Abstract
Changes in l-arginine metabolism, including increased arginase levels and decreased nitric oxide production, are involved in the pathophysiology of asthma. In this study, using an intranasal mite-induced NC/Nga mouse model of asthma, we examined whether administration of l-arginine ameliorated airway hyperresponsiveness and inflammation by altering l-arginine metabolism. Experimental asthma was induced in NC/Nga mice via intranasal administration of mite crude extract (50 µg/day) on 5 consecutive days (days 0–4, sensitization) and on day 11 (challenge). Oral administration of l-arginine (250 mg/kg) was performed twice daily on days 5–10 for prevention or on days 11–13 for therapy. On day 14, we evaluated the inflammatory airway response (airway hyperresponsiveness, the number of cells in the bronchoalveolar lavage fluid, and the changes in pathological inflammation of the lung), arginase expression and activity, l-arginine bioavailability, and the concentration of NOx, the end products of nitric oxide. Treatment with l-arginine ameliorated the mite-induced inflammatory airway response. Furthermore, l-arginine administration attenuated the increases in arginase expression and activity and elevated the NOx levels by enhancing l-arginine bioavailability. These findings indicate that l-arginine administration may contribute to the improvement of asthmatic symptoms by altering l-arginine metabolism.
Keywords: l-arginine, asthma, arginase, nitric oxide, l-arginine paradox
Introduction
Asthma is a chronic inflammatory disease of the lung that is characterized by variable airflow obstruction, airway hyperresponsiveness (AHR), and airway inflammation, and frequent and spontaneous exacerbations and remissions.(1,2) Thus, it is desirable to effectively prevent these exacerbations and treat prolonged and severe asthmatic attacks.
Accumulating evidence suggests that changes in l-arginine (Arg) metabolism are involved in the pathogenesis of asthma. Arg is catabolized by nitric oxide synthase (NOS) and arginase.(3–5) The catabolism of Arg by NOS produces NO and l-citrulline (Cit). NO plays an important role in inflammation, host defense, and the regulation of the airway bronchomotor and pulmonary vascular tone by inducing the relaxation of smooth muscle. Moreover, NO deficiency caused by reduced Arg bioavailability for NOS-mediated metabolism is related to the development of asthma.(3,4,6) Arginase is the enzyme involved in the urea cycles and consists of the two isoforms I and II. Arginase catalyzes Arg hydrolysis into urea and l-ornithine (Orn). The metabolites of Orn, polyamine and l-proline, are involved in airway remodeling by promoting cell proliferation and collagen synthesis.(3–5,7) Our and other studies have shown an increased level of arginase I in an experimental asthma model and in asthmatic patients.(8–11) Interestingly, we have demonstrated that the inhibition of arginase attenuated the decrease in the concentration of NOx in the asthmatic state and ameliorated airway allergic reactions and inflammation.(12) Studies from other groups have also shown that the inhibition of arginase alleviates the allergic characteristics of asthma.(9,13) Given these findings, it is likely that consumption of Arg by arginase inhibits the production of NO by reducing the Arg bioavailability to NOS, thereby contributing to the development of asthma, including AHR, inflammation, and remodeling.
It is known that the administration of exogenous Arg elicits an increase in NO production, even when excess levels of Arg are available, a circumstance referred to as the ”Arg paradox”.(14–16) Interestingly, ex vivo treatment with Arg reduced AHR in tracheae isolated from ovalbumin-challenged guinea-pigs, an asthma model.(17,18) Furthermore, in vivo oral administration of Arg alleviated the AHR and airway inflammation in an ovalbumin-induced murine model of asthma.(19) Taken together, Arg administration may ameliorate airway allergic reactions by modulating Arg metabolism via a mechanism that involves arginase, Arg bioavailability, and NO.
In this study, using a mite-challenged NC/Nga mouse model,(8,12,20) we addressed the preventive or therapeutic effect of Arg administration on the clinical parameters of experimental asthma before and after challenge with mites. We also investigated the alterations in arginase expression and activity, Arg availability and NOx following Arg supplementation.
Materials and Methods
Animals
Male 7-week-old NC/Nga mice were obtained from Charles River Laboratories Japan (Yokohama, Japan). The care and handling of the animals were in accordance with the Guidelines for the Care and Use of Laboratory Animals at Shikata Campus of Okayama University and were approved by the Okayama University Institutional Animal Care and Use Committee.
Induction of asthma and treatment with Arg
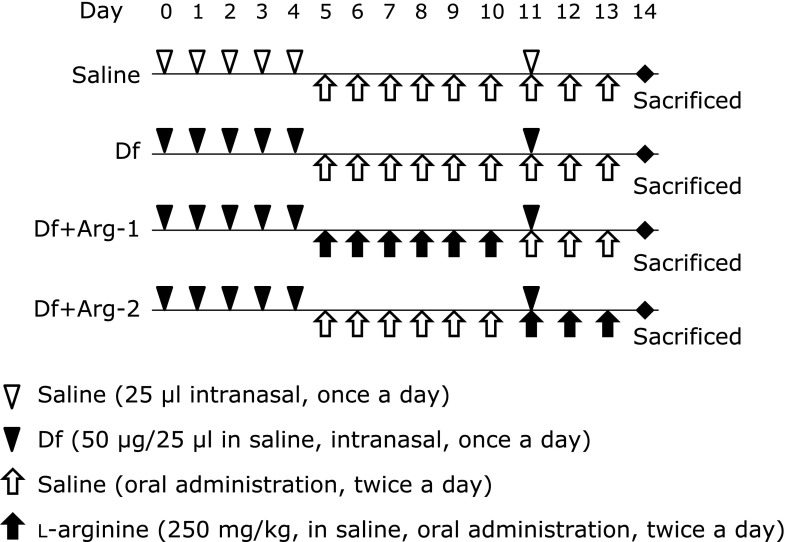
Induction of experimental asthma was performed on NC/Nga mice via sensitization and challenge using mite crude extract from Dermatophagoides farinae (Df) (Cosmo Bio, Tokyo, Japan) as previously described.(20,21) Briefly, Df crude extract (50 µg/25 µl in saline, Df group) or saline (25 µl, saline group) was intranasally administered via on 5 consecutive days (day 0–4, sensitization) and on day 11 (challenge) under anesthesia. In addition to administrating the Df crude extract, Arg (250 mg/kg, dissolved in saline) was orally administered twice daily on days 5–10 (Df + Arg-1 group; prevention group) or on days 11–13 (Df + Arg-2 group; therapy group). The dose of Arg was selected based on a previous study.(19) The mice in the Df and saline groups received oral saline alone on the same days. The schematic protocol is shown in Fig. 1.
Fig. 1.
Schematic illustration of the experiment.
Assessment of AHR
On day 14, AHR to acetylcholine was measured as described previously.(12,21) Dose-response curves for acetylcholine (from 62.5 to 4,000 µg/kg) in anesthetized, mechanically ventilated mice were obtained. Bronchoconstriction was expressed as the respiratory overflow volume provoked by acetylcholine as a percentage of the maximal overflow volume (100%) obtained by completely occluding the tracheal cannula.
Collection of bronchoalveolar lavage fluid (BALF) and plasma
Immediately after the assessment of acetylcholine-induced AHR, bronchoalveolar lavage was performed. BALF samples were processed and used to determine the numbers of total cells and of different cell types as described previously.(12,21) Blood samples were collected, and plasma was separated for further analysis.
Preparation of lung specimens
After the collection of BALF and plasma, lung tissue was harvested as described previously.(12,21) A portion of the lung tissue was fixed in 10% neutral phosphate-buffered formalin for morphological examination. The remaining lung tissue was homogenized in buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and complete protease inhibitor mixture tablets (Roche, Mannheim, Germany) with or without 1% Triton X-100. The lung samples treated with 1% Triton X-100 were ultracentrifuged, and the supernatants were used for further analysis.
Histopathological evaluation
The fixed lung tissues were embedded in paraffin. Sections were sliced and stained with hematoxylin and eosin to assess the degree of inflammation. The levels of inflammation in the peribronchial and perivascular spaces of the lung were determined using an ordinal scale ranging from 0 to 3, as described previously.(21,22)
Western blot analysis
To examine the arginase protein expression level, Western blot analysis was performed as described previously.(12,21) Equal amounts of protein from the lung tissue samples treated with 1% Triton X-100 or plasma were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). These membranes were incubated in primary antibodies against arginase I, arginase II (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or the internal control β-actin (Abcam, Cambridge, UK). Corresponding horseradish peroxidase-conjugated secondary antibodies were used. Signals were visualized using an enhanced chemiluminescence Western blot detection system (Perkin-Elmer, Boston, MA). Each band was quantified using Image J software. The expression level of each arginase isoform in the lung tissue was normalized to that of β-actin. The data are expressed as the fold-change in expression.
Measurement of arginase activity
Arginase activity was measured as described previously,(12,21) with minor modifications. Briefly, endogenous urea in the lung samples treated with 1% Triton X-100 was removed using an Amicon Ultra-0.5 centrifugal filter device (10K molecular weight cutoff; Millipore). The samples were incubated in the presence of Arg and MnCl2 at 37°C for 60 min. The amount of the reaction product urea was measured using a urea assay kit (BioChain Institute, Inc., Hayward, CA) according to the manufacturer’s instructions.
Measurement of Arg, Cit, and Orn
The concentration of Arg, Cit, and Orn in the lung samples was quantified via high-performance liquid chromatography followed by fluorescence detection as described previously,(23) with minor modifications. Briefly, 10 µl of the lung sample treated without 1% Triton X-100 was mixed with 50 µl of 1.5 M HClO4. After 2 min, 1 ml of H2O, 25 µl of 2 M K2CO3, and 125 µl of 100 µM monomethylarginine, an internal standard, were added. This mixture was vortexed and centrifuged to obtain the supernatant for analysis. The supernatant was mixed with an equal amount of derivatization reagent (1 mg/ml ortho-phthaldialdehyde, 2% methanol, and 0.1% 3-mercaptopropionic acid in 200 mM borate buffer, pH 8.5) and was equilibrated for 30 min at room temperature. This sample was injected into the high-performance liquid chromatography system consisting of a solvent delivery system and fluorometer (HITACHI Ltd., Tokyo, Japan). An Ascentis® C18 (15 cm × 4.6 mm, 3 µm; Supelco Inc., Bellefonte, PA) and an Ascentis® C18 SupelguardTM (2 cm × 4.0 mm, 3 µm; Supelco Inc.) were used as the analytical column and the guard column, respectively. Mobile phase A consisted of 0.1 M sodium acetate, pH 7.2, containing 9% methanol and 0.5% tetrahydrofuran, and mobile phase B was 100% methanol. The following elution conditions were used: flow rate of 1.1 ml/min; column temperature of 30°C; 0–15 min, isocratic elution using 91% mobile phase A and 9% mobile phase B; 15–20 min, linear gradient to 70% mobile phase A and 30% mobile phase B; 20–30 min, linear gradient to 65% mobile phase A and 35% mobile phase B; 30–35 min, isocratic elution using 65% mobile phase A and 35% mobile phase B; 35–38 min, linear gradient to 50% mobile phase A and 50% mobile phase B; and 38–50 min, linear gradient to 45% mobile phase A and 55% mobile phase B. The total run time was 60 min including the time for column regeneration: 51–54 min, isocratic elution using 100% mobile phase B; 54.1–60 min, isocratic elution using 91% mobile phase A and 9% mobile phase B. The fluorescence excitation and emission wavelengths were 340 and 455 nm, respectively.
Measurement of the NOx concentrations
To estimate NO production in the lung, the concentration of NOx, consisting of nitrite (NO2−) and nitrate (NO3−), was determined using a NO analyzer (Model-280i NOA with a Purge Vessel; Sievers, Boulder, CO) as described previously.(12,21) Briefly, the lung sample treated without 1% Triton X-100 was treated with nitrate reductase (Sigma, St. Louis, MO) to convert NO3− to NO2− at room temperature for 30 min. After removing proteins via precipitation with acetonitrile, NO2− in the supernatant was further reduced to NO in a Purge Vessel containing the reducing agent potassium iodide in acetic acid. Subsequently, NO was detected using the ozone-chemiluminescence method.
Statistical analysis
All results are expressed as the means ± SEM. If the difference based on via one-way or two-way analysis of variance (ANOVA) was significant, Bonferroni’s post hoc test or Tukey’s multiple comparison test was used for paired comparisons using GraphPad Prism for Windows (GraphPad Software, Inc., San Diego, CA). The results were considered to be significantly different at p<0.05.
Results
Effect of Arg on AHR to acetylcholine
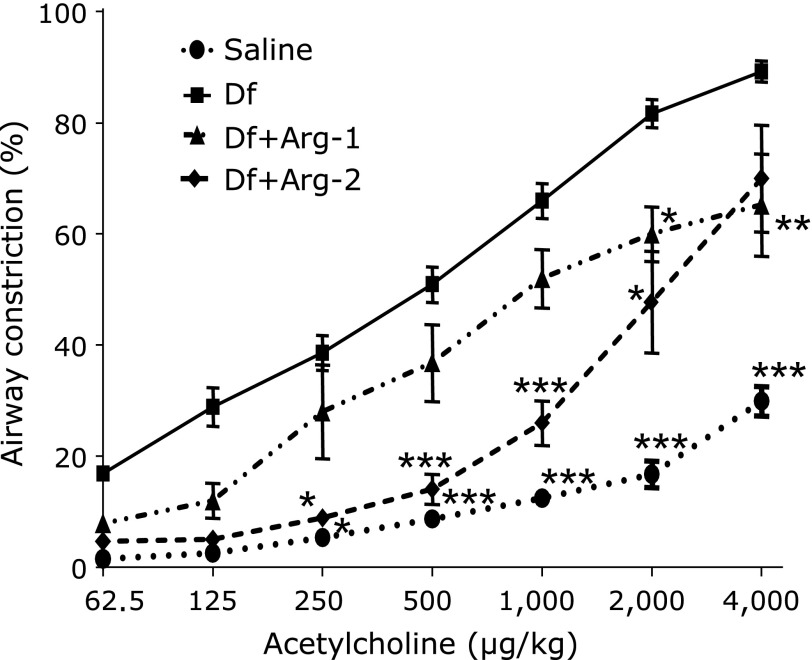
We measured AHR to acetylcholine. Compared with the saline group, a significant increase in AHR in response to acetylcholine was found in the Df group. However, remarkably attenuated AHR were observed in the Df + Arg-1 and the Df + Arg-2 groups compared to the Df group (Fig. 2).
Fig. 2.
The effect of Arg on AHR to acetylcholine in mite-induced asthma. Two-way ANOVA revealed a significant effect of the acetylcholine dose, p<0.0001; group, p<0.0001; acetylcholine dose × group, p<0.0001. *p<0.05, **p<0.01, and ***p<0.001 vs the Df group based on one-way ANOVA followed by Bonferroni’s post hoc test after confirming an interaction between the acetylcholine dose and the groups via two-way ANOVA (n = 5 to 8 mice/group).
Effect of Arg on the cell counts in the BALF
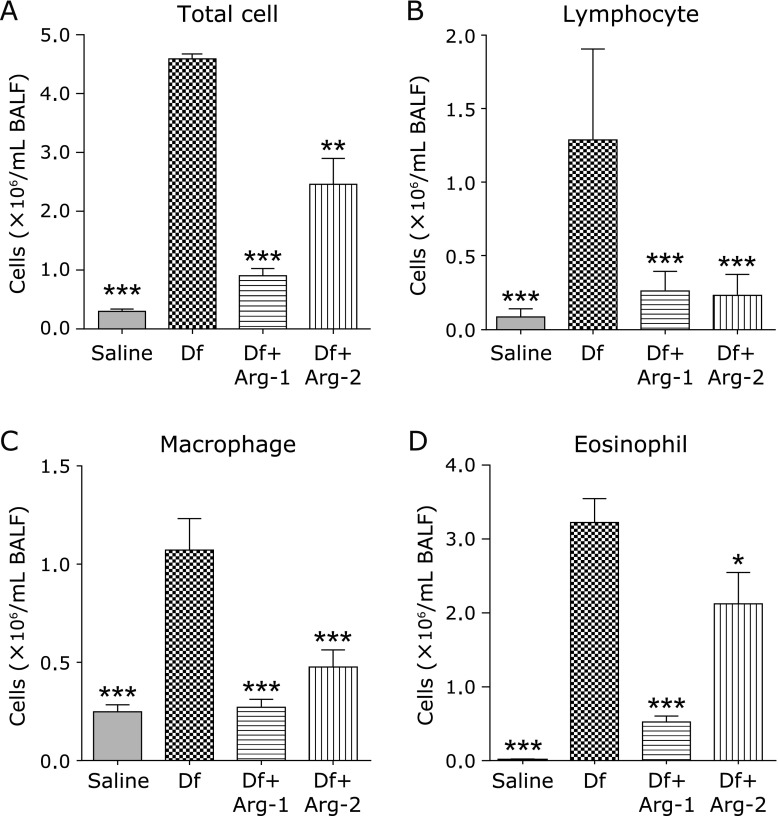
We examined the accumulation of inflammatory cells in the BALF. The numbers of inflammatory cells, including total cells, lymphocytes, macrophages, and eosinophils, in BALF were significantly increased in the Df group than in the saline group. However, significant decreased numbers of inflammatory cells in the BALF were detected in the Df + Arg-1 and the Df + Arg-2 groups than in the Df group (Fig. 3A–D).
Fig. 3.
The effect of Arg on BALF cell numbers in mite-induced asthma. The numbers of total cell (A) lymphocyte (B), macrophage (C), and eosinophil (D) in the BALF are shown. *p<0.05, **p<0.01, and ***p<0.001 vs the Df group based on one-way ANOVA followed by Tukey’s multiple comparison test (n = 5 to 8 mice/group).
Effect of Arg on the scores of lung inflammation
We investigated the histopathological changes in the lung. Compared with the saline group, significantly higher inflammation scores in the perivascular area and the peribronchial space were found in the Df group. However, inflammation scores in the Df + Arg-1 and the Df + Arg-2 group were significant lower than those of the Df group (Fig. 4A–E).
Fig. 4.
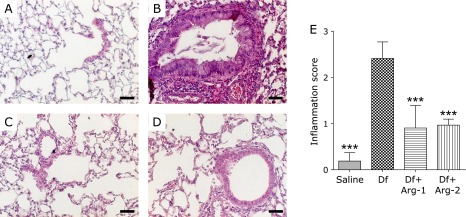
The effect of Arg on histopathological inflammation in the mite-induced asthmatic lung. Representative images of hematoxylin and eosin-stained lung tissue from the saline (A), Df (B), Df + Arg1 (C), and Df + Arg2 groups (D), and inflammation scores (E) are shown. The bars indicate 50 µm. ***p<0.001 vs the Df group based on one-way ANOVA followed by Tukey’s multiple comparison test (n = 8 mice/group).
Effect of Arg on the protein expression and activity of arginase
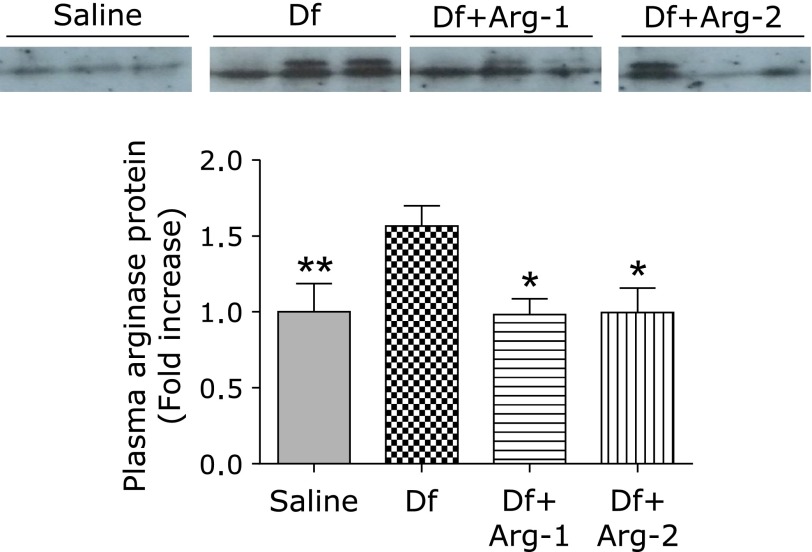
We assessed the protein expression and activity of arginase. Quantitative Western blot analysis revealed higher expression of arginase I and arginase II in the lung tissue from the Df group than that from the saline group. Interestingly, treatment with Arg significantly attenuated the mite-induced expression of arginase I, although no significant difference in the expression of arginase II was detected between the Df group and either the Df + Arg-1 or Df + Arg-2 group (Fig. 5A). The increased activity of arginase in the lung from the Df group was significantly attenuated in that from the Df + Arg-1 and Df + Arg-2 groups (Fig. 5B). In addition, interestingly, arginase I expression in plasma was higher in the Df group than in the saline group. Importantly, the mite-induced expression of arginase I in plasma was significantly attenuated by the administration of Arg (Fig. 6). These data suggest that Arg supplementation attenuated the increases in the expression and activity of arginase I in mite-induced asthma.
Fig. 5.
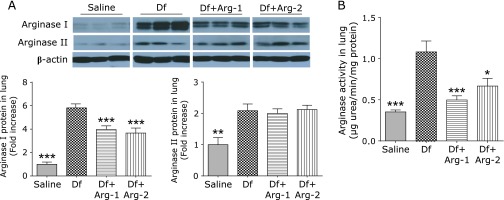
The effect of Arg on the protein expression and activity of arginase in the mite-induced asthmatic lung. Representative images and quantification after normalization to β-actin of Western blots for arginase I and arginase II (A), and the activity of arginase (B) in the lung tissue are shown. *p<0.05, **p<0.01, and ***p<0.001 vs the Df group based on one-way ANOVA followed by Tukey’s multiple comparison test (n = 7 to 8 mice/group).
Fig. 6.
The effect of Arg on the protein expression of plasma arginase I in mite-induced asthma. Representative images and quantification of Western blots for arginase I in plasma are shown. *p<0.05, and **p<0.01 vs the Df group based on one-way ANOVA followed by Tukey’s multiple comparison test (n = 6 to 8 mice/group).
Effect of Arg on Arg bioavailability and the NOx levels
We evaluated the Arg bioavailability ratio for cellular utilization, characterized by the ratio of Arg to Orn plus Cit, which are products generated from the enzymatic catabolism of Arg,(24,25) and the NOx concentration in the lung tissue. No significantly difference in the concentration of Arg was detected between the groups (Fig. 7A). However, the Arg availability ratio, Arg/(Orn + Cit), was slightly higher in the Df + Arg-1 (p = 0.015) and Df + Arg-2 (p = 0.081) groups than in the Df group (Fig. 7B). The concentration of NOx was significantly higher in the Df + Arg-2 group than in the other groups (Fig. 7C). These results suggest that Arg administration increases the production of NO by enhancing Arg bioavailability.
Fig. 7.
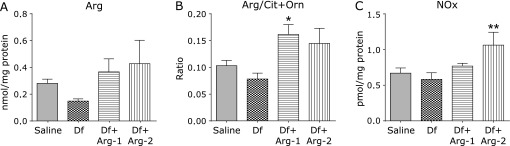
The effect of Arg on Arg bioavailability and the NOx levels in the mite-induced asthmatic lung. The concentration of Arg (A), the Arg bioavailability ratio, expressed as Arg/Orn + Cit (B), and the concentrations of NOx (C) are shown. *p<0.05 and **p<0.01 vs the Df group based on one-way ANOVA followed by Tukey’s multiple comparison test (n = 5 to 8 mice/group).
Discussion
The data from this study demonstrate that, in terms of asthma prevention and therapy, Arg administration ameliorated AHR and inflammation in a mite-induced NC/Nga mouse model of asthma. We also found that treatment with Arg attenuated the increases in the protein expression and activity of arginase and induced changes in Arg metabolism, including the enhancement of Arg bioavailability and the increase in the concentration of NOx.
In this study, we used an experimental asthmatic model of NC/Nga mice subjected to intranasal administration of mite allergen, a common allergen, without adjuvant. This model is considered to closely resemble human asthma.(20) Our previous studies using this model have demonstrated that increased levels of arginase I and concomitantly decreased production of NO may be implicated in the development of asthma.(8,12,21)
We found that Arg administration ameliorated the pathophysiologic allergic changes induced by mites, as demonstrated by the amelioration of AHR, the decrease in the number of inflammatory cells in the BALF, and the reduction in inflammation in the lung tissue. We also found that Arg administration attenuated the increase in the mRNA expression of Th2-related cytokines, such as inteleukin-4 and inteleukin-5, in the asthmatic lung (unpublished data). Furthermore, the increased expression and activity of arginase in the mite-induced asthmatic lung tissue and plasma were attenuated by Arg supplementation. Given that arginase contributes to the pathophysiology of asthma,(7,9,12,13) we propose that this Arg administration-mediated decrease in the expression and activity of arginase might be responsible for the amelioration of pathophysiologic allergic reactions in asthma.
Arg supplementation was performed before or after mite challenge because we aimed to assess its preventive and therapeutic effects, respectively. The therapeutic administration of Arg (Df + Arg-2 group) resulted in the enhancement of Arg bioavailability and the increase in the NOx levels. Considering that Arg is catabolized by both arginase and NOS as a substrate,(3–5) increased Arg bioavailability may be, at least in part, due to the decreased levels of arginase. Furthermore, no change in the mRNA expression of NOS2 was detected in the lung tissue from the asthmatic or Arg-administered asthmatic mice compared to that from the saline-treated mice (unpublished data). Collectively, we propose that the restoration of Arg bioavailability to NOS, independently of NOS expression, by Arg administration may improve asthmatic symptoms via increased NO production.
Similar to therapeutic Arg administration, preventive therapeutic administration of Arg (Df + Arg-1 group) resulted in attenuated levels of arginase, enhanced Arg bioavailability, and ameliorated asthmatic symptoms, although no change in the NOx levels was detected. This finding regarding the NOx levels may be due to timing of Arg administration (on days 5–10) and sample collection (day 14). Given the ”Arg paradox”, in which acute exogenous administration of Arg increases NO production despite apparently saturating the intracellular level of the substrate,(14–16) the NOx levels may be increased by enhancing Arg bioavailability to NOS, independently of NOS expression, during the preventive administration of Arg (on days 5–10) and surrounding mite-challenge (on day 11). Therefore, we speculate that the increased level of NO mediated by preventive treatment with Arg may partially ameliorate airway allergic reactions and inflammation, although we did not examine in this hypothesis in detail.
The mechanism by which Arg supplementation decreases the expression of arginase I remains unclear. The expression of arginase I is regulated by various stimuli, including Th2-related cytokines, such as inteleukin-4, growth factors, oxygen tension, and reactive oxygen species (ROS).(3,7) The upregulation of arginase I by Th2-related cytokines is regulated by the transcription factors CCAAT-enhancer binding protein and STAT6.(26) Thus, it is likely that the attenuated levels of Th2-related cytokines caused by Arg administration accounts for the decreased expression of arginase I. Alternatively, our population studies have shown that induction of arginase I expression was associated with increased oxidative stress.(27–29) Oxidative stress is involved in the pathophysiology of asthma. Overproduction of ROS activates redox-sensitive transcription factors, such as nuclear factor-κB and activator protein-1, and induces the expression of pro-inflammatory genes.(30,31) Considering that NO attenuates oxidative stress by counteracting ROS, the decreased expression of arginase may be attributed to the changes in the redox regulation of transcription via increased NO production. Therefore, further studies should elucidate the exact mechanisms by which Arg administration regulates the expression of arginase.
This study clearly showed that Arg administration alleviated asthmatic symptoms. These findings were consistent with a study indicating the beneficial effect of Arg administration at the same dose (250 mg/kg) in an ovalbumin-induced murine model of asthma,(19) although there was a difference in the timing of Arg administration. Conversely, it has been reported that Arg administration potentiated airway inflammation in an ovalbumin-induced murine model of asthma;(32) however, there was a difference in the dose (12.5 mg/kg) of Arg administered.
In conclusion, the present study proposes that Arg administration may be useful for the prevention or treatment of asthma by attenuating the expression and activity of arginase and by modulating the metabolism of Arg. Further studies are required to elucidate the precise mechanisms by which Arg supplementation ameliorates asthmatic symptoms, modulates arginase expression and activity, and alters Arg metabolism.
Acknowledgments
This work was supported in part by Grant-in-Aid for Science Research (B) (No. 23390163, 26293152) from the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese Government.
Abbreviations
- AHR
airway hyperresponsiveness
- ANOVA
analysis of variance
- Arg
l-arginine
- BALF
bronchoalveolar lavage fluid
- Cit
l-citrulline
- Df
Dermatophagoides farinae
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOx
nitrate and nitrite
- NO2−
nitrite
- NO3−
nitrate
- Orn
l-ornithine
- ROS
reactive oxygen species
Conflict of Interest
Ikuo Murakami is employed by Otsuka Pharmaceutical Co., Ltd. The remaining authors report no conflicts.
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Maarsingh H, Zaagsma J, Meurs H. Arginine homeostasis in allergic asthma. Eur J Pharmacol. 2008;585:375–384. doi: 10.1016/j.ejphar.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 4.Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson RC, Hardy KA, Morris CR. Arginase and arginine dysregulation in asthma. J Allergy (Cairo) 2011;2011:736319. doi: 10.1155/2011/736319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redington AE. Modulation of nitric oxide pathways: therapeutic potential in asthma and chronic obstructive pulmonary disease. Eur J Pharmacol. 2006;533:263–276. doi: 10.1016/j.ejphar.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 7.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol. 2009;158:652–664. doi: 10.1111/j.1476-5381.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takemoto K, Ogino K, Shibamori M, et al. Transiently, paralleled upregulation of arginase and nitric oxide synthase and the effect of both enzymes on the pathology of asthma. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1419–L1426. doi: 10.1152/ajplung.00418.2006. [DOI] [PubMed] [Google Scholar]
- 9.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L911–L920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 10.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 11.Ogino K, Obase Y, Takahashi N, et al. High serum arginase I levels in asthma: its correlation with high-sensitivity C-reactive protein. J Asthma. 2011;48:1–7. doi: 10.3109/02770903.2010.528496. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Ogino K, Takemoto K, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L17–L24. doi: 10.1152/ajplung.00216.2009. [DOI] [PubMed] [Google Scholar]
- 13.Maarsingh H, Zuidhof AB, Bos IS, et al. Arginase inhibition protects against allergen-induced airway obstruction, hyperresponsiveness, and inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- 14.Kurz S, Harrison DG. Insulin and the arginine paradox. J Clin Invest. 1997;99:369–370. doi: 10.1172/JCI119166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dioguardi FS. To give or not to give? Lessons from the arginine paradox. J Nutrigenet Nutrigenomics. 2011;4:90–98. doi: 10.1159/000327777. [DOI] [PubMed] [Google Scholar]
- 16.Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab. 2012;303:E1177–E1189. doi: 10.1152/ajpendo.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boer J, Duyvendak M, Schuurman FE, Pouw FM, Zaagsma J, Meurs H. Role of l-arginine in the deficiency of nitric oxide and airway hyperreactivity after the allergen-induced early asthmatic reaction in guinea-pigs. Br J Pharmacol. 1999;128:1114–1120. doi: 10.1038/sj.bjp.0702882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maarsingh H, Bossenga BE, Bos IS, Volders HH, Zaagsma J, Meurs H. l-arginine deficiency causes airway hyperresponsiveness after the late asthmatic reaction. Eur Respir J. 2009;34:191–199. doi: 10.1183/09031936.00105408. [DOI] [PubMed] [Google Scholar]
- 19.Mabalirajan U, Ahmad T, Leishangthem GD, et al. Beneficial effects of high dose of l-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 2010;125:626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 20.Shibamori M, Ogino K, Kambayashi Y, Ishiyama H. Intranasal mite allergen induces allergic asthma-like responses in NC/Nga mice. Life Sci. 2006;78:987–994. doi: 10.1016/j.lfs.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Ogino K, Kubo M, Takahashi H, Zhang R, Zou Y, Fujikura Y. Anti-inflammatory effect of arginase inhibitor and corticosteroid on airway allergic reactions in a Dermatophogoides farinae-induced NC/Nga mouse model. Inflammation. 2013;36:141–151. doi: 10.1007/s10753-012-9529-3. [DOI] [PubMed] [Google Scholar]
- 22.Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000;30:79–85. doi: 10.1046/j.1365-2222.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008;440:177–189. doi: 10.1016/S0076-6879(07)00810-5. [DOI] [PubMed] [Google Scholar]
- 24.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–2067. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Ogino K, Takahashi N, Takigawa T, Obase Y, Wang DH. Association of serum arginase I with oxidative stress in a healthy population. Free Radic Res. 2011;45:147–155. doi: 10.3109/10715762.2010.520318. [DOI] [PubMed] [Google Scholar]
- 28.Ogino K, Murakami I, Wang DH, et al. Evaluation of serum arginase I as an oxidative stress biomarker in a healthy Japanese population using a newly established ELISA. Clin Biochem. 2013;46:1717–1722. doi: 10.1016/j.clinbiochem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Ogino K, Wang DH, Kubo M, et al. Association of serum arginase I with l-arginine, 3-nitrotyrosine, and exhaled nitric oxide in healthy Japanese workers. Free Radic Res. 2014;48:137–145. doi: 10.3109/10715762.2013.842979. [DOI] [PubMed] [Google Scholar]
- 30.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Zuo L, Otenbaker NP, Rose BA, Salisbury KS. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol. 2013;56:57–63. doi: 10.1016/j.molimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Takano H, Lim HB, Miyabara Y, Ichinose T, Yoshikawa T, Sagai M. Oral administration of l-arginine potentiates allergen-induced airway inflammation and expression of interleukin-5 in mice. J Pharmacol Exp Ther. 1998;286:767–771. [PubMed] [Google Scholar]