recent discoveries on the glycosylation steps that confer ECM-binding properties to dystroglycan. First, we provide an overview of the receptor function of dystroglycan and of the enzymes that are required for its posttranslational processing. Second, we discuss the ECM-binding structure—a novel glycosaminoglycan (GAG) subspecies—and the biosynthetic pathways that contribute to its production. The key components of the latter are the bifunctional glycosyltransferase LARGE and several O-mannosyl glycans, in particular a phosphorylated structure that links the ECM-binding motif to dystroglycan (platform structure).
α-Dystroglycan: an ECM receptor in skeletal muscle
Dystroglycan was originally identified as a component of the dystrophin–glycoprotein complex (DGC) of skeletal muscle (Ervasti et al. 1990). The DGC plays an important role in muscle maintenance, since mutations in multiple components, the dystrophin and sarcoglycans, cause Duchenne/Becker muscular dystrophy and limb-girdle muscular dystrophies (LGMDs), respectively (see more in reviews; Ozawa et al. 1995; Cohn and Campbell 2000; Nowak and Davies 2004). Biochemical analyses revealed that a single polypeptide encoded by DAG1 is cleaved into α- and β-dystroglycan subunits by autoproteolysis of its SEA (sea urchin sperm protein, enterokinase and agrin) module (Ibraghimov-Beskrovnaya et al. 1992; Deyst et al. 1995; Smalheiser and Kim 1995; Akhavan et al. 2008). Within the extracellular space, α-dystroglycan binds directly to several non-collagenous proteins including laminin, perlecan and agrin—components of the basement membrane that are essential for its formation. β-Dystroglycan is the transmembrane subunit and its cytoplasmic C-terminal region associates with dystrophin, which in turn binds to the F-actin cytoskeleton. Its N-terminal region, on the other hand, associates with the C-terminal region of α-dystroglycan, securing this soluble subunit to the outer surface of the cell. By bridging the cell membrane and binding to both dystrophin and extracellular proteins, dystroglycan physically links the cytoskeleton to the basement membrane.
Among the ECM proteins so far found to bind to α-dystroglycan, the laminins have been characterized most extensively. These ligands contain multiple domains to which other laminins, entactin/nidogen, integrins, α-dystroglycan, heparin, heparan sulfate and sulfatides can bind. Thus, laminins play a central role in establishing networks among ECM proteins in the basement membrane, and connect this membrane to the surfaces of adjacent cells. Five laminin globular (LG) domains at the C-termini of the α-chain of laminin-111 are the sites of interaction with cell-surface receptors of various types. In particular, the LG4–5 domains interact with α-dystroglycan, heparin and galactosyl sulfatide (Taraboletti et al. 1990), and the LG1–3 domains interact with integrins (Sung et al. 1993). The crystal structure of LG4–5 within the laminin α1 chain revealed that these two domains fold into curved β-sandwiches, each of which is built from two antiparallel sheets and contains a metal ion, likely a calcium ion under physiological conditions (Harrison et al. 2007). A combination of site-directed mutagenesis and in vitro binding analysis further demonstrated that α-dystroglycan and heparin bind to partially overlapping basic amino acid patches near the Ca2+-folding region on the LG4–5 domain surface. These data may explain why the dystroglycan–laminin interaction is sensitive to EDTA and heparin. Laminin-211 (Pall et al. 1996), laminin-511, laminin-521 (Yu and Talts 2003), agrin (Bowe et al. 1994), neurexin (Sugita et al. 2001), perlecan (Peng et al. 1998), pikachurin (Sato et al. 2008) and Slit (Wright et al. 2012) also have highly conserved LG-like domains and are known to bind α-dystroglycan in a Ca2+-dependent manner.
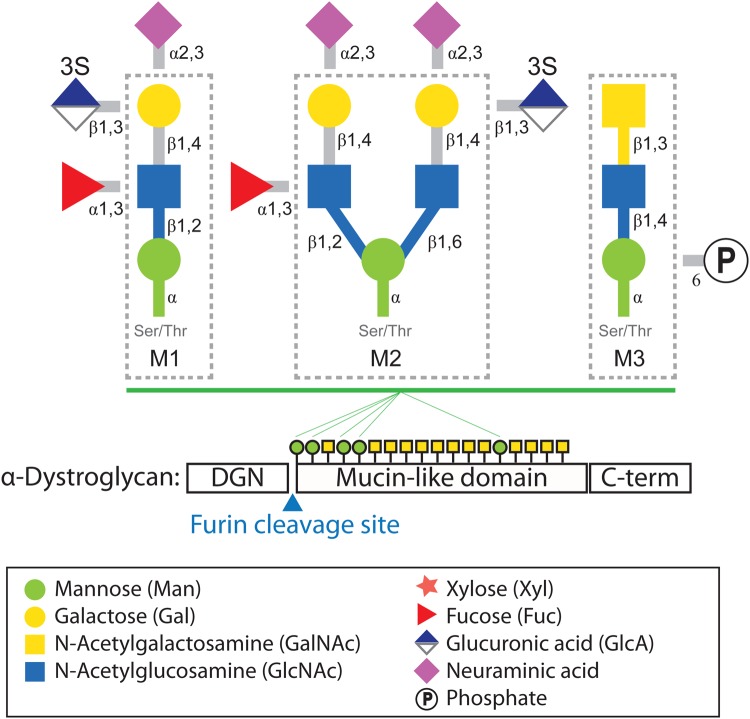
The ability of α-dystroglycan to function as a receptor relies on posttranslational modifications, especially glycosylation. α-Dystroglycan is comprised of three domains: the α-dystroglycan N-terminal (DGN) domain (1–312 amino acid: aa), a serine–threonine-rich mucin-like domain (313–485 aa) and a C-terminal domain (486–653 aa) (Figure 1). Analyses by mass spectrometry have shown that the N- and C-terminal domains have several N-glycosylation sites, and that the mucin-like domain contains at least 21 O-glycosylation sites (Nilsson et al. 2010; Stalnaker et al. 2010). O-Glycosylation at residues Thr-317 and Thr-319, which fall within the highly conserved first 18 amino acids of the mucin-like domain, is thought to be crucial for α-dystroglycan's ability to function as a receptor (Hara, Kanagawa, et al. 2011), although the complete structure of the LG domain-binding glycan that is linked to these sites has not yet been determined. It is clear that synthesis of this functional moiety depends on the presence of the DGN domain, which is cleaved by a furin-like proprotein convertase during the posttranslational processing of α-dystroglycan (Kanagawa et al. 2004). However, other than contributing to glycosylation of the neighboring mucin-like domain, the physiological roles of the DGN domain remain largely unknown.
Fig. 1.
O-Mannosyl glycans identified on α-dystroglycan. The O-mannosyl glycan structures designated as cores M1, M2 and M3 are shown surrounded by dotted lines. The modifications outside each box are those known to enable extension of the respective core glycan. 3S represents 3-O-sulfation. The three domains of α-dystroglycan are indicated below the glycan structures. Green circles and yellow squares indicate O-mannose and O-GalNAc-initiated glycans on the mucin-like domains, respectively (the numbers and the order of glycosylation sites do not follow the published mapping studies precisely). Symbolic representations of monosaccharides and other molecules are described in the box at the bottom. DGN stands for the N-terminus of α-dystroglycan.
The glycosylation status of α-dystroglycan is strictly regulated with respect to both developmental stage and tissue, with its apparent molecular weight (MW; as determined by western blotting) varying from 100 to 200 kDa across tissues. In the brain, heart, skeletal muscle and kidney, α-dystroglycan is modified such that it can function as an ECM-receptor. Monoclonal antibody (mAb) IIH6 (available from the Developmental Studies Hybridoma Bank at the University of Iowa) is widely accepted to recognize specifically an LG domain-binding modification on α-dystroglycan because it can inhibit the binding of laminin (Ervasti and Campbell 1993). Another glyco-specific α-dystroglycan mAb (2238) of potential importance was recently developed; it binds to the brain, but not muscle, form of α-dystroglycan (McDearmon et al. 2006). Notably, the brain form of α-dystroglycan has a higher affinity for laminin-511/521 than its muscle counterpart, and the brain-specific mAb is thought to recognize the modification that binds to the LG domains of the laminin α5 chain. The development of this antibody suggests that the glycan modification on α-dystroglycan is heterogeneous among tissues, and that the tissue-dependent glycosylation fine-tunes the protein's affinity for the ECM ligands that are most relevant in that context.
Genes that are disrupted in cells defective for α-dystroglycan receptor function
The discovery of a group of genetic disorders in which the receptor function of α-dystroglycan is disrupted has greatly accelerated our knowledge of the posttranslational processing pathway that enables α-dystroglycan to bind LG domains. Collectively termed dystroglycanopathies, these disorders include various forms of LGMD and congenital muscular dystrophy (CMD), with or without ocular and brain abnormalities. The CMDs include Fukuyama CMD (FCMD), muscle-eye-brain disease (MEB) and Walker–Warburg syndrome (WWS). Hallmarks include: a reduction, or complete loss, of the laminin-binding ability of α-dystroglycan in skeletal muscle; a loss of α-dystroglycan immunoreactivity to mAb IIH6; and a reduction in MW of the α-dystroglycan produced by skin fibroblasts and tissues derived from the patients (Willer et al. 2012). The brain abnormalities associated with these disorders include hydrocephalus, cobblestone lissencephaly, complete or partial absence of the corpus callosum, cerebellar hypoplasia, flattening of the brainstem, the presence of cerebellar cysts and/or abnormalities in white matter signal (as assessed by MRI; for more detail, see Topaloglu and Talim 2007). Eye abnormalities can include congenital glaucoma, microphthalmia, buphthalmos, congenital cataracts, retinal malformations, myopia and anterior-chamber defects. These abnormalities underscore the importance of dystroglycan's ability to function as a receptor during development of the brain and eye, as well as in the context of muscle maintenance.
To date, 17 genes have been identified as causative in “secondary dystroglycanopathies” (so named because the affected gene is not DAG1, which encodes dystroglycan) (Table I). Numerous genetic studies of these secondary disorders have revealed that clinical severity does not necessarily correlate with genotype. For example, defects in the FKTN gene, which was originally discovered in FCMD patients, can also cause WWS or a mild LGMD phenotype (LGMD2M). Early studies suggested that the severity of the phenotype was inversely associated with the degree of functional glycosylation on α-dystroglycan (Kanagawa et al. 2009). This notion was supported by a correlation between clinical severity and the extent to which glycosyltransferase activity was reduced in cells from patients with POMT1 mutation-associated LGMD and CMD (Lommel et al. 2010). Furthermore, flow cytometry analysis of skin fibroblasts from 21 dystroglycanopathy patients revealed an inverse correlation between clinical severity and the amount of IIH6-reactive glycan at the cell surface (Stevens et al. 2012). Whereas an attempt to correlate the genotype to the pathological phenotype among a cohort of 24 patients carrying mutations in POMT1, POMT2, POMGNT1, FKTN or FKRP demonstrated a good correlation between overall clinical severity and the glycosylation status of α-dystroglycan in patients with mutations in the first three genes, this was not the case in patients with FKTN or FKRP mutations (Jimenez-Mallebrera et al. 2009). However, it is possible that some of the proteins whose defects cause secondary dystroglycanopathies have additional substrates besides α-dystroglycan (discussed in greater detail in section: Significance of POMGNT1-mediated O-mannosyl glycan synthesis). The aberrant function of proteins with such pleiotropic roles may contribute to the observed ambiguities in the correlation between clinical severity and the glycosylation status of α-dystroglycan.
Table I.
Secondary dystroglycanopathy genes
| Gene name | OMIM | Protein function(s) |
|---|---|---|
| Protein-O-mannosyl transferase 1 (POMT1) (Beltran-Valero de Bernabe et al. 2002) | 607423 | Protein-O-mannosyl transferase (Manya et al. 2004) |
| Protein-O-mannosyl transferase 2 (POMT2) (van Reeuwijk et al. 2005) | 607439 | Protein-O-mannosyl transferase Manya et al. (2004) |
| Protein-O-mannose β2-N-acetylglucosaminyltransferase 1 (POMGNT1) (Manya et al. 2003) | 606822 | Protein-O-mannose β2-N-acetylglucosaminyltransferase (Manya et al. 2003) |
| Fukutin (FKTN) (Kobayashi et al. 1998) | 607440 | Not determined |
| Fukutin-related protein (FKRP) (Brockington et al. 2001) | 606596 | Not determined |
| Protein O-linked mannose N-acetylglucosaminyltransferase 2 (POMGNT2) (Manzini et al. 2012) | 614828 | Protein O-linked mannose β4-N-acetylglucosaminyltransferase (Yoshida-Moriguchi et al. 2013) |
| Transmembrane protein 5 (TMEM5) (Vuillaumier-Barrot et al. 2012) | 605862 | Not determined |
| Like-acetylglucosaminyltransferase (LARGE) (Longman et al. 2003) | 603590 | β3-Glucuronylltransferase (Inamori et al. 2012); α3-xylosyltransferase (Inamori et al. 2012) |
| β3-N-Acetylgalactosaminyltransferase 2 (B3GALNT2) (Stevens et al. 2013) | 610194 | β3-N-Acetylgalactosaminyltransferase (Hiruma et al. 2004) |
| β3-N-Acetylglucosaminyltransferase 1 (B3GNT1) (Buysse et al. 2013); β4-glucuronyltransferase 1 (B4GAT1) (Willer et al. 2014; Praissman et al. 2014) | 605517 | β3-N-Acetylglucosaminyltransferase (Sasaki et al. 1997); β4-glucuronyltransferase (Willer et al. 2014; Praissman et al. 2014) |
| Protein-O-mannose kinase (POMK) (Jae et al. 2013) | 615247 | Protein O-linked mannose kinase (Yoshida-Moriguchi et al. 2013) |
| Isoprenoid synthase domain containing (ISPD) (Roscioli et al. 2012, Willer et al. 2012) | 614631 | Not determined |
| GDP-mannose pyrophosphorylase B (GMPPB) (Carss et al. 2013) | 615320 | GDP-mannose pyrophosphorylase (Ning and Elbein 2000) |
| Dolichol kinase (DOLK) (Lefeber et al. 2011) | 610746 | Dolichol kinase (Fernandez et al. 2002) |
| Dolichyl-phosphate mannosyltransferase polypeptide 1 (DPM1) (Yang et al. 2013) | 603503 | Dolichyl-phosphate mannosyltransferase (Maeda et al. 2000) |
| Dolichyl-phosphate mannosyltransferase polypeptide 2 (DPM2) (Barone et al. 2012) | 603564 | Dolichyl-phosphate mannosyltransferase (Maeda et al. 2000) |
| Dolichyl-phosphate mannosyltransferase polypeptide 3 (DPM3) (Lefeber et al. 2009) | 605951 | Dolichyl-phosphate mannosyltransferase (Maeda et al. 2000) |
Thus far, two cases of primary dystroglycanopathy have been reported. One is a patient with LGMD accompanied by cognitive impairment. In this case, a homozygous C-to-T missense mutation at position c.575 of DAG1 causes a threonine-to-methionine substitution at amino acid residue 192 (T192M) within the DGN domain (Dincer et al. 2003; Hara, Balci-Hayta, et al. 2011). As noted above, this domain is not present in the mature form of α-dystroglycan, due to processing by a furin-like protease (Figure 1). However, this prodomain binds directly to LARGE, an event that is a prerequisite for functional modification of the mucin-like domain of α-dystroglycan (Kanagawa et al. 2004). Studies using knock-in mice and in vitro binding assays demonstrated that the T192M mutation affects the LG-binding modification of α-dystroglycan by disrupting its interaction with LARGE (i.e., formation of the enzyme–substrate complex). In the second case of a primary dystroglycanopathy, a homozygous missense mutation (c.2006G>T) was found in two siblings with an MEB-like phenotype and multicystic leukodystrophy (Geis et al. 2013). This mutation, which results in a cysteine-to-phenylalanine substitution at amino acid residue 669 (C669P) in the extracellular portion of β-dystroglycan, is predicted to disrupt a disulfide bond between this cysteine and another at amino acid residue 713, and to concomitantly alter the tertiary structure of the β-dystroglycan ectodomain. Currently, it is not clear if the primary α-dystroglycan defect caused by this mutation is a deficiency in its association with the plasma membrane or in its posttranslational modification.
Dystroglycan also serves as a primary receptor for the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV), the highly pathogenic Lassa virus (LASV), the African arenaviruses Mopeia and Mobala, and the Clade C New World arenaviruses (Cao et al. 1998; Spiropoulou et al. 2002; Oldstone and Campbell 2011). Initial characterization of the virus–receptor interaction revealed that high-affinity binding of the envelope glycoproteins (GPs) of all known arenaviruses to α-dystroglycan depends on modification by LARGE (Kunz et al. 2005; Rojek et al. 2007) and that this interaction is essential for virus entry into the cell. The similarity in the recognition of α-dystroglycan by arenavirus GPs and ECM proteins suggested that the molecular mechanisms used by the pathogens for binding closely mimic those whereby the host-derived ligands are recognized by the receptor. A recent study employed recombinant vesicular stomatitis virus (VSV) expressing LASV-derived GP to perform a haploid screen for factors required for LASV entry (Jae et al. 2013). In addition to DAG1 and LARGE, this robust screen implicated 10 genes already known to cause dystroglycanopathies in LASV entry into the host cell (ISPD, FKTN, FKRP, POMT1, POMT2, DPM3, GTDC2, TMEM5, B3GNT1 and B3GALNT2). Moreover, using LASV GP as a molecular probe for α-DG glycosylation, this study revealed a new causative gene (called SGK196 at the time) for the disease, emphasizing the striking similarities between the virus and ECM binding to DG. Additional genes isolated by this screen encode proteins required to synthesize UDP-xylose, UDP-glucuronic acid and dolichol-phosphate mannose (e.g., UDP-glucose 6-dehydrogenase, UDP-glucuronate decarboxylase 1 and DPM1) and proteins that maintain function of the Golgi apparatus (e.g., subunits of the conserved oligomeric Golgi complex). Interestingly, genome-wide screens for positive selection in human populations revealed strong positive selection for specific LARGE alleles in Western African populations from regions where 20–50% of the population is seropositive for LASV (Sabeti et al. 2007; Andersen et al. 2012). Although correlative at the moment, these population genetics data likely reflect the importance of DG and its LARGE-dependent modification for human LASV infection.
Posttranslational modifications that confer adhesive properties to α-dystroglycan
Protein O-mannosylation: building a platform for addition of the laminin-binding moiety
Protein O-mannosylation is a modification that is highly conserved among many species, ranging from fungi to mammals (Lommel and Strahl 2009; Panin and Wells 2014). In the case of mammals, α-dystroglycan was the first protein confirmed to receive this modification (Chiba et al. 1997). In Figure 1, we depict the O-mannosyl glycans that have been characterized on α-dystroglycan isolated from various tissues including peripheral nerve (bovine) (Chiba et al. 1997), brain (sheep) (Smalheiser et al. 1998) and skeletal muscle (rabbit and human) (Sasaki et al. 1998; Nilsson et al. 2010; Stalnaker et al. 2010), as well as from cultured cells (Yoshida-Moriguchi et al. 2010). To simplify the description of the individual O-mannosyl glycans in this review, we have designated the three subtypes cores M1, M2 and M3 (Figure 1) (Yoshida-Moriguchi et al. 2013).
Although the sialylated mucin type O-glycan (O-GalNAc) is the most prevalent O-glycan detected on α-dystroglycan, it is the O-mannosyl glycans that appear to be essential for ECM binding given that mutations in the genes encoding POMT1, POMT2 and POMGNT1 are those that cause dystroglycanopathies (Yoshida et al. 2001; Beltran-Valero de Bernabe et al. 2002; van Reeuwijk et al. 2005). POMT1 and POMT2 form a complex within the ER, where they initiate O-mannosyl glycan synthesis by adding mannose to Ser and Thr residues (Figure 2) (Manya et al. 2004). POMGNT1 acts in the Golgi, where it transfers β2-linked GlcNAc residues during synthesis of the core M1 and M2 glycans from the mannose residues added by POMT1 and POMT2 (Figure 2) (Yoshida et al. 2001). However, the sialylated core M1 glycan (Figure 1) appears not to be the moiety to which the ECM ligands bind directly, because removing the Siaα2-3Galβ1-4GlcNAcβ1-terminus from this structure by applying a combination of glycosidases does not reduce the affinity of muscle α-dystroglycan for its ligands (Combs and Ervasti 2005). Rather, as demonstrated recently, the LG-domain-binding modification requires the core M3 glycan with a phosphate at the C6 position of its O-mannose (Yoshida-Moriguchi et al. 2010). This structure was originally found on α-dystroglycan produced in HEK293 cells, as an incomplete form of the LG-binding moiety. Subsequent analyses revealed that GTDC2 (also known as AGO61) and B3GALNT2—deficiencies in which have been implicated in dystroglycanopathies—contribute to synthesis of the moiety by catalyzing the transfer of GlcNAc and GalNAc, respectively (Figure 2) (Yoshida-Moriguchi et al. 2013). Moreover, labeling studies using patient fibroblasts and [32P]orthophosphate indicated that mutations in GTDC2 and B3GALNT2 prevent generation of the phosphorylated structure on α-dystroglycan. This finding led to identification of SGK196 as a kinase capable of phosphorylating the 6-OH residue of an O-mannose that is modified by GTDC2 and B3GALNT2 prior to the reaction (Figure 2). Based on these assignments of enzymatic properties, from herein we refer to GTDC2 as protein O-linked mannose N-acetylglucosaminyltransferase 2 (POMGNT2) and to SGK196 as protein O-mannose kinase (POMK).
Fig. 2.
O-Linked glycosylation of α-dystroglycan's mucin-like domain, and enzymes in the protein processing pathway that are known or predicted to contribute. The left-hand box indicates sequential O-glycosylation steps that occur in the ER. The right-hand box indicates how the nascent dystroglycan can be modified by various glycosyltransferases (GTs) in the Golgi after being processed in the ER. Currently, it is unclear how a defect in POMGNT1 perturbs the post-phosphoryl modification on the phosphorylated core M3 glycan in the Golgi. Defects in DOLK, GMPPB, ISPD and DPM1, 2 and 3 are predicted to perturb POMT activity by reducing the availability of the donor substrate (Table I) or co-factor(s) (Willer et al. 2012). The structure of the moiety that links the phosphorylated core M3 glycan to matriglycan (indicated as [?]) has not yet been solved. Symbols are colored as in Figure 1.
POMK is an unusual kinase in that it shares homology with protein kinases, yet it does not contain certain amino acids, within three highly conserved motifs, that are considered critical for kinase activity: the Lys within VALK, which positions the α- and β-phosphates of ATP; the Asp of HRD, which lies in the catalytic loop; and the Asp within DFG, which stabilizes the bound ATP (Manning et al. 2002). These findings had prompted speculation that POMK is a catalytically inactive protein kinase (pseudokinase). However, the above-described demonstration that POMK is required for phosphorylation of α-dystroglycan indicates that this protein is, in fact, catalytically active but uses an unusual mechanism for phosphotransfer. This finding raises the exciting possibility that other kinase-like proteins that lack the known consensus domains are perhaps likewise capable of phosphotransfer—to as yet unknown substrates.
Matriglycan: the ECM-binding motif of dystroglycan
Treatment with cold aqueous hydrofluoric acid (which hydrolyzes phosphoester linkages irrespective of whether they are the monoester or the diester form) but not alkaline phosphatase (hydrolyzes only monoester-linked phosphate) significantly reduces the MW of skeletal muscle-derived α-dystroglycan. This suggests that the distal hydroxyl residue of the phosphate at the six position of O-linked mannose is further modified and therefore forms a diester linkage during the production of α-dystroglycan with receptor function. Although the moiety that is attached directly to the phosphate is not yet known, the results of an experiment in which patient cells were labeled with [32P]orthophosphate indicate that at least FKTN and LARGE are involved in this post-phosphoryl modification (Figure 2) (Yoshida-Moriguchi et al. 2010).
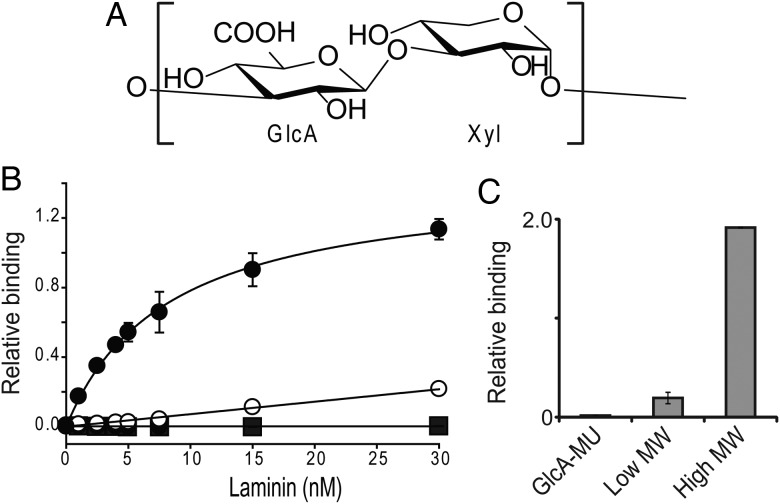
Multiple studies, carried out in cultured cells and mouse skeletal muscle, have demonstrated that forced expression of LARGE increases the MW of α-dystroglycan and its binding ability to LG domains (Barresi et al. 2004; Patnaik and Stanley 2005). LARGE is a type II transmembrane protein that contains two distinct domains: one with homology to β3GNT1, and another with homology to proteins belonging to glycosyltransferase family 8. Consistent with the implications of this structure, a recent in vitro study demonstrated that LARGE possesses two glycosyltransferase activities: an α3-xylosyltransferase activity and a β3-glucuronyltransferase activity (Inamori et al. 2012). Furthermore, this study demonstrated that LARGE can synthesize a polysaccharide composed of the repeating disaccharide [Xylα1-3GlcAβ1-3] (Figure 3A) by alternating between transfers of these two monosaccharides. Earlier studies on LARGE had suggested that N- and O-glycans on non-dystroglycan proteins receive the laminin-binding modification when LARGE is overexpressed (Patnaik and Stanley 2005; Zhang and Hu 2012). Because LARGE can use both β-linked GlcA and α-linked Xyl as acceptors, it may extend the non-reducing ends of certain glycans with these two monosaccharides when it is overexpressed. Notably, the LARGE paralog, LARGE2, possesses the same enzymatic function, although its optimal pH and pattern of expression differ from those of LARGE (Ashikov et al. 2013; Inamori et al. 2013).
Fig. 3.
The polysaccharide (matriglycan) synthesized by LARGE. (A) The chemical structure of matriglycan. (B) Solid-phase assay testing binding to laminin-111. ELISA plates were coated with biotinylated high-MW matriglycan (>13 disaccharide repeats, closed circles), low-MW matriglycan (<13 disaccharide repeats, open circles) or GlcA (negative control, closed square). (C) Quantitation of data from a solid-phase assay testing the binding of sugars that are described in (B) by mAb IIH6. (B) and (C) are modified from Figure 3 and extended data from Figure 7, respectively, in Goddeeris et al. (2013), and the error bars indicate s.e.m. (n = 3).
The extent to which LARGE modifies individual α-dystroglycan molecules seems to have functional consequences. This notion was supported by a solid-phase binding assay using laminin-111, which demonstrated that ligand binding is proportional to polysaccharide-chain length (Figure 3B) (Goddeeris et al. 2013). However, it is not yet clear how many repeating units are needed to bind one molecule of laminin. Harrison et al. (2007) previously showed, by point mutagenesis and crystallography approaches, that three surface-exposed basic patches in the LG4–5 domains of laminin-111 contribute to its interaction with α-dystroglycan. It is likely that the negatively charged polysaccharide synthesized by LARGE binds to these basic patches through electrostatic associations when α-dystroglycan approaches the ligand. This notion is supported by the fact that one of the patches also contributes to binding with heparin, which contains GlcA as well and is able to compete with α-dystroglycan for binding to the ligand (Harrison et al. 2007). Considering the linear structure of the LARGE modification, its incorporation of GlcA, and the fact that it is synthesized by a polymerizing enzyme that possesses dual glycosyltransferase activities, this novel polysaccharide may fit into the category of GAGs. In light of confirmation by ELISA that both mAb IIH6 and laminin-111 directly recognize this GAG-like structure (Figure 3B and C) (Goddeeris et al. 2013), we propose to assign the name “matriglycan” to the polysaccharide composed of the repeating disaccharide [Xylα1-3GlcAβ1-3].
Currently, it is not known if, like most GAGs, LARGE glycan is further modified, for example by sulfation and epimerization. However, an in vitro study using human natural killer (HNK)-1ST, the enzyme responsible for synthesizing the HNK-1 glyco-epitope in brain, indicated that this enzyme can transfer a sulfate to a GlcA at the non-reducing end of the post-phosphoryl modification on α-dystroglycan (Nakagawa et al. 2012, 2013). The fact that both HNK-1ST and LARGE (via its xylosyltransferase activity) modify the 3-OH position of β-linked GlcA suggests that competition between these two enzymatic activities may determine the length of the LARGE glycan chain on α-dystroglycan in certain tissues. This may be the case in brain, which is known to produce an α-dystroglycan form that is recognized by the HNK-1 antibody. Since the degree of glycosylation of α-dystroglycan and the ability of this protein to bind LG domains appear to be regulated very precisely over time and across tissues, it is likely that specialized mechanisms determine the length of matriglycan in a given context. Notably, during myogenesis the MW of α-dystroglycan and the expression levels of DAG1 and LARGE increase simultaneously (Brockington et al. 2010). Also, in both cultured cells and mice, ectopic expression of LARGE leads to significant increases in the degree of glycosylation of α-dystroglycan and its ability to bind ligands within the ECM (Barresi et al. 2004; Patnaik and Stanley 2005; Brockington et al. 2010). These findings support the notion that the level of LARGE expression may be a determinant of the length of the LARGE glycan chain.
Currently, the most important unresolved question in the field of dystroglycan biology is how the LARGE glycan is linked to its platform (i.e., the phosphorylated core M3 glycan). An enzymatic assay using this phosphorylated glycan as the acceptor demonstrated that LARGE alone is not sufficient to transfer either Xyl or GlcA to this moiety (Yoshida-Moriguchi et al. 2013), suggesting that either (i) LARGE requires another enzyme(s) to modify the phosphate before it can add sugars to it or (ii) LARGE requires a chaperone(s) or co-factor(s) to recognize this structure as an acceptor. In support of the former notion, two recent in vitro studies showed that β3GnT1, which had previously been implicated in synthesis of a linear poly-N-acetyllactosamine in vitro (Sasaki et al. 1997), instead has β4-glucuroniltransferase activity toward xylose. This led to the proposal to rename this enzyme B4GAT1 (Praissman et al. 2014; Willer et al. 2014). The fact that LARGE can extend fluorescently tagged products of B4GAT1 (GlcA-β4-Xyl-α-pNP and GlcA-β4-Xyl-β-MU) with matriglycan in vitro strongly indicates that this enzyme synthesizes a part of the structure that links the phosphorylated core M3 glycan to matriglycan. This finding further indicates that matriglycan synthesis has similarity to the synthesis of GAG, which requires a polymerizing enzyme with dual glycosyltransferase activities like those of LARGE and a priming glycosyltransferase(s) that adds one of the monosaccharides found in the repeating structure (e.g. EXTs (exostosins) and EXTLs (exostosin-likes) in the case of heparan sulfate synthesis). The roles of three dystroglycanopathy-causing proteins that contribute to synthesis of the LG-binding moiety (FKRP, FKTN and TMEM5) are largely unknown. Notably, TMEM5, which was originally identified as a gene mutated in a patient with cobblestone lissencephaly (Vuillaumier-Barrot et al. 2012), encodes a protein that shares homology with an exostosin 1 domain (218–353 aa) and also contains a DxD motif. This structure is consistent with its participation in the synthesis of a glycan that links matriglycan to the phosphate on core M3, in cooperation with B4GAT1.
It should also be noted that both FKTN and FKRP feature an LicD domain. This domain is also present in the bacterial cholinephosphotransferase, an enzyme that transfers phosphocholine to hexose residues of teichoic acid and glycerophospholipids, using CDP-choline as substrate (Ishida et al. 2009). Insight into the molecular functions of FKRP and FKTN may be gained from studies on the synthetic pathway of mannose-6-phosphate in yeast. This pathway requires a protein known as Mnn4p, which also features an LicD domain and is believed to facilitate the addition, by a mannosylphosphate transferase (Mnn6p), of a phosphate residue to the C6-position of mannose on yeast N- and O-glycans (Jigami and Odani 1999). Although the mechanism underlying this facilitatory role is unknown, these studies suggest that FKTN and FKRP may act as regulatory units rather than glycosyltransferases, possibly protecting the phosphate linked to the core M3 glycan from phosphatase-mediated hydrolysis or presenting it to modifiers. A better understanding of the role of Mnn4p is expected to help to pinpoint the molecular functions of FKTN and FKRP with respect to the post-phosphoryl modification on α-dystroglycan.
Significance of POMGNT1-mediated O-mannosyl glycan synthesis
Discovery of the phosphorylated core M3 glycan as a platform that is ultimately extended by the LARGE-dependent matriglycan structure raised questions about how the activity of POMGNT1, which is specific for synthesis of the core M1 and M2 glycans, contributes to production of an α-dystroglycan form that is capable of functioning as a receptor. The available data are consistent with a number of explanations. One is that POMGNT1 activity is not limited to M1 and M2, but additionally modifies the phosphorylated core M3 glycan synthesized in the ER, and that the resulting highly branched moiety is required as a platform for addition and extension of the LG-binding polysaccharide. Thus far, we know that mannose-6-phosphate attached to α-dystroglycan-derived peptide (aa 374–383) does not function as an acceptor for POMGNT1 in vitro (Mo et al. 2011), but this remains to be tested in the context of the phosphorylated core M3 glycan. A second potential explanation is supported by the observation that certain POMGNT1 mutations in dystroglycanopathy patients cause the protein to localize to the ER (vs. the Golgi) without significantly affecting its in vitro catalytic activity (Voglmeir et al. 2011; Pereira et al. 2014). Considering that POMGNT2 acts within the ER to add GlcNAc to an O-mannose during synthesis of the core M3 structure, it is possible that POMGNT1 mis-localized to the ER competes with POMGNT2 for the acceptor substrate (i.e. O-mannose), and thus interferes with proper modification of α-dystroglycan. A third possibility is supported by binding assays carried out in skeletal-muscle lysates using immobilized metal affinity chromatography beads. The latter can capture non-functional α-dystroglycan carrying the phosphorylated core M3 but lacking the post-phosphoryl modification, but cannot capture the functional protein whose phosphate is extended with the LG domain-binding motif (Yoshida-Moriguchi et al. 2010). These studies revealed that, in patients with POMGNT1 mutations, α-dystroglycan is heterogeneous, with only a subpopulation of the protein possessing laminin-binding activity and the post-phosphoryl modification. This result suggests that lack of the core M1 structure on the mucin-like domain hinders processing of the phosphorylated core M3 within the Golgi.
The question of why a POMGNT1-dependent modification is required for α-dystroglycan to become a receptor for basement membrane proteins may be illuminated by identification of the sites that are decorated with the core M1 and M3 glycans. Mapping studies based on mass spectrometry analysis of skeletal muscle-derived α-dystroglycan have identified >20 Ser and Thr residues in the mucin-like domain as sites of O-GalNAc- or O-mannose-initiated glycosylation (Nilsson et al. 2010; Stalnaker et al. 2010). These studies failed to identify an obvious consensus recognition sequence for O-mannosylation. However, a study using deletion and point-mutation α-dystroglycan constructs indicated that functional modification, which promotes laminin clustering on the cell surface, requires a specific pair of Thr residues (317-TPT-319) located at the very N-terminus of the α-dystroglycan mucin-like domain, and that these residues are highly conserved (Hara, Kanagawa, et al. 2011). Although the ability to bind laminin was reduced when either Thr was replaced with Ala, recent mass spectrometry analysis using α-dystroglycan produced in COS7 cells revealed that the phosphorylated core M3 structure is produced only on Thr-317 (Yagi et al. 2013). These studies suggest that modifiers involved in the post-phosphoryl modification of α-dystroglycan may require the presence of a core M1 structure C-terminal to the acceptor moiety. In other words, the lack of a core M1 structure at Thr-319 may prevent post-phosphoryl modification of core M3 at Thr-317, which would account for detection of the incomplete form of the LG-binding moiety in this mapping analysis. This notion is supported by the results of another mass spectrometry-based analysis, which showed that in the case of another TPT motif (379-TPT-381) within the mucin-like domain of α-dystroglycan, the N-terminal Thr was modified with the phosphorylated core M3 glycan and the C-terminal side of Thr (381 aa) was decorated with a simple O-linked mannose (Yoshida-Moriguchi et al. 2010). Notably, a tryptic glycopeptide derived from the highly conserved N-terminal region of the mucin-like domain (313-QIHATPTPVTAIGPPTTAIQEPPSR-337) has not been covered in most mapping analyses performed on α-dystroglycan. Identification of sites within this region that are modified by POMGNT1 and POMGNT2 might help to explain how defects in POMGNT1 perturb the maturation of α-dystroglycan into a functional receptor for ECM proteins.
The transfer of GlcNAc by POMGNT1 is a prerequisite for addition, by the β1-6-N-acetylglucosaminyltransferase GnT-IX (also called GnT-Vb), of a GlcNAc residue during synthesis of core M2, which is found predominantly on α-dystroglycan in the brain (Inamori et al. 2004; Stalnaker et al. 2010). GnT-Vb/IX was cloned as a paralog of β1-6-N-acetylglucosaminyltransferase V (GnT-V or Mgat5) and is expressed exclusively in neural tissues (Inamori et al. 2003; Kaneko et al. 2003). Although GnT-Vb/IX can transfer GlcNAc to an N-glycan in vitro, a comparison of GnT-V and GnT-Vb/IX null mice revealed that the function of the latter is limited to synthesis of the branched O-mannosyl glycan (i.e., core M2), and thus that it cannot compensate for GnT-V (Lee et al. 2012). The same study revealed that GnT-V, on the other hand, can compensate for a deficiency of GnT-Vb/IX in the brain. Since α-dystroglycan in the GnT-Vb/IX-null brain retains the ability to bind to laminin, defects in this enzyme are unlikely to interfere with the generation of an LG-binding moiety in the same way that defects in POMGNT1 do (Lee et al. 2012). In the brain, a 3-sulfoglucuronyl residue caps a small subpopulation of the core M1 and M2 glycans, as well as N-glycans, thereby forming the HNK-1 glyco-epitope mentioned above (Yuen et al. 1997). This epitope is present in several neuronal cell-adhesion molecules (including NCAM, P0 and L1), and the analysis of mice deficient for it suggests that it plays a role in synaptic plasticity and memory formation (Morita et al. 2008). Western blot analysis revealed that α-dystroglycan in the brain, but not muscle, features the HNK-1 epitope (McDearmon et al. 2006). A fucosylated O-mannose core M1 structure known as the Lewis X epitope has also been detected in brain α-dystroglycan (Smalheiser et al. 1998). This epitope has a high affinity for certain C-type lectins, including scavenger receptor C-type lectin and dendritic cell-specific intercellular adhesion molecule-3-Grabbing non-integrin (Taylor and Drickamer 2007). It is likely that in the nervous system, these adhesive O-mannosyl glycans enable α-dystroglycan to facilitate cell–cell and cell–substrate associations, in addition to anchoring the cell to the basement membrane via the LG-binding modification.
Is α-dystroglycan the only protein that contributes to the pathologies observed among dystroglycanopathy patients with defective O-mannosyl glycosylation? Mass spectrometry analysis revealed that the ratio of O-mannose glycans to O-GalNAc glycans among entire O-glycans prepared from brain is 1 : 3 (Chai et al. 1999). Given that O-GalNAc glycans are present in multiple mammalian proteins, these data indicate that α-dystroglycan may not be the only glycoprotein in the brain that is subject to O-mannosyl modification. In support of this notion, in brains from mice in which DAG1 was knocked out using the Cre/loxP recombination system, the amounts of O-mannosyl glycan detected in the glycoprotein pool were similar to those in the brains of wild-type mice (Stalnaker et al. 2011). Also, mass spectrometry has shown that, within the brain, the cell-adhesion molecule CD24 is modified by core M1 (Bleckmann et al. 2009). The receptor tyrosine phosphatase β appears to be decorated with the HNK-1-positive core M2 glycan, because its recognition by mAb Cat-315 is reduced in GnT-Vb/IX-deficient mice (Kanekiyo et al. 2013). Finally, a robust proteomics study using human cultured cells in which POMGNT1 was deleted by a zinc finger nuclease recently revealed that proteins belonging to two families of membrane receptors (the cadherins and plexins), a disulfide-isomerase (PDIA3), and a membrane protein of unknown function (KIAA1549) are subject to O-mannosylation (Vester-Christensen et al. 2013). In addition, O-mannosylation was confirmed to be crucial for E-cadherin-mediated adhesion in cell aggregation assays (Lommel et al. 2013). Considering that cadherins and plexins mediate cell adhesion, these studies indicate that some of the abnormalities observed in patients with deficiencies for POMT1, POMT2 and POMGNT1 may arise from defects in cell adhesion and cell–cell communication resulting from aberrant O-mannosylation of these adhesive proteins. Although dystroglycan-deficient mice recapitulate phenotypes that are present in the brain and muscle of dystroglycanopathy patients (Kanagawa et al. 2004; Satz et al. 2008; Nguyen et al. 2013), these findings suggest that the clinical manifestations observed among patients with mutations in POMT1, POMT2 and POMGNT1 may differ slightly from those observed in dystroglycanopathy patients with mutations that affect other glycosyltransferases. This notion is supported by the discovery of a patient who had a POMGNT1 mutation and exhibited profound cognitive deficiencies as well as structural defects in the brain and eyes, but had only mild myopathic changes without clear signs of dystrophy (Raducu et al. 2014).
Future directions
Identification of the genes that confer receptor function to α-dystroglycan was recently accelerated by several approaches: genetic studies of patients with CMD (Manzini et al. 2012; Roscioli et al. 2012; Willer et al. 2012; Buysse et al. 2013; Stevens et al. 2013) and severe cobblestone lissencephaly (Vuillaumier-Barrot et al. 2012); murine forward-genetics screens for factors that are involved in axon guidance (Wright et al. 2012) and responsible for congenital hydrocephalus (Vogel et al. 2012); and the implementation of a gene screening method using Lassa virus and haploid cells (Jae et al. 2013). The subsequent discoveries of the enzymatic functions of the disease-implicated proteins shed light on a novel pathway of mammalian posttranslational modification (Figure 2). This pathway is unique in that phosphorylation at the 6-OH of mannose is catalyzed directly by a kinase rather than being the consequence of two enzymatic reactions as is the case for Man-6-phosphate on N-glycans; in that case the phosphate is transferred as part of a GlcNAc-1-phosphate (a UDP-GlcNAc serves as the donor) and the GlcNAc moiety is subsequently removed (Varki and Kornfeld 1980; Reitman and Kornfeld 1981).
Which O-mannose-linked Ser/Thr will mature into the LG-binding moiety is determined within the ER, through the collective efforts of POMGNT2, B3GALNT2 and POMK. POMGNT2 and B3GALNT2 are required to synthesize the a GalNAcβ1-3GlcNAcβ1-terminus of core M3 (Figure 2, left), which serves as the substrate recognition motif for POMK, enabling it to phosphorylate the O-mannose to produce a platform that can ultimately be extended by LARGE within the Golgi (Figure 2, right). A better understanding of the determinants of this site will require knowledge of whether POMGNT2 recognizes the peptide region adjacent to the target O-mannose. It is likely that after certain O-mannose residues have been modified by these three enzymes within the ER, the hydroxyl residue on the distal side of the phosphate is further modified by Golgi-resident proteins such as FKRP, FKTN and B4GAT1, and ultimately extended with matriglycan. Given that forced expression of LARGE in cells led to a significant increase in the ability of α-dystroglycan to bind its ligands, and even ameliorated defects in receptor function of the α-dystroglycan produced by fibroblasts from dystroglycanopathy patients, this enzyme has received a great deal of attention. The feasibility of carrying out gene therapy for muscular dystrophy using the LARGE-encoding AAV9 vector was tested on mouse models in which LARGE, POMGNT1 or FKRP was defective (Yu et al. 2013; Vannoy et al. 2014). In all cases, gene delivery resulted in enhancement of both α-dystroglycan immunoreactivity to IIH6 and its ability to bind laminin. In the cases of both the LARGE- and POMGNT1-deficient mice, this treatment also led to improvements in the distance the mice were able to run and ameliorated fibrosis. In the skeletal muscle of FKRP-mutated mice, the percentage of centrally nucleated fibers was reduced. However, a recent study demonstrated that crossing a transgenic mouse in which the LARGE gene is overexpressed (under control of the CAG promoter) with laminin α2- and fukutin-deficient mice aggravated rather than ameliorated the dystrophic phenotype, and that this was because overexpression of LARGE suppresses muscle regeneration via downregulation of insulin-like growth factor 1 (Saito et al. 2014). Similarly, the muscle pathology in FKRP-deficient mice was exacerbated by crosses with a LARGE transgenic mouse (Whitmore et al. 2014). Goddeeris et al. (2013) demonstrated that the length of the matriglycan chain synthesized by LARGE varies over the course of muscle regeneration, suggesting that the structure to which the ECM ligands bind directly may have to be precisely regulated in vivo.
Recent progress in identifying the enzymatic functions of LARGE and other glycosyltransferases that have been implicated in the dystroglycanopathies has significantly improved our knowledge of how α-dystroglycan maintains tight connections between the muscle cell and the basement membrane. It also supports the potential of developing a therapeutic approach based on LARGE function. Finally, it underscores the need to understand the structure of the matriglycan present on α-dystroglycan in native tissues—with respect to chain length, the number and location of modification sites, and whether it is further modified by sulfation and/or epimerization.
Funding
This work was supported in part by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (1U54NS053672) and a Muscular Dystrophy Association Grant (238219). K.P.C. is an investigator of the Howard Hughes Medical Institute. Funding to pay the Open Access publication charges for this article was provided by the Howard Hughes Medical Institute.
Conflict of interest statement
The authors have no conflict of interest.
Abbreviations
CMD, congenital muscular dystrophy; DGC, dystrophin–glycoprotein complex; DGN, α-dystroglycan N-terminal; ECM, extracellular matrix; FCMD, Fukuyama CMD; GAG, glycosaminoglycan; GPs, glycoproteins; HNK, human natural killer; LG, laminin globular; LGMDs, limb-girdle muscular dystrophies; mAb, monoclonal antibody; MW, molecular weight; POMK, protein O-mannose kinase; VSV, vesicular stomatitis virus; WWS, Walker–Warburg syndrome.
Acknowledgements
We thank Harry Schachter, Tamao Endo, Stefan Kunz, Ajit Varki, Jamey Marth, Juan Carlos de la Torre and James Ervasti for fruitful discussions. We also thank members of the Campbell laboratory and Christine Blaumueller for substantial contributions. We apologize to our colleagues whose work we could not cite due to space constraints and the narrow focus of this review.
References
- Akhavan A, Crivelli SN, Singh M, Lingappa VR, Muschler JL. 2008. SEA domain proteolysis determines the functional composition of dystroglycan. FASEB J. 22:612–621. [DOI] [PubMed] [Google Scholar]
- Andersen KG, Shylakhter I, Tabrizi S, Grossman SR, Happi CT, Sabeti PC. 2012. Genome-wide scans provide evidence for positive selection of genes implicated in Lassa fever. Philos Trans R Soc Lond B Biol Sci. 367:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikov A, Buettner FF, Tiemann B, Gerardy-Schahn R, Bakker H. 2013. LARGE2 generates the same xylose- and glucuronic acid-containing glycan structures as LARGE. Glycobiology. 23:303–309. [DOI] [PubMed] [Google Scholar]
- Barone R, Aiello C, Race V, Morava E, Foulquier F, Riemersma M, Passarelli C, Concolino D, Carella M, Santorelli F, et al. 2012. DPM2-CDG: A muscular dystrophy-dystroglycanopathy syndrome with severe epilepsy. Ann Neurol. 72:550–558. [DOI] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang WL, Schachter H, Dumanski JP, et al. 2004. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 10:696–703. [DOI] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, et al. 2002. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 71:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann C, Geyer H, Lieberoth A, Splittstoesser F, Liu Y, Feizi T, Schachner M, Kleene R, Reinhold V, Geyer R. 2009. O-Glycosylation pattern of CD24 from mouse brain. Biol Chem. 390:627–645. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Deyst KA, Leszyk JD, Fallon JR. 1994. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: A heteromeric complex related to the dystroglycans. Neuron. 12:1173–1180. [DOI] [PubMed] [Google Scholar]
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, et al. 2001. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 69:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Torelli S, Sharp PS, Liu K, Cirak S, Brown SC, Wells DJ, Muntoni F. 2010. Transgenic overexpression of LARGE induces alpha-dystroglycan hyperglycosylation in skeletal and cardiac muscle. PLoS ONE. 5:e14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse K, Riemersma M, Powell G, van Reeuwijk J, Chitayat D, Roscioli T, Kamsteeg EJ, van den Elzen C, van Beusekom E, Blaser S, et al. 2013. Missense mutations in beta-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum Mol Genet. 22:1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus [see comments]. Science. 282:2079–2081. [DOI] [PubMed] [Google Scholar]
- Carss KJ, Stevens E, Foley AR, Cirak S, Riemersma M, Torelli S, Hoischen A, Willer T, van Scherpenzeel M, Moore SA, et al. 2013. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of alpha-dystroglycan. Am J Hum Genet. 93:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Yuen CT, Kogelberg H, Carruthers RA, Margolis RU, Feizi T, Lawson AM. 1999. High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem. 263:879–888. [DOI] [PubMed] [Google Scholar]
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. 1997. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem. 272:2156–2162. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Campbell KP. 2000. Molecular basis of muscular dystrophies. Muscle Nerve. 23:1456–1471. [DOI] [PubMed] [Google Scholar]
- Combs AC, Ervasti JM. 2005. Enhanced laminin binding by alpha-dystroglycan after enzymatic deglycosylation. Biochem J. 390:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyst KA, Bowe MA, Leszyk JD, Fallon JR. 1995. The alpha-dystroglycan-beta-dystroglycan complex. Membrane organization and relationship to an agrin receptor. J Biol Chem. 270:25956–25959. [DOI] [PubMed] [Google Scholar]
- Dincer P, Balci B, Yuva Y, Talim B, Brockington M, Dincel D, Torelli S, Brown S, Kale G, Haliloglu G, et al. 2003. A novel form of recessive limb girdle muscular dystrophy with mental retardation and abnormal expression of alpha-dystroglycan. Neuromuscul Disord. 13:771–778. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 122:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. 1990. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 345:315–319. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Shridas P, Jiang SM, Aebi M, Waechter CJ. 2002. Expression and characterization of a human cDNA that complements the temperature-sensitive defect in dolichol kinase activity in the yeast sec59–1 mutant: The enzymatic phosphorylation of dolichol and diacylglycerol are catalyzed by separate CTP-mediated kinase activities in Saccharomyces cerevisiae. Glycobiology. 12:555–562. [DOI] [PubMed] [Google Scholar]
- Geis T, Marquard K, Rodl T, Reihle C, Schirmer S, von Kalle T, Bornemann A, Hehr U, Blankenburg M. 2013. Homozygous dystroglycan mutation associated with a novel muscle-eye-brain disease-like phenotype with multicystic leucodystrophy. Neurogenetics. 14:205–213. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Wu B, Venzke D, Yoshida-Moriguchi T, Saito F, Matsumura K, Moore SA, Campbell KP. 2013. LARGE glycans on dystroglycan function as a tunable matrix scaffold to prevent dystrophy. Nature. 503:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltran-Valero de Bernabe D, Gundesli H, Willer T, Satz JS, Crawford RW, Burden SJ, et al. 2011. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 364:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Kanagawa M, Kunz S, Yoshida-Moriguchi T, Satz JS, Kobayashi YM, Zhu Z, Burden SJ, Oldstone MB, Campbell KP. 2011. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc Natl Acad Sci USA. 108:17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D, Hussain SA, Combs AC, Ervasti JM, Yurchenco PD, Hohenester E. 2007. Crystal structure and cell surface anchorage sites of laminin alpha1LG4–5. J Biol Chem. 282:11573–11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, et al. 1998. Mutations in the integrin alpha 7 gene cause congenital myopathy. Nat Genet. 19:94–97. [DOI] [PubMed] [Google Scholar]
- Hiruma T, Togayachi A, Okamura K, Sato T, Kikuchi N, Kwon YD, Nakamura A, Fujimura K, Gotoh M, Tachibana K, et al. 2004. A novel human beta1,3-N-acetylgalactosaminyltransferase that synthesizes a unique carbohydrate structure, GalNAcbeta1–3GlcNAc. J Biol Chem. 279:14087–14095. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. 1992. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 355:696–702. [DOI] [PubMed] [Google Scholar]
- Inamori K, Endo T, Gu J, Matsuo I, Ito Y, Fujii S, Iwasaki H, Narimatsu H, Miyoshi E, Honke K, et al. 2004. N-Acetylglucosaminyltransferase IX acts on the GlcNAc beta 1,2-Man alpha 1-Ser/Thr moiety, forming a 2,6-branched structure in brain O-mannosyl glycan. J Biol Chem. 279:2337–2340. [DOI] [PubMed] [Google Scholar]
- Inamori K, Endo T, Ide Y, Fujii S, Gu J, Honke K, Taniguchi N. 2003. Molecular cloning and characterization of human GnT-IX, a novel beta1,6-N-acetylglucosaminyltransferase that is specifically expressed in the brain. J Biol Chem. 278:43102–43109. [DOI] [PubMed] [Google Scholar]
- Inamori K, Hara Y, Willer T, Anderson ME, Zhu Z, Yoshida-Moriguchi T, Campbell KP. 2013. Xylosyl- and glucuronyltransferase functions of LARGE in alpha-dystroglycan modification are conserved in LARGE2. Glycobiology. 23:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. 2012. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 335:93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Irikura D, Matsuda K, Sato S, Sone T, Tanaka M, Asano K. 2009. Molecular cloning and expression of a novel cholinephosphotransferase involved in glycoglycerophospholipid biosynthesis of Mycoplasma fermentans. Curr Microbiol. 58:535–540. [DOI] [PubMed] [Google Scholar]
- Jae LT, Raaben M, Riemersma M, van Beusekom E, Blomen VA, Velds A, Kerkhoven RM, Carette JE, Topaloglu H, Meinecke P, et al. 2013. Deciphering the glycosylome of dystroglycanopathies using haploid screens for Lassa virus entry. Science. 340:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigami Y, Odani T. 1999. Mannosylphosphate transfer to yeast mannan. Biochim Biophys Acta. 1426:335–345. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Torelli S, Feng L, Kim J, Godfrey C, Clement E, Mein R, Abbs S, Brown SC, Campbell KP, et al. 2009. A comparative study of alpha-dystroglycan glycosylation in dystroglycanopathies suggests that the hypoglycosylation of alpha-dystroglycan does not consistently correlate with clinical severity. Brain Pathol. 19:596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Nishimoto A, Chiyonobu T, Takeda S, Miyagoe-Suzuki Y, Wang F, Fujikake N, Taniguchi M, Lu Z, Tachikawa M, et al. 2009. Residual laminin-binding activity and enhanced dystroglycan glycosylation by LARGE in novel model mice to dystroglycanopathy. Hum Mol Genet. 18:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, et al. 2004. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 117:953–964. [DOI] [PubMed] [Google Scholar]
- Kanekiyo K, Inamori K, Kitazume S, Sato K, Maeda J, Higuchi M, Kizuka Y, Korekane H, Matsuo I, Honke K, et al. 2013. Loss of branched O-mannosyl glycans in astrocytes accelerates remyelination. J Neurosci. 33:10037–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Alvarez-Manilla G, Kamar M, Lee I, Lee JK, Troupe K, Zhang W, Osawa M, Pierce M. 2003. A novel beta(1,6)-N-acetylglucosaminyltransferase V (GnT-VB)(1). FEBS Lett. 554:515–519. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, et al. 1998. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 394:388–392. [DOI] [PubMed] [Google Scholar]
- Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MB. 2005. Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 79:14282–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Matthews RT, Lim JM, Swanier K, Wells L, Pierce JM. 2012. Developmental expression of the neuron-specific N-acetylglucosaminyltransferase Vb (GnT-Vb/IX) and identification of its in vivo glycan products in comparison with those of its paralog, GnT-V. J Biol Chem. 287:28526–28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeber DJ, de Brouwer APM, Morava E, Riemersma M, Schuurs-Hoeijmakers JHM, Absmanner B, Verrijp K, van den Akker WMR, Huijben K, Steenbergen G, et al. 2011. Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet. 7:e10002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeber DJ, Schonberger J, Morava E, Guillard M, Huyben KM, Verriip K, Grafakou O, Evangelioi A, Preijers FW, Manta P, et al. 2009. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am J Hum Genet. 85:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel M, Cirak S, Willer T, Hermann R, Uyanik G, van Bokhoven H, Korner C, Voit T, Baric I, Hehr U, et al. 2010. Correlation of enzyme activity and clinical phenotype in POMT1-associated dystroglycanopathies. Neurology. 74:157–164. [DOI] [PubMed] [Google Scholar]
- Lommel M, Strahl S. 2009. Protein O-mannosylation: Conserved from bacteria to humans. Glycobiology. 19:816–828. [DOI] [PubMed] [Google Scholar]
- Lommel M, Winterhalter PR, Willer T, Dahlhoff M, Schneider MR, Bartels MF, Renner-Muller I, Ruppert T, Wolf E, Strahl S. 2013. Protein O-mannosylation is crucial for E-cadherin-mediated cell adhesion. Proc Natl Acad Sci USA. 110:21024–21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, et al. 2003. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 12:2853–2861. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tanaka S, Hino J, Kangawa K, Kinoshita T. 2000. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. EMBO J. 19:2475–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. 2002. The protein kinase complement of the human genome. Science. 298:1912–1934. [DOI] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. 2004. Demonstration of mammalian protein O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA. 101:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manya H, Sakai K, Kobayashi K, Taniguchi K, Kawakita M, Toda T, Endo T. 2003. Loss-of-function of an N-acetylglucosaminyltransferase, POMGnT1, in muscle-eye-brain disease. Biochem Biophys Res Commun. 306:93–97. [DOI] [PubMed] [Google Scholar]
- Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, Shianna KV, Stevens CR, Partlow JN, Barry BJ, et al. 2012. Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker-Warburg syndrome. Am J Hum Genet. 91:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDearmon EL, Combs AC, Sekiguchi K, Fujiwara H, Ervasti JM. 2006. Brain alpha-dystroglycan displays unique glycoepitopes and preferential binding to laminin-10/11. FEBS Lett. 580:3381–3385. [DOI] [PubMed] [Google Scholar]
- Meilleur KG, Zukosky K, Medne L, Fequiere P, Powell-Hamilton N, Winder TL, Alsaman A, El-Hattab AW, Dastgir J, Hu Y, et al. 2014. Clinical, pathologic, and mutational spectrum of dystroglycanopathy caused by LARGE mutations. J Neuropath Exp Neur. 73:425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, et al. 2002. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 418:417–422. [DOI] [PubMed] [Google Scholar]
- Mo KF, Fang T, Stalnaker SH, Kirby PS, Liu MA, Wells L, Pierce M, Live DH, Boons GJ. 2011. Synthetic, structural, and biosynthetic studies of an unusual phospho-glycopeptide derived from alpha-dystroglycan. J Am Chem Soc. 133:14418–14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen JG, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. 2002. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 418:422–425. [DOI] [PubMed] [Google Scholar]
- Morita I, Kizuka Y, Kakuda S, Oka S. 2008. Expression and function of the HNK-1 carbohydrate. J Biochem. 143:719–724. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Manya H, Toda T, Endo T, Oka S. 2012. Human natural killer-1 sulfotransferase (HNK-1ST)-induced sulfate transfer regulates laminin-binding glycans on alpha-dystroglycan. J Biol Chem. 287:30823–30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa N, Takematsu H, Oka S. 2013. HNK-1 sulfotransferase-dependent sulfation regulating laminin-binding glycans occurs in the post-phosphoryl moiety on alpha-dystroglycan. Glycobiology. 9:1066–1074. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Ostendorf AP, Satz JS, Westra S, Ross-Barta SE, Campbell KP, Moore SA. 2013. Glial scaffold required for cerebellar granule cell migration is dependent on dystroglycan function as a receptor for basement membrane proteins. Acta Neuropathol Commun. 1:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Nilsson J, Larson G, Grahn A. 2010. Characterization of site-specific O-glycan structures within the mucin-like domain of alpha-dystroglycan from human skeletal muscle. Glycobiology. 20:1160–1169. [DOI] [PubMed] [Google Scholar]
- Ning BT, Elbein AD. 2000. Cloning, expression and characterization of the pig liver GDP-mannose pyrophosphorylase – Evidence that GDP-mannose and GDP-Glc pyrophosphorylases are different proteins. Eur J Biochem. 267:6866–6874. [DOI] [PubMed] [Google Scholar]
- Nowak KJ, Davies KE. 2004. Duchenne muscular dystrophy and dystrophin: Pathogenesis and opportunities for treatment. EMBO Rep. 5:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB, Campbell KP. 2011. Decoding arenavirus pathogenesis: Essential roles for alpha-dystroglycan-virus interactions and the immune response. Virology. 411:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa E, Yoshida M, Suzuki A, Mizuno Y, Hagiwara Y, Noguchi S. 1995. Dystrophin-associated proteins in muscular dystrophy. Hum Mol Genet. 4:1711–1716. [DOI] [PubMed] [Google Scholar]
- Pall EA, Bolton KM, Ervasti JM. 1996. Differential heparin inhibition of skeletal muscle alpha-dystroglycan binding to laminins. J Biol Chem. 271:3817–3821. [DOI] [PubMed] [Google Scholar]
- Panin VM, Wells L. 2014. Protein O-mannosylation in metazoan organisms. Curr Protoc Protein Sci., 75:Unit 12 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. 2005. Mouse large can modify complex N- and mucin O-glycans on alpha-dystroglycan to induce laminin binding. J Biol Chem. 280:20851–20859. [DOI] [PubMed] [Google Scholar]
- Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. 1998. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 5:475–489. [DOI] [PubMed] [Google Scholar]
- Pereira NA, Pu HX, Goh H, Song Z. 2014. Golgi phosphoprotein 3 mediates the Golgi localization and function of protein O-linked mannose beta-1,2-N-acetlyglucosaminyltransferase 1. J Biol Chem. 289:14762–14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praissman JL, Live DH, Wang S, Ramiah A, Chinoy ZS, Boons GJ, Moremen KW, Wells L. 2014. B4GAT1 is the priming enzyme for the LARGE-dependent functional glycosylation of α-dystroglycan. eLife. 03943. [DOI] [PMC free article] [PubMed]
- Raducu M, Cotarelo RP, Simon R, Camacho A, Rubio-Fernandez M, Hernandez-Lain A, Cruces J. 2014. Clinical features and molecular characterization of a patient with muscle-eye-brain disease: A novel mutation in the POMGNT1 gene. J Child Neurol. 29:289–294. [DOI] [PubMed] [Google Scholar]
- Reitman ML, Kornfeld S. 1981. UDP-N-acetylglucosamine:glycoprotein N-acetylglucosamine-1-phosphotransferase. Proposed enzyme for the phosphorylation of the high mannose oligosaccharide units of lysosomal enzymes. J Biol Chem. 256:4275–4281. [PubMed] [Google Scholar]
- Roberts DD, Rao CN, Magnani JL, Spitalnik SL, Liotta LA, Ginsburg V. 1985. Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci USA. 82:1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek JM, Campbell KP, Oldstone MBA, Kunz S. 2007. Old world Arenavirus infection interferes with the expression of functional alpha-dystroglycan in the host cell. Mol Biol Cell. 18:4493–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioli T, Kamsteeg EJ, Buysse K, Maystadt I, van Reeuwijk J, van den Elzen C, van Beusekom E, Riemersma M, Pfundt R, Vissers LE, et al. 2012. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of alpha-dystroglycan. Nat Genet. 44:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, Xie X, Byrne EH, McCarroll SA, Gaudet R, et al. 2007. Genome-wide detection and characterization of positive selection in human populations. Nature. 449:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Kanagawa M, Ikeda M, Hagiwara H, Masaki T, Ohkuma H, Katanosaka Y, Shimizu T, Sonoo M, Toda T, et al. 2014. Overexpression of LARGE suppresses muscle regeneration via down-regulation of insulin-like growth factor 1 and aggravates muscular dystrophy in mice. Hum Mol Genet. 23:4543–4558. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, et al. 2003. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 38:747–758. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Kurata-Miura K, Ujita M, Angata K, Nakagawa S, Sekine S, Nishi T, Fukuda M. 1997. Expression cloning of cDNA encoding a human beta-1,3-N-acetylglucosaminyltransferase that is essential for poly-N-acetyllactosamine synthesis. Proc Natl Acad Sci USA. 94:14294–14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. 1998. Detection of O-mannosyl glycans in rabbit skeletal muscle alpha-dystroglycan. Biochim Biophys Acta. 1425:599–606. [DOI] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, et al. 2008. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 11:923–931. [DOI] [PubMed] [Google Scholar]
- Satz JS, Barresi R, Durbeej M, Willer T, Turner A, Moore SA, Campbell KP. 2008. Brain and eye malformations resembling Walker-Warburg syndrome are recapitulated in mice by dystroglycan deletion in the epiblast. J Neurosci. 28:10567–10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Haslam SM, Sutton-Smith M, Morris HR, Dell A. 1998. Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in cranin (Dystroglycan) purified from sheep brain. J Biol Chem. 273:23698–23703. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Kim E. 1995. Purification of cranin, a laminin binding membrane protein. Identity with dystroglycan and reassessment of its carbohydrate moieties. J Biol Chem. 270:15425–15433. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Modderman PW, Hogervorst F. 1988. Laminin receptor on platelets is the integrin Vla-6. Nature. 336:487–489. [DOI] [PubMed] [Google Scholar]
- Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. 2002. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J Virol. 76:5140–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker SH, Aoki K, Lim JM, Porterfield M, Liu M, Satz JS, Buskirk S, Xiong Y, Zhang P, Campbell KP, et al. 2011. Glycomic analyses of mouse models of congenital muscular dystrophy. J Biol Chem. 286:21180–21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker SH, Hashmi S, Lim JM, Aoki K, Porterfield M, Gutierrez-Sanchez G, Wheeler J, Ervasti JM, Bergmann C, Tiemeyer M, et al. 2010. Site mapping and characterization of O-glycan structures on alpha-dystroglycan isolated from rabbit skeletal muscle. J Biol Chem. 285:24882–24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, Tambunan DE, Yau S, Brodd L, Sewry CA, et al. 2013. Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of alpha-dystroglycan. Am J Hum Genet. 92:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E, Torelli S, Feng L, Sewry C, Muntoni F. 2012. Flow cytometry in the assessment of functional alpha-dystroglycan glycosylation in dystroglycanopathy patient fibroblasts. Neuromuscular Disord. 22:S11. [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. 2001. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 154:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung U, O'Rear JJ, Yurchenco PD. 1993. Cell and heparin binding in the distal long arm of laminin: Identification of active and cryptic sites with recombinant and hybrid glycoprotein. J Cell Biol. 123:1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G, Rao CN, Krutzsch HC, Liotta LA, Roberts DD. 1990. Sulfatide-binding domain of the laminin A chain. J Biol Chem. 265:12253–12258. [PubMed] [Google Scholar]
- Taylor ME, Drickamer K. 2007. Paradigms for glycan-binding receptors in cell adhesion. Curr Opin Cell Biol. 19:572–577. [DOI] [PubMed] [Google Scholar]
- Topaloglu H, Talim B. 2007. Lissencephaly type II. Handb Clin Neurol. 87:219–234. [DOI] [PubMed] [Google Scholar]
- van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, et al. 2005. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 42:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannoy CH, Xu L, Keramaris E, Lu P, Xiao X, Lu QL. 2014. Adeno-associated virus-mediated overexpression of LARGE rescues alpha-dystroglycan function in dystrophic mice with mutations in the Fukutin-related protein. Hum Gene Ther Methods. 25:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Kornfeld S. 1980. Identification of a rat liver alpha-N-acetylglucosaminyl phosphodiesterase capable of removing “blocking” alpha-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J Biol Chem. 255:8398–8401. [PubMed] [Google Scholar]
- Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H. 2013. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci USA. 110:21018–21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Read RW, Hansen GM, Payne BJ, Small D, Sands AT, Zambrowicz BP. 2012. Congenital hydrocephalus in genetically engineered mice. Vet Pathol. 49:166–181. [DOI] [PubMed] [Google Scholar]
- Voglmeir J, Kaloo S, Laurent N, Meloni MM, Bohlmann L, Wilson IB, Flitsch SL. 2011. Biochemical correlation of activity of the alpha-dystroglycan-modifying glycosyltransferase POMGnT1 with mutations in muscle-eye-brain disease. Biochem J. 436:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillaumier-Barrot S, Bouchet-Seraphin C, Chelbi M, Devisme L, Quentin S, Gazal S, Laquerriere A, Fallet-Bianco C, Loget P, Odent S, et al. 2012. Identification of mutations in TMEM5 and ISPD as a cause of severe cobblestone lissencephaly. Am J Hum Genet. 91:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore C, Fernandez-Fuente M, Booler H, Parr C, Kavishwar M, Ashraf A, Lacey E, Kim J, Terry R, Ackroyd MR, et al. 2014. The transgenic expression of LARGE exacerbates the muscle phenotype of dystroglycanopathy mice. Hum Mol Genet. 23:1842–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer T, Inamori K, Venzke D, Harvey C, Morgensen G, Hara Y, Beltrán Valero de Bernabé D, Yu L, Wright KM, Campbell KP. 2014. The glucuronyltransferase B4GAT1 is required for initiation of LARGE-mediated α-dystroglycan functional glycosylation. eLife. 03941. [DOI] [PMC free article] [PubMed]
- Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DB, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T, et al. 2012. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat Genet. 44:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Lyon KA, Leung HW, Leahy DJ, Ma L, Ginty DD. 2012. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron. 76:931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Nakagawa N, Saito T, Kiyonari H, Abe T, Toda T, Wu SW, Khoo KH, Oka S, Kato K. 2013. AGO61-dependent GlcNAc modification primes the formation of functional glycans on alpha-dystroglycan. Sci Rep. 3:3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Ng BG, Moore SA, Rush J, Waechter CJ, Raymond KM, Willer T, Campbell KP, Freeze HH, Mehta L. 2013. Congenital disorder of glycosylation due to DPM1 mutations presenting with dystroglycanopathy-type congenital muscular dystrophy. Mol Genet Metab. 110:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Willer T, Anderson ME, Venzke D, Whyte T, Muntoni F, Lee H, Nelson SF, Yu L, Campbell KP. 2013. SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science. 341:896–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, et al. 2001. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 1:717–724. [DOI] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. 2010. O-Mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 327:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Talts JF. 2003. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem J. 371:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, He YL, Wang KJ, Zhang P, Zhang SL, Hu HY. 2013. Adeno-associated viral-mediated LARGE gene therapy rescues the muscular dystrophic phenotype in mouse models of dystroglycanopathy. Hum Gene Ther. 24:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen CT, Chai W, Loveless RW, Lawson AM, Margolis RU, Feizi T. 1997. Brain contains HNK-1 immunoreactive O-glycans of the sulfoglucuronyl lactosamine series that terminate in 2-linked or 2,6-linked hexose (mannose). J Biol Chem. 272:8924–8931. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hu HY. 2012. Differential glycosylation of alpha-dystroglycan and proteins other than alpha-dystroglycan by like-glycosyltransferase. Glycobiology. 22:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]