Abstract
Oral lichen planus is a chronic inflammatory disease that affects the mucous membrane of the oral cavity and can contribute to the development of other diseases. Inflammation in oral lichen planus is a T-cell-mediated autoimmune disease that acts through cytotoxic CD8+ T cells to trigger apoptosis of keratinocytes. However, the specific cause of oral lichen planus remains unknown and no effective medical treatment has yet been established. Astaxanthin is a carotenoid pigment with capacity for anti-inflammatory and anti-oxidant activities. In this study, we evaluated whether astaxanthin could be used to improve the pathology of oral lichen planus by reducing inflammation. In particular, the anti-inflammatory effects of astaxanthin on the chronic inflammation caused by lipopolysaccharide derived from Escherichia coli O55 in human gingival keratinocytes (NDUSD-1) were evaluated. Following astaxanthin treatment, localization of nuclear factor κB/p65 and the level of inflammatory cytokines (interleukin-6, tumor necrosis factor-α) tended to decrease, and cell proliferation significantly increased in vitro. These results suggest that astaxanthin could be useful for improving chronic inflammation such as that associated with oral lichen planus.
Keywords: human gingival keratinocyte, chronic inflammation, astaxanthin, inflammatory cytokine, nuclear factor κB/p65
Introduction
The oral mucosa has been demonstrated to play several important biological functions, including the preservation of homeostasis and characteristic features that distinguish epithelial cells from the other somatic cells, maintenance of the mechanical resistance of the oral epithelium, and creation of a selective chemical barrier.(1) Oral lichen planus (OLP) is a chronic inflammatory disease that affects the mucous membrane of the oral cavity.(2) The pathogenesis of this disorder involves, a T-cell-mediated autoimmune reaction in which cytotoxic CD8+ T cells trigger apoptosis of the keratinocytes.(3) One report indicated that approximately 1% of OLP patients may develop oral squamous cell carcinoma,(4) resulting in a decreased quality of life because of the accompanying pain produced by mastication and swallowing. In addition, the cytokine-mediated lymphocyte homing mechanism plays an important role in the pathogenesis of OLP. Some of these cytokines [interleukin (IL)-1, IL-2, IL-6, IL-8, and tumor necrosis factor (TNF)-α] contribute to the up-regulation of adhesion molecules.(5) OLP has also been associated with viral or fungal infections such as hepatitis C virus and human immunodeficiency virus,(6) oral candidosis,(7) or allergies to dental metals such as amalgam or palladium.(8–11) In a recent clinical report, levels of oxidative stresses such as nitric oxide, hydrogen peroxide, and malondialdehyde in the serum or saliva were found to be significantly increased in OLP patients compared with healthy subjects, and activities of antioxidants such as superoxide dismutase, catalase, and glutathione were decreased significantly.(3,12,13) However, the cause of OLP remains unknown and no medical treatment has yet been established;(14) therefore, only symptomatic therapy is currently provided for OLP patients. Symptomatic therapy for bacterial, fungal, or viral infections involves antimicrobial agents, antifungal drugs, or antiviral agents, and for patients with an underlying metal allergy, removal of the metal is the primary treatment.(15,16) The main therapy for chronic mechanical stimulation of the oral mucosa is the use of a modified, generic form of a crown. When such treatments do not result in improvement of these symptoms, immunosuppressive drugs (predominantly glucocorticoids) are commonly selected.(14,16) However, glucocorticoid drugs could also lead to major or minor side effects, including severe infection,(17) osteoporosis, and diabetes mellitus, or moon face, skin symptoms, and blood pressure elevation, respectively.(18) Moreover, the use of immunosuppressive drugs results in a slower response because OLP disease is more common in elderly patients who are often taking other types of drugs for underlying diseases. Astaxanthin (AX; 3,3-dihydroxy-beta, beta-carotene-4,4-dione) is a carotenoid pigment similar to β-carotene or lycopene, which is classified into the xanthophyll group of red pigments. Since AX cannot be generated de novo in the body, it must be obtained through the consumption of vegetables or fruits. AX is also widely and naturally distributed among marine organisms, including crustaceans such as shrimps and crabs, and fish such as salmon and sea bream.(19) In addition, AX is currently used as a supplement, and clinical and basic research has shown that extracts of this naturally derived pigment can defend the body against oxidative stress such as lipid peroxidation,(20,21) singlet oxygen,(22,23) and ultraviolet rays,(24,25) indicating that AX has antioxidant or anti-inflammatory effects. Therefore, the aim of this study was to evaluate the preventive or curative anti-inflammatory effects of AX in vitro to improve lipopolysaccharide (LPS)-induced inflammation in the human gingival keratinocyte line NDUSD-1.
Materials and Methods
Reagents
LPS from Escherichia coli O55:B5 was purchased from Wako Pure Chem. Ind., Ltd. (Osaka, Japan). After dissolving LPS with phosphate-buffered saline (PBS; Wako Pure Chem. Ind.), it was adjusted to a concentration of 100 µg/ml in keratinocyte serum-free medium (K-SFM; GIBCO, Carlsbad, CA). Base-free AX was obtained from AstaReal Corporation (Tokyo, Japan). After dissolving it with 99.0% dimethyl sulfoxide (Wako Pure Chem. Ind.), it was adjusted to a concentration of 10 µM in K-SFM (AX solution). In addition, a control group without AX-contained medium was established.
Cell culture
An immortalized human gingival keratinocyte cell line (NDUSD-1, obtained from the Pharmacology Department of the Nippon Dental University School of Life Dentistry at Tokyo) was used.(26) Cell culture was performed as previously reported.(27) Briefly, NDUSD-1 cells were seeded at a density of 5.0 × 104 cells/ml on 24-well multi-plates (Corning Incorporated, Corning, NY) until initial adhesion was reached (day 1). The time course of the experiment is shown in Fig. 1a and b. In addition, examination of the preventive anti-inflammatory effects of AX (Cont. group vs Pre-AX group) or that of the curative effects (Cont. group vs Post-AX group) was performed by the separate experimental system, respectively.
Fig. 1.
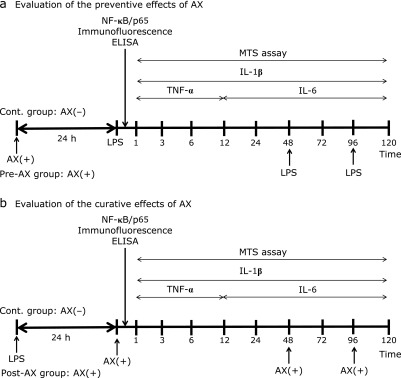
Time course of the experimental design. (a) Time course of evaluation of the preventive effect of astaxanthin (AX). The timings of medium changes, addition of each reagent, and the course of the experiment from initial cell adhesion (day 1) onward are indicated. A control group was established with only dimethyl sulfoxide (DMSO) in keratinocyte serum-free medium (K-SFM), and the Pre-AX group was established with AX added to the medium. Each medium was changed to lipopolysaccharide (LPS)-containing medium after treatment by each reagent for 0, 48, and 96 h. (b) Time course of the evaluation of the curative effect of AX, showing the timing of the experiment from initial cell adhesion (day 1) onward. A control group was established with only DMSO in K-SFM and a Post-AX group was established with AX in the medium. After treatment with LPS-containing medium for 24 h, each group was changed to DMSO-only medium or AX-added medium for 0, 48, and 96 h.
Immunocytochemistry staining of nuclear factor κB (NF-κB)/p65
NDUSD-1 cells were seeded on a PLL-coated culture cover glass (diameter, 13 mm; thickness, 0.12–0.17 mm; Matsunami Glass Ind., Ltd., Osaka, Japan) into 24-well multi-plates at a density of 5.0 × 104 cells/ml. After initial adhesion, the cells were treated with AX for 24 h (Pre-AX group) or with LPS (Post-AX group) for 30 min. Subsequently, each group of cells was treated with LPS for 30 min or AX for 24 h, respectively. In each experimental condition, the cells were fixed with 4% paraformaldehyde (Wako Pure Chem. Ind.) for 15 min. Cells were blocked using Blocking One Histo (Nacalai Tesque, Inc., Kyoto, Japan) for 10 min and were then incubated with a 0.1% Tween 20/PBS solution for 5 min at room temperature. Cells were then incubated with the primary antibody NF-κB p65 (D14E12) XP Rabbit mAb (1:50; Cell Signaling Technology, Inc., Danvers, MA) for 24 h at 4°C. Cells were washed 3 times with PBS and incubated with the secondary antibody anti-rabbit IgG (H + L), F(ab’)2 fragment (Alexa Fluor 488 Conjugate; 1:1000; Cell Signaling Technology, Inc.) for 1 h. After the cells were washed using PBS 3 times, they were mounted with mounting medium (4'-6-diamidino-2-phenylindole, DAPI) for H-1400 fluorescence (Vector Laboratories, Inc., Burlingame, CA). Fluorescence was visualized under a confocal laser microscope (LSM700, Carl Zeiss, Oberkochen, Germany). In addition, this experiment was performed twice at least.
Quantity of NF-κB/p65 in the cytoplasm and nucleus
A total of 4.0 × 106 NDUSD-1 cells were seeded in 60-mm cell culture dishes (FARCON; Becton Dickinson, Franklin Lakes, NJ). After initial adhesion, in each experimental condition the same as ”Immunocytochemistry staining of NF-κB/p65”, the cells were handled using Nuclear Extraction Kit (IMGENEX Co., San Diego, CA), and the cytoplasmic and nuclear fractions were collected and stored at 4°C or −80°C until analysis. Each fraction was treated with the NF-κB/p65 ActivELISA Kit (IMGENEX Co.) according to the manufacturer’s protocol. Analysis of NF-κB/p65 in the cytoplasmic fraction and nuclear fraction was determined based on absorbance at 450 nm a microplate reader (Bio-Rad Laboratories, Inc., Tokyo, Japan).
Cytokine assay
To evaluate the anti-inflammatory effects of AX, the levels of inflammatory cytokines (TNF-α, IL-1β, and IL-6) were measured with an enzyme-linked immunosorbent assay (ELISA) kit (BioLegend, Inc., San Diego, CA). According to the schedule and the protocol (Fig. 1), the supernatant fluid was collected from each cell culture and stored at −80°C until analysis. The absorbance of the supernatant was measured, and a standard curve was prepared from the absorbance readings obtained for the standard solution at each concentration at a wavelength of 450 nm on a micro-plate reader. Based on the standard curve, the concentrations of TNF-α, IL-1β, and IL-6 were determined for each sample.
Cell proliferation assay
Cell proliferation was determined by an MTS assay using a cell proliferation reagent (CellTiter96; Promega Co., Madison, WI). After the cells had attached to the 24-well plates, they were cultured for 1, 3, 6, 12, 24, 72, and 120 h, according to the schedule (Fig. 1a and b). The absorbance of the supernatant was measured at a wavelength of 490 nm on a micro-plate reader.
Statistical analysis
Statistical significance (p values) was calculated using unpaired t tests. The level of statistical significance was set to p<0.05.
Results
Immunocytochemistry for NF-κB/p65
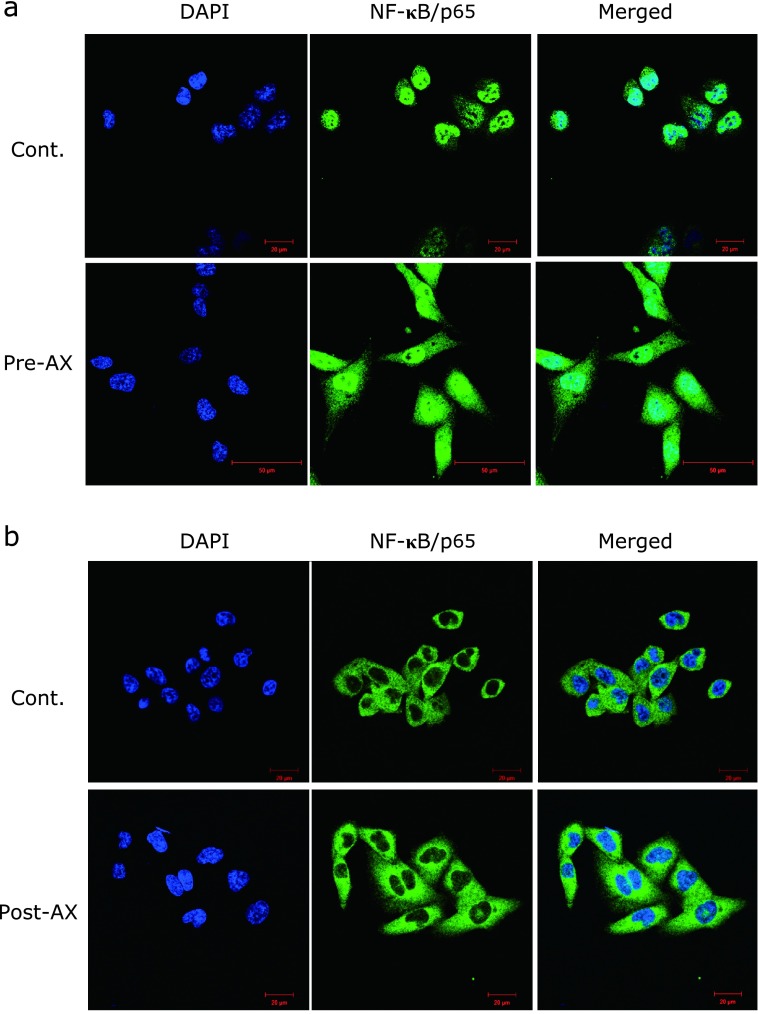
NF-κB/p65-positive staining was detected in the cytoplasm and the nucleus of NDUSD-1 cells. The control group tended to show increased translocation of NF-κB/p65 in the nucleus compared to the Pre-AX group (Fig. 2a). In contrast, expression of NF-κB/p65 in the Pre-AX group was observed mostly in the cytoplasm (Fig. 2a). In the Post-AX group, NF-κB/p65 was mostly localized in the cytoplasm and was hardly observed in the nucleus, similar to the control group (Fig. 2b).
Fig. 2.
Immunocytochemical analysis of the effect of astaxanthin (AX) on the localization of NF-κB/p65 in the nucleus and cytoplasm. (a) Immunocytochemical expression of DAPI (blue) and NF-κB/p65 (green) when AX was administered to cells preventatively. NF-κB/p65 was localized in the nucleus in both the control group and the Pre-AX group. However, NF-κB/p65 showed greater localization in the cytoplasm in the Pre-AX group than the control group (n = 2) (scale bar: 20 or 50 µm, magnification: ×40). (b) Immunocytochemical expression of DAPI (blue) and NF-κB/p65 (green) when AX was administered as curative treatment following inflammation. Localization of NF-κB/p65 was similar in both the cytoplasm and the nucleus between the control group and the Post-AX group (n = 2) (scale bar: 20 µm, magnification: ×40).
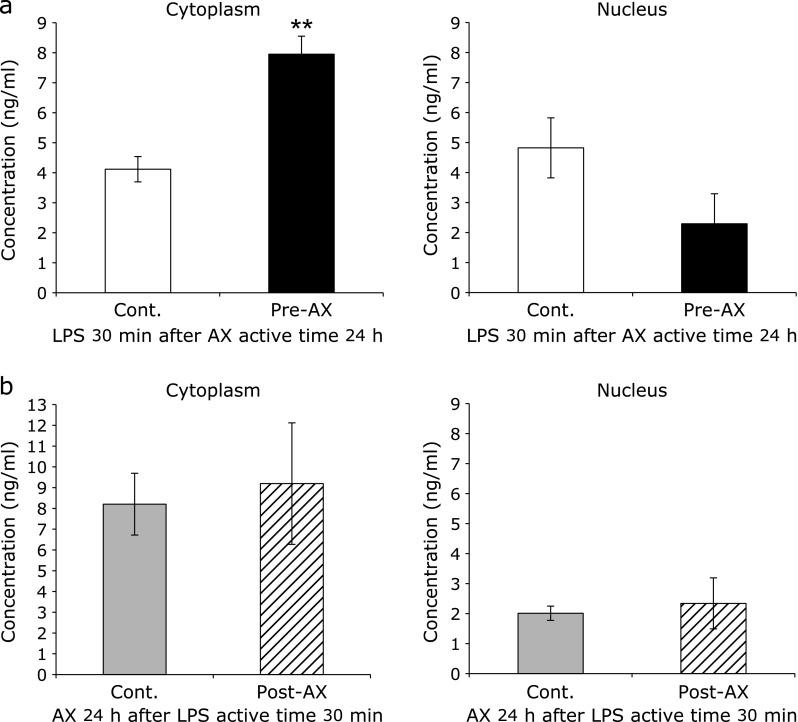
NF-κB/p65 expression in the cytoplasm and nucleus
The expression level of NF-κB/p65 in the cytoplasm was increased significantly (p<0.05) in the Pre-AX group compared with control group (Fig. 3a). In contrast, NF-κB/p65 expression in the nucleus tended to decrease in the Pre-AX group compared with control group (Fig. 3a). There was no significant difference in the expression level of NF-κB/p65 in the cytoplasm or nucleus between the control group and the Post-AX group (Fig. 3b). (Supplemental Table 1 and 2*)
Fig. 3.
Effect of astaxanthin (AX) on the nuclear and cytoplasmic localization of NF-κB/p65. After 30 min of lipopolysaccharide (LPS) treatment, the quantity of NF-κB/p65 in the cytoplasm and the nucleus was evaluated using an enzyme-linked immunosorbent assay. (a) When administered preventatively, AX significantly increased the NF-κB/p65 content in the cytoplasm (Pre-AX group) compared with the control group (**p<0.01) (left). In addition, NF-κB/p65 in the nucleus was decreased in the Pre-AX group compared to the control group, although the difference was not significant (right) (n = 5). (b) When AX was administered as a curative treatment after inflammation stimulation, there was no significant difference in NF-κB/p65 content in the cytoplasm and the nucleus from the control group (left, right) (n = 5).
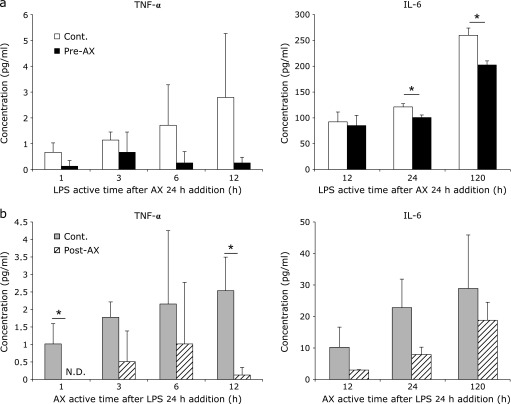
Cytokine assay
IL-6 production of the Pre-AX group was decreased significantly (p<0.05) from 24 to 120 h compared with that of the control group (Fig. 4a). Although the difference was not significant, TNF-α production in the Pre-AX group was decreased compared with that of the control group (Fig. 4a). TNF-α production of the Post-AX group was decreased significantly (p<0.05) for 1 and 12 h compared with that of the control group (Fig. 4b). IL-6 production in the Post-AX group was decreased compared with that in the control group, although the difference was not significant (Fig. 4b). IL-1β production was below the detection limit value for all groups (data not shown).
Fig. 4.
Effect of astaxanthin (AX) on TNF-α (left) and IL-6 (right) content. (a) An enzyme-linked immunosorbent assay (ELISA) showed that when treated preventatively (Pre-AX group), AX resulted in a significant decrease in IL-6 (*p<0.05) from 24 h of incubation to 120 h (right). Although there was no significant difference in TNF-α between the control group and the Pre-AX group, TNF-α production of the Pre-AX group tended to down-regulated (left) (n = 3). (b) ELISA results indicated that when AX was administered as a curative treatment for inflammation (Post-AX group), TNF-α decreased significantly (**p<0.01) for 1 and 12 h of incubation compared with the control group (left). Although there was no significant difference in IL-6 production between the control group and the Post-AX group, IL-6 production in the Post-AX group tended to be down-regulated (right) (n = 3).
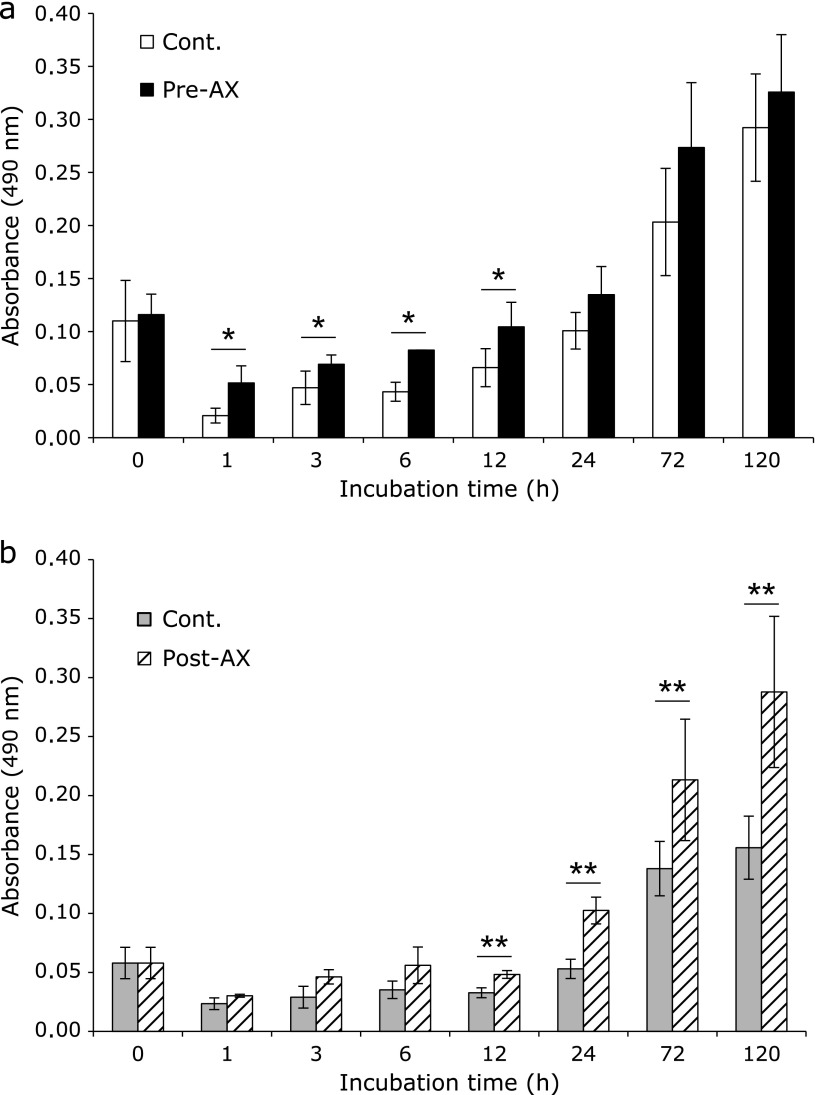
Cell proliferation assay
Cell proliferation of the Pre-AX group was increased significantly (p<0.05) from 1 to 12 h compared with that of the control group (Fig. 5a). Proliferation was also increased significantly in the Post-AX group (p<0.01) from 12 to 120 h compared with that of the control group (Fig. 5b).
Fig. 5.
Effect of astaxanthin (AX) on cell proliferation. (a) When cells were preventatively treated with AX (Pre-AX), proliferation increased significantly compared with that of control group from 1 h of cultivation to 12 h (*p<0.05) (n = 4). (b) When AX was administered to the cells as a curative treatment following inflammation stimulation (Post-AX), proliferation increased significantly compared with that of the control group from 12 h of cultivation to 24 h (**p<0.01) (n = 4).
Discussion
NF-κB is located in the cytoplasm of different cell types, where it is bound to inhibitory molecules such as IκBα and IκBβ, which prevent it from entering the nucleus.(28) Upon cell activation by numerous signals, including inflammatory cytokines, viruses, oxidizing agents, and protein kinase C,(29) NF-κB is released from inhibitory complexes and translocates to the nucleus, where it binds to the promoter regions of different genes encoding immune and pro-inflammatory mediators such as TNF-α, IL-1β, leukocyte and vascular adhesion molecules, and inducible nitric oxide synthase.(29,30) These products can activate NF-κB, and this type of positive regulatory feedback loop may exacerbate and perpetuate local inflammatory reactions.(29,30) Recently, several positive effects of AX have been reported such as anti-inflammatory and anti-oxidant activities and improvement of blood flow.(31–34) Accordingly, AX causes improvement in the case of systemic or local conditions such as choroidal neovascularization in age-related macular degeneration,(33,35) vocal fold wounds,(36) arteriosclerosis,(37) diabetes mellitus,(38) and nonalcoholic fatty liver disease.(39) Therefore, we evaluated whether AX could be used to improve the inflammation response associated with OLP. The chemical structure of AX is C40H52O4 and it contains two hydroxyl groups and two carbonyl groups.(40) Therefore, AX is functionalized on the surface of the cell membrane and the intracellular membrane.(40) Furthermore, it has been reported that AX does not change the structure of the cell membrane, as is the case for most other carotenoids.(40,41) The biological membrane contains cell membrane proteins such as a receptor, a transporter, and an ion channel. These are useful for the maintenance of important life activities such as signal transduction, taking in of required substances, excretion of dispensable substances, and maintenance of osmotic pressure and homeostasis. Maintenance of the cell membrane structure is considered to be useful for the prevention and treatment of various diseases by protecting the cell from damage caused by oxidative stress such as reactive oxygen species (ROS) and inflammatory stimulation. In a separate report, AX protects mesangial cells from hyperglycemia-induced oxidative stress such as ROS production, the activation of nuclear transcription factors such as NF-κB and activator protein-1, and the expression/production of transforming growth factor-beta 1 and monocyte chemoattractant protein-1.(42) In addition, it has been reported that the expression of Toll like receptor-4 (TLR-4) was increased significantly in OLP subjects.(43) TLR-4 detects gram-negative bacteria by recognizing the lipid A moiety of LPS.(44,45) LPS (a TLR-4 agonist) induces the homodimerization of TLR-4.(45,46) Indeed, Ge et al.(47) showed that the TLR-4 and the NF-κB signaling pathway might be associated with the perpetuation process of OLP.(47) Specifically, NF-κB translocation in keratinocytes may induce the production of inflammatory cytokines (such as TNF-α, IL-1β, and IL-6) and chemokines (IL-8) that have been shown to be up-regulated in OLP.(48) To control this inflammation response, topical steroids have been widely used as anti-inflammatory reagents for treating OLP and other inflammatory conditions.(49,50) For example, the use of topical cyclosporine as a second-line drug to treat symptomatic OLP showed satisfactory clinical efficacy.(51) However, Ge et al.(47) did not conclusively demonstrate the anti-inflammatory mechanism of topical steroids and cyclosporine on OLP.(47) Therefore, in this study, we investigated the potential preventive and curative anti-inflammatory effects of AX in vitro in a human gingival keratinocyte line, NDUSD-1, with LPS-induced inflammatory stimulation. In addition, since the simultaneous evaluation of the preventive or curative effects of AX was difficult, we divided the experimental system for convenience and performed the evaluations separately. In NF-κB/p65 immunocytochemistry, NDUSD-1 cells were stimulated with LPS-containing medium for 30 min, according to the peak levels indicated in the NF-κB/p65 ActivELISA Kit protocol (IMGENEX Co.). This preventive treatment with AX resulted in a decrease of the nuclear localization of NF-κB/p65. In addition, IL-6 production decreased significantly after 24 h. When AX was administered following inflammatory stimulation, as a curative treatment, there was no difference in the nuclear or cytoplasmic localization of NF-κB/p65 from the control group. Possibly activity was lost, when medium replacement was carried out and 24 h passed after the stimulus for LPS 30 min. However, the control group after a LPS 24 h stimulus, TNF-α production continued the increasing and that of the Post-AX group were decreased significantly for 1 and 12 h. Furthermore, cell proliferation was increased significantly by the anti-inflammatory effect of AX when administered preventatively by inhibiting the transcriptional activity of NF-κB/p65 in NDUSD-1 cells. In either preventive or curative administration of AX, there was minimal nuclear transfer of NF-κB/p65, indicating that AX was likely activated on the cell membrane surface or on the intracellular membrane. We developed the following hypothesis from these results. Administering AX preventively might control the inflammatory stimulus by conferring protection to a biomembrane prior to stimulation, resulting in an increase in cell proliferation at early stages. Curative administration of AX might suppress the vicious cycle of the inflammatory stimulus which has already acted on a biomembrane, resulting in an increase in cell proliferation in later stages. In summary, AX was found to suppress LPS-induced inflammation in the human gingival keratinocyte line NDUSD-1 in vitro when administered in either a preventive or curative manner. Therefore, the results of the present study suggest that AX might be useful to improve the chronic inflammation associated with OLP.
Acknowledgments
We wish to thank Professor Emeritus Takeki Tsutsui, Department of Pharmacology at The Nippon Dental University, School of Life Dentistry at Tokyo, for providing the NDUSD-1 cell line. We also wish to thank Associate Professor Masanori Nasu of the Research Center for Odontology at The Nippon Dental University, School of Life Dentistry at Tokyo, and Eiji Yamashita, the General Manager of AstaReal Co., Ltd., for their generous technical support.
Abbreviations
- AX
astaxanthin
- DAPI
4'-6-diamidino-2-phenylindole
- K-SFM
keratinocyte serum-free medium
- LPS
lipopolysaccharide
- NF-κB/p65
nuclear factor κB/p65
- OLP
oral lichen planus
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- TLR-4
Toll like receptor-4
- TNF
tumor necrosis factor
- IL
interleukin
Conflict of Interest
No potential conflicts of interest are disclosed.
Supplementary Material
References
- 1.Zapala J, Zarzecka J, Drukala J. Role of keratinocytes in preservation of oral mucosa epithelium integrity. Part I. Przegl Lek. 2005;62:72–75. (in Polish) [PubMed] [Google Scholar]
- 2.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus--a review. J Oral Pathol Med. 2010;39:729–734. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 3.Scrobotă I, Mocan T, Cătoi C, Bolfă P, Mureşan A, Băciuţ G. Histopathological aspects and local implications of oxidative stress in patients with oral lichen planus. Rom J Morphol Embryol. 2011;52:1305–1309. [PubMed] [Google Scholar]
- 4.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. 2008;14:229–243. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 5.Femiano F, Scully C. Functions of the cytokines in relation oral lichen planus-hepatitis C. Med Oral Patol Oral Cir Bucal. 2005;10:E40–E44. [PubMed] [Google Scholar]
- 6.Giuliani M, Lajolo C, Sartorio A, Scivetti M, Capodiferro S, Tumbarello M. Oral lichenoid lesions in HIV-HCV-coinfected subjects during antiviral therapy: 2 cases and review of the literature. Am J Dermatopathol. 2008;30:466–471. doi: 10.1097/DAD.0b013e31817e23af. [DOI] [PubMed] [Google Scholar]
- 7.Lodi G, Tarozzi M, Sardella A, et al. Miconazole as adjuvant therapy for oral lichen planus: a double-blind randomized controlled trial. Br J Dermatol. 2007;156:1336–1341. doi: 10.1111/j.1365-2133.2007.07883.x. [DOI] [PubMed] [Google Scholar]
- 8.Lomaga MA, Polak S, Grushka M, Walsh S. Results of patch testing in patients diagnosed with oral lichen planus. J Cutan Med Surg. 2009;13:88–95. doi: 10.2310/7750.2008.08017. [DOI] [PubMed] [Google Scholar]
- 9.McParland H, Warnakulasuriya S. Oral lichenoid contact lesions to mercury and dental amalgam--a review. J Biomed Biotechnol. 2012;2012:589569. doi: 10.1155/2012/589569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch P, Bahmer FA. Oral lesions and symptoms related to metals used in dental restorations: a clinical, allergological, and histologic study. J Am Acad Dermatol. 1999;41:422–430. doi: 10.1016/s0190-9622(99)70116-7. [DOI] [PubMed] [Google Scholar]
- 11.Lundström IM. Allergy and corrosion of dental materials in patients with oral lichen planus. Int J Oral Surg. 1984;13:16–24. doi: 10.1016/s0300-9785(84)80051-4. [DOI] [PubMed] [Google Scholar]
- 12.Ergun S, Troşala SC, Warnakulasuriya S, et al. Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. J Oral pathol Med. 2011;40:286–293. doi: 10.1111/j.1600-0714.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 13.Aly DG, Shahin RS. Oxidative stress in lichen planus. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:3–11. [PubMed] [Google Scholar]
- 14.Bogdán S, Németh Z. The characteristics of oral lichen planus. Fogorv Sz. 2012;105:35–42. (in Hungarian) [PubMed] [Google Scholar]
- 15.Dunsche A, Kästel I, Terheyden H, Springer IN, Christophers E, Brasch J. Oral lichenoid reactions associated with amalgam: improvement after amalgam removal. Br J Dermatol. 2003;148:70–76. doi: 10.1046/j.1365-2133.2003.04936.x. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki G, Yokozeki H, Katayama I, Nishioka K. Three cases of linear lichen planus caused by dental metal compounds. J Dermatol. 1996;23:890–892. doi: 10.1111/j.1346-8138.1996.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 17.Berthelot JM, Le Goff B, Maugars Y. Side effects of corticosteroid injections: what’s new? Joint Bone Spine. 2013;80:363–367. doi: 10.1016/j.jbspin.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14:203–207. [PMC free article] [PubMed] [Google Scholar]
- 19.Tominaga K, Hongo N, Karato M, Yamashita E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim Pol. 2012;59:43–47. [PubMed] [Google Scholar]
- 20.Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992;1126:178–184. doi: 10.1016/0005-2760(92)90288-7. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Shibata T, Hisaka S, Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009;1254:18–27. doi: 10.1016/j.brainres.2008.11.076. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Bai SK, Lee KS, et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol Cells. 2003;16:97–105. [PubMed] [Google Scholar]
- 24.Woodall AA, Lee SW, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biochim Biophys Acta. 1997;1336:33–42. doi: 10.1016/s0304-4165(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty AK, Funasaka Y, Slominski A, et al. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 26.Kubo C, Tsutsui TW, Tamura Y, Kumakura S, Tsutsui T. Immortalization of normal human gingival keratinocytes and cytological and cytogenetic characterization of the cells. Odontology. 2009;97:18–31. doi: 10.1007/s10266-008-0089-9. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu A, Satoh T, Wakabayashi H, Ikeda F. Effects of bovine lactoferrin to oral Candida albicans and Candida glabrata isolates recovered from the saliva in elderly people. Odontology. 2013 doi: 10.1007/s10266-013-0135-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 29.Barnes PJ. Nuclear factor-kappa B. Int J Biochem Cell Biol. 1997;29:867–870. doi: 10.1016/s1357-2725(96)00159-8. [DOI] [PubMed] [Google Scholar]
- 30.Santoro A, Majorana A, Bardellini E, Festa S, Sapelli P, Facchetti F. NF-kappaB expression in oral and cutaneous lichen planus. J Pathol. 2003;201:466–472. doi: 10.1002/path.1423. [DOI] [PubMed] [Google Scholar]
- 31.Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules. 2012;17:2030–2048. doi: 10.3390/molecules17022030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anarjan N, Nehdi IA, Tan CP. Protection of astaxanthin in astaxanthin nanodispersions using additional antioxidants. Molecules. 2013;18:7699–7710. doi: 10.3390/molecules18077699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito M, Yoshida K, Saito W, et al. Astaxanthin increases choroidal blood flow velocity. Graefes Arch Clin Exp Ophthalmol. 2012;250:239–245. doi: 10.1007/s00417-011-1843-1. [DOI] [PubMed] [Google Scholar]
- 34.Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5:333–342. doi: 10.2217/fca.09.19. [DOI] [PubMed] [Google Scholar]
- 35.Izumi-Nagai K, Nagai N, Ohgami K, et al. Inhibition of choroidal neovascularization with an anti-inflammatory carotenoid astaxanthin. Invest Ophthalmol Vis Sci. 2008;49:1679–1685. doi: 10.1167/iovs.07-1426. [DOI] [PubMed] [Google Scholar]
- 36.Mizuta M, Hirano S, Hiwatashi N, et al. Effect of astaxanthin on vocal fold wound healing. Laryngoscope. 2014;124:E1–E7. doi: 10.1002/lary.24197. [DOI] [PubMed] [Google Scholar]
- 37.Riccioni G, Speranza L, Pesce M, Cusenza S, D'Orazio N, Glade MJ. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition. 2012;28:605–610. doi: 10.1016/j.nut.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Chan KC, Pen PJ, Yin MC. Anticoagulatory and antiinflammatory effects of astaxanthin in diabetic rats. J Food Sci. 2012;77:H76–H80. doi: 10.1111/j.1750-3841.2011.02558.x. [DOI] [PubMed] [Google Scholar]
- 39.McCarty MF. Full-spectrum antioxidant therapy featuring astaxanthin coupled with lipoprivic strategies and salsalate for management of non-alcoholic fatty liver disease. Med Hypotheses. 2011;77:550–556. doi: 10.1016/j.mehy.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 40.Goto S, Kogure K, Abe K, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta. 2001;1512:251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 41.Anarjan N, Tan CP. Physico-chemical stability of astaxanthin nanodispersions prepared with polysaccharides as stabilizing agents. Int J Food Sci Nutr. 2013;64:744–748. doi: 10.3109/09637486.2013.783001. [DOI] [PubMed] [Google Scholar]
- 42.Manabe E, Handa O, Naito Y, et al. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J Cell Biochem. 2008;103:1925–1937. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- 43.Janardhanam SB, Prakasam S, Swaminathan VT, Kodumudi KN, Zunt SL, Srinivasan M. Differential expression of TLR-2 and TLR-4 in the epithelial cells in oral lichen planus. Arch Oral Biol. 2012;57:495–502. doi: 10.1016/j.archoralbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 45.Kim YS, Park ZY, Kim SY, Jeong E, Lee JY. Alteration of Toll-like receptor 4 activation by 4-hydroxy-2-nonenal mediated by the suppression of receptor homodimerization. Chem Biol Interact. 2009;182:59–66. doi: 10.1016/j.cbi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Saitoh S, Akashi S, Yamada T, et al. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16:961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- 47.Ge Y, Xu Y, Sun W, et al. The molecular mechanisms of the effect of Dexamethasone and Cyclosporin A on TLR4 /NF-κB signaling pathway activation in oral lichen planus. Gene. 2012;508:157–164. doi: 10.1016/j.gene.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 48.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 49.Donjerković D, Mueller CM, Scott DW. Steroid- and retinoid-mediated growth arrest and apoptosis in WEHI-231 cells: role of NF-kappaB, c-Myc and CKI p27(Kip1) Eur J Immunol. 2000;30:1154–1161. doi: 10.1002/(SICI)1521-4141(200004)30:4<1154::AID-IMMU1154>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 50.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Setterfield JF, Black MM, Challacombe SJ. The management of oral lichen planus. Clin Exp Dermatol. 2000;25:176–182. doi: 10.1046/j.1365-2230.2000.00607.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.