Abstract
Ageing is associated with a chronic low-grade inflammatory profile (CLIP). Physical exercise could circumvent the deleterious effects of CLIP by influencing circulating inflammatory mediators and neurotrophic growth factors. This study aimed at assessing whether 12 weeks of progressive strength training (PST) influences circulating brain-derived neurotrophic factor (BDNF), interleukin (IL)-6 and IL-10 in elderly individuals. Forty community-dwelling persons aged 62–72 years participated. Twenty participants were assigned to 12-week PST (70–80 % of maximal strength, three times per week). Matched control individuals (n = 20) maintained daily activity levels. Serum was collected for BDNF, IL-6 and IL-10 assay from all participants before and after 12 weeks (for PST subjects 24–48 h after the last training). In PST, muscle strength was significantly improved (+49 % for leg extension, p = 0.039), and basal IL-6 levels significantly reduced (p = 0.001), which remained unchanged in control (p = 0.117). No significant change in BDNF was observed in PST subjects (p = 0.147) or control (p = 0.563). IL-10 was below the detection limit in most subjects. Gender and health status did not influence the results. Our results show that after 12-week PST, muscle performance improved significantly, and basal levels of IL-6 were significantly decreased in older subjects. However, serum BDNF was not altered. The lack of an observable change in BDNF might be due to a short-lived BDNF response, occurring acutely following exercise, which might have been washed out when sampling. Furthermore, blood levels of BDNF may not reflect parallel increases that occur locally in the brain and muscle. These hypotheses need confirmation by further studies.
Keywords: Brain-derived neurotrophic factor, Interleukin-6, Interleukin-10, Strength training, Exercise, Ageing
Introduction
Ageing has been associated with an increase in pro-inflammatory mediators in the circulation, corresponding to a chronic low-grade inflammatory profile (CLIP) (Beyer et al. 2012), one of the characteristics of immunosenescence. Elderly presenting more pronounced CLIP are more prone to frailty and mortality (Giovannini et al. 2011). One of the concepts regarding immunosenescence is “Inflammaging/anti-inflammaging” (Franceschi et al. 2007). Inflammaging describes a highly responsive immune system with good resistance to infections at young, but higher CLIP at old age. Anti-inflammaging refers to low inflammatory responses and higher susceptibility to infections at young, but lower CLIP and better survival at old age (Franceschi et al. 2007). There is, indeed, growing evidence for the involvement of CLIP in most age-related diseases (Simpson et al. 2012). To circumvent the deleterious effects of CLIP, several intervention strategies have been investigated, including physical exercise. The available evidence indicates that participation in physical exercise not only protects against some of the pathological conditions associated with CLIP (Corsonello et al. 2010), but may also prevent cognitive dysfunction at higher age (Sofi et al. 2011; Marx 2005). Contracting skeletal muscle is reported to secrete myokines which can have a beneficial effect on other organs and can play a potential role in countering CLIP (Raschke and Eckel 2013). Contraction is the major avenue that induces skeletal muscles to activate intra-cellular pathways (Pedersen and Febbraio 2012), giving rise to several myokines in the blood circulation in a dose–response-related manner (i.e. higher release following more intensive and/or longer muscle activity). The favourable effect of physical exercise on CLIP in elderly persons is thought to be mediated in part via the release of the cytokine interleukin (IL)-6 (Pedersen and Febbraio 2012; Petersen and Pedersen 2005; Mathur and Pedersen 2008). We previously described that a single bout of intensive strength training induces a significant immune response in elderly individuals (Bautmans et al. 2005). Following exercise of sufficient load, circulating levels of IL-6 increase very rapidly (Steensberg et al. 2002) and return back to baseline within 24 h (Mendham et al. 2011). This acute exercise-induced IL-6 response can stimulate the release of interleukin-1 receptor antagonist (IL-1Ra) and IL-10, with an anti-inflammatory effect. IL-10 inhibits the production of key pro-inflammatory cytokines (IL-1α, IL-1β and TNF-α) as well as some chemokines such as CXCL8 (Pedersen and Bruunsgaard 2003; Simpson et al. 2012). IL-10 could thus downregulate adaptive immune responses and reduce inflammation-induced tissue damage (Gleeson et al. 2011).
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophic growth factor family and an important molecular mediator of brain neuroplasticity. It was reported to play a crucial role in neuronal protection and survival, axonal and dendritic growth and remodelling, neuronal differentiation and synaptic plasticity (Coelho et al. 2013; Knaepen et al. 2010; Voss et al. 2013). The concentration of BDNF was reported to decrease with age, both at the cellular and extracellular levels, which is related to neuronal loss (Ziegenhorn et al. 2007). In addition, the circulating BDNF level is also reported to decrease in elderly individuals suffering from depression (Pereira et al. 2013; Laske et al. 2010), neurodegenerative diseases (Frazzitta et al. 2014; Baker et al. 2010) and frailty (Coelho et al. 2012). Physical exercise has been recently mentioned as an important intervention triggering BDNF-induced brain neuroplasticity (Knaepen et al. 2010; Voss et al. 2013; Coelho et al. 2013). The origin of the exercise-induced BDNF release in the extracellular space is still a subject of debate. The brain is thought to be the main BDNF source, responsible for almost 75 % of BDNF in the circulation (Rasmussen et al. 2009; Seifert et al. 2010). There is, however, evidence that BDNF crosses the blood–brain barrier in both directions, which suggests that the peripheral sources of BDNF could influence the brain (Pan et al. 1998). Other potential sources of BDNF in the blood circulation include platelets (Yamamoto and Gurney 1990) and vascular endothelial cells (Nakahashi et al. 2000). BDNF production by blood monocytes is also upregulated by IL-6 and TNF-α stimulation (Schulte-Herbruggen et al. 2005), and BDNF would also be released from contracting muscle, thus acting as a myokine (Pedersen and Febbraio 2012). However, this latter hypothesis remains unclear since an earlier in vitro study of the same group (Matthews et al. 2009) showed significant exercise-induced increases of intra-myocellular BDNF messenger RNA (mRNA) and protein expression but failed to demonstrate significant increases in extra-myocellular BDNF.
To understand the exercise-induced changes in circulating BDNF-levels, one must distinguish acute effects (i.e. changes in concentration of circulating BDNF during and immediately following the exercise bout) and effects on basal levels (i.e. changes in concentration of circulating BDNF when the acute exercise-induced changes have been washed out, e.g. after an overnight resting period). In addition, it appears that exercise-induced changes in circulating BDNF-levels in elderly persons might be different according to the type of physical exercise (aerobic exercise or strength training) as well as the clinical condition of the participants.
An acute (immediately post exercise) increase in circulating BDNF following aerobic exercise has been recorded in elderly women with osteoarthritis (67 ± 4.41 years) (Gomes et al. 2013) and in elderly persons with major depression (aged 61 ± 7 years), but not in healthy older adults (aged 58 ± 6 years) (Laske et al. 2010). Participants with major depression showed a significantly lower basal circulating BDNF concentration prior to exercise, which immediately after exercise increased up to comparable levels as in the healthy controls and decreased back to baseline levels after 30-min rest. Several studies have shown increased basal circulating BDNF levels following 1 to 6-month aerobic exercise both in elderly residents of independent living facilities, without neurologic disorders (Anderson-Hanley et al. 2012) as well as in elderly patients suffering from osteoarthritis (Gomes et al. 2013) or neurodegenerative diseases (Frazzitta et al. 2014; Baker et al. 2010). Interestingly, Babaei et al. (2013) reported a higher baseline BDNF concentration in older (57 ± 6 years) patients with metabolic syndrome compared with their healthy counterparts. Following 6-week endurance training, they found a significant decrease in basal circulating BDNF for patients with metabolic syndrome but a significant increase for healthy participants. Frazzitta et al. reported a significant increase in basal circulating BDNF following 10-day exercise in elderly Parkinson patients (aged 61–72 years) (Frazzitta et al. 2014) which remained elevated during the 28-day exercise intervention period. Despite these positive outcomes of aerobic exercise on basal circulating BDNF levels in older adults, other studies found no change following intervention in healthy older community dwellers (Voss et al. 2013) and adults with type 2 diabetes with a broad age range (30–75 years) (Swift et al. 2012).
The effects of strength training on circulating BDNF levels in elderly persons are scarcely studied. To our knowledge, acute effects following strength training is not yet reported in the literature for elderly persons. Levinger et al. (2008) found that 10 weeks of resistance training did not affect basal circulating BDNF levels in middle-aged subjects (51 ± 6 years) with mixed risk factors for metabolic syndrome. Similarly, Swift et al. (2012) found no significant change in basal circulating BDNF levels after 9 months of resistance exercise in adults with type 2 diabetes with a broad age range (30–75 years). In contrast, in a large randomized clinical trial (strength versus aerobic exercise) involving elderly women with depressive symptoms (n = 451; aged 65–89 years), Pereira et al. (2013) showed that basal circulating BDNF levels increased significantly following 10 weeks of strength training, but not following aerobic training. Also, Coelho et al. (2012) reported lower basal levels of circulating BDNF in pre-frail compared to non-frail elderly women (aged 73 ± 4 and 71 ± 5 years, respectively). However, basal circulating BDNF increased significantly in both groups following 10 weeks of progressive resistance training. In summary, the effects of strength training on circulating BDNF levels in elderly persons are still unclear. Given the high impact of strength training on muscle mass, strength and functionality in the elderly, it is important to further investigate its role in neurobiology.
Interestingly, circulating levels of inflammatory markers and BDNF seem to be interrelated. Recently, circulating BDNF was reported to be correlated with inflammatory cytokines in the perioperative period in patients undergoing major abdominal surgery (Chimienti et al. 2012). Furthermore, inflammatory cytokines have also been shown to enhance BDNF secretion by monocytes, probably reflecting a neuroprotective role of BDNF in inflammatory conditions (Schulte-Herbruggen et al. 2005). Therefore, the aim of our study was to assess whether 12-week progressive strength training can influence basal serum levels of BDNF, IL-6 and IL-10 in elderly community-dwelling individuals.
Participants and methods
Participants
In this prospective, non-randomized controlled study, 20 elderly volunteers were allocated to 12 weeks of progressive strength training (PST) and matched with 20 non-exercising controls based on age (maximal age difference of 5 years) and gender (male/female) (see Table 1). Recruitment of participants was conducted on a voluntary basis from different senior’s associations through lectures given by the last author. After each inclusion of a participant for the PST group, a matched control subject was recruited until a total number of 20 participants in each group were reached. In order to have a representative sample, all persons older than 60 years who were living independently in the community and who were sufficiently fit for strength training were eligible to participate (n = 44). Subjects were excluded when performing currently or within the past 6 months on a regular basis physical exercise at higher intensities than habitual daily activity (e.g. fitness class, strengthening exercises, cycling club; n = 2). Prior to the study, all participants allocated to the strength training intervention underwent a thorough medical examination by a geriatrician (questioning and physical examination) in order to estimate any possible risk associated with intensive strength training. Co-morbidity was not an exclusion criterion per se, but to be eligible, the participants needed to be free of cognitive deterioration (Mini Mental State Examination score ≥ 24/30) (Folstein et al. 1975), physical impairments interfering with the exercise procedures or unstable medical conditions. Subjects using medication with anti-inflammatory effect (corticosteroids (n = 1) or non-steroidal anti-inflammatory drugs (n = 1)) were excluded. As shown in Table 2, the participants are classified into health categories, based on modified SENIEUR criteria and according to the risk for complications during physical training as described previously by Bautmans et al. (2004).
Table 1.
Characteristics of PST and control participants at baseline
| PST n = 20 | Control n = 20 | Difference between groups (p value) | |
|---|---|---|---|
| Gender male/female | 11/9 | 11/9 | |
| Age (years) | 65.69 (61.09,72.57) | 67.14 (63.03, 72.67) | 0.779 |
| Height (m) | 1.71 (1.59, 1.73) | 1.63 (1.57, 1.73) | 0.925 |
| BMI (kg/m2) | 24.70 (23.78, 29.40) | 27.30 (23.33, 31.49) | 0.640 |
| WHR | 0.93 (0.82, 0.98) | 0.90 (0.85, 0.95) | 0.779 |
| Weight (kg) | 72.25 (60.88, 84.75) | 79.00 (65.00, 87.00) | 0.565 |
| HGS (kPa) | 77.00 (53.50,101.25) | 63.00 (50.00, 95.00) | 0.301 |
| YPAS-ADS | 42.00 (28.25, 72.25) | 39.00 (35.00, 58.00) | 0.199 |
| Walking index | 12.00 (8.00, 16.00) | 16.00 (12.00, 28.00) | 0.131 |
| IL-6 (pg/mL) | 1.59 (0.84, 2.63) | 1.02 (0.55, 1.22) | 0.072 |
| BDNF (pg/mL) | 78.50 (57.50,116.75) | 83.00 (80.00, 90.00) | 0.879 |
Except indicated data are median (interquartile range)
BMI body mass index, HGS hand grip strength, IL-6 interleukin 6, BDNF brain-derived neurotrophic factor, WHR waist–hip ratio, YPAS-ADS Yale Physical Activity Survey
Table 2.
Participant’s health status
| Health category | Description | PST group(n = 20) | Control group (n = 20) | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| A1 | Completely healthy; no medication | 1 (9.1 %) | 1 (11.1 %) | 3 (27.3 %) | 1 (11.1 %) |
| A2 | Completely healthy; using only preventive medication | 1 (9.1 %) | 0 (0.0 %) | 0 (0.0 %) | 1 (11.1 %) |
| B1 | Functioning normally; presence of stabilized, non-cardiovascular disease; absence of cardiovascular abnormalities | 2 (18.2 %) | 3 (33.3 %) | 2 (18.2 %) | 3 (33.3 %) |
| B2 | Functioning normally; using medication with cardiovascular effect, no overt cardiovascular disease other than normalized arterial hypertension | 2 (18.2 %) | 4 (44.4 %) | 3 (27.3 %) | 4 (44.4 %) |
| C | History of cardiovascular pathology or abnormal ECG | 5 (45.5 %) | 1 (11.1 %) | 3 (27.3 %) | 0 (0.0 %) |
The study protocol was approved by the local ethical committee, and all participants gave written informed consent.
Strength training program
The PST training program took place in a commercial fitness centre located on the medical campus of the Vrije Universiteit Brussel. The exercises (leg press, leg abductor, leg adductor, vertical traction, chest press and shoulder press) were performed on Technogym™ devices, designed for strength training. The details of the training program are similar to those described previously (Bautmans et al. 2005). Briefly, before the start of the training program, the maximal muscle strength (1 repetition maximum (RM)) of the participants was evaluated for each exercise. On each device, a warming up of 20 repetitions at 30 % of 1RM preceded the training, which consisted in three series of 10 repetitions. The exercise intensity was progressively increased by 10 % of 1RM every two sessions from 50 up to70–80 % of 1RM. Every six sessions, the 1RM was reassessed and the training weights adapted.
A complete training session lasted for about 1 h and finished by stretching the involved muscle groups (see Table 3 for an overview of the strength training program). Participants were encouraged to follow three training sessions a week on non-consecutive days. The training was supervised by experienced coaches. Control individuals (n = 20) were asked to maintain their daily activity levels and not to engage in any additional form of physical exercise for the duration of the study.
Table 3.
Twelve-week progressive strength training program (PST)
| Warming up | Training1 | Cooling down | Phase | |
|---|---|---|---|---|
| Sessions 1–3 | 1 × 20 rep 30 % of 1RM | 3 × 10 rep 50 % of 1RM | Muscle stretching | Adaptation |
| Session 4–5 | 3 × 10 rep 60 % of 1RM | |||
| Session 6 | 3 × 10 rep 70 % of 1RM | |||
| Session 7 and later | 3 × 10 rep 70–80 % of 1RM | Intensive training |
1On each training device a warming up of 20 repetitions at 30 % of 1RM preceded the training, which consisted in three series of 10 repetitions. The exercise intensity was progressively increased by 10 % of 1RM every two sessions from 50 % up to 70-80 % of 1RM
Measurements
At baseline, the daily physical activity profile of all participants was evaluated using the Yale Physical Activity Survey (YPAS) for older adults (Dipietro et al. 1993), and the Activity Dimensions Summary (YPAS-ADS) score was calculated, reflecting the subject’s physical activity (vigorous activity, leisure walking, moving and sitting) over the last month on a scale from 0 (no activity at all) to 177 (maximal activity). YPAS-derived walking index was also evaluated at baseline for all participants. Additionally, for the participants in the control group, the physical activity level was reassessed monthly.
At baseline, the maximum grip strength was measured using the Martin vigorimeter (Elmed, Addison, I11, USA) as outlined previously (Bautmans and Mets 2005); the highest score of three consecutive attempts was registered (in kilopascal) for the dominant hand.
For the participants assigned to PST, maximal leg press performance (1RM) was recorded at baseline and after 12 weeks to document muscle strength adaptations following the training.
Cytokine and BDNF assays
Before starting with exercising or control (T0) and at the end (at least 24 h and maximum 48 h after the last training session in order to capture only effects on basal levels and to avoid bias due to acute exercise effects) of the 12 week strength training program or control period (TE), serum samples were collected from all participants and stored at −20 °C until determination (simultaneously for both time points) of cytokines and BDNF. IL-6 and IL-10 measurements were performed using commercially available ELISA kits (Biosource International, Nijvel, Belgium) according to the manufacturer’s instructions. The detection limits for IL-6 and IL-10 were <2 and 1 pg/mL, respectively. The intra-assay coefficient of variations (CVs) for IL-6 and IL-10 were determined by the manufacturer for low (L), normal (N) and high (H) standards: IL-6 CV-L = 7.7 %, CV-N = 5.7 % and CV-H = 5.1 %; IL-10 CV-L = 2.9 %, CV-N = 2.9 % and CV –H = 4.8 %, respectively.
BDNF was analyzed using a commercially available ELISA kit (ChemiKine™ BDNF ELISA kit, Millipore™, Temecula, CA, USA) following the manufacturer’s instructions. The ELISA kit has a detection range from 7.8 to 500 pg/mL. The intra-assay CV was ± 3.7 % (125 pg/mL).
Statistical analysis
Referring to our previous study, the minimal required sample size of each group is N = 18 in order to find relevant changes in CLIP following PST with a power of 80 % at two-sided p < 0.05 (see (Bautmans et al. 2005) for calculation details). For exercise-induced changes in basal circulating BDNF, sample size calculation using G-power 3 software (Faul et al. 2007) revealed that a minimal required sample size of each group of N = 19 is necessary to detect similar changes following PST as reported by Coelho et al. (2012) with a power of 80 % for two-sided p < 0.05.
Statistical analysis was performed using IBM SPSS statistics 22.0.0 software and GraphPad PRISM™ version 4.0. Given the non-normal distribution of the cytokine data (Kolmogorov–Smirnov goodness of fit test p < 0.05) and ordinal scale of some outcomes (YPAS subscales), non-parametric analyses were used. The Mann–Whitney test was used to assess the differences in continuous data and chi-square test for differences in categorical data between groups. The Wilcoxon signed-rank test was used to compare the variables at baseline and after 12 weeks. Friedman test was used to detect any difference in YPAS-ADS and walking index in the control group. Differences were considered to be significant for two-sided p < 0.05. All data are presented as median (interquartile range) except if otherwise indicated.
Results
Baseline
The baseline characteristics of the PST and control groups were similar. As can be seen in Table 1, there are no significant differences for the anthropometric variables nor for the levels of IL-6 (p = 0.072) and BDNF (p = 0.879) between the two groups. For most subjects (16 control and 16 exercise participants), IL-10 levels were below the detection limit of the kit used. In addition, there was no significant difference in handgrip strength between control and PST groups.
Follow-up
All participants in the PST group completed at least 32 sessions of strength training (average of 2.6 sessions per week), and no complications or adverse events were recorded. After 12 weeks of training, muscle strength improved significantly, illustrated by an increase of 49 % in leg muscle strength (1RM on leg press, 87.5 kg (63.0, 111.0) and 130.0 kg (70.0, 155.0)), before and after 12 weeks of PST; p = 0.039). No significant change was recorded in the physical activity status among the control participants over the entire study period as demonstrated by YPAS-ADS (baseline, 39.0 (33.0, 53.5); 1 month, 56.0 (35.0, 79.0); 2 months, 57.0 (41.5, 75.0); 3 months, 46.0 (34.5, 68.0); p = 0.177) and walking index (baseline, 16.0 (12.0, 32.0); 1 month, 24.0 (16.0, 24.0); 2 months, 24.0 (16.0, 24.0); 3 months, 24.0 (16.0, 24.0); p = 0.926).
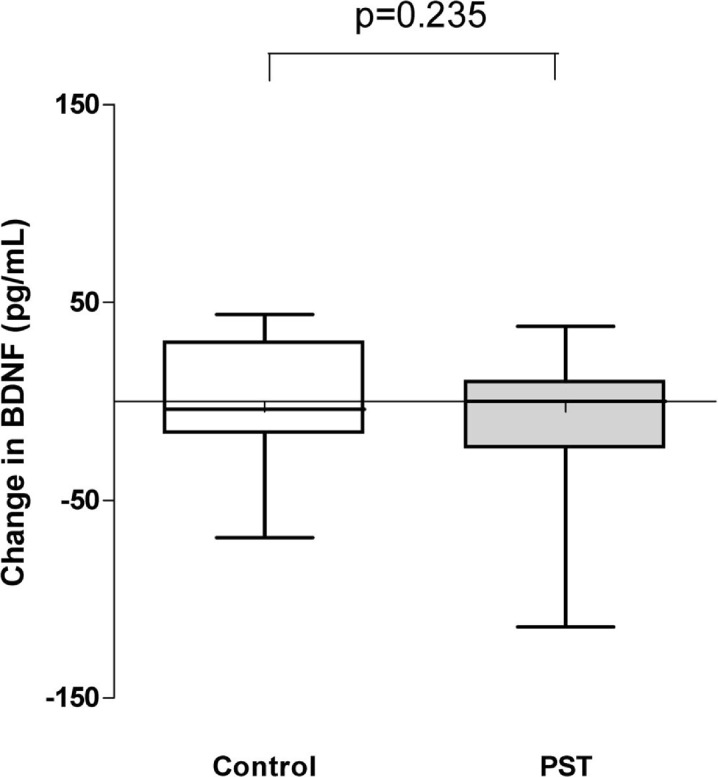
Circulating BDNF concentration was not significantly affected neither in the PST nor in the control condition, and no significant difference was observed when comparing the two groups for changes in BDNF levels after 12 weeks (p = 0.235, see Fig. 1). In addition, comparison of male versus female for changes in BDNF-levels over 12 weeks (for PST and control separately) showed no significant gender differences (data not shown).
Fig. 1.
Change in BDNF concentrations in PST and control groups between baseline and 12 weeks. There was no significant change in BDNF in both groups at 12 weeks compared to baseline (p = 0.235)
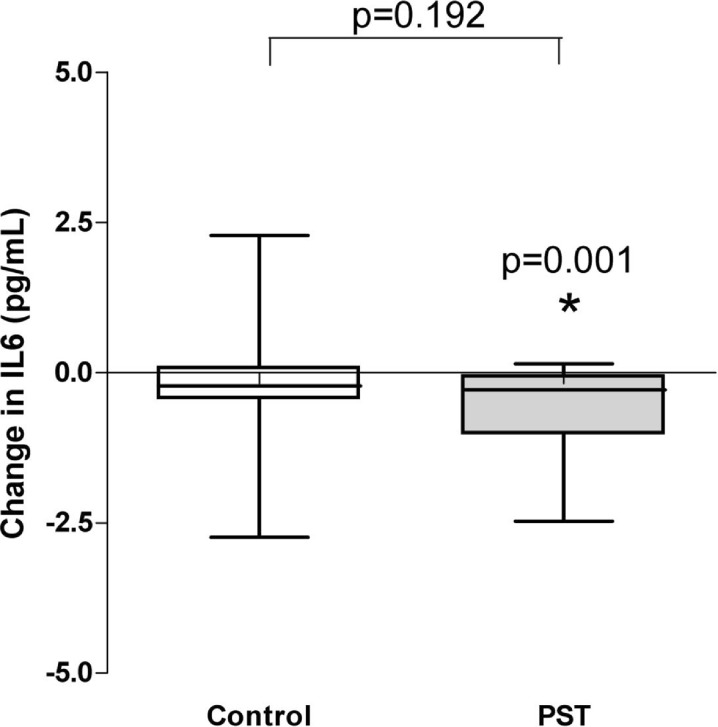
Basal IL-6 decreased significantly in the PST group, whereas no significant change was found in the control group. However, we did not observe a statistically significant difference in changes of IL-6 concentration after 12 weeks when the PST and control groups were compared (p = 0.192) (see Fig. 2).
Fig. 2.
Change in IL-6 concentrations in PST and control groups between baseline and 12 weeks. A significant change (decrease) in IL-6 levels was observed in the PST (p = 0.001), but not in the control group (p = 0.192). There was no significant difference in evolution at 12 weeks between the groups
In most participants (32 participants out of 40), values for IL-10 were below the detection limit (at baseline and at 12 weeks), and thus, no statistical analyses were performed for this cytokine. We further compared changes in IL-6 and BDNF between the control and PST groups after 12 weeks stratified according to health categories, but this analysis revealed no significant difference (all p > 0.05, data not shown).
Discussion
The present study investigated whether 12 weeks of PST affect serum levels of BDNF, IL-6 and IL-10 in community-dwelling older persons. In our exercise cohort, we recorded a median increase of 49 % in lower limb muscle strength (p = 0.039). This improvement in muscle strength is in line with previous reports (Ogawa et al. 2010; Greiwe et al. 2001; Schiffer et al. 2009; Bruunsgaard et al. 2004; Bautmans et al. 2005) and confirms that the exercise intensity was sufficient to induce exercise-induced muscle adaptations. The control participants maintained a constant physical activity level across the 12-week study period as illustrated by physical activity questionnaires. Basal IL-6 levels significantly decreased in the exercise group (p = 0.001) despite the presence of mixed co-morbidity in several participants. Our results indicate that co-morbidity was not a hindrance to lowering CLIP through strength training in these elderly individuals. The possible lack of difference in IL-6 over time between both groups might be due to the relatively small sample size and the existing co-morbidity, which might have counteracted larger exercise-induced effects. Our results support the hypothesis that following acute exercise, IL-6 provokes the production of anti-inflammatory cytokines such as IL-1Ra, soluble tumour necrosis factor receptor (sTNF-R) and IL-10 which will reduce CLIP. The acute cytokine response to exercise differs from that of sepsis and infectious diseases in that the classical pro-inflammatory cytokines, TNF-α and IL-1β, do not increase (Petersen and Pedersen 2005). During exercise, the earliest cytokine present in the circulation is IL-6 (with a concentration increase up to 100-fold) which is later followed by anti-inflammatory cytokines such as IL-1Ra, sTNF-R and IL-10. The increase in systemic IL-6 levels is acute, returning progressively to its initial value within 24 h (Steensberg et al. 2002). In this context, IL-6 exerts rather an anti-inflammatory effect. However, a chronically increased IL-6 level reflects low-grade chronic inflammation (CLIP). Skeletal muscle was reported as a major production site for the exercise-induced IL-6 increase, which is known to mediate the beneficial effects of exercise. In our study, we were unable to report IL-10 data since most of the values were below the detection limits of the kit used (1 pg/mL). This result is in contrast with the report by Jankord and Jemiolo (2004) and earlier work of our group (Bautmans et al. 2005). These studies showed that regular physical activity led to a significant decrease in IL-6 and a significant increase in IL-10. However, it must be noted that the participants in the study by Jankord and Jemiolo (2004) were healthy males corresponding to the SENIEUR criteria with a high level of physical activity (which is related to higher levels of circulating IL-10) and that the sensitivity of their ELISA kit was higher (0.5 pg/mL) than ours (1 pg/mL). On the other hand, other groups also failed to detect circulating IL-10 in most of their community-dwelling participants using ELISA kits with a sensitivity of 3 pg/mL (Fayad et al. 2001; Nemunaitis et al. 2001). Nevertheless, the decrease of IL-6 that we found here is a confirmation of the findings of an earlier study by our group in which we reported a significant acute elevation of IL-6 in elderly women and men after one bout of strengthening exercise and a significant decrease in basal IL-6 levels after 6-week strength training (Bautmans et al. 2005). Also Phillips et al. (2010) reported a reduction in the inflammatory milieu and the reduction of circulating IL-6 following strength training in previously sedentary women.
In this study, we did not find any significant change in circulating BDNF concentration after a period of 12 weeks of PST or control. Currently, data are limited concerning the effect of strength training on circulating BDNF levels in the elderly. Our result is supported by data from Swift et al. (2012) and Levinger et al. (2008) who found no increase in basal circulating BDNF levels following resistance training in middle-aged adults with respectively mixed risk factors for metabolic syndrome and type 2 diabetes. Contrastingly, Coelho et al. and Pereira et al. reported significantly increased basal circulating BDNF following resistance training in elderly participants (Coelho et al. 2012; Pereira et al. 2013). The difference between our study and previous studies that found an association between circulating BDNF levels and strength training may be due to differences in the study population. The participants in our study were non-frail elderly persons living independently in the community. Although several subjects showed mixed co-morbidity, all were free of diagnosed depression, and only two had type 2 diabetes. Pereira et al. (2013) have demonstrated in elderly (aged 65–89 years) women with depressive symptoms that 10 weeks of strength training increased basal circulating BDNF levels but observed no significant effect resulting from aerobic exercise. Similarly, Coelho et al. (2012) found lower levels of circulating BDNF in pre-frail elderly women compared to non-frail women but showed that strength training increased significantly basal circulating levels of BDNF in both groups. In this work by Coelho et al. (2012), pre-frail elderly women presented with less muscular strength which might have been as a result of sarcopenia. Moreover, Coelho et al. and Pereira et al. measured BDNF levels in plasma (Coelho et al. 2012; Pereira et al. 2013), whereas in our study, serum levels were assessed. Recent reports by Cho et al. (2012) and Gilder et al. (2014) showed that in young adults, acute changes in circulating BDNF following one bout of endurance exercise are higher in serum compared to plasma, as well that subjects with higher fat free mass show more important exercise-induced changes in serum BDNF levels. The differences in serum and plasma BDNF kinetics following exercise are probably due to the role of platelets, which have an important capacity for uptake and storage of BDNF. However, future studies are needed to elucidate whether this interferes with changes in BDNF levels following strength training in elderly persons.
The lack of significant change in basal circulating BDNF levels in our study does not exclude the possibility of an exercise-induced change in onsite production in the muscle (Matthews et al. 2009) or the brain (Rasmussen et al. 2009). Matthews et al. in an in vitro study showed significant exercise-induced acute increases of intra-myocellular BDNF mRNA and protein expression but failed to demonstrate significant increases in extra-myocellular BDNF (Matthews et al. 2009). However, we did not study skeletal muscle gene expression or protein levels after exercise, and an undetected upregulation cannot be excluded. Also, it cannot be excluded that exercise-induced acute changes in circulating BDNF occurred, which might have been washed out at the moment of sampling. In fact, our participants were sampled at least 24 h and maximum 48 h after the last training session in order to capture only effects on basal levels and to avoid bias due to acute exercise effects. Strength training has been an effective measure against sarcopenia in the elderly, and given the relevance of this type of intervention, new studies are needed to investigate its appropriateness to modulate neurobiology in the elderly. Based on the current literature, many diseases of the CNS including neurodegenerative diseases and psychiatric disorders and metabolic diseases are partly associated to decreased expression of neurotrophins (Pedersen and Febbraio 2012; Zoladz and Pilc 2010). In addition, some recent studies have reported a link between BDNF and a pro-inflammatory state (Chimienti et al. 2012; Schulte-Herbruggen et al. 2005), but the direction of the exercise-induced changes in BDNF is not clear and could be different based on the clinical condition and age of the subjects. As a result, further studies investigating the physiological importance of the training-induced changes in circulating levels of BDNF in individuals with different forms of co-morbidities are imperative. Compared with prior research on the effects of strength training on basal circulating BDNF, this study addresses a gap in the literature by reporting on community-dwelling elderly persons with some forms of co-morbidities, which we compared with controls having a similar health status.
This study had some limitations. Firstly, our study design, although involving a control group, was not randomized. Also, the study population was a convenience sample; thus, caution is warranted when interpreting the results. However, the comparison of the two groups of community-dwelling older persons with comparable co-morbidity, rather than highly selected healthy subjects, constitutes a novelty and strength. As we addressed older persons with co-morbidity, our results might be more representative of real-life community-dwelling older subjects. The basal IL-6 level was somewhat, although not significantly, higher for the PST group than for the control group (p = 0.072). It cannot be excluded that this might have affected our results. Secondly, although unlikely, it cannot be excluded that the lack of significant change in BDNF was due to a type II error. In fact, our study sample size (N = 40, 20 participants per group) was a priori calculated in order to be able to detect exercise-induced changes in basal circulating BDNF levels of similar magnitude as previously reported by Coelho et al. (2012) with 80 % power and alpha = 0.05. On the other hand, the absence of any significant change in BDNF levels could also result from the long washout period (blood was drawn 24 to 48 h after last training session in order to capture only effects on basal levels and to avoid bias due to acute exercise effects). Thirdly, it cannot be excluded that the changes we observed in the PST group could have been influenced by other confounding factors (such as dietary habits and/or vitamin D intake (De Vita et al. 2014)) that we did not examine here and for which we did not control. Finally, although the detection limit of the ELISA kit used was 1 pg/mL, most participants showed undetectable IL-10 levels, and consequently, we were unable to perform statistical analyses for this cytokine.
Conclusion
Our results demonstrate that 12-week PST in older subjects, despite the presence of co-morbidities, was associated with decreased basal levels of IL-6 which remained unchanged in the control group. Despite this positive change in CLIP, the strength training did not influence circulating BDNF levels when compared with matched controls. These results are in line with observations from other studies, although the literature remains controversial about it. As physical exercise should be encouraged in all older individuals whether healthy or affected by chronic diseases and because neurodegenerative disease is and will remain a major public health issue, the type and intensity of exercise that yield optimal effects on the neuroprotective growth factor BDNF are of utmost importance and should be further investigated.
References
- Anderson-Hanley C, Arciero PJ, Brickman AM, Nimon JP, Okuma N, Westen SC, Merz ME, Pence BD, Woods JA, Kramer AF, Zimmerman EA. Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med. 2012;42(2):109–119. doi: 10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Babaei P, Azali Alamdari K, Soltani Tehrani B, Damirchi A. Effect of six weeks of endurance exercise and following detraining on serum brain derived neurotrophic factor and memory performance in middle aged males with metabolic syndrome. J Sports Med Phys Fitness. 2013;53(4):437–443. [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautmans I, Mets T. A fatigue resistance test for elderly persons based on grip strength: reliability and comparison with healthy young subjects. Aging Clin Exp Res. 2005;17(3):217–222. doi: 10.1007/BF03324600. [DOI] [PubMed] [Google Scholar]
- Bautmans I, Lambert M, Mets T. The six-minute walk test in community dwelling elderly: influence of health status. BMC Geriatr. 2004;4:6. doi: 10.1186/1471-2318-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautmans I, Njemini R, Vasseur S, Chabert H, Moens L, Demanet C, Mets T. Biochemical changes in response to intensive resistance exercise training in the elderly. Gerontology. 2005;51(4):253–265. doi: 10.1159/000085122. [DOI] [PubMed] [Google Scholar]
- Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin in Clin Nutr Metab Care. 2012;15(1):12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Bjerregaard E, Schroll M, Pedersen BK. Muscle strength after resistance training is inversely correlated with baseline levels of soluble tumor necrosis factor receptors in the oldest old. J Am Geriatr Soc. 2004;52(2):237–241. doi: 10.1111/j.1532-5415.2004.52061.x. [DOI] [PubMed] [Google Scholar]
- Chimienti G, Mezzapesa A, Rotelli MT, Lupo L, Pepe G (2012) Plasma concentrations but not serum concentrations of brain-derived neurotrophic factor are related to pro-inflammatory cytokines in patients undergoing major abdominal surgery. Clin Biochem 45 (9):631–636. doi: 10.1016/j.clinbiochem.2012.02.025 [DOI] [PubMed]
- Cho HC, Kim J, Kim S, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO (2) max performance in healthy college men. Neurosci Lett. 2012;519(1):78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Coelho FM, Pereira DS, Lustosa LP, Silva JP, Dias JM, Dias RC, Queiroz BZ, Teixeira AL, Teixeira MM, Pereira LS. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr. 2012;54(3):415–420. doi: 10.1016/j.archger.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56(1):10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Corsonello A, Garasto S, Abbatecola AM, Rose G, Passarino G, Mazzei B, Pranno L, Guffanti EE, Bustacchini S, Lattanzio F. Targeting inflammation to slow or delay functional decline: where are we? Biogerontology. 2010;11(5):603–614. doi: 10.1007/s10522-010-9289-0. [DOI] [PubMed] [Google Scholar]
- De Vita F, Lauretani F, Bauer JM, Bautmans I, Shardell M, Cherubini A, Bondi G, Zuliani G, Bandinelli S, Perdazzoni M, Dall’ Aglio E, Ceda GP, Maggio M (2014) Relationship between vitamin D and inflammatory markers in older individuals. AGE 36: in press. doi:10.1007/s11357-014-6964-4 [DOI] [PMC free article] [PubMed]
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical-activity among older adults. Med Sci Sports Exerc. 1993;25(5):628–642. doi: 10.1249/00005768-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Fayad L, Keating MJ, Reuben JM, O’Brien S, Lee BN, Lerner S, Kurzrock R. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97(1):256–263. doi: 10.1182/blood.V97.1.256. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Frazzitta G, Maestri R, Ghilardi MF, Riboldazzi G, Perini M, Bertotti G, Boveri N, Buttini S, Lombino FL, Uccellini D, Turla M, Pezzoli G, Comi C. Intensive rehabilitation increases BDNF serum levels in parkinsonian patients: a randomized study. Neurorehabil Neural Repair. 2014;28(2):163–168. doi: 10.1177/1545968313508474. [DOI] [PubMed] [Google Scholar]
- Gilder M, Ramsbottom R, Currie J, Sheridan B, Nevill AM. Effect of fat free mass on serum and plasma BDNF concentrations during exercise and recovery in healthy young men. Neurosci Lett. 2014;560:137–141. doi: 10.1016/j.neulet.2013.12.034. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Onder G, Liperoti R, Russo A, Carter C, Capoluongo E, Pahor M, Bernabei R, Landi F (2011) Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc 59 (9):1679–1685. doi: 10.1111/j.1532-5415.2011.03570.x [DOI] [PMC free article] [PubMed]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Gomes WF, Lacerda AC, Mendonca VA, Arrieiro AN, Fonseca SF, Amorim MR, Teixeira AL, Teixeira MM, Miranda AS, Coimbra CC, Brito-Melo GE. Effect of exercise on the plasma BDNF levels in elderly women with knee osteoarthritis. Rheumatol Int. 2013 doi: 10.1007/s00296-013-2786-0. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. Faseb J. 2001;15(2):475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36(6):960–964. doi: 10.1249/01.MSS.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity— exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40(9):765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, Fritsche A, Hipp A, Niess A, Eschweiler GW. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. . The International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13(5):595–602. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- Levinger I, Goodman C, Matthews V, Hare DL, Jerums G, Garnham A, Selig S. BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med Sci Sports Exerc. 2008;40(3):535–541. doi: 10.1249/MSS.0b013e31815dd057. [DOI] [PubMed] [Google Scholar]
- Marx J. Neuroscience preventing Alzheimer’s: a lifelong commitment? Science. 2005;309(5736):864–866. doi: 10.1126/science.309.5736.864. [DOI] [PubMed] [Google Scholar]
- Mathur N, Pedersen BK (2008) Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. doi: Artn 109502; doi:10.1155/2008/109502 [DOI] [PMC free article] [PubMed]
- Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, Akerstrom T, Yfanti C, Broholm C, Mortensen OH, Penkowa M, Hojman P, Zankari A, Watt MJ, Bruunsgaard H, Pedersen BK, Febbraio MA. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52(7):1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- Mendham AE, Donges CE, Liberts EA, Duffield R. Effects of mode and intensity on the acute exercise-induced IL-6 and CRP responses in a sedentary, overweight population. Eur J Appl Physiol. 2011;111(6):1035–1045. doi: 10.1007/s00421-010-1724-z. [DOI] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470(2):113–117. doi: 10.1016/S0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Fong T, Shabe P, Martineau D, Ando D. Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Investig. 2001;19(3):239–247. doi: 10.1081/CNV-100102550. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Sanada K, Machida S, Okutsu M, Suzuki K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediat Inflamm. 2010;2010:171023. doi: 10.1155/2010/171023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. 2003;13(1):56–62. doi: 10.1034/j.1600-0838.2003.20218.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pereira DS, de Queiroz BZ, Miranda AS, Rocha NP, Felicio DC, Mateo EC, Favero M, Coelho FM, Jesus-Moraleida F, Gomes Pereira DA, Teixeira AL, Maximo Pereira LS. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women–a randomized clinical trial. Arch Phys Med Rehabil. 2013;94(8):1443–1450. doi: 10.1016/j.apmr.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc. 2010;42(2):314–325. doi: 10.1249/MSS.0b013e3181b11ab7. [DOI] [PubMed] [Google Scholar]
- Raschke S, Eckel J. Adipo-myokines: two sides of the same coin–mediators of inflammation and mediators of exercise. Mediat Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Schiffer T, Schulte S, Hollmann W, Bloch W, Struder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res. 2009;41(3):250–254. doi: 10.1055/s-0028-1093322. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol. 2005;160(1–2):204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11(3):404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, Macchi C. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(6):E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, Church TS. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS One. 2012;7(8):e42785. doi: 10.1371/journal.pone.0042785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wojcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. The official journal of the Society for Neuroscience. 1990;10(11):3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins—a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28(9):1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. An official journal of the Polish Physiological Society. 2010;61(5):533–541. [PubMed] [Google Scholar]