Abstract
Background
The environmental pollution is one of the factors contributing to the decrease of sperm quality for human beings. The aim of this study was to assess cadmium (Cd), chromium (Cr), and copper (Cu) concentration of man in environmental pollution site, and explore relationships between men exposure to Cd, Cr, and Cu and semen-quality parameters in environmental pollution site.
Methods
Ninety five men were recruited through pollution area and controls in 2011. We measured semen quality using Computer-aided Semen Quality Analysis, and Cd, Cr, and Cu levels in seminal plasma using Graphite Gurnace Atomic Absorption Spectroscopy. Spearman rank correlation analysis was used to evaluate the correlation between Cd, Cr and Cu concentration in seminal plasma and semen quality.
Results
The mean of seminal plasma Cd, Cr, and Cu values in pollution area was higher than the controls. Seminal plasma Cr values displayed a significant negative correlation with total motility and normomorph sperm rate. Seminal plasma Cu values also displayed a negative correlation with normomorph sperm rate.
Conclusions
Male reproductive health may be threatened by environmental pollution, and it may be influence local population diathesis.
Keywords: Environmental pollution, Semen quality, Cadmium, Chromium, Copper, China
Introduction
The male reproductive system is very sensitive to environment. The environmental pollution is one of the factors contributing to the decrease of sperm quality for human beings (1, 2). In recent years, declining sperm counts and quality become an important health issue because environmental pollution (3-5). Some researchers have reported that low levels of cadmium (Cd) accumulation in semen may contribute to male infertility by reducing sperm quality (6). Chromiumis (Cr) is an essential nutrient required for sugar and fat metabolism, excessive Cr can affect human oxidation-reduction and hydrolysis reactions, lead to denaturation of protein, precipitation of nucleic acid, andinterfere with normal enzymatic activity (7). Cr acts as cofactors for a variety of important enzymes, have been associated with reduced semen quality in rodents and in humans (8). Copper (Cu) is also an essential element required for animal and human health, but excessive Cu may be harmful. Cu is cofactors for a variety of important enzymes, and Cu might be mediators of the effects of oxidative damage and plays an essential role in spermatogenesis and male infertility (9, 10).
Rapid development of modern science and technology has lead to newest period of electronic products, thus large quantities of electronic waste (e-waste) are generated. The dismantling and disposal of e-waste in China causes concern because of its impacts on the environment and risks to human health. Uncontrolled family-run e-waste recycling often damage the environment and local people's health (11-13). There are more than 1000 different substances in e-waste, many of which are highly toxic. One of the most materials in e-waste is lead (Pb). Pb exerts known reproductive toxicity. We found that Pb exposure had some correlation with semen quality of men in e-waste environmental pollution site (14). Cd is found in some switches, soldered joints, rechargeable batteries, ultraviolet stabilizers in older PVC cables, and “phosphor” coatings in older cathode ray tubes. Cr is one of the most widely used in electronic devices for various purposes including preventing rust and beautification of steel (15). Cu is widely used in electronics products due to its high electrical conductivity, primarily as a pure metal, or as part of mixture (alloys) with other metal, for example from wires and cables. And there are many other metals in e-waste, including barium, cobalt, gold, mercury, nickel, silver and zinc etc. When e-waste is improperly dismantled and recycled, toxic heavy metal such as Cd, Cr and Cu can be released to the environment. Elevated level of dissolved Cd and Cu was found in river within e-waste pollution area (16). Some river sediments in e-waste pollution site were also contaminated with Cd (n.d.–10.3 mg/kg), the maximum concentration of which was 17.8 times higher than the US Environmental Protection Agency-defined threshold effect level of Cd (0.58 mg/kg) (17,18). Sampling chemical examination showed that Cr concentration in river sediments of e-waste pollution site is 1,338 times higher than the soil-pollution-risk threshold issued by the U.S. environmental protection agency (17).
In this study we presumed that male reproductive health might be influenced by local e-waste environmental pollution. We investigated Cd, Cr and Cu level and sperm quality of man in e-waste environmental pollution site, and explored the effect of e-waste environmental pollutions on male reproductive health.
Materials and Methods
Study Population
Volunteers were recruited from an e-waste environmental polluted area (exposure group) in 2011. Volunteers are workers and other staffs in factory that were engaged in the dealing of e-waste. For control groups volunteers were recruited from towns which is 100 km away (control group one) and 200 km away (control group two) from pollution area, and does not have e-waste factory. The population, traffic density, cultural background, lifestyle, and socioeconomic status of the three sites were similar. This study excluded the adult men that have congenital diseases, hypertension, diabetes mellitus, infection symptom and male reproductive disease. The study was approved by the Human Ethics Committee of Medical School of Ningbo University.
Sample Collection and Storage
Sperm samples were collected from thirty individuals in environmental pollution area, thirty two and thirty three individuals from control sites of control group 1 and control group 2, relatively. Participants had been directed to abstain from ejaculation for 3 to 5 days before providing the semen. Semen was collected via masturbation into separate sterile glass beaker, incubated at 37°C for 30 min, allowing the sperms to stay active. Then semen was diluted and obtained a concentration for computer-aided sperm analysis. After that, the samples were subjected to 1,000 ×g centrifugation for 25 minutes to break the seminal plasma. Seminal plasma was stored at -20°C for detecting Cd, Cr and Cu concentration.
Computer-aided Semen Quality Analysis
After liquefaction and within 1 h of ejaculation, the samples were analyzed for semen volume and pH. Each specimen was diluted at least 1:1 with phosphate buffered saline and loaded into one chamber of a 20-micron-deep chamber, placed on a stage warmer set to 37°C, and observed. The sperm count and motility were determined by an eyepiece reticule and bright-field light microscopy at ×400 total magnification (Sperm analytical system MX7.5). Sperm motility was defined as WHO motility grade A (rapidly progressive motility), grade B (progressive motility), grade C (non progressive motility), or grade D (immotile) by systematic visual scanning of the microscopic field. Each analysis was conducted in duplicate for each specimen on the same microcell by the same technician. Sperm concentration values were measured using the improved Neubauer hemocytometer method according to the WHO 1999 guidelines.
Heavy Metal Concentration Analysis
Semen was collected by masturbation into sterile heavy metal-free plastic containers. After that, the samples were subjected to 1,000 ×g centrifugation for 25 minutes to break the seminal plasma. Seminal plasma were added to portions of optima grade concentrated nitric acid in a glass centrifuge tube at 80°C for removing protein in fume cupboard. The resulting aqueous digests were diluted 5-fold with 2% nitric acid to provide sufficient volume of each sample to determine several heavy metals by Graphite Furnace Atomic Absorption Spectroscopy (SHIMADZU GFA-7000, AA-70000, Japan). The main parameters used for Cd determination were wavelength 228.8 nm, current 2 mA, slit width 1.2 nm, drying at 90, 105, 120°C, ashing at 300°C, and atomization at 1,300°C. The main parameters used for Cr determination were wavelength 357.9 nm, current 4 mA, slit width 0.8 nm, drying at 90, 105, 120°C, ashing at 1,100°C, and atomization at 2,400°C. The main parameters used for Cu determination were wavelength 324.8 nm, current 2 mA, slit width 0.8 nm, drying at 90, 105, 120°C, ashing at 800°C, and atomization at 1,800°C. The relative standard deviation was less than 10%. Recovery rate was between 89.4% and 91.2%.
Statistical Analysis
All analyses were performed using SPSS v13.0 (SPSS Inc., Chicago, IL, USA). Student-Newman-Keuls analysis of One-way ANOVA was used to compare intergroup values of semen quality and Cd, Cr and Cu concentration. Spearman rank correlation analysis was used to evaluate the correlation between Cd, Cr and Cu concentration in seminal plasma and semen quality. A P<0.05 was considered statistically significant.
Results
Cd, Cr and Cu Concentration in Seminal Plasma
Each seminal plasma specimen was assayed in quadruplicate for Cd, Cr and Cu (Table 1). The mean of seminal plasma Cd, Cr and Cu values in exposure group were significantly higher than the controls, populations not occupationally exposed to Cd, Cr and Cu (6.328 µg/L, 3.354 µg/L, 1.356 µg/L).
Table 1.
Seminal plasma heavy metal levels [µg/L Mean (SD)]
| District | Cd | Cr | Cu |
|---|---|---|---|
| Exposure | 0.185(0.340) | 67.969(161.329) | 986.00(3166.477) |
| Control one | 0.102(0.109) | 56.005(128.063) | 405.01(874.626) |
| Control two | 0.058(0.056) | 8.140(5.291) | 80.15(135.858) |
| P value | <0.05 |
Correlation between Semen Quality and Heavy Metal Concentration in Seminal Plasma
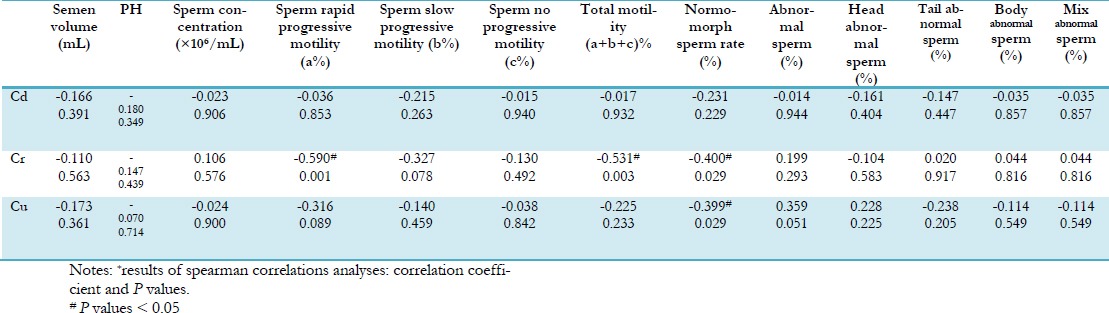
We had previously measured semen quality of the men in e-waste environmental pollution area (14). Table 2 showed the correlation between semen quality and Cd, Cr and Cu concentration in seminal plasma. Seminal plasma Cr values displayed a significant negative correlation with normmorph sperm rate (correlation coefficient: -0.231, P<0.05), motility (correlation coefficient: -0.531, P<0.05), and sperm rapid progressive motility (correlation coefficient: -0.590, P<0.05). Seminal plasma Cu values also displayed a negative correlation with normomorph sperm rate (correlation coefficient: -0.399, P<0.05). Cd did not display a significant correlation with semen quality.
Table 2.
Relationship between seminal plasma heavy metals levels and semen quality*
Discussion
This study indicated that the mean of seminal plasma Cd, Cr and Cu value in e-waste pollution area was higher than the controls. Seminal plasma Cr values displayed a significant negative correlation with sperm motility, sperm rapid progressive motility and norm morph sperm rate. Cu values also displayed a negative correlation with normomorph sperm rate. Cd did not display a significant correlation with semen quality.
High concentrations Cd affect semen quality (19). Even low concentrations of Cd were associated with sperm concentration, motility, morphology, and head pathologic sperms (20, 21). But some studies showed no correlation between higher and low Cd concentrations and sperm concentration, motility, impairment of morphology (22-24). Moreover, this study demonstrated no effect of Cd concentrations in spermatic fluid on sperm density and motility.
Cr exists in two stable states: Cr (III) and Cr (VI). The toxicity of Cr for the different valences is quite different. Cr (III) is a necessary trace element of the human body, but excessive intake of Cr (III) may bring about health damage. Cr (VI) is generally considered 1,000 times more toxic than Cr (III). Cr (III) is not very soluble and is immobilized by precipitation as hydroxides. Cr (VI) is toxic, soluble, and easily transported in water resources (9). The experimental study suggested that Cr may cause testicular atrophy and reduce sperm count and motility (25-27). A reduction in sperm count was seen in male mice fed with 15.1 mg hexavalent Cr/kg/d for 7 wk (28). Occupational group with exposure to Cr have some adverse effects on sperm morphology, motility and physiologic functions (29). Danadevi et al. showed deterioration in sperm quality among welders exposed to Cr, there was a significant negative collocation with blood Cr levels and sperm concentrations (30). Li et al. observed a reduction in the sperm count and motility among Cr exposed workers as compared to controls (31). Cr exposure has some effect on human sperm morphology (32). The present study found a significant association between deterioration in sperm motility, sperm rapid progressive motility, norm morph sperm rate and seminal plasma Cr values.
Cu levels in seminal plasma in the subfertile male group were significantly higher than those in the fertile male group (33). However another study could not observe differences in the levels of Cuin seminal plasma between fertile and subfertile males, and found a significant positive correlation between blood Cu concentrations and sperm motility (34). Li et al. showed a statistically significant negative correlation was observed between the levels of Cu and the sperm concentrations (35). Jockenhövel et al. demonstrated a significant correlation between Cu concentrations and sperm count, motility and normal morphology (36). Ackerman et al. found an effect of high concentrations of Cu on sperm morphology (37). In our study, the concentration of Cu was higher in the pollution group and displayed a negative correlation with normomorph sperm rate.
Conclusion
Seminal plasma Cd, Cr and Cu values in e-waste pollution area were higher than the controls, and there is correlation between the Cr and Cu values of seminal plasma and semen quality. Our results suggest that male reproductive health may be threatened by environmental pollution, and it may be influence local population diathesis.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This work was supported by the Science and Technology Project of Ningbo City, China (2013C50054); the Scientific Innovation Team Project of Ningbo (No.2011B82014); the Scientific Research Fund of Zhejiang Provincial Education Department (Y201120665); the Academic Discipline Project of Ningbo University (XKL11D2112); the Natural Science Foundation of Zhejiang Province, China (LY12H04002). The research was sponsored by K.C. Wong Magna Fund in Ningbo University. The authors declare that there is no conflict of interest.
References
- Hernßndez-Ochoa I, García-Vargas G, López-Carrillo L, Rubio-Andrade M, Morßn-Martínez J, Cebrißn ME, Quintanilla-Vega B (2005). Low lead environmental exposure alters semen quality and sperm chromatin condensation in northern Mexico. Reprod Toxicol, 20(2):221–228. [DOI] [PubMed] [Google Scholar]
- Sharpe RM (2010). Environmental/lifestyle effects on spermatogenesis. Philos Tra R Soc Lond B Biol Sci, 365(1546):1697–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Liu J, Li P, Cao J (2010). Male reproductive and behavior toxicity in rats after subchronic exposure to organic extracts from Jialing River of Chongqing, China. Birth Defects Res B Dev Reprod Toxico, 89(1):34–42. [DOI] [PubMed] [Google Scholar]
- Hammoud A, Carrell DT, Gibson M, Sanderson M, Parker-Jones K, Peterson CM (2010). Decreased sperm motility is associated with air pollution in Salt Lake City. Fertil Steril, 93(6):1875–1879. [DOI] [PubMed] [Google Scholar]
- Wu J, Hu G, Wang X, Li D, Yu H, Han X (2010). The reproductive toxicity of organic compounds extracted from drinking water sources on Sprague Dawley rats: an in vitro study. Environ Toxicol, 25(3):284–293. [DOI] [PubMed] [Google Scholar]
- Wu HM, Lin-Tan DT, Wang ML, Huang HY, Wang HS, Soong YK, Lin JL (2009). Cadmium level in seminal plasma may affect the pregnancy rate for patients undergoing infertility evaluation and treatment. Reprod Toxicol 25(4):481–484. [DOI] [PubMed] [Google Scholar]
- Yu-gang J, Jing L, Wei P (2006). Advances in the safety of chromium. Foreign Medical Sciences (Section of Medgeography), 27:97–99. [Google Scholar]
- Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ (2008). Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116(11):1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir B, Kiziler AR, Onaran I, Alici B, Ozkara H, Akyolcu MC (2006). Impact of Cu and Fe concentrations on oxidative damage in male infertility. Biol Trace Elem Res 112(3):193–203. [DOI] [PubMed] [Google Scholar]
- Sakhaee E, Emadi L, Abshenas J, Kheirandish R, Azari O, Amiri E (2012). Evaluation of epididymal sperm quality following experimentally induced copper poisoning in male rats. Andrologia, 44 Suppl 1:110–116. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu X, Liu J, Wu K, Gu C, Shao G, Chen S, Chen G, Huo X (2008). The Hazard of Chromium Exposure to Neonates in Guiyu of China. Sci Total Environ, 403(1–3):99–104. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu X, Wu K, Chen G, Liu J, Chen S, Gu C, Zhang B, Zheng L, Zheng M, Huo X (2008). Monitoring of Lead Load and its Effect on Neonatal Behavioral Neurological Assessment Scores in Guiyu, an Electronic Waste Recycle Town in China. J Environ Monit 10(10):1233–1238. [DOI] [PubMed] [Google Scholar]
- Li Y, Huo X, Liu J, Peng L, Li W, Xu X (2011). Assessment of cadmium exposure for neonates in Guiyu, an electronic waste pollution site of China. Environ. Monit Assess, 177(1–4):343–351. [DOI] [PubMed] [Google Scholar]
- Li Y, Li M, Li M, Gao X, Gao Q. Semen Quality and Lead Concentration of Men in Electronic Waste Environmental Pollution Site, China (2013). Polish J Environ Stud 22(2): 119–123. [Google Scholar]
- Musson SE, Vann KN, Jang YC, Mutha S, Jordan A, Pearson B, ownsend TG (2006). RCRA toxicity characterization of discarded electronic devices. Environ Sci Technol 40(8):2721–2726. [DOI] [PubMed] [Google Scholar]
- Wong CS, Duzgoren-Aydin NS, Aydin A, Wong MH (2007b).Evidence of excessive releases of metals from primitive e-waste processing in Guiyu, China. Environ Pollut 148(1):62–72. [DOI] [PubMed] [Google Scholar]
- Wong CS, Wu SC, Duzgoren-Aydin NS, Aydin A, Wong MH (2007a). Trace metal contamination of sediments in an e-waste processing village in China. Environ Pollut 145(2):434–442. [DOI] [PubMed] [Google Scholar]
- Ingersoll CG, Haverland PS, Brunson EL (1996). Calculation and evaluation of sediment effect concentrations for the amphipod hyalella azteca and the midge chironomus riparius. J Great Lakes Res, 22:602–623. [Google Scholar]
- Akinloye O, Arowojolu AO, Shittu OB, Anetor JI (2006). Cadmium toxicity: a possible cause of male infertility in Nigeria. Reprod Biol 6(1):17–30. [PubMed] [Google Scholar]
- Benoff S, Hauser R, Marmar JL, Hurley IR, Napolitano B, Centola GM (2009). Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers. Mol Med, 15(7–8):248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telisman S, Cvitković P, Jurasović J, Pizent A, Gavella M, Rocić B (2000). Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect 108(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Moreno JM, Roca M, Vergara-Jußrez N, Martínez-García MJ, García-Sßnchez A, Elvira-Rendueles B, Moreno-Grau S, López-Espín JJ, Ten J, Bernabeu R, Torres-Cantero AM (2001). Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health 10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O, Venäläinen ER, Kuusimäki L, Heikkilä J, Hirvi T, Reima I (1998). Aluminium, lead and cadmium concentrations in seminal plasma and spermatozoa, and semen quality in Finnish men. Hum Reprod 13(1):115–119. [DOI] [PubMed] [Google Scholar]
- Chia SE, Xu B, Ong CN, Tsakok FM, Lee ST (1994). Effect of cadmium and cigarette smoking on human semen quality. Int J Fertility Menopausal Studies 39(5):292–298. [PubMed] [Google Scholar]
- Saxena DK, Murthy RC, Lal B, Srivastava RS, Chandra SV (1990). Effect of hexavalent chromium on testicular maturation in the rat. Reprod Toxicol 4(3):223–228. [DOI] [PubMed] [Google Scholar]
- Ernst E (1990). Testicular toxicity following short-term exposure to tri- and hexavalent chromium: an experimental study in the rat. Toxicol Lett 51(3):269–275. [DOI] [PubMed] [Google Scholar]
- Ernst E, Bonde JP (1992). Sex hormones and epididymal sperm parameters in rats following sub-chronic treatment with hexavalent chromium. Hum Exp Toxicol 11(4):255–258. [DOI] [PubMed] [Google Scholar]
- Zahid ZR, AI-Hakkak ZS, Kadhim AHH (1990). Comparative effects of trivalent and hexavalent chromium on spermatogenesis of the mouse. Toxicol Environ Chem, 25:131–136. [Google Scholar]
- Kumar S, Zaidi SS, Gautam AK, Dave LM, Saiyed HN (2003). Semen quality and reproductive hormones among welders -A preliminary study. Environ Health Prev Med 8(2):64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danadevi K, Rozati R, Reddy PP, Grover P (2003). Semen quality of Indian welders occupationally exposed to nickel and chromium. Reprod Toxicol 17(4):451–456. [DOI] [PubMed] [Google Scholar]
- Li H, Chen Q, Li S, Yao W, Li L, Shi X, Wang L, Castranova V, Vallyathan V, Ernst E, Chen C (2001). Effect of Cr (VI) exposure on sperm quality: human and animal studies. Ann Occup Hyg 45(7): 505–511. [PubMed] [Google Scholar]
- Kumar S, Sathwara NG, Gautam AK, Agarwal K, Shah B, Kulkarni PK, Patel K, Patel A, Dave LM, Parikh DJ, Saiyed HN (2005). Semen quality of industrial workers occupationally exposed to chromium. Occup Health 47(5):424–430. [DOI] [PubMed] [Google Scholar]
- Aydemir B, Kiziler AR, Onaran I, Alici B, Ozkara H, Akyolcu MC (2006). Impact of Cu and Fe concentrations on oxidative damage in male infertility. Biol Trace Elem Res 112(3):193–203. [DOI] [PubMed] [Google Scholar]
- Wong WY, Flik G, Groenen PM, Swinkels DW, Thomas CM, Copius-Peereboom JH, Merkus HM, Steegers-Theunissen RP (2001). The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol 15(2):131–136. [DOI] [PubMed] [Google Scholar]
- Li P, Zhong Y, Jiang X, Wang C, Zuo Z, Sha A (2012). Seminal Plasma Metals Concentration with Respect to Semen Quality. Biol Trace Elem Res 148(1):1–6. [DOI] [PubMed] [Google Scholar]
- Jockenhövel F, Bals-Pratsch M, Bertram HP, Nieschlag E (1990). Seminal lead and copper in fertile and infertile men. Andrology 22(6):503–511. [PubMed] [Google Scholar]
- Ackerman DJ, Reinecke AJ, Els HJ, Grobler DG, Reinecke SA (1999). Sperm abnormalities associated with high copper levels in impala. Ecotoxicol Environ 43(3):261–266. [DOI] [PubMed] [Google Scholar]


