Abstract
Direct conversion of mammalian fibroblasts into induced neuronal (iN) cells has been attained by forced expression of pro-neural transcriptional factors, or by combining defined factors with either microRNAs or small molecules. Here, we show that neuronal cells can be converted from postnatal human fibroblasts into cell populations with neuronal purities of up to >80% using a combination of six chemical compounds. The chemical compound-induced neuronal cells (CiNCs) express neuron-specific proteins and functional neuron markers. The efficiency of CiNCs is unaffected by either the donor’s age or cellular senescence (passage number). We propose this chemical direct converting strategy as a potential approach for highly efficient generation of neuronal cells from human fibroblasts for such uses as in neural disease modeling and regenerative medicine.
Keywords: direct conversion, human fibroblasts, neuronal cells, chemical compounds
Introduction
The generation of induced pluripotent stem cells (iPSCs) from somatic cells has been attained by forced expression of a set of transcription-factor-encoding genes,(1) indicating that terminally differentiated cells can be induced to undergo cell fate change by the epigenetic modifications. In fact, recently, by introducing pro-neural transcriptional factors, or by combining defined factors with either microRNAs or small molecules, direct conversion of fibroblasts to neurons, which are termed induced neurons (iN) without going through the pluripotent state has been established.(2,3) However, induction of the conversion is still complicated and takes a few months with low efficiencies of success.
On the other hand, small molecules specifically modifying key signaling pathway provide a powerful tool to enhance or even replace defined genes. For iPSCs from mouse somatic cells by small molecule compounds or under chemical cocktails with hypoxic conditions for induced neural progenitor cells (iNPCs) without introducing exogenous factors has been demonstrated.(4,5) Thus, a direct conversion of somatic cells to iN cells (iNCs) by small molecule compounds without the introduction of ectopic genes should provide another desirable alternative to recent available strategies.
Inhibition of SMAD signaling and glycogen synthase kinase-3β (GSK-3β) with small molecules in combination with forced expression of two pro-neural genes, Ascl1 and Ngn2, significantly improves the efficiency of neuronal conversion of human fibroblasts.(6) In addition, the MEK-ERK pathway is downstream of TGF-β signaling,(7) and its inhibition promotes various reprogramming steps and accelerates mesenchymal-to-epithelial transition (MET).(6–8) Previously, Forskolin was confirmed to stimulate adenylate cyclase activity and increase cyclic AMP (cAMP), and its capability potentiates neuron differentiation.(9) Moreover, p53 is a tumor suppressor gene and a key factor in cell cycle arrest and apoptosis. p53 is known also as a regulator of neural cell differentiation, and expression of p53 blocks the neuronal conversion of human somatic cells.(10) Consequently, these lines of evidence led us to reason that a combination of chemical inhibitors including SMAD, GSK-3β, MEK-ERK and p53 pathway with cAMP stimulation would serve as a novel method for generating transgene-free iNCs.
Materials and Methods
Cell culture and generation of CiNCs
Four human primary fibroblasts were purchased via DS Pharma Medical (Osaka, Japan). Information on the fibroblasts is shown in Table 1. Cells were seeded at 8 × 104 cells in a 35-mm dish in DMEM high-glucose medium (Gibco, Grand Island, NY) supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. To generate induced neurons, on day 3, cells were switched from DMEM medium to neuronal medium containing DMEM/F12 [1% (vol/vol) N2 supplement, Gibco] and Neurobasal [2% (vol/vol) B27 supplement, Gibco] mixed at a 1:1 ratio. When indicated, small molecules were added to neuronal medium to reach the following final concentrations: SB-431542 (2 µM, Wako, Osaka, Japan), LDN-193189 (1 µM, Wako), CHIR99021 (1 µM, Wako), PD0325901 (1 µM, Wako), Pifithrin-α (5 µM, Wako) and Forskolin (7.5 µM, Wako).
Table 1.
Information on human fibroblasts used for direct conversion
| Catalog# | Lot# | Passage | BMI | Donor ages | Gender | Site | Abbreviation |
|---|---|---|---|---|---|---|---|
| BBDFF | DFMF112410B | 4 | U | 0 (6 months) | M | Foreskin | Neo |
| BBDFF | DFM062509 | 3 | 29 | 22 | M | Breast | Hum22 |
| BBDFF | DFM1002511 | 2 | 22 | 42 | F | Abdomen | Hum42 |
| BBDFF | DFM021610 | 3 | 28 | 55 | F | Abdomen | Hum55 |
Immunofluorescence staining
Immunocytochemistry was performed using standard protocol. Briefly, cells were fixed in 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO), washed three times with PBS, and then incubated in PBS containing 0.1% Triton X-100 for 10 min and 3% skim milk in PBS for 1 h at room temperature. Samples were then incubated with the following primary antibodies diluted in 3% skim milk in PBS at 4°C overnight: mouse anti-βIII-tub (Tuj1, Covance, Princeton, NJ, MMS-435P; 1:1,000), rabbit anti-MAP2ab (AB5622, Millipore, Temecula, CA, 1:1,000), rabbit anti-Synapsin I (Millipore, AB1543; 1:1,000), rabbit anti-GFAP (Dako, Glostrup, Denmark, Z0334; 1:500), rabbit anti-vGLUT1 (Synaptic Systems, Gottingen, Germany, 1:250), rabbit anti-GABA (Sigma Aldrich, A2052; 1:500), rabbit anti-serotonin (Sigma Aldrich, S5545; 1:800), rabbit anti-tyrosine hydroxylase (Millipore, AB152; 1:500). After washing three times with PBS, cells were incubated with secondary antibodies diluted in 3% skim milk in PBS: Alexa Fluor 488 goat anti-mouse IgG (Invitrogen-Molecular Probes, Carlsbad, CA, 1:1000) and/or Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen-Molecular Probes, 1:1000) for 2 h at room temperature. Nuclei were detected by propidium iodide (PI) (Vector Laboratories, Inc., Burlingame, CA) staining. Images were analyzed by confocal microscopy (FV1000; Olympus, Tokyo, Japan).
Results and Discussion
To successfully induce iNCs from differentiated cells without introducing exogenous transcriptional factors, four human postnatal fibroblast lines (Neo, Hum22, Hum42 and Hum55) derived from donors ranging in age from newborn to 55 years old (Table 1) were cultured with neuronal medium containing a cocktail of chemicals including 2 µM TGF-β inhibitor SB-431542 (S), 1 µM bone morphogenic protein (BMP) inhibitor LDN-193189 (L), 1 µM GSK-3β inhibitor CHIR99021 (G), 1 µM MEK-ERK inhibitor PD0325901 (M), 5 µM p53 inhibitor Pifithrin-α (P) and 7.5 µM Forskolin (F). By 3 weeks after treatment, the cultures comprised cells with immunoreactivity for βIII-tubulin (Tuj1+) and with short or long unipolar, bipolar or elongated processes and arborized neurites with mature neuronal morphology (Fig. 1A). To study in more detail the dynamics of this conversion process, we cultured Hum22 cells with the cocktail for 1, 2, 3, or 4 weeks and performed βIII-tubulin staining, respectively. The percentage of cells in cultures treated for 1 week was 8.4 ± 2.7%; the proportion increased to 58.5 ± 4.1% and 88.2 ± 3.9% for 2- and 3-week exposure. Notably, the proportion increased to 89.2 ± 2.1% for 4-week exposure, suggesting that a maximum increase of Tuj1+ cells was attained at 3 weeks after treatment with the six compounds (Fig. 1B and C).
Fig. 1.
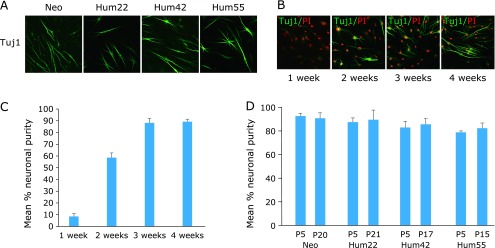
Highly efficient direct conversion of CiNCs from four samples of human fibroblasts by a chemical cocktail of six compounds. (A) Representative morphologies of transdifferentiated cells stained with neuronal marker Tuj1 from the fibroblasts at 3 weeks exposure to the chemical cocktail. (B) Immunofluorescence analysis of CiNCs in Hum22 fibroblasts for staining with Tuj1 antibody. PI, propidium iodide. (C) Quantification of Tuj1+ CiNCs in Hum22 fibroblasts. (D) Conversion efficiencies of different passage (P) number of the fibroblasts cultured with a 3-week exposure to the chemical cocktail expressed as neuronal purity.
Using the above protocol, at 3 weeks after treatment, we obtained a large number of CiNCs, i.e., cell populations with neuronal purities of 92.4 ± 2.3%, 87.3 ± 3.5%, 82.7 ± 5.3% and 78.6 ± 1.3% for Neo, Hum22, Hum42 and Hum55 at passage 5 (P5), respectively (Fig. 1D; based on Tuj1+ cells). The efficiency of CiNC generation was unaffected by cellular senescence (passages 15–21, Fig. 1D). However, compared with cells exposed to the cocktail of the six compounds, a remarkable decrease of CiNC generation was observed when cells were cultured with combinations of five compounds; in particular, no detectable Tuj1+ cells could be observed when cells were cultured with a medium from which p53 inhibitor Pifithrin-α was eliminated from the cocktail, at two weeks after treatment (quantified in Hum22; Fig. 2), suggesting that inhibition of p53 is a crucial step for neuronal direct conversion of human somatic cells. Moreover, no immunoreactivity for βIII-tubulin could be detected when cells were cultured with one compound, or with a cocktail of combinations of two, three or four compounds (data not shown).
Fig. 2.
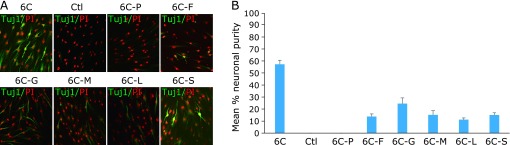
Quantification of CiNCs from Hum22 fibroblasts cultured with combinations of five compounds at two weeks after treatment. (A) Immunofluorescence analysis of CiNCS in Hum22 fibroblasts for staining with Tuj1 antibody. 6C: the six compounds; Ctl: Control; 6C-P, -F, -G, -M, -L, and -S denote that one compound, i.e., P, F, G, M, L or S was eliminated from the cocktail of the six compounds (6C). PI, propidium iodide. (B) Quantification of Tuj1+ CiNCs in Hum22 fibroblasts.
We conducted immunofluorescence studies to characterize the phenotype of the CiNCs. At three weeks after treatment with the cocktail, CiNCs converted from Neo, Hum22, Hum42 and Hum55 cell lines were all positive for the immunostaining of Tuj1 and mature neuronal marker MAP2 (Fig. 3A). We found that CiNCs were positive for the immunostaining of synapsin and showed synapsin-positive puncta that labeled the vesicles in the presynaptic nerve terminal (Fig. 3B). Thus, our study demonstrated that highly efficient direct conversion of human fibroblasts into neurons can be attained by using chemical compounds without the introduction of exogenous factors.
Fig. 3.
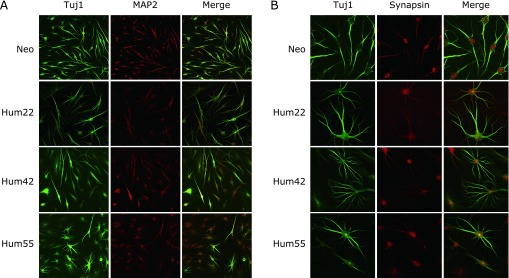
Characterization of highly efficient direct conversion of mature CiNCs from four samples of human fibroblasts by a chemical cocktail of six compounds. (A) Double-immunofluorescence analysis for Tuj1 and mature neuronal marker MAP2 of the fibroblasts cultured after a 3-week exposure to the chemical cocktail. (B) Expression of Tuj1 and Synapsin in CiNCs from the fibroblasts cultured with a 3-week exposure to the chemical cocktail.
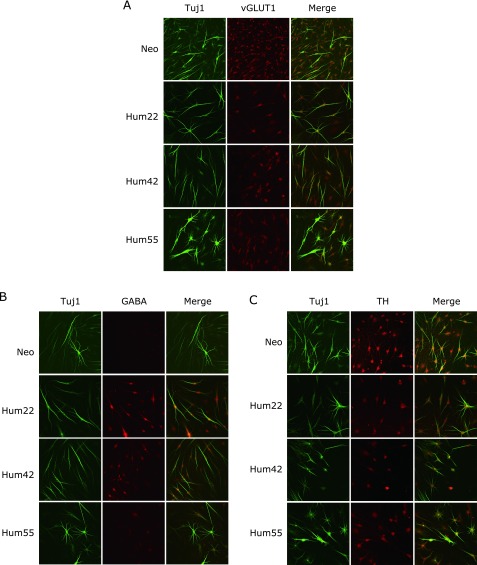
We further characterized the functional aspects of CiNCs induced by the six compounds from the four human fibroblasts. At three weeks after treatment, CiNCs were positive for the immunostaining of vesicular glutamate transporter 1 (vGLUT1, glutamatergic neuron marker, Fig. 4A) and GABA (GABAergic neuron marker, Fig. 4B) and tyrosine hydroxylase (TH, dopaminergic neuron marker, Fig. 4C), suggesting that CiNCs could be either excitatory or inhibitory, as previously reported.(3,6,10) CiNCs were negative in the immunostaining for serotonin (serotonergic neuron marker, data not shown) or glial fibrillary acidic protein (GFAP, data not shown).
Fig. 4.
Highly efficient direct conversion of functional CiNCs from four samples of human fibroblasts by a chemical cocktail of six compounds. (A) Three weeks after treatment with the chemical cocktail, Tuj1-positive CiNCs co-expressed vesicular glutamine transporter 1 (vGLUT1). (B) Tuj1-positive CiNCs co-expressed GABAergic neuron marker (GABA) at three weeks after treatment with the chemical cocktail. (C) Co-expression of Tuj1 and tyrosine hydroxylase (TH) in CiNCs from the fibroblasts cultured with a 3-week exposure to the chemical cocktail.
In summary, we show that neuronal cells can be converted from postnatal human fibroblasts into cell populations with neuronal purities of up to >80% using a combination of six chemical compounds. CiNCs express neuron-specific proteins and functional neuron markers. The efficiency of CiNCs is unaffected by either the donor’s age or cellular senescence (passage number). We propose this chemical direct converting strategy as a potential approach for highly efficient generation of neuronal cells from human fibroblasts for such uses as in neural disease modeling and regenerative medicine.
Acknowledgments
We thank Dr. Toshimasa Kusaka (Takara Bio Inc., Otsu, Japan) and Dr. Ming-Wei Wang (Chinese National Compound Library, Shanghai, China) for confirming our experiments, and Dr. Keimei Nakano of the Department of Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine, for technical assistance with figure preparation.
Author Contributions
P.D. designed the experiments and conducted the experiments in this study; Y.H. gave technical support and conceptual advice; P.D. and T.T. wrote the manuscript.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng L, Hu W, Qiu B, et al. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24:665–679. doi: 10.1038/cr.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 6.Ladewig J, Mertens J, Kesavan J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Takanashi S, Zhang Q, et al. Reversine increases the plasticity of lineage-committed mammalian cells. Proc Natl Acad Sci U S A. 2007;104:10482–10487. doi: 10.1073/pnas.0704360104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladewig J, Koch P, Brüstle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat Rev Mol Cell Biol. 2013;14:225–236. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- 9.Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CK, Zhou D, Zhang Z, et al. Senescence impairs direct conversion of human somatic cells to neurons. Nat Commun. 2014;5:4112. doi: 10.1038/ncomms5112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]