Abstract
Background:
To compare the accuracy of five major risk stratification systems (RSS) in classifying the risk of recurrence and nodal metastases in early-stage endometrial cancer (EC).
Methods:
Data of 553 patients with early-stage EC were abstracted from a prospective multicentre database between January 2001 and December 2012. The following RSS were identified in a PubMed literature search and included the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC-1), the Gynecologic Oncology Group (GOG)-99, the Survival effect of para-aortic lymphadenectomy (SEPAL), the ESMO and the ESMO-modified classifications. The accuracy of each RSS was evaluated in terms of recurrence-free survival (RFS) and nodal metastases according to discrimination.
Results:
Overall, the ESMO -modified RSS provided the highest discrimination for both RFS and for nodal metastases with a concordance index (C-index) of 0.73 (95% CI, 0.70–0.76) and an area under the curve (AUC) of 0.80 (0.78–0.72), respectively. The other RSS performed as follows: the PORTEC1, GOG-99, SEPAL, ESMO classifications gave a C-index of 0.68 (0.66–0.70), 0.65 (0.63–0.67), 0.66 (0.63–0.69), 0.71 (0.68–0.74), respectively, for RFS and an AUC of 0.69 (0.66–0.72), 0.69 (0.67–0.71), 0.68 (0.66–0.70), 0.70 (0.68–0.72), respectively, for node metastases.
Conclusions:
None of the five major RSS showed high accuracy in stratifying the risk of recurrence or nodal metastases in patients with early-stage EC, although the ESMO-modified classification emerged as having the highest power of discrimination for both parameters. Therefore, there is a need to revisit existing RSS using additional tools such as biological markers to better stratify risk for these patients.
Keywords: endometrial cancer, prediction, stratification, recurrence, lymph node
Endometrial cancer (EC) is a major cause of mortality for patients worldwide. Although its incidence differs throughout the world, it is estimated to be the most common cancer of the female genital tract and the fourth most common cancer in North America and Europe (Jemal et al, 2010; Colombo et al, 2013).
Early-stage EC restricted to the uterus represents nearly 80% of all cases (Creasman et al, 1987, 2006; Colombo et al, 2013). The estimated 5-year overall survival for these patients is 95% but decreases substantially to 67.0% and 15.9% for local and distant disease, respectively (Creutzberg et al, 2000a; Randall et al, 2006; Benedetti Panici et al, 2008; ASTEC study group et al, 2009). Moreover, the recurrence rate for early-stage EC is widely variable ranging from 2 to 26% (Creutzberg et al, 2000a; Benedetti Panici et al, 2008; ASTEC study group et al, 2009; Nout et al, 2010; Todo et al, 2010; Nugent et al, 2012). In this specific setting, many epidemiological and histological factors such as increasing age, depth of myometrial invasion, histological tumour type and grade, presence of lymphovascular space invasion (LVSI) and the International Federation of Gynecology and Obstetrics (FIGO) classification (Pecorelli, 2009) have been reported to be correlated with a higher risk of recurrence and nodal metastases (Creasman et al, 1987; Mariani et al, 2002; Keys et al, 2004; Nout et al, 2010; Todo et al, 2010; Nugent et al, 2012; Colombo et al, 2013).
Over the last decade, these criteria have been aggregated into several risk stratification systems (RSS) that are currently used worldwide to guide decision-making and clinical trial design (Creutzberg et al, 2000a; Keys et al, 2004; Todo et al, 2010; Colombo et al, 2013; Bendifallah et al, 2014). The assumption is based on defining recurrence risk groups, which can help identify clinical situations where multimodality therapy and/or nodal staging should be proposed for high-risk patients or, conversely, single modality or wait-and-see strategies for low-risk patients. Although the core variables of these RSS are very similar (Creutzberg et al, 2000a; Keys et al, 2004; Todo et al, 2010; Colombo et al, 2013; Bendifallah et al, 2014), finally, it appears that for major RSS: (i) most have never been externally validated; (ii) accuracy is not reported and (iii) no simultaneous comparisons using the same cohort have been performed.
Hence, the aim of this study was to compare five major RSS (Creutzberg et al, 2000a; Keys et al, 2004; Todo et al, 2010; Colombo et al, 2013; Bendifallah et al, 2014) in a multicenter cohort of patients with early-stage EC with regard to their discriminative performance in stratifying the risk of recurrence and nodal metastases.
Materials and Methods
Study population
The data of 553 patients with apparent early-stage EC, who received primary surgical treatment between January 2001 and December 2012, were abstracted from five institutions with maintained EC databases in France (Tenon University Hospital, Reims University Hospital, Dijon Cancer Center, Creteil hospital and Jeanne de Flandre University Hospital) and from the Senti-Endo trial (Ballester et al, 2011). All patients had undergone a preoperative endometrial biopsy. All enrolled patients underwent a preoperative MRI unless contraindicated, in which case a CT scan was performed. Patients with histologically proven EC were staged on the basis of final pathological findings according to the 2009 FIGO classification (Pecorelli, 2009). Clinical and pathologic variables included patient age, surgical procedure, 2009 FIGO stage and final pathological analysis (histological type and grade, depth of myometrial invasion and LVSI status). A tumour was considered LVSI-positive when tumour emboli were found within a space clearly lined by endothelial cells (Tsuruchi et al, 1995). The research protocol was approved by the institutional review board of the French college of obstetricians and gynecologists (CEROG 2014-GYN-020).
Treatment and follow-up
We included all women who underwent primary surgical treatment including at least total hysterectomy with bilateral salpingo-oophorectomy, with or without nodal staging (pelvic ± paraaortic lymphadenectomy) according to the current guidelines (Querleu et al, 2011; Colombo et al, 2013) and to the surgeon's discretion. Sentinel lymph node biopsies (SLNB) were performed by a dual intracervical injection based on the histological validation of SLN by Delpech et al (2007). A para-aortic lymphadenectomy was recommended for women with metastatic pelvic SLN on intraoperative histology or after final histology. Systematic pelvic and para-aortic lymphadenectomy was also recommended for patients with type 2 EC (clear-cell, serous EC and carcinosarcoma) and type 1, grade 3 with a depth of myometrial invasion >50%. Adjuvant therapy was administered on an individual basis at the discretion of a multidisciplinary committee according to international guidelines (Colombo et al, 2013) and involved vaginal brachytherapy and/or external beam radiotherapy (EBRT) and/or chemotherapy. Clinical follow-up consisted of physical examinations and the use of imaging techniques depending on the findings. Follow-up sessions were conducted every 3 months during the first 2 years, every 6 months during the following 3 years and once a year thereafter.
RSS description
Five major RSS related to the risk stratification of early-stage EC were identified in the medical literature using PubMed: the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC)-1 classification (Creutzberg et al, 2000a), the Gynecologic Oncology Group (GOG) 99 classification (Keys et al, 2004), the Survival effect of para-aortic lymphadenectomy (SEPAL) in EC classification (Todo et al, 2010), the ESMO (Colombo et al, 2013) and ESMO-modified (Bendifallah et al, 2014) classifications. RSS were selected with respect to their acceptance in the literature and clinical applicability. Table 1 describes the criteria for each RSS.
Table 1. Description of five risk recurrence systems.
| RSS | Year | Number of patients | Criteria |
|---|---|---|---|
| PORTEC-1 (Creutzberg et al, 2000b) | 2000 | 715 | Low risk Endometrial adenocarcinoma stage Ia, grade 1 Intermediate risk Endometrial adenocarcinoma Stage I based on uterine factors Grade 1 histology and myometrial invasion of ⩾50% Grade 2 histology with any myometrial invasion Grade 3 histology with myometrial invasion <50% High-intermediate risk Age >60 years with grade 1 or 2 histology and myometrial invasion >50% Age >60 with grade 3 histology and myometrial invasion <50% High-risk Stage III–IV disease Uterine serous carcinoma or clear cell carcinoma of any stage |
| GOG-99 (Keys et al, 2004) | 2004 | 382 | Low risk Grade 1 or 2, endometrioid cancers confined to the endometrium stage IA Low-intermediate risk Age ⩽50 years + ⩽2 pathologic risk factors Age 50–69 years + ⩽1 pathologic risk factor Age ⩾70 years + no pathologic risk factors (Risk factors (1) grade 2 or 3 histology; (2) positive lymphovascular space invasion; (3) myometrial invasion to outer 1/3) High-intermediate risk (HIR) Any age + 3 pathologic risk factors Age 50–69 years + ⩾2 pathologic risk factors Age ⩾70 years + ⩾1 pathologic risk factor (Risk factors (1) grade 2 or 3 histology; (2) positive lymphovascular space invasion; (3) myometrial invasion to outer 1/3) High-risk Stage III–IV disease, regardless of histology or grade Uterine serous carcinoma or clear cell carcinoma of any stage |
| SEPAL (Todo et al, 2010) | 2010 | 671 | Low risk Stage IA IB, endometrioid type, LVSI negative Intermediate risk Stage IA grade 3 endometrioid adenocarcinoma; any grade of non-endometrioid carcinoma (serous adenocarcinoma, clear cell adenocarcinoma or other type of carcinoma), any LVSI Stage IB, grade 1–2 endometrioid adenocarcinoma, LVSI positive Stage IB, grade 3 endometrioid adenocarcinoma; any grade of non- endometrioid carcinoma (serous adenocarcinoma, clear cell adenocarcinoma or other type of carcinoma), any LVSI Stage IC, stage II, any grade, any LVSI High risk Stage III–IV, any grade, any LVSI |
| ESMO (Colombo et al, 2013) | 2013 | — | Low risk Stage IA (grade 1 and grade 2) with endometrioid type Intermediate risk Stage IA grade 3 with endometrioid type Stage IB (grade 1 and grade 2) with endometrioid type High risk Stage IB grade 3 with endometrioid type All stages with non-endometrioid type |
| ESMO modified (Bendifallah et al, 2014) | 2014 | 496 | Low-risk ESMO/LVSI- Low-risk ESMO/LVSI+ Intermediate-risk ESMO/LVSI- Intermediate-risk ESMO/LVSI+ High-risk ESMO/LVSI- High-risk ESMO/LVSI+ |
Abbreviations: ESMO=European Society for Medical Oncology; LVSI=lymphovascular space invasion.
Recurrence events and recurrence-free survival (RFS)
The clinical end point was recurrence. Disease recurrence was diagnosed by biopsy or imaging studies and defined as a relapse without differentiating between their local or distant nature. RFS was defined as the time from surgery to the date of recurrence. Estimates were produced using the Kaplan–Meier method.
Statistical analysis
Stratification accuracy
The receiver operating characteristic area under the curve (ROC-AUC) as well as the concordance index (C-index) indicate the discriminatory properties and quantify the stratification accuracy (i.e., whether the relative ranking of individual stratification was in the correct order) (Hanley and McNeil, 1982; Heagerty et al, 2000; Heagerty and Zheng, 2005). The AUC requires binary outcomes (presence or absence of the event) and is reserved for binary logistic regression models. The c-index represents an adaptation of the AUC for censored data and is necessary when time-to-event data are used. In the current analysis, the accuracy of each RSS for RFS (censored data) was conducted using the Cox Proportional Hazards Model. Similarly to quantify the discriminatory properties of each RSS with regard to the risk for LNM, a binary logistic regression model was performed. The AUC, as well as the c-index of 0.5, represents no discriminating ability, and a value of 1.0 represents perfect discrimination.
RSS diagnostic accuracy
Sensitivity, specificity, negative predictive values, positive predictive values and the overall diagnostic accuracy (ODA) (i.e., the probability of a patient being correctly classified by the RSS) with 95% CI were calculated to study the diagnostic ability of each RSS to classify patients at low risk and those at high risk of recurrence and nodal metastases.
Others analysis
Statistical analysis was based on Student's t-test and the Mann–Whitney test for parametric and nonparametric continuous variables, respectively, and the χ2-test or Fisher's exact test, as appropriate, for categorical variables. Values of P<0.05 were considered to denote significant differences. Data were managed with an Excel database (Microsoft, Redmond, WA, USA) and analysed using R 2.15 software, available online.
Results
Characteristics of the study population
During the study period 553 patients with EC were documented as having received primary surgical treatment according to the following distribution: Dijon Cancer Center (n=122; 22%), Creteil Hospital (n=83; 15%), Reims University Hospital (n=87; 16%), Tenon University Hospital (n=70; 13%), Jeanne de Flandre University Hospital (n=97; 17%) and Senti-Endo trial (n=94; 17%). The demographics and clinicopathological characteristics of the whole cohort are reported in Table 2. The median age of the patients was 65.0 years (range: 31–98 years).
Table 2. Characteristics of the whole population.
| Overall population n=553 | No recurrence n=462 | Recurrence n=91 | P-valuea | |
|---|---|---|---|---|
| Age-mean (range) | 64.9 (31–98) | 64.4 (31–98) | 67.8 (32–88) | 0.0033 |
|
Histological grade | ||||
| I | 48.6% (269) | 52.4% (242) | 29.7% (27) | |
| II | 27.5% (152) | 29.2% (135) | 18.7% (17) | |
| III | 23.9% (132) | 18.4% (85) | 51.6% (47) | <0.0001 |
|
Pathological type | ||||
| 1 | 86.6% (479) | 89.2(412) | 73.6% (67) | |
| 2 | 13.4% (74) | 10.8(50) | 26.7% (24) | 0.0001 |
|
Myometrial invasion | ||||
| <50% | 54.3% (300) | 58.4% (270) | 32.9% (30) | |
| ⩾50% | 45.7% (253) | 41.6% (192) | 67.1% (61) | <0.0001 |
|
Lymphovascular space invasion | ||||
| No | 66.4% (367) | 70.4% (325) | 46.1% (42) | |
| Yes | 25.3% (140) | 21.2% (98) | 46.1% (42) | |
| NA | 8.3% (46) | 8.4% (39) | 7.8% (7) | <0.0001 |
|
FIGO stage | ||||
| I | 78.1% (432) | 81.8% (378) | 59.3% (54) | |
| II | 7.6% (42) | 6.3% (29) | 14.3% (13) | |
| IIIc | 14.3% (79) | 11.9% (55) | 26.4% (24) | <0.0001 |
| Nodal staging (P/PAL) | 86.6% (479/553) | 87.1% (402/462) | 84.6% (77/91) | 0.0001 |
| Nodal metastasis | 16.5% (79/479) | 13.7% (55/402) | 31.2% (24/77) | 0.0001 |
|
PORTEC-1 (Creutzberg et al, 2000a) | ||||
| Low risk | 32% (175) | 35% (163) | 13% (12) | |
| Intermediate risk | 19% (106) | 21% (97) | 10% ( 9) | |
| High-intermediate risk | 24% (134) | 23% (105) | 32% ( 29) | |
| High risk | 25% (138) | 21% (97) | 45% ( 41) | — |
|
GOG-99 (Keys et al, 2004) | ||||
| Low risk | 51% (280) | 55% (255) | 27% (25) | |
| Low-intermediate risk | 2% (13) | 2% (10) | 3% (3) | |
| High-intermediate risk | 23% (129) | 23% (106) | 25% (23) | |
| High risk | 24% (131) | 20% (91) | 44% ( 40) | — |
|
SEPAL (Todo et al, 2010) | ||||
| Low risk | 43% (238) | 48% (221) | 19% (17) | |
| Intermediate risk | 43% (236) | 40% (186) | 55% (50) | — |
| High risk | 14% (79) | 12% (55) | 26% (24) | |
|
ESMO (Colombo et al, 2013) | ||||
| Low risk | 45.1% (249) | 50.4% (233) | 17.6% (16) | |
| Intermediate risk | 34.5% (191) | 34.0% (157) | 37.4% (34) | |
| High risk | 20.4% (113) | 15.6% (72) | 45.0% (41) | — |
|
ESMO/LVSI (Bendifallah et al, 2014) | ||||
| Low-risk ESMO/LVSI− | 37.6% (208) | 41.8% (193) | 16.5% (15) | |
| Low-risk ESMO/LVSI+ | 2.7% (15) | 3.2% (15) | 0% (0) | |
| Intermediate-risk ESMO/LVSI− | 18.8% (104) | 19.6% (90) | 15.4% (14) | |
| Intermediate-risk ESMO/LVSI+ | 13.2% (73) | 12.1% (56) | 18.7% (17) | |
| High-risk ESMO/LVSI− | 9.9% (55) | 9.1% (42) | 14.3% (13) | |
| High-risk ESMO/LVSI+ | 9.4% (52) | 5.8% (27) | 27.4% (25) | — |
| NA | 8.4% (46) | 8.4% (39) | 7.7% (7) | |
|
Adjuvant therapy | ||||
| No adjuvant therapy | 18.1% (100) | 20.1% (93) | 7.7% (7) | — |
| EBRT ± brachytherapy | 34.7% (192) | 30.8% (142) | 54.9% (50) | |
| Brachytherapy | 30.1% (166) | 34.8% (161) | 5.5% (5) | |
| Chemotherapy | 2.3% (13) | 0.9% (4) | 9.9% (9) | |
| Multimodal therapy | 4.9% (27) | 3.5% (16) | 12.1% (11) | |
| NA | 9.9% (55) | 9.9% (46) | 9.9% (9) | |
Abbreviations: EBRT=External beam radiotherapy; ESMO=European Society for Medical Oncology; FIGO=Federation of Gynecology and Obstetrics; GOG=Gynecologic Oncology Group; LVSI=lymphovascular space invasion; NA=not applicable; P/PAL=pelvic and/or paraaortic lymphadenectomy; PORTEC=Post Operative Radiation Therapy in Endometrial Carcinoma; SEPAL=Survival effect of para-aortic lymphadenectomy.
Univariate logistic regression.
RFS according to each RSS
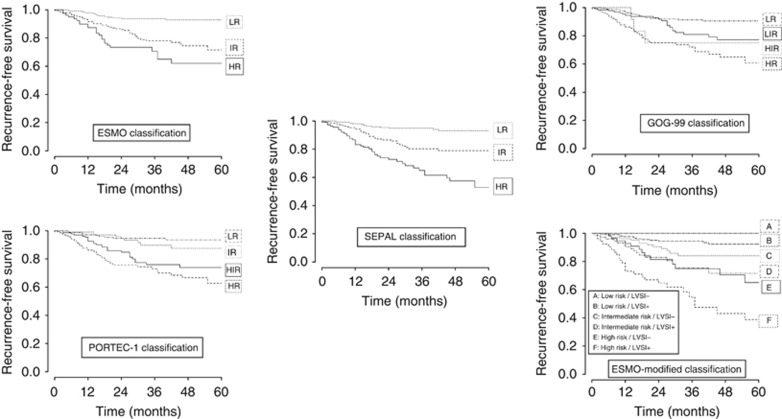
The median follow-up was 32 (range: 2–165) months and the median time to initial recurrence was 29 (range: 1–165) months. Overall 3-year RFS and 3-year recurrence rates were 83.9% (95% CI, 80.6–87.4) and 16.4%, respectively. Loco-regional, nodal and distant recurrences were observed in 20% (18/91), 24% (22/91) and 56% (51/91) of cases, respectively. The respective 3-year RFS according to each RSS are reported in Figure 1.
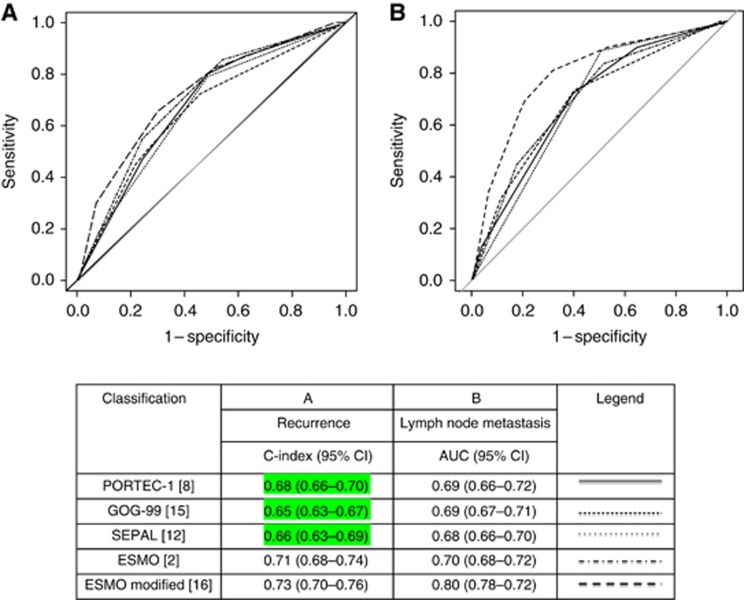
Figure 1.
Discrimination of each RSS for recurrence and nodal metastases.
Discrimination and diagnostic accuracy of each RSS system for recurrence
The discrimination of each RSS is reported in Figure 2A. The RSS with the highest discrimination was the ESMO-modified classification (C-index=0.73 (95% CI, 0.70–0.76)). The diagnostic accuracy of each RSS is reported in Table 3. The RSS with the highest ODA to select patients at low risk of recurrence was the PORTEC-1 classification with 56% of patients correctly stratified. The RSS with the highest ODA to select patients at increased risk of recurrence was the ESMO-modified classification with 78% of patients correctly assigned.
Figure 2.
Recurrence-free survival curves according to each risk classification.
Table 3. Diagnostic accuracy for recurrence.
|
Low risk group (compared with other groups) |
High risk group (compared with other groups) |
||||||
|---|---|---|---|---|---|---|---|
| RSS | Diagnostic accuracy statistics | Value | Low 95% CI | High 95% CI | Value | Low 95% CI | High 95% CI |
| PORTEC-1 (Creutzberg et al, 2000a) | Sensitivity | 0.132 | 0.074 | 0.216 | 0.451 | 0.357 | 0.545 |
| Specificity | 0.647 | 0.636 | 0.664 | 0.790 | 0.772 | 0.809 | |
| PPV | 0.069 | 0.039 | 0.112 | 0.297 | 0.236 | 0.360 | |
| NPV | 0.791 | 0.777 | 0.811 | 0.880 | 0.859 | 0.900 | |
| ODA | 0.562 |
0.734 |
|||||
| GOG-99 (Keys et al, 2004) | Sensitivity | 0.275 | 0.193 | 0.371 | 0.440 | 0.347 | 0.534 |
| Specificity | 0.448 | 0.432 | 0.467 | 0.803 | 0.785 | 0.822 | |
| PPV | 0.089 | 0.063 | 0.121 | 0.305 | 0.241 | 0.371 | |
| NPV | 0.758 | 0.731 | 0.790 | 0.879 | 0.859 | 0.899 | |
| ODA | 0.420 |
0.743 |
|||||
| SEPAL (Todo et al, 2010) | Sensitivity | 0.187 | 0.118 | 0.278 | 0.264 | 0.187 | 0.350 |
| Specificity | 0.522 | 0.508 | 0.540 | 0.881 | 0.866 | 0.898 | |
| PPV | 0.071 | 0.045 | 0.106 | 0.304 | 0.215 | 0.403 | |
| NPV | 0.765 | 0.745 | 0.791 | 0.859 | 0.844 | 0.875 | |
| ODA | 0.457 |
0.769 |
|||||
| ESMO (Colombo et al, 2013) | Sensitivity | 0.176 | 0.109 | 0.266 | 0.451 | 0.359 | 0.542 |
| Specificity | 0.496 | 0.482 | 0.513 | 0.844 | 0.826 | 0.862 | |
| PPV | 0.064 | 0.040 | 0.097 | 0.363 | 0.289 | 0.437 | |
| NPV | 0.753 | 0.733 | 0.780 | 0.886 | 0.867 | 0.905 | |
| ODA | 0.467 |
0.773 |
|||||
| ESMO modifieda (Bendifallah et al, 2014) | Sensitivity | 0.179 | 0.109 | 0.273 | 0.452 | 0.357 | 0.548 |
| Specificity | 0.508 | 0.494 | 0.527 | 0.837 | 0.818 | 0.856 | |
| PPV | 0.067 | 0.041 | 0.103 | 0.355 | 0.280 | 0.430 | |
| NPV | 0.757 | 0.736 | 0.785 | 0.885 | 0.865 | 0.905 | |
| ODA | 0.453 | 0.776 | |||||
Abbreviations: ESMO=European Society for Medical Oncology; GOG=Gynecologic Oncology Group; NPV=negative predictive values; ODA=overall diagnostic accuracy; PORTEC=Post Operative Radiation Therapy in Endometrial Carcinoma; PPV=positive predictive values; SEPAL=Survival effect of para-aortic lymphadenectomy.
Intermediate-risk ESMO/LVSI+ and high risk groups compared with intermediate risk ESMO/LVSI- and low risk.
Discrimination and diagnostic accuracy of each RSS systems for nodal metastases
Overall, 86.6% (479/553) of the patients underwent systematic nodal staging and 16.5% (79/479) of these had nodal metastases (Table 2). Discrimination of each RSS is reported in Figure 2B. The RSS with the highest discrimination was the ESMO-modified classification (AUC=0.80 (95% CI, 0.78–0.82)). The diagnostic accuracy of each RSS is reported in Table 3. The RSS with the highest ODA to select patients at low risk of nodal metastases was the PORTEC-1 classification with 56% of patients correctly stratified. The RSS with the highest ODA to select patients at increased risk of metastases was the ESMO-modified system with 77% of patients correctly assigned.
Discussion
To our knowledge, this is the first study to provide a comparison of five major RSS applied to a multicenter population with early-stage EC. The results suggest that these five RSS have a poor-to-moderate discrimination for recurrence and nodal metastases. In addition, the clinical diagnostic accuracy to distinguish subgroups of patients at low- and high-risk of recurrence or nodal metastases appears to be limited and heterogeneous.
Management of women with early-stage EC remains controversial and practice patterns vary widely among gynecologic oncologists (Creutzberg et al, 2000a; Keys et al, 2004; ASTEC study group et al, 2009; Nout et al, 2010; Colombo et al, 2013; Ko et al, 2013). This is mainly because there are several criteria defining risk groups for recurrence, unstandardised protocols for surgical staging and different indications for adjuvant therapies (Creutzberg et al, 2000a; Keys et al, 2004; ASTEC study group et al, 2009; Nout et al, 2010; Colombo et al, 2013; Ko et al, 2013). To overcome these limitations and guide clinicians in their decision-making and in providing patient information, several authors have developed RSS to create a common nomenclature (Creutzberg et al, 2000a; Keys et al, 2004; Creasman et al, 2006; Querleu et al, 2011; Colombo et al, 2013; Bendifallah et al, 2014). Although all of these RSS include similar variables, the combination of variables differs substantially between the United States and European countries leading to widely differing practice patterns for adjuvant therapies and indications for nodal staging (Creutzberg et al, 2000a; Keys et al, 2004; ASTEC study group et al, 2009; Nout et al, 2010; Colombo et al, 2013; Ko et al, 2013). The potential of ROC curves in medical diagnostic testing was recognised as early as 1960 (LUSTED, 1960) as the most relevant statistical tool to describe diagnostic performance (Hanley and McNeil, 1982; DeLong et al, 1988). Classically, the predictive accuracy of a classification is based on the assumption that all patients within a given risk group are equal. However, in practice, heterogeneity in both biological parameters and patients' characteristics within each risk subgroup has been reported, especially for women with early-stage EC (Creutzberg et al, 2000a; Keys et al, 2004; Ballester et al, 2011, 2013; Nugent et al, 2012), leading to incorrect risk assignment. Our results confirm that the ESMO-modified classification (Bendifallah et al, 2014) was the RSS with the highest discrimination according to recurrence with a C-index of 0.72. We also found that the PORTEC-1 classification (Creutzberg et al, 2000a) was the most accurate in selecting patients at low risk of recurrence with an ODA of 55% and the ESMO-modified classification (Bendifallah et al, 2014) more accurate in selecting patients at increased risk with an ODA of 78%. These results also suggest that these RSS are heterogeneous in terms of classification performance. Moreover, it highlights the high rate of misclassified patients whatever the RSS used and the potential risk of inadequate surgical staging and over- or under-treatment. Finally, these results underline that new biological markers or stratification tools are probably needed to improve discrimination of such classifications, resulting in a more adapted surgical staging and adjuvant treatment.
Despite a reported good overall survival, almost 15% of patients with localised disease experience recurrence during the first 2 years following initial treatment (Creasman et al, 2006; Benedetti Panici et al, 2008; ASTEC study group et al, 2009; Bendifallah et al, 2014). It is therefore essential to distinguish patients at increased risk of recurrence who require systematic adjuvant EBRT and/or chemotherapy. A debate exists regarding the optimal adjuvant therapy for patients with early-stage EC. Published trials involve a wide variety of patients with different characteristics, rendering interpretation of the results somewhat difficult (Creutzberg et al, 2000a; Keys et al, 2004; Nout et al, 2010; Ko et al, 2013). Moreover, there are several differences in surgical staging from one study to another; in some trials, lymphadenectomy was systematically performed (Kuoppala et al, 2008; Reed et al, 2008; Susumu et al, 2008), whereas in others it was not required (Creutzberg et al, 2000a; Maggi et al, 2006; Randall et al, 2006; ASTEC/EN.5 Study Group et al, 2009; Nout et al, 2010) or performed only in case of suspicious lymph nodes (Morrow et al, 1990; Sorbe et al, 2009, 2012). This gives rise to an important confounding bias. Three randomised trials on adjuvant pelvic radiation versus a wait-and-see approach have shown significantly improved loco-regional control in case of additional EBRT, with no impact on overall survival (Aalders et al, 1980; Creutzberg et al, 2000a; Keys et al, 2004). Indeed, when focusing on the high-risk cohorts, the reported loco-regional recurrence rates vary from 13 to 23% with no adjuvant EBRT (Aalders et al, 1980; Creutzberg et al, 2000a; Keys et al, 2004) versus 5% when adjuvant EBRT is administered systematically (Aalders et al, 1980; Creutzberg et al, 2000a; Keys et al, 2004). This underlines the importance of accurate risk stratification in selecting the most adapted treatment option. Similarly, few data exist on the role of chemotherapy in early-stage EC. In high-risk EC, the Cochrane meta-analysis showed a positive impact of chemotherapy on overall survival, disease-free survival and distant metastasis (Johnson et al, 2011). However, these results may be biased by the inclusion of patients with more advanced disease once again rendering interpretation somewhat difficult (Randall et al, 2006).
Selecting patients who might benefit from systematic nodal staging is a major issue to guide postoperative treatment in patients with early-stage EC (Benedetti Panici et al, 2008; ASTEC study group et al, 2009; Ballester et al, 2011; Nugent et al, 2012). In this setting, a meta-analysis of two randomised trials on the impact of systematic lymphadenectomy in early-stage EC showed no benefit on overall and recurrence-free survival (Benedetti Panici et al, 2008; ASTEC study group et al, 2009). In contrast, in the SEPAL study Todo et al (2010) reported a survival benefit for systematic pelvic and para-aortic lymphadenectomy especially in patients with intermediate- and high-risk EC. These results highlight that the intermediate-risk group, as currently defined by the major classifications, is a heterogeneous group of patients in terms of nodal metastases rendering indications for complete surgical staging and adjuvant therapies somewhat blurred. Moreover, in a retrospective study on the rate of nodal metastases in clinical stage 1 type 1 EC according to the PORTEC 1 (Creutzberg et al, 2000a) and GOG-99 criteria (Keys et al, 2004) for high-intermediate risk patients Nugent et al (2012) reported that patients have substantial risk of nodal involvement and recurrence, suggesting that complete nodal staging is crucial for this subgroup. Our results confirm that the ESMO-modified classification has the highest discrimination for nodal metastases. Moreover, we found that the PORTEC-1 RSS (Creutzberg et al, 2000a) was the most accurate to select patients at low risk with an ODA of 56%, whereas the ESMO-modified RSS (Bendifallah et al, 2014) was the most accurate to select patients at high-risk with an ODA of 77%. These results underline the need in the future for precise quantification of the risk of nodal metastases using a complementary approach based on individualized prediction models such as nomograms (Bendifallah et al, 2012; AlHilli et al, 2013). In this specific setting, AlHilli et al (2013) developed two nomograms in patients with surgically treated stage I–IV endometrioid EC to predict the probability of lymph node metastases. However, the definition of an optimal threshold to decide whether to perform secondary lymphadenectomy is lacking. Finally, the authors did not focus on women with early-stage disease, which is the subgroup with the most discrepancies in terms of nodal metastases.
Some limitations of the present study deserve to be mentioned. First, it included patients treated for early-stage EC over a relatively long period. During the data collection period, modifications in staging modalities (FIGO classification (Pecorelli, 2009)) and surgical techniques (LN staging) were introduced. For example, SLNB was introduced and shown to be a possible first-line treatment for patients with early-stage EC. Indeed, Raimond et al (2014) recently demonstrated that SLN mapping and ultrastaging improved staging and made it possible to adapt adjuvant therapy to the risk of recurrence. Second, our cohort included patients from several centers and discrepancies in patient management might have affected our results in part. However, all included centers were regional referral centers applying the current French guidelines. Third, although the ESMO-modified classification seems to be associated to higher stratification accuracy, an external and independent validation study of the current results is needed. Fourth, although the multicentre nature of this study provides an overview of clinical practice during a long period, the overall survival analysis could not be performed. Finally, central pathology review was not available. However, dedicated pathologists from tertiary referral centers assessed all biopsies and specimens.
In conclusion, we demonstrate here that none of five major RSS shows high accuracy to stratify recurrence risk and nodal metastases in women with early-stage EC. Therefore, there is a need to revisit existing RSS using additional tools such as biological markers to better stratify patient risk in this setting. Moreover, several promising prognostic in situ biomarkers such as DNA ploidy, expression of P53, oestrogen and progesterone receptors have been identified (Ballester et al, 2013; Murali et al, 2014). These biomarkers could be used in clinical practice for a more individualised management in EC. At last, the therapeutic challenge for early-stage EC lies in promoting a personalized therapeutic strategy to avoid over- or under-treatment.
Acknowledgments
We thank Professeur Pierre-Yves Boelle who spent time reading and making comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- AlHilli MM, Podratz KC, Dowdy SC, Bakkum-Gamez JN, Weaver AL, McGree ME, Keeney GL, Cliby WA, Mariani A. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol Oncol. 2013;131:103–108. doi: 10.1016/j.ygyno.2013.06.037. [DOI] [PubMed] [Google Scholar]
- ASTEC study group. Kitchener H, Swart AMC, Qian Q, Amos C, Parmar MKB. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTEC/EN.5 Study Group. Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M, Tu D, Parmar MKB, Amos C, Murray C, Qian W. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester M, Canlorbe G, Cortez A, Gonin J, Laas E, Bendifallah S, Graesslin O, Daraï E. Histological and immunohistochemical profiles predict lymph node status in women with low-intermediate risk endometrial cancer. Gynecol Oncol. 2013;130:457–462. doi: 10.1016/j.ygyno.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, Querleu D, Golfier F, Leblanc E, Rouzier R, Daraï E. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- Bendifallah S, Canlorbe G, Raimond E, Hudry D, Coutant C, Graesslin O, Touboul C, Huguet F, Cortez A, Daraï E, Ballester M. A clue towards improving the European Society of Medical Oncology risk group classification in apparent early stage endometrial cancer? Impact of lymphovascular space invasion. Br J Cancer. 2014;110 (11:2640–2646. doi: 10.1038/bjc.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendifallah S, Genin AS, Naoura I, Chabbert Buffet N, Clavel Chapelon F, Haddad B, Luton D, Darai E, Rouzier R, Koskas M. A nomogram for predicting lymph node metastasis of presumed stage I and II endometrial cancer. Am J Obstet Gynecol. 2012;207 (197:e1–e8. doi: 10.1016/j.ajog.2012.06.080. [DOI] [PubMed] [Google Scholar]
- Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, Angioli R, Tateo S, Mangili G, Katsaros D, Garozzo G, Campagnutta E, Donadello N, Greggi S, Melpignano M, Raspagliesi F, Ragni N, Cormio G, Grassi R, Franchi M, Giannarelli D, Fossati R, Torri V, Amoroso M, Crocè C, Mangioni C. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C, ESMO Guidelines Working Group Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 (Suppl 6:vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz APM, Ngan HYS, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 (Suppl 1:S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Delpech Y, Cortez A, Coutant C, Callard P, Uzan S, Darai E, Barranger E. The sentinel node concept in endometrial cancer: histopathologic validation by serial section and immunohistochemistry. Ann Oncol. 2007;18:1799–1803. doi: 10.1093/annonc/mdm334. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Johnson N, Bryant A, Miles T, Hogberg T, Cornes P.2011Adjuvant chemotherapy for endometrial cancer after hysterectomy Cochrane Database Syst Rev CD003175doi: 10.1002/14651858.CD003175.pub2 [DOI] [PMC free article] [PubMed]
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG, Gynecologic Oncology Group A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Ko EM, Funk MJ, Clark LH, Brewster WR. Did GOG99 and PORTEC1 change clinical practice in the United States. Gynecol Oncol. 2013;129:12–17. doi: 10.1016/j.ygyno.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Kuoppala T, Mäenpää J, Tomas E, Puistola U, Salmi T, Grenman S, Lehtovirta P, Fors M, Luukkaala T, Sipilä P. Surgically staged high-risk endometrial cancer: randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecol Oncol. 2008;110:190–195. doi: 10.1016/j.ygyno.2008.03.020. [DOI] [PubMed] [Google Scholar]
- LUSTED LB. Logical analysis in roentgen diagnosis. Radiology. 1960;74:178–193. doi: 10.1148/74.2.178. [DOI] [PubMed] [Google Scholar]
- Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, Colombo A, Fossati R. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani A, Webb MJ, Keeney GL, Lesnick TG, Podratz KC. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002;87:274–280. doi: 10.1006/gyno.2002.6836. [DOI] [PubMed] [Google Scholar]
- Morrow CP, Bundy BN, Homesley HD, Creasman WT, Hornback NB, Kurman R, Thigpen JT. Doxorubicin as an adjuvant following surgery and radiation therapy in patients with high-risk endometrial carcinoma, stage I and occult stage II: a Gynecologic Oncology Group Study. Gynecol Oncol. 1990;36:166–171. doi: 10.1016/0090-8258(90)90166-i. [DOI] [PubMed] [Google Scholar]
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–e278. doi: 10.1016/S1470-2045(13)70591-6. [DOI] [PubMed] [Google Scholar]
- Nout RA, VTHBM Smit, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, LCHW Lutgens, van der Steen-Banasik EM, Mens JWM, Slot A, Kroese MCS, van Bunningen BNFM, Ansink AC, van Putten WLJ, Creutzberg CL, PORTEC Study Group Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- Nugent EK, Bishop EA, Mathews CA, Moxley KM, Tenney M, Mannel RS, Walker JL, Moore KN, Landrum LM, McMeekin DS. Do uterine risk factors or lymph node metastasis more significantly affect recurrence in patients with endometrioid adenocarcinoma. Gynecol Oncol. 2012;125:94–98. doi: 10.1016/j.ygyno.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Querleu D, Planchamp F, Narducci F, Morice P, Joly F, Genestie C, Haie-Meder C, Thomas L, Quénel-Tueux N, Daraï E, Dorangeon P-H, Marret H, Taïeb S, Mazeau-Woynar V, Institut National du Cancer. Societe Francaise d'Oncologie Gynecologique Clinical practice guidelines for the management of patients with endometrial cancer in France: recommendations of the Institut National du Cancer and the Société Française d'Oncologie Gynécologique. Int J Gynecol Cancer. 2011;21:945–950. doi: 10.1097/IGC.0b013e31821bd473. [DOI] [PubMed] [Google Scholar]
- Raimond E, Ballester M, Hudry D, Bendifallah S, Daraï E, Graesslin O, Coutant C. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: results of a retrospective multicenter study. Gynecol Oncol. 2014;133 (3:506–511. doi: 10.1016/j.ygyno.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA, Gynecologic Oncology Group Study Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- Reed NS, Mangioni C, Malmström H, Scarfone G, Poveda A, Pecorelli S, Tateo S, Franchi M, Jobsen JJ, Coens C, Teodorovic I, Vergote I, Vermorken JB, European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44:808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Sorbe B, Horvath G, Andersson H, Boman K, Lundgren C, Pettersson B. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma—a prospective randomized study. Int J Radiat Oncol Biol Phys. 2012;82:1249–1255. doi: 10.1016/j.ijrobp.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Sorbe B, Nordström B, Mäenpää J, Kuhelj J, Kuhelj D, Okkan S, Delaloye J-F, Frankendal B. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: a controlled randomized study. Int J Gynecol Cancer. 2009;19:873–878. doi: 10.1111/IGC.0b013e3181a6c9df. [DOI] [PubMed] [Google Scholar]
- Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, Kudo R, Japanese Gynecologic Oncology Group Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- Tsuruchi N, Kaku T, Kamura T, Tsukamoto N, Tsuneyoshi M, Akazawa K, Nakano H. The prognostic significance of lymphovascular space invasion in endometrial cancer when conventional hemotoxylin and eosin staining is compared to immunohistochemical staining. Gynecol Oncol. 1995;57:307–312. doi: 10.1006/gyno.1995.1148. [DOI] [PubMed] [Google Scholar]