Abstract
Pruni cortex, the bark of Prunus jamasakura Siebold ex Koidzumi, has been used in the Japanese systems of medicine for many years for its anti-inflammatory, antioxidant and antitussive properties. In this study, we investigated the effect of pruni cortex on atopic dermatitis NC/Nga mouse model. Atopic dermatitis-like lesion was induced by the application of house dust mite extract to the dorsal skin. After induction of atopic dermatitis, pruni cortex aqueous extract (1 g/kg, p.o.) was administered daily for 2 weeks. We evaluated dermatitis severity, histopathological changes and cellular protein expression by Western blotting for nuclear and cytoplasmic high mobility group box 1, receptor for advanced glycation end products, nuclear factor κB, apoptosis and inflammatory markers in the skin of atopic dermatitis mice. The clinical observation confirmed that the dermatitis score was significantly lower when treated with pruni cortex than in the atopic dermatitis group. Similarly pruni cortex inhibited hypertrophy and infiltration of inflammatory cells as identified by histopathology. In addition, pruni cortex significantly inhibited the protein expression of cytoplasmic high mobility group box 1, receptor for advanced glycation end products, nuclear p-nuclear factor kappa B, apoptosis and inflammatory markers. These results indicate that pruni cortex may have therapeutic potential in the treatment of atopic dermatitis by attenuating high mobility group box 1 and inflammation possibly through the nuclear factor κB pathway.
Keywords: High mobility group box protein 1, inflammation, pruni cortex, nuclear factor κB, atopic dermatitis
Introduction
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease characterized by various factors, including immunological abnormalities and exposure to allergens that contribute to the pathogenesis and development of skin lesions.(1) Activation of T lymphocytes, dendritic cells, macrophages, keratinocytes, mast cells, and eosinophils is characteristic of AD skin inflammatory responses. Atopic patients exposed to house dust mite Dermatophagoides farinae (DfE) develop potent inflammatory diseases such as allergic asthma, perennial rhinitis, and AD.(2)
High mobility group box (HMGB)1, is a member of a subfamily of the high mobility group protein,which is either passively released from injured or necrotic cells or actively secreted by immune cells stimulated by cytokine and endotoxin.(3) Although the role of HMGB1 in the nucleus is not completely understood, its extracellular function has been found to be associated with inflammatory responses. HMGB1 is expressed by almost all cells, and usually located in the nucleus. Accumulative evidences have shown that extracellular HMGB1 is a critical proinflammatory cytokine, which can bind to particular receptors, including receptor for advanced glycation end products (RAGE), toll like receptor (TLR)2 and TLR4 on target cells to induce the production of proinflammatory cytokines, chemokines, adhesion molecules and reactive oxygen species (ROS), leading to inflammation and injury.(4) Activation of these receptors results in the activation of nuclear factor κB (NFκB), which accelerates the production of pro-inflammatory cytokines. NFκB signaling is also tightly linked with Janus kinase/Signal Transducer and Activator of Transcript (JAK/STAT) and phosphatidylinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling. Although PI3K/Akt signaling pathways are important for cellular responses, aberrant activation of these signaling pathways can induce chronic inflammation and inflammatory diseases.(5)
Pruni cortex (PC) (Rosaceae), which is called as the bark of ”sakura” and have been used in Japan and other Asian countries as a traditional medicine to treat several diseases. Folk medicine uses as a cough remedy and several flavonoids (such as flavanone xyloside), have seen isolated from its bark.(6) Five unique constituents (i.e., sakuranetin, naringenin, genistein, genkwanin and arctigenin) were detected in PC.(7) Another constituent, sakuranin, has been shown to have antioxidant and antiproliferative activity.(8) The bark is used for the treatment of food poisoning and also as an antitussive. The water extracts of PC, exhibited a binding effect on estrogen receptor beta (ERβ).(7) In spite of its use in several ailments in traditional medicine, there were no studies on AD or other skin diseases using PC. Hence, the present study was aimed to investigate the ameliorative potential of PC extract on skin inflammation. In addition to explore its mode of action, we have studied its effect on HMGB1 release and NFκB signaling pathway in DfE induced AD mouse model.
Materials and Methods
Materials
Biostir-AD, a cream containing the extract of the DfE was purchased from Biostir, Inc. (Kobe, Japan). Phosphatase arrest-III was purchased from G-Biosciences, St. Louis, MO. Trizma base, sodium chloride, sodium fluoride, sodium orthovanadate, 2-mercaptoethanol, bovine serum albumin (BSA) and Tween 20 were purchased from Wako Pure Chem. Ind., Ltd., Osaka, Japan. Naringenin and genistein were purchased from LKT Laboratories, Inc., Phalen Blvd, St. Paul, MN; Sakuranetin was purchased from Extrasynthese, BP 62-69726 Genay Cedex, France. Unless otherwise stated, all other reagents were of analytical grade and were purchased from Sigma (Tokyo, Japan).
Plant extraction
PC extract was gifted from Kracie Pharma Ltd., Kampo Research Laboratories, Toyama, Japan. The cut crude drug (1.5 kg) of PC was soaked in 15,000 ml water and boiled for 1 h, then centrifuged to remove the herbal medicine residue. Spray drying was done to concentrate the extracted material and the final dry brown color crude extract (180 g) was stored in 4°C until experiment.
Preparation of standard (Std) and sample solutions for high performance liquid chromatography (HPLC)
Stock solutions of naringenin, genistein and sakuranetin were prepared at concentration (conc) of 1,000 µg/ml immediately before use and used as reference Std. The hot water extract of PC was dissolved in water, to obtain a conc. of 2 mg/ml and used as sample solution. All sample solutions were filtrated through a 0.45 µm PVDF disposable syringe filter (Millipore Corporation, Bedford 01730, MA) and injected directly.
Finger print analysis of PC extract by HPLC
The components of PC were determined through an HPLC system, 10AD liquid chromatograph, DGU-20A3R degasser (LC-SHIMADZU, Kyoto, Japan). Reverse-phase chromatographic analysis was carried out in isocratic conditions using a shim-pack CLC column (SHIMADZU), (CLC-ODS(M), 250 × 4.6 mm internal diameter, particle size 5 µm, at 26°C. Running conditions included: injection volume 20 µl; mobile phase for naringenin and genistein, phosphate buffer (pH 7): acetonitrile (70:30), detection at 280 nm; for sakuranetin, methanol: water (6:4);(9) flow rate, 1 ml/min and the chromatogram monitored at 280.0 nm.(10) The chromatographic peaks of the analytes were confirmed by comparing their retention time and UV spectra with those of the reference Std. The naringenin, genistein and sakuranetin (1 to 100 µg/ml) Std solutions were injected into the HPLC and peak height responses obtained. Std graphs were prepared by plotting conc vs peak height. Quantification was carried out from integrated peak height of the samples using the corresponding Std graph.
Animals
Specific pathogen free female 6 weeks old NC/Nga mice were obtained from Charles River Japan (Yokohama, Japan). The animals were maintained in controlled room (temperature 23 ± 2°C, 12 h lighting cycle). After 1 week, the mice (7 weeks old) were randomly divided into 3 groups, untreated group (Normal, n = 6); DfE cream treated mice (100 mg/mouse) were divided into two groups and each received either vehicle (1% methyl cellulose) (AD, n = 6) or PC aqueous extract (1 g/kg/day at evening, per oral) (AD + PC, n = 6) and they were allowed free access to water and chow throughout the period of study (4 weeks). The mice were weighed once a week. Food intake was estimated every second day, always at the same time of the day. The animal experiments were performed in accordance with national guidelines for the use and approved by the animal care committee of Niigata University of Pharmacy and Applied Life sciences.
Induction of AD in NC/Nga mice
AD-like skin lesions were induced in NC/Nga mice using DfE cream, as described previously.(11) Briefly, the hair on the upper back was shaved and 150 µl of 4% (w/v) sodium dodecyl sulfate was applied to the shaved dorsal skin and both surfaces of each ear for barrier disruption. After 3 h, 100 mg of DfE cream was applied topically. This procedure was carried out twice weekly for 2 weeks. PC aqueous extract (1 g/kg/day at evening, per oral, tenfold human dose used in clinical setting (6–9 kg/day/person), which was found be effective during our preliminary studies without any adverse effects) treatment was started after second week of AD induction and continued for 2 weeks (4 weeks total period). The duration of study was chosen on the basis of previous reports.(12) By the end of the study period (4th week), mice were weighted and, sacrificed and skin tissues were harvested for semi-quantitative immunoblotting and immunohistochemical studies.
Evaluation of Dermatitis Severity
The relative dermatitis severity was assessed macroscopically every week according to the eczema area and severity index scoring system using the following scoring procedure: 0, no symptoms; 1, mild symptoms; 2, moderate symptoms; 3, severe symptoms. The dermatitis score was defined as the sum of scores for erythema/haemorrhage, edema, excoriation/erosion, and scaling/dryness.(13)
Histopathological studies
The half of the skin was immediately snap frozen in liquid nitrogen for subsequent protein extraction assays. The remaining excised skin were cut into about 2 mm-thick transverse slices and fixed in 10% formalin. Sections of 3–5 µm thickness were stained with haematoxylin and eosin (HE) for histological examination. A histomorphological evaluation of all the skin sections was carried out in a blinded fashion.
Homogenization and fractionation of skin tissue
Briefly, 100 mg of skin was homogenized in 0.5 ml of buffer A containing 10 mM HEPES, pH 7.8, 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.1 mM PMSF, 1 µM pepstatin, and 1 mM p-aminobenzamidine using a tissue homogenizer for 20 s. Homogenates were kept on ice for 15 min, and then 125 µl of a 10% Triton X-100 solution was added and mixed for 15 s and the mixture was centrifuged for 2 min at 12,000 rpm. The supernatant containing cytosolic proteins was collected. The pelleted nuclei were washed once with 200 µl of buffer A plus 25 µl of 10% Triton X-100, centrifuged, then suspended in 50 µl of buffer B containing 50 mM HEPES, pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 10% v/v glycerol, mixed for 20 min, and centrifuged for 5 min at 12,000 rpm. The supernatant containing nuclear proteins was stored at −80°C.(14) The protein conc of the resulting solution were determined by the bicinchoninic acid method.(15)
Protein analysis by Western blotting
Protein samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. Membranes were blocked in 5% BSA in Tris-buffered saline with Tween (0.2% Tween 20 in 1× Tris-buffered saline) (TBS-T) and incubated using the following antibodies: Antibodies against HMGB1, RAGE, p-NFκB, cleaved caspase7, caspase12, p22phox, Tumor necrosis factor (TNF)α, TNF receptor-associated factor (TRAF)2, p-PI3K, C/Ebp-Homologous Protein (CHOP) and Interleukin1 beta (IL1β) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) or Cell Signaling Technology, Inc. (Danvers, MA) and used at a dilution of 1:1,000. After washing for three times with TBS-T, incubation with appropriate horseradish-peroxidase-conjugated secondary antibodies was performed for 1 h at room temperature. Further, the membranes were washed three times with 1X Tris-buffered saline and then developed using a chemiluminescence detection system (Amersham Biosciences, Buckinghamshire, UK). The signals were quantified with densitometric analysis using Scion Image program (Epson GT-X700, Tokyo, Japan). Antibodies to Lamin A and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as housekeeping proteins for nuclear and cytosolic target proteins, respectively.
Statistical analysis
Data are presented as mean ± SEM and were analyzed using one-way analysis of variance (ANOVA) followed by Tukey multiple comparison test or two-tailed t test when appropriate. A value of p<0.05 was considered statistically significant. For statistical analysis, GraphPad Prism 5 software (San Diego, CA) was used.
Results
HPLC analysis
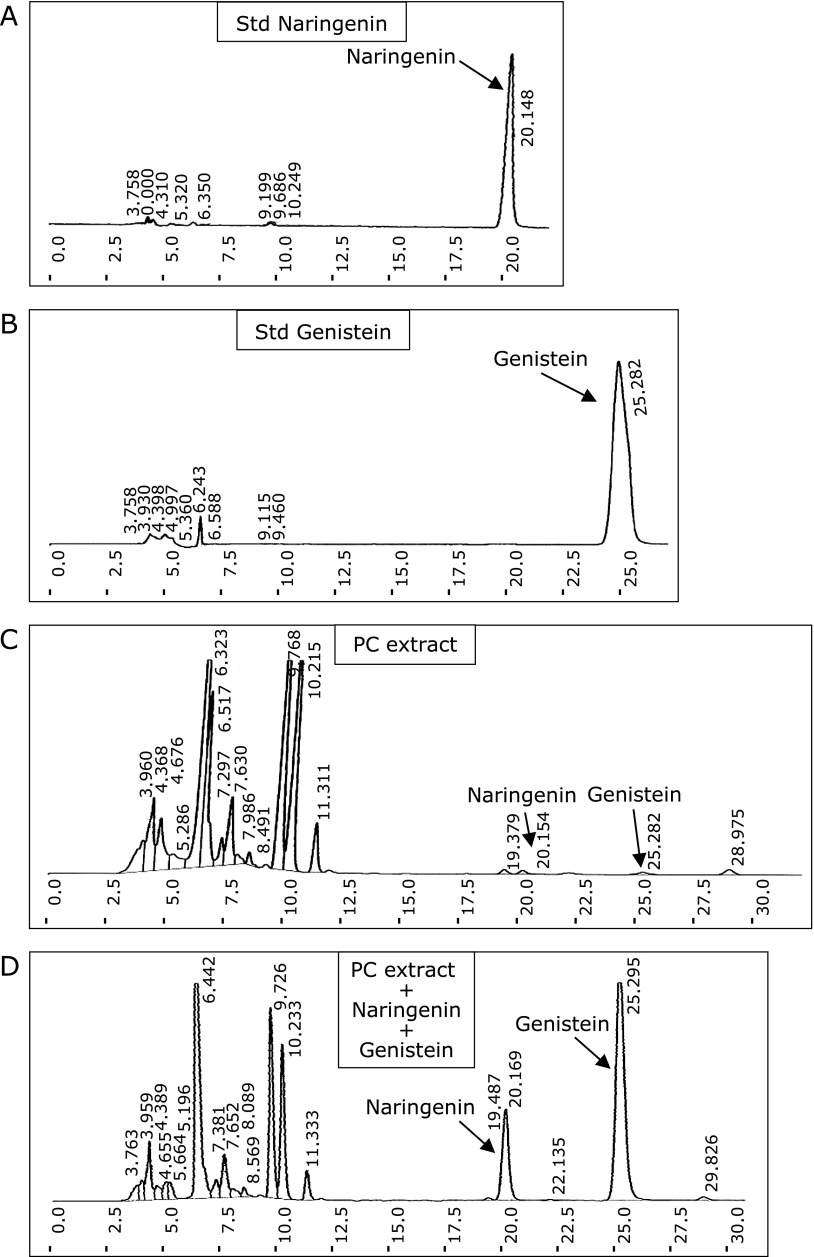
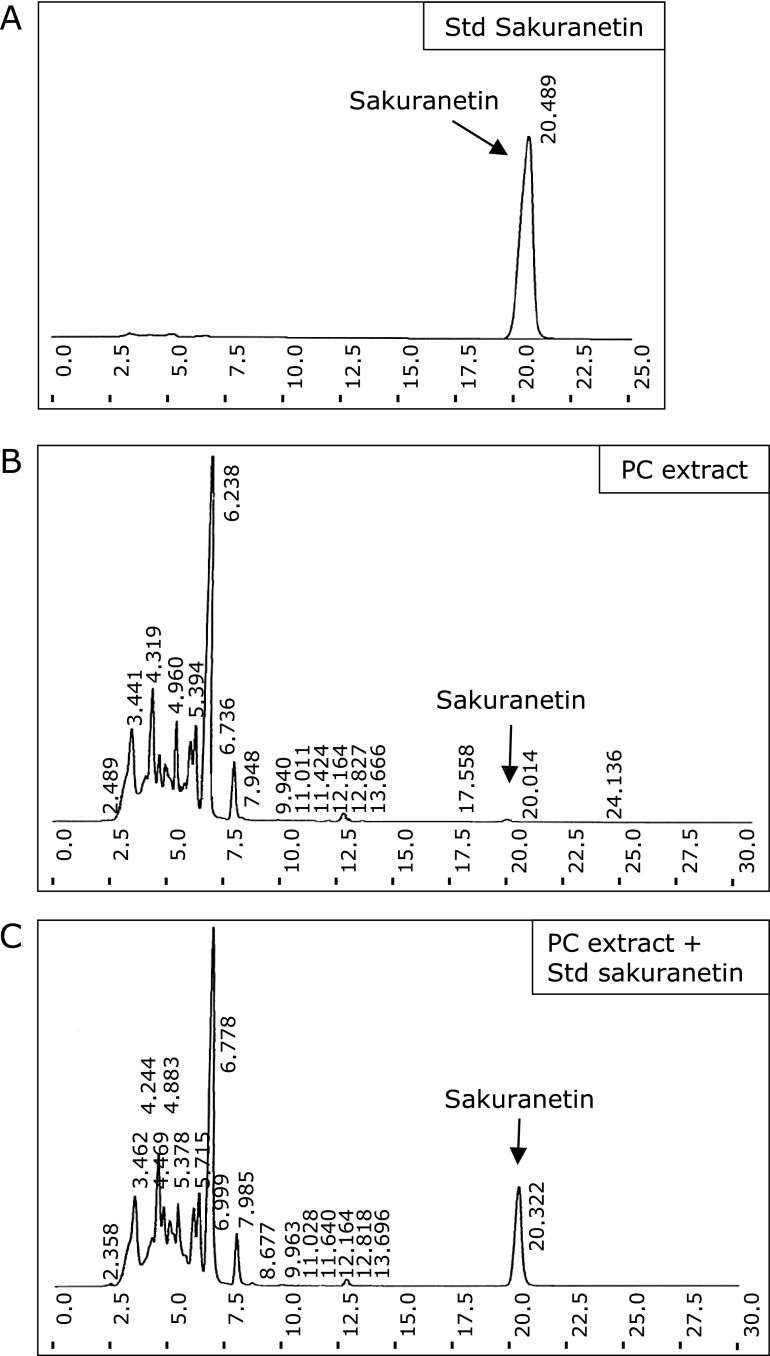
To confirm the extract used in the study possess the major active constituent, PC extract (200 µg/ml) as subjected to HPLC fingerprint and the peak of major active constituent naringenin, genistein and sakuranetin were identified with UV absorption at 280 nm. It was confirmed comparing with the reference Std (10 µg/ml of naringenin, genistein or sakuranetin) and reference Std mixed with PC extract (similar conc). The amount of naringein, genistein and sakuranetin in PC were found to be 4.68 mg/g, 1.15 mg/g and 1.55 mg/g respectively (Fig. 1A–D and Fig. 2A–C).
Fig. 1.
HPLC fingerprint of PC aqueous extract was performed as described in method section. (A), Std naringenin (10 µg/ml). (B), Std genistein (10 µg/ml). (C), PC extract (200 µg/ml). (D), PC extract with Std naringenin and genistein mixture (similar conc). Reference Std of naringenin and genistein were used to compare the obtained peak.
Fig. 2.
HPLC fingerprint of PC aqueous extract was performed as described in method section. (A), Std sakuranetin (10 µg/ml). (B), PC extract (200 µg/ml). (C), PC extract with Std sakuranetin mixture (similar conc). Reference Std of sakuranetin was used to compare the obtained peak.
Effects of PC on dermatitis score and body weight
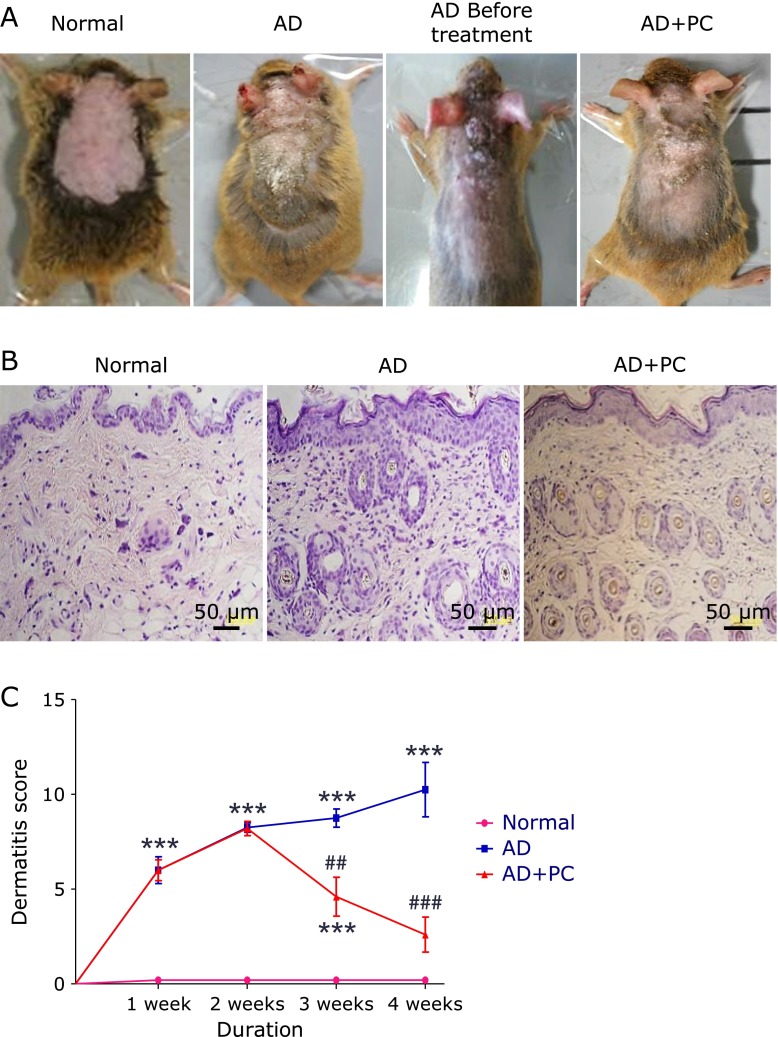
Cutaneous application of DfE cream resulted in immediate itching, erythema, and haemorrhage on the ear and back that was followed by edema, superficial erosion, deep excoriation, scaling and dryness of the skin. The clinical signs and symptoms of dermatitis significantly developed during the course of the study. Whereas the oral administration of PC extract markedly improved these phenotypes in AD + PC group mice (Fig. 3A and C). In addition, we measured mice body weight and food intake, but no significant changes in normal, AD and AD + PC group (data not shown). These results clearly suggest that PC has an ability to modulate skin inflammatory diseases, such as AD.
Fig. 3.
(A), Development of AD-like skin lesions after DfE application in NC/Nga mice. (B), Haematoxylin and eosin staining of the cross-sectional tissue slices of skin depicting hypertrophy, intracellular edema and infiltration of inflammatory cells (400×). (C), Dermatitis score. Each bar represents mean ± SEM. Normal, age-matched normal NC/Nga mice (4th week); AD, AD induced NC/Nga mice (4th week); AD before treatment, AD induced NC/Nga mice before treatment of PC aqueous extract (2nd week), AD + PC, AD induced mice administered with PC aqueous extract (4th week). ***p<0.001 vs Normal; ##p<0.01 and ###p<0.001 vs AD.
Effects of PC on histopathological analysis
HE staining of the dorsal skin sections revealed hypertrophy, intracellular edema and infiltration of inflammatory cells into the upper dermis of AD mice. These observations were all suppressed by PC treatment. These results suggest that PC decreases AD symptoms in NC/Nga mice (Fig. 3B).
PC attenuated AD-induced increases in cytoplasmic HMGB1 levels in the AD skin
To better establish the altered distribution of HMGB1 in lesional skin of AD mice, Western blot was performed to investigate the HMGB1 expression in cytoplasm and nuclear fractions extracted from the lesional skin of AD and normal mice. The ratio of HMGB1 expression in cytoplasm protein to nuclear protein was measured by densitometrical analysis. As presented in Fig. 4A, increased HMGB1 expression was seen in the cytosolic fraction than in the nuclear fraction in group AD mice. In contrast, PC treatment significantly attenuated the translocation of HMGB1 from the nucleus to the cytoplasm in the skin of AD mice.
Fig. 4.
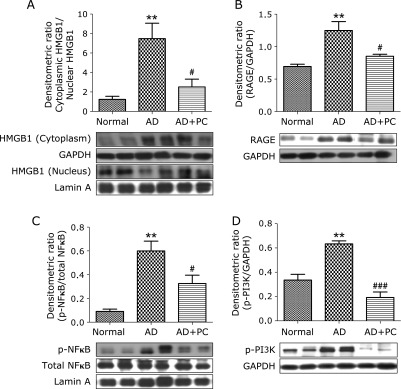
PC effects on cytoplasmic HMGB1, RAGE, nuclear p-NFκB and p-PI3K. Western blots show specific bands for (A) The expression of nuclear and cytoplasmic HMGB1, expressed as a ratio relative to that of Lamin A and GAPDH, The ratio of HMGB1 expression in cytoplasmic fraction to nuclear fraction was measured by densitometric quantification, (B) The expression of RAGE, expressed as a ratio relative to that of GAPDH, (C) The expression of nuclear p-NFκB, expressed as a ratio relative to that of NFκB, (D) The expression of p-PI3K, expressed as a ratio relative to that of GAPDH, Each bar represents mean ± SEM. Normal, age-matched normal NC/Nga mice; AD, AD induced NC/Nga mice; AD + PC, AD induced mice administered with PC aqueous extract. **p<0.01 vs Normal; #p<0.05 and ###p<0.001 vs AD.
PC attenuated AD-induced increases in RAGE, nuclear NFκB and p-PI3K expression in the skin
HMGB1 signaling via RAGE can lead to the activation of NFκB. So, we also examined protein expression of RAGE and nuclear p-NFκB levels. Both of them were significantly increased in AD mice, when compared with normal mice. In contrast, treatment with PC decreased it levels compared with that in group AD mice. (Fig. 4B and C). In addition, protein expression of p-PI3K was significantly up regulated in AD mice. These changes were significantly attenuated by PC treatment (Fig. 4D). These results clearly suggest that the RAGE and NFκB signaling pathway is affected by PC, which makes it a useful anti-inflammatory therapy.
Effects of PC on TNFα, TRAF2, IL1β expression in AD skin
Cytokines are playing an important role in the progression of AD. Using Western blot analysis we found that skin TNFα, TRAF2 and IL1β protein expression were significantly increased in the skin of AD mice, compared with that in the normal mice. This increase in skin TNFα, TRAF2 and IL1β protein expression were markedly suppressed by PC treatment in the AD mice (Fig. 5A–C) These results clearly indicate PC can inhibit inflammatory cytokines.
Fig. 5.
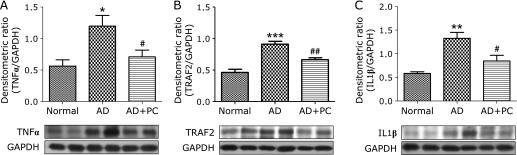
Effects of PC on TNFα, TRAF2 and IL1β. (A–C) Densitometric data of protein analysis. The mean density values of TNFα, TRAF2 and IL1β (expressed as a ratio relative to that of GAPDH) with representative Western blots showing specific bands for TNFα, TRAF2 and IL1β. GAPDH was used as an internal control. Each bar represents mean ± SEM. Normal, age-matched normal NC/Nga mice; AD, AD induced NC/Nga mice; AD + PC, AD induced mice administered with PC aqueous extract. *p<0.05, **p<0.01 and ***p<0.001 vs Normal; #p<0.05 and ##p<0.01 vs AD.
Effects of PC on p22phox, CHOP and apoptosis markers expression in lesional skin
The protein levels of p22phox, CHOP, cleaved caspase7, and caspase12 were significantly up-regulated in the group AD, compared with those in group normal. In contrast, treatment with PC significantly decreased these changes. (Fig. 6A–D).
Fig. 6.
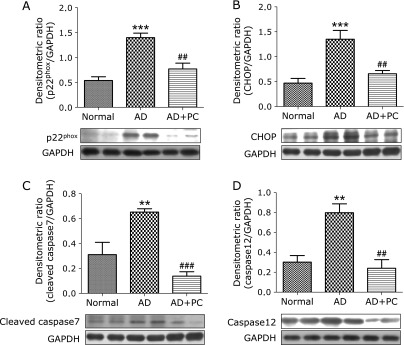
Skin expression of p22phox, CHOP, cleaved caspase7 and caspase12. (A–D) Densitometric data of protein analysis. The mean density values of p22phox, CHOP, cleaved caspase7 and caspase12 (expressed as a ratio relative to that of GAPDH) with representative Western blots showing specific bands for p22phox, CHOP, cleaved caspase7 and caspase12. GAPDH was used as an internal control. Each bar represents mean ± SEM. Normal, age-matched normal NC/Nga mice; AD, AD induced NC/Nga mice; AD + PC, AD induced mice administered with PC aqueous extract. **p<0.01 and ***p<0.001 vs Normal; ##p<0.01 and ###p<0.001 vs AD.
Discussion
PC has been used in Japan and other Asian countries as a traditional medicine to treat several diseases. It is used for infectious diarrhea, food poisoning and catarrhal gastritis. The active agent octacosyl ferulate, isolated from the active fraction of PC, inhibited tumor promotion by 9,10-dimethylbenz (alpha) anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) in mouse skin.(16) Sakuranetin was named after being first isolated as the aglycone of sakuranin from PC, which is affectionately associated with cherry blossoms (sakura). In our work, naringenin, genistein and sakuranetin were identified in the crude extract by the HPLC analysis (Fig. 1 and 2). The results are in agreement with previous reported works that the water extract of PC contains naringenin, genistein, sakuranetin, genkwanin and arctigenin.(7) The major active constituents naringenin and genistein suppresses development of AD in NC/Nga mice model is well documented.(17,18) Another study reported that, sakuranetin treatment attenuated T helper cells (Th)2 pro-inflammatory cytokines and control NFκB activation in murine model.(19) PC was reported to possess anti-inflammatory, antioxidant, antianalgesic, antidiabetic and antiasthmatic effects.(20) More over PC is one of ingredients of kampo formula, Jumi-haidoku-to and used in the treatment of skin disease such as acne (acne vulgaris).(7) In this study, we characterized detailed pharmacological effects of PC aqueous extract showing its antioxidant and anti-inflammatory activities in AD mice model.
AD as characterized as a chronic inflammatory skin disease, which is one of the many diseases induced by environmental elements such as antigens and the incidences of AD have sharply increased recently.(1) The exact etiology of AD remains unknown, and a cure for AD is not currently available. One of the experimental animal models of AD uses DfE treatment in NC/Nga mice to simulate the clinical features of human AD-like skin symptoms, such as erythema, haemorrhage, and edema;(21) all of these abnormalities were ameliorated by PC treatment in DfE-induced AD mice. To the best of our knowledge, our study is the first that identifies the protective effect of treatment with PC aqueous extract against AD induced by DfE.
In this study, histological analysis clearly revealed that the application of DfE increased skin inflammation in NC/Nga mice. Previous clinical study suggests that histologically, acute eczematous lesions in AD patients exhibit hypertrophy and infiltration of inflammatory cells.(22) In present study, hypertrophy and infiltration of inflammatory cells in the dermis were observed in the AD mice when compared with the normal mice (Fig. 3B). These changes were associated with oxidative stress and inflammation in skin tissues. Oral administration of PC suppressed DfE-induced skin edema, morphological changes and dermal infiltration of inflammatory cells, suggesting that treatment with PC can exert anti-inflammatory effects in AD skin.
DfE are the source of molecules that can influence innate and immune responses in human skin. Numerous studies have shown that molecules in DfE can affect the function of human fibroblasts, keratinocytes, skin microvascular postcapillary endothelial cells, dendritic cells and macrophages.(23) Moreover HMGB1, initially described as a nonhistone nuclear protein with transcriptional regulatory properties, is now recognized as a late mediator in septic shock as well as a pro-inflammatory cytokine.(24) It is actively released from activated cells such as monocytes and macrophages in response to pro-inflammatory cytokines.(25) It is expressed by almost all cells, and usually located in the nucleus. However, it has been reported that HMGB1 can be translocated to the cytosol and then released into the extracellular space.(26) Recently, increased presence of extracellular HMGB1 was detected in experimentally photo provoked and spontaneously occurring skin lesions in lupus, psoriasis vulgaris and AD suggesting a possible role of HMGB1 in skin lesion development.(26,27) In this study, we confirmed that the skin levels of cytoplasmic HMGB1 was increased in AD mice, and treatment with PC mediated the down-regulation of cytoplasmic HMGB1 (Fig. 4A). It has been reported that the activated HMGB1 binds to the cell surface receptor RAGE. Interaction between HMGB1 and RAGE might elicit various cellular responses including chemotaxis, cellular movement and the production of various pro-inflammatory cytokines.(28) In addition, the RAGE ligand HMGB1 has been recognized as a potent innate ”danger signal” for inflammation response.(29) Several lines of evidence demonstrate that the effects of HMGB1 are mediated by binding to RAGE. These findings suggested that RAGE is the major functional receptor mediating the proinflammatory effects of HMGB1 in skin cells. Interestingly, we could observe an increase in protein levels of RAGE and which was normalized by PC treatment.
RAGE ligation was observed to activate NFκB and PI3K pathway, leading to induction of pro-inflammatory cytokines and enhancing reactive species production and oxidative stress related cell damage.(30) Although PI3K signaling pathways are important for cellular responses, aberrant activation of these signaling pathways can induce chronic inflammation and inflammatory skin diseases.(31) Moreover, it has been well proven that the activation of HMGB1 signaling through RAGE promotes chemotaxis and the production of cytokines in a process that involves the activation of the transcription factor nuclear NFκB.(32) Furthermore, activated NFκB, which is one of the first responses of keratinocytes to alterations in epidermal barrier function and is often associated with increased epidermal proliferation, is considered a critical event in the progression and maintenance of AD.(33) Recent study suggested that RAGE promoter contains binding sites for nuclear NFκB that contributes to this receptor up-regulation.(34) Consistent with these previous reports, we have also observed that p-PI3K and nuclear p-NFκB levels were increased in AD mice and these changes were ameliorated by PC treatment. These data suggest that HMGB1 translocation could be regulated by the RAGE, PI3K and NFκB activation pathway.
Activated NFκB by RAGE can produce proinflammatory cytokines and chemokines such as TNFα, and IL1β.(35) In addition, HMGB1 increases their receptor RAGE expression as well as secretion of proinflammatory cytokines such as TNFα.(36) In agreement with the results, we demonstrated that PC effectively suppressed the expression of pro-inflammatory cytokines such as TNFα, TRAF2 and IL1β.
Many observations suggest that oxidative stress plays an important role in the pathogenesis of AD in humans.(37) Besides direct damaging effects of unregulated ROS production, dysregulation of several pro-inflammatory pathways, like NFκB, has been considered to contribute to psoriasis etiology. To address the role of oxidative stress, we next measured in the AD skin protein levels of p22phox, which was one of the subunit of NADPH oxidase, by Western blotting. Our results showed that AD increased the expression level of p22phox significantly and that inhibited by treatment with PC. Moreover, the endoplasmic reticulum (ER) is the main organelle responsible for protein folding and post-translational modification. Several recent studies have revealed that ER stress may mediate the inflammatory response. Growth arrest and DNA damage inducible gene (GADD153)/CHOP plays an important role in activation of ER stress mediated apoptosis.(38) Cell apoptosis can proceed through caspase12, which is localized in the ER and activated by ER stress.(39) CHOP, cleaved caspase7 and caspase12 expression levels were significantly suppressed in PC treated AD mice. (Fig. 6A–D). These results suggest that PC treatment can protect the animals from skin damage induced by oxidative stress and apoptosis following AD.
In conclusion, progressive skin inflammation in the DfE induced mice is associated with upregulation of inflammation, oxidative stress and apoptosis. The present study suggested that PC prevents development of skin lesion, oxidative stress, inflammation, and inhibits HMGB1 release and NFκB signaling pathways. Our results suggest that PC aqueous extract may be a promising medicine for treatment of AD. Further investigation is necessary to determine in-depth investigation with various fractions to identify the active principle of this extract and to elucidate the mechanism of action.
Acknowledgments
This research was supported by a Yujin Memorial Grant, Ministry of Education, Culture, Sports and Technology of Japan and by a grant from the Promotion and Mutual Aid Corporation for Private Schools, Japan. The authors have no conflict of interest.
References
- 1.Boguniewicz M, Schmid-Grendelmeier P, Leung DY. Atopic dermatitis. J Allergy Clin Immunol. 2006;118:40–43. doi: 10.1016/j.jaci.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Arshad SH. Does exposure to indoor allergens contribute to the development of asthma and allergy? Curr Allergy Asthma Rep. 2010;10:49–55. doi: 10.1007/s11882-009-0082-6. [DOI] [PubMed] [Google Scholar]
- 3.Vande Walle L, Kanneganti TD, Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2:162–165. doi: 10.4161/viru.2.2.15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Han D, Zhang Y, et al. A novel hypothesis: up-regulation of HO-1 by activation of PPARγ inhibits HMGB1-RAGE signaling pathway and ameliorates the development of ALI/ARDS. J Thorac Dis. 2013;5:706–710. doi: 10.3978/j.issn.2072-1439.2013.08.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi S, Xin Y, Guo Y, et al. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int Immunopharmacol. 2012;12:278–287. doi: 10.1016/j.intimp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Yoshinari K, Shimazaki N, Sashida Y, Mimaki Y. Flavanone xyloside and lignans from Prunus jamasakura bark. Phytochem. 1990;29:1675–1678. [Google Scholar]
- 7.Tohno H, Horii C, Fuse T, Okonogi A, Yomoda S. Evaluation of estrogen receptor Beta binding of pruni cortex and its constituents. Yakugaku Zasshi. 2010;130:989–997. doi: 10.1248/yakushi.130.989. [DOI] [PubMed] [Google Scholar]
- 8.Ugocsai K, Varga A, Molnár P, Antus S, Molnár J. Effects of selected flavonoids and carotenoids on drug accumulation and apoptosis induction in multidrug-resistant colon cancer cells expressing MDR1/LRP. In Vivo. 2005;19:433–438. [PubMed] [Google Scholar]
- 9.Ishihara A, Hashimoto Y, Tanaka C, et al. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008;54:481–495. doi: 10.1111/j.1365-313X.2008.03441.x. [DOI] [PubMed] [Google Scholar]
- 10.Takemoto JK, Remsberg CM, Yáñez JA, Vega-Villa KR, Davies NM. Stereospecific analysis of sakuranetin by high-performance liquid chromatography:pharmacokinetic and botanical applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:136–141. doi: 10.1016/j.jchromb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365–371. doi: 10.1016/j.jep.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Seo CS, Ha H, et al. Effect of Alpinia katsumadai Hayata on House Dust Mite-Induced Atopic Dermatitis in NC/Nga Mice. Evid Based Complement Alternat Med. 2012;2012:705167. doi: 10.1155/2012/705167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 14.Soetikno V, Sari FR, Lakshmanan AP, et al. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57:1649–1659. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- 15.Arumugam S, Thandavarayan RA, Veeraveedu PT, et al. Beneficial effects of edaravone, a novel antioxidant, in rats with dilated cardiomyopathy. J Cell Mol Med. 2012;16:2176–2185. doi: 10.1111/j.1582-4934.2012.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasukawa K, Dimitrijevic SM, Evans FJ, Kawabata S, Takido M. Inhibitory effect of Prunus Cortex extract and its component, octacosyl ferulate, on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Phytother Res. 1998;12:261–265. [Google Scholar]
- 17.Kim TH, Kim GD, Ahn HJ, CHo JJ, Park YS, Park CS. The inhibitory effect of naringenin on atopic dermatitis induced by DNFB in NC/Nga mice. Life Sci. 2013;93:516–524. doi: 10.1016/j.lfs.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Sakai T, Kogiso M, Mitsuya K, Komatsu T, Yamamoto S. Genistein suppresses development of spontaneous atopic-like dermatitis in NC/Nga mice. J Nutr Sci Vitaminol (Tokyo) 2006;52:293–296. doi: 10.3177/jnsv.52.293. [DOI] [PubMed] [Google Scholar]
- 19.Toledo AC, Sakoda CP, Perini A, et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br J Pharmacol. 2013;168:1736–1749. doi: 10.1111/bph.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yáñez JA, Remsberg CM, Takemoto JK, et al. Polyphenols and flavonoids: an overview. In: Davies NM, Yáñez JA, editors. Flavonoid Pharmacokinetics: Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety and Toxicology. Hoboken, NJ: John Wiley & Sons, Inc.; 2012. DOI: 10.1002/9781118468524.ch1 [Google Scholar]
- 21.Pokharel YR, Lim SC, Kim SC, Heo TH, Choi HK, Kang KW. Sopungyangjae-Tang inhibits development of dermatitis in Nc/Nga mice. Evid Based Complement Alternat Med. 2008;5:173–180. doi: 10.1093/ecam/nem015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakam Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arlian LG, Morgan MS, Peterson KT. House dust and storage mite extracts influence skin keratinocyte and fibroblast function. Int Arch Allergy Immunol. 2008;145:33–42. doi: 10.1159/000107464. [DOI] [PubMed] [Google Scholar]
- 24.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Li J, Ochani M, et al. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol. 2004;76:994–1001. doi: 10.1189/jlb.0404242. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Guo ZP, Li L, et al. Increased HMGB1 serum levels and altered HMGB1 expression in patients with psoriasis vulgaris. Arch Dermatol Res. 2013;305:263–267. doi: 10.1007/s00403-013-1330-0. [DOI] [PubMed] [Google Scholar]
- 27.Barkauskaite V, Ek M, Popovic K, Harris HE, Wahren-Herlenius M, Nyberg F. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 2007;16:794–802. doi: 10.1177/0961203307081895. [DOI] [PubMed] [Google Scholar]
- 28.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 29.Volz HC, Kaya Z, Katus HA, Andrassy M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 2010;36:185–194. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- 30.Touré F, Zahm JM, Garnotel R, et al. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem J. 2008;416:255–261. doi: 10.1042/BJ20080054. [DOI] [PubMed] [Google Scholar]
- 31.Jeon YJ, Kim BH, Kim S, et al. Rhododendrin ameliorates skin inflammation through inhibition of NF-κB, MAPK, and PI3K/Akt signaling. Eur J Pharmacol. 2013;714:7–14. doi: 10.1016/j.ejphar.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 32.Feng L, Zhu M, Zhang M, et al. Amelioration of compound 4,4'-diphenylmethane-bis(methyl)carbamate on high mobility group box1-mediated inflammation and oxidant stress responses in human umbilical vein endothelial cells via RAGE/ERK1/2/NF-κB pathway. Int Immunopharmacol. 2013;15:206–216. doi: 10.1016/j.intimp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Chervet L, Galichet A, McLean WH, et al. Missing C-terminal filaggrin expression, NFkappaB activation and hyperproliferation identify the dog as a putative model to study epidermal dysfunction in atopic dermatitis. Exp Dermatol. 2010;19:e343–e346. doi: 10.1111/j.1600-0625.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 35.Wang XC, Saban R, Kaysen JH, et al. Nuclear factor kappa B mediates lipopolysaccharide-induced inflammation in the urinary bladder. J Urol. 2000;163:993–998. [PubMed] [Google Scholar]
- 36.Fiuza C, Bustin M, Talwar S, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 37.Nakai K, Yoneda K, Maeda R, et al. Urinary biomarker of oxidative stress in patients with psoriasis and atopic dermatitis. J Eur Acad Dermatol Venerol. 2009;23:1405–1408. doi: 10.1111/j.1468-3083.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- 38.Anand S, Chakrabarti E, Kawamura H, Taylor CR, Maytin EV. Ultraviolet light (UVB and UVA) induces the damage-responsive transcription factor CHOP/gadd153 in murine and human epidermis: evidence for a mechanism specific to intact skin. J Invest Dermatol. 2005;125:323–333. doi: 10.1111/j.0022-202X.2005.23784.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Liu G, Song T, et al. Upregulation of GRP78 and caspase-12 in diastolic failing heart. Acta Biochimi Pol. 2008;55:511–516. [PubMed] [Google Scholar]