Abstract
Cardiovascular disease is the second leading cause of death (9.1 %) in Taiwan. Heart function deteriorates with age at a rate of 1 % per year. As society ages, we must study the serious problem of cardiovascular disease. SIRT1 regulates important cellular processes, including anti-apoptosis, neuronal protection, cellular senescence, aging, and longevity. In our previous studies, rats with obesity, high blood pressure, and diabetes exhibiting slowed myocardial performance and induced cell apoptosis were reversed via sports training through IGF1 survival signaling compensation. This study designed a set of experiments with rats, in aging and exercise groups, to identify changes in myocardial cell signaling transduction pathways. Three groups of three different aged rats, 3, 12, and 18 months old, were randomly divided into aging groups (C3, A12, and A18) and exercise groups (E3, AE12, and AE18). The exercise training consisted of swimming five times a week with gradual increases from the first week from 20 to 60 min for 12 weeks. After the sports training process was completed, tissue sections were taken to observe cell organization (hematoxylin and eosin (H&E) stain) and apoptosis (terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays) and to observe any changes in the myocardial tissues and proteins (Western blotting). The experimental results show that cardiomyocyte apoptotic pathway protein expression increased with age in the aging groups (C3, A12, and A18), with improvement in the exercise group (E3, AE12, and AE18). However, the expression of the pro-survival p-Akt protein decreased significantly with age and reduced performance. The IGF1R/PI3K/Akt survival pathway in the heart of young rats can indeed be increased through exercise training. As rats age, this pathway loses its original function, even with increasing upstream IGF1. However, levels of SIRT1 and its downstream target PGC-1α were found to increase with age and compensatory performance. Moreover, exercise training enhanced the SIRT longevity pathway compensation instead of IGF1 survival signaling to improve cardiomyocyte survival.
Keywords: Aging, Exercise training, Apoptosis, SIRT1, IGF1 survival signaling
Introduction
Cardiovascular disease involves the pathological damage of the heart and major blood vessels near the heart (Shoucri 1991). The Department of Health, the Executive Yuan, R.O.C. (Taiwan), published a report on the top ten leading causes of death, indicating that cardiovascular disease has become the second major cause (9.1 %). Other heart related diseases and complications, such as cerebrovascular disease and hypertension, are the third (9.3 %) and tenth (1.3 %) causes of death, respectively.
As people age, heart function deteriorates at 1 % each year. In old age, human blood circulation and cardiovascular compensatory mechanisms deteriorate, enhancing the risk for high cholesterol, hypertension, diabetes, cerebrovascular disease, obstructive sleep apnea (OSA), ventricular hypertrophy, ischemic heart disease, myocardial infarction, and related cancers (Cheitlin 2003; Khang et al. 2008). These are all serious issues that middle-aged people in modern society must face as they age. More research should concentrate on the relationship between aging and cardiovascular disease. There are various reasons for cardiovascular disease, including heredity, obesity and excessive weight, hypertension, diabetes mellitus, hyperlipidemia, alcoholism, smoking, gender difference, and aging (Hobi et al. 2006).
Previous studies have indicated that exercise training is an integral part of cardiac rehabilitation and is effective both in young and old people. Exercise training has been proven to be very effective for both the prevention and management of many diseases, especially metabolic syndrome (Conti et al. 2012). In this study, we predicted that exercise training can effectively improve cardiac function in naturally aging rats. We expected that exercise training could reduce cardiomyocyte apoptosis caused by natural aging in the rat heart. We also expected the Fas receptor and FADD in the death-receptor-dependent apoptotic pathway and the mitochondria-dependent apoptotic pathway to be effectively reduced through proper exercise training in aging rat heart tissue. In contrast, IGF and IGF1R in the survival pathway could be compensated and increased through exercise training in aging rat heart tissue. Additionally, which is associated with the anti-aging SIRT1 pathway, we expected exercise training to effectively preserve the function of cardiomyocytes.
Apoptosis is involved in the regulation of many intracellular physiological and pathological conditions, including the development of embryos, tissue and organ formation, and immune system generation (Schwartzman and Cidlowski 1993). In mammalian cells, there are two major apoptotic pathways divided into intrinsic mitochondrial and extrinsic death receptor activation (Crow et al. 2004; Fischer et al. 2004). Extrinsic death receptor activation is a predisposing factor for inducing apoptosis in other immune system cells.
In vitro, the cell releases predisposing factors in conjunction with the cell membrane receptor via aggregation to become a composite body (Czerski and Nuñez 2004). The FASL/FAS/TNF-α pathway incorporates adaptive proteins such as the Fas-associated death domain (FADD), which uses the death domain (DD) to bind death receptors, such as the pro-caspase-8 or pro-caspase-10 interaction platform to activate caspase-8 or caspase-10 molecules, respectively, that activate other proteins in the downstream signaling pathway (Bishopric et al. 2001). This activation reaction series has a positive singling network called a positive feedback mechanism. Mitochondria signaling pathways activated by caspase-8 strengthen and stabilize the signal transmission to induce apoptosis (Riedl and Shi 2004).
Insulin-like growth factor 1 (IGF1) has important survival roles in myocardial cells to promote survival. Obese rats have reinforced obesity regulation functions, and coincidentally, insulin and IGF1 networks may be adjusted by the signaling protein inositol phospholipid 3-kinase, PI3K. Myocardial cells provide multi-signaling pathways in cell regulation. These multiple protein regulations contain phosphatidylinositol 3-kinase (PI3K), Akt, protein kinase B (PKB), and calcium ions (Catalucci and Condorelli 2006; Khoynezhad et al. 2004; Lai et al. 2003; Latronico et al. 2004). Animal experiments have confirmed that IGF1 in the heart increases anti-apoptotic protein (Bcl-xL) mitochondrial performance in vivo (Yamamura et al. 2001). Over-expression of cardiac-specific IGF1 (Torella et al. 2004) has an effect on anti-apoptosis. At the same time, it was found that IGF1 plays a role by increasing apoptosis in IGF1-deficient rats during myocardial infarction (Palmen et al. 2001). IGF1 is very important in determining the rate of the aging process (Eli and Fasciano 2006). This study explores how aging is highly correlated with cardiovascular disease mortality for 51- to 98-year-old men and women. Based on the above results, IGF1 is beneficial for the aging heart and will increase the number and growth of cardiac stem cells, thus promoting muscle cell differentiation and function (Torella et al. 2004).
Silent information regulator 2 (Sir2) was first discovered in yeast and encoded a 63-kDa protein (Brachmann et al. 1995). All proteins of this class contain a conserved core catalytic domain, which corresponds to the COOH-terminal region of yeast Sir2p (aa 245–459). A highly conserved region between amino acids 255 to 275, with the conserved GAGxSxxxG sequence, has a weak similarity to part of the NAD+ binding domain in 6-phosphogluconate dehydrogenases (Bisercić et al. 1991). Sir2-like proteins (sirtuins) are present in all species of the animal kingdom. The most highly related mammalian homolog of yeast Sir2p is called Sirtuin 1 (SIRT1) (Frye 1999).
In the process of aging, many oxidant molecules and reactive oxygen species (ROS) are released into the body. Moreover, many of these molecules accumulate in various cells and tissues. An important mechanism involved in this process is represented by SIRT1, the activity of which is reduced by aging (Corbi et al. 2012). SIRT1 is involved in the oxidative stress response and in aging. It has been shown that SIRT1 activity increases in antioxidant capacity after moderate and prolonged exercise training in the hearts of aged rats (Ferrara et al. 2008).
Exercise training will activate the muscle cells of the AMP-activated protein kinase (AMPK), and AMPK will further promote downstream pathways of SIRT1 and peroxisome-proliferator-activated receptor coactivators (PGC-1α) (Suchankova et al. 2009). PGC-1α effects glucose transport regulation and mitochondrial biogenesis (Jäger et al. 2007; Lagouge et al. 2006). In previous laboratory studies, it was proven that PGC-1α exacerbated myocardial apoptosis in rats with diabetes, obesity, and high blood pressure. After exercise training, the heart muscle in rats with diabetes, obesity, and high blood pressure activated the survival pathway, thereby inhibiting the myocardial apoptosis pathway (Huang et al. 2012; Lee et al. 2012; Cheng et al. 2013). When an individual suffers from obesity, hypertension, and diabetes, this may be referred to as metabolic syndrome. Metabolic syndrome is a complex of interrelated risk factors for cardiovascular disease (CVD) (DeFronzo and Ferrannini 1991). Metabolic syndrome occurs in modern society and is more common among middle-aged people and the elderly. The SIRT1 regulatory mechanism de-acetylates PPAR-γ coactivator-1a (PGC-1a) and activates PGC-1a. PGC-1a functions as a coactivator with pleiotropic (Knutti and Kralli 2001; Lin et al. 2005). Most importantly, SIRT1 can control muscle function and mitochondrial biogenesis (Lin et al. 2002).
Therefore, this study involved three groups with different ages of Sprague–Dawley (SD) rats to investigate relevant myocardial functions and signaling molecules and whether exercise training can reduce cardiovascular disease in rats of different ages. We further attempted to understand the myocardial cell molecular regulation mechanisms in aging with or without exercise.
Methods and materials
Animal models
The study was performed using 26 Sprague–Dawley (SD) rats. The rat ages and numbers were ten 3-month-aged rats, ten 12-month-aged rats, and six 18-month-aged rats. Each age group was randomly divided into two groups: aging rats (A) and exercise training aging rats (AE). Exercise training was 20 min per session in the first 2 weeks, 30 min in the 3rd week, and 60 min in the 4th to 12th weeks, with five sessions per week for a total of 12 weeks. Ambient temperature was maintained at 22–24 °C, and the animals were kept on an artificial 12-h light–dark cycle. The light period began at 7:00 in the morning. The rats were provided with standard laboratory chow (Lab Diet 5001; PMI Nutrition International Inc., Brentwood, MO, USA) and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee of China Medical University, Taichung, Taiwan, and the principles of laboratory animal care (NIH publication) were followed.
Exercise training
The 12-week exercise training protocol was implemented according to the study of Chen Hi et al. (1996). At the beginning of exercise training, rats from the AE group had to swim 20 min per day, five times per week in the first 2 weeks. In the third week, the training time was increased to 30 min per day, five times a week. In the 4th to 12th weeks, the swimming training time was increased to 1 h per day. After the exercise training, heart tissues were collected, the left ventricle tissue was isolated, and the tibial length was measured. The experimental data were statistically analyzed.
Histological analysis
We removed tissue from the left ventricles, washed the samples using 1× phosphate-buffered saline (PBS), and then fixed them for 12 h using formalin. After 12 h, the tissues were dehydrated. The prepared tissue samples were immersed in PBS for 30 min and then in 0.85 % NaCl(aq) for another 30 min. Under different alcohol concentrations (70, 85, 95, and 100 %), the samples were soaked for 15, 30, 30, and 30 min, respectively. Each step in this process was repeated two times to dehydrate the samples completely. The dehydrated tissue samples were then embedded. The samples were soaked in 100 % xylene two times for 30 min, then in a xylene and paraffin solution (v/v = 1/1) for 45 min at 60 °C and, finally, in 100 % paraffin three times for 20 min at 60 °C. These embedded samples were cut into sections and sorted, with a thickness of approximately 0.5 μm and then analyzed.
Hematoxylin and eosin staining
The tissue sections were dyed using hematoxylin and eosin (H&E). First, the sections were de-waxed and then dyed using hematoxylin for 3 min. After 3 min, the sections were washed two times in double-distilled water (DDW) and then soaked in 85 % alcohol for 1 min. We then dyed the sections with eosin for 5 min and immersed them in 70, 80, and 90 % alcohol, successively. The sections were then soaked in 100 % alcohol for 2 min and two times in xylene for 30 s. The sections were then sorted and photographically analyzed.
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling assay
We placed the tissue sections in the upper side of a hybridization incubator at 58 °C overnight or 60 °C for 30 min to dissolve the wax. The sections were then soaked in 100 % xylene and shaken for 5 min. The process was conducted three times to de-wax the samples. The de-waxed sections were soaked in 100, 90, 85, and 75 % alcohol for 5 min, successively, followed by shaking in DDW for 5 min. After completely re-hydrating the sections, we carefully dried the surfaces using paper. A Dako pen was used to depict the section ranges. It was important that the sections remained wet. We covered the sections with proteinase K for 25 min and then washed them in 1× PBS two times for 4 min and dried the section surfaces again. A 0.1 % sodium citrate solution was poured over the sections and left for 8 min, and the sections were then washed in 1× PBS two times for 5 min and dried. Blocking buffer (0.1 M Tris–HCl + 3 % BSA) was poured onto the sections and left for 30 min, and the sections were then washed two times in 1× PBS for 5 min. A 1:9 enzyme solution was then poured over the sections. The samples were kept in a hybridization incubator under reduced light for 1 h and then washed in 1× PBS two times for 5 min followed with photographic analysis.
DAPI stain
The terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling (TUNEL) assay DAPI dye was diluted using 1× PBS to DAPI: 1× PBS = 1:10,000 and kept on the slides for 5 min under reduced light, and then, the slides were washed in 1× PBS two times for 5 min. We observed the sections under fluorescent microscopy.
Tissue grinding
The collected left ventricle tissues of the A (A3, A12, and A18, the numbers indicating the age in months) and AE (AE3, AE12, AE18) groups were washed in PBS buffer and then weighed. Approximately 0.1 g tissue was added by 1 mL lysis buffer into the mixture, and the mixture was homogenized for 20 min, at 1,200 rpm and 4 °C. After stirring, we extracted a clean upper layer suspension. The homogenization was repeated to extract a clean upper layer suspension.
Western blotting
The protein samples and 5× loading dye were mixed and boiled for 10 min and then analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The upper SDS-PAGE layer was 4 % stacking gel, and the bottom layer was 10 or 12 or 14 % separating gel. The prepared SDS-PAGE was fixed onto a vertical electrophoresis system, and the electrode buffer was reloaded into the bank. The prepared protein samples were then loaded into U-type cannelures. The gel electrophoresis worked continuously under 75–100 V, and the voltage was chosen by the acquired time. After the electrophoresis was completed, the gel was removed from the tank, smoothed out upon wet Whatman 3 M filter paper, and covered with an alcohol-immersed polyvinylidene difluoride (PVDF) membrane and another 3 M filter paper. A glass rod was used to strip the bubbles away from the membrane. The gel and the papers were placed into a transfer holder and then soaked in transfer buffer in an electrotransfer tank for 1.5 h, under 100 transferred volts and at 4 °C. One and one-half hours later, we placed the PVDF membrane into a 5 % blocking buffer/Tris-buffered saline (TBS)-non-fat milk powder solution and shook it for 1 h at RT. After shaking, the PVDF membrane was incubated with the primary antibody overnight in a 4 °C refrigerator. The next day, the PVDF membrane was washed with washing buffer (1× TBS) three times for 10 min. The buffer was then poured out. After washing, the secondary antibody (2 μL/mL 1× TBS buffer) was added and incubated for 2 h in 4 °C, and then, the membrane was washed again with washing buffer. Finally, the membrane was colored with A/B solution (1:1).
Statistics
The experimental data were given as the mean ± SEM. The differences between two groups were analyzed using Student’s t test. The difference between multiple groups were analyzed using one-way ANOVA, and p < 0.05 was considered significant.
Result
Body weight and cardiac characteristics
The SD rat heart tissues were used to analyze the differences between groups in the experiments. First, we weighed the tissue and measured the tibia length of each group, and then we dissected whole heart samples and removed the residual adipose tissue and the blood vessels from the specimen. Excess specimen water was dried using paper towels pressed gently against the tissue before weighing the whole heart weight. This was followed by separating the left ventricle and weighing it (g). Statistical analysis was applied to determine the significance of different relationship factors. We studied the relationship between left ventricular weight and tibia length. This value agrees more closely with the estimates based on tibial length than with those based on left ventricular weight. To predict the left ventricular weight in aging rats, a regression equation using left ventricular weight, age, and tibial length was derived (Yin et al. 1982). By quantifying and comparing the ratio, it was found that the ratio in SD rats changed with left ventricular weight and influenced the cardiac index data. The experimental results shown in (Table 1) indicate that the left ventricular weight and heart chamber increase proportionally with age. Without exercise training, rat (C3, A12, and A18) left ventricular weight indexes increased with age. The left ventricular weight of the exercise training groups (E3, AE12, and AE18) improved significantly compared to the no exercise training groups. The ratio of left ventricular weight to tibia length is proportional to age.
Table 1.
Experimental results for Sprague–Dawley (SD) rats and aging rats with or without exercise training (A and AE)
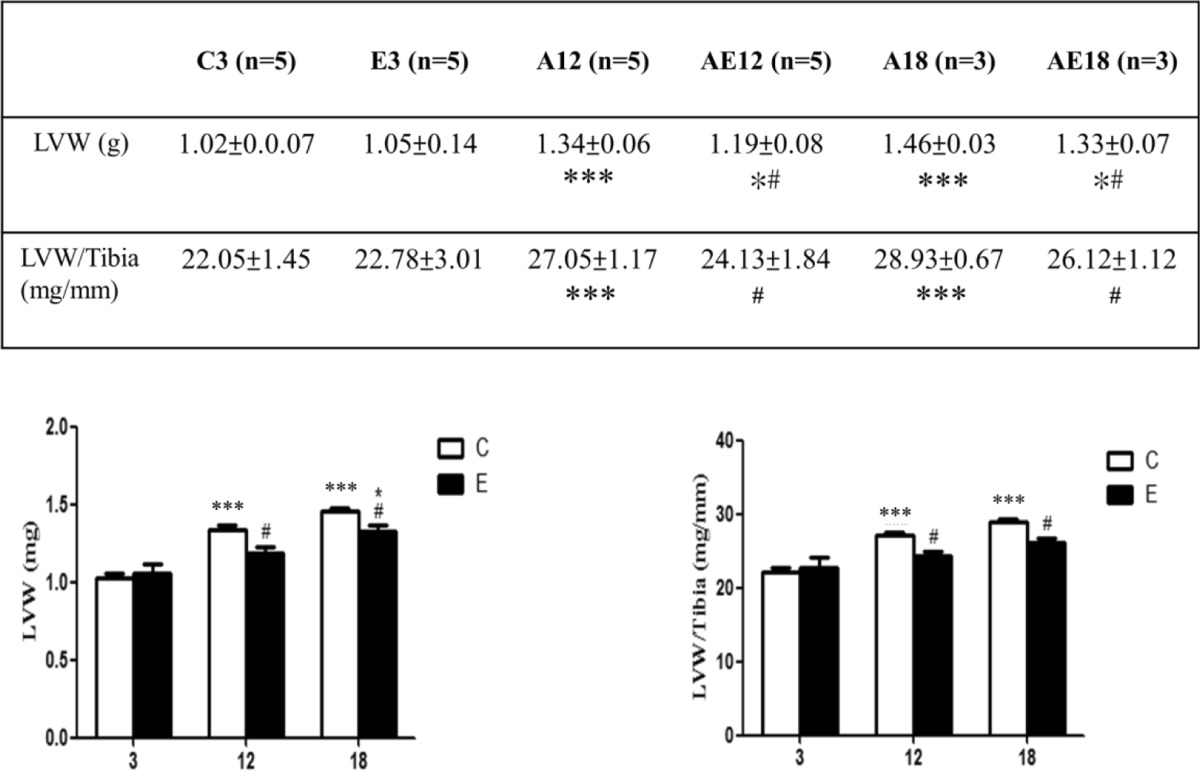
Values are means ± SD
LVW left ventricular weight
*p < 0.05; **p < 0.01; ***p < 0.001, significant differences between the C3 and A12 or A18 groups
# p < 0.05; ## p < 0.01; ### p < 0.001, significant differences between the aging (C3, A12, and A18) and exercise (E3, AE12, and AE18) groups
TUNEL apoptotic cells in cardiac tissues
We investigated whether DNA breakage could have serious effects during aging in cardiomyocytes. DNA fragmentation in apoptosis is usually associated with structural changes in cellular morphology and can be examined using the TUNEL assay. The in situ staining of DNA strand breaks detected by the TUNEL assay and subsequent visualization by light microscopy provides biologically significant data regarding DNA damage relating to apoptosis.
We performed a histopathological analysis of ventricular tissues stained with hematoxylin and eosin (Fig. 1). After viewing ×400 magnified images, we observed that, of the aging groups (C3, A12, and A18), C3 had normal myocardial cell architecture and volume. However, the A12 and A18 groups exhibited abnormal myocardial architecture and volume due to aging. This phenomenon was improved in all the exercise groups (E3, AE12, and AE18), and the experimental results are shown in Table 1.
Fig. 1.
Hematoxylin and eosin staining (H&E staining). Cardiac tissue sections stained with hematoxylin and eosin. The myocardial architecture images were magnified ×400. The scale bar is 100 μm
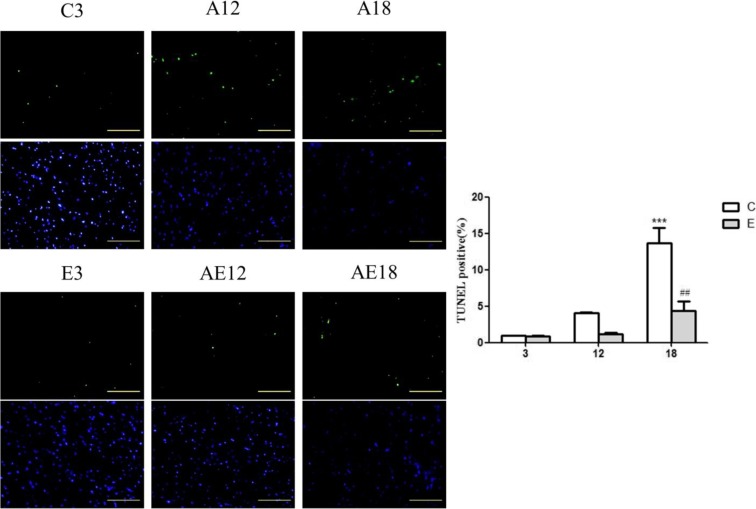
The apoptotic cells and total cells were quantified using the TUNEL assay and DAPI staining, respectively, to view the apoptotic activity in cardiac tissues from the aging (C3, A12, and A18) and exercise (E3, AE12, and AE18) groups. Viewing images magnified ×400, we observed that the left ventricles of the aging groups (A12 and A18) stained with the TUNEL assay had a greater number of TUNEL-positive cardiac cells than those in the C3 group. Additionally, the left ventricles of the exercise groups (E3, AE12, and AE18) had fewer TUNEL-positive cells than those in C3, A12, and A1 (Fig. 2a). Moreover, the ImageJ software analyzed the number of apoptotic cells with one-way analysis of variance (ANOVA) statistical analysis, showing differences with p < 0.001 for C3:18 and p < 0.01 for A18:AE18. The results in Fig. 2b show significant differences.
Fig. 2.
a Representative apoptotic cells in cardiac sections from the left ventricles of SD rats, in the aging (C3, A12, and A18) and exercise (E3, AE12, and AE18) groups, measured using DAPI staining (upper panels, blue spots) and the TUNEL assay (lower panels, green spots). The images were magnified ×400. The scale bar is 100 μm. b Bars present the percentage of TUNEL-positive cells relative to total cells. *p < 0.05; **p < 0.01; ***p < 0.001, significant differences between the C3 and A12 or A18 groups. # p < 0.05; ## p < 0.01; ### p < 0.001, significant differences between the aging (C3, A12, and A18) and exercise (E3, AE12, and AE18) groups
Immunoblotting analysis
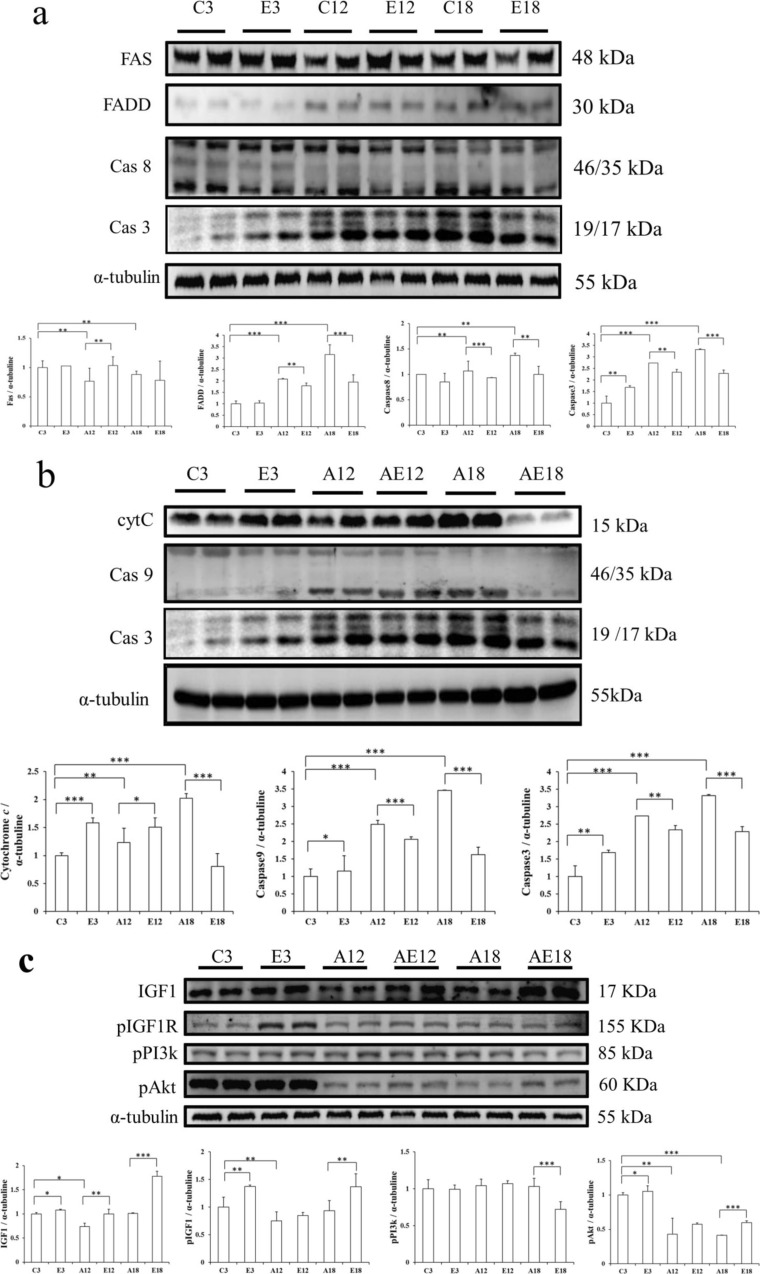
To investigate the upstream components of the cardiac Fas-receptor-dependent apoptotic signaling pathway in aging rats after exercise training, we measured Fas, FADD, caspase-8, and caspase-3 (Fig. 3a). The aging groups (C3, A12, and A18) displayed myocardial apoptosis, but in the exercise groups (E3, AE12, and AE18), apoptosis was suppressed. We also investigated the mitochondria-dependent pathway signal proteins, cytochrome c, caspase-9, and caspase-3 (Fig. 3b). In the aging groups (C3, A12, and A18), protein expression increased with age. However, the exercise groups (E3, AE12, and AE18) showed improvement when compared to the aging group.
Fig. 3.
a The representative protein products of the Fas receptor, FADD, caspase-8, and caspase-3 extracted from the left ventricles of two rats in each group, SD rats (C3), aging rats (A12, A18), and aging, exercise-trained rats (E3, AE12, AE18), measured using Western blotting. b The representative cytochrome c, caspase-9, and caspase-3 protein products extracted from the left ventricles of two rats in each group, SD rats (C3), aging rats (A12, A18), and aging, exercise-trained rats (E3, AE12, AE18), measured using Western blotting. c The representative protein products of IGF1, pIGF1R, pPI3K, and pAkt extracted from the left ventricles of two rats in each group, SD rats (C3), aging rats (A12, A18), and aging, exercise-trained rats (E3, AE12, AE18), measured using Western blotting. α-Tubulin was used as an internal control. *p < 0.05; **p < 0.01; ***p < 0.001, significant differences between the aging (C3, A12, and A18) and exercise (E3, AE12, and AE18) groups
To investigate the correlation between apoptosis and the survival pathway, we tested IGF1, pIGF1R, pPI3K, and pAKT from the survival pathway (Fig. 3c). The IGF1, pIGF1R, and pPI3K signals from the three signaling molecules in the aging group (C3, A12, and A18) did not change significantly, but the pAKT level was greatly reduced in the aging process. Comparing the aging groups with the exercise groups, each exercise group’s pAKT expression level was slightly greater than that of the respective aging group. The expression showed increasing upstream IGF1, with the survival pathway losing its original compensative function in the heart with or without exercise training.
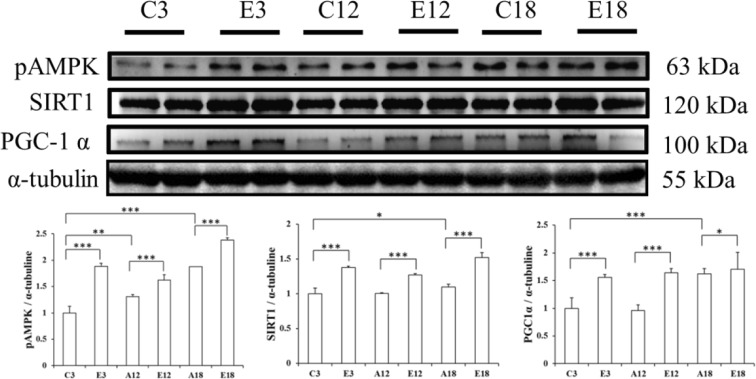
Our experiments also looked at the longevity-related signaling molecules, pAMPK, SIRT1, and PGC-1α (Fig. 4). Interestingly, the SIRT1 pathway protein increased with age. pAMPK, SIRT1, and PGC-1α were significantly lower with age in the aging groups (A12 and A18). Comparing the exercise groups (E3, AE12, and AE18) to aging groups (C3, A12, and A18), in which pAMPK, SIRT1, and PGC-1α were lower, a compensatory increase was demonstrated in this longevity-related signaling pathway.
Fig. 4.
The representative protein products of pAMPK, SIRT1, and PGC-1α extracted from the left ventricles of two rats in each group, SD rats (C3), aging rats (A12, A18), and aging, exercise-trained rats (E3, AE12, AE18) were measured using Western blotting. α-Tubulin was used as an internal control. *p < 0.05; **p < 0.01; ***p < 0.001, significant differences between the aging (C3, A12, and A18) and exercise (E3, AE12, and AE18) groups
Discussion
As an individual ages, cardiomyocyte mitosis gradually slows down. Any of the simulated factors can promote the compensatory mechanism of myocardial cells. More myocardial cells are produced to reduce the workload in response to a gradual increase in workload. Unfortunately, myocardial cells express no re-differentiation in their lives, and any attempt to solve the insufficient function problem leads to cardiac hypertrophy, which supports the function of the defective heart more efficiently (Sugden and Clerk 1998). Hypertrophy of the cardiomyocyte includes changes in cell size and appearance, which are referred to as called remodeling (Aiello and Binotto 2007). The after-load and pre-load or any pressure increase triggers cardiomyocyte remodeling, with increases in cell length and width in the early period. This is known as physiological hypertrophy and supplies the body with blood pumped from the heart to cope with a sudden increase in pressure. Heart physiological hypertrophy is an urgent adaptive response to stress. If the pressure is not reduced by physiological hypertrophy, myocardial cells will not withstand an excessive load for an extended period. Recurrent excessive heart loads will create pathological hypertrophy, and this phenomenon is irreversible in the heart (Tezuka and Takahashi 1976). After long-term injury, myocardial cells will cease to withstand the excessive strain, bringing about exhaustion and apoptosis.
Our previous experiment found that poor health factors can cause heart disease, such as high blood pressure, obesity, diabetes, and even secondhand smoke in rats. The apoptotic pathway exhibits increased levels with a reduced survival pathway in these situations. Exercise training and eating purple sweet potato yogurt can help to prevent heart failure and apoptosis (Cheng et al. 2013; Kuo et al. 2005; Lin et al. 2013; van Tol et al. 2006; Huang et al. 2012). Spontaneously hypertensive rats (SHR) of advanced age exhibit depressed myocardial contractile function and ventricular fibrosis, and as a result, stable compensatory hypertrophy progresses to heart failure. In spontaneously hypertensive rats of advanced age, the contraction ability of heart decreases (Boluyt et al. 1995). The left ventricular weight increased, and the mean cardiomyocyte number increased slightly with age to cause hypertrophy. However, hypertrophy improved after exercise training (Table 1).
As shown in Fig. 3, the expression of caspase-3 and caspase-9 in rat hearts increased with age, as did mean cell apoptosis. This can be reduced by exercise training. Our previous reports revealed an anti-hypertrophic effect in rat hearts through exercise training (Chang et al. 2013; Lee et al. 2013; Lee et al. 2008; Huang et al. 2012). This aging model expression after stimulation is weakened with increasing age. We speculate that the aging rat cardiac tissue repair capacity is decreased so that the survival pathway cannot return to the same vigor as that in young rats after exercise training (Fig. 3a). The TUNEL assay of biopsied samples did not detect increased apoptosis. This may suggest that the apoptotic cells are not cardiomyocytes and that exercise training does indeed regulate heart remodeling in young rat hearts (Trask et al. 2012). In other words, remodeling caused by exercise has favorable effects on cardiac rehabilitation (Giallauria et al. 2009).
In the heart, the IGF1 survival pathway is more easily stimulated and susceptible to increased expression of upstream IGF1 with age and without exercise training. However, downstream pAkt only increased in young rats after exercise training (Fig. 3c). When ROS accumulated in vivo, Sirt1 increased cellular stress resistance by increasing insulin sensitivity. A decrease in circulating free fatty acids and insulin-like growth factors (IGF1), increased AMPK activity, increased PGC-1a activity, and an increase in mitochondria occurred (Corbi et al. 2013). Our experimental data also showed similar events. In the A18 group, IGF1 expression decreased and SIRT1 expression increased (Figs. 3c and 4).
Although there are other proteins that help the heart survive through compensatory performance, SIRT1 for example (Fig. 4), aging is the main factor of survival. In the aging rat heart, upstream pAMPK decreased due to aging and downstream PGC-1 activation was increased. However, no age-related significant increase of SIRT1 occurred. Even more surprising is the fact that after exercise training, pAMPK, SIRT1, and PGC-1 expression levels were highly increased in aging rats, just as with young rats given the same exercise training (Figs. 4 and 5).
Fig. 5.
The summary of SIRT1 regulating the apoptotic upstream pathways within a cardiomyocyte in exercise-trained SD rats
In conclusion, the IGF1-R/PI3K/Akt survival pathway in the young rat heart can be increased through exercise training. As the rat ages, this pathway loses its original function. This study showed that SIRT1 and its downstream PGC-1α constitute a novel alternative survival pathway for the heart. With increasing age and compensatory performance, exercise training can increase SIRT longevity pathway performance instead of IGF1 survival signaling and increase the chance for cardiomyocyte survival.
Acknowledgments
This study is supported in part by the Taiwan Department of Health Clinical Trial and Research Center for Excellence (DOH102-TD-B-111-004).
References
- Aiello VD, Binotto MA. Myocardial remodeling in congenital heart disease. Arq Bras Cardiol. 2007;88(6):185–186. doi: 10.1590/S0066-782X2007000600013. [DOI] [PubMed] [Google Scholar]
- Bisercić M, Feutrier JY, Reeves PR. Nucleotide sequences of the gnd genes from nine natural isolates of Escherichia coli: evidence of intragenic recombination as a contributing factor in the evolution of the polymorphic gnd locus. J Bacteriol. 1991;173(12):3894–3900. doi: 10.1128/jb.173.12.3894-3900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopric NH, Andreka P, Slepak T, Webster KA. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol. 2001;1(2):141–150. doi: 10.1016/S1471-4892(01)00032-7. [DOI] [PubMed] [Google Scholar]
- Boluyt M, Bing O, Lakatta E. The ageing spontaneously hypertensive rat as a model of the transition from stable compensated hypertrophy to heart failure. Eur Heart J. 1995;16(suppl N):19–30. doi: 10.1093/eurheartj/16.suppl_N.19. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9(23):2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- Catalucci D, Condorelli G. Effects of Akt on cardiac myocytes location counts. Circ Res. 2006;99(4):339–341. doi: 10.1161/01.RES.0000239409.90634.a9. [DOI] [PubMed] [Google Scholar]
- Chang C, Zhang C, Zhao X, Kuang X, Tang H, Xiao X (2013) Differential regulation of mitogen-activated protein kinase signaling pathways in human with different types of mitral valvular disease. Journal of Surgical Research 181 (1):49–59. doi:http://dx.doi.org/10.1016/j.jss.2012.05.028 [DOI] [PubMed]
- Cheitlin MD. Cardiovascular physiology—changes with aging. Am J Geriatr Cardiol. 2003;12(1):9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- Chen Hi H, Chiang IP, Jen CJ. Exercise training increases acetylcholine-stimulated endothelium-derived nitric oxide release in spontaneously hypertensive rats. J Biomed Sci. 1996;3(6):454–460. doi: 10.1007/BF02258049. [DOI] [PubMed] [Google Scholar]
- Cheng SM, Ho TJ, Yang AL, Chen IJ, Kao CL, Wu FN, Lin JA, Kuo CH, Ou HC, Huang CY, Lee SD. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;167(2):478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Conti V, Russomanno G, Corbi G, Filippelli A. Exercise training in aging and diseases. Transl Med UniSa. 2012;3:74. [PMC free article] [PubMed] [Google Scholar]
- Corbi G, Conti V, Russomanno G, Rengo G, Vitulli P, Ciccarelli AL, Filippelli A, Ferrara N. Is physical activity able to modify oxidative damage in cardiovascular aging? Oxidative Med Cell Longev. 2012 doi: 10.1155/2012/728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbi G, Conti V, Russomanno G, Longobardi G, Furgi G, Filippelli A, Ferrara N. Adrenergic signaling and oxidative stress: a role for sirtuins? Front Physiol. 2013;4:324. doi: 10.3389/fphys.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Mani K, Nam Y-J, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95(10):957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- Czerski L, Nuñez G. Apoptosome formation and caspase activation: is it different in the heart? J Mol Cell Cardiol. 2004;37(3):643–652. doi: 10.1016/j.yjmcc.2004.04.016. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E (1991) Insulin resistence. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atheroclerotic cardiovascular disease. Diabetes Care 14(3):173–194 [DOI] [PubMed]
- Eli R, Fasciano JA. An adjunctive preventive treatment for heart disease and a set of diagnostic tests to detect it: Insulin-like growth factor-1 deficiency and cell membrane pathology are an inevitable cause of heart disease. Med Hypotheses. 2006;66(5):964–968. doi: 10.1016/j.mehy.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Fischer U, Steffens S, Frank S, Rainov NG, Schulze-Osthoff K, Kramm CM. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2004;24(7):1231–1243. doi: 10.1038/sj.onc.1208290. [DOI] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260(1):273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Giallauria F, Cirillo P, Lucci R, Pacileo M, D'agostino M, Maietta P, Vitelli A, Chiarielo M, Vigorito C (2009) Effects of excercise-based cardiac rehabilitation on high mobility group box-1 levels after acute myocardial infarction: rationale and design. J Cardiovasv Med (Hagerstown) 10(8):659–663 [DOI] [PubMed]
- Hobi A, Roy S, Vuille C, Perdrix J, Darioli R. Evolution of cardiac risk factors management among patients aged 65 years and older with coronary artery disease] Rev Méd Suisse. 2006;2(56):658. [PubMed] [Google Scholar]
- Huang CY, Yang AL, Lin YM, Wu FN, Lin JA, Chan YS, Tsai FJ, Tsai CH, Kuo CH, Lee SD. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J Appl Physiol. 2012;112(5):883–891. doi: 10.1152/japplphysiol.00605.2011. [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, Pierre JS, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials. 2008;29(8):970–983. doi: 10.1016/j.biomaterials.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Khoynezhad A, Jalali Z, Tortolani AJ. Apoptosis: pathophysiology and therapeutic implications for the cardiac surgeon. Ann Thorac Surg. 2004;78(3):1109–1118. doi: 10.1016/j.athoracsur.2003.06.034. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12(8):360–365. doi: 10.1016/S1043-2760(01)00457-X. [DOI] [PubMed] [Google Scholar]
- Kuo W-W, Wu C-H, Lee S-D, Lin JA, Chu C-Y, Hwang J-M, Ueng K-C, Chang M-H, Yeh Y-L, Wang C-J. Second-hand smoke–induced cardiac fibrosis is related to the Fas death receptor apoptotic pathway without mitochondria-dependent pathway involvement in rats. Environ Health Perspect. 2005;113(10):1349. doi: 10.1289/ehp.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lai H-C, Liu T-J, Ting C-T, Sharma PM, Wang PH. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol Cell Endocrinol. 2003;205(1):99–106. doi: 10.1016/S0303-7207(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Latronico MVG, Costinean S, Lavitrano ML, Peschle C, Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann N Y Acad Sci. 2004;1015(1):250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- Lee S-D, Kuo W-W, Ho Y-J, Lin A-C, Tsai C-H, Wang H-F, Kuo C-H, Yang A-L, Huang C-Y, Hwang J-M. Cardiac Fas-dependent and mitochondria-dependent apoptosis in ovariectomized rats. Maturitas. 2008;61(3):268–277. doi: 10.1016/j.maturitas.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Lee S-D, Shyu W-C, Cheng I-S, Kuo C-H, Chan Y-S, Lin Y-M, Tasi C-Y, Tsai C-H, Ho T-J, Huang C-Y (2012) Effects of exercise training on cardiac apoptosis in obese rats. Nutr Metab Cardiovasc Dis [DOI] [PubMed]
- Lee S-D, Lai TW, Lin S-Z, Lin C-H, Hsu Y-H, Li C-Y, Wang H-J, Lee W, Su C-Y, Yu Y-L, Shyu W-C. Role of stress-inducible protein-1 in recruitment of bone marrow derived cells into the ischemic brains. EMBO Mol Med. 2013;5(8):1227–1246. doi: 10.1002/emmm.201202258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang C-Y, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lin P-P, Hsieh Y-M, Kuo W-W, Lin Y-M, Yeh Y-L, Lin C-C, Tsai F-J, Tsai C-H, Huang C-Y, Tsai C-C. Probiotic-fermented purple sweet potato yogurt activates compensatory IGF‑IR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int J Mol Med. 2013;32(6):1319–1328. doi: 10.3892/ijmm.2013.1524. [DOI] [PubMed] [Google Scholar]
- Palmen M, Daemen MJAP, Bronsaer R, Dassen WRM, Zandbergen HR, Kockx M, Smits JFM, van der Zee R, Doevendans PA. Cardiac remodeling after myocardial infarction is impaired in IGF-1 deficient mice. Cardiovasc Res. 2001;50(3):516–524. doi: 10.1016/S0008-6363(01)00237-1. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Shoucri R. Pump function of the heart as an optimal control problem. J Biomed Eng. 1991;13(5):384–390. doi: 10.1016/0141-5425(91)90019-4. [DOI] [PubMed] [Google Scholar]
- Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier M-S, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun. 2009;378(4):836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med. 1998;76(11):725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- Tezuka F, Takahashi T (1976) Pathology of cardiac hypertrophy in pressure overload. Jpn Circ J 40(10) [DOI] [PubMed]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94(4):514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- Trask AJ, Delbin MA, Katz PS, Zanesco A, Lucchesi PA (2012) Differential coronary resistance microvessel remodeling between type 1 and type 2 diabetic mice: impact of excercise training. Vascul Pharmacol 57(5–6):187–193 [DOI] [PMC free article] [PubMed]
- van Tol BAF, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Otani H, Nakao Y, Hattori R, Osako M, Imamura H. IGF-I differentially regulates Bcl-xL and Bax and confers myocardial protection in the rat heart. Am J Physiol-Heart Circ Physiol. 2001;280(3):H1191–H1200. doi: 10.1152/ajpheart.2001.280.3.H1191. [DOI] [PubMed] [Google Scholar]
- Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol. 1982;243(6):H941–H947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]