Abstract
Purpose.
To measure the change in peripapillary retinal blood flow in response to hyperoxia by using optical coherence tomography (OCT) angiography.
Methods.
One eye of each healthy human participants (six) was scanned with a commercial high-speed (70 kHz) spectral OCT. Scans were captured twice after 10-minute exposures to normal breathing (baseline) and hyperoxia. Blood flow was detected by the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm. Peripapillary retinal blood flow index and vessel density were calculated from en face maximum projections of the retinal layers. The experiment was performed on 2 separate days for each participant. Coefficient of variation (CV) was used to measure within-day repeatability and between-day reproducibility. Paired t-tests were used to compare means of baseline and hyperoxic peripapillary retinal blood flow.
Results.
A decrease of 8.87% ± 3.09% (mean ± standard deviation) in flow index and 2.61% ± 1.50% in vessel density was observed under hyperoxia. The within-day repeatability CV of baseline measurements was 5.75% for flow index and 1.67% for vessel density. The between-day reproducibility CV for baseline flow index and vessel density was 11.1% and 1.14%, respectively. The between-day reproducibility of the hyperoxic response was 3.71% and 1.67% for flow index and vessel density, respectively.
Conclusions.
Optical coherence tomography angiography with SSADA was able to detect a decrease in peripapillary retinal blood flow in response to hyperoxia. The response was larger than the variability of baseline measurements. The magnitude of an individual's hyperoxic response was highly variable between days. Thus, reliable assessment may require averaging multiple measurements.
Keywords: optical coherence tomography, peripapillary retina, angiography, hyperoxia
OCT angiography with SSADA is able to detect a decrease in peripapillary retinal blood flow in response to hyperoxia.
The complexity in structure and function of retinal tissue makes it one of the most metabolically active locations in the body.1 The retina must maintain a high oxygen concentration that remains stable despite changes in hemodynamics and atmospheric partial pressures.2–6 Retinal blood vessels are unique in that they can regulate vascular tone, and therefore blood flow, without the presence of an autonomic nerve supply.7 This autoregulation is essential to preserving the function of retinal tissue.8 Uncontrolled hyperfusion during the early stages of diabetic retinopathy suggests that deficits in the autoregulatory response have an important role in pathogenesis.9–11 Quantification of retinal blood flow change due to autoregulation may thus be useful in a clinical setting.
Several techniques have been implemented to investigate blood flow in the healthy eye. Blue field entopic phenomenon12,13 and scanning laser Doppler flowmetry14 have both been successfully implemented to investigate retinal blood flow variation. These methods have shown that in order to maintain relatively constant retinal oxygen levels, retinal blood velocity decreases with an increase in arterial oxygen partial pressure (hyperoxia). Other techniques using laser Doppler velocimetry15 and Doppler optical coherence tomography (OCT)16 have been able to measure decreases in retinal blood vessel diameter and blood flow during hyperoxia.
Optical coherence tomography is well established in clinical ophthalmology for structural imaging and evaluation of diseases.17 The recent development of OCT angiography has allowed for the mapping of ocular circulation down to the capillary level. We have developed an efficient OCT angiography algorithm called “split-spectrum amplitude-decorrelation angiography” (SSADA)18–20 that greatly reduces the scan time and improves the signal-to-noise ratio of flow detection, making it possible to obtain high-quality angiograms with a commercial OCT retinal scanner.
Optical coherence tomography angiography has great potential for quantifying microvascular change as a response to physiologic changes. The purpose of this study was to investigate the change of the peripapillary retinal blood flow in healthy eyes before and after hyperoxia.
Methods
Study Population
This study was conducted at the Casey Eye Institute at the Oregon Health & Science University. The research adhered to the tenets of the Declaration of Helsinki in the treatment of human participants. The study protocol was approved by the Institutional Review Board. Six healthy participants (age, average ± standard deviation, 33 ± 6.0 years) were recruited to participate in the study. One eye from each of the participants was scanned and analyzed throughout the study (six eyes). All scans were obtained within the time frame of 1 month.
System Setup
The experiment was conducted by using a commercial Fourier-domain OCT system (RTVue-XR, Optovue, Fremont, CA, USA) to obtain images for quantification of the peripapillary retinal blood flow. This instrument has an A-scan rate of 70K scans per second, using a light source centered on 840 nm and a bandwidth of 45 nm. The tissue resolution is 5 μm axially, and there is 22-μm beam width. The exposure power at the pupil of 750 μW was used in accordance with the safety limits set by the American National Standards Institute.21
Data Acquisition
Participants were asked to sit and breathe normally for 10 minutes. Immediately following this baseline condition, systemic blood pressure and heart rate were recorded. Participants were then asked to fix their gaze on an internal fixation target that centers the scan on the optic disc. The OCT angiography scans of the disc were then acquired, during which time the normal breathing condition (baseline) was maintained. Following the baseline scan, participants were fitted with a nasal mask (OxyMask, Southmedic, Barrie, Ontario, Canada) and delivered supplemental oxygen at a rate of 15 L/min, for 10 minutes. This equates to an approximate 60% hyperoxic condition. Systemic blood pressure and heart rate were recorded at the end of the 10-minute hyperoxia exposure. The hyperoxic breathing condition was sustained throughout the scanning.
Two sets of OCT angiography scans were obtained during each breathing condition. Each imaging session included two sets of scans. Each set contains a horizontal-priority and vertical-priority volumetric raster scan (Fig. 1A). In the fast transverse scan direction, the B-scan contained 304 A-lines covering 3 mm. In the slow transverse scan direction, 304 B-scan locations covering 3 mm were used. Two consecutive B-scans were done at each location to compute inter–B-scan decorrelation with the SSADA algorithm. Altogether, each volumetric scan consists of 608 B-scans acquired in 2.9 seconds.
Figure 1.
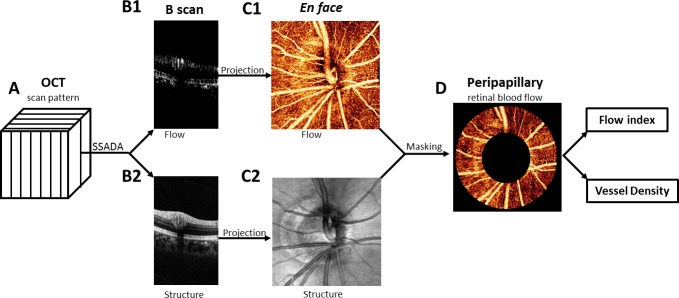
The horizontal and vertical scans were registered and merged to obtain one motion- corrected 3D data cube (A). The SSADA algorithm was used to compute flow (B1) and structural (B2) B-scan images. The en face OCT angiogram (C1) is produced by maximum flow projection of the retinal layer and the optic disc. The en face OCT structure (C2) is produced by averaging the signal intensity in each axial scan. The peripapillary region was delineated on the OCT structural image as a 700-μm-wide elliptical annulus extending outward from the optic disc boundary (C2). The peripapillary region of the en face OCT angiogram (D) was used to compute the flow index and vessel density index.
Data Processing
In each scan, the SSADA algorithm was used to compute flow map (Fig. 1B1). The SSADA algorithm incorporates three other steps to reduce motion artifacts.18 Cross-sectional image frames corrupted by saccadic eye movements were removed. The horizontal-priority and vertical-priority scans were combined by using an orthogonal registration algorithm that removes bulk motion and produces a merged OCT image volume with almost no residual motion artifacts.22 The retinal layer was segmented between the inner limiting membrane and the retinal pigment epithelium layer on the basis of OCT structural image (Fig. 2B2). The en face angiogram (Fig. 1C1) was then produced by maximum decorrelation (flow) projection within the segmented retinal slab. Within the optic disc, maximum flow was projected along the full depth range. The disc boundary was delineated by the authors using the OCT structural en face image (Fig. 1C2). Within the disc, sections of the major retinal vessels that contain significant axial flow velocity component exhibited loss of OCT signal (dark patches, Fig. 2B) due to the interferometric fringe washout artifact.23 This artifact is not seen in the peripapillary area, where the blood vessels are nearly perpendicular to the OCT beam. Therefore, flow statistics can be more reliably assessed in the peripapillary region. The peripapillary region was defined as a 700-μm-wide elliptical annulus extending outward from the optic disc boundary (Fig. 1D).
Figure 2.
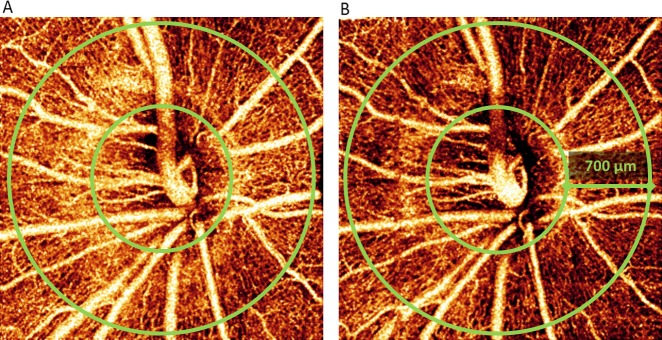
Optic disc en face maximum decorrelation projecton angiograms from a participant with a large response to hyperoxia at baseline (A) and under hyperoxia (B). The images represent a 3 × 3–mm area. The peripapillary region extends from the optic disc (inner green ring) outward for 700 μm (outer green ring). The angiogram after hyperoxia exposure (B) shows a 17% decrease in flow index and a 4% decrease in vessel density.
Peripapillary retinal flow index was defined as the average decorrelation value in the peripapillary region of the en face retinal angiogram. The vessel density is the percentage area occupied by blood vessels, with the blood vessels being defined as pixels having decorrelation values above threshold level (two standard deviations above noise).
Data Analysis
The experiment was performed on 2 separate days for each participant. The peripapillary retinal blood flow mean and standard deviation of all baseline and hyperoxia scans were calculated from the combined testing days. Paired t-tests were used to compare means of baseline and hyperoxic peripapillary retinal blood flow. Change in blood flow during hyperoxia was calculated as the average percentage change and standard deviation of all participants during both testing days. Coefficient of variation (CV) was used to measure within-day repeatability and between-day reproducibility. The population variation in hyperoxic response CV was calculated for the between-day percentage change of blood flow during hyperoxia.
Results
Hemodynamic Response
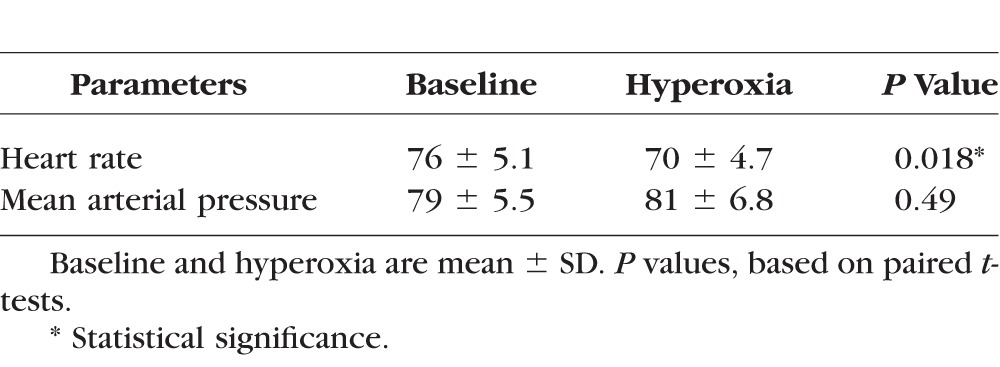
Under hyperoxia, there was a significant decrease in heart rate but no significant change in mean arterial pressure (Table 1).
Table 1.
Characteristics of Healthy Participants
Peripapillary Retinal Angiograms
All scans had adequate signal strength and were void of excessive motion error; therefore, no scans were excluded from the data set.
Detailed peripapillary microvasculature could be visualized on en face OCT angiograms both under room air (Fig. 2A) and hyperoxic (Fig. 2B) conditions. The angiograms appear darker after exposure to hyperoxia because of lower decorrelation (flow) values in the capillaries. These are quantified by flow index below.
Peripapillary Retinal Flow Index and Vessel Density
Compared to the baseline mean, there was a greater percentage reduction in flow index than vessel density after exposure to hyperoxia, both of which were statistically significant (Table 2). The population variation in the hyperoxic response was smaller for the flow index than vessel density (Table 2). All six participants showed reduction in both flow index (Fig. 3A) and vessel density (Fig. 3B) during hyperoxia on each experimental day and when results from the 2 experimental days were averaged.
Table 2.
Optical Coherence Tomography Peripapillary Perfusion Measurements
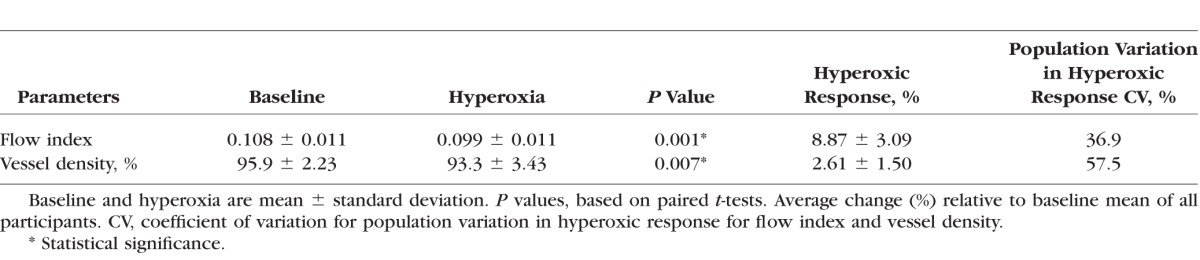
Figure 3.
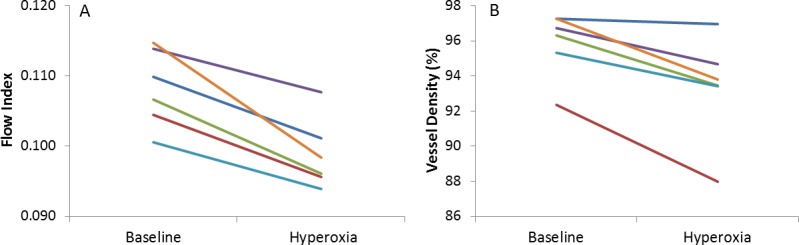
Line graph displaying the flow index (A) and vessel density (B) average of all peripapillary retinal blood flow measurements from the six participants at baseline and under hyperoxic breathing. Results from the 2 experimental days were averaged.
Repeatability and Reproducibility
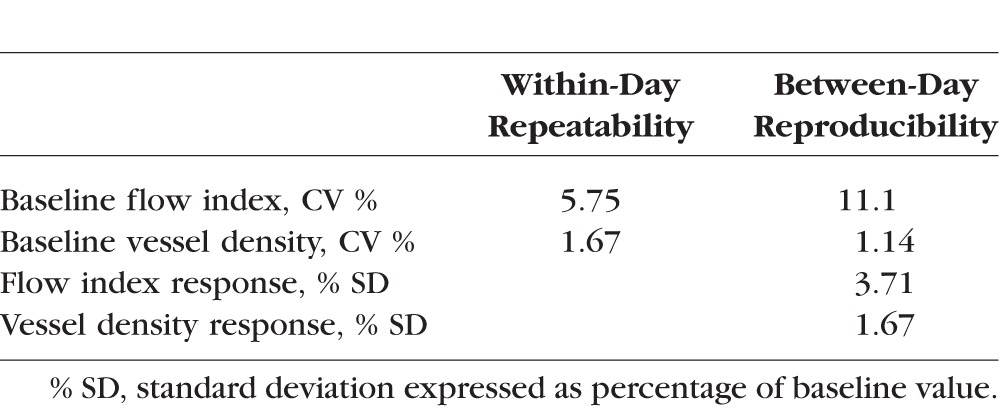
As assessed by CV, vessel density had better within-session repeatability than flow index (Table 3). Vessel density also had better between-day reproducibility. The hyperoxic response for vessel density was also more reproducible than the hyperoxic response for flow index in terms of CV (Table 3).
Table 3.
Variability of OCT Peripapillary Retinal Perfusion Measurements
Discussion
Optical coherence tomography has become the standard imaging modality for evaluating ocular structures in ophthalmology. A growing body of evidence suggests the importance of assessing blood flow in the eye.9–11 Optical coherence tomography angiography is a novel technology that detects blood flow by using intrinsic motion contrast within the microcirculatory network and does not need any intravenous contrast. The SSADA is an efficient algorithm that makes it possible to obtain OCT angiograms on a commercial OCT scanner and provide quantitative flow indices to assess the microcirculation of the retina and optic nerve.18–20,24
The SSADA algorithm has been used to assess the parafoveal retinal blood flow index response to light stimulation.25 In this study we measured the change in flow index and vessel density during hyperoxia. The flow index response found here was greater in magnitude and had a smaller standard deviation than the maximal response (7.2% ± 5.1%) during light stimulation.25 This difference in response magnitude may be due to the different types of stimulus or location of measurements.
Previous studies have shown that an increase in breathable oxygen (hyperoxia) causes a decrease in both the retinal vascular caliber and blood flow. Using Doppler OCT, Bower et al.16 have found a 6% decrease in total retinal blood flow after 5 minutes of hyperoxia. Furthermore, Riva et al.15,26 have used laser Doppler velocimetry (LDV) to measure a 12% vasoconstriction response in the large retinal blood vessels. The LDV method has been implemented at both the macula and optic disc to show a similar hyperoxic-induced blood flow response.8 Investigations done at the macula, using blue field entropic phenomenon12,13 and laser Doppler flowmetry,27 have shown that blood flow decreases by 30% during hyperoxia. In our study, the average 8.9% reduction in flow index measured with OCT angiography is within the range of previously measured responses to hyperoxia. The flow index is the average decorrelation value within a region of interest. The decorrelation value increases with flow velocity up to a level, after which it saturates. Generally, the decorrelation value varies within the capillaries and reaches the saturation value in larger blood vessels.18,19 Thus, the flow index contains information on capillary flow velocity in addition to vessel density.
In our study, we detected reduction in both flow index and vessel density during hyperoxia. The reduction in vessel density could be attributed to a reduction in vessel caliber, because we defined vessel density as the percentage area occupied by flow pixels. The flow index contains information on capillary flow velocity in addition to vessel density. Comparing the two indices, the flow index showed 3.4 times larger hyperoxic response (Table 2). The between-day reproducibility for hyperoxic response was 2.2 times worse for the flow index than the vessel density (Table 3). Overall, the flow index was a more sensitive metric for detecting hyperoxic response. This makes sense because the flow index contains additional information on capillary flow velocity.
The relatively small response magnitude is the primary limitation of using OCT angiography to assess blood flow response to hyperoxia or other stimuli. In this study, the hyperoxic response in flow index reduction was 8.9%, which was only 2.4 times larger than the between-day standard deviation. Thus, reliable assessment of response magnitude based on this technique would require averaging of several trials, with each trial taking at least 10 minutes to reach stable physiologic state after changing breathing conditions. The time required may pose a practical limitation as a clinical test. Additional limitations include the need for stable fixation and good signal strength to obtain high-quality OCT angiography. Thus, the method may be difficult to apply for patients with poor cooperation, poor visual acuity, nystagmus, or opacity of the ocular media.
Another limitation of our approach is that the flow index does not provide a volumetric measure of retinal blood flow. Because the decorrelation values produced by the SSADA saturates at velocity levels in vessels larger than capillaries, the flow index is likely to underestimate blood flow changes. Furthermore, because capillary diameters (5–10 μm) are generally finer than the transverse resolution of the OCT beam (∼15 μm), the SSADA vessel density index is likely to overestimate actual vessel density while underestimating change. However, both flow index and vessel density had good repeatability and varied within a relatively narrow range in a healthy population (Table 3). Therefore, they offer sufficient precision to detect difference between individuals and changes between physiologic states. The level precision compared favorably to other methods of assessing retinal perfusion. However, caution must be exercised in comparing the magnitude of response measured by OCT angiography and other methods.
In summary, we have demonstrated that SSADA OCT angiography could detect blood flow and vascular changes in response to hyperoxia. This might be useful in the investigation of vascular autoregulation in the eye.
Acknowledgments
Oregon Health & Science University (OHSU), Yali Jia, and David Huang have a significant financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. David Huang receives royalties on an optical coherence tomography patent licensed by the Massachusetts Institute of Technology (MIT) to Carl Zeiss Meditec, Inc. Other authors do not have financial interest in the subject of this article.
Supported by National Institutes of Health Grants R01EY023285, R01EY024544, DP3 DK104397, Clinical & Translational Science Awards grant (UL1TR000128), T32EY23211, and an unrestricted grant from Research to Prevent Blindness.
Disclosure: A.D. Pechauer, None; Y. Jia, Optovue, Inc. (F), P; L. Liu, None; S.S. Gao, None; C. Jiang, None; D. Huang, Optovue, Inc. (F, I), P
References
- 1. Vanderkooi JM,, Erecinska M,, Silver IA. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol. 1991; 260: C1131–C1150. [DOI] [PubMed] [Google Scholar]
- 2. Harris A,, Arend O,, Kopecky K, et al. Physiological perturbation of ocular and cerebral blood flow as measured by scanning laser ophthalmoscopy and color Doppler imaging. Surv Ophthalmol. 1994; 38 (suppl): S81–S86. [DOI] [PubMed] [Google Scholar]
- 3. Hill DW. The regional distribution of retinal circulation. Ann R Coll Surg Engl. 1977; 59: 470–475. [PMC free article] [PubMed] [Google Scholar]
- 4. Gilmore ED,, Hudson C,, Venkataraman ST,, Preiss D,, Fisher J. Comparison of different hyperoxic paradigms to induce vasoconstriction: implications for the investigation of retinal vascular reactivity. Invest Ophthalmol Vis Sci. 2004; 45: 3207–3212. [DOI] [PubMed] [Google Scholar]
- 5. Kiss B,, Dallinger S,, Polak K,, Findl O,, Eichler HG,, Schmetterer L. Ocular hemodynamics during isometric exercise. Microvasc Res. 2001; 61: 1–13. [DOI] [PubMed] [Google Scholar]
- 6. Sponsel WE,, DePaul KL,, Zetlan SR. Retinal hemodynamic effects of carbon dioxide hyperoxia, and mild hypoxia. Invest Ophthalmol Vis Sci. 1992; 33: 1864–1869. [PubMed] [Google Scholar]
- 7. Ye XD,, Laties AM,, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990; 31: 1731–1737. [PubMed] [Google Scholar]
- 8. Schmetterer L,, Wolzt M,, Lexer F, et al. The effect of hyperoxia and hypercapnia on fundus pulsations in the macular and optic disc region in healthy young men. Exp Eye Res. 1995; 61: 685–690. [DOI] [PubMed] [Google Scholar]
- 9. Kohner EM,, Patel V,, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995; 44: 603–607. [DOI] [PubMed] [Google Scholar]
- 10. Evans DW,, Harris A,, Danis RP,, Arend O,, Martin BJ. Altered retrobulbar vascular reactivity in early diabetic retinopathy. Br J Ophthalmol. 1997; 81: 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmetterer L,, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999; 42: 387–405. [DOI] [PubMed] [Google Scholar]
- 12. Fallon TJ,, Maxwell D,, Kohner EM. Measurement of autoregulation of retinal blood flow using the blue field entoptic phenomenon. Trans Ophthalmol Soc U K. 1985; 104 (pt 8): 857–860. [PubMed] [Google Scholar]
- 13. Fallon TJ,, Maxwell D,, Kohner EM. Retinal vascular autoregulation in conditions of hyperoxia and hypoxia using the blue field entoptic phenomenon. Ophthalmology. 1985; 92: 701–705. [DOI] [PubMed] [Google Scholar]
- 14. Strenn K,, Menapace R,, Rainer G,, Findl O,, Wolzt M,, Schmetterer L. Reproducibility and sensitivity of scanning laser Doppler flowmetry during graded changes in PO2. Br J Ophthalmol. 1997; 81: 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riva CE,, Grunwald JE,, Sinclair SH. Laser doppler velocimetry study of the effect of pure oxygen breathing on retinal blood flow. Invest Ophthalmol Vis Sci. 1983; 24: 47–51. [PubMed] [Google Scholar]
- 16. Bower BA,, Zhao M,, Zawadzki RJ,, Izatt JA. Real-time spectral domain Doppler optical coherence tomography and investigation of human retinal vessel autoregulation. J Biomed Opt. 2007; 12: 041214. [DOI] [PubMed] [Google Scholar]
- 17. Huang D,, Swanson EA,, Lin CP, et al. Optical coherence tomography. Science. 1991; 254: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jia Y,, Bailey ST,, Wilson DJ,, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014; 121: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jia Y,, Morrison JC,, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012; 3: 3127–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia Y,, Wei E,, Wang X,, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014; 121: 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American National Standard for Safe Use of Lasers. ANSI Z136.1e2007 Orlando, FL: Laser Institute of America; 2007. [Google Scholar]
- 22. Kraus MF,, Potsaid B,, Mayer MA, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express. 2012; 3: 1182–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hendargo HC,, McNabb RP,, Dhalla AH,, Shepherd N,, Izatt JA. Doppler velocity detection limitations in spectrometer-based versus swept-source optical coherence tomography. Biomed Opt Express. 2011; 2: 2175–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia Y,, Bailey ST,, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. PNAS. In press. [DOI] [PMC free article] [PubMed]
- 25. Wei E,, Jia Y,, Tan O,, et al. Parafoveal retinal vascular response to pattern visual stimulation assessed with OCT angiography. PLoS One. 2013; 8: e81343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riva C,, Ross B,, Benedek GB. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Invest Ophthalmol. 1972; 11: 936–944. [PubMed] [Google Scholar]
- 27. Langhans M,, Michelson G,, Groh MJ. Effect of breathing 100% oxygen on retinal and optic nerve head capillary blood flow in smokers and non-smokers. Br J Ophthalmol. 1997; 81: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


