Abstract
Exercise training is considered a benefit to heart function, but the benefit in aging hearts remains unknown. Activation of the PI3K-Akt survival pathway and suppression of Fas/FADD/caspase-8 apoptotic signaling by exercise training in hearts from young subjects have been described in our previous studies. However, the mechanisms are still unclear and need to be explored in aging hearts. Thus, 18-month-old rats were used as a model and underwent swimming exercise training, resveratrol treatment (15 mg/kg/day), or exercise training with resveratrol treatment for 1 month. The results showed that heart function in each group improved. However, the 18-month-old rats in the exercise-only group experienced the slightly inevitable impact of increased TNF-α, cell apoptosis, and fibrosis. In the protein analysis, the PI3K-Akt pathway was slightly increased with exercise training and resveratrol treatment, but Sirtuin 1 (SIRT1) was only highly activated with resveratrol treatment in the aged rat hearts. Moreover, the exercise training plus resveratrol group benefited from SIRT1 and PI3K-Akt dual pathways and blocked FOXO3 accumulation. Our experimental results strongly suggest that resveratrol treatment improves the beneficial effects of exercise training in aging rat hearts.
Keywords: Aging, Exercise training, Resveratrol, Sirtuin 1
Introduction
Cardiovascular diseases (CVDs) are the number one cause of death globally; more people die annually from CVDs than any other cause. An estimated 17.3 million people died from CVDs in 2008, representing 30 % of all global deaths (Alwan 2011). Several studies have shown a positive relationship between aging and chronic heart failure (Shioi and Inuzuka 2012; Piccirillo et al. 2013). The pathogenic factors of CVDs include smoking habits, alcohol consumption, and lack of exercise, especially in the elder population (Blondon et al. 2013; Fernhall 2013; Gargiulo et al. 2013).
Some factors are highly associated with heart failure in aging, such as oxidative stress and inflammation (Madamanchi and Runge 2013). Oxidative stress is due to metabolic function degradation causing reactive oxygen species (ROS) accumulation (Ahmed and Tang 2012). This long-term oxidative stress is usually accompanied by inflammation in the elderly. There is a report that mitochondrial damage will trigger mitophagy in cardiomyocytes through HSP90 and CDC37 activation, but oxidative stress inhibits HSP90 activation and causes cell death with FOXO3 accumulation in the nuclei. FOXO3 is a transcription factor with a function in Fas/FADD signaling pathway protein production and cell death (Joo et al. 2011; Paraiso et al. 2012; Hsu and Chung 2012).
Moreover, resveratrol has been reported to be a direct SIRT1 activator. SIRT1 is a deacetylase that can remove the acetyl group from FOXO3 and NFκB (Frazzi et al. 2013; Lakshminarasimhan et al. 2013; Li et al. 2013). FOXO3 and NFκB deacetylation causes their transcription to fail and inhibits the downstream regulation of cell death by inflammation proteins, such as Bim, Fas, and TRAIL (Oellerich and Potente 2012). Thus, SIRT1 activation is beneficial to cell survival. There is a report that appropriate long-term exercise training can protect the heart through SIRT1 activation and decreased ROS (Zarzuelo et al. 2013). Our previous study showed a similar result; Fas-FADD/caspase-8 expression in hypertrophic rat hearts was reversed by exercise training.
Furthermore, we also found that appropriate exercise training can enhance the PI3K-Akt pathway (Lee et al. 2008; Huang et al. 2012; Cheng et al. 2013; Lee et al. 2013). In FOXO3, the three sites phosphorylated by Akt are Thr32, Ser253, and Ser315. FOXO3 associates with 14-3-3 proteins upon phosphorylation by Akt and is retained in the cytoplasm (Jang et al. 2007; Dobson et al. 2011). In the absence of survival factors, FOXO3 is dephosphorylated and translocates to the nucleus and triggers cell death by a Fas ligand-dependent mechanism (Behzad et al. 2007). In our study, an 18-month-old rat model was used for 4 weeks of swimming training with or without resveratrol treatment. Our analysis reveals that SIRT1 and PI3K-Akt activation during exercise training with resveratrol co-treatment helps FOXO3 phosphorylation and retention in the cytoplasm. Thus, resveratrol augments the effect of exercise to protect heart function and avoid the inevitable impact of aging on the heart.
Methods and materials
Animal model
All Sprague-Dawley (SD) rats were 5 months of age and purchased from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan). Then the SD rats were raised at animal center of China Medical University until 18 months of age and divided into four groups (n = 6 each). Group I was the control, and group II was the exercise training group. Exercise training consisted of swimming for 30 min in warm water at a temperature of 25 °C for 4 weeks. Group III received resveratrol treatment (15 mg/kg/day) and was given for 4 weeks. Group IV underwent both exercise training and resveratrol treatment (15 mg/kg/day) for 4 weeks. The animal use experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (No. 101-263-B).
Hematoxylin and eosin staining
The rat hearts from each group were soaked in formalin, dehydrated through graded alcohols, and embedded in paraffin wax. Then, 0.2-μm-thick paraffin sections were cut from these paraffin-embedded tissue blocks. The tissue sections were deparaffinized by immersing in xylene and rehydrated. All slices were dyed with hematoxylin and eosin and then rinsed with water. Each slide was dehydrated through graded alcohols. Finally, they were soaked in xylene twice. Photomicrographs were obtained using a Zeiss Axiophot microscope.
Masson’s trichrome
The rat hearts from each group were soaked in formalin, dehydrated through graded alcohols, and embedded in paraffin wax. Then, 0.2-μm-thick paraffin sections were cut from these paraffin-embedded tissue blocks. The tissue sections were deparaffinized by immersing in xylene and rehydrated. All slices were dyed with Masson’s trichrome and then rinsed with water. Each slide was dehydrated through graded alcohols. Finally, they were soaked in xylene twice. Photomicrographs were obtained using a Zeiss Axiophot microscope.
DAPI and TUNEL staining
For the terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay, the sections were incubated with proteinase K, washed in phosphate-buffered saline (PBS), incubated with permeabilization solution and then blocking buffer, and washed two times with PBS. The terminal deoxynucleotidyl transferase and fluorescein isothiocyanate-dUTP for 60 min at 37 °C were detected using an apoptosis detection kit (Roche Applied Science, Indianapolis, IN). TUNEL-positive nuclei (fragmented DNA) were fluoresced by bright green light at 460 nm. The 4,6-diamidino-2-phenylindole (DAPI) was dissolved in PBS at 0.1 μg/ml and added to the slides for 5 min, and the nuclei were fluoresced by blue light at 454 nm. Photomicrographs were obtained using a Zeiss Axiophot microscope.
Tissue protein extraction
Cardiac tissue extracts from six rats in each group were obtained by homogenizing left ventricle samples in a lysis buffer at a ratio of 100 mg tissue/1 ml buffer. The homogenates were placed on ice and then centrifuged at 12,000g for 40 min. The supernatant was collected and stored at −80 °C for further experiments.
Western blot
The protein concentrations of the cardiac tissue extracts were determined using the Lowry protein assay. Protein samples (40 μg/lane) were separated by 12 % SDS polyacrylamide gel electrophoresis (SDS-PAGE) at a constant voltage of 75 V. The proteins were then transferred to Hybond-C membranes using 50 V for 3 h. PVDF membranes were incubated in 3 % bovine serum albumin (BSA) in TBS buffer. Primary antibodies, including p-PI3K, SIRT1, p-FOXO3, FOXO3, BNP, β-actin, TNF-α, Fas/L, FADD, caspase-8, caspase-3, and PARP (Santa Cruz Biotechnology, Santa Cruz, CA), were added to the membranes to recognize the corresponding proteins. Finally, horseradish peroxidase-labeled antibodies were used, and pictures were then taken using Fujifilm LAS-3000 (GE Healthcare).
Heart echocardiography
M-mode echocardiographic examination was performed using a 6–15-MHz linear transducer (15–6 L) via a parasternal long axis approach. Left ventricular M-mode measurements at the level of the papillary muscles included left ventricular internal end-diastolic dimensions diastolic (LVIDd), left ventricular internal end-systolic dimensions (LVIDs), and fractional shortening (FS%). FS% was calculated according to the following equation: [(LVIDd − LVIDs)/LVIDd] × 100.
Statistical analysis
The results shown are the means ± SD of six independent experiments. Statistical analysis was performed by one-way analysis of variance. For paired samples, Student’s t test was applied.
Results
Cardiac echocardiography
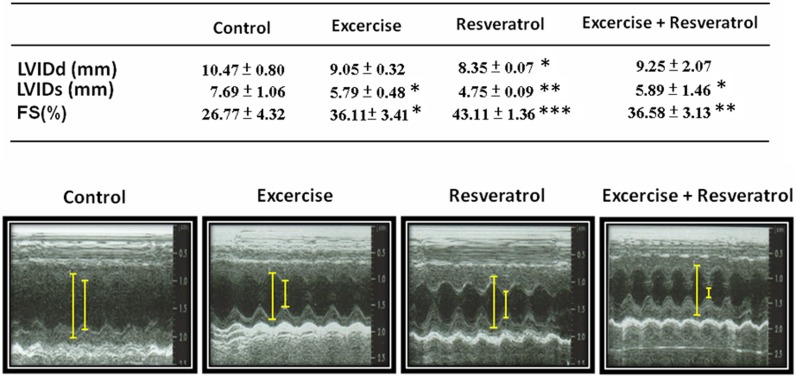
Based on the results of the echocardiographic analysis, heart function evaluated by FS% in the group II rats was 36.11 %, which was better than the control group (Fig. 1). Moreover, FS% in the rats in groups III and IV was better at 43.11 and 36.85 %, respectively. LVIDd only decreased in the resveratrol treatment group, and LVIDs decreased in the exercise training, resveratrol treatment, and exercise training co-treated with resveratrol groups.
Fig. 1.
Echocardiographic assessments of cardiovascular structure and function. Fractional shortening (FS, unit = %) was calculated by (LVIDd − LVIDs)/LVIDd × 100. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group
Heart biopsy
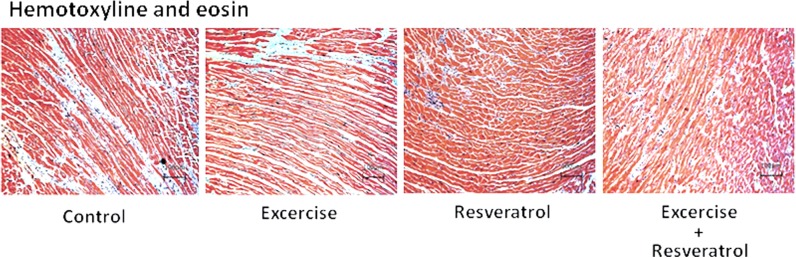
Hematoxylin and eosin staining of tissue slides was performed to image cardiomyocytes (Fig. 2). Cardiomyocytes from the control rats were disordered with more space between the cells; this disordered arrangement improved in the exercise training group. Moreover, the resveratrol treatment and exercise training and resveratrol treatment groups both had significantly reduced disordered arrangement and space between cardiomyocytes (Fig. 2).
Fig. 2.
Hematoxylin and eosin staining of heart slides. Cell nuclei are stained blue, and other structures are stained pink
Masson’s trichrome
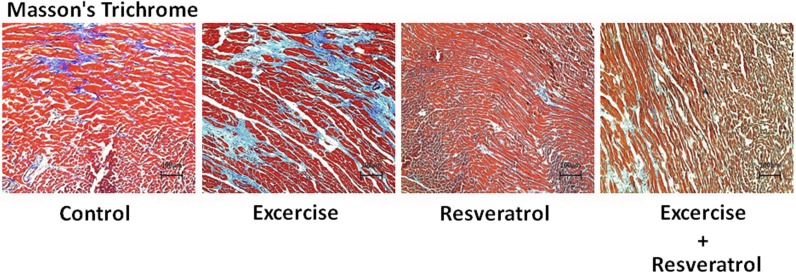
Masson’s trichrome staining of the heart tissue slides was performed for collagen accumulation (in blue) (Fig. 3). In the controls, collagen accumulation was severe and indicated fibrosis in the heart. Collagen accumulation in the exercise training group rat hearts slightly decreased with a lower collagen concentration in the fibrotic areas. Moreover, Masson’s trichrome staining in the resveratrol treatment and exercise training and resveratrol treatment rat hearts showed no collagen accumulation.
Fig. 3.
Masson’s trichrome staining of heart slides. The cells are stained red, and collagen is stained blue
Nucleic acid stain
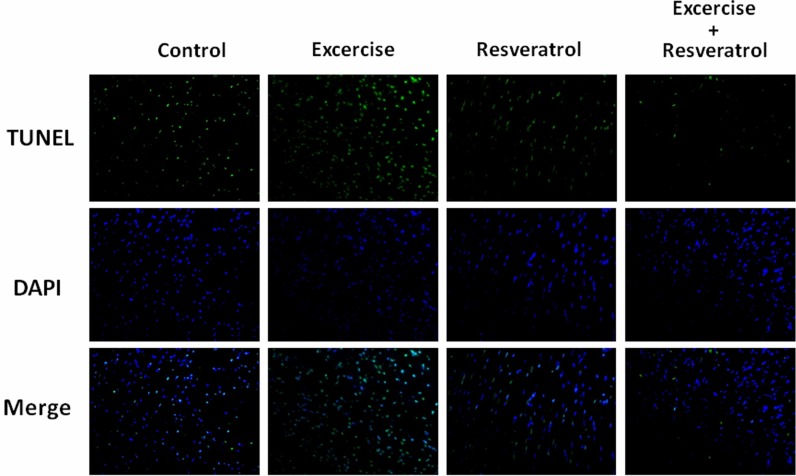
Cell nuclei were stained with DAPI (blue), and specific DNA fragments caused by caspase cleavage during apoptosis were stained by TUNEL (green). DAPI/TUNEL dual staining provided an image of several apoptotic cell nuclei in the control group heart slides (Fig. 4). In the exercise training group, the number of apoptotic cells did not significantly decrease. In the resveratrol treatment and exercise training and resveratrol treatment groups, apoptosis was significantly inhibited.
Fig. 4.
DAPI and TUNEL staining of heart slides. Cell nuclei are stained blue using DAPI, and specific DNA fragments caused by caspase cleavage during apoptosis are stained green using TUNEL
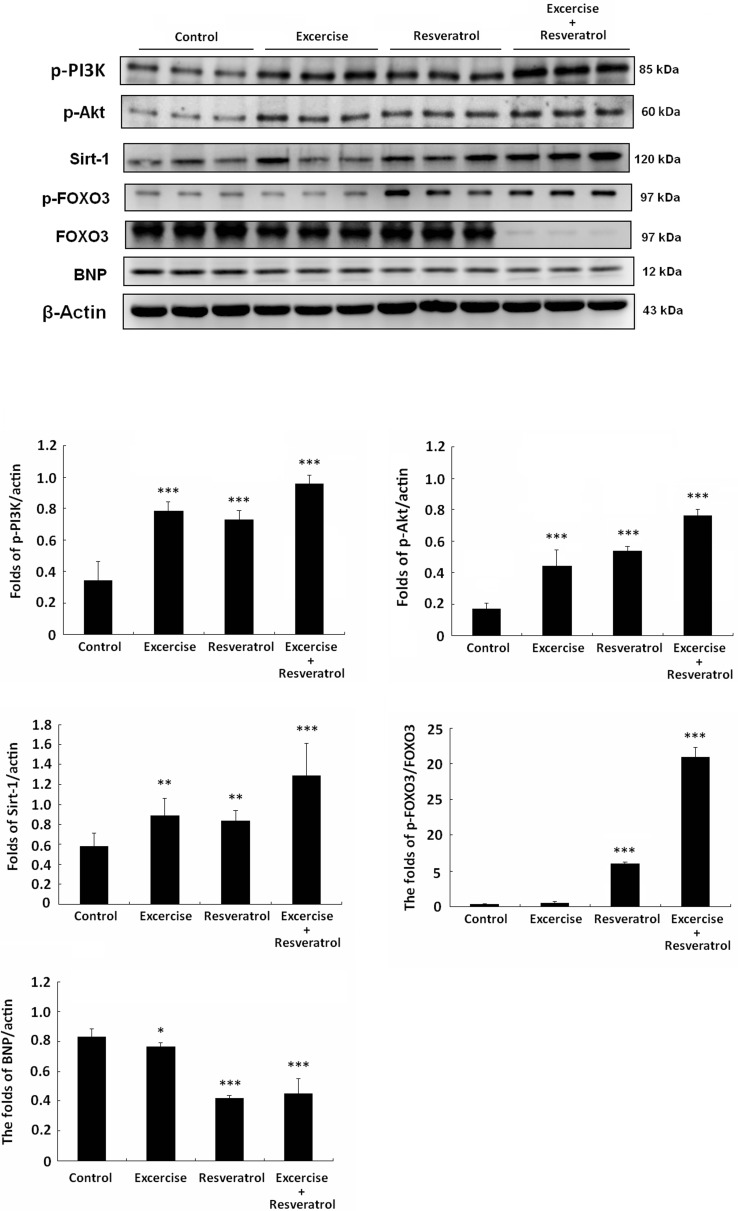
Protein analysis
Western blotting was used for heart protein analysis. PI3K survival signaling pathway was low in the control group, and only exercise training or resveratrol treatment slightly increased PI3K signaling (Fig. 5). However, SIRT1 expression was high in the resveratrol treatment and exercise training and resveratrol treatment rat hearts. FOXO3 is a downstream protein phosphorylated through PI3K signaling and dysfunction of SIRT1 deacetylase. As shown, p-FOXO3 expression was low in the control and exercise training groups. However, p-FOXO3 expression was higher in the resveratrol treatment and exercise training and resveratrol treatment groups. In particular, FOXO3 expression was higher in each group compared with that of the exercise training with resveratrol treatment group. Additionally, the heart failure biomarker BNP was lower in the exercise and resveratrol treatment group and efficiently inhibited in exercise training and resveratrol treatment group.
Fig. 5.
PI3K-Akt signaling pathway analysis. All protein samples from each rat group were analyzed by Western blotting (n = 3). The protein expression folds were normalized with β-actin. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group
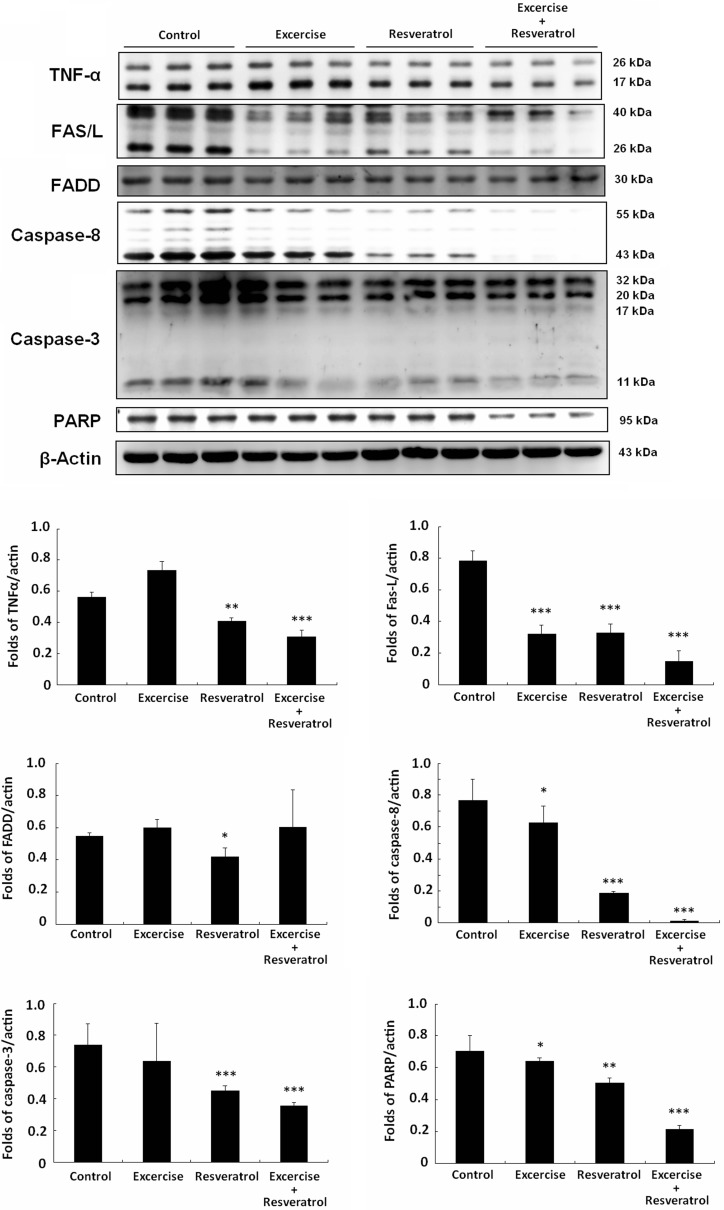
Also, TNF-α and Fas-FADD-caspase-8 protein expression in the control aging rat hearts was much higher than that in the exercise training and resveratrol treatment groups (Fig. 6). Moreover, exercise training combined with resveratrol treatment significantly decreased TNF-α and Fas protein expression and also inhibited caspase-8 and caspase-3 activation in the rat hearts.
Fig. 6.
Fas-FADD signaling pathway analysis. All protein samples for each rat group were analyzed by Western blotting (n = 3). The protein expression folds were normalized with β-actin. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group
Discussion
A normal rat heart FS% is greater than 40, and FS% is greater than 30 in healthy humans (Gudmundsson et al. 2005; Regitz-Zagrosek et al. 2007; He et al. 2009; Soetikno et al. 2012; Allen et al. 2013). In this study, FS% in the aged rat hearts was 26.77 %, and FS% in the other groups increased from 36.11 to 43.11 % (Fig. 1). These data suggest that exercise training, resveratrol treatment, and exercise training combined with resveratrol treatment can all be cardioprotective.
Our previous report revealed an antihypertrophic effect in rat hearts from exercise training (Lee et al. 2008; Huang et al. 2012; Cheng et al. 2013; Lee et al. 2013). Indeed, the collagen accumulation in the exercise training rat hearts was not as severe as that in the control group (Figs. 2 and 3). In particular, the collagen concentration in cardiomyocytes from the exercise training group rat was slightly lower than that from the controls. Moreover, in the resveratrol treatment and exercise training combined with resveratrol treatment group, the rat hearts did not show the same collagen accumulation (Fig. 3). Thus, we are curious to determine the mechanism.
TUNEL/DAPI nucleic acid staining is an efficiency assay for identifying cell apoptosis (Gavrieli et al. 1992). The green spots from TUNEL staining show apoptotic cells, and exercise training did not prevent cell apoptosis in this aging rat model (Fig. 4). Actually, given that exercise will increase the ROS level, which fortunately can be metabolized by SOD, catalase GPX, and ferritin or can be blocked directly by redox cycling compounds, such as resveratrol (Zarzuelo et al. 2013). Therefore, only the resveratrol treatment and exercise training combined with resveratrol treatment groups promoted cardiomyocyte survival in aging hearts.
Resveratrol is a compound with strong antioxidant activity, which might directly inhibit O2 oxidation and prevent ROS production during aging or exercise training. Resveratrol is also a SIRT1 activator (Frazzi et al. 2013; Lakshminarasimhan et al. 2013; Li et al. 2013). When SIRT1 is activated, SIRT1 will block FOXO3 accumulation in the nucleus and inhibit cell death. If FOXO3 translocates into the nucleus, downstream proteins regulate inflammation, and cell death will occur through their functions. PI3K-Akt activation can also help phosphorylate FOXO3 at threonine 32, serine 253, and serine 315 and cause the nuclear export of FOXO3 (Jang et al. 2007; Dobson et al. 2011).
Here, a similar mechanism for p-FOXO3 export from the nuclei of cardiomyocytes has also been demonstrated in the hearts from rats undergoing exercise training and resveratrol treatment (Fig. 5). Exercise can promote PI3K-Akt pathway activation and maintain FOXO3 phosphorylation; resveratrol treatment induces SIRT1 activation, leading to FOXO3 dysfunction. The benefits described above, exercise training combined with resveratrol, avoid ROS damage and further promote a synergetic cardioprotective effect.
Furthermore, FOXO3 accumulation in nuclei will also induce the FAS/FADD/Caspase-8 signaling pathway and cause cell apoptosis. Although exercise training and resveratrol treatment reduced FAS/FADD/Caspase-8 signaling, exercise training combined with resveratrol demonstrated a novel, strong protection in aging rat hearts (Fig. 6).
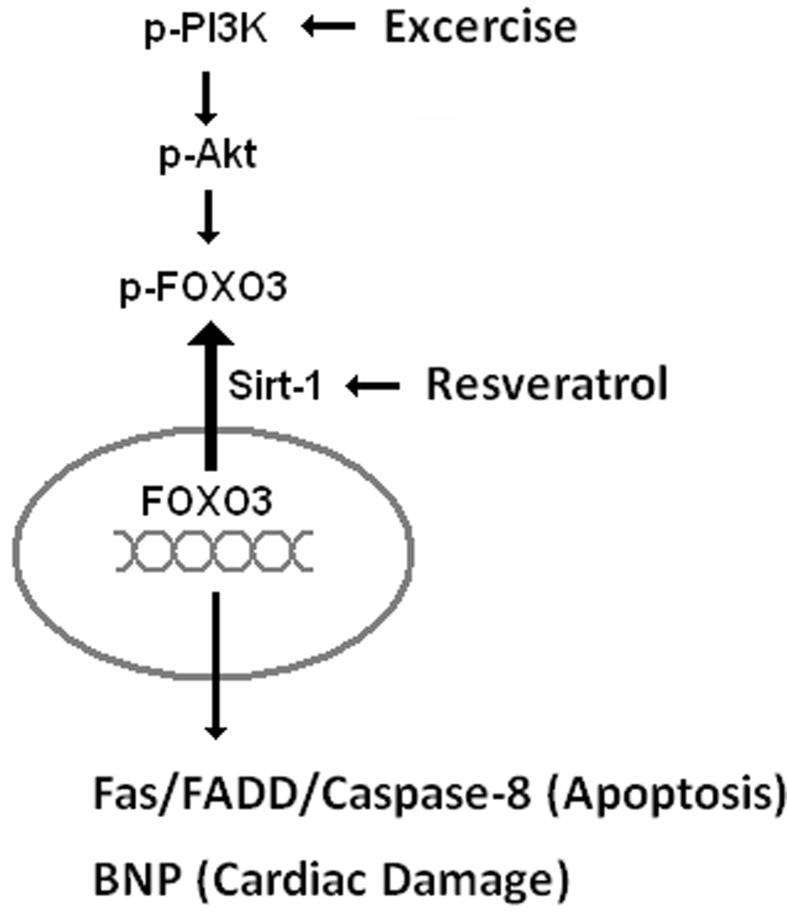
In conclusion, the major effect of exercise training is PI3K survival pathway activation (Cheng et al. 2013; Fernhall 2013; Morikawa et al. 2013; Hsu et al. 2013). Resveratrol treatment can help ROS elimination and SIRT1 activation (Fig. 7). Taken together, exercise training and resveratrol treatment provide the benefits of two pathways and block FOXO3 accumulation. These data suggest that resveratrol treatment augments the cardioprotective effect of exercise training in aging rat hearts.
Fig. 7.
The mechanism of exercise training and resveratrol mediated cardioprotection during aging. Exercise training activated PI3K-Akt signaling and induced FOXO3 phosphorylation. Resveratrol treatment activated SIRT1, causing FOXO3 dysfunction and nuclear export of FOXO3. The exercise training and co-treatment with resveratrol provided the benefits of both pathways to protect the heart from the apoptosis induced by ROS and Fas-FADD signaling and therefore prevent heart failure
Acknowledgments
This study is supported in part by the Taiwan Department of Health Clinical Trials and Research Center of Excellence (DOH102-TD-B-111-004), China medical university (CMU99-NTU-01), and National Science Council (NSC101-2410-H-029-055).
Footnotes
Chih-Yang Huang and Wan-Teng Lin contributed equally.
Contributor Information
Chih-Yang Huang, Phone: +886-4-22053366, Email: cyhuang@mail.cmu.edu.tw.
Wan-Teng Lin, Phone: +886-4-23506053, Email: 040770@thu.edu.tw.
References
- Ahmed Z, Tang WH. Pharmacologic strategies to target oxidative stress in heart failure. Curr Heart Fail Rep. 2012;9:14–22. doi: 10.1007/s11897-011-0081-5. [DOI] [PubMed] [Google Scholar]
- Allen LA, Magid DJ, Gurwitz JH, Smith DH, Goldberg RJ, Saczynski J, Thorp ML, Hsu G, Sung SH, Go AS. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ Heart Fail. 2013;6:635–646. doi: 10.1161/CIRCHEARTFAILURE.112.000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan A (2011) Global status report on noncommunicable diseases 2010. World Health Organization, Geneva
- Behzad H, Jamil S, Denny TA, Duronio V. Cytokine-mediated FOXO3a phosphorylation suppresses FasL expression in hemopoietic cell lines: investigations of the role of Fas in apoptosis due to cytokine starvation. Cytokine. 2007;38:74–83. doi: 10.1016/j.cyto.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Blondon M, Wiggins KL, McKnight B, Psaty BM, Rice KM, Heckbert SR, Smith NL. The association of smoking with venous thrombosis in women. A population-based, case–control study. Thromb Haemost. 2013;109:891–896. doi: 10.1160/TH12-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SM, Ho TJ, Yang AL, Chen IJ, Kao CL, Wu FN, Lin JA, Kuo CH, Ou HC, Huang CY, Lee SD. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;167:478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, Tzivion G. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim Biophys Acta. 2011;1813:1453–1464. doi: 10.1016/j.bbamcr.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernhall B. Long-term aerobic exercise maintains peak VO (2), improves quality of life, and reduces hospitalisations and mortality in patients with heart failure. J Physiother. 2013;59:56. doi: 10.1016/S1836-9553(13)70149-8. [DOI] [PubMed] [Google Scholar]
- Frazzi R, Valli R, Tamagnini I, Casali B, Latruffe N, Merli F. Resveratrol-mediated apoptosis of Hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int J Cancer. 2013;132:1013–1021. doi: 10.1002/ijc.27748. [DOI] [PubMed] [Google Scholar]
- Gargiulo G, Testa G, Cacciatore F, Mazzella F, Galizia G, Della-Morte D, Langellotto A, Pirozzi G, Ferro G, Ferrara N, Rengo F, Abete P. Moderate alcohol consumption predicts long-term mortality in elderly subjects with chronic heart failure. J Nutr Health Aging. 2013;17:480–485. doi: 10.1007/s12603-012-0430-4. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–401. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson P, Rydberg E, Winter R, Willenheimer R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Inter J Cardiol. 2005;101:209–212. doi: 10.1016/j.ijcard.2004.03.027. [DOI] [PubMed] [Google Scholar]
- He KL, Burkhoff D, Leng WX, Liang ZR, Fan L, Wang J, Maurer MS. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55 % versus 40 % to 55 % versus <40 % Am J Cardiol. 2009;103:845–851. doi: 10.1016/j.amjcard.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Chung JG. Anticancer potential of emodin. Bio Med. 2012;2:108–116. doi: 10.1016/j.biomed.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Lin JH, Weng SW, Chueh FS, Yu CC, Lu KW, Wood WG, Chung JG. Crude extract of Rheum palmatum inhibits migration and invasion of U-2 OS human osteosarcoma cells by suppression of matrix metalloproteinase-2 and -9. Bio Med. 2013;3:120–129. [Google Scholar]
- Huang CY, Yang AL, Lin YM, Wu FN, Lin JA, Chan YS, Tsai FJ, Tsai CH, Kuo CH, Lee SD. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J Appl Physiol. 2012;112:883–891. doi: 10.1152/japplphysiol.00605.2011. [DOI] [PubMed] [Google Scholar]
- Jang SW, Yang SJ, Srinivasan S, Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem. 2007;282:30836–30844. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- Joo JH, Dorsey FC, Joshi A, Hennessy-Walters KM, Rose KL, McCastlain K, Zhang J, Iyengar R, Jung CH, Suen DF, Steeves MA, Yang CY, Prater SM, Kim DH, Thompson CB, Youle RJ, Ney PA, Cleveland JL, Kundu M. Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell. 2011;43:572–585. doi: 10.1016/j.molcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan M, Rauth D, Schutkowski M, Steegborn C. Sirt1 activation by resveratrol is substrate sequence-selective. Aging (Albany NY) 2013;5:151–154. doi: 10.18632/aging.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Kuo WW, Ho YJ, Lin AC, Tsai CH, Wang HF, Kuo CH, Yang AL, Huang CY, Hwang JM. Cardiac Fas-dependent and mitochondria-dependent apoptosis in ovariectomized rats. Maturitas. 2008;61:268–277. doi: 10.1016/j.maturitas.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Lee SD, Shyu WC, Cheng IS, Kuo CH, Chan YS, Lin YM, Tasi CY, Tsai CH, Ho TJ, Huang CY. Effects of exercise training on cardiac apoptosis in obese rats. Nutr Metab Cardiovasc Dis. 2013;23:566–573. doi: 10.1016/j.numecd.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q, Sun J, Lin Y, Zhang M, Huang R, Cheng J, Cao Y, Xiang G, Zhang J, Wu Q. Resveratrol reduces acute lung injury in a LPS? induced sepsis mouse model via activation of Sirt1. Mol Med Rep. 2013;7:1889–1895. doi: 10.3892/mmr.2013.1444. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Runge MS. Redox signaling in cardiovascular health and disease. Free Radic Biol Med. 2013;61C:473–501. doi: 10.1016/j.freeradbiomed.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Mizuno Y, Harada E, Katoh D, Kashiwagi Y, Morita S, Yoshimura M, Uemura S, Saito Y, Yasue H. Aerobic interval exercise training in the afternoon reduces attacks of coronary spastic angina in conjunction with improvement in endothelial function, oxidative stress, and inflammation. Coron Artery Dis. 2013;24:177–782. doi: 10.1097/MCA.0b013e32835cbef5. [DOI] [PubMed] [Google Scholar]
- Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. 2012;110:1238–1251. doi: 10.1161/CIRCRESAHA.111.246488. [DOI] [PubMed] [Google Scholar]
- Paraiso KH, Haarberg HE, Wood E, Rebecca VW, Chen YA, Xiang Y, Ribas A, Lo RS, Weber JS, Sondak VK, John JK, Sarnaik AA, Koomen JM, Smalley KS. HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clin Cancer Res. 2012;18:2502–2514. doi: 10.1158/1078-0432.CCR-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo G, Moscucci F, Pascucci M, Pappadà MA, D’Alessandro G, Rossi P, Quaglione R, Di Barba D, Barillà F, Magrì D. Influence of aging and chronic heart failure on temporal dispersion of myocardial repolarization. Clin Interv Aging. 2013;8:293–300. doi: 10.2147/CIA.S41879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Shioi T, Inuzuka Y. Aging as a substrate of heart failure. J Cardiol. 2012;60:423–428. doi: 10.1016/j.jjcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata M, Takagi R, Watanabe K. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC–MAPK signaling pathway. Eur J Pharm Sci. 2012;47:604–614. doi: 10.1016/j.ejps.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]