Abstract
Purpose.
To characterize the function and mechanisms of cdc42 and sec10 in eye development in zebrafish.
Methods.
Knockdown of zebrafish cdc42 and sec10 was carried out using antisense morpholino injection. The phenotype of morphants was characterized by histology, immunohistology, and transmission electron microscopy (TEM). To investigate a synergistic genetic interaction between cdc42 and sec10, we titrated suboptimal doses of cdc42 and sec10 morpholinos, and coinjected both morpholinos. To study trafficking, a melanosome transport assay was performed using epinephrine.
Results.
Cdc42 and sec10 knockdown in zebrafish resulted in both abnormal eye development and increased retinal cell death. Cdc42 morphants had a relatively normal retinal structure, aside from the absence of most connecting cilia and outer segments, whereas in sec10 morphants, much of the outer nuclear layer, which is composed of the photoreceptor nuclei, was missing and RPE cell thickness was markedly irregular. Knockdown of cdc42 and sec10 also resulted in an intracellular transport defect affecting retrograde melanosome transport. Furthermore, there was a synergistic genetic interaction between zebrafish cdc42 and sec10, suggesting that cdc42 and sec10 act in the same pathway in retinal development.
Conclusions.
We propose a model whereby sec10 and cdc42 play a central role in development of the outer segment of the retinal photoreceptor cell by trafficking proteins necessary for ciliogenesis.
Keywords: zebrafish, connecting cilium, cdc42, sec10, retinal degeneration, protein trafficking
Cdc42 and sec10 knockdown in zebrafish leads to increased retinal cell death, as well as photoreceptor and transport defects. There was a genetic interaction between cdc42 and sec10, suggesting they act in the same pathway. These data show that sec10 and cdc42 are necessary for retinal development.
Cilia are rod-like, microtubule-based organelles found on most mammalian cell types. Cilia can be classified as motile or nonmotile (primary) cilia. Motile cilia function mainly as motor organelles, whereas primary cilia are sensory organelles.1–3 Dysfunction of primary cilia results in human disorders, such as Bardet-Biedl (BBS), Joubert, and Senior-Loken syndromes, that affect multiple organs, resulting in central nervous system malformation, cystic kidney disease, and retinal dystrophy.4–5 Zebrafish knockdown models for several ciliopathies, including cep290, cc2d2a, inpp5e, ift57, ift88, and ift172, have kidney and retinal phenotypes that suggest insight into the mechanisms underlying these defects.6–9
The zebrafish eye is a well-laminated structure similar to the eyes of other vertebrates. Eye morphogenesis in the zebrafish starts at 11.5 hours post fertilization (hpf) and the eyecup is well-formed by 24 hpf. By 48 hpf, retinal lamination occurs in most of the retina.10 The vertebrate retina is organized into three laminae: the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL). The photoreceptor cell in the ONL has a specialized morphology consisting of an inner segment (IS) and outer segment (OS) linked by connecting cilium, and is a highly polarized and light-sensitive cell. The IS, where protein synthesis occurs, is connected by the cilium to the OS, which consists of a microtubule-based axoneme and membrane disc stacks containing opsin required for phototransduction.11–13 The connecting cilia and basal body in the IS are observed at 50 hpf, and the OS is visible by 54 hpf. The first visual responses are present at approximately 70 hpf, and photoreceptor cells reach adult size by 576 hpf (24 days).10,14
The development and maintenance of photoreceptors requires intracellular trafficking in both photoreceptor and RPE cells. In photoreceptor cells, vesicles containing proteins destined for the OS traffic from the trans-Golgi network to the base of the connecting cilium via vesicular transport.15–17 In RPE cells, intracellular trafficking controls the phagocytosis of disk membranes shed from the tip of the OS.18 To replace lost membrane, the photoreceptor inner segments continuously provide material to the OSs.9 The connecting cilium, therefore, plays an important role as the bridge between the IS and OS.
One of the proteins implicated in vesicular trafficking from the Golgi to the cilium is the small GTPase, Rab8.19 Disruption of Rab8 in Xenopus photoreceptor cells blocks rhodopsin-bearing post-Golgi vesicle trafficking, and results in the abnormal accumulation of rhodopsin carrier vesicles at the base of connecting cilium.15,16 Rab proteins perform functions through downstream effectors, such as the exocyst, a highly conserved eight-protein trafficking complex.20,21 We previously demonstrated that the exocyst is required for ciliogenesis in MDCK cells, due to its role in targeting and docking vesicles carrying ciliary proteins.22 We also showed that Cdc42, another small GTPase, localizes with the exocyst at the primary cilium, and biochemically and genetically interacts with exocyst Sec10.23 Although the roles of Cdc42 and Sec10 in epithelial cell biology are now better understood, their potential functions in eye development are still unknown. Interestingly, we recently found that knockdown of both cdc42 and sec10 in zebrafish resulted in small eyes, and knockdown of cdc42 led to loss of photoreceptor cilia.24,25
Here, we describe the role of cdc42 and sec10 in eye development using histological, functional, and embryonic manipulations in zebrafish. We find that cdc42 and sec10 knockdown results in increased retinal cell death, photoreceptor defects, and intracellular transport defects. We also demonstrate a synergistic genetic interaction between cdc42 and sec10, suggesting that cdc42 and sec10 act in the same pathway in retinal development. These findings indicate that sec10 and cdc42 play a central role in trafficking ciliary proteins to the photoreceptor cell for ciliogenesis.
Methods
Ethics Statement
Wild-type zebrafish embryos were provided by the University of Pennsylvania Zebrafish Core, and were raised at 28.5°C until the appropriate stages. All the zebrafish experiments were approved by the Institutional Animal Care and Use Committees at the University of Pennsylvania and the Philadelphia VAMC, and conform to the ARVO Animal Statement guidelines.
Microinjection for Knockdown and Rescue
Embryos were injected at the one- to two-cell stage, and morpholinos were diluted with phenol red tracer (P0290; Sigma-Aldrich Corp., St. Louis, MO, USA) at 0.05% and injected at 500 pL or 1 nL/embryo. The cdc42 and sec10 morpholinos, designed against zebrafish cdc42 and sec10, were purchased from Gene Tools, LLC (Philomath, OR, USA): cdc42MO (5′-CAACGACGCACTTGATCGTCTGCAT-3′), sec10MO (5′-AATATTCTGTAACTCACTTCTTAGG-3′). We described the cdc42MO and sec10 morpholinos in previous publications.24,25 Morpholinos were injected either as single doses of 1, 2, or 3 ng cdc42MO (designated in the text as “cdc42MO”) or a combined dose of 1 or 2 ng cdc42MO + 7.5 ng sec10MO (designated in the text as “2 ng cdc42MO + 7.5 ng sec10MO”) per embryo. For the rescue experiments, capped human SEC10 full-length mRNA was synthesized using the mMessage mMachine T7 kit per the instructions of the manufacturer (AM1344; Ambion, Grand Island, NY, USA); 25 to 150 pg of SEC10 mRNA was coinjected with the morpholinos into two- to four-cell stage embryos.
Quantification of Eye/Body Size
To compare eye-to-body ratio between injection controls and morphant zebrafish embryos, the diameter of zebrafish eyes and the body length were measured with Fiji (ImageJ) software, version 1.47g (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) using images of whole embryos collected with a Leica M205 C microscope (Leica Microsystems, Wetzlar, Germany) and a DFC450 digital camera (Leica Microsystems). The eye-to-body length ratios were determined by collecting one image at the area of widest diameter from each morphant or wild-type embryo.
Histological Analysis
Zebrafish embryos were fixed in 4% paraformaldehyde in 1× PBS at 4°C overnight. After gradual dehydration into ethanol, embryos were embedded in paraffin, and were sectioned at 4-μm thickness. For immunohistochemistry, the sections were deparaffinized and epitope retrieval was performed by heating the sections at 95°C in 10 mM sodium citrate buffer pH 6.0 for 10 minutes. After treating in 0.5% hydrogen peroxide for 5 minutes at room temperature, the sections were blocked by normal serum according to the instructions for the VECTASTAIN Elite ABC kit (PK-6101 and PK-6102; Vector Laboratories, Burlingame, CA, USA). Rabbit anti-cleaved caspase-3 (9661; Cell Signaling, Danvers, MA, USA) was used to detect apoptosis, and hematoxylin was used for counterstaining.
Western Blot Analysis
Dechorionated zebrafish embryos at 120 hpf were homogenized in SDS sample buffer containing protease inhibitor cocktail (P2714; Sigma-Aldrich Corp.) and phosphatase inhibitor (78420; Thermo, Rockford, IL, USA) to perform Western blot analysis. The homogenized lysates were boiled for 5 minutes at 95°C followed by centrifugation at 17,136g for 20 minutes at 4°C, and the supernatants were collected and mixed with 3× Laemmli sample buffer for the protein electrophoresis. The protein samples were separated on NuPage 4% to 12% Bis-Tris gels (NP0336; Novex, Carlsbad, CA, USA) and then transferred to a nitrocellulose membrane (LC2000; Novex). The antibodies used in this study were mouse monoclonal anti-Cdc42 (610929; BD Transduction Laboratories, San Jose, CA, USA), mouse monoclonal anti-γ tubulin (ab11316; Abcam, Cambridge, MA, USA), mouse monoclonal anti-acetylated α-tubulin (T6793; Sigma-Aldrich Corp.), mouse anti-rhodopsin [1D4] (ab5417; Abcam), and mouse anti-Sec8 (ADI-VAM-SV016; Enzo, Farmingdale, NY, USA). Secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA, USA) and Thermo Fisher Scientific (Waltham, MA, USA).
Transmission Electron Microscopy (TEM)
Injection control and morphant larvae were fixed in a solution containing 2.5% glutaraldehyde, 2% paraformaldehyde, and postfixed with 2% osmium tetroxide. The fixed tissue was sectioned and rinsed with 100 mM cacodylate buffer, dehydrated through a graded ethanol series, and infiltrated with Epon resin. Samples were processed by the Electron Microscopy Resource Laboratory at the University of Pennsylvania.
Melanosome Transport Assay
The melanosome transport assay was performed as described.26,27 Briefly, 120 hpf embryos were exposed to epinephrine (50 mg/mL) at a final concentration of 2 mg/mL in a dark room, and melanosome retractions were observed under the bright field microscope. The aggregation endpoint was scored when all melanosomes in the head and trunk were perinuclear.
Imaging
All images were captured in TIF format and processed in Adobe Photoshop CS5.1 (Adobe Systems, Inc., San Jose, CA, USA). For immunofluorescence, zebrafish embryos were imaged on an Olympus BX41 (Tokyo, Japan) and a Zeiss Axio Observer D1m (Oberkochen, Germany). Histology samples were imaged using a Leica M205C light microscope.
Statistical Analysis
We compared the frequency of the small-eye phenotype across different exposures using logistic regression, clustered by trial. Synergistic effects and phenotype-rescue were explored using Fisher's exact test. These tests were performed using Stata software v12.1 (Stata Corp., College Station, TX, USA). For comparison of means, a Student's t-test was performed using Microsoft Office Excel (2007) (Microsoft Corp., Redmond, WA, USA). For all tests, P values less than 0.05 were considered statistically significant.
Results
Knockdown of cdc42 and sec10 Reduces Eye Size in Zebrafish
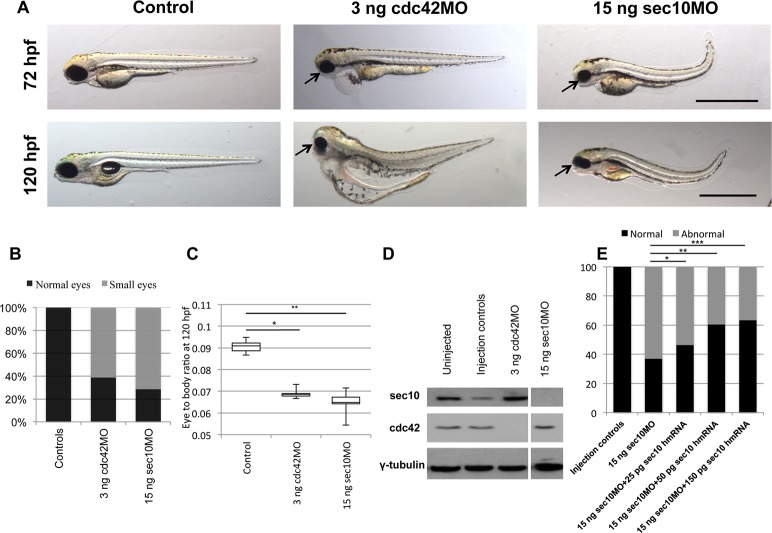
We previously showed that the knockdown of cdc42 and sec10 in zebrafish is associated with ciliary defects, including tail curvature, glomerular expansion, and MAPK pathway activation.24,25 The phenotypic defects occur at varying levels of severity in cdc42 and sec10 morphants; however, the eye phenotype was very similar in the cdc42 and sec10 morphants at 72 hpf and 120 hpf (Fig. 1A). Most cdc42 and sec10 knockdown morphants had small eyes in comparison to their wild-type siblings; 0% of wild-type, 61.4% of 3 ng cdc42MO, and 71.4% of 15 ng sec10MO had small eyes (n = 311). To investigate how cdc42 and sec10 affect eye development, we measured eye size and body length at 28 hpf, 52 hpf, 72 hpf, and 120 hpf in cdc42 and sec10 morphants (Supplementary Fig. S1). Compared with the embryos that were injected with dye only (controls), embryos injected with cdc42 and sec10 morpholinos displayed significantly smaller eyes from 28 hpf onward (Supplementary Fig. S1A). Interestingly, the body length of cdc42 and sec10 knockdown embryos was also somewhat shorter at the same time points (Supplementary Fig. S1B). Nevertheless, after normalizing the eye size with the body length of the embryos, the cdc42 and sec10 morphants still had a significantly smaller eye/body ratio compared with the controls after 72 hpf (data not shown). By 120 hpf, the eye/body ratio was further reduced in cdc42 and sec10 morphants (Fig. 1C).
Figure 1.
Small eyes are seen following knockdown of cdc42 and sec10. (A) Live images of 72 hpf and 120 hpf injection control, cdc42, and sec10 morphant embryos. Small eyes were seen in the cdc42 and sec10 morphants (arrows). (B) Percentages of control, cdc42, and sec10 morphant embryos with small eyes at 120 hpf. (C) A boxplot of eye/body size ratios of control, cdc42, and sec10 knockdown larvae at 120 hpf. (D) The Cdc42 and Sec10 proteins at 120 hpf were undetectable by Western blot in 3 ng cdc42 and 15 ng sec10 morphants, respectively. (E) The sec10MO morphants were rescued by coinjecting zebrafish sec10 mRNA and human SEC10 mRNA (hmRNA), which is resistant to the zebrafish sec10MO, due to a difference in primary base pair structure. *P = 0.2964; **P = 0.0087; ***P = 0.0012. Scale bars: 1 mm.
Cdc42 and Sec10 protein levels at 120 hpf were undetectable by Western blot in the cdc42 and sec10 morphant embryos (Fig. 1D). To rule out off-target effects of sec10MO, we added human SEC10 mRNA, which is resistant to the zebrafish sec10MO due to a difference in primary base pair structure, to zebrafish embryos to see whether this could rescue the defect in sec10 morphants. After injection of different amounts of human SEC10 mRNA, eye size was partially rescued (Fig. 1E). The specificity of cdc42MO was confirmed in our previous study,24 in which cdc42MO embryos were rescued by coinjecting with mouse Cdc42 mRNA, which is resistant to the zebrafish cdc42MO.
Knockdown of cdc42 and sec10 Results in Retinal Degeneration
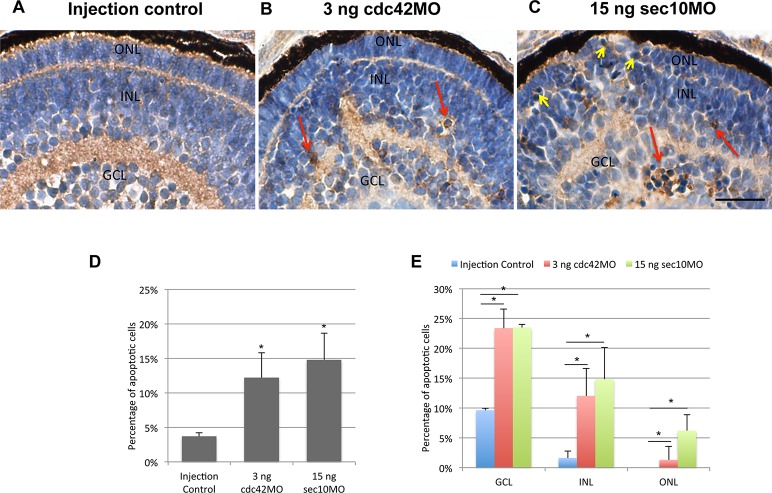
We next investigated the eye phenotype seen in Figure 1 in more detail by histological analysis. Examination of hematoxylin and eosin–stained histological sections at 120 hpf revealed a significant reduction in retinal size and cell number in cdc42 and sec10 morphants when compared with control embryos (Fig. 2). Cdc42 morphants had relatively normal retinal structure except for the ONL thickness (Figs. 2A, 2B, arrow), which is composed of the nuclei of photoreceptor cells. By contrast, we found that in sec10 morphants, much of the ONL was missing and the RPE cell thickness was irregular (Fig. 2C). We next evaluated whether the reduced cell number was associated with cell death by performing immunohistochemistry on fixed retinal sections using antibody against activated caspase-3 (Fig. 3). High rates of apoptosis were seen in the retina of both cdc42 and sec10 morphants at 72 hpf (Figs. 3B, 3C). Whereas levels of apoptosis were low in control embryos (3.7%), 12.2% of cells stained positive for activated caspase-3 in the retinas of cdc42 morphants, and 14.8% in the retinas of sec10 morphants (Fig. 3D). In injection controls, the caspase-3–positive cells were mostly observed in the GCL (9.6%) and INL (1.6%), but were not found in the ONL (Fig. 3E). This is similar to previous studies, which showed that cell death in the GCL is highest at 72 hpf.28 Most apoptotic cells in cdc42 morphant retinas were located in the GCL (23.4%) and INL (12.0%), with only a few apoptotic cells found in ONL cells (1.3%). In sec10 morphant retinas, the apoptotic cells were found not only in GCL cells (23.5%) and INL (14.1%) cells, but also in ONL cells (6.2%). Interestingly, 72 hpf sec10 morphants had relatively normal ONL structure (Fig. 3C) compared with 120 hpf morphants (Fig. 2C), although we found several apoptotic cells in sec10 morphant ONL cells (Fig. 3C, yellow arrows). These results indicate that loss of sec10 results in disruption of the ONL after 72 hpf. The cell number and apoptosis data, taken together, suggest that loss of cdc42 and sec10 results in lower retinal cell numbers due, at least in part, to increased apoptosis, leading to a smaller eye size.
Figure 2.
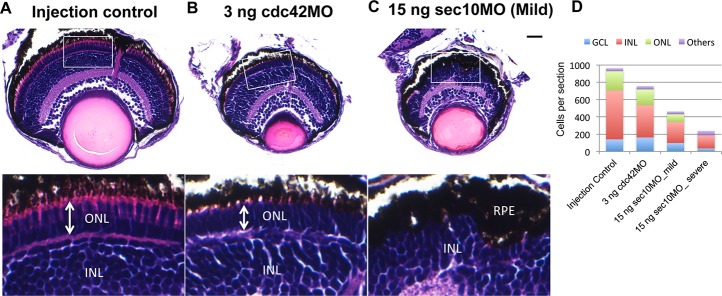
Knockdown of cdc42 and sec10 results in decreased retinal cell numbers in the developing zebrafish. (A–C) Hematoxylin-eosin (H&E) staining of transverse retinal paraffin sections of control, cdc42, and sec10 morphants at 120 hpf. The cdc42 morphants had relatively normal retinal structure except for the decreased ONL thickness (arrow). The OS is almost completely absent in sec10 morphants. (D) Retinal cell number is reduced in cdc42 and sec10 morphants at 120 hpf. Scale bar: 10 μm.
Figure 3.
Increased apoptosis is seen the retina of cdc42 and sec10 morphants at 72 hpf. (A–C) Casepase-3 staining reveals increased apoptotic cells within the retina of cdc42 and sec10 morphants. (D) The percentage of apoptotic retinal cells was significantly increased in cdc42 and sec10 morphants. (E) The frequency of apoptotic cells in the ONL, INL, and GCL of control, cdc42, and sec10 morphants is shown. *P < 0.001. Scale bars: 10 μm.
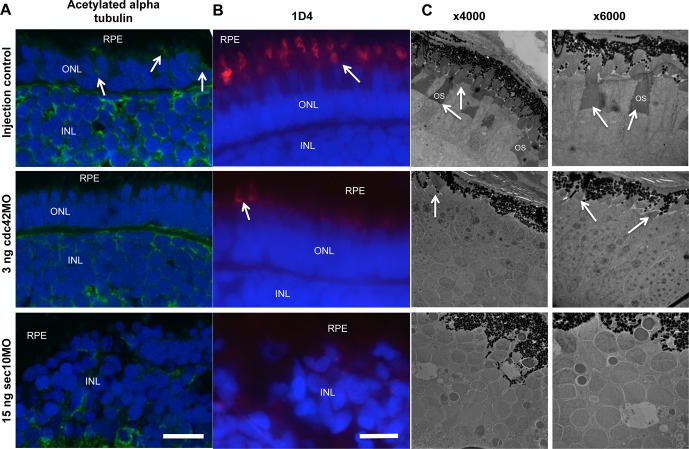
To better evaluate the effects of the cdc42 and sec10 morpholinos on photoreceptor development, we performed immunofluorescence and TEM analysis on 120 hpf embryos. The connecting cilia of the OS of photoreceptor cells were clearly absent in cdc42 and sec10 morphant retinas when probed using antibody against acetylated alpha tubulin, which stains primary cilia (Fig. 4A). We next examined OSs with the anti-rhodopsin antibody, 1D4. In controls, photoreceptor OSs were adjacent to the RPE (Fig. 4B, upper panel). In contrast, the OSs of cdc42 morphant retinas were shorter and less numerous (Fig. 4B, middle and arrows). Transmission electron microscopy analysis of cdc42 morphant embryos was significant for the absence of most ISs and OSs, along with a reduction of the remaining OS size (Fig. 4C, middle, arrows). In the sec10 morphant embryos, OSs were completely absent due to the loss of ONL cells (Figs. 4B, 4C, bottom).
Figure 4.
The cdc42 and sec10 morphant embryos display abnormal OS development. (A, B) Immunofluorescence analysis of control, cdc42, and sec10 morphant zebrafish at 120 hpf. (A) Acetylated alpha tubulin (green), a marker for connecting cilia, localized to the OS region in control (arrow), but not cdc42 and sec10 morphants. (B) A marker for long double cones, 1D4 (red), localized to the OS region in control and cdc42 mutants. Staining was rarely observed in shorter OSs of cdc42 morphants (arrow), and no staining was observed in sec10 morphants. (C) Transmission electron micrographs of transverse sections along the dorsal-ventral axis of control, cdc42, and sec10 morphant zebrafish at 120 hpf. Low- and high-magnification images revealed longer OSs (arrow) in control embryos. Photoreceptor OSs were present in cdc42 morphants, but were significantly shorter and smaller in number than in controls. The OS was not observed in sec10 morphants. Scale bars: 10 μm.
To determine the expression pattern of cdc42 and sec10 gene in zebrafish retina, immunofluorescence was carried out on embryos at 72 and 120 hpf. Unfortunately, the Cdc42 antibody and the Sec10 antibody, which we made, do not work well for immunofluorescence (despite working quite well for Western blot). We, therefore, performed immunofluorescence with exocyst Sec8 antibody, and demonstrated clear staining at the IS region, near the base of the cilium (Supplementary Fig. S2). The exocyst is thought to act as a holocomplex, so Sec8 staining should be a good surrogate for Sec10 staining, and indeed the entire exocyst complex.
cdc42 and sec10 Genetically Interact During Eye Development
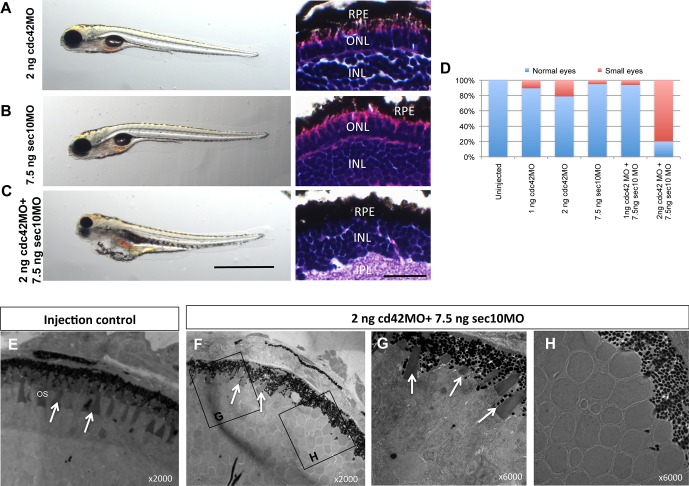
Our previous in vitro and in vivo studies supported a link between sec10 and cdc42.22–24 The ciliary phenotypes shared between cdc42 and sec10 morphant embryos also have been observed on knockdown of other ciliary proteins.29,30 We wanted to directly test for a specific genetic interaction in eye phenotype between these two genes. We titrated dosages of the cdc42 and sec10 morpholinos, and found suboptimal doses that did not result in gross phenotypes on their own (Figs. 5A, 5B, 5D). Importantly, when we coinjected both morpholinos at these reduced doses, we observed a synergistic effect on eye size reduction (Figs. 5C, 5D), and an increased number of apoptotic cells (data not shown). Disrupted photoreceptor OSs were readily observed in 2 ng cdc42MO + 7.5 ng sec10MO morphant retinas by TEM. At 120 hpf, only a small number of OSs were observed in 2 ng cdc42MO + 7.5 ng sec10MO morphant retinas (Figs. 5F, 5G, arrows), which was similar to the phenotype seen with 3 ng of cdc42 morpholino (Fig. 4C). Moreover, low- (Fig. 5F) and high-magnification (Fig. 5H) images revealed regions of missing ONL, as seen in sec10 morphant retinas. These results demonstrate a genetic interaction between cdc42 and sec10, and suggest that sec10 and cdc42 act in the same pathway in retina development.
Figure 5.
cdc42 and sec10 genetically interact. (A–C) Phenotype and H&E staining at 120 hpf shows a severe phenotype in the zebrafish injected with suboptimal doses of both cdc42 and sec10 morpholinos, whereas no effect is observed when the suboptimal doses were injected alone. Scale bar for left: 10 mm. Scale bar for right: 10 μm. (D) The histograms quantified the effect of the MOs. (E–H) Transmission electron micrographs of transverse sections along the dorsal-ventral axis of control and 2 ng cdc42MO+7.5 ng sec10MO at 120 hpf.
Knockdown of cdc42 and sec10 Delays Retrograde Intracellular Transport
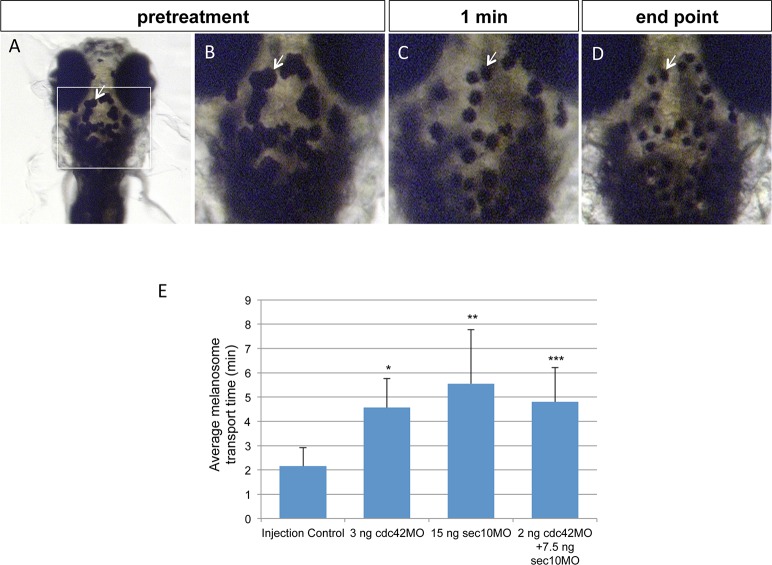
Melanosome movement is an excellent method to examine microtubule-based ciliary transport. Melanosomes are lysosome-related organelles whose bidirectional movements, toward the cell center (aggregation, retrograde transport) or toward the cell periphery (dispersion, anterograde transport), are carried out by different ciliary motor proteins.26 Anterograde transport is accomplished by kinesin II motors, and retrograde transport is performed by dynein motors.31,32 Many ciliary proteins, such as BBS family members, are involved in retrograde melanosome transport in zebrafish.26 Retrograde and anterograde trafficking of melanosomes is known to be stimulated by epinephrine and caffeine, respectively, due to their effects on cyclic AMP levels.31 In our analysis, embryos at 120 hpf displayed melanophore dispersion after overnight dark adaptation (Figs. 6A, 6B). When the injection control embryos were treated with epinephrine, the melanosomes rapidly aggregated and the area of pigmentation was reduced (Fig. 6C, arrow). At the maximum aggregation endpoint, all pigment granules showed perinuclear accumulation of melanosomes (Fig. 6D). The time to reach this pigment aggregation endpoint was measured as the readout. Completion of melanosome transport in wild-type embryos averaged 2.56 minutes (n = 11), whereas 3 ng cdc42MO (n = 11), 15 ng sec10MO (n = 13), and 2 ng cdc42MO+7.5 ng sec10MO (n = 15) morphant embryos showed a statistically significant delay with an average of 5.25 minutes, 7.50 minutes, and 4.94 minutes, respectively (Fig. 6E, n = 50). These data suggest that the cdc42 and sec10 are involved in retrograde transport.
Figure 6.
Knockdown of cdc42 and sec10 delays retrograde intracellular transport. (A) Dorsal view of the head melanocytes of a 120-hpf dark-adapted embryo (pretreatment). The boxed region encompasses the area of high magnification seen in (B–D). (C) Embryos were treated with epinephrine, and the melanosomes rapidly decreased in size. (D) The endpoint occurred when the pigment granules showed perinuclear localization, and the size was maximally decreased. (E) Quantification of the response time to the endpoint for epinephrine treatment in control and cdc42, sec10, and cdc42+sec10 morphants is shown. *P = 0.0177; **P = 0.0154; ***P = 0.0008. This experiment was repeated three times with similar results.
Discussion
In retinal degeneration, photoreceptor cell death is a common endpoint that is reached through a diverse range of cellular dysfunctions. Interestingly, almost one-quarter of known retinal degeneration genes in the Retinal Information Network (RetNet) are associated with ciliary function and trafficking.33 Retinal ciliopathies seem to express their phenotypes through abnormalities in ciliary structure and trafficking, rather than defects in signaling or polarity. For example, proteins involved in Usher and Bardet-Biedl syndromes seem to affect ciliary transport through defects in the docking and loading of vesicles coming from the Golgi complex to the base of the connecting cilium.5,34,35 Primary ciliary dyskinesia, Joubert syndrome, and Senior-Loken syndrome/nephronophthisis involve proteins that are thought to function in microtubule associated protein transport in primary cilia and disk morphogenesis.36–39
One factor implicated in vesicular transport to the connecting cilium is small GTPases that mediate the transport, docking, and fusion of carrier vesicles. These small GTPases are divided into several families, including, Rab, Rho, Ral, and Arf. The Rab family GTPases, Rab8 and Rab11, bind rhodopsin directly and traffic it to the cilium.40 The Rho family GTPase, Rac1, has been implicated in light-induced photoreceptor degeneration as a proapoptotic factor, and also has been linked to the tethering and fusion of rhodopsin-bearing transport carriers with moesin, actin, and Rab8.41,42 Another Rho family GTPase, Cdc42 is expressed in corneal epithelial cells and photoreceptor cells in mice,43 and is also associated with photoreceptor cilia formation in the zebrafish retina.24 Another eye-specific Cdc42 knockdown mouse model showed that lack of Cdc42 affected organization and cell survival in the developing eye, but that mature rod photoreceptors do not need intracellular Cdc42 for normal function.44,45 These studies suggest that Cdc42 is important during eye development, but its mechanism is still uncertain.
Another important component in vesicle trafficking is the exocyst, a highly conserved eight-protein tethering complex, that we showed localizes to cilia,22,46 and is regulated by multiple Rab and Rho family GTPases.47,48 In a recent study, a mutation in an exocyst component, EXO84, was found in a family with Joubert syndrome, a nephronophthisis-related disorder.49 In addition, Sec10, a central component of the eight-protein exocyst complex,22 is necessary for ciliogenesis, and also is associated with eye development in zebrafish.25 Interestingly, Sec10 interacts with Cdc42 in vitro and in vivo,23,24 and Sec10 and Cdc42 were identified in a photoreceptor sensory cilium proteomics study.50 Together, these data strongly suggest that sec10 and cdc42 colocalize to the photoreceptor cell sensory cilium.
Here, we show that knockdown of cdc42 in zebrafish resulted in a relatively normal retinal structure, but led to increased cell death and the absence of most connecting cilia and OSs. This suggests that the absence of cilia might be caused by defects in ciliary protein trafficking, which results in the loss of OSs. Lack of sec10 resulted in severe and progressive degeneration of retinal cells, especially of photoreceptor cells. The 72 hpf sec10 morphants still had ONL structure, but 120 hpf sec10 morphants were completely missing photoreceptor cells, due to apoptosis. Interestingly, we also found a genetic interaction between cdc42 and sec10 that is necessary for retinal development. Suboptimal doses of cdc42 and sec10 morpholinos, that alone had no effect, caused a severe retinal defect when given together. This synergistic result suggests that cdc42 and sec10 act in the same pathway. Retinal defects, similar to what we saw in cdc42 and sec10 morphants, have been similarly described in ciliopathy zebrafish models of intraflagellar transport. The rpgr and inpp5e mutants, which are associated with ciliogenesis and maintenance, have small eyes, cell death, connecting cilium defects, and short or absent OSs.6,27,51,52
In this study, knockdown of cdc42 and sec10 resulted in disruption of the retrograde movement of skin melanosomes along microtubules. Importantly, some ciliary proteins capable of causing retinal degeneration, such as BBS1-8 and Rpgr, have been implicated in the regulation of retrograde melanosome transport by dynein motors, which also are associated with rhodopsin transport.26,27 Rhodopsin requires the dynein light chain, Tctex-1, which binds directly to the dynein intermediate chain and rhodopsin, targeting these proteins to the apical surface.53 A recent study in Xenopus showed retrograde trafficking at the early stages of the rod OS, in which OSs are still cone-shaped.54 This study suggests that opsin is trafficked in a retrograde fashion toward the basal portion of the OS in the early development of rod photoreceptors. In the zebrafish, OSs first appear at 60 hpf (cone-shaped) and the first indication of rod formation was evidenced at 192 hpf.14 This is very similar to OS development in Xenopus. Together, these data raise the possibility that cdc42 and sec10 are involved in retrograde transport, and OS formation, in the developing zebrafish.
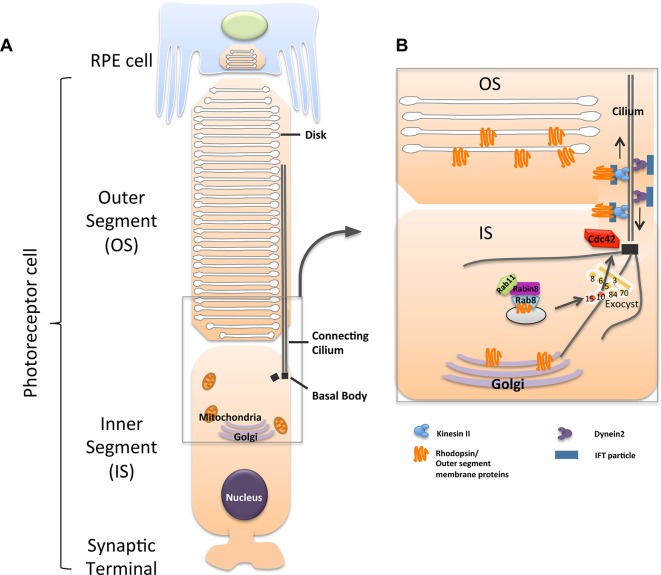
Together with our previous studies,23–25 our findings suggest a model in which cdc42 and sec10 cooperate in the transport and trafficking of proteins necessary for normal retina structure and function in photoreceptor cells (Fig. 7).
Figure 7.
Model for the role of Cdc42 and exocyst Sec10 in delivery of proteins. The connecting cilium originates from the basal body in the IS, and the axoneme extends into the OS. (A) An illustration of the photoreceptor and retinal pigmented cell structures. (B) The illustration focuses on the connecting cilium, a region of disk morphogenesis and protein transport. Vesicles containing rhodopsin/OS membrane proteins express Rab8 on the vesicle surface, and Rab8, in turn, is regulated by Rabin8 and Rab11. The exocyst complex is localized to the primary cilium by Cdc42, and then targets and docks vesicles carrying rhodopsin/OS membrane proteins. This occurs because exocyst Sec15 interacts with Rab8 found on the vesicle surface. Sec10 then binds to Sec15, and pulls the vesicle to the rest of the exocyst complex located at the cilium.
This study is the first to implicate ciliogenesis and photoreceptor development in cdc42- and sec10-related retinal degeneration. This study also confirms a role for cdc42 and sec10 in the development and function of the retina, and suggests trafficking mechanisms that may underlie retinal degeneration. Nevertheless, further studies regarding the association among retrograde trafficking, the exocyst, cdc42, and ciliogenesis are needed to clarify the precise role of cdc42 and sec10 in these processes.
Supplementary Material
Acknowledgments
The University of Pennsylvania Biomedical Imaging Core Facility of Cancer Center is acknowledged for providing histology services, and the University of Pennsylvania Zebrafish Core for providing fish maintenance services.
Supported in part by Department of Veterans Affairs Merit Award I01 and 2I01 BX000820 (JHL), National Institutes of Health Grants DK069909 and DK070980 (JHL), Research to Prevent Blindness, Inc., the F.M. Kirby Foundation, and the Paul and Evanina Bell MacKall Foundation Trust (JLD).
Disclosure: S.Y. Choi, None; J.-I. Baek, None; X. Zuo, None; S.-H. Kim, None; J.L. Dunaief, None; J.H. Lipschutz, None
References
- 1. Bhogaraju S,, Engel BD,, Lorentzen E. Intraflagellar transport complex structure and cargo interactions. Cilia. 2013; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badano JL,, Mitsuma N,, Beales PL,, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006; 7: 125–148. [DOI] [PubMed] [Google Scholar]
- 3. Cardenas-Rodriguez M,, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet. 2009; 151: 263–280. [DOI] [PubMed] [Google Scholar]
- 4. Baker K,, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009; 151: 281–295. [DOI] [PubMed] [Google Scholar]
- 5. Blacque OE,, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 2006; 63: 2145–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo N,, Lu J,, Sun Y. Evidence of a role of inositol polyphosphate 5-phosphatase INPP5E in cilia formation in zebrafish. Vision Res. 2012; 75: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baye LM,, Patrinostro X,, Swaminathan S, et al. The N-terminal region of centrosomal protein 290 (CEP290) restores vision in a zebrafish model of human blindness. Hum Mol Genet. 2011; 20: 1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bachmann-Gagescu R,, Phelps IG,, Stearns G,, et al. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum Mol Genet. 2011; 20: 4041–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sukumaran S,, Perkins BD. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res. 2009; 49: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitt EA,, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999; 404: 515–536. [PubMed] [Google Scholar]
- 11. Besharse JC,, Baker SA,, Luby-Phelps K,, Pazour GJ. Photoreceptor intersegmental transport and retinal degeneration: a conserved pathway common to motile and sensory cilia. Adv Exp Med Biol. 2003; 533: 157–164. [PubMed] [Google Scholar]
- 12. Tsujikawa M,, Malicki J. Genetics of photoreceptor development and function in zebrafish. Int J Dev Biol. 2004; 48: 925–934. [DOI] [PubMed] [Google Scholar]
- 13. Liu Q,, Zhang Q,, Pierce EA. Photoreceptor sensory cilia and inherited retinal degeneration. Adv Exp Med Biol. 2010; 664: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Branchek T,, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984; 224: 107–115. [DOI] [PubMed] [Google Scholar]
- 15. Moritz OL,, Tam BM,, Hurd LL,, Peranen J,, Deretic D,, Papermaster DS. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001; 12: 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deretic D,, Huber LA,, Ransom N,, Mancini M,, Simons K. Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995; 108: 215–224. [DOI] [PubMed] [Google Scholar]
- 17. Deretic D,, Papermaster DS. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci. 1993; 106: 803–813. [DOI] [PubMed] [Google Scholar]
- 18. Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967; 33: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nachury MV,, Loktev AV,, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007; 129: 1201–1213. [DOI] [PubMed] [Google Scholar]
- 20. Das A,, Guo W. Rabs and the exocyst in ciliogenesis tubulogenesis and beyond. Trends Cell Biol. 2011; 21: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hutagalung AH,, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011; 91: 119–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zuo X,, Guo W,, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009; 20: 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuo X,, Fogelgren B,, Lipschutz JH. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem. 2011; 286: 22469–22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi SY,, Chacon-Heszele MF,, Huang L, et al. Cdc42 deficiency causes ciliary abnormalities and cystic kidneys. J Am Soc Nephrol. 2013; 24: 1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fogelgren B,, Lin SY,, Zuo X,, et al. The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet. 2011; 7: e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yen HJ,, Tayeh MK,, Mullins RF,, Stone EM,, Sheffield VC,, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet. 2006; 15: 667–677. [DOI] [PubMed] [Google Scholar]
- 27. Shu X,, Zeng Z,, Gautier P, et al. Zebrafish Rpgr is required for normal retinal development and plays a role in dynein-based retrograde transport processes. Hum Mol Genet. 2010; 19: 657–670. [DOI] [PubMed] [Google Scholar]
- 28. Biehlmaier O,, Neuhauss SC,, Kohler K. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Res. 2001; 306: 199–207. [DOI] [PubMed] [Google Scholar]
- 29. Kramer-Zucker AG,, Olale F,, Haycraft CJ,, Yoder BK,, Schier AF,, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005; 132: 1907–1921. [DOI] [PubMed] [Google Scholar]
- 30. Serluca FC,, Xu B,, Okabe N, et al. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning. Development. 2009; 136: 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nascimento AA,, Roland JT,, Gelfand VI. Pigment cells: a model for the study of organelle transport. Annu Rev Cell Dev Biol. 2003; 19: 469–491. [DOI] [PubMed] [Google Scholar]
- 32. Kimmel CB,, Ballard WW,, Kimmel SR,, Ullmann B,, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995; 203: 253–310. [DOI] [PubMed] [Google Scholar]
- 33. Wright AF,, Chakarova CF,, Abd El-Aziz MM,, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010; 11: 273–284. [DOI] [PubMed] [Google Scholar]
- 34. Reiners J,, Nagel-Wolfrum K,, Jurgens K,, Marker T,, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res. 2006; 83: 97–119. [DOI] [PubMed] [Google Scholar]
- 35. Williams DS. Usher syndrome: animal models retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008; 48: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arts HH,, Doherty D,, van Beersum SE,, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007; 39: 882–888. [DOI] [PubMed] [Google Scholar]
- 37. Adams NA,, Awadein A,, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007; 28: 113–125. [DOI] [PubMed] [Google Scholar]
- 38. Moore A,, Escudier E,, Roger G, et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006; 43: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roepman R,, Letteboer SJ,, Arts HH,, et al. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci U S A. 2005; 102: 18520–18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deretic D,, Wang J. Molecular assemblies that control rhodopsin transport to the cilia. Vision Res. 2012; 75: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haruta M,, Bush RA,, Kjellstrom S, et al. Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc Natl Acad Sci U S A. 2009; 106: 9397–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deretic D,, Traverso V,, Parkins N,, Jackson F,, Rodriguez de Turco EB,, Ransom N. Phosphoinositides ezrin/moesin and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol Biol Cell. 2004; 15: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitchell DC,, Bryan BA,, Liu JP,, et al. Developmental expression of three small GTPases in the mouse eye. Mol Vis. 2007; 13: 1144–1153. [PMC free article] [PubMed] [Google Scholar]
- 44. Heynen SR,, Tanimoto N,, Joly S,, Seeliger MW,, Samardzija M,, Grimm C. Retinal degeneration modulates intracellular localization of CDC42 in photoreceptors. Mol Vis. 2011; 17: 2934–2946. [PMC free article] [PubMed] [Google Scholar]
- 45. Heynen SR,, Meneau I,, Caprara C, et al. CDC42 is required for tissue lamination and cell survival in the mouse retina. PLoS One. 2013; 8: e53806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rogers KK,, Wilson PD,, Snyder RW,, et al. The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun. 2004; 319: 138–143. [DOI] [PubMed] [Google Scholar]
- 47. Knodler A,, Feng S,, Zhang J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010; 107: 6346–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lipschutz JH,, Mostov KE. Exocytosis: the many masters of the exocyst. Curr Biol. 2002; 12: R212–R214. [DOI] [PubMed] [Google Scholar]
- 49. Dixon-Salazar TJ,, Silhavy JL,, Udpa N, et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med. 2012; 4: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Q,, Tan G,, Levenkova N,, et al. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007; 6: 1299–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Insinna C,, Pathak N,, Perkins B,, Drummond I,, Besharse JC. The homodimeric kinesin Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008; 316: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsujikawa M,, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004; 42: 703–716. [DOI] [PubMed] [Google Scholar]
- 53. Yeh TY,, Peretti D,, Chuang JZ,, Rodriguez-Boulan E,, Sung CH. Regulatory dissociation of Tctex-1 light chain from dynein complex is essential for the apical delivery of rhodopsin. Traffic. 2006; 7: 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tian G,, Lodowski KH,, Lee R,, Imanishi Y. Retrograde intraciliary trafficking of opsin during the maintenance of cone-shaped photoreceptor outer segments of Xenopus laevis. J Comp Neurol. 2014; 522: 3577–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.