Abstract
The aim of this study was to determine the outcomes of oestrogen and melatonin treatments following long-term ovarian hormone depletion on neuroinflammation and apoptotic processes in dentate gyrus of hippocampi. Forty-six female Wistar rats of 22 months of age were used. Twelve of them remained intact, and the other 34 were ovariectomized at 12 months of age. Ovariectomized animals were divided into three groups and treated for 10 weeks with oestrogens, melatonin or saline. All rats were killed by decapitation at 24 months of age, and dentate gyri were collected. A group of 2 month-old intact female rats was used as young control. The levels of pro-inflammatory cytokines and heat shock protein 70 (HSP 70) were analysed by ELISA. The expressions of TNFα, IL1β, GFAP, nNOS, iNOS, HO-1, NFκB, Bax, Bad, AIF, Bcl2 and SIRT1 genes were detected by real-time (RT)-PCR. Western blots were used to measure the protein expression of NFκB p65, NFκB p50/105, IκBα, IκBβ, p38 MAPK, MAP-2 and synapsin I. We have assessed the ability of 17β-oestradiol and melatonin administration to downregulate markers of neuroinflammation in the dentate gyrus of ovariectomized female rats. Results indicated that 17β-oestradiol and melatonin treatments were able to significantly decrease expression of pro-inflammatory cytokines, iNOS and HO-1 in the hippocampus when compared to non-treated animals. A similar age- and long-term ovarian hormone depletion- related increase in GFAP was also attenuated after both melatonin and oestradiol treatments. In a similar way to oestradiol, melatonin decreased the activation of p38 MAPK and NFκB pathways. The treatments enhanced the levels of synaptic molecules synapsin I and MAP-2 and have been shown to modulate the pro-antiapoptotic ratio favouring the second and to increase SIRT1 expression. These findings support the potential therapeutic role of melatonin and oestradiol as protective anti-inflammatory agents for the central nervous system during menopause.
Keywords: Ovariectomy, Dentate gyrus, Ageing, Neuroinflammation, Apoptosis, Oestrogens, Melatonin
Introduction
Dramatic changes in the endocrine activity of the female gonads ensue in ageing females, which are thought to underlie the emergence of a spectrum of physiological and psychological health complications. Menopause, whether occurring naturally or induced surgically, is thereby affecting the functional capacity of certain brain regions involved in learning, memory, and cognitive ability (Buckler 2005).
Meng et al. (2010) showed that decreases in learning and memory functions in ovariectomized rats were associated with degenerative changes in hippocampal neurons. Oestrogen deprivation also enhanced apoptotic cell death and decreased expression of the anti-apoptotic protein Bcl2. How apoptosis causes neurons to die is still a matter of debate; however, the principal mechanism is by triggering the release of intercellular signals which induce phagocytic cells to take in the neurons. Astrocytes and microglia express membrane receptors that recognize molecules released by neurons, leading to phagocytosis of ‘altered’ cells and neuronal debris (Harrison et al. 1998; Noda et al. 2011). Chronic activation of microglia is believed to trigger and maintain an inflammatory response, which may ultimately lead to neuronal cell death (Tan et al. 1999).
In the past few years, it has been increasingly clear that neuroinflammation negatively impacted on neuronal plasticity and specifically LTP was decreased when microglial activation and/or inflammatory cytokine production were increased in hippocampus (Hall et al. 1998; Lyons et al. 2009; Lynch 2010). The loss of ovarian oestrogens in postmenopausal women may exacerbate central and peripheral inflammatory responses that occur with normal ageing. One of the most prominent changes in the brain's neuroinflammatory response during ageing is the increased and/or poorly regulated production of pro-inflammatory mediators (Franceschi et al. 2000).
Previous work has shown that oestrogens are able to repress the transcription of numerous pro-inflammatory cytokine genes, suggesting that these hormones could be used to prevent and/or treat inflammatory conditions associated with menopause (Cvoro et al. 2011). Furthermore, mechanisms by which oestrogens prevent neuronal death involve the inhibition of apoptosis by increasing the level of anti-apoptotic proteins or repressing the level of pro-apoptotic proteins (Meda et al. 2000; Amantea et al. 2005). In a previous publication, we have demonstrated that the number of neurons in the hilus of the dentate gyrus was significantly decreased during ageing and that 22-month-old ovariectomized females chronically treated with oestradiol showed a significantly higher number of BrdU-immunoreactive cells in the GCL/SGZ than control animals treated with vehicle (Perez-Martin et al. 2005). Other groups have also confirmed that oestrogen treatments were able to enhance neurogenesis in the dentate gyrus of the hippocampus in adult animals (Brinton 2009; Pawluski et al. 2009).
Reiter et al. (1981) reported a marked decline in nocturnal pineal melatonin levels with ageing in female rats. Okatani et al. (2000) observed that although nocturnal serum melatonin levels in premenopausal women declined moderately from 17 to 45 years of age, it showed an increase during the period from 46 to 50 years of age. However, among postmenopausal women, a steep, age-related decline in nocturnal melatonin secretion was found for up to 15 years of postmenopause, followed by an extremely gradual decline thereafter. Some experimental and clinical studies have demonstrated positive effect of melatonin administration that was able to delay the characteristic endocrine changes that occur during the course of menopause (Diaz and Llaneza 2008) and that significantly increased serum levels of HDL cholesterol (Tamura et al. 2008). Ciortea et al. (2011) have shown that the addition of melatonin to oestrogen replacement treatment is associated with a decrease in endometrial proliferation and prevents the appearance of cellular atypia. These results suggested that melatonin supplementation might play an important role in the prophylaxis of endometrial cancer in menopause.
Since melatonin and oestrogen are potent anti-oxidants, as well as anti-apoptotic and anti-inflammatory substances, the decrease in melatonin synthesis in the elderly, together with changes in hormone levels that occur with increased age, may account for the increased pathology in aged people, including neurodegenerative diseases (Harrod et al. 2005).
The aim of this study was to determine the outcomes of oestrogen and melatonin treatments following long-term ovarian hormone depletion in female rats on neuroinflammation and apoptotic processes in dentate gyrus of hippocampi.
Materials and methods
Animals and treatment
Forty-six female Wistar rats of 22 months of age were used in the present study. Twelve of them remained intact, and the other 34 were ovariectomized at 12 months of age, according to the following procedure: Rats were anaesthetized with Equithesin, and two small incisions (8 mm) were made through the skin and the muscle back walls in parallel with the animal body line. The ovaries were then located, and a silk thread (Silkam®, Braun-Aesculap, Germany) was tightly tied around the oviduct, including the ovarian blood vessels. The oviduct was sectioned, and the ovary was removed, taking good care in leaving the knot intact. The muscle wall was then sutured with a synthetic absorbable thread (Safil®, Braun-Aesculap, Germany) and the skin with metallic clips. The animals were given a standard laboratory rat diet (A.04; Panlab, Barcelona, Spain) and water ad libitum, in a light and temperature controlled room. Ovariectomized animals were divided into three groups: non-treated, treated with oestrogens (oestradiol valerianate, Sigma, St. Louis, Missouri, USA; 125 μg/week, s.c, diluted in sunflower seed oil) and treated with melatonin (melatonin, Sigma, St. Louis, Missouri, USA, was given in the drinking water at a dose of 1 mg/kg/day). A fresh melatonin solution was prepared three times per week, depending on the water consumption and the weight of the animals to obtain a daily melatonin dose of 1 mg/kg. Water bottles were covered with aluminium foil to be protected from light, and the drinking fluid was changed three times weekly. After 10 weeks of treatment, rats were sacrificed by decapitation, and dentate gyrus were collected as published before13 and processed as described later. All non-treated animals received the correspondent vehicle doses. Six of the intact rats were submitted to sham operation following the same procedure, without removing ovaries at 12 month of age. A group of 2-month-old female rats was used as young control (n = 12). All the animals received humane care according to the Guidelines for Ethical Care of Experimental Animals of the European Union. The study was approved by the ethical committee of the Complutense University of Madrid.
Cytokine determination
Dentate gyrus were dissected and frozen in liquid nitrogen. Samples were homogenized in 0.05 M Tris-HCl buffer with protease inhibitors (0.1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin) for 30 s with an electrical homogenizer (Polytron; Brinkmann Instruments, Westminster, NY, USA) and later centrifuged at 7,000×g (10 min, 4 °C). The supernatant was collected and was stored at −80 °C until the determination of cytokines. Tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL1β) and interleukin 6 (IL6) were measured in dentate gyrus homogenates collected from aged, young controls and treated rats with an ELISA kit according to the manufacturer’s instructions (Bio-NOVA Cientifica Ltd., Madrid, Spain).
Extraction of tissue samples and determination of HSP 70
Frozen dentate gyrus samples were transferred to 5-ml polypropylene tubes containing 1× Extraction reagent (4 °C) with protease inhibitors (0.1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin). Samples were homogenized for 30 s with an electrical homogenizer (Polytron; Brinkmann Instruments, Westminster, NY, USA) and later centrifuged at 21,000×g (10 min, 4 °C). The supernatant was collected and was stored at −80 °C until assayed for the quantitative presence of heat shock protein 70 (HSP 70).
HSP 70 was measured with an ELISA kit according to the manufacturer’s instructions (Assay Designs, Stressgen, MI, USA, catalog number EKS-700B). HSP 70 concentrations from the sample are quantitated by interpolating absorbance reading from a standard curve generated with the calibrated HSP 70 protein standard provided.
Western blotting analysis
Western blots were used to measure the protein expression of NFκB p65, NFκB p50/105, IκBα, IκBβ, mitogen-activated protein kinase (MAPK) p38, synapsin I and microtubule-associated protein 2 (MAP-2). Briefly, dentate gyrus samples after homogenization with lysis buffer were sonicated, boiled with gel-loading buffer (0.100 M Tris-Cl, 4 % sodium dodecyl sulphate (SDS), 20 % glycerol, 0.1 % bromophenol blue) in the ratio 1:1, and the concentration of protein solutions were measured using a BCA kit and a microplate reader. Total protein equivalents (25–30 μg) for each sample were separated by SDS-polyacrylamide gel electrophoresis (PAGE) by using 10 % acrylamide gels and were transferred onto nitrocellulose membrane in a semi-dry transfer system. The membrane was immediately placed into blocking buffer containing 5 % non-fat milk in 20 mM Tris, pH 7.5; 150 mM NaCl and 0.01 % Tween-20. The blot was allowed to block at 37 °C for 1 h. The membrane was incubated with rabbit polyclonal antibody (1:1,000) for 2 h at 25–27 °C, followed by incubation in an anti-rabbit horseradish peroxidise-conjugated antibody (1:4,000). After washing with T-TBS, the membranes were incubated with ECL Plus detection reagents (Amersham Life Science Inc., Buckinghamshire, UK), exposed to X-ray film. The films were scanned with densitometer (BioRad GS 800) to determine the relative optical densities. Pre-stained protein markers were used for molecular weight determinations. As an internal control, the expression of β-actin was also determined simultaneously by re-blotting the membranes with the antibody for β-actin (1:5,000) (Santa Cruz, CA). Expression levels were then normalized to the young control animals (controls were set to 100 %).
RNA isolation and RT-PCR
RNA was isolated from dentate gyrus samples of female rats using the TRI Reagent Kit (Molecular Research Center, Inc., Cincinnati, OH), following the manufacturer’s protocol. The purity of the RNA was estimated by 1.5 % agarose gel electrophoresis, and RNA concentration was determined by spectrophotometry (260 nm). Reverse transcription of 2 μg RNA for complementary DNA (cDNA) synthesis was performed using the Reverse Transcription System (Promega, Madison, WI, USA) and a pd(N)6 random hexamer. Real-time (RT)-PCR was performed in an Applied Biosystems 7300 apparatus using the SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) and 300 nM concentrations of specific primers (Table 1). The thermocycling profile conditions used were 50 °C for 2 m, 95 °C for 10 m, 95 °C for 15 s, 60 °C for 1 m, 95 °C for 15 s, 60 °C for 30 s and 95 °C for 15 s. For the normalization of cDNA loading in the PCR reaction, the amplification the 18S rRNA for every sample was used. Relative changes in gene expression were calculated using the 2−ΔΔCT method. Relative differences between groups were expressed as relative increases or decreases, with the young group set as 1.00.
Table 1.
Primers used in real-time PCR experiments
| Primers | Sequence (5′-3′) |
|---|---|
| 18S | Forward GGTGCATGGCCGTTCTTA |
| Reverse TCGTTCGTTATCGGAATTAACC | |
| TNFα | Forward ATGAGAAGTTCCCAAATGGC |
| Reverse CTCCACTTGGTGGTTTGCTA | |
| IL1β | Forward TGTGATGAAAGACGGCACAC |
| Reverse CTTCTTCTTTGGGTATTGTTTGG | |
| Bcl2 | Forward CAGGTATGCACCCAGAGTGA |
| Reverse GTCTCTGAAGACGCTGCTCA | |
| Bad | Forward GCCCTAGGCTTGAGGAAGTC |
| Reverse CAAACTCTGGGATCTGGAACA | |
| Bax | Forward GTGAGCGGCTGCTTGTCT |
| Reverse GGTCCCGAAGTAGGAGAGGA | |
| AIF | Forward AGTCCTTATTGTGGGCTTATCAAC |
| Reverse TTGGTCTTCTTTAATAGTCTTGTAGGC | |
| iNOS | Forward CTTTGCCACGGACGAGAC |
| Reverse TCATTGTACTCTGAGGGCTGAC | |
| nNOS | Forward AGATGAGGCACCCCAACTC |
| Reverse CCTTTACGGGGAAAGAAACG | |
| eNOS | Forward CCAGTGCCCTGCTTCATC |
| Reverse GCAGGGCAAGTTAGGATCAG | |
| NFκB1 | Forward CAGCTCTTCTCAAAGCAGCA |
| Reverse TCCAGGTCATAGAGAGGCTCA | |
| NFκB2 | Forward TGGAACAGCCCAAACAGC |
| Reverse CACCTGGCAAACCTCCAT | |
| GFAP | Forward ACAGACTTTCTCCAACCTCCAG |
| Reverse CCTTCTGACACGGATTTGGT | |
| HO-1 | Forward GTCAAGCACAGGGTGACAGA |
| Reverse ATCACCTGCAGCTCCTCAAA | |
| HO-2 | Forward TACGGCACCAGAAAAGGAAA |
| Reverse GTGCTTCCTTGGTCCCTTC | |
| Sirtuin 1 | Forward TCGTGGAGACATTTTTAATCAGG |
| Reverse GCTTCATGATGGCAAGTGG |
18S was used as a housekeeping gene to compare the samples
Statistical analyses
The results were statistically analyzed with the ANOVA method, with a confidence level of 95 % (p ˂ 0.05) that was considered significant. Results were expressed as the mean ± SEM. Mean comparison was done by the ANOVA analysis of variance followed by a Fisher’s test.
Results
The gene expressions of TNFα and IL1β were found to be increased in the dentate gyrus of old females as compared with young ones, and this elevation was more evident in ovariectomized animals. Treatments with oestradiol and melatonin were able to downregulate the messenger RNA (mRNA) expression of these pro-inflammatory cytokines in the group of castrated old females (Fig. 1). As shown on Table 2, the levels of pro-inflammatory cytokines TNFα, IL1β and IL6 were also significantly elevated during ageing and ovariectomy. No statistically significant differences were observed between TNFα levels of intact and castrated old female rats. However, significant increases in IL1β and IL6 levels in ovariectomized versus the old intact group were observed. Administration of oestradiol and melatonin to the ovariectomized groups significantly lowered the levels of TNFα, IL1β and IL6 (Table 2).
Fig. 1.
Expression of mRNA TNFα and IL1β in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. *p < 0.01 compared with 2 months, Δp < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for TNFα (F = 13.2, p = 0.0001 for age; F = 12.48, p = 0.0014 for treatments), for IL1β (F = 13.62, p = 0.0001 for age; F = 15.04, p = 0.0006 for treatments)
Table 2.
Effect of ovariectomy and treatments with oestogens and melatonin on the levels of pro-inflammatory cytokines in dentate gyrus of female rats
| Cytokines pg/mg of proteins | Young (2 month) | Old intact (24 month) | Ovariectomized animals (24 month) | ||
|---|---|---|---|---|---|
| Without treatment | +EOS | +MEL | |||
| TNFα | 8.6 ± 0.5 | 13.9 ± 1.4a | 14.1 ± 1.7a | 9.3 ± 0.7d | 7.7 ± 0.5e |
| IL1β | 63.7 ± 5.9 | 120.8 ± 7.7b | 161.2 ± 8.2b,c | 89.5 ± 10e | 93.4 ± 13e |
| IL6 | 20.6 ± 2.9 | 33.9 ± 4.2a | 44.5 ± 0.9b,c | 34.1 ± 3.5e | 37.6 ± 1.7d |
a p ˂ 0.05, b p ˂ 0.01 compared with 2 months, c p ˂ 0.05 compared with 24 months (intact), d p ˂ 0.05, e p ˂ 0.01 compared with ovariectomized
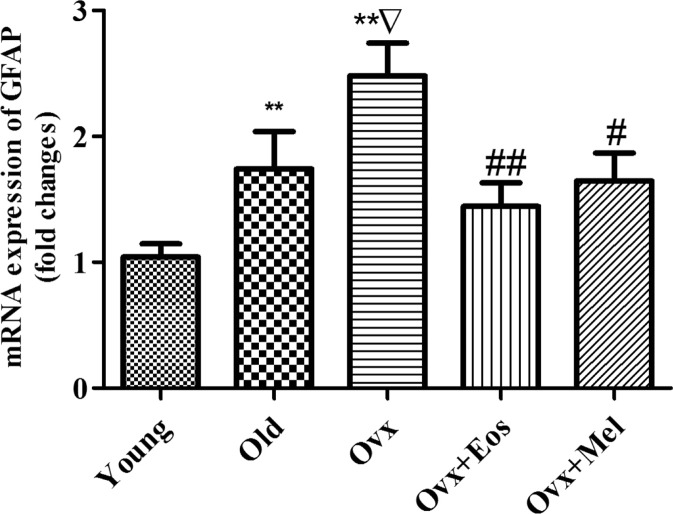
The dentate gyrus of aged rats showed an increase in mRNA expression of glial fibrillary acidic protein (GFAP) as compared with young females. Ovariectomy was able to significantly further increase the expression of this parameter. These changes were significantly blunted by melatonin and oestradiol treatments, and the expression of GFAP mRNA was also reduced (Fig. 2).
Fig. 2.
Expression of mRNA GFAP in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. **p < 0.01 compared with 2 months, Δ p < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for GFAP (F = 12.99, p = 0.0001 for age; F = 12.18, p = 0.0016 for treatments)
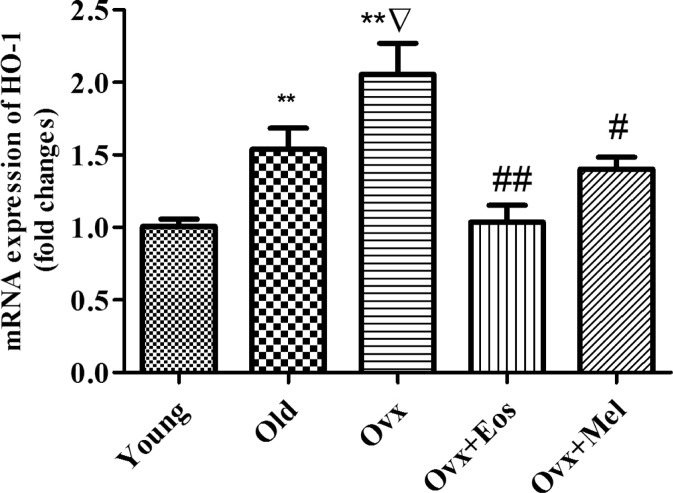
Ageing significantly increased inducible nitric oxide synthase (iNOS) gene expression, and this increase was more marked in ovariectomized animals as compared to both intact old females and young controls. The enhanced expression of iNOS was significantly attenuated after oestradiol and melatonin replacement in the group of old castrated females (Fig. 3). However, mRNA expression of neuronal nitric oxide synthase (nNOS) did not change in the dentate gyrus during ageing and following long-term ovarian hormone depletion. Melatonin was able to significantly increase mRNA expression of nNOS in the group of ovariectomized females, but oestrogen treatment did not produce any effect on this parameter (Fig. 3). An elevation of the heme oxygenase-1 (HO-1) gene expression was induced by ageing and ovariectomy and was extremely high in the group of castrated animals as compared with young rats. Osstradiol and melatonin replacement reversed these enhancements in ovariectomized rats (Fig. 4). Expression of the constitutive enzymes HO-2 and endothelial NOS did not differ significantly between intact and ovariectomized females (data not presented).
Fig. 3.
Expression of mRNA iNOS and nNOS in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. Δp < 0.001 compared with 2 months, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for iNOS (F = 10.47, p = 0.0005 for age; F = 15.7, p = 0.0005 for treatments), for nNOS (F = 0.87, p = 0.4303 for age; F = 4.18, p = 0.0501 for treatments)
Fig. 4.
mRNA expression of HO-1 in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. **p < 0.01 compared with 2 months, Δ p < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for HO-1 (F = 13.69, p = 0.0001 for age; F = 16.27, p = 0.0003 for treatments)
HSP 70 levels were decreased in the dentate gyrus of intact females and ovariectomized rats as compared with young ones. When ovariectomized old females were treated with oestradiol or melatonin, the levels of HSP 70 were significantly increased as compared with untreated rats, with melatonin showing a more marked effect (Fig. 5).
Fig. 5.
Level of HSP 70 in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. *p < 0.05, **p < 0.01 compared with 2 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for HSP 70 (F = 1.83, p = 0.182 for age; F = 14.64, p = 0.0008 for treatments)
The mRNA expression of NFκB1 (p105/50) was increased in the dentate gyrus of 24-month-old animals as compared to 2-month female rats, but this was not the case in NFκB2 (p100/52). Ovariectomy was able to further increase significantly the expression of both NFκB1 and NFκB2 genes as compared with intact old and with young animals. Following melatonin and oestradiol administration, a significant reduction in the expressions was observed (Fig. 6).
Fig. 6.
Expression of mRNA NFκB 1 (p105/50) and NFκB 2 (p100/52) in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. **p < 0.01 compared with 2 months, Δp < 0.001 compared with 2 months, *p < 0.05 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for NFκB1 (F = 13.47, p = 0.0001 for age; F = 15.7, p = 0.0005 for treatments), for NFκB2 (F = 13.71, p = 0.0001 for age; F = 20.27, p = 0.0001 for treatments)
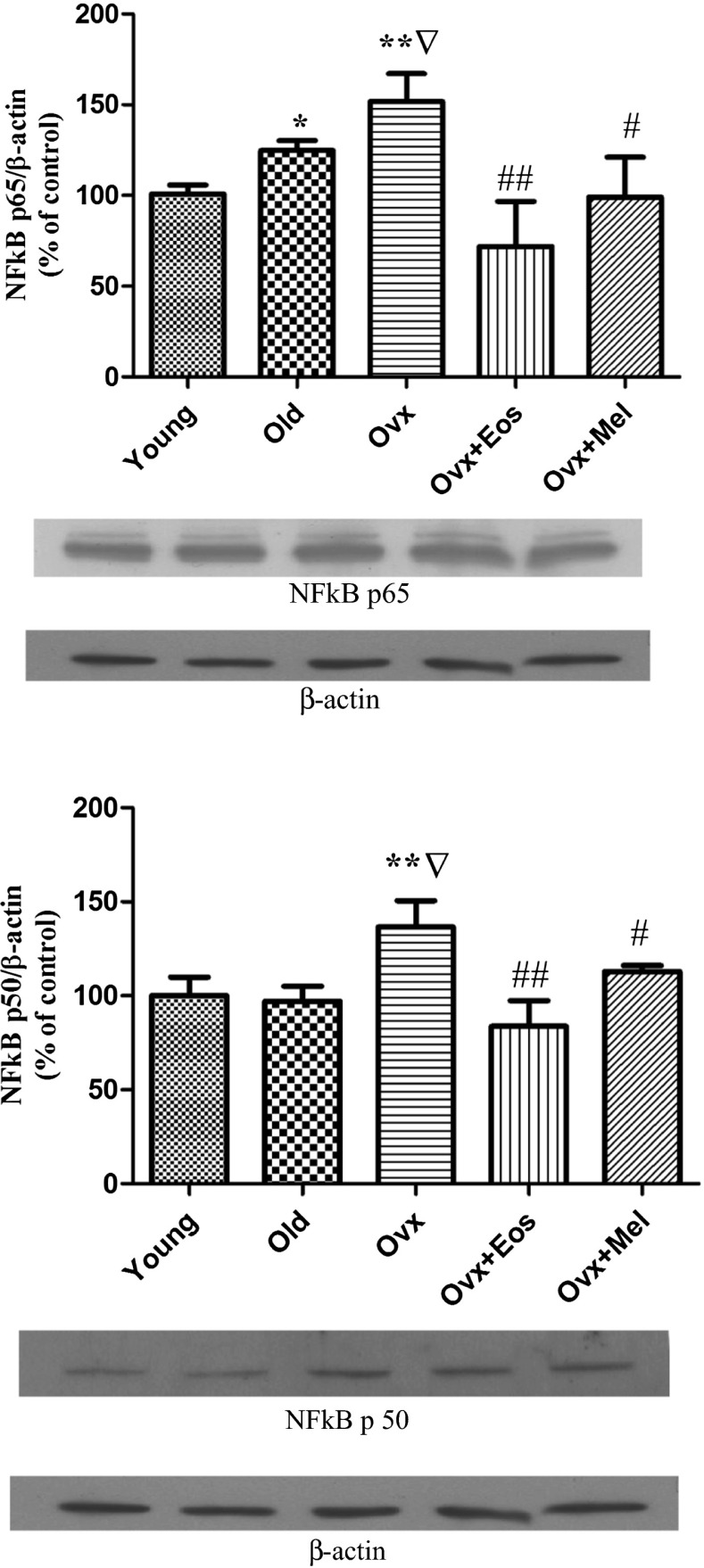
Ageing induced a significant elevation in the expression of NFκB p65 protein, and ovariectomy showed an additional increase. Treatment with oestradiol and melatonin significantly decreased this parameter in old castrated females (Fig. 7). Expression of NFκB p105 subunits did not changed in the groups of intact and ovariectomized old females as compared with the young controls (data not presented). Expression of NFκB p50 was found to be increased in the dentate gyrus of ovariectomized old females, whereas this parameter did not show any change in the group of intact females as compared with the young controls. Treatment with oestradiol and melatonin to ovariectomized females was able to significantly reduce the NFκB p50 protein expression values in the dentate gyrus (Fig. 7). Expressions of IκBα and IκBβ proteins were dramatically decreased in the dentate gyrus of ovariectomized females as compared with young and old intact animals (Fig. 8). In the group of old intact females, we observed only a significantly decreased expression of IκBα protein but not of IκBβ as compared with young animals. When old ovariectomized animals were treated with melatonin, a significant upregulation of both proteins could be observed. However, treatment with oestradiol to old ovariectomized females was only able to increase IκBα but not IκBβ protein in the dentate gyrus (Fig. 8).
Fig. 7.
NFκB p65 and NFκB p50 expressions in the dentate gyrus of young, old intact and ovariectomized female’s and effect of administration of melatonin and oestrogens. The upper part of figures illustrates expression of NFκB p65 and NFκB p50 by representative Western blots from each group compared to β-actin. The columns show the mean ± SEM from each group. **p < 0.01 compared with 2 months, Δp < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized
Fig. 8.
IκBα and IκBβ expressions in the dentate gyrus of young, old intact and ovariectomized female’s and effect of administration of melatonin and oestrogens. The upper part of figures illustrates expression of IκBα and IκBβ by representative Western blots from each group compared to β-actin. The columns show the mean ± SEM from each group. **p < 0.01 compared with 2 months, Δp < 0.001compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized
Western blotting analyses demonstrated activation of p38 MAPK in the dentate gyrus of aged intact and ovariectomized females. So, the ratio phospho-p38/p38 MAPK was higher in the groups of intact and castrated old female rats as compared with young animals. After chronic treatment with oestradiol and melatonin, a very marked decrease in the ratio phospho-p38/p38 MAPK was detected (Fig. 9).
Fig. 9.
Intensities of p38 MAPK phosphorylation in the dentate gyrus of young, old intact and ovariectomized female’s and effect of administration of melatonin and oestrogens are presented. The intensity of phosphorylation is expressed by the ratio of p-MAPK/MAPK (see also the representative Western blots above the columns). **p < 0.01 compared with 2 months, Δp < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized
The gene expressions of pro-apoptotic markers like Bcl-2-associated X protein (Bax) and Bcl-2-associated death promoter (Bad) were significantly upregulated in the dentate gyrus of old ovariectomized females (Fig. 10). In the group of old intact females, we could observe only an increase in the expression of Bad mRNA but not in Bax as compared with young animals. Treatment with oestradiol blocked Bad and Bax induction in the dentate gyrus of ovariectomized females (Fig. 10). However, melatonin administration was only able to downregulate the expression of Bad genes but did not affect the mRNA expression of Bax (Fig. 10). A significant upregulation of apoptosis inducing factor (AIF) was also observed in the intact old and ovariectomized animals, and both oestradiol or melatonin therapy led to a significant downregulation of this parameter (Fig. 12). In the case of Bcl-2, we were not able to demonstrate any significant differences in its expression between young, intact old and ovariectomized females (Fig. 11). However, treatments with melatonin and oestradiol were able to significantly upregulate the expression of Bcl-2 in the group of ovariectomized animals (Fig. 11).
Fig. 10.
Expression of mRNA Bax and Bad in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. Δp < 0.01 compared with 2 months, *p < 0.05 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for Bax (F = 9.7, p = 0.0008 for age; F = 3.29, p = 0.0818 for treatments), for Bad (F = 16.40, p = 0.00001 for age; F = 28.37, p = 0.00001 for treatments)
Fig. 12.
mRNA expression of AIF in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. Δp < 0.01 compared with 2 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for AIF (F = 4.87, p = 0.0153 for age; F = 9.57, p = 0.0045 for treatments)
Fig. 11.
mRNA expression of Bcl-2 in the dentate gyrus of young, old intact and ovariectomizedfemale rats and effect of administration of melatonin and estrogens. Data represent mean ± SEM.#- p<0.05; ##- p<0.01-compared with ovariectomized.Two-way ANOVA analysis for Bcl-2 (F=0.51; p=0.6078 for age; F=17.32; p=0.0003 for treatments)
In addition, we also demonstrated that the expression of sirtuin 1 (SIRT1) was dramatically decreased in the dentate gyrus of old intact and castrated rats and that treatments with oestradiol and melatonin were able to significantly increase this parameter (Fig. 13). MAP-2 immunoreactivity was not influenced by ageing but was significantly decreased in the group of ovariectomized females. The concentration of synapsin I declined with age and was more markedly decreased in ovariectomized animals (Fig. 14). The expression of MAP-2 was significantly upregulated after oestrogenic replacement, but melatonin did not produce any effect on this parameter in the group of ovariectomized rats. Synapsin I was significantly increased in the group of castrated females that received oestrogenic and melatonin treatments (Fig. 14).
Fig. 13.
mRNA expression of SIRT1 in the dentate gyrus of young, old intact and ovariectomized female rats and effect of administration of melatonin and oestrogens. Data represent mean ± SEM. **p < 0.01 compared with 2 months, Δp < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05, ##p < 0.01 compared with ovariectomized. Two-way ANOVA analysis for SIRT1 (F = 10.19, p = 0.0004 for age; F = 33.66, p = 0.00001 for treatments)
Fig. 14.
MAP-2 and synapsin-I expressions in the dentate gyrus of young, old intact and ovariectomized female’s and effect of administration of melatonin and oestrogens. The upper part of figures illustrates expression of MAP-2 and synapsin-I by representative Western blots from each group compared to β-actin. The columns show the mean ± SEM from each group. **p < 0.01 compared with 2 months, Δp < 0.001 compared with 2 months, **p < 0.01 compared with 24 months, #p < 0.05 compared with ovariectomized
Discussion
In this study, we have investigated the possibility that oestrogen and melatonin may reduce in a similar way neuroinflammation and apoptosis and its molecular signalling pathways, which may, in turn, prevent neuronal loss and/or connectivity, during ageing and long-term ovarian hormone depletion in the dentate gyrus of female rats.
The levels of pro-inflammatory cytokines and the gene expressions were increased in the dentate gyrus of old females as compared with young animals. These data confirm previous studies, which showed that ageing led to increases in IL1β, TNFα, IL6, MHCII, CD68, and CD11b mRNA in hippocampal tissue (Murphy et al. 2012). As we had previously demonstrated, ageing by itself was able to induce alterations in hippocampus, but when females with long-term oestrogen deprivation were investigated, the damages observed were due to the combination of ovariectomy and ageing combined and could be regarded as a model for menopause. The present study demonstrates also that long-term ovariectomy further negatively affects the already altered neuroinflammatory status of the dentate gyrus in old intact female rats. So, castration induced additional significant increases of IL1β and TNFα gene expression and in IL6 and IL1β levels as compared to old intact animals. Other authors have also demonstrated that ovariectomy was able to further increase the already enhanced age-dependent accumulation of mRNAs encoding inflammatory mediators (TNFα, IL1β and macrophage inflammatory protein-2) and to induce changes in the morphology of astroglia and microglia (Benedusi et al. 2012). Santizo and Pelligrino (1999) reported that chronic oestrogen depletion did enhance leucocyte adhesion in rat cerebral circulation, and this has been suggested to underlie the more marked brain damage observed in ovariectomized females as compared to intact animals of the same age.
In our data, the administration of oestradiol or melatonin was accompanied by an improvement in the inflammatory status of ovariectomized female rats. As a result, oestradiol and also melatonin treatments were able to decrease pro-inflammatory cytokine (TNFα, IL1β and IL6) levels and to induce also reductions in the gene expressions of TNFα and IL1β. In different experimental models, other authors have also demonstrated protective effects of melatonin and oestrogen treatments. So, melatonin reduced in a concentration-dependent manner IL6 secretion in amyloid β peptide-treated brain slices (Clapp-Lilly et al. 2001). Administration of melatonin in doses of 5 and 10 mg/kg were able to decrease lipopolysaccharide (LPS)-induced pro-inflammatory cytokines (TNFα, IL1β) and oxidative stress in different brain regions, including the hippocampus (Tyagi et al. 2010). Choi et al. (2008) reported that oestrogen-induced protection was associated with a decrease in IL1β and an increase in interleukin-1 receptor antagonist (IL1ra) expression in the ischemic hippocampus during early reperfusion periods, which suggests that the modulation of IL1β/IL1ra might be part of the anti-inflammatory effects of oestrogens. Oestrogen were also able to increase IL-10 levels while decreasing TNFα and IFNγ release from both basal and LPS-stimulated N9 (murine microglial) cells (Dimayuga et al. 2005).
Like microglia, astrocytes become activated with age—a process known as ‘astrogliosis’, which is characterized by altered gene expression, increased expression of gliosis marker molecules (i.e. GFAP and vimentin) and also by hypertrophy and proliferation (Ridet et al. 1997) of this tissue. Activated astrocytes release also a wide array of immune mediators such as cytokines, chemokines, and growth factors, contributing either to the neuroprotective or to the neurotoxic effects raised by microglia (Farinas et al. 2007). In the current study, the activated astrocytes were identified by the enhanced expression of GFAP and were also demonstrated by the positive correlations between this marker, the ageing process and ovarian hormone depletion. It has been also shown that with increasing age, the staining for GFAP showed a rise (e.g. labelling for astrocytes), and age-related enhancement in GFAP mRNA content has also been demonstrated in the mouse brain (Hayakawa et al. 2007). Previous studies have shown that pharmacological doses of oestrogens can suppress ageing-associated reactive gliosis in 20 to 24-month-old mice (Lei et al. 2003). Interestingly, oestradiol significantly was able to suppress GFAP expression by 49 days following ovariectomy but not during the period between 5 and 28 days (McAsey et al. 2006) after castration. The present results showed that the pharmacological administration of melatonin and oestradiol for 10 weeks prevented the elevation of GFAP content in the dentate gyrus of old chronically ovariectomized female rats.
Cytokines released from microglia and astrocytes activated MAPKs and NFκB (McCoy and Tansey 2008), and these pathways were important in the production of many inflammatory proteins (O’neill 2006) and were able to increase NADPH oxidase activation (Kozuka et al. 2005). Our data have shown for the first time that ageing together with long-term ovarian hormone depletion induced phosphorylation of p38 MAPK in the dentate gyrus. On the other hand, we have also demonstrated that mRNA expression of NFκB 1 and 2 and levels of p50 and p65 proteins were also increased in the dentate gyrus of ovariectomized females when compared with old intact animals. Elevated levels of NFκB were accompanied with low expression of IκBα and IκBβ proteins. These data closely correlated with high levels of pro-inflammatory cytokines in the group of ovariectomized animals. Upstream MAPK signalling mediated both the transcriptional and post-transcriptional regulation of iNOS in activated microglia and astrocytes (Bhat et al. 1998; Marcus et al. 2003). On the other side, NFκB responded to p38 signalling and was also involved in iNOS induction (Bhat et al. 2002), suggesting that there was an interplay between signalling pathways, transcription factors and cytokine production in determining the neuroinflammatory response of the CNS. Oestrogen receptor (ER) and NFκB family members have been shown to influence each other’s transcriptional activity. Much work has been done to delineate the multiple mechanisms by which ER could repress NFκB action to exert an anti-inflammatory effect (Kalaitzidis and Gilmore 2005; Cerciat et al. 2010). Similarly, there were several pieces of evidence indicating that NFκB could repress ER expression and transcriptional activity (Feldman et al. 2007; Mahmoodzadeh et al. 2009). In the present investigation, administration of oestradiol and melatonin to ovariectomized females significantly abolished iNOS expression, by decreasing expression of NFκB genes and proteins and by inhibiting the p38 mitogen-activated kinase. In an animal model of transient cerebral ischemia, administration of pharmacological doses of oestrogens produced strong neuroprotective effects by reducing the negative elements associated with inflammatory responses, namely IκB phosphorylation, NFκB activation and iNOS over-expression in the cortex during the reperfusion phase (Wen et al. 2004). In vitro studies showed that pre-treatment with oestradiol was able to prevent neuronal death by inhibiting the activation of p38 MAPK after the treatment of cells with Aβ (Valles et al. 2008). Eijo et al. (2011) described that oestradiol was able to decrease TNFα-induced, NFκB p65 and p50 nuclear translocation in primary cultures of anterior pituitary cells from ovariectomized rats. Inhibition of NFκB by melatonin has been also previously reported in other experimental models that showed how melatonin prevented NFκB activation by oxidative stress (Veneroso et al. 2009). Previous studies also showed that melatonin inhibited phosphorylation of p38 MAPK and to a lesser extent of ERK1/2 thus reducing iNOS mRNA expression and rescuing neurons from apoptosis after acute traumatic SCI (Xu et al. 2006).
During ageing (Rastogi et al. 2012) and ovariectomy (Martins et al. 2012), the enhanced generation of ROS induced chronic oxidative stress in the brain. This condition caused gradual accumulation of damaged proteins leading to a functional decline in the brain’s endogenous defence system (Rumora et al. 2007; Min et al. 2008) and leading to the activation of stress kinases (JNK and p38 MAPK) (Gabai et al. 1997). Hou et al. (2010) demonstrated that oestrogen deficiency induced by ovariectomy was able to exacerbate the impairment of heat stress-induced brain HSP 70 expression in female mice during ageing and resulted in reduced thermotolerance. Oestrogen supplementation restored HSP 70 expression by increasing the binding activity of heat shock transcriptional factor (HSF1) and heat shock element (HSE) and subsequently enhanced thermotolerance thus attenuating heat stress-induced brain damage. Our results clearly indicated that levels of HSP 70 were decreased in the dentate gyrus of old and ovariectomized female rats and that the administrations of oestrogens or melatonin were able to increase its levels. Previous studies also suggested that oestrogen could regulate the expression of heat shock proteins in neuronal cells (Manthey and Behl 2006) and oestrogen deficiency might accelerate brain ageing in (postmenopausal) oestrogen-deprived or castrated animals (Sherwin and Henry 2008; Shuster et al. 2008). Since ageing and menopause are accompanied with increased oxidative stress, induction of HO-1 may have a compensatory action caused by its antioxidant feature. On the other hand, free iron and CO released during heme degradation might, under certain circumstances, perpetuate intracellular oxidative stress and predispose the mitochondrial compartment to free radical damage (Zhang and Piantadosi 1992; Frankel et al. 2000). Interestingly, increased hippocampal astroglial HO-1 expression was associated with lower scores for global cognition, semantic memory and perceptual speed (Schipper et al. 2006). Our results clearly indicated that HO-1 expression was significantly increased in both old intact and castrated females, as compared to young animals, and these data correlated with a parallel increase in the level of pro-inflammatory cytokines and expression of iNOS. These findings are in consonance with the results obtained by Lee et al. (2005), in which HO-1 protein expression in heart was induced by ovariectomy and was extremely high 2–6 weeks after castration when compared with the sham-operated group. 17β-Oestradiol replacement after ovariectomy was able to reverse these changes in rats.
IL1β and TNFα caused cell death by the activation of a signal transduction pathway leading to apoptosis in hippocampal and neocortical cells (Kajta et al. 2006). Cerbai et al. (2012) demonstrated that astrocytes and microglia in the hippocampus of aged and adult LPS-treated rats participated in the clearance of neuronal debris associated with programmed cell death and the phagocytosis of apoptotic neurons. We have demonstrated that Bcl-2 gene did not change its expression in the dentate gyrus of female Wistar rats, neither during ageing nor with ovariectomy. However, the Bax/Bcl-2 ratio was significantly higher in the group of ovariectomized animals as compared with intact ones, indicating a higher level of apoptosis. Oestradiol and melatonin administrations were able to increase the expression of Bcl2 and decreased pro-apoptotic markers like Bad and/or Bax. Results obtained in our research were in accordance with data demonstrating that chronic administration of E2 prevented the ovariectomy-induced downregulation of Bcl-2 and upregulation of Bax expression while restoring the Bcl-2/Bax ratio as observed in the hippocampus of intact rats. Furthermore, terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay demonstrated a decline in the percentage of TUNEL positive cells in E2-treated groups (Sharma and Mehra 2008). Sales et al. (2010) also found that ovariectomy decreased Bcl-2 expression and increased Bax expression and the also the number of apoptotic cells. Replacement with 17β-oestradiol (21 days) throughout the post-ovariectomy period reduced the number of apoptotic cells to control levels and prevented the enhancing effects of ovariectomy on Bax expression but only partially restored Bcl-2 expression. It has been shown that oestradiol reduced mRNA levels of the pro-apoptotic Bad in PC12 cells transfected with ERα (Gollapudi and Oblinger 1999). Nopparat et al. (2010) have demonstrated, in the SK-N-SH dopaminergic cell line, a novel role of melatonin in protecting cells from autophagic cell death triggered by the Bcl-2/Beclin 1 pathway, by inhibiting the activation of the JNK1 (c-Jun amino-terminal kinase), Bcl-2 upstream pathway. Melatonin increased Bcl-2 and augmented expression of XIAP in ethanol-treated HN2-5 (mouse hippocampal neuron-derived) cells (Shetha et al. 2009). Our results also demonstrated an increase in the mRNA expression of AIF in the dentate gyrus of old and ovariectomized rats, and this could suggest that this brain area was following a different apoptotic pathway. In addition, we have also observed that treatment with melatonin or oestrogens was able to decrease mRNA expression of AIF. Our data were in agreement with previous reports in which melatonin was able to prevent the insult-related release of AIF from mitochondria (Liang et al. 2012). Bethea et al. (Bethea et al. 2009) showed that AIF gene expression was significantly decreased by hormone therapy (E and E + P-treated animals) in laser-captured serotoninergic neurons.
One of the most important changes that we have observed for the first time is that ageing and more markedly ovariectomy decreased the expression of SIRT1 in the dentate gyrus. Previous results have shown that SIRT1 is associated with a reduction in apoptosis and could regulate other pathways. So, Kolthur-Seetharam et al. (2006) showed that SIRT1 modulates poly(ADP-ribose)polymerase-1 (PARP-1) activity following DNA damage. Indeed, these authors showed that SIRT1-null cells exposed to H2O2 undergo AIF-mediated programmed cell death due to a dysregulation in PARP-1 expression. Yeung et al. (2004) demonstrated that SIRT1 can physically interact with the p65/RelA protein in the NFκB complex, specifically to deacetylate the lysine-310 of p65 protein. The cleavage and removal of the acetyl group thereafter inhibited the transactivation efficiency of the NFκB-dependent complex since acetylated lysine-310 is a powerful promoter of its transactivation. The absence of SIRT1 also impaired cognitive abilities, including immediate memory, classical conditioning, and spatial learning. Michan et al. (2010) found that the cognitive deficits in SIRT1 knock-out (KO) mice were associated with defects in synaptic plasticity without alterations in basal synaptic transmission or NMDA receptor function.
The expression of SIRT1 gene was significantly upregulated after oestrogen or melatonin replacement therapy in the dentate gyrus of ovariectomized females. In addition, we also observed that both melatonin and oestradiol induced an enhancement of protein levels of MAP-2 and synapsin I that might have been partially responsible for the improved learning and memory performance in ageing rats, since they were end-stage elements in synaptic plasticity.
Summing up, our study indicated that long-term ovarian hormone depletion and ageing induced a neuroinflammatory process (with increased levels of pro-inflammatory cytokines, and enhanced expression of iNOS, HO-1) in the dentate gyrus. Increased production of pro-inflammatory cytokines is associated with the activation of stress kinases and NFκB. Using ovariectomy model in old rats, we have found that reduced levels of synaptic plasticity markers (synapsin I and MAP-2) in the dentate gyrus were associated with low levels of SIRT1 expression and high levels of neuroinflammation. Treatments with oestradiol or melatonin have been shown to modulate the pro/anti-apoptotic ratio favouring the second and to increase SIRT1 expression. Other neuroprotective effects after both melatonin and oestradiol administrations were clearly demonstrated by the normalization of the levels of pro-inflammatory cytokines and by the decrease of p38 MAPK phosphorylation and gene and protein expressions of NFκB. Oestradiol or melatonin treatments were also able to increase the expression of synapsin I and MAP-2 in dentate gyrus of old ovariectomized females. Since these proteins might have been an index of synaptic integrity, this implied that melatonin might be beneficial in the preservation of synaptic performance during senescence and that melatonin might also have applications in the clinical practice for menopausal women.
Acknowledgment
Thanks are given to D. Campon for his technical support.
Conflict of interest
The authors declare no conflict of interest.
References
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Benedusi V, Meda C, Della Toree S, Monteleone G, Vegeto E, Maggi A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology. 2012;153:2777–2788. doi: 10.1210/en.2011-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30(2):212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Feinstein DL, Shen Q, Bhat AN. p38 MAPK mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30(4):212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler H. The menopause transition: endocrine changes and clinical symptoms. J Br Menopause Soc. 2005;11:61–65. doi: 10.1258/136218005775544525. [DOI] [PubMed] [Google Scholar]
- Cerbai F, Lana D, Nosi D, et al. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS ONE. 2012;7(9):e45250. doi: 10.1371/journal.pone.0045250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2010;58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Kim SJ, Shin JA, Lee KE, Park EM. Effects of estrogen on temporal expressions of IL-1β and IL-1ra in rat organotypic hippocampal slices exposed to oxygen–glucose deprivation. Neurosci Lett. 2008;438:233–237. doi: 10.1016/j.neulet.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Ciortea R, Costin N, Braicu I, Haragâş D, Hudacsko A, Bondor C, Mihu D, Mihu CM. Effect of melatonin on intra-abdominal fat in correlation with endometrial proliferation in ovariectomized rats. Anticancer Res. 2011;31(8):2637–2643. [PubMed] [Google Scholar]
- Clapp-Lilly KL, Smith MA, Perry G, Duffy LK. Melatonin reduces interleukin secretion in amyloid-beta stressed mouse brain slices. Chem Biol Interact. 2001;134:101–107. doi: 10.1016/S0009-2797(00)00319-7. [DOI] [PubMed] [Google Scholar]
- Cvoro A, Yuan C, Paruthiyil S, Miller OH, Yamamoto KR, Leitman DC. Cross talk between glucocorticoid and estrogen receptors occurs at a subset of proinflammatory genes. J Immunol. 2011;186(7):4354–4360. doi: 10.4049/jimmunol.1002205. [DOI] [PubMed] [Google Scholar]
- Diaz BL, Llaneza PC. Endocrine regulation of the course of menopause by oral melatonin: first case report. Menopause. 2008;15(2):388–392. doi: 10.1097/gme.0b013e31812503f2. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Eijo G, Zarate S, Jaita G, Ferraris J, Magri ML, Zaldivar V, Radl D, Boti V, Pisera D, Seilicovich A. Inhibition of nuclear factor-kappa B sensitises anterior pituitary cells to tumor necrosis factor-α- and lipopolysaccharide-induced apoptosis. J Neuroendocrinol. 2011;23(8):651–659. doi: 10.1111/j.1365-2826.2011.02157.x. [DOI] [PubMed] [Google Scholar]
- Farinas C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Feldman I, Feldman GM, Mobarak C, Dunkelberg JC, Leslie KK. Identification of proteins within the nuclear factor-kappa B transcriptional complex including estrogen receptor-alpha. Am J Obstet Gynecol. 2007;196(4):394–e1-11. doi: 10.1016/j.ajog.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Frankel D, Mehindate K, Schipper HM. Role of heme oxygenase-1 in the regulation of manganese superoxide dismutase gene expression in oxidatively-challenged astroglia. J Cell Physiol. 2000;185:80–86. doi: 10.1002/1097-4652(200010)185:1<80::AID-JCP7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272(29):18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Gollapudi L, Oblinger MM. Stable transfection of PC12 cells with estrogen receptor (ERalpha): protective effects of estrogen on cell survival after serum deprivation. J Neurosci Res. 1999;56:99–108. doi: 10.1002/(SICI)1097-4547(19990401)56:1<99::AID-JNR13>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(SICI)1098-1136(199807)23:3<249::AID-GLIA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod CG, Bendok BR, Batjer H. Interactions between melatonin and estrogen may regulate cerebrovascular function in women: clinical implications for the effective use of HRT during menopause and aging. Med Hypotheses. 2005;64:725–735. doi: 10.1016/j.mehy.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Hayakawa N, Kato H, Araki T. Age-related changes of astrocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev. 2007;128:311–316. doi: 10.1016/j.mad.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Hou Y, Wei H, Luo Y, Liu G. Modulating expression of brain heat shock proteins by estrogen in ovariectomized mice model of aging. Exp Gerontol. 2010;45:323–330. doi: 10.1016/j.exger.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Kajta M, Trotter A, Lasoń W, Beyer C. Impact of 17β-estradiol on cytokine-mediated apoptotic effects in primary hippocampal and neocortical cell cultures. Brain Res. 2006;1116:64–74. doi: 10.1016/j.brainres.2006.07.105. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-κB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- Kozuka N, Itofusa R, Kudo Y, Morita M. Lipopolysaccharide and proinflammatory cytokines require different astrocyte states to induce nitric oxide production. J Neurosci Res. 2005;82:717–728. doi: 10.1002/jnr.20671. [DOI] [PubMed] [Google Scholar]
- Lee YM, Cheng PY, Hong SF, Chen SY, Lam KK, Sheu JR, Yen MH. Oxidative stress induces vascular heme oxygenase-1 expression in ovariectomized rats. Free Radic Biol Med. 2005;39:108–117. doi: 10.1016/j.freeradbiomed.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Lei DL, Long JM, Hengemihle J, O’Neill J, Manaye KF, Ingram DK, Mouton PR. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003;121:659–666. doi: 10.1016/S0306-4522(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Liang YL, Zhang ZH, Liu XJ, Liu XQ, Tao L, Zhang YF, Wang H, Zhang C, Chen X, Xu DX. Melatonin protects against apoptosis-inducing factor (AIF)-dependent cell death during acetaminophen-induced acute liver failure. PLoS ONE. 2012;7(12):e51911. doi: 10.1371/journal.pone.0051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, McQuillan K, Deighan BF, O’Reilly JA, Downer EJ, Murphy AC, Watson M, Piazza A, O’Connell F, Griffin R, Mills KH, Lynch MA. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav Immun. 2009;23:1020–1027. doi: 10.1016/j.bbi.2009.05.060. [DOI] [PubMed] [Google Scholar]
- Mahmoodzadeh S, Fritschka S, Dworatzek E, Pham TH, Becher E, Kuehne A, Davidson MM, Regitz-Zagrosek V. Nuclear factor-kappaB regulates estrogen receptor-alpha transcription in the human heart. J Biol Chem. 2009;284:24705–24714. doi: 10.1074/jbc.M109.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey D, Behl C. From structural biochemistry to expression profiling: neuroprotective activities of estrogen. Neuroscience. 2006;138:845–850. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Marcus JS, Karackattu SL, Fleegal MA, Sumners C. Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-kappaB. Glia. 2003;41:152–160. doi: 10.1002/glia.10168. [DOI] [PubMed] [Google Scholar]
- Martins DB, Mazzanti CM, França RT, Pagnoncelli M, Costa MM, Martins de Souza E, Gonçalves J, et al. 17-β estradiol in the acetylcholinesterase activity and lipid peroxidation in the brain and blood of ovariectomized adult and middle-aged rats. Life Sci. 2012;90:351–359. doi: 10.1016/j.lfs.2011.12.006. [DOI] [PubMed] [Google Scholar]
- McAsey ME, Cady C, Jackson LM, Li M, Randall S, Nathan BP, Struble RG. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol. 2006;197(1):197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda C, Vegeto E, Pollio G, Ciana P, Patrone C, Pellicciari C, Maggi A. Oestrogen prevention of neural cell death correlates with decreased expression of mRNA for the pro-apoptotic protein nip-2. J Neuroendocrinol. 2000;12:1051–1059. doi: 10.1046/j.1365-2826.2000.00541.x. [DOI] [PubMed] [Google Scholar]
- Meng Y, Wang R, Yang F, Ji Z, Fang L, Sheng S. Amyloid precursor protein 17-mer peptide ameliorates hippocampal neurodegeneration in ovariectomized rats. Neurosci Lett. 2010;468:173–177. doi: 10.1016/j.neulet.2009.07.058. [DOI] [PubMed] [Google Scholar]
- Michan S, Li Y, Meng-Hsiu Chou M, Parrella E, Ge H, Long JM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JN, Whaley RA, Sharpless NE, Lockyer P, Portbury AL, Patterson C. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol Cell Biol. 2008;28:4018–4025. doi: 10.1128/MCB.00296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N, Cowley TR, Blau CW, Dempsey CN, Noonan J, Gowran A, Tanveer R, Olango WM, Finn DP, Campbell VA, Lynch MA. The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J Neuroinflammation. 2012;9:79. doi: 10.1186/1742-2094-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, et al. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem. 2011;286:2308–2319. doi: 10.1074/jbc.M110.169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopparat C, Porter JE, Ebadi M, Govitrapong P. The mechanisms for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. J Pineal Res. 2010;49(4):382–389. doi: 10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- O’neill L. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Morioka N, Wakatsuki A. Changes in nocturnal melatonin secretion in perimenopausal women: correlation with endogenous estrogen concentrations. J Pineal Res. 2000;28(2):111–118. doi: 10.1034/j.1600-079X.2001.280207.x. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LAM. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30(3):343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Salazar V, Castillo C, Ariznavarreta C, Azcoitia I, Garcia-Segura LM, Tresguerres JAF. Estradiol and soy extract increase the production of new cells in the dentate gyrus of old rats. Exp Gerontol. 2005;40:450–453. doi: 10.1016/j.exger.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Rastogi M, Ojha RP, Prabu PC, Devi BP, Agrawal A, Dubey GP. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 2012;13:183–195. doi: 10.1007/s10522-011-9367-y. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Craft CM, Johnson JE, King TS, Richardson BA, Vaughan GM, Vaughan MK. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology. 1981;109:1295–1297. doi: 10.1210/endo-109-4-1295. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/S0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rumora L, Lovric J, Sairam MR, Maysinger D. Impairments of heat shock protein expression and MAPK translocation in the central nervous system of follitropin receptor knockout mice. Exp Gerontol. 2007;42:619–628. doi: 10.1016/j.exger.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Sales S, Ureshino RP, dos Tavares do Santos Pereira R, et al. Effects of 17β-estradiol replacement on the apoptotic effects caused by ovariectomy in the rat hippocampus. Life Sci. 2010;86:832–838. doi: 10.1016/j.lfs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Santizo R, Pelligrino DA. Estrogen reduces leukocyte adhesion in the cerebral circulation of female rats. J Cereb Blood Flow Metab. 1999;19:1061–1065. doi: 10.1097/00004647-199910000-00001. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Sharma K, Mehra RD. Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res. 2008;1204:1–15. doi: 10.1016/j.brainres.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shetha DS, Tajuddin NF, Druse MJ. Antioxidant neuroprotection against ethanol-induced apoptosis in HN2-5 cells. Brain Res. 2009;1285:14–21. doi: 10.1016/j.brainres.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Narimatsu A, Yamagata Y, Takasaki A, Reiter RJ, Sugino N. Melatonin treatment in peri- and postmenopausal women elevates serum high-density lipoprotein cholesterol levels without influencing total cholesterol levels. J Pineal Res. 2008;45(1):101–105. doi: 10.1111/j.1600-079X.2008.00561.x. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, Yu H, Humphrey J, Mullan M. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J Neuroimmunol. 1999;97:77–85. doi: 10.1016/S0165-5728(99)00053-3. [DOI] [PubMed] [Google Scholar]
- Tyagi E, Agrawal R, Nath C, Shukla R. Effect of melatonin on neuroinflammation and acetylcholinesterase activity induced by LPS in rat brain. Eur J Pharmacol. 2010;640:206–210. doi: 10.1016/j.ejphar.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Valles SL, Borras C, Gambini J, Furriol J, Ortega A, Sastre J, Pallardo FV, Viña J. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7(1):112–118. doi: 10.1111/j.1474-9726.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- Veneroso C, Tuñón MJ, González-Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J Pineal Res. 2009;47(2):184–191. doi: 10.1111/j.1600-079X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-κB activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang BR, Wang X, Kuang F, Duan XL, Jiao XY, Ju G. Erk1/2 and p38 mitogen activated protein kinase mediate iNOS-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci. 2006;79:1895–1905. doi: 10.1016/j.lfs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Investig. 1992;90:1193–1199. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]