Abstract
Long-living Ames dwarf mice (df/df) characterized by growth hormone (GH) deficiency are widely used in aging research because of their 40–60 % lifespan extension compared to normal (N) littermates. Importantly, these mice not only live longer but are also protected from age-related diseases including insulin resistance. Several studies demonstrate that df/df mice have enhanced insulin signaling in different insulin-sensitive tissues and suggest that this is a mechanism for extended lifespan. However, it is unknown whether the enhanced insulin signaling in df/df mice translates to improved insulin action on hepatic glucose production and tissue glucose uptake. We performed hyperinsulinemic-euglycemic clamps to assess tissue-specific insulin action in vivo for the first time in these small long-living dwarfs. Our results demonstrate that the glucose infusion rate required to maintain euglycemia was ∼2-fold higher in df/df mice compared to N controls. Insulin-mediated glucose production was completely suppressed in dwarf mice, and stimulation of gastrocnemius and vastus muscle and adipose tissue glucose uptake was also enhanced in df/df mice (100, 86, and 65 %, respectively). These findings show that improved insulin signaling in df/df mice is associated with enhanced tissue-specific insulin action in vivo. This improved functionality of insulin action and glucose homeostasis may play a key role in promoting healthy aging and longer lifespan in df/df mice.
Keywords: Ames dwarf, Insulin signaling, Adipose tissue, Clamp, Aging
Introduction
Enhanced insulin action and glucose homeostasis seems to be an important factor for maintaining healthy aging (Barbieri et al. 2003; Masternak et al. 2009; Wijsman et al. 2012). Disrupted insulin and glucose homeostasis is mostly related to increased obesity. However, aging is associated with a high risk for development of metabolic syndrome and insulin resistance which in turn increases the risk of type 2 diabetes, cardiovascular disease, promotes chronic inflammation, and since recently has also been associated with development of cancer (Kintscher et al. 2008; Lago et al. 2007; Weisberg et al. 2003; Willerson and Ridker 2004; Nelson et al. 2004; Pan et al. 2006; Pradhan et al. 2001). It is well established that alteration in insulin and insulin-like growth factor-1 (IGF-1) signaling extends the lifespan and delay aging in nematodes, flies, and rodents (Tatar et al. 2003) suggesting this signaling pathway as a main factor regulating healthy aging. Importantly, the role of this signaling pathway on aging extends also to humans. It is known that in humans, a decline in glucose tolerance begins in the third decade and continues throughout one’s entire adult life (DeFronzo 1981). Studies with centenarians correlate human longevity with a low degree of insulin resistance (Barbieri et al. 2003). This suggests that exceptionally long-living people are insulin sensitive throughout their lifespan and are genetically protected from an age-related decline of insulin action. Ames dwarf (Prop1df, df/df) mice, characterized by growth hormone (GH), prolactin (PRL), and thyroid-stimulating hormone (TSH) deficiency live 40–60 % longer than their normal (N) controls (Bartke and Brown-Borg 2004; Bartke et al. 2001; Brown-Borg et al. 1996). Most importantly, these animals show hypersensitivity to injected insulin, have low fasting insulin and glucose levels, high serum adiponectin, and overall are healthier as they age revealing similar characteristics as the one observed in centenarians (Barbieri et al. 2003; Masternak et al. 2009, 2010). Our study of insulin signaling pathways in different organs and basic glucose and insulin tolerance tests supports our hypothesis that improved insulin signaling strongly correlates with life extension in these animals (Masternak et al. 2004, 2005, 2009, 2010; Masternak and Bartke 2007; 2012; Wang et al. 2006). Beside known mutations, there are established interventions that extend the lifespan in laboratory animals. One of the most powerful intervention used in laboratories is calorie restriction (CR) which extends the longevity and improves insulin sensitivity (Masternak et al. 2009; Bartke and Brown-Borg 2004; Bartke et al. 2001). There is not enough evidence that CR would extend the lifespan in humans. However, it is known that the reduction of calorie intake improves glucose homeostasis, insulin sensitivity, and promotes healthy metabolism in humans. On the other hand, pharmacological intervention with rapamycin that suppresses the action of mammalian target of rapamycin gene (mTOR) indicated successful life extension in mice (Harrison et al. 2009; Miller et al. 2011). Interestingly, it is also shown that this pro-longevity treatment does not improve insulin sensitivity (Miller et al. 2008) but may rather promote glucose intolerance and insulin resistance (Blagosklonny 2011). However, prolonged treatment with rapamycin promotes beneficial adaptations and metabolic switching by increasing insulin sensitivity and also extending longevity (Fang and Bartke 2013). All of these observations suggest healthy insulin and glucose metabolism as a key factor for longevity, but it is not yet determined if whole-body insulin sensitivity or just tissue-specific action is crucial for healthy aging.
In the present study, we determined whether the improved insulin signaling in df/df mice translates to enhanced tissue-specific insulin action in vivo. We performed hyperinsulinemic-euglycemic clamps combined with tracer methods to assess whole-body and tissue-specific insulin action in vivo for the first time in these long-living small dwarf mice. The hyperinsulinemic-euglycemic clamp is widely considered as the “gold standard” for assessing insulin action in vivo (Tam et al. 2012; Ayala et al. 2011; Berglund et al. 2008; McGuinness et al. 2009). During this procedure, insulin is infused at a constant rate to achieve physiological or pharmacological hyperinsulinemia. Euglycemia is achieved by infusing glucose at a variable rate that is determined by frequent measurement of blood glucose and subsequent adjustment of the glucose infusion rate. This allows for the measurement of insulin action independently of changes in circulating glucose. Radioactively-labeled glucose analogs are administered to measure tissue-specific insulin action, including suppression of hepatic glucose production and stimulation of tissue glucose uptake.
Results
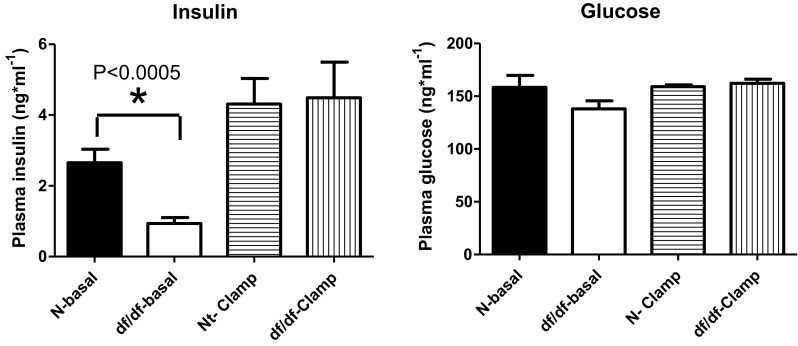
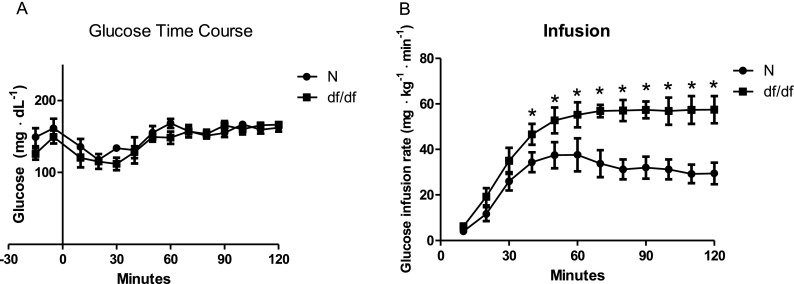
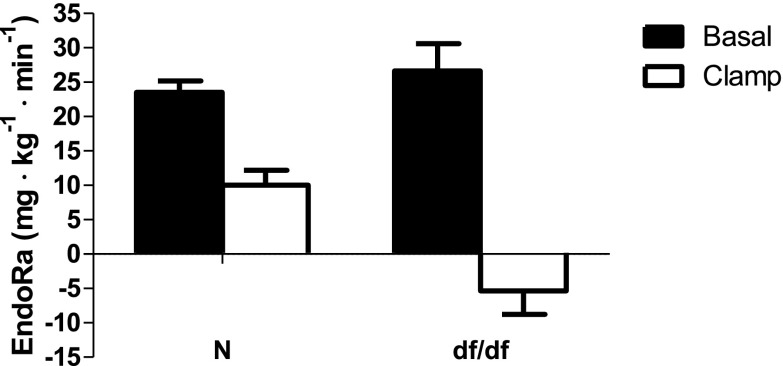
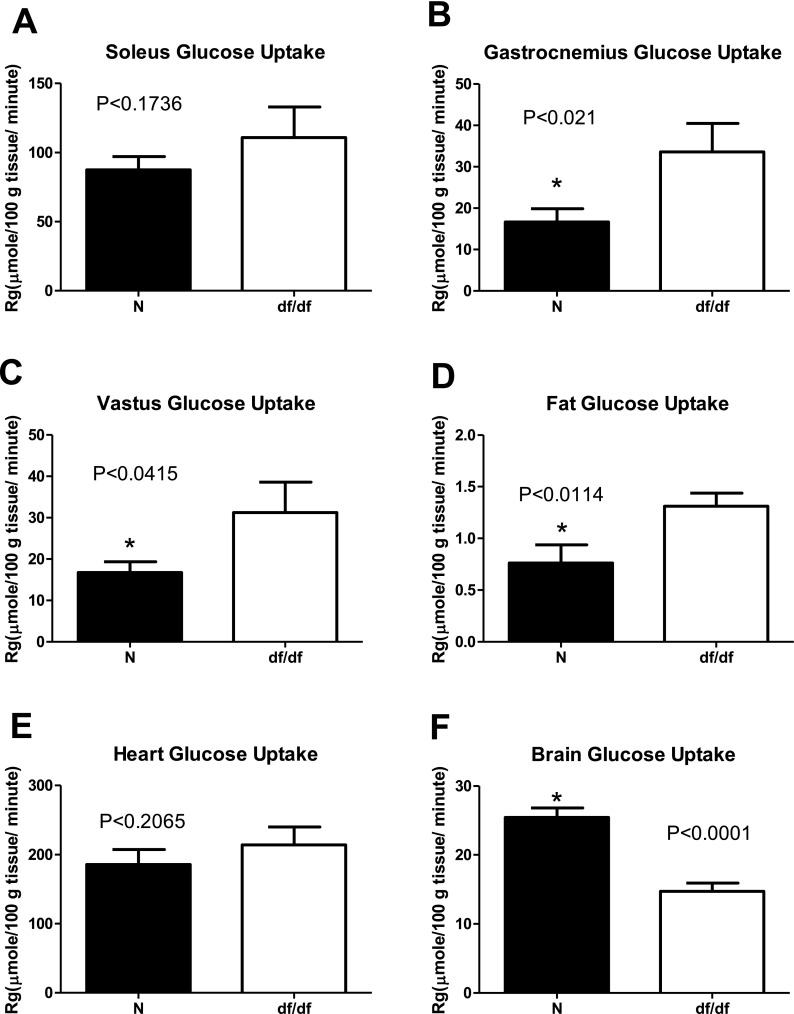
We did not observe any differences in insulin sensitivity and glucose regulation between males and females, which corresponds to the lack of sex differences in the lifespan in these animals (Bartke and Brown-Borg 2004; Brown-Borg et al. 1996). This allowed us to pool together results from males and females. Our initial testing indicated significantly lower fasting insulin levels in df/df mice compared to N controls (P < 0.0005; F = 5.329; df = 14), while fasting glucose levels were not altered between genotypes (P < 0.08; F = 2.826; df = 14) (Fig. 1). During a hyperinsulinemic-euglycemic clamp, the rate of glucose infusion required to maintain euglycemia (∼160 mg·dL−1) (Fig. 2a) was ∼2-fold higher in GH-deficient df/df mice compared to N littermates (Fig. 2b). The rate of endogenous glucose appearance (endoRa), which is indicative of hepatic glucose production, was not different between df/df and N mice during fasted, baseline conditions. However, endoRa was completely suppressed during the clamp in df/df mice whereas it was only suppressed by ∼60 % in N littermates (Fig. 3). This demonstrates that df/df mice display enhanced hepatic insulin sensitivity. In vivo analysis of skeletal muscle tissue indicated greater glucose uptake in the gastrocnemius and superficial vastus lateralis of df/df mice compared to N animals (P < 0.021; F = 4.588; df = 14 and P < 0.0415; F = 7.850; df = 14, respectively) (Fig. 4). However, there was no difference in soleus muscle and heart glucose uptake when comparing df/df and N mice (P < 0.1736; F = 5.23; df = 14 and P < 0.2065; F = 1.435; df = 14, respectively). Importantly, analysis of visceral adipose tissue also indicated greater glucose uptake in df/df mice compared to N mice (P < 0.0114; F = 1.911; df = 14) (Fig. 4). Surprisingly, the glucose uptake in the brain was lower in df/df mice compared to N mice (P < 0.0001; F = 1.403; df = 14) (Fig. 4).
Fig. 1.
Insulin and glucose level of normal and df/df mice before and during clamp. n = 8 animals per genotype. *P < 0.05
Fig. 2.
Arterial glucose (a) and glucose infusion rate (b) during insulin clamp in normal (N) and Ames dwarf (df/df) mice. n = 8 mice per genotype. *P < 0.05
Fig. 3.
EndoRa during basal conditions (black bars) and during insulin clamps (white bars) in normal (N) and Ames dwarf (df/df) mice
Fig. 4.
Glucose metabolic index (Rg) in soleus (a), gastrocnemius (b), superficial vastus lateralis (c), fat (d), heart (e), and brain (e) during insulin clamps in normal (N) and Ames dwarf (df/df) mice. n = 8 mice per genotype. *P < 0.05
Discussion
GH-deficient df/df mice are widely used for aging research because of their exceptional longevity (Masternak et al. 2009; Tatar et al. 2003; Bartke and Brown-Borg 2004; Brown-Borg et al. 1996; Masternak et al. 2004, 2005, 2010; Masternak and Bartke 2007, 2012; Wang et al. 2006). At the same time, there is a strong indication that insulin signaling is enhanced in these mice, making them a useful model for studying insulin and glucose metabolism (Masternak et al. 2009; Wang et al. 2006). Our published data show that replacing GH in these animals reverses their improvements in insulin signaling and shortens their lifespan (Masternak et al. 2010; Panici et al. 2010). This supports our hypothesis that GH deficiency rather than PRL or TSH deficiency is the main player regulating the lifespan and metabolic status in df/df mice. As shown in Fig. 1, these animals have significantly lower fasting insulin levels than N mice yet still are able to maintain normal fasting glucose levels. Our previous observation indicated that during young life and adulthood, there is no difference between glucose levels in df/df and normal control mice. However, as these animals age, the long-living df/df mice maintain lower glucose levels than their N controls (Masternak et al. 2004, 2005, 2009, 2010; Panici et al. 2010; Louis et al. 2010; Menon et al. 2014). Healthy humans also do not show any problems with raising fasting glucose levels, glucose intolerance, or insulin resistance. However, with age at third decade of the life, there is gradual decline in glucose tolerance in normal aging populations with exception for centenarians that maintain high insulin sensitivity through their entire lifespan (Barbieri et al. 2003; DeFronzo 1981). Our clamp results showed that df/df mice required a ∼2-fold greater glucose infusion rate to maintain the same glycemia as N controls indicating enhanced insulin sensitivity in these long-living animals. Tracer methods revealed that df/df mice display improved hepatic insulin action compared to N controls as demonstrated by a complete suppression of endogenous rates of glucose appearance. These data support our earlier observations showing improved insulin sensitivity at the levels of the insulin receptor (IR) and IR substrate-1 (IRS1) in the liver of df/df mice (Masternak et al. 2004, 2009, 2010; Panici et al. 2010; Louis et al. 2010). Phosphorylation of the IR after acute insulin stimulation was significantly higher in df/df mice, and this effect was attenuated with GH replacement (Masternak et al. 2010; Panici et al. 2010). This data indicate the important role of liver in regulating insulin sensitivity in df/df mice which may contribute to extended longevity. However, as we previously showed, the responses to injected insulin (Masternak et al. 2009) and the efficiency of glucose clearance (Masternak et al. 2010; Menon et al. 2014) in these animals probably require multiorgan action. Our present studies also demonstrate enhanced insulin-stimulated gastrocnemius and vastus muscle glucose uptake in df/df mice. This may also play an important role in maintaining normal glucose levels in df/df animals characterized by very low levels of circulating insulin as the skeletal muscle represent main organs responsible for glucose clearance. Importantly, in response to insulin stimulation, the df/df adipose tissue also showed increased glucose uptake compared to adipose tissue from N animals. This data supports our previous findings that suggest an important role for adipose tissue in the regulation of whole-body insulin sensitivity by GH. We have shown that surgical removal of visceral fat improves insulin sensitivity in N mice, while the same procedure either impaired insulin sensitivity or had no effect in either GH-deficient df/df or GH-resistant Laron dwarf mice (Menon et al. 2014; Masternak et al. 2012). This shows an important role of visceral fat, known as “bad” metabolic fat, in maintaining healthy glucose level and high insulin sensitivity in these long-living mutants. It also suggests that suppression of GH signaling significantly alters the function of adipose tissue on improved insulin sensitivity, and this may be mediated via effects on adipose tissue glucose uptake. Our previous findings indicated enhanced insulin-stimulated IR and IRS1 phosphorylation in adipose tissue of df/df mice (Masternak et al. 2010) suggesting more efficient activation of insulin signaling pathway at cellular levels in adipose tissue. This enhanced insulin signaling pathway is necessary to maintain a healthy glucose level with a chronically suppressed level of insulin in df/df mice.
The most surprising observation was that rates of brain glucose uptake were reduced in df/df mice. Typically, reduced glucose levels in the brain are associated with increased hepatic glucose production and decreased muscle and adipose glucose uptake, neither of which are observed in df/df mice. Furthermore, decreased brain glucose availability is also associated with cognitive deficiencies, but df/df mice actually maintain cognitive function longer than control mice (Kinney et al. 2001). Thus, the mechanism and consequences of decreased brain glucose uptake in df/df mice remain to be explored. However, we could speculate that this phenomenon represents an adaptation to low glucose levels in df/df animals. Importantly, whole-body insulin sensitivity does not need to be supported by increased insulin signaling action in all insulin target organs. It could provide the evidence that some blockade or suppression of the cellular signal may be beneficial for health and aging as well as enhancement of the same signal. It was reported that over-expression of Klotho gene extends longevity of mice but at the same time produces insulin resistance (Kurosu et al. 2005; Kuro-o et al. 1997). Similarly, rapamycin treatment extends longevity and suppresses rather than increases insulin sensitivity and glucose tolerance indicating that suppression of insulin signaling or parts of the signal can be also beneficial at some stages of life for healthy aging (Fang and Bartke 2013; Kurosu et al. 2005; Kuro-o et al. 1997; Bartke 2006). We speculate that the adaptation to low glucose uptake in df/df brain could resemble the condition present during CR or starvation which may be necessary to preserve the healthy function of the brain during long life and aging. In summary, hyperinsulinemic-euglycemic clamp studies demonstrate improved insulin sensitivity in long-living Ames dwarf mice. This effect is mediated via effects on hepatic, muscle, and adipose tissue insulin action. These functional data correlate with our previous findings showing enhanced insulin signaling in these insulin-sensitive tissues (Masternak et al. 2004, 2005, 2009, 2010; Wang et al. 2006; Panici et al. 2010; Louis et al. 2010; Menon et al. 2014). Taken together, our results suggest that enhanced insulin sensitivity in multiple organ systems and the subsequent improvement in overall metabolic status may contribute to the extended lifespan of Ames dwarf mice (Menon et al. 2014; Bartke and Westbrook 2012; Westbrook et al. 2009, 2014).
Materials and methods
Animals
Ames dwarf and normal controls were provided from NIA Aged Rodent Colonies (http://www.nia.nih.gov/research/dab/aged-rodent-colonies-handbook) at the age of 12 months. N and df/df males and females were subjected to clamp study (n = 4/sex/genotype).
Hyperinsulinemic-euglycemic clamp
N and df/df mice were surgically implanted with a catheter in the right jugular vein for infusions as previously described (Ayala et al. 2006). Due to their small size and difficulty of the surgery, a catheter was not inserted into the carotid artery for sampling. Instead, blood samples were obtained during the clamp in unrestrained mice via the tip of the tail. Following a 5-day recovery from the surgery, mice were fasted for 5 h prior to the onset of a hyperinsulinemic-euglycemic clamp at t = 0 min. At t = −90 min, mice received a primed, continuous infusion of HPLC-purified [3-3H]glucose (1 μCi prime, 0.05 μCi/min continuous) during a 90 min equilibration period. Blood samples were taken at −15 min (25 μl) and −5 min (50 μl) for measurements of fasting glucose, insulin, and plasma [3-3H]glucose. At t = 0 min, a continuous infusion of insulin (2.5 mU·kg−1·min−1) and a variable glucose infusion was begun. Blood glucose was measured every 10 min (Accu-Chek Aviva, 1 μl samples), and the rate of glucose infusion was adjusted accordingly to maintain euglycemia (∼160 mg·dL−1). Blood samples were obtained at t = 80, 90, 100, 110, and 120 min (50 μl) for measurements of steady-state clamp glucose and plasma [3-3H]glucose. Clamp insulin levels were obtained from blood samples at t = 120 min (additional 25 μl). A 12 μCi bolus of 2[14C]deoxyglucose (2[14C]DG) was then administered. Blood samples (25 μl) were obtained at t = 2, 15, 25, and 35 min after the bolus for measurements of plasma 2[14C]DG. Mice were then anesthetized with an intravenous bolus of sodium pentobarbital. The soleus, gastrocnemius, superficial vastus lateralis, gonadal adipose tissue, heart, and brain were harvested, immediately frozen and stored at −80 °C until analyzed.
Processing of plasma and tissues
Plasma [3-3H]glucose and 2[14C]DG was determined from deproteinized samples by the method of Somogyi as previously described (Ayala et al. 2006). Phosphorylated 2[14C]DG in tissue samples was obtained by homogenizing tissues in 0.5 % PCA, neutralizing the supernatant with 5 N KOH and deproteinizing the supernatant with Ba(OH)2 and ZnSO4 as previously described (Ayala et al. 2008).
Calculations
Rates of glucose appearance (Ra) and disappearance (Rd) were calculated using Steele’s non-steady-state equations (Debodo et al. 1963; Steele et al. 1956). The rate of endogenous glucose appearance (endoRa) was determined by subtracting the glucose infusion rate from the Ra. The glucose metabolic index (Rg), an index of glucose uptake, was calculated as previously described (Ayala et al. 2008). Rg was normalized to tissue weight.
Statistical analysis
One-tailed t test was performed between the groups followed by F-statistics. All statistical tests were conducted using Prism 5.04 (GraphPad Software, San Diego, CA). Alpha is set to 0.05. All values are reported as mean ± standard error of the mean (SEM) throughout the text and figures.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG032290 and P01AG031736.
References
- Ayala JE, et al. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- Ayala JE, et al. Insulin action in the double incretin receptor knockout mouse. Diabetes. 2008;57(2):288–297. doi: 10.2337/db07-0704. [DOI] [PubMed] [Google Scholar]
- Ayala JE et al. (2011) Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp (57) [DOI] [PMC free article] [PubMed]
- Barbieri M, et al. Glucose regulation and oxidative stress in healthy centenarians. Exp Gerontol. 2003;38(1–2):137–143. doi: 10.1016/S0531-5565(02)00153-5. [DOI] [PubMed] [Google Scholar]
- Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006;17(2):33–35. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Westbrook R. Metabolic characteristics of long-lived mice. Front Genet. 2012;3:288. doi: 10.3389/fgene.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, et al. Extending the lifespan of long-lived mice. Nature. 2001;414(6862):412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Berglund ED, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57(7):1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Rapamycin-induced glucose intolerance: hunger or starvation diabetes. Cell Cycle. 2011;10(24):4217–4224. doi: 10.4161/cc.10.24.18595. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, et al. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Debodo RC, et al. On the hormonal regulation of carbohydrate metabolism; studies with C14 glucose. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4(4):493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- Fang Y, Bartke A. Prolonged rapamycin treatment led to beneficial metabolic switch. Aging (Albany NY) 2013;5(5):328–329. doi: 10.18632/aging.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney BA, et al. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39(4):277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Kintscher U, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28(7):1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago F, et al. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3(12):716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- Louis A, Bartke A, Masternak MM. Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65(4):344–352. doi: 10.1093/gerona/glq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A. PPARs in calorie restricted and genetically long-lived mice. PPAR Res. 2007;2007:28436. doi: 10.1155/2007/28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A (2012) Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis:2 [DOI] [PMC free article] [PubMed]
- Masternak MM, et al. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004;59(8):784–788. doi: 10.1093/gerona/59.8.B784. [DOI] [PubMed] [Google Scholar]
- Masternak MM, et al. Effect of every other day feeding diet on gene expression in normal and in long-lived Ames dwarf mice. Exp Gerontol. 2005;40(6):491–497. doi: 10.1016/j.exger.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Masternak MM, et al. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, et al. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11(1):73–81. doi: 10.1111/j.1474-9726.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness OP, et al. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297(4):E849–E855. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V et al. (2014) The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell [DOI] [PMC free article] [PubMed]
- Miller AM, et al. Rapamycin does not improve insulin sensitivity despite elevated mammalian target of rapamycin complex 1 activity in muscles of ob/ob mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1431–R1438. doi: 10.1152/ajpregu.90428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WG, et al. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172(5 Pt 2):S6–S11. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- Pan SY, et al. Obesity, high energy intake, lack of physical activity, and the risk of kidney cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2453–2460. doi: 10.1158/1055-9965.EPI-06-0616. [DOI] [PubMed] [Google Scholar]
- Panici JA, et al. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24(12):5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Steele R, et al. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Tam CS, et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61(4):323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook R, et al. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64(4):443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook R, et al. Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. J Gerontol A Biol Sci Med Sci. 2014;69(1):25–33. doi: 10.1093/gerona/glt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman CA, et al. Responsiveness of the innate immune system and glucose concentrations in the oldest old. Age (Dordr) 2012;34(4):983–986. doi: 10.1007/s11357-011-9292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]