Abstract
Background:
Follow-up care in breast cancer is still an issue of debate. Diagnostic methods are more sensitive, and more effective therapeutic options are now available. The risk of recurrence is not only influenced by tumour stage but also by the different molecular subtypes. This study was performed to evaluate the use of whole-body imaging combined with tumour marker monitoring for the early detection of asymptomatic metastatic breast cancer (MBC).
Methods:
This analysis was performed as part of a follow-up study evaluating 813 patients with a median follow-up of 63 months. After primary therapy, all patients underwent tumour marker monitoring for CEA, CA 15-3 and CA 125 at 6-week intervals within an intensified diagnostic aftercare algorithm. A reproducible previously defined increase was considered as a strong indicator of MBC. From 2007 to 2010, 44 patients with tumour marker increase underwent whole-body magnetic resonance imaging and/or an FDG-PET/CT scan. Histological clarification and/or imaging follow-up were done.
Results:
Metastases were detected in 65.9% (29/44) of patients, 13.6% (6/44) had secondary malignancies besides breast cancer and 20.5% (9/44) had no detectable malignancy. Limited disease was found in 24.1% (7/29) of patients. Median progression-free survival of MBC was 9.2 months and median overall survival was 41.1 months. The 3- and 5-year survival rates were 64.2% and 40.0%, respectively.
Conclusions:
A reproducible tumour marker increase followed by whole-body imaging is highly effective for early detection. By consequence, patients might benefit from earlier detection and improved therapeutic options with a prolonged survival.
Keywords: breast cancer, tumour marker increase, follow-up, computed tomography, positron emission tomography, whole-body imaging
Breast cancer is the most frequent malignancy and most common cause of cancer-related death in women worldwide (Parkin et al, 2005). Despite advances in the treatment of early breast cancer, approximately 20– 30% of patients will relapse with distant metastases (EBCTCG, 2005). Metastatic breast cancer is a heterogeneous disease with a variety of clinical presentations ranging from a single metastatic lesion to diffuse and multiple organ involvement. The risk of recurrence and the distinct patterns of metastatic spread are not only influenced by stage at initial presentation, but are also associated with the molecular subtype of the primary tumour (Kennecke et al, 2010).
In the last decade, the advances in chemotherapy, hormone therapy and HER-2-targeted therapy are gradually improving the survival of patients with metastatic breast cancer. However, according to literature, the median overall survival (OS) of all patients with metastatic breast cancer irrespective of their molecular subtypes is only 24 months and the primary goal of treatment is to prolong survival while maintaining a good quality of life (Cardoso et al, 2002; Dawood et al, 2008). Regarding the median OS in accordance to molecular subtypes, patients with luminal A tumours achieved the longest survival with 26–40 months compared with the other subtypes like luminal B (19–32 months), HER2-enriched (8–32 months) and triple-negative tumours (10–22 months) (Kennecke et al, 2010; Metzger-Filho et al, 2013; Seah et al, 2014). A minority of patients with metastatic breast cancer (5–10%) survives more than 5 years and 2–5% even obtain long-term survival (>10 years) (Iwata, 2012; Kobayashi et al, 2012). Long-term survivors are usually young with an excellent performance status and have only limited disease. Metastatic breast cancer with only a few metastatic lesions is classified in oligometastatic state. This group represents only 1–3% of patients (Hellman and Weichselbaum, 1995; Tait et al, 2005).
Therefore, early diagnosis of recurrent breast cancer is important to identify patients with limited disease who potentially could benefit from a more aggressive and multidisciplinary approach (Pagani et al, 2010).
The current surveillance guidelines for follow-up of breast cancer recommend regular mammography and physical examinations as well as symptom-orientated further investigations like laboratory tests and imaging (Khatcheressian et al, 2013). These guidelines by large are based on data from clinical trials performed in the early 90s, which did not show any survival benefit with the early detection of distant metastases (Ghezzi et al, 1994; Rosselli Del Turco et al, 1994). Unfortunately, this approach mainly comprised imaging modalities with known poor sensitivity (e.g., chest radiographs), examinations with examiner-dependent variation of sensitivity (e.g., abdominal ultrasound) or procedures with limited specificity, like bone scintigraphy, and did not include tumour marker investigations.
In follow-up, tumour markers like carcinoembryonal antigen (CEA) and cancer antigen (CA) 15-3 have been shown to detect 40–60% of breast cancer recurrences before clinical or radiological evidence of disease with a lead-time between 2 and 18 months. Simultaneous use of both serum markers allows the early diagnosis of metastasis in up to 60–80% of patients with breast cancer (Nicolini et al, 1991; Molina et al, 1999, 2005). Cancer antigen 125 is also a biomarker with sensitivity in breast cancer (Ertl et al, 2009). Mainly owing to the lack of knowledge, experience and confidence in tumour markers, the current recommendation is to observe tumour marker kinetics rather than use them as an indicator to perform imaging.
Advances in radiological examination techniques allow the very early detection of distant metastases. Whole-body imaging modalities, such as fluorodeoxyglucose positron emission tomography (FDG-PET)-computed tomography (CT) or whole-body magnetic resonance imaging (WB-MRI) appear as new promising tools to detect tumour recurrence with high accuracy in its initial stage and to provide more effective therapeutic strategies to the patient. It has been reported that FDG-PET/CT is of clinical value when searching for breast cancer metastases, especially when suggested by the presence of clinical symptoms or by a progressive increase in biochemical markers (Suarez et al, 2002; Gallowitsch et al, 2003; Murakami et al, 2012). MRI, with its lack of ionising radiation, high soft tissue contrast and spatial resolution is a useful application for tumour detection and staging of malignant disease. A high sensitivity has been reported for the detection of organ metastases, especially for tumours frequently metastasising to the liver, bone or the brain, like breast cancer (Engelhard et al, 2004; Lauenstein et al, 2004).
The purpose of the present study was to assess the use of whole-body imaging using FDG-PET/CT and WB-MRI for the early detection of asymptomatic tumour recurrence in breast cancer patients with defined tumour marker increase. In contrast to other previous studies, only truly asymptomatic patients with tumour marker increase from individual baseline value were included into this trial.
Patients and methods
Patients
From 1998 to December 2010, a prospective follow-up study was performed in the Institute of Clinical Chemistry of the University Hospital Klinikum Grosshadern, Munich. Approval of the institutional review board and written patient consent were obtained. In this follow-up study, 813 patients were included, and they underwent regular laboratory tests of tumour markers (CEA, CA 15-3 and CA 125) in 6-week intervals in addition to standard follow-up care, with periodic visits for history and physical examinations and regular mammograms. All patients entered into the study after the end of adjuvant therapy, which included chemotherapy and radiotherapy, and were primarily treated with a curative approach. Patients with a history of metastatic disease (lymph node and organ metastases) and/or patients under palliative treatment were not incorporated into this aftercare algorithm.
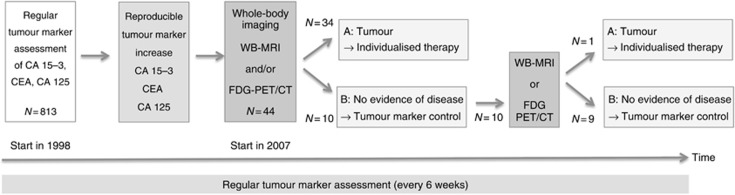
We determined a reproducible defined increase as an indicator for recurrent disease, and imaging was performed. Initially, the modality and the time point of imaging were determined by the discretion of the attending physicians. From May 2007 to December 2010, patients with a reproducible defined increase of at least one tumour marker underwent whole-body imaging with whole-body MRI and FDG-PET/CT. Figure 1 shows an overview of the study design.
Figure 1.
Study design for an intensified aftercare algorithm for breast cancer patients using tumour marker monitoring combined with whole-body imaging. Since 2007, 44 patients met inclusion criteria. Twenty-eight patients showed tumour recurrence at initial exam, 6 had a secondary malignancy besides breast cancer and 10 patients showed no malignancies. In one patient, a liver metastasis was detected 6 months later in follow-up. In 2 patients with no evidence of disease, no follow-up could be acquired.
In the present evaluation, 44 asymptomatic female patients with defined tumour marker increase were included. Median age at the time of primary diagnosis of breast cancer was 53 years (range 28–76 years). Tumour size using the UICC classification was mostly T2 in 24 (54.6%) and T1 in 12 (27.3%) patients. Of the patients, 65.9% had positive lymph nodes. Immunohistochemistry assays showed overexpression of HER2/neu in 7 (15.9%) patients and hormone receptors in 33 (75.0%) patients including positive for oestrogen receptor and/ or positive for progesterone receptor.
All patients had no previous history of metastatic tumour recurrence, nine patients had a previous history of curatively treated local tumour recurrence or contralateral tumour. Breast cancer subtypes were defined as follows: luminal A (oestrogen receptor (ER)-positive and progesterone receptor (PR)-positive and HER2-negative), luminal B (ER-positive and/or PR-neagtive and/or HER2-positive), HER2-enriched (ER-negative and PR-negative and HER2-positive), and triple-negative breast cancer (ER-negative, PR-negative, HER2-negative). The histopathological information was retrospectively obtained by reviewing medical records. Thus, data about Ki-67, EGFR or CK5/6 were not available. Data of one patient were completely missing.
Detailed information about patient characteristics is shown in Table 1.
Table 1. Clinical characteristics of 44 patients.
| All (N=44) | M1 (N=29) | |
|---|---|---|
| Age at primary diagnosis, median (range), years | 53 (28–76) | 54 (30–76) |
|
Tumour size, n (%) | ||
| T0 | 1 (2.3) | 1 (3.5) |
| T1 | 12 (27.3) | 7 (24.1) |
| T2 | 24 (54.6) | 18 (62.1) |
| T3 | 2 (4.6) | 2 (6.9) |
| T4 | 1 (2.3) | 0 (0.0) |
| Unknown | 4 (9.1) | 1 (3.5) |
|
Axillary lymph nodes, n (%) | ||
| N− | 14 (31.8) | 5 (17.2) |
| N+ | 29 (65.9) | 24 (72.8) |
| Unknown | 1 (2.3) | 0 (0.0) |
|
Grading, n (%) | ||
| G1 | 4 (9.1) | 2 (6.9) |
| G2 | 18 (40.9) | 13 (44.8) |
| G3 | 18 (40.9) | 13 (44.8) |
| Unknown | 4 (9.1) | 1 (3.5) |
|
Hormone receptors, n (%) | ||
| ER- and/ or PR-positive | 33 (75.0) | 22 (75.9) |
| Both negative | 11 (25.0) | 7 (24.1) |
| Unknown | 0 (0.0) | 2 (6.4) |
|
HER2/neu-status, n (%) | ||
| Negative (0/1+ or 2+/FISH−) | 31 (70.5) | 23 (79.3) |
| Positive (3+ or 2+/FISH+) | 7 (15.9) | 5 (17.2) |
| Unknown | 6 (13.6) | 1 (3.5) |
|
Breast cancer subtype, n (%) | ||
| Luminal A | 21 (47.7) | 14 (48.3) |
| Luminal B | 9 (20.5) | 7 (24.1) |
| HER2-enriched | 5 (11.4) | 3 (10.3) |
| Triple negative | 4 (9.1) | 4 (13.8) |
| Unknown | 5 (11.4) | 1 (3.5) |
| Local recurrence/contralateral tumour before study entry, n (%) | 9 (20.5) | 7 (24.1) |
|
Neo−/adjuvant treatment, n (%) | ||
| Chemotherapy | 33 (75.0) | 25 (86.2) |
| − Anthracycline +/− Taxane | 30 (68.2) | 23 (79.3) |
| Hormonal therapy | 33 (75.0) | 21 (72.4) |
| Irradiation | 34 (77.3) | 24 (82.8) |
| None | 1 (2.3) | 0 (0.0) |
Abbreviations: ER=oestrogen receptor; PR=progesterone receptor.
All these patients underwent whole-body imaging with either both FDG-PET/CT and whole-body MRI (N=39), only whole-body MRI (N=4) or only with FDG-PET/CT (N=1). The median time from early breast cancer to first tumour marker increase was 75.2 months ranging from 13 to 276 months. The median follow-up after tumour marker increase was 46.6 months.
Tumour marker monitoring
Carcinoembryonal antigen was quantified using a microparticle immunoenzymometric assay (Abbott Laboratories, Chicago, IL, USA) on the AxSYM system. The serum levels of CA 15-3 and of CA 125 were determined by an electrochemiluminescent immunoenzymometric assay (Roche Diagnostics, Mannheim, Germany) on the Elecsys system.
Patients were screened for CEA, CA 15-3 and CA 125 after curative treatment of primary tumour or local recurrence, and individual baseline values were determined. The individual baseline value was defined as the mean value of the first three tumour marker measurements, measured at least 4 weeks after the end of adjuvant irradiation and/or chemotherapy, and every 6 weeks thereafter. In 2 of the 44 patients, only two measurements were used for calculating the baseline value because one marker began to rise. On the basis of these values, follow-ups were performed in 6-week intervals. A reproducible, previously defined increase (CEA 100%, CA 15-3 75%, CA 125 150%) of single or combined markers compared with the baseline value was considered as a strong indicator of recurrent disease. A substantial marker increase was confirmed by a second measurement within 4 weeks before initiating whole-body imaging procedures (Figure 1). A singular, irreproducible marker increase was considered insufficient for whole-body imaging initiation.
Whole-body imaging with WB-MRI and/or FDG-PET/CT
If a reproducible tumour marker increase was observed, whole-body imaging with WB-MRI and/ or FDG-PET/CT within an average of 5.6 days (median 2.5, range 0–23) was done.
Whole-body magnetic resonance imaging was performed on a 1.5 Tesla whole-body scanner (Magnetom Avanto, Siemens Healthcare, Erlangen/Germany) using matrix coil technology with 76 combinable coil elements and 32 receiver channels. After a single positioning, the system allows whole-body imaging with a total field of view of 205 cm. Coils used were head-, neck-, body-, customised extremity and integrated spine coil. The patients were imaged from head to the proximal calves with STIR- and T1-weighted sequences at four body levels in coronal orientation. The lung was examined with HASTE- and STIR-sequences, followed by HASTE of the abdomen and a free breathing T2w-fat saturated-TSE scan of the liver. Then, imaging of the complete spine with T1-weighted-TSE and STIR sequences was performed. After application of Gadolinium-DTPA (0.1 mmol per kg body mass, Magnevist®, Bayer Schering Pharma AG, Berlin/Germany), a dynamic axial 3D-VIBE liver scan was performed including a late venous scan of the breast/lung level. Then, a fat-saturated T1w-GRE-sequence of the abdomen as well as T1w- and T2w-TSE imaging of the brain were carried out.
Positron emission tomography/computed tomography examinations were performed on a 64-detector row PET/CT-scanner (Siemens Biograph 64, Siemens Healthcare) after injection of an average of 400 MBq [18F]-fluoro-2-deoxy-D-glucose. Patients were asked to fast for at least 6 h before examination to assure blood glucose levels were below 150 mg dl−1. Twenty milligrams of Butylscopolamine (BS Inj. Carino, Carinopharm GmbH, Elze, Germany) were given intravenously to avoid a first-pass uptake of FDG into smooth muscle. Additionally, 20 mg of Furosemide were given to increase renal excretion of the tracer. After the PET emission scan (5-6 bed positions, FOV 11cm, 144 × 144 matrix), a diagnostic contrast-enhanced CT from skull base to the pelvis was conducted (120 mAs, 160 kV, collimation 2 × 5 mm, pitch 1) with application of 120 ml of i.v.-contrast agent (Ultravist 300, Bayer Schering Pharma AG, Berlin/Germany) in venous phase (80 s delay).
Examination time for FDG-PET/CT was 83 min (60 min patient preparation, scan time 23 min). Total scan time for WB-MRI at 1.5 Tesla was 52 min, mean in room time was 60 min. Both FDG-PET/CT and WB-MRI were well tolerated by all patients.
Radiological data analysis
Imaging evaluation was performed by a consensus panel of one radiologist with more than 10 years of experience for MRI and another radiologist/nuclear medicine physician with 9 years of experience for PET/CT. Both were fully blinded to the other modality without information on previous or current diagnostic imaging results. Established region-specific size criteria were applied to determine tumour involvement when assessing lymph nodes (Glazer et al, 1985; Dorfman et al, 1991; Som et al, 2000; Suzuma et al, 2001). Criteria indicating lesion malignancy in both modalities were defined: aggressive expansion, infiltration of neighbouring anatomical structures or signs of necrosis. In MRI, additionally, established sequence-specific signal changes and classic abnormal static (CT) or dynamic (MRI) contrast uptake characteristics were assessed (Vanel et al, 1998; Danet et al, 2003). In addition, malignancy in PET/CT was assessed by a focally increased glucose uptake using the maximum ‘standard uptake value' (SUVmax) as a reference (Boellaard et al, 2010). A progressive change in size/number of a lesion within the follow-up period or an increase of pathological tracer uptake was considered as criteria indicating malignancy (Wahl et al, 2009). Response of therapy was assessed by standard WHO criteria (WHO, 1979).
The anatomical distribution of observed malignant lesions and their number by organ was recorded.
Limited disease was defined as metastatic disease confined to a single organ with at most three lesions as maximum cut-off.
Verification of initially observed findings was done by biopsy (N=9), or histology after surgical intervention (n=6), or follow-up examinations (n=27) within 6 months. Depending on their nature and anatomical location, patients underwent WB-MRI (n=11), FDG-PET/CT (n=5), CT scan (n=8) and dedicated MRI (n=3) for follow-up. In two patients with no evidence of disease, no follow-up could be done, as patients did not give consent for the follow-up examination. Mean time interval between initial imaging findings and confirmation was 4.1 months.
Statistical analysis
Data were analysed using the statistical package SAS (V 9.2, SAS Inc., Cary, NC, USA). A P-value of <0.05 was considered to indicate statistical significance. Relapse-free survival (RFS) was defined as the duration from primary diagnosis of breast cancer to detection of distant metastasis. Progression-free survival was defined as the duration from initiation of treatment for metastatic disease to the point when disease progression was detected. Overall survival was defined as the duration from first distant metastasis to last visit or death. Survival curves and 3- and 5-year survival rates were estimated by the Kaplan–Meier method, and analysed using the log-rank test. Relapse-free survival and OS were regarded in relation to breast cancer subtypes.
Results
Tumour marker values at baseline and time of increase
Table 2 gives an overview about the baseline values for CEA, CA 15-3 and CA 125 for all patients. The medians were similar to healthy individuals and 10 patients had a value above the reference range (1 for CEA (>3 ng ml−1), 6 for CA 15-3 (> 28 U ml−1), 2 for CA 125 (>35 U ml−1), and 1 patient for both CA 15-3 and CA 125). All patients (N=44) showed a reproducible marker increase of at least one tumour marker. Carcinoembryonal antigen was the most frequently increased single tumour marker (18/44; 40.9%), followed by CA 15-3 in 34.1% (15/44) of cases and in 18.2% (8/44) of patients for CA 125. In three patients, a combination of two tumour markers were significantly elevated (two patients with CA 15-3 and CA 125 and one patient with CA 15-3 and CEA). In 11 patients, the increase was completely within the reference ranges: 6 for CEA (<3 ng ml−1), 3 for CA 15-3 (<28 U ml−1) and 2 for CA 125 (<35 U ml−1). Of these, nine patients showed malignancies. For one patient with secondary malignancy, CEA increased within the reference range while CA 15-3 started within and exceeded reference range at time of increase. In 27 patients, the baseline values were within the reference ranges and by increase, they exceeded the reference ranges (11 for CEA, 9 for CA 15-3, 5 for CA 125 and 2 for both CA15-3 and CA 125). Five patients had baseline values above the reference range and also showed an increase.
Table 2. Tumour marker values at baseline and time of increase.
| Parameter | Median | Range | 95th percentile | Above the reference range (N) |
|---|---|---|---|---|
|
Baseline values (N=44) | ||||
| CEA (ng ml−1) | 1.3 | 1.0–5.4 | 2.9 | 1 |
| CA 15-3 (U ml−1) | 19.5 | 5.9–50.3 | 33.4 | 7 |
| CA 125 (U ml−1) | 11.7 | 2.9–90.8 | 60.9 | 3 |
|
Values at time of increase | ||||
| CEA (ng ml−1) (N=19) | 3.1 | 2.1–11.1 | 11.1 | 12 |
| CA 15-3 (U ml−1) (N=18) | 41.7 | 13.2–90.0 | 90.0 | 15 |
| CA 125 (U ml−1) (N=10) | 51.7 | 25.5–189.0 | 189.0 | 8 |
Abbreviations: CA=cancer antigen; CEA=carcinoembryonal antigen.
Reproducible increases: 100% for CEA, 75% for CA 15-3 and 150% for CA 125.
Reference ranges: CEA<3 ng ml−1, CA 15-3<28 U ml−1 and CA 125<35 U ml−1.
Findings with WB-MRI and/or FDG-PET/CT at the time of tumour marker increase
Whole-body magnetic resonance imaging and/or FDG-PET/CT detected malignancies in 77.3% (34/44) of patients, as confirmed by pathological examination (N=15) and radiological follow-up (N=27). In 63.7% (28/44) of cases, metastatic lesions were found (Figure 2A shows an example). In 13.6% (6/44) of patients, secondary malignancies besides breast cancer were detected: two ovarian (Figure 2B), one uterine, one gastric, one lung, one parotid cancer and one multiple myeloma (Table 3). One patient had both local recurrence and a secondary tumour. Of the patients, 22.7% (10/44) had no detectable malignancy whether in WB-MRI or in FDG-PET/CT at the time of initial examination. One of these patients was shown to have liver metastases in follow-up imaging after 6 months (Figure 2C). Thus, 29 of 44 patients suffered from metastatic disease.
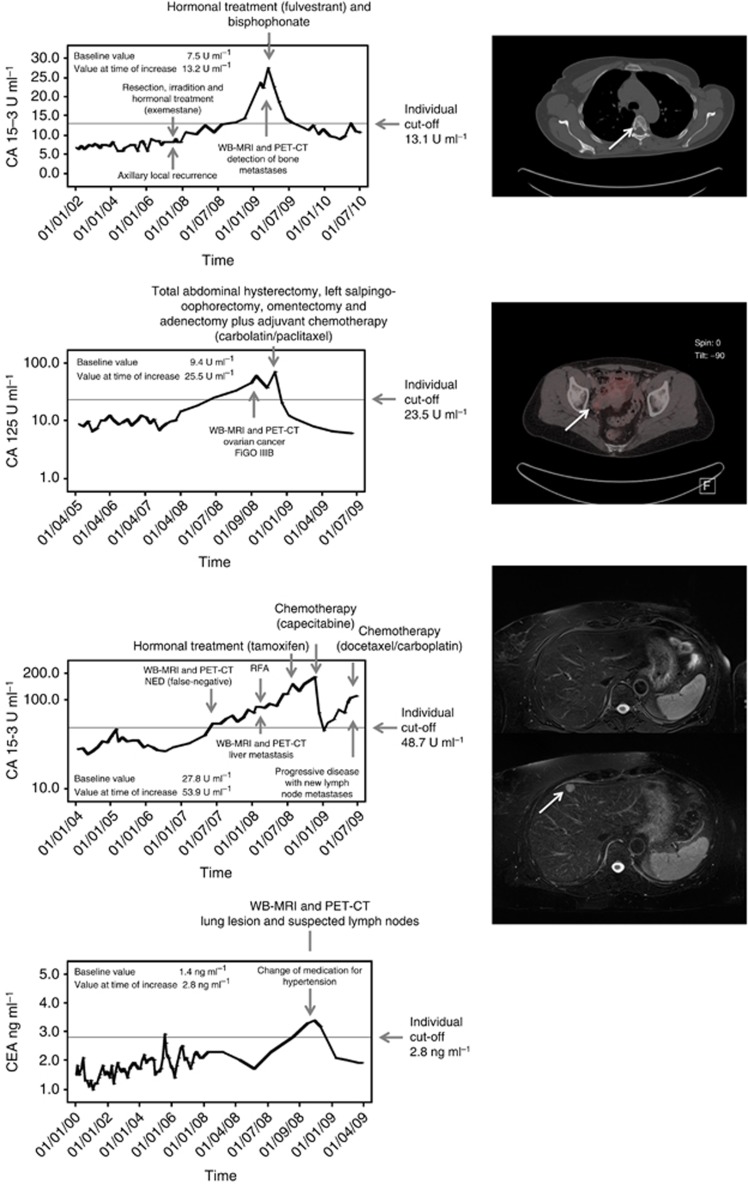
Figure 2.
Biochemical course of tumour markers and findings by whole-body imaging. (A) A 47-year-old patient with history of breast cancer (1998) and axillary local recurrence in June 2007. Metastases to the bone were detected in February 2009. At time of tumour marker increase, CT scan shows multiple bone metastases. One osteolytic metastasis is shown here at the fifth thoracic vertebral body. (B) A 53-year-old patient with history of breast cancer (2003) and primary diagnosis of ovarian cancer in September 2008. Fluorodeoxyglucose positron emission tomography/CT showed an increased uptake in the right ovary region susceptive of ovarian cancer. The patient was treated by resection and chemotherapy. The ovarian cancer was pathologically confirmed. (C) A 58-year-old patient with history of breast cancer (2001) and a tumour marker increase in July 2007, but no detectable malignancy whether in WB-MRI nor in FDG-PET/CT at the time of initial examination; liver metastasis were finally detected in follow-up imaging after 6 months. Whole-body-MRI at initial tumour marker increase did not show any morphologic suspected lesion in the whole body. After 6 months (control MRI), the axial T2-w fat saturated sequence shows a new focal metastatic lesion in segment 4a. Computed tomography-guided biopsy confirmed metastasis. (D) An 81-year-old patient with history of breast cancer (January 1995) and tumour marker increase in October 2009. In whole-body imaging, no correlate could be found. But, at time of tumour marker increase, the patient changed her medication for hypertension. After a while, CEA levels decreased to the individual baseline.
Table 3. Distribution of findings by imaging at the time of tumour marker increase.
| Patients | |
|---|---|
| Detected lesions, N (%) | N=44 (100) |
| Distant metastasesa | 29 (65.9) |
| Local recurrenceb | 1 (2.3) |
| Secondary malignancies | 6 (13.6) |
| Benign lesions | 9 (20.5) |
| Metastatic sites, N (%) | N=29 (100) |
| Bone | 18 (62.1) |
| Liver | 8 (27.6) |
| Lung | 7 (24.1) |
| Lymph node | 12 (41.4) |
| Others | 11 (37.9) |
| Visceral diseasec | 12 (41.4) |
| Only bone lesions | 10 (34.5) |
| Numbers of metastatic sites, N (%) | N=29 (100) |
| 1 organ | 14 (48.3) |
| 2 organs | 7 (24.1) |
| >2 organs | 8 (27.6) |
| Limited diseased | 7 (24.1) |
| Diffuse metastatic disease | 20 (69.0) |
| Secondary malignancies, N (%) | N=6 (100) |
| Ovarian cancere | 2 (33.3) |
| Uterine cancer | 1 (16.7) |
| Gastric cancer | 1 (16.7) |
| Non-small cell lung cancer | 1 (16.7) |
| Multiple myeloma | 1 (16.7) |
| Carcinoma of the parotid gland | 1 (16.7) |
| Benign lesions, N (%) | N=9 (100) |
| Benign lesion of the lung | 1 (11.1) |
| Benign lymph node | 1 (11.1) |
| Breast implant rupture | 2 (22.2) |
| Pancreatitis | 1 (11.1) |
| Cyst of the ovarian | 1 (11.1) |
| Diarrhoea | 1 (11.1) |
| No evidence of disease | 2 (22.2) |
The liver metastases of one patient were detected 6 months later in follow-up imaging.
One patient had both local recurrence and a secondary malignancy.
Involvement of the lung and/or liver.
Less than four malignant lesions to a single organ.
One patient had both an primary cancer of the uterine and an ovarian cancer.
The anatomical distribution of metastatic disease showed lymph node metastases in 41.4% (12/29), bone metastases in 62.1% (18/29), lung metastases in 24.1% (7/29) and liver metastases in 27.6% (8/29) of the patients. In 37.9% (11/29), metastases were found in further localisations, such as the adrenal glands, pleura, peritoneum and brain (Table 3).
Limited metastatic disease, which was defined as at most three lesions confined to a single organ, was found in 24.1% (7/29) of patients. Overall, 48.3% (14/29) of patients had metastases spread to a single organ only. Multifocal metastatic disease affected 51.7% of patients (15/29) with the following distribution: in 24.1% (7/29) of patients, metastases were confined to two, in 13.8% (4/29) to three, in 6.9% (2/29) to four, in 3.4% (1/29) to five and 3.4% (1/29) to six different organs.
In 20.5% (9/44) patients, no malignancies could be detected. Two patients showed a breast implant rupture (one patient with increase of CA 15-3, one patient with increase of CA 125), one patient had a benign lesion of the lung with hilar suspected lymph nodes (CEA), one patient had a cyst of the ovarian (CA 125) and another one had benign lymph node (CEA). In one case, there was a pancreatitis detected (CA 125) and another patient suffered from diarrhoea (CEA). In two cases, evidence of disease could not be found, but one of them had a change in medication for hypertension, and 4 weeks later, the CEA value normalised to baseline (Figure 2D).
Treatments after detecting tumour recurrence or secondary malignancy besides breast cancer
Patients with secondary malignancies were treated by resection and/or chemotherapy with curative approach (N=3). The patients with gastric cancer, ovarian plus uterine cancer and multiple myeloma underwent palliative treatment. In the group of patients with limited metastatic disease, five patients underwent a loco-regional therapy like radiofrequency ablation (N=1), cyber knife (N=1) and/or irradiation (N=4) in combination with systemic treatment. Of the patients, 44.8% (13/29) could be treated by endocrine therapy like an aromatase inhibitor (N=7) or an antioestrogen (N=6); 48.3% (14/29) had to be treated by chemotherapy (monochemotherapy (N=3), polychemotherapy (N=2) or with a combination of chemotherapy with targeted therapies like trastuzumab (N=4), lapatinib (N=2) or bevacizumab (N=3).
Relapse-free survival and OS
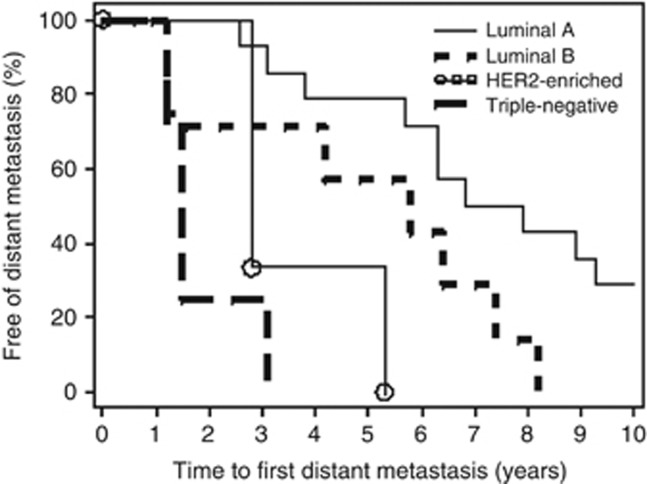
Only patients with metastatic breast cancer (N=29) were included in this evaluation. Breast cancer subtypes based on IHC or FISH findings were known for 28 patients. Among these, 50.0% were luminal A, 25.0% were luminal B, 10.7% were HER2-enriched and 14.3% were triple-negative breast cancer (TNBC). These subtypes showed distinct differences in the times from primary diagnosis to detection of distant metastasis (RFS). As shown in Figure 3, the survival curves for RFS differed significantly, with luminal A patients achieving the longest RFS (median 88.4 months), followed by luminal B (69.4 months), HER2-enriched (34.0 months) and TNBC (17.5 months; P<0.0001).
Figure 3.
Relapse-free survival curves are presented by breast cancer subtype (N=28).
Median progression-free survival and OS from time of first distant metastasis of all patients irrespective of breast cancer subtype (N=29) were 9.2 months and 41.1 months, respectively. The survival rates at 1, 2, 3 and 5 years were as follows: 86.2%, 79.3%, 64.2% and 40.0%.
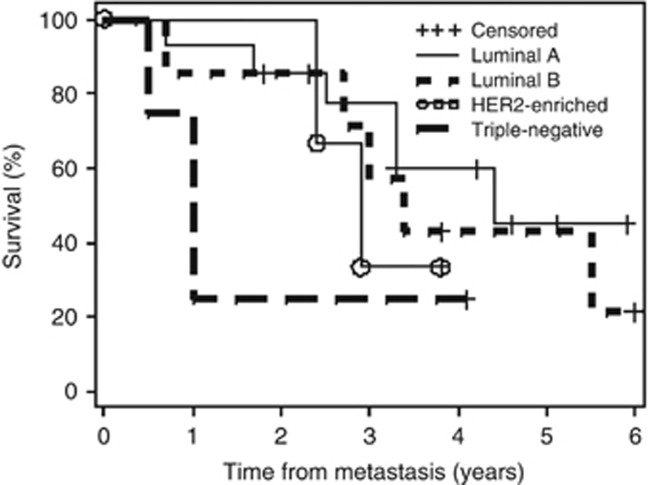
Median OS according to subtype also differed with the longest survival for luminal A patients (52.4 months), followed by luminal B (41.1 months), HER2-enriched (34.3 months) and TNBC (12.2 months; Figure 4). These differences were statistically not significant.
Figure 4.
Overall survival from time of first distant metastasis according to breast cancer subtype.
Comparing OS in accordance to extent of metastatic disease the 3- and 5-year survival rates of patients with limited disease (⩽3 metastases to a single organ) were 71.4% and 53.6%, respectively. They were higher compared with the 3- (56.7%) and 5-year survival rates (34.8%) of patients with disseminated metastatic disease, but this difference was not significant.
Discussion
The current surveillance guidelines for follow-up of breast cancer recommend only regular mammography and physical examinations as basic follow-up. Only in symptomatic patients, further investigations like laboratory tests and imaging are recommended (Khatcheressian et al, 2013). This strategy has the reason in studies from the 90s where intensified aftercare did not lead to a prolonged survival of breast cancer patients (1994; Rosselli Del Turco et al, 1994). However, at this time, no tumour markers and only relatively insensitive imaging techniques have been used, such as X-ray of the thorax, scintigraphy and ultrasound of the abdomen. It is well known from the literature that new techniques such as whole-body MRI as well as FDG-PET/CT can lead to a much higher diagnostic accuracy for the detection of primary and secondary tumours (Suarez et al, 2002; Gallowitsch et al, 2003; Murakami et al, 2012). In addition, molecular subtypes of breast cancers have been identified with different OS rates and risk for developing metastasis (Kennecke et al, 2010; Metzger-Filho et al, 2013; Minicozzi et al, 2013). Furthermore, new loco-regional therapy regimes, such as radiofrequency ablation, SIRT of the liver as well as systemic new therapies showed a significant impact on disease progression and OS (Livraghi et al, 2001; Andre et al, 2004; Mack et al, 2004; Baselga et al, 2012a, 2012b; Berghoff et al, 2013). Thus, our rationale was to test the most sensitive laboratory tests, that is, tumour marker increase from individual baseline values as well as high-resolution whole-body imaging with MRI and FDG-PET/CT for the detection of early metastasis in primarily asymptomatic patients at an early stage.
We examined a special cohort of patients who were primarily not metastasised and asymptomatic in a regular tumour marker follow-up. Forty-four consecutive breast cancer patients showed a defined increase of at least one of the three tumour markers CEA, CA 15-3 and CA 125 compared with individual baseline values. Most studies that deal with the role of tumour markers in follow-up care use fixed, but inconsistent cut-off values to evaluate the sensitivity and specificity of a tumour marker. This is problematic, as low cut-off values lead to many false-positive results (low specificity) and high cut-off points constrain sensitivity (Molina et al, 1995). Therefore, the use of cut-off values limits inevitably the sensitivity of a tumour marker. A tumour marker increase is not detected when the increase is still below the reference range. In addition, baseline values can differ significantly among patients. Therefore, baseline values give important information for the interpretation of tumour marker increase. In our cohort, 11 patients showed an increase completely within the reference range: 6 with CEA (<3 ng ml−1), 3 with CA 15-3 (<28 U ml−1) and 2 patients with CA 125 (<35 U ml−1). However, nine of these patients showed malignancies.
In whole-body imaging, a significant number of patients (63.3%) with tumour marker increase showed metastases. In our cohort, bone (62.1%) was the most frequent anatomic site, followed by liver (27.6%) and lung metastases (24.1%). Previously unknown brain metastases were shown in two patients in whole-body MRI but not in FDG-PET/CT. Owing to the high FDG uptake of the CNS in FDG-PET/CT, cerebral pathologies are usually missed. One patient developed a liver metastasis in the further course, which was not seen at the time point of tumour marker increase in WB-MRI as well as in FDG-PET/CT.
There are two other studies dealing with tumour marker increase and FDG-PET/CT. Radan et al (2006) examined 46 women with a history of breast cancer and elevated tumour markers with FDG-PET/CT for follow-up. They reported a similarly high tumour recurrence in 65% of patients and an accuracy of 81% for FDG-PET/CT. However, methodological questions arise as the exact tumour marker inclusion criteria in this study remain unclear and obviously a population including previously metastasised patients was retrospectively analysed. Another report describes an even better performance for PET/CT with a sensitivity of 98% (Piperkova et al, 2007). Yet again, patient history was heterogeneous, consisting of women referred for staging, restaging as well as evaluation of treatment response. This fact limits comparison of those results with our patient cohort, which is unique in the literature.
Ten (22.7%) of our patients with a reproducible tumour marker increase showed no morphologically detectable malignant tumour in imaging. Only one of these patients exhibited visible liver metastases in follow-up imaging after 6 months. In this case, biochemical tumour detection obviously preceded morphological tumour manifestation as described in literature (Nicolini et al, 1991; Molina et al, 1999, 2005). The remaining nine patients did not suffer from tumour recurrence or other malignancies neither in the first examination nor in the follow-up examination after 6 months. Except for one patient, in all other patients, a reason for the tumour marker increase could be identified. Six patients showed a morphological correlate of benign findings, one had diarrhoea and another patient had a change in her hypertension medication. None of the patients had a renal dysfunction nor started off with smoking. The follow-up period of these patients was in median 20.4 months, ranging from 5.2 to 42.8 months. In eight patients, the marker levels declined to baseline, but in three of them, the values fluctuated for the rest of the observation period.
It is known that non-malignant, inflammatory and malignant diseases other than breast might release CA 15-3, CEA and CA 125 (Stieber et al, 2003, 2005). Elevated plasma levels are also associated with liver, biliary and renal function (Ruibal Morell, 1992). In one patient, medication for hypertension and anticoagulation was changed which means that the metabolism of CEA concentration could be influenced for a while. In contrast to our results, Murakami et al (2012) reported in their study that 47% (22/47) of patients had no evidence of disease in PET/CT scan. Indication for imaging was elevated tumour markers and/or suspicious findings on conventional morphological imaging modality studies. But, in this study too, the exact tumour marker inclusion criterion remains unclear. These findings point out that it is of high interest to choose the right way of tumour marker assessment and interpretation to eliminate false-positive results and to finally avoid uncertainty of the affected patient.
Another important finding of our study was the relative high amount of secondary malignancies (13.6%, n=6) besides breast cancer. These were detected by tumour marker increase and localised by whole-body imaging. There were two ovarian, one uterine, one gastric, one lung and one parotid cancer, and one multiple myeloma (Table 3). Three out of six patients were diagnosed at an early stage and could be cured by resection followed by chemotherapy.
One important rationale to combine biochemical tumour marker monitoring with comprehensive imaging procedures was to identify metastatic tumour spread in its very early stage, long before the patient becomes symptomatic and therefore potentially benefits from improved therapeutic options. Nicolini et al (2003) could show that a ‘tumour marker guided' salvage treatment can delay disease progression of relapsing breast cancer patients responsive to treatment. They started a study where the survival of relapsed patients treated at the time of elevated serum markers (CEA, TPA and/or CA 15-3) and negative findings was compared with that of relapsed patients treated conventionally at the time of definite positive radiological and/or clinical findings. In fact, the 3-year survival rate was significantly higher in the group with ‘tumour marker guided' treatment compared with the group treated conventionally (27.8% vs 9.4%).
Although metastatic breast cancer is a systemic disease in most patients, there is a recognised smaller subgroup of patients with limited disease and potentially resectable metastases. Limited disease, defined in our cohort as at most three metastases confined to a single organ, was present in a substantial proportion of our surveillance cohort (24.1%). After verification of disease and stabilisation by systemic therapy, loco-regional treatment could be applied. Furthermore, new therapeutic approaches are introduced for patients with non-resectable limited disease, such as radiofrequency ablation or laser-induced thermotherapy, including reports on 3- and 5-year survival rates of 63% and 41%, respectively (Mack et al, 2004).
In our cohort, seven patients had limited disease. Five patients underwent loco-regional therapies in combination with systemic treatment; the remaining two patients were primarily only treated with endocrine therapies. The 3- and 5-year survival rates of patients with limited disease (⩽ 3 metastases to a single organ) in our cohort were 71.4% and 53.6%, respectively. They were higher when compared with the 3- (56.7%) and 5-year survival rates (34.8%) of patients with disseminated metastatic disease, but this difference was not significant. This could be explained by the fact that there was only a very small proportion of patients with limited disease (n=7) and a small number of events. Carlini et al (2002) obtained a 5-year survival rate of almost 46% in breast cancer after liver resection. Kim et al (2014) recently referred from 1- and 3-year OS rates of patients after surgery of isolated liver metastasis of 83.3% and 66.7%. The overall 3- and 5-year survival rates of all metastatic breast cancer patients in our cohort were 64.2% and 40.0%, respectively. Thus, the survival rates of our patient group were similar to the patients treated with loco-regional therapies as reported by Carlini et al (2002) and Mack et al (2004).
Regarding the median OS after distant metastases in accordance to molecular subtypes, the longest survival in our cohort were patients with luminal A tumours with 4.4 years, followed by luminal B with 3.4 years, HER2-enriched with 2.9 years. The shortest median OS of 1.0 year had patients with TNBC. In accordance to literature, Kennecke et al (2010) also described a different median duration of survival from time of first distant metastasis according to subtypes. In their analysis, patients with luminal A tumours also achieved the longest survival with 2.2 years followed by luminal B (1.6 years), HER2-enriched (0.7 years) and TNBC patients (0.9 years; P<0.001). However, several more recent studies demonstrated that patients with luminal A tumours achieved the longest survival compared with other subtypes like TNBC (Metzger-Filho et al, 2013; Seah et al, 2014).
By interpreting our good survival data in metastatic breast cancer, it has to be considered that besides the large proportion of luminal A tumours in our cohort (50%, n=14), a very long median RFS of 69.4 months could be observed. In general, patients with a long disease-free survival have a better prognosis than patients with a short disease-free survival (Clark et al, 1987). Patients with luminal A tumours had the longest RFS with a median of 88.4 months and patients with TNBC had the shortest with only 17.5 months. Kennecke et al (2010) found similar results with distinct differences in the timing of relapse, whereas all relapses occurred within the first 5 years among TNBC and HER2-enriched breast cancer types. Luminal A subtypes experienced continued relapses between 5 and 15 years. Minicozzi et al (2013) also demonstrated that patients with luminal A tumours had the longest disease-free survival and they could show that cancer subtype was an independent prognostic factor for relative and disease-free survival.
One limitation is the lack of a histological proof as a true reference standard for some of the detected lesions. With a reference standard based on imaging, false-negatives may arise in small or slowly growing lesions in the absence of substantial morphological changes. On the other hand, comparable with numerous studies of similar design, obtaining multiple biopsies for tissue verification would have been impracticable and ethically unacceptable (Dirisamer et al, 2010; Murakami et al, 2012). Another limitation of our study is that our patient cohort represents mostly a favourable group with luminal A tumours in 50% and a long RFS over 60 months which could also have a major impact on the good survival data apart from the early detection of metastatic disease in our asymptomatic patients.
Furthermore, our sample size was relatively small, resulting from the defined clinical focus in our patient cohort. Finally, our study was not a randomised trial that compared our patient cohort with controls undergoing standard follow-up care.
Conclusion
Summarising our findings, a reproducible tumour marker increase based on individual baseline values followed by whole-body imaging is highly effective for early detection and localisation of tumour recurrence in clinically asymptomatic breast cancer patients. We could also show that patients (except patients with TNBC) developed distant metastases more than 60 months after primary diagnosis, which probably could result in continuing follow-up care beyond 5 years in a more intensified way. Whether patients might benefit from earlier and more accurate tumour detection and improved therapeutic options with a prolonged survival has to be investigated in further studies. Therefore, a large prospective randomised trial will be needed to draw any firm conclusions.
The authors have declared no conflicts of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- WHO 1979. WHO handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization Offset Publication No. 48.
- Ghezzi P, Magnanini S, Rinaldini M, Berardi F, Di Biagio G, Testare F, Tavoni N, Schittulli F, D'Amico C, Pedicini T, Fumagalli M, Gritti G, Braga M, Marini G, Zaniboni A, Cosentino D, Epifani C, Gini G, Perroni D, Peradotto D, Indelli M, Santini A, Isa L, Aitini E, Cavazzini G, Smerieri F, Nascimben O, Busolin R, Papaccio G, Locatelli E, Monti M, Ghislandi E, Gottardi O, Majno M, Pluchinotta A, Armaroli L, Confalonieri C, Viola P, Galletto L, Sussio M, Trolli B, Biasio M, Rolfo A, Vaudano G, Giolito MR, Ambrosini G, Busana L, Molteni M, Richetti A, Marubini E, Piffanelli A, Salvadori B, Tognoni G, Zola P, Liberati A, Fossati R, Meyerowitz BE, Torri V, Apolone G, Mosconi P, Marsoni S, Liberati MC, Alexanian A, Grilli R, Nicolucci A, Monferroni N. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA. 1994;271 (20:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R, Delaloge S, Spielmann M. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22 (16:3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366 (6:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366 (2:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS, Bago-Horvath Z, Dubsky P, Rudas M, Pluschnig U, Wiltschke C, Gnant M, Steger GG, Zielinski CC, Bartsch R. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19 (2:149–155. doi: 10.1111/tbj.12070. [DOI] [PubMed] [Google Scholar]
- Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37 (1:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, Di LA, Lohrisch C, Bernard C, Ferreira F, Piccart MJ. Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades. Ann Oncol. 2002;13 (2:197–207. doi: 10.1093/annonc/mdf101. [DOI] [PubMed] [Google Scholar]
- Carlini M, Lonardo MT, Carboni F, Petric M, Vitucci C, Santoro R, Lepiane P, Ettorre GM, Santoro E. Liver metastases from breast cancer. Results of surgical resection. Hepatogastroenterology. 2002;49 (48:1597–1601. [PubMed] [Google Scholar]
- Clark GM, Sledge GW, Jr, Osborne CK, McGuire WL. Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987;5 (1:55–61. doi: 10.1200/JCO.1987.5.1.55. [DOI] [PubMed] [Google Scholar]
- Danet IM, Semelka RC, Leonardou P, Braga L, Vaidean G, Woosley JT, Kanematsu M. Spectrum of MRI appearances of untreated metastases of the liver. AJR Am J Roentgenol. 2003;181 (3:809–817. doi: 10.2214/ajr.181.3.1810809. [DOI] [PubMed] [Google Scholar]
- Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26 (30:4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirisamer A, Halpern BS, Flory D, Wolf F, Beheshti M, Mayerhoefer ME, Langsteger W. Integrated contrast-enhanced diagnostic whole-body PET/CT as a first-line restaging modality in patients with suspected metastatic recurrence of breast cancer. Eur J Radiol. 2010;73 (2:294–299. doi: 10.1016/j.ejrad.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180 (2:319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- EBCTCG EBCTCG (2005Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials Lancet 365(94721687–1717. [DOI] [PubMed] [Google Scholar]
- Engelhard K, Hollenbach HP, Wohlfart K, von Imhoff E, Fellner FA. Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol. 2004;14 (1:99–105. doi: 10.1007/s00330-003-1968-7. [DOI] [PubMed] [Google Scholar]
- Ertl I, Heinemann V, Laessig D, Nagel D, Seidel D, Stieber P. CA 125 in the early detection of metastatic breast cancer. J Clin Oncol. 2009;27 (suppl:abstr e12015. [Google Scholar]
- Gallowitsch HJ, Kresnik E, Gasser J, Kumnig G, Igerc I, Mikosch P, Lind P. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol. 2003;38 (5:250–256. doi: 10.1097/01.RLI.0000063983.86229.f2. [DOI] [PubMed] [Google Scholar]
- Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144 (2:261–265. doi: 10.2214/ajr.144.2.261. [DOI] [PubMed] [Google Scholar]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13 (1:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- Iwata H. Future treatment strategies for metastatic breast cancer: curable or incurable. Breast Cancer. 2012;19 (3:200–205. doi: 10.1007/s12282-011-0267-4. [DOI] [PubMed] [Google Scholar]
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28 (20:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, Vogel VG, Wolff AC, Somerfield MR, Davidson NE. Breast cancer follow-up and management after primary treatment: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31 (7:961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park JS, Lee SA, Kim JK, Jeong J, Yoon DS, Lee HD. Does liver resection provide long-term survival benefits for breast cancer patients with liver metastasis? A single hospital experience. Yonsei Med J. 2014;55 (3:558–562. doi: 10.3349/ymj.2014.55.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ichiba T, Sakuyama T, Arakawa Y, Nagasaki E, Aiba K, Nogi H, Kawase K, Takeyama H, Toriumi Y, Uchida K, Kobayashi M, Kanehira C, Suzuki M, Ando N, Natori K, Kuraishi Y. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19 (3:218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- Lauenstein TC, Goehde SC, Herborn CU, Goyen M, Oberhoff C, Debatin JF, Ruehm SG, Barkhausen J. Whole-body MR imaging: evaluation of patients for metastases. Radiology. 2004;233 (1:139–148. doi: 10.1148/radiol.2331030777. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg SN, Solbiati L, Meloni F, Ierace T, Gazelle GS. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220 (1:145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- Mack MG, Straub R, Eichler K, Sollner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy—local tumor control rate and survival data. Radiology. 2004;233 (2:400–409. doi: 10.1148/radiol.2332030454. [DOI] [PubMed] [Google Scholar]
- Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31 (25:3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minicozzi P, Bella F, Toss A, Giacomin A, Fusco M, Zarcone M, Tumino R, Falcini F, Cesaraccio R, Candela G, La Rosa F, Federico M, Sant M. Relative and disease-free survival for breast cancer in relation to subtype: a population-based study. J Cancer Res Clin Oncol. 2013;139 (9:1569–1577. doi: 10.1007/s00432-013-1478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, Goike H, Lamerz R, Nap M, Soletormos G, Stieber P. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol. 2005;26 (6:281–293. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- Molina R, Jo J, Filella X, Zanon G, Farrus B, Munoz M, Latre ML, Pahisa J, Velasco M, Fernandez P, Estape J, Ballesta AM. C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer Res. 1999;19 (4A:2551–2555. [PubMed] [Google Scholar]
- Molina R, Zanon G, Filella X, Moreno F, Jo J, Daniels M, Latre ML, Gimenez N, Pahisa J, Velasco M, Ballesta AM. Use of serial carcinoembryonic antigen and CA 15.3 assays in detecting relapses in breast cancer patients. Breast Cancer Res Treat. 1995;36 (1:41–48. doi: 10.1007/BF00690183. [DOI] [PubMed] [Google Scholar]
- Murakami R, Kumita S, Yoshida T, Ishihara K, Kiriyama T, Hakozaki K, Yanagihara K, Iida S, Tsuchiya S. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta Radiol. 2012;53 (1:12–16. doi: 10.1258/ar.2011.110245. [DOI] [PubMed] [Google Scholar]
- Nicolini A, Carpi A, Michelassi C, Spinelli C, Conte M, Miccoli P, Fini M, Giardino R. "Tumour marker guided" salvage treatment prolongs survival of breast cancer patients: final report of a 7-year study. Biomed Pharmacother. 2003;57 (10:452–459. doi: 10.1016/j.biopha.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Nicolini A, Colombini C, Luciani L, Carpi A, Giuliani L. Evaluation of serum CA15-3 determination with CEA and TPA in the post-operative follow-up of breast cancer patients. Br J Cancer. 1991;64 (1:154–158. doi: 10.1038/bjc.1991.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured. J Natl Cancer Inst. 2010;102 (7:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55 (2:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Piperkova E, Raphael B, Altinyay ME, Castellon I, Libes R, Sandella N, Heiba S, Abdel-Dayem H. Impact of PET/CT in comparison with same day contrast enhanced CT in breast cancer management. Clin Nucl Med. 2007;32 (6:429–434. doi: 10.1097/RLU.0b013e31805375e0. [DOI] [PubMed] [Google Scholar]
- Radan L, Ben-Haim S, Bar-Shalom R, Guralnik L, Israel O. The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer. 2006;107 (11:2545–2551. doi: 10.1002/cncr.22292. [DOI] [PubMed] [Google Scholar]
- Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994;271 (20:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- Ruibal Morell A. CEA serum levels in non-neoplastic disease. Int J Biol Markers. 1992;7 (3:160–166. doi: 10.1177/172460089200700307. [DOI] [PubMed] [Google Scholar]
- Seah DS, Luis IV, Macrae E, Sohl J, Litsas G, Winer EP, Lin NU, Burstein HJ. Use and duration of chemotherapy in patients with metastatic breast cancer according to tumor subtype and line of therapy. J Natl Compr Canc Netw. 2014;12 (1:71–80. doi: 10.6004/jnccn.2014.0008. [DOI] [PubMed] [Google Scholar]
- Som PM, Curtin HD, Mancuso AA. The new imaging-based classification for describing the location of lymph nodes in the neck with particular regard to cervical lymph nodes in relation to cancer of the larynx. ORL J Otorhinolaryngol Relat Spec. 2000;62 (4:186–198. doi: 10.1159/000027745. [DOI] [PubMed] [Google Scholar]
- Stieber P, Heinemann V, Schalhorn A.2005[Tumor markers—how they should be applied] MMW Fortschritte der Medizin 147(203537-9. [PubMed] [Google Scholar]
- Stieber P, Molina R, Chan DW, Fritsche HA, Beyrau R, Bonfrer JM, Filella X, Gornet TG, Hoff T, Jager W, van Kamp GJ, Nagel D, Peisker K, Sokoll LJ, Troalen F, Untch M, Domke I. Clinical evaluation of the Elecsys CA 15-3 test in breast cancer patients. Clin Lab. 2003;49 (1-2:15–24. [PubMed] [Google Scholar]
- Suarez M, Perez-Castejon MJ, Jimenez A, Domper M, Ruiz G, Montz R, Carreras JL. Early diagnosis of recurrent breast cancer with FDG-PET in patients with progressive elevation of serum tumor markers. Q J Nucl Med. 2002;46 (2:113–121. [PubMed] [Google Scholar]
- Suzuma T, Sakurai T, Yoshimura G, Umemura T, Tamaki T, Naito Y. A mathematical model of axillary lymph node involvement considering lymph node size in patients with breast cancer. Breast Cancer. 2001;8 (3:206–212. doi: 10.1007/BF02967510. [DOI] [PubMed] [Google Scholar]
- Tait CR, Waterworth A, Loncaster J, Horgan K, Dodwell D. The oligometastatic state in breast cancer: hypothesis or reality. Breast. 2005;14 (2:87–93. doi: 10.1016/j.breast.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Vanel D, Bittoun J, Tardivon A. MRI of bone metastases. Eur Radiol. 1998;8 (8:1345–1351. doi: 10.1007/s003300050549. [DOI] [PubMed] [Google Scholar]
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 (Suppl 1:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]