Abstract
Purpose.
Dry eye disease (DED) produces ocular pain and irritation, yet a detailed characterization of ocular sensitivity in a preclinical model of DED is lacking. The aim of the present study was to assess nociceptive behaviors in an aqueous tear deficiency model of DED in the rat.
Methods.
Spontaneous blinking, corneal mechanical thresholds, and eye wipe behaviors elicited by hypertonic saline (5.0 M) were examined over a period of 8 weeks following the unilateral excision of either the exorbital lacrimal gland or of the exorbital and infraorbital lacrimal glands, and in sham surgery controls. The effect of topical proparacaine on spontaneous blinking and of systemic morphine (0.5–3.0 mg/kg, subcutaneous [SC]) on spontaneous blinking and eye wipe responses were also examined.
Results.
Lacrimal gland excision resulted in mechanical hypersensitivity and an increase in spontaneous blinking in the ipsilateral eye over an 8-week period that was more pronounced after infra- and exorbital gland excision. The time spent eye wiping was also enhanced in response to hypertonic saline (5.0 M) at both 1- and 8-week time-points, but only in infra- and exorbital gland excised animals. Morphine attenuated spontaneous blinking, and the response to hypertonic saline in dry eye animals and topical proparacaine application reduced spontaneous blinking down to control levels.
Conclusions.
These results indicate that aqueous tear deficiency produces hypersensitivity in the rat cornea. In addition, the increase in spontaneous blinks and their reduction by morphine and topical anesthesia indicate the presence of persistent irritation elicited by the activation of corneal nociceptors.
Keywords: corneal sensitivity, dry eyes, lacrimal gland excision
Corneal sensitivity was examined after exorbital and infraorbital lacrimal gland excision in the rat. Hypersensitivity to mechanical and hyperosmotic stimuli was found, as well as evidence for persistent irritation.
Dry eye disease (DED) represents a significant clinical problem that can severely impact the physical, social, and psychological functioning of an individual.1–4 While DED likely results from an inadequate or altered tear film on the surface of the eye, its etiology is often unknown. Potential causes include damage to tear secreting glandular tissues or an inability of sensory neurons innervating the cornea to sufficiently drive tear secretion.5–11 The resulting absence of adequate lubrication causes sensations of ocular dryness, grittiness, irritation, and burning pain.12 Treatment options for DED are limited and mainly palliative, most frequently consisting of repetitive application of artificial tears or other lubricants, silicone plugs, or cauterization to block lacrimal drainage ducts.
Ocular pain and irritation is a common complaint in those suffering from DED, and an overall reduction in pain and irritation is an important clinical outcome measurement when testing potential treatments.13 Corneal sensitivity, however, is not typically assessed in animal models of DED. Instead, tear secretion, corneal epithelial barrier disruption, and inflammatory markers are often used to determine the severity of dry eye and treatment efficacy in preclinical models.14–21 One shortcoming to this approach is that an apparent improvement in the condition of the corneal epithelium does not necessarily indicate relief from pain and irritation associated with DED.
Aqueous tear deficiency in the rat has been induced by either excision of the exorbital lacrimal gland (single lacrimal gland excision, LGE) or by excision of both the exorbital and infraorbital lacrimal glands (double LGE),16,18,22–25 and more recently following exorbital excision in the mouse.26 Over the course of 6 to 8 weeks, both single and double LGE have been reported to reduce tear secretion and produce damage to the corneal epithelium, as indicated by an increase in corneal fluorescein scores, yet no direct comparison has been made between these two models.
Recently, the sensitization of corneal cold receptors was reported after double LGE in the rat 8 weeks.25 An increase in blinking over the 8-week period was also noted in the eye ipsilateral to gland excision. While an increase in spontaneous blinking might be a sign of irritation, changes in corneal sensitivity to mechanical or chemical stimuli were not determined. In the present study, the effects of single and double LGE on spontaneous blinking and corneal sensitivity to mechanical and hyperosmotic stimuli were determined in order to evaluate ocular sensitivity in a model of aqueous tear deficiency.
Methods
Animals
A total of 150 male Sprague-Dawley rats (200–250 g) were obtained from Charles River Laboratories (Cambridge, MA, USA) and housed in an environment with free access to food and water and a controlled 12-hour light/dark cycle. Animals were treated according to the policies and recommendations of the National Institutes of Health guidelines for the handling and use of laboratory animals and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All procedures were approved by the committee on animal research at the University of New England.
Surgery
Unilateral lacrimal gland excision was performed under 2% to 3% isoflurane anesthesia. The left exorbital lacrimal gland was removed in animals receiving single LGE surgery, whereas double LGE included the removal of both the left exorbital and infraorbital lacrimal glands.27 For sham surgeries, incisions were made and glands were partially exposed on the left side. Lidocaine (lidocaine HCl 1% and epinephrine 1:100,000) was injected subcutaneously into the cheek prior to gland excision to attenuate bleeding. Carprofen was administered subcutaneously (5 mg/kg) once per day for 3 days to provide postsurgical analgesia.
Tear Measurements
Tears were measured starting 1-week post surgery for a total of 8 weeks. Using fine forceps, cotton phenol red threads (Zone-Quick; FCI Ophthalmic, Pembroke, MA, USA) were placed in the lateral canthus of the eye for 30 seconds in unanesthetized animals.25,28 One measurement was taken from each eye. After removal, the length of color change on the phenol red threads was measured under a microscope to the nearest 0.1 mm.
Blink Measurements
Blinks were monitored every week beginning the first week post surgery in animals. Rats were placed in a 24 × 45 × 20 cm (L × W × H) chamber to habituate for 15 minutes. Spontaneous blinks in each eye were quantified for 5 minutes by an observer in real time using a manual counter.
Eye Wipe Behaviors
Following a 10-minute acclimation period, 40 μL of either normal saline or 5 M saline was placed into the eye. The total time spent wiping the eye was recorded over a 3-minute period. Eye wipe behavior consisted of either front or hind paw swiping directed toward the eye in which the saline was applied. Normal facial grooming behavior was not included. Animals were euthanized after testing.
Fluorescein Staining
Corneal fluorescein staining was performed in order to assess the degree of corneal damage. We applied 10 μL of 1% fluorescein solution to the cornea in isoflurane anesthetized animals. After 3-minutes, the eye was flushed with artificial tears to remove excess fluorescein and examined using cobalt blue light from an ophthalmic slit lamp handheld model scope (Hai Laboratories, Inc., Lexington, MA, USA). The degree of staining was scored based on a 0 to 4 grading system.25,29 A score of 0 indicated an absence of punctate fluorescein staining. A score of 1 indicated one-eighth or less of the corneal surface showed staining. A score of 2 indicated one-eighth to one-fourth of the corneal surface showed staining. A score of 3 indicated one-quarter to one-half of the corneal surface showed staining. A score of 4 was given when greater than one-half of the corneal surface was stained. Corneas were examined every week beginning the first week after surgery.
Corneal Mechanical Thresholds
In a subset of animals, corneal mechanical thresholds were determined using a Cochet-Bonnet esthesiometer (Western Ophthalmics, Lynnwood, WA, USA) according to the methods of Wakuta et al.30 While holding the animal, a nylon filament was applied to the cornea and a positive response was indicated by a clear stimulus–evoked blink and retraction of the eye into the ocular orbit. While on some trials the stimulus also elicited head withdrawal motion, this reaction did not occur without the standard blink and eye retraction response. Measurements were made starting with the filament fully extended to 60 mm so as to apply the least amount of pressure, and then incrementally shortened in 5-mm steps until a response was obtained. If a response was obtained, the filament was lengthened again and measurements were repeated.
Drugs
Morphine (gift from National Institute on Drug Abuse) was administered subcutaneously 30 minutes prior to eye wipe measurements. Proparacaine hydrochloride ophthalmic solution (0.5%, 10 μL, Alcon, Fort Worth, TX, USA) was topically applied to the eye.
Statistical Analysis
Multiple group means of parametric data sets were compared using either a one- or two-way ANOVA after it was determined that data conformed to a normal distribution with equal variances. A Tukey post hoc test was performed if an overall significance was found. The Kruskal-Wallis one-way ANOVA on ranks test with post hoc comparisons (Dunn's method) was applied to nonparametric data sets (fluorescein scores and mechanical thresholds) and in parametric data when tests for normality or equal variance failed. Analyses were performed using commercial software (Sigma Stat version 3.5; Systat Software, Chicago, IL, USA). All results are expressed as mean ± SEM. Values of P < 0.05 were considered to be statistically significant.
Results
Tear Levels
Tear measurements were conducted weekly after sham surgery, single and double LGE. While sham surgery had no effect on tear levels (P > 0.05), a significant reduction was found after both single and double LGE (Fig. 1). After single LGE, a two-way ANOVA with repeated measures indicated significant effects of both time after surgery (F [7.84] = 8.06, P < 0.001) and side of gland excision (F [1.84] = 25.64, P < 0.001) but no interaction between these two factors (F [7.84] = 2.06, P = 0.06). Post hoc analysis revealed lower tear levels on the side of the gland excision during 4 of the 8 weeks (Fig. 1B). Single LGE produced a 42% to 64 % reduction in tear levels over the first 2 weeks after surgery; however, this reduction was not consistently maintained in subsequent weeks. The removal of both the infra- and exorbital lacrimal glands also affected tears levels. A two-way ANOVA with repeated measures indicated a significant effect of side of the gland excision (F [1.98] = 31.65, P < 0.001), but no effect of time after surgery (F [7.98] = 0.67, P = 0.70) and no interaction between these factors (F [7.98] = 0.780, P = 0.59). Post hoc analysis revealed significantly lower tear levels in the ipsilateral eye over each of the 8 weeks examined (Fig. 1C). Compared with the contralateral side, tear levels were reduced by 42% to 86%, with the greatest reduction observed during the first 2 weeks after surgery.
Figure 1.
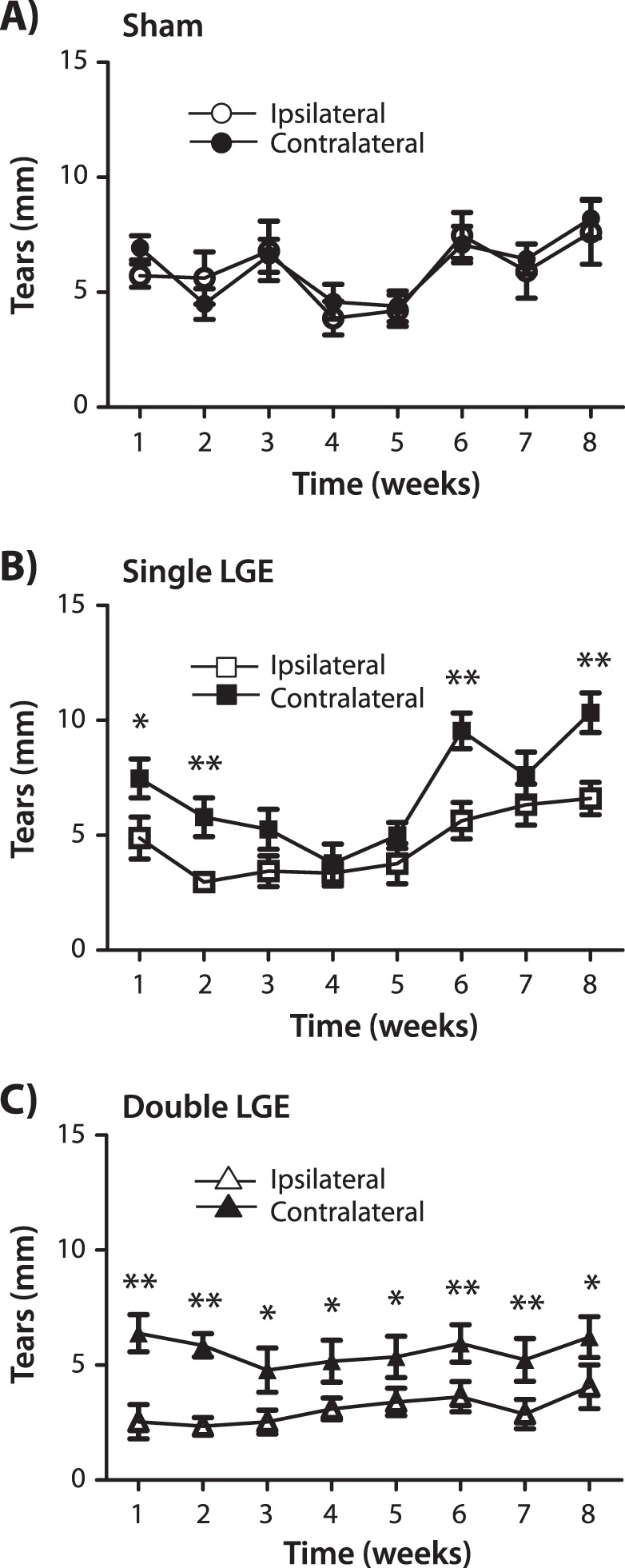
The effect of lacrimal gland excision on tearing. Tear levels were quantified each week in both the ipsi- and contralateral eye after (A) sham surgery; (B) unilateral excision of the exorbital lacrimal gland (single LGE); and (C) unilateral excision of the infraorbital and exorbital lacrimal glands (double LGE). The length of the phenol red cotton filament was measured after placement in the lateral canthus of the eye for 30 seconds (n = 13 to 15 for all treatment groups). *P < 0.05. **P < 0.01 versus the contralateral side.
Fluorescein Staining
The ability of lacrimal gland excision to affect the corneal epithelium was examined using a slit lamp ophthalmoscope after the application of fluorescein (Fig. 2). Following double LGE, cornea fluorescein scores escalated throughout the duration of the study, from an initial score of 1.0 ± 0.2 recorded 1 week after surgery to 2.8 ± 0.2 observed at week 8. The increase in fluorescein scores between 1 and 8 weeks after double LGE was statistically significant (Mann-Whitney sum rank test, P < 0.001). While not as severe, single LGE also produced significant fluorescein staining, which increased from 0.14 ± 0.1 one week after surgery and peaked at 1.14 ± 0.18 after 8 weeks (Mann-Whitney sum rank test, P < 0.05). Sham surgery had no effect on cornea fluorescein scores (P = 0.93). At the 1-week time point, a one-way ANOVA on ranks test revealed a significant difference between treatment groups (P < 0.01) with post hoc analysis indicating lower fluorescein scores after single LGE when compared with double LGE (Fig. 2). In addition, at the 8-week time point, a one-way ANOVA on ranks indicated a significant difference between treatment groups (P < 0.001) with post hoc analysis showing lower fluorescein scores after single LGE when comparing with double LGE (Fig. 2).
Figure 2.
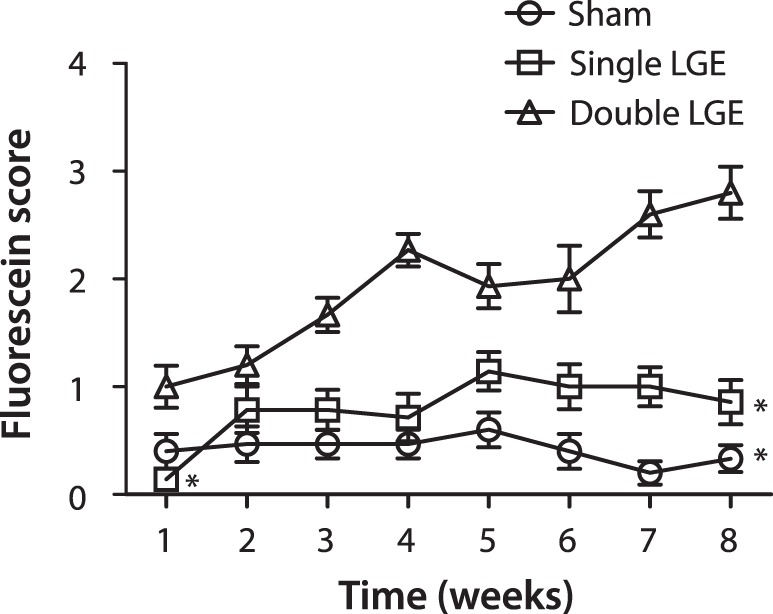
Corneal fluorescein scores following lacrimal gland excision. Fluorescein was applied to the ipsilateral cornea in sham (circles), exorbital lacrimal gland excised (squares), and infraorbital and exorbital lacrimal gland excised (triangles) animals. Scores ranged from 0 to 4 based on the severity of the staining (n = 14 to 15 for all treatment groups). *P < 0.05 versus double lacrimal gland excision at the same time point.
Eye Blinking
Spontaneous eye blinking was quantified over the 8-week observation period in animals undergoing single LGE, double LGE, and sham surgery. In the case of sham-treated animals, a two-way ANOVA revealed no significant effect of surgery side (P = 0.18), no effect of time after surgery (P = 0.50), and no interaction (P = 0.60; Fig. 3A). In contrast, after single LGE, a two-way ANOVA indicated a significant effect of surgery side (F [1.91] = 9.88, P < 0.01) and interaction (F [7.91] = 2.60, P < 0.05), but no effect of time after surgery (F [7.91] = 1.36, P = 0.23). Post hoc analysis indicated significantly higher eye blinks ipsilateral to the side of gland excision at 1, 3, and 5 weeks post surgery when compared with the contralateral side (Fig. 3A). While single LGE produced a greater than 2-fold increase in ipsilateral eye blinks over the first few weeks, the effect of double LGE on the number of eye blinks was even more dramatic (Fig. 3C). A two-way ANOVA indicated a significant effect of surgery side (F [1.98] = 53.76, P < 0.001), but no effect of time after surgery (F [7.98] = 2.03, P > 0.05) and no interaction (F [7.98] = 1.77, P = 0.102). Post hoc analysis revealed a significant elevation in ipsilateral eye blinks across the entire 8-week period after double LGE when compared with the contralateral side (P < 0.001).
Figure 3.
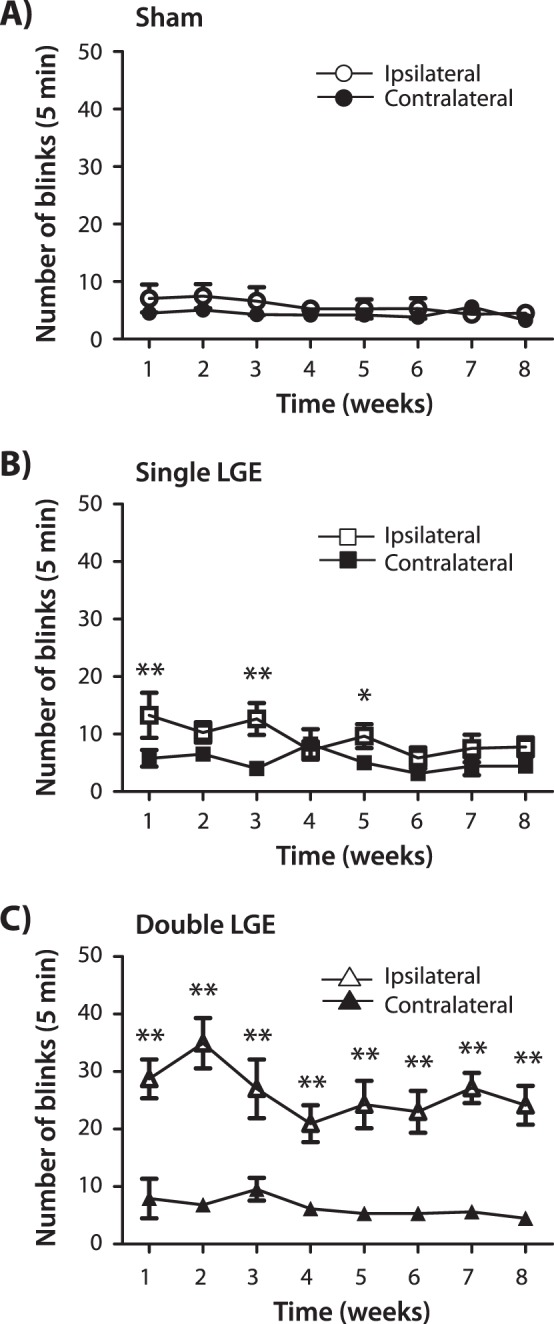
Spontaneous blinking following unilateral lacrimal gland excision. Total number of blinks recorded over a 5-minute period from the ipsilateral and contralateral eye. (A) Sham surgery. (B) Excision of the exorbital lacrimal gland (single LGE). (C) Excision of the infraorbital and exorbital glands (double LGE; n = 14 to 15 for all treatment groups). *P < 0.05. ***P < 0.001 versus the contralateral side.
In order to determine the contribution of primary afferent neuronal activity in driving the increase in blinking, the topical anesthetic proparacaine (10 μL) was applied to the eye 8-weeks after animals had undergone double LGE. A 2-way ANOVA indicated a significant effect of time after application of drugs (F [2.12] = 1.77, P < 0.001) and a significant interaction (F [2.12] = 6.77, P < 0.05) but no effect of drug (F [1.12] = 0.62, P < 0.46) (Fig. 4). Post hoc analysis demonstrated that proparacaine produced a significant reduction in the number of eye blinks when compared to both baseline and the animals treated with vehicle control (P < 0.05). The effect was short lasting, with blinking coming back up to control levels by 25–30 minutes after drug application.
Figure 4.
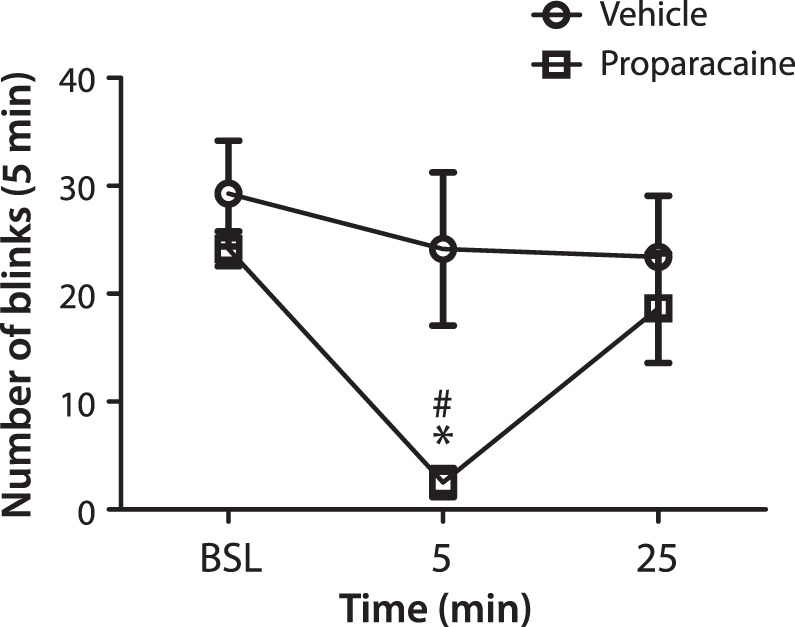
Reduction in blinking produced by the application of a topical anesthetic 8 weeks after double lacrimal gland excision. Baseline (BSL) blinking was recorded over a 5-minute period, followed by either vehicle (circles) or proparacaine hydrochloride (squares) application to the eye. Spontaneous blinking was then recorded 5 to 10 minutes and 25 to 30 minutes post treatment. n = XXX per treatment group. *P < 0.05 versus baseline. #P < 0.05 versus vehicle control.
Corneal Sensitivity
Mechanical sensitivity of the corneal surface was examined using a Cochet-Bonnet esthesiometer. Baseline thresholds were obtained prior to surgery, with thresholds for all treatment groups averaging between 4.3 and 5.5 g/mm2 (Fig. 5A). In animals receiving both single and double LGE, mechanical thresholds were reduced beginning the second week after surgery (P < 0.01, Kruskal-Wallis one-way ANOVA; Fig. 5A). By week 6, these differences were no longer significant, although still numerically lower than baseline. In sham-treated animals, a decrease in corneal thresholds was observed 1 week after surgery; however, this decrease did not reach significance, and values increased toward baseline values in subsequent weeks.
Figure 5.
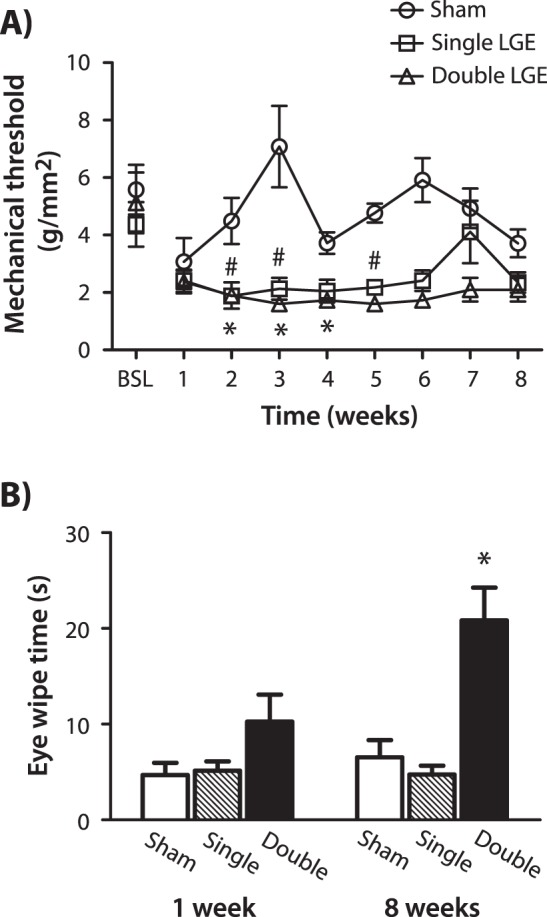
Sensitivity of the cornea after lacrimal gland excision. (A) Mechanical sensitivity assessed using an esthesiometer applied to the cornea. Mechanical thresholds were measured prior to (baseline) and each week following the excision of the exorbital lacrimal gland (single LGE), both the infra- and exorbital gland (double LGE) or sham surgery. *P < 0.05 for single LGE. #P < 0.05 for double LGE versus BSL. (B) Eye wipe behaviors elicited by 5 M saline quantified 1 week and 8 weeks after sham surgery, single LGE, and double LGE. *P < 0.05 for double LGE versus sham and single LGE at the 8-week time point (n = 8 to 10 for all treatment groups).
Sensitivity of the cornea to hypertonic saline was assessed by quantifying wipe behaviors in response to the application of 5 M saline to the eye at 1 and 8 weeks post surgery in separate groups of animals. At the 1-week time point, a one-way ANOVA did not indicate a significant difference between treatment groups (P = 0.34, Kruskal-Wallis one-way ANOVA). However, at the 8-week time point, double LGE produced a significant increase in eye wipe behaviors when compared with sham and single LGE (P < 0.05, Kruskal-Wallis one-way ANOVA; Fig. 5B).
Effect of Morphine on Blinking and Eye Wipe Behavior
Spontaneous blinking and 5 M saline-evoked eye wipe behaviors were quantified after the systemic administration of morphine in sham and double LGE animals (Fig. 6). Morphine produced a dose-dependent decrease in spontaneous blinking in double LGE animals, reaching significance after 2.0 mg/kg (P < 0.05, Kruskal-Wallis one-way ANOVA; Fig. 6A). In sham-treated animals, morphine did not produce a significant difference in the number of blinks (P = 0.36). In these same animals, morphine suppressed eye wipe behavior in both sham surgery and double LGE-treated animals (P < 0.05, Kruskal-Wallis one-way ANOVA; Fig. 6B).
Figure 6.
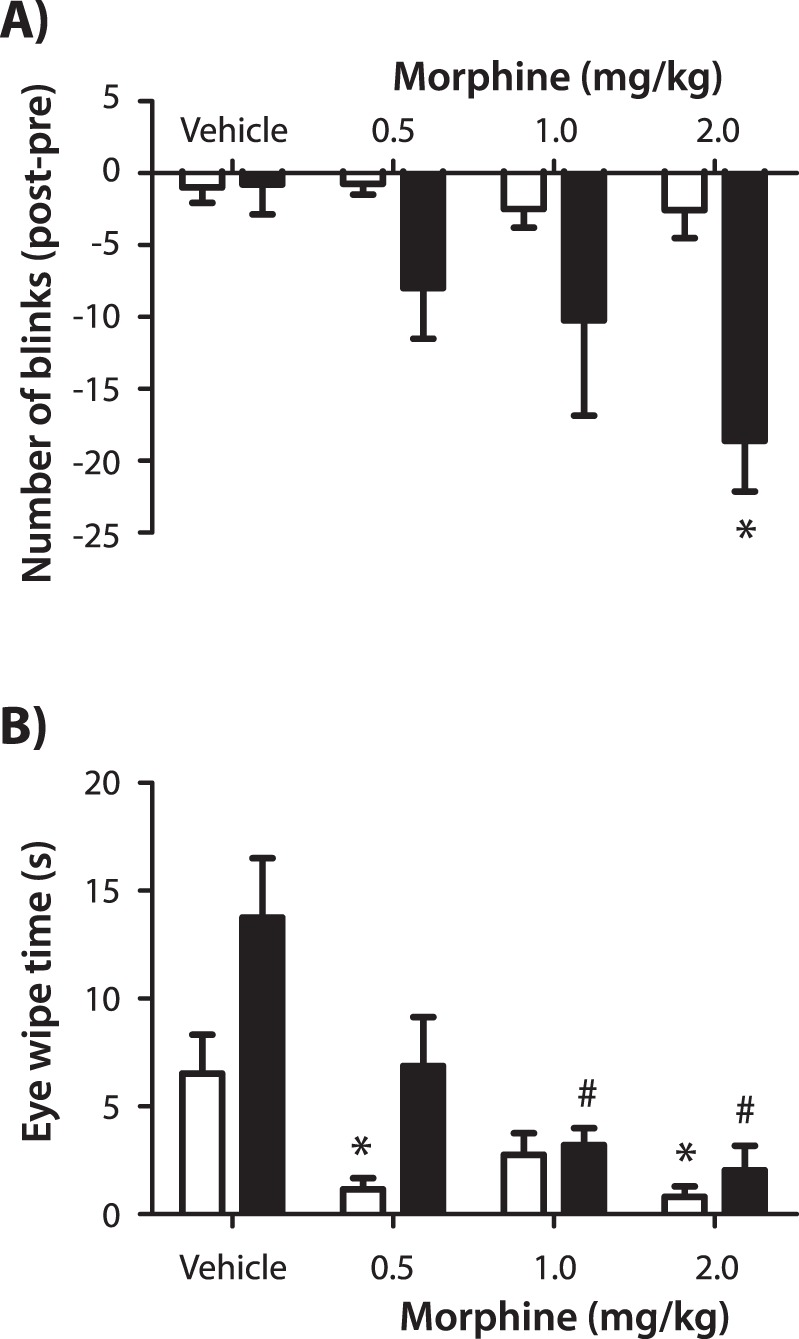
The effect of morphine on spontaneous blinking and hypertonic saline evoked eye wipe behaviors. (A) The difference in spontaneous blinking measured before and 15 to 20 minutes after administration of vehicle or morphine in sham (open bars) and double LGE (filled bars) animals. (B) Eye wipe behaviors quantified after the application of 5 M saline in sham (open bars) and double LGE (filled bars) animals. *P < 0.05 versus the sham vehicle control. #P < 0.05 versus the LGE vehicle control (n = 8 per treatment group).
Discussion
While LGE has been used to produce aqueous tear deficiency in rodents, this is the first study to directly compare the effects of single and double LGE on tear levels and corneal pathology associated with DED. Tear measurements decreased and corneal fluorescein scores increased in a graded fashion, with greater signs of epithelial cell damage associated with double LGE. The excision of both the infra- and exorbital glands also produced a greater increase in spontaneous blinking when compared with single LGE and sham treatment throughout the 8-week observation period. This increase in blinking was reduced by the application of the topical anesthetic proparacaine and systemic morphine. Furthermore, LGE increased sensitivity to mechanical stimuli and hyperosmotic saline applied to the cornea, indicating the sensitization of nociceptive neurons in this model of DED.
The composition of tears includes water, electrolytes, mucins, and other glycoproteins and proteins.31 The lacrimal gland is the major supplier of the aqueous components, including water, electrolytes, and proteins, whereas conjunctival goblet cells are the primary source of mucins.11,31,32 Dry eye disease can result from multiple factors, including reduced or altered composition of tears secreted from glandular tissues. The reduction in tears produced by lacrimal gland excision likely results in aqueous tear deficiency, a specific subclass of DED marked by inadequate tear volume.11,33 Evaporative DED, in contrast, may result from decreased lipid content.33–36
Previous studies have examined ocular conditions following excision of the exorbital lacrimal gland in rats, using this model of aqueous tear deficiency to test the effectiveness of various potential therapies for DED.16,18,22,23,37 In these studies, fluorescein staining was commonly used as the primary endpoint to determine the overall condition of the ocular surface. To this end, compounds that have targeted oxidative stress and purinergic receptors, and the nonsteroidal anti-inflammatory drug diclofenac were all demonstrated to reduce ocular surface damage produced by LGE. The severity of DED symptoms after excision of the exorbital gland—as measured by corneal fluorescein staining—in these studies appears to be greater than that found in the present study. The reasons for these differences are unclear, but may be related to rat age or relative humidity levels in the vivarium. In the present study, the severity of dry eye was much greater after double LGE. Furthermore, the effects of double LGE were also more sustained throughout the 8-week observation period, perhaps indicating that the infraorbital gland may have the ability to compensate for the loss of the exorbital gland over time. If this is the case, then excision of the exorbital lacrimal gland may be an ideal model for studying plasticity in the infraorbital lacrimal gland.
The reduction in blinking following the application of a topical anesthetic in double LGE animals indicates that although these blinks are often referred to as “spontaneous,” they are driven by the activation of corneal afferents.24 The class of afferent neurons responsible for eliciting eye blinks under this condition remains unknown. At least three types of corneal afferents have been characterized according to their receptive field properties.38–48 Mechanoreceptive afferents respond exclusively to mechanical stimulation, polymodal nociceptive afferents are activated by mechanical, noxious thermal, and chemical (e.g., low pH, capsaicin) stimulation, and cold receptive afferents are sensitive to innocuous cooling. All three categories of corneal afferents project to the brainstem region in the trigeminal nucleus that regulates tearing and blinking through projections to preganglionic parasympathetic neurons.5,49–54
Cold receptors are responsible for the regulation of secretions and possibly blinking that occurs in response to the constant evaporative cooling on the ocular surface.43,46 Mechanoreceptors and polymodal nociceptors are more likely involved in noxious stimulation evoked reflexive tearing and blinking as well as the perception of irritation and pain.48,55,56 Lacrimal gland excision has been shown to sensitize corneal cold receptors to cooling and menthol stimulation, suggesting that these neurons may be involved in the increased blinking observed in dry eye animals.25 The suppression by morphine, however, may implicate the activation of nociceptors in blinking following LGE. Morphine has been demonstrated to increase the activity cold cells recorded in the trigeminal nucleus region that regulates tearing and blinking as well as in second-order neurons located in the spinal dorsal horn.57,58
Corneal cold receptors are sensitized following LGE, yet the properties of corneal mechanoreceptors and polymodal nociceptors have not been examined under similar conditions. The lower mechanical thresholds and increased responses to hypertonic saline are signs that corneal nociceptors may also be sensitized in this model of DED. This result is in contrast to reports on corneal sensitivity in human subjects with DED, in which decreased sensitivity to mechanical, thermal, and acidic conditions has been reported.59–61 In contrast to these findings, other studies have observed lower mechanical thresholds in subjects with DED.62–66 Possible reasons for the discrepancy in these results include potential differences in the severity or etiology of the disease among the study participants, as well as differences in the methods used for assessing corneal sensitivity.
Comparing the results from human studies with the present findings in rats is complicated by several factors, including the cause and duration of the dry eye and the absence of any treatment provided to the animals. One possibility is that corneal hypersensitivity occurs during the initial phase of DED, with nerve degeneration and hypoesthesia taking place during later stages of the disease. In order to explore this possibility, changes in corneal sensitivity would need to be carried out over a longer period of time and correlated with morphological alterations in corneal nerves following LGE. Alterations in corneal nerve morphology, including reduced nerve fiber density in the subbasal nerve plexus, have previously been reported in individuals with DED and may be correlated with the occurrence of corneal hypoesthesia.60,64,67–70
Lacrimal gland excision and other preclinical models of DED have been utilized in evaluating the efficacy of novel therapies.16,18,23 These studies typically examine tear levels, tear break-up time, and the overall condition of the corneal epithelium. Signs of ocular discomfort and pain are not generally quantified, despite the importance of treating these symptoms in patients with DED and their common use as endpoints in clinical studies.71–73 The assessment of corneal sensitivity following LGE now provides an opportunity to examine preclinically the effectiveness of potential therapies in treating ocular pain and irritation. Importantly, these treatments should be tested under conditions in which corneal nociceptors have become sensitized, providing an advantage over previous studies that have examined acute nociceptive responses under healthy ocular conditions.74–76
In summary, unilateral excision of the exorbital lacrimal gland or removing both the infraorbital and exorbital lacrimal glands produced graded symptoms of DED in the rat. These symptoms included increased corneal fluorescein staining and spontaneous eye blinks on the side ipsilateral to gland excision. In addition, signs of persistent sensitization developed, including a decrease in corneal mechanical thresholds and increased eye wipe behaviors in response to hypertonic saline over the course of 8 weeks. These features indicate that LGE may provide a useful model for preclinical testing of potential treatments for DED and lead to a more mechanistic approach in the development of novel therapeutics for ocular pain.
Acknowledgments
Supported by the National Eye Institute Grant R01EY021230 and the National Institute of General Medicine Grant P20GM103643 (IDM). The authors declare no competing financial interests.
Disclosure: I.D. Meng, None; S.T. Barton, None; N.E. Mecum, None; M. Kurose, None
References
- 1. Miljanovic B,, Dana R,, Sullivan DA,, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007; 143: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pflugfelder SC. Prevalence burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008; 14: S102–106. [PubMed] [Google Scholar]
- 3. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- 4. Mertzanis P,, Abetz L,, Rajagopalan K,, et al. The relative burden of dry eye in patients' lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005; 46: 46–50. [DOI] [PubMed] [Google Scholar]
- 5. Meng ID,, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res. 2013; 117: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan DA,, Krenzer KL,, Sullivan BD,, Tolls DB,, Toda I,, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999; 40: 1261–1265. [PubMed] [Google Scholar]
- 7. Javadi MA,, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011; 6: 192–198. [PMC free article] [PubMed] [Google Scholar]
- 8. Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. Clao J. 2000; 26: 159–165. [PubMed] [Google Scholar]
- 9. Mantelli F,, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008; 8: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Bijsterveld OP,, Kruize AA,, Bleys RL. Central nervous system mechanisms in Sjogren's syndrome. Br J Ophthalmol. 2003; 87: 128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009; 28: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- 13. Galor A,, Levitt RC,, Felix ER,, Martin ER,, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2014; 29: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barabino S,, Chen W,, Dana MR. Tear film and ocular surface tests in animal models of dry eye: uses and limitations. Exp Eye Res. 2004; 79: 613–621. [DOI] [PubMed] [Google Scholar]
- 15. Barabino S,, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004; 45: 1641–1646. [DOI] [PubMed] [Google Scholar]
- 16. Fujihara T,, Murakami T,, Fujita H,, Nakamura M,, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42: 96–100. [PubMed] [Google Scholar]
- 17. Fabiani C,, Barabino S,, Rashid S,, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009; 89: 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higuchi A,, Takahashi K,, Hirashima M,, Kawakita T,, Tsubota K. Selenoprotein P controls oxidative stress in cornea. PLoS One. 2010; 5: e9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu L,, Shen J,, Zhang C, et al. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Mol Vis. 2009; 15: 250–258. [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu L,, Zhang C,, Chuck RS. Topical steroid and non-steroidal anti-inflammatory drugs inhibit inflammatory cytokine expression on the ocular surface in the botulinum toxin B-induced murine dry eye model. Mol Vis. 2012; 18: 1803–1812. [PMC free article] [PubMed] [Google Scholar]
- 21. Lin Z,, Liu X,, Zhou T, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011; 17: 257–264. [PMC free article] [PubMed] [Google Scholar]
- 22. Kaminer J,, Powers AS,, Horn KG,, Hui C,, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci. 2011; 31: 11256–11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higuchi A,, Inoue H,, Kawakita T,, Ogishima T,, Tsubota K. Selenium compound protects corneal epithelium against oxidative stress. PLoS One. 2012; 7: e45612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dauvergne C,, Evinger C. Experiential modification of the trigeminal reflex blink circuit. J Neurosci. 2007; 27: 10414–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurose M,, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J Neurophysiol. 2013; 110: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stevenson W,, Chen Y,, Lee SM, et al. Extraorbital lacrimal gland excision: a reproducible model of severe aqueous tear-deficient dry eye disease. Cornea. 2014; 33: 1336–1341. [DOI] [PubMed] [Google Scholar]
- 27. Venable JH,, Grafflin AL. Gross anatomy of the orbital glands in the albino rat. J Mammal. 1940; 21: 66–71. [Google Scholar]
- 28. Dursun D,, Wang M,, Monroy D, et al. Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv Exp Med Biol. 2002; 506: 647–655. [DOI] [PubMed] [Google Scholar]
- 29. Suwan-apichon O,, Rizen M,, Rangsin R,, et al. Botulinum toxin B-induced mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2006; 47: 133–139. [DOI] [PubMed] [Google Scholar]
- 30. Wakuta M,, Morishige N,, Chikama T,, Seki K,, Nagano T,, Nishida T. Delayed wound closure and phenotypic changes in corneal epithelium of the spontaneously diabetic Goto-Kakizaki rat. Invest Ophthalmol Vis Sci. 2007; 48: 590–596. [DOI] [PubMed] [Google Scholar]
- 31. Tiffany JM. The normal tear film. Dev Ophthalmol. 2008; 41: 1–20. [DOI] [PubMed] [Google Scholar]
- 32. Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004; 78: 173–185. [DOI] [PubMed] [Google Scholar]
- 33. O'Brien PD,, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004; 4: 314–319. [DOI] [PubMed] [Google Scholar]
- 34. Bron AJ,, Yokoi N,, Gafney E,, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009; 7: 78–92. [DOI] [PubMed] [Google Scholar]
- 35. Borchman D,, Foulks GN,, Yappert MC,, Mathews J,, Leake K,, Bell J. Factors affecting evaporation rates of tear film components measured in vitro. Eye Contact Lens. 2009; 35: 32–37. [DOI] [PubMed] [Google Scholar]
- 36. Foulks GN,, Borchman D. Meibomian gland dysfunction: the past, present, and future. Eye Contact Lens. 2010; 36: 249–253. [DOI] [PubMed] [Google Scholar]
- 37. Sawazaki R,, Ishihara T,, Usui S, et al. Diclofenac protects cultured human corneal epithelial cells against hyperosmolarity and ameliorates corneal surface damage in a rat model of dry eye. Invest Ophthalmol Vis Sci. 2014; 55: 2547–2556. [DOI] [PubMed] [Google Scholar]
- 38. Giraldez F,, Geijo E,, Belmonte C. Response characteristics of corneal sensory fibers to mechanical and thermal stimulation. Brain Res. 1979; 177: 571–576. [DOI] [PubMed] [Google Scholar]
- 39. Belmonte C,, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981; 321: 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacIver MB,, Tanelian DL. Structural and functional specialization of A-d and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci. 1993; 13: 4511–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacIver MB,, Tanelian DL. Free nerve ending terminal morphology Is fiber type specific for A∂ and C fiber innervating rabbit corneal epithelium. J Neurophysiol. 1993; 69: 1779–1783. [DOI] [PubMed] [Google Scholar]
- 42. Hirata H,, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010; 51: 3969–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robbins A,, Kurose M,, Winterson BJ,, Meng ID. Menthol activation of corneal cool cells induces trpm8-mediated lacrimation but not nociceptive responses in rodents. Invest Ophthalmol Vis Sci. 2012; 53: 7034–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belmonte C,, Gallar J,, Pozo MA,, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J Physiol. 1991; 437: 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gallar J,, Pozo MA,, Tuckett RP,, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol. 1993; 468: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parra A,, Madrid R,, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010; 16: 1396–1399. [DOI] [PubMed] [Google Scholar]
- 47. Acosta MC,, Tan ME,, Belmonte C,, Gallar J. Sensations evoked by selective mechanical chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001; 42: 2063–2067. [PubMed] [Google Scholar]
- 48. Belmonte C,, Aracil A,, Acosta MC,, Luna C,, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004; 2: 248–253. [DOI] [PubMed] [Google Scholar]
- 49. Kurose M,, Meng ID. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J Neurophysiol. 2013; 109: 2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hirata H,, Okamoto K,, Tashiro A,, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004; 24: 4224–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okamoto K,, Tashiro A,, Thompson R,, Nishida Y,, Bereiter DA. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci. 2012; 36: 3492–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Henriquez VM,, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007; 179: 691–702. [DOI] [PubMed] [Google Scholar]
- 53. Toth IE,, Boldogkoi Z,, Medveczky I,, Palkovits M. Lacrimal preganglionic neurons form a subdivision of the superior salivatory nucleus of rat: transneuronal labelling by pseudorabies virus. J Auton Nerv Syst. 1999; 77: 45–54. [DOI] [PubMed] [Google Scholar]
- 54. Hirata H,, Takeshita S,, Hu JW,, Bereiter DA. Cornea-responsive medullary dorsal horn neurons: modulation by local opioids and projections to thalamus and brain stem. J Neurophysiol. 2000; 84: 1050–1061. [DOI] [PubMed] [Google Scholar]
- 55. Acosta MC,, Peral A,, Luna C,, Pintor J,, Belmonte C,, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004; 45: 2333–2336. [DOI] [PubMed] [Google Scholar]
- 56. Acosta MC,, Belmonte C,, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001; 534: 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meng ID,, Hu JW,, Bereiter DA. Differential effects of morphine on corneal-responsive neurons in rostral versus caudal regions of spinal trigeminal nucleus in the rat. J Neurophysiol. 1998; 79: 2593–2602. [DOI] [PubMed] [Google Scholar]
- 58. Craig AD,, Hunsley SJ. Morphine enhances the activity of thermoreceptive cold-specific lamina I spinothalamic neurons in the cat. Brain Res. 1991; 558: 93–97. [DOI] [PubMed] [Google Scholar]
- 59. Bourcier T,, Acosta MC,, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005; 46: 2341–2345. [DOI] [PubMed] [Google Scholar]
- 60. Benitez-Del-Castillo JM,, Acosta MC,, Wassfi MA,, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007; 48: 173–181. [DOI] [PubMed] [Google Scholar]
- 61. Stapleton F,, Hayward KB,, Bachand N, et al. Evaluation of corneal sensitivity to mechanical and chemical stimuli after LASIK: a pilot study. Eye Contact Lens. 2006; 32: 88–93. [DOI] [PubMed] [Google Scholar]
- 62. De Paiva CS,, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004; 137: 109–115. [DOI] [PubMed] [Google Scholar]
- 63. Chen J,, Simpson TL. A role of corneal mechanical adaptation in contact lens-related dry eye symptoms. Invest Ophthalmol Vis Sci. 2011; 52: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 64. Tuisku IS,, Konttinen YT,, Konttinen LM,, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren's syndrome. Exp Eye Res. 2008; 86: 879–885. [DOI] [PubMed] [Google Scholar]
- 65. Situ P,, Simpson TL,, Jones LW,, Fonn D. Conjunctival and corneal hyperesthesia in subjects with dryness symptoms. Optom Vis Sci. 2008; 85: 867–872. [DOI] [PubMed] [Google Scholar]
- 66. Situ P,, Simpson TL,, Fonn D,, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008; 49: 2971–2976. [DOI] [PubMed] [Google Scholar]
- 67. Erdelyi B,, Kraak R,, Zhivov A,, Guthoff R,, Nemeth J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 39–44. [DOI] [PubMed] [Google Scholar]
- 68. Villani E,, Galimberti D,, Viola F,, Mapelli C,, Ratiglia R. The cornea in Sjogren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007; 48: 2017–2022. [DOI] [PubMed] [Google Scholar]
- 69. Tuominen IS,, Konttinen YT,, Vesaluoma MH,, Moilanen JA,, Helinto M,, Tervo TM. Corneal innervation and morphology in primary Sjogren's syndrome. Invest Ophthalmol Vis Sci. 2003; 44: 2545–2549. [DOI] [PubMed] [Google Scholar]
- 70. Zhang M,, Chen J,, Luo L,, Xiao Q,, Sun M,, Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005; 24: 818–824. [DOI] [PubMed] [Google Scholar]
- 71. Amparo F,, Dastjerdi MH,, Okanobo A, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. 2013; 131: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meerovitch K,, Torkildsen G,, Lonsdale J,, et al. Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase 2 clinical trial in patients with dry eye. Clin Ophthalmol. 2013; 7: 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Celebi AR,, Ulusoy C,, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. 2014; 252: 619–626. [DOI] [PubMed] [Google Scholar]
- 74. Price TJ,, Patwardhan A,, Akopian AN,, Hargreaves KM,, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004; 141: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Farazifard R,, Safarpour F,, Sheibani V,, Javan M. Eye-wiping test: a sensitive animal model for acute trigeminal pain studies. Brain Res Brain Res Protoc. 2005; 16: 44–49. [DOI] [PubMed] [Google Scholar]
- 76. Urban L,, Campbell EA,, Panesar M,, et al. In vivo pharmacology of SDZ 249-665, a novel, non-pungent capsaicin analogue. Pain. 2000; 89: 65–74. [DOI] [PubMed] [Google Scholar]


