Abstract
In periodontitis, production of reactive oxygen species (ROS) by neutrophils induces oxidative stress and deteriorates surrounding tissues. Antioxidants reduce damage caused by ROS and are used to treat diseases involving oxidative stress. This study summarizes the different effects of resveratrol, quercetin, and N-acetylcysteine (NAC) on human gingival fibroblasts (HGFs) under oxidative stress induced by hydrogen peroxide. Real-time cytotoxicity analyses reveals that resveratrol and quercetin enhanced cell proliferation even under oxidative stress. Of the antioxidants tested, resveratrol is the most effective at inhibiting ROS production. HGFs incubated with resveratrol and quercetin up-regulate the transcription of type I collagen gene after 3 h, but only resveratrol sustained this up-regulation for 24 h. A measurement of the oxygen consumption rate (OCR, mitochondrial respiration) shows that resveratrol generates the highest maximal respiratory capacity, followed by quercetin and NAC. Simultaneous measurement of OCR and the extracellular acidification rate (non-mitochondrial respiration) reveals that resveratrol and quercetin induce an increase in mitochondrial respiration when compared with untreated cells. NAC treatment consumes less oxygen and enhances more non-mitochondrial respiration. In conclusion, resveratrol is the most effective antioxidant in terms of real-time cytotoxicity analysis, reduction of ROS production, and enhancement of type I collagen synthesis and mitochondrial respiration in HGFs.
Keywords: human gingival fibroblasts, periodontitis, resveratrol, quercetin, N-acetylcysteine
Introduction
Chronic periodontitis is an infectious disease associated with Gram-negative anaerobic or facultative anaerobic bacteria. After bacterial infection, the initial inflammatory response activates the immune system through cytokines and chemokines. Additionally, lipid mediators of inflammation are released by macrophages, which recruit neutrophils and polymorphonuclear cells. Neutrophils are the first line of defense and produce a variety of toxic products at the site of infection including reactive oxygen species (ROS).(1,2) ROS are rapidly generated, short-lived, and diffusible. ROS have been proposed as second messengers to mediate cellular responses, making them essential to many normal biologic processes. At low concentrations, ROS stimulate the growth of gingival fibroblasts and epithelial cells in culture, but at higher concentrations they induce tissue damage by initiating free radical chain reaction.(3–6) Under normal conditions, protective antioxidants (enzymes and redox molecules) rapidly repair the damage induced by ROS.(3,7) Antioxidants can be categorized according to their mode of action (preventative or scavenging), location (intra-, extracellular, or membrane associated), solubility (water or lipid soluble), and their origin/source (natural or synthetic).(8) An imbalance between ROS and antioxidants may be a key factor in the initiation and development of periodontal disease.(9) The enhanced and continuous production of ROS and/or impaired function of antioxidants leads to oxidative stress, which progressively induces the destruction of periodontal structure, alveolar bone, and connective tissue.(1,2,4,9,10) In current treatments, antioxidants are used as an alternative to complement the traditional therapies to treat diseases involving oxidative stress, yet their mechanism of action are not clearly understood.(11)
Polyphenols are reactive metabolites abundant in plant-derived foods, particularly fruits, seeds, and leaves. In the tissues of the digestive tract, particularly the oral mucosa, active polyphenols reach their highest concentration.(9) Polyphenols are now attracting attention as potential sources of agents capable of inhibiting, reversing, or retarding the progression of diseases caused by oxidative stress and inflammatory processes.(12–17)
Among the wide range of polyphenols, resveratrol (trans-3,4',5-trihyrodoxystilbene) is widely available and found in red wine, among other sources.(18) Resveratrol is thought to have cardio-protective, anti-inflammatory, and anti-aging properties in animal model systems.(19) The mechanism of action has been widely debated and attributed to many targets, including SIRT1, cyclooxygenase 1, and AMP-activated protein kinase.(20,21) Resveratrol induces enzymes such as superoxide dismutase and glutathione S-transferase.(22) Quercetin is another polyphenol universally present in many natural sources such as onions, apples, broccoli, berries, and capers.(23) Quercetin represents a major polyphenol in the human diet. In vitro studies indicate that quercetin has diverse biological effects, including induction of apoptosis, anti-mutagenesis properties, and modulation of the cell cycle. Also, quercetin inhibits the activation of protein kinase C (PKC) and the release of histamine. (14) Another antioxidant is the thiol N-acetylcysteine (NAC), a glutathione (GSH) precursor.(17) NAC has a wide range of protective effects against DNA damage and carcinogenesis. These effects are related to the ability of NACs to modulate metabolism, gene expression, and signal transduction pathways and to regulate antioxidants and anti-inflammatory activities.(24,25)
Human gingival fibroblasts (HGFs) are the major constituents of gingival tissues and are responsible to maintain homeostasis by regulating collagen metabolism. Therefore, the promotion of collagen synthesis and suppression of ROS may contribute to the integrity of gingival tissues and prevention of periodontitis.(26) Few in vitro studies have identified a way to avoid or delay the establishment of periodontitis at early stages, when the clinical outcomes are still not evident. Most in vivo studies are focused on bone loss, which is the latest clinical outcome of periodontitis. We hypothesize that the use of antioxidants will lessen the damage caused by ROS in response to oxidative stress and retard the initiation of periodontitis. The aim of our study is to compare the biological effects of three antioxidants: resveratrol, quercetin, and NAC on cultured HGFs under oxidative stress induced by exposure to H2O2. We investigated whether the antioxidants induced cellular proliferation or cytotoxicity through dynamic monitoring of HGF cells. Further, we verified the ability of each antioxidant to limit the production of ROS in HGFs following oxidative stress. Real-time quantitative reverse transcription-PCR (qRT-PCR) assays were performed to determine if the antioxidants were capable of maintaining or restoring the functioning of HGFs during short and long periods of oxidative stress. Finally, to confirm whether the antioxidants had a direct effect on HGFs’ cellular machinery, we measured oxygen consumption rates (OCR, mitochondrial respiration) and extracellular acidification rates (ECAR, non-mitochondrial respiration) during oxidative stress.
Materials and Methods
Cell culture
The HGF-1 cell line was purchased from ATCC® (CRL-2014TM; Manassas, VA). Cells were plated in cell culture flasks (Cole Parmer International, Vernon Hills, IL), and suspended in Dulbecco’s Modified Eagle Medium (DMEM; Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10% heat-inactivated fetal bovine serum (MP Biomedicals LLC, Santa Ana, CA), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Sigma Aldrich, St. Louis, MO) and kept in a humidified incubator at 37°C, with an atmosphere of 5% CO2. Subculture was performed before cells reached confluence. Cells were washed with phosphate-buffered saline and briefly trypsinized (0.25% trypsin-0.2% EDTA; Life Technologies, Carlsbad, CA) to detach the cells from the flasks. HGF-1 cells were used for assays from the 6th to the 12th passage.(27)
Dynamic monitoring of cell viability and proliferation assay
The xCELLigence system (ACEA Biosciences, San Diego, CA) was used following the manufacturer’s protocol. The data representing the cell status are based on the measured relative change in electrode impedance and are expressed as cell index (CI), a unit-less parameter. The presence of more cells on the top of the electrodes affects the local ionic environment at the electrode/solution interface, increasing the CI. The CI also can vary based on the quality of the cell interaction with the electrodes. If the cells are more attached or covering a larger area of the electrodes, they will induce a greater change in the CI. HGF-1 cells were suspended in cell culture medium and adjusted to 10,000 cells per well and seeded into E-plate 16. The plates were connected to the RTCA single plate station. HGFs were incubated until reaching the stationary growth phase for 24 h, and CI values were recorded, normalized, and termed ”Base value”. After that, HGF-1 cells were stimulated with H2O2 (final concentration: 0.23 mM) for 48 h to induce oxidative stress. At the same time, designated groups were treated with various concentrations of resveratrol (Yamada bee Co., Okayama, Japan), quercetin (Extrasynthese, Genay, France), and NAC (Sigma Aldrich). Controls were treated with medium only. The CI value of each group was monitored and recorded every 15 min for 48 h.(28)
The effectiveness (as percentage value) of each antioxidant was calculated using the following formula: Effectiveness (%) = (Sample CIt – H2O2 CIt) / (Control CIt – H2O2 CIt) × 100, where Effectiveness is antioxidants’ effectiveness, CI corresponds to Cell Index, and t represents a time point during the incubation period.
ROS production
HGF-1 cells were subcultured onto a glass-bottomed dish (Matsunami Glass Ind. Ltd., Osaka, Japan) with 5,000 cells per well. H2O2 (0.23 mM) was added to induce oxidative stress. Simultaneously, designated groups were treated with resveratrol (50 µM), quercetin (15 µM), or NAC (1.5 mM). The cells were incubated for 30 min at 37°C. CellROX® Green Reagent (Life Technologies) was used to identify intracytoplasmic ROS and NucBlueTM Live Cell Stain (Life Technologies) was used as a nuclear counterstain. ROS production by HGF-1 cells was viewed under a confocal laser microscope (Nikon, Tokyo, Japan).(29)
qRT-PCR
To observe how the cell cycle and functioning are arrested during oxidative stress, HGFs were subcultured (10,000 cells/well) and stimulated with H2O2 (1 mM) to induce oxidative stress in the presence or absence of resveratrol (50 µM), quercetin (15 µM), or NAC (1.5 mM) and incubated at 37°C, with an atmosphere of 5% CO2 for 3 h. Cells were then washed with phosphate-buffered saline, fresh culture medium was added to the wells, and the plates were incubated for 3, 12, 24, 48, or 72 h. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed using RevertTra Ace (Toyobo, Osaka, Japan). Real-time PCR was performed using SYBR Green (Bio-Rad Laboratories, Foster City, CA) and a real-time PCR system (Bio-Rad Laboratories) according to the following cycling parameters: initial denaturation at 95°C for 1 min, 40 cycles of denaturation at 95°C for 20 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The following primers were used for mRNA amplification: type I collagen (forward): 5'-GTGGAAATGATGGTGCTACT-3', (reverse): 5'-TTAGCACCAGTGTCTCCTTT-3'. GAPDH (forward): 5'-GTA TTGGGCGCCTGGTCACC-3', (reverse): 5'-CGCTCCTGGAAG ATGGTGATGG-3'. mRNA levels were calculated by determining the relative copy number compared with GAPDH and the Ct of the control group was normalized to 1.(26,30)
Mitochondrial oxygen consumption
OCR and ECAR were measured in human gingival fibroblasts with a XF 24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA). HGF cells were seeded in every well of a XF 24-well cell culture microplate (Seahorse Bioscience) at a density of 10,000 cells/well in 200 µl of DMEM and incubated for 24 h at 37°C in 5% CO2. After replacing the growth medium with bicarbonate-free DMEM XF assay medium (containing 1% serum) supplemented with 5.5 mM glucose and 4 mM l-glutamine and pre-warmed at 37°C, selected wells were treated with resveratrol (50 µM), quercetin (15 µM), or NAC (1.5 mM). HGFs were pre-incubated at 37°C without CO2 for 30 min before starting the assay.
The detailed protocol is as follows. 1) Basal OCR reading, 2) addition of 75 µl H2O2 through port A to each well, 3) addition of 75 µl oligomycin (ATP synthase inhibitor) through port B to each well, 4) addition of 75 µl of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; mitochondrial uncoupler) through port C and, 5) addition of 75 µl of antimycin A with rotenone (complex I and III inhibitors, respectively) through port D. Each step had three cycles with cycles consisting of 3 min mixing, 2 min waiting and, 3 min measurement.(31)
Statistical analysis
All data were expressed as mean ± SEM. For statistical analysis, differences between the groups were analyzed with one-way analysis of variance using SPSS 19.0 (SPSS Inc., Tokyo, Japan).
Results
Dynamic monitoring of antioxidant effects on cell viability and proliferation
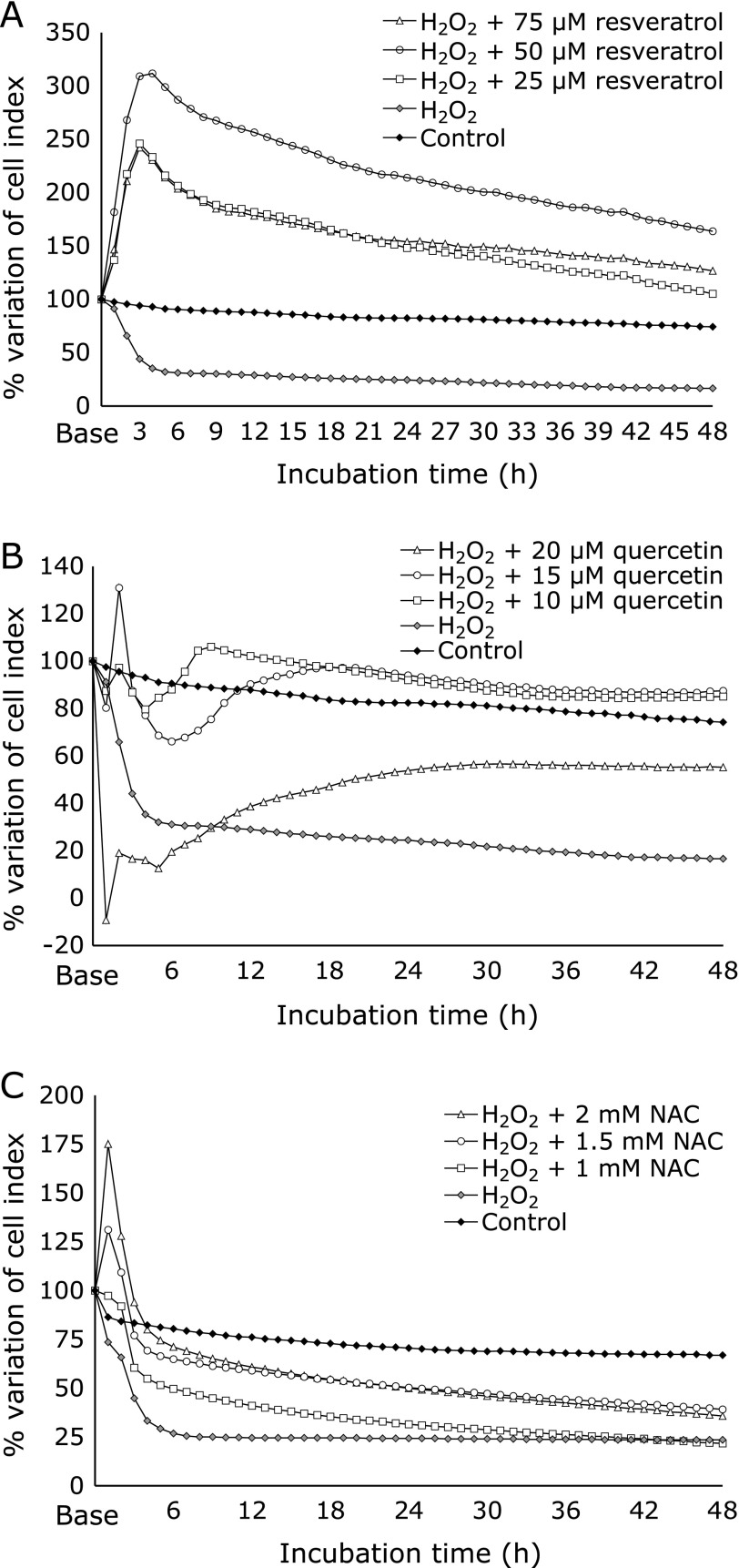
We compared the effects of three antioxidants against ROS by recurrent measurements with xCELLigence in three independent experiments. In comparison with control cells, after 48 h of incubation, resveratrol stimulated the highest percentage of cell growth and proliferation, expressed as CI variation (Fig. 1A, Table 1). Quercetin also stimulated cell growth and proliferation, but levels were close to control group (Fig. 1B). NAC-treated cells did not stimulate cell growth or proliferation, reaching 40% of CI variation (Fig. 1C).
Fig. 1.
Dynamic monitoring of cell viability and proliferation expressed as a cell index (CI) by xCELLigence system. HGFs (10,000 cells/well) were exposed to H2O2 (0.23 mM) to induce oxidative stress and treated with different concentrations of (A) resveratrol, (B) quercetin, and (C) NAC for 48 h. The percentage of variation of CI represents the changes recorded in CI values compared to the Base value. The graph shown is the result of the mean of three independent experiments (n = 6).
Table 1.
Effectiveness derived from real-time analysis of three antioxidants at different concentrations under oxidative stress induced by H2O2 (0.23 mM) on HGFs
| Concentration | Incubation Time | |||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 36 h | 48 h | ||
| Resveratrol | 75 µM | 103a | 88 | 75 | 73 | 63 |
| 50 µM | 152 | 134 | 110 | 100 | 85 | |
| 25 µM | 104 | 90 | 72 | 65 | 51 | |
| Quercetin | 20 µM | –7 | 6 | 17 | 22 | 22 |
| 15 µM | 21 | 36 | 40 | 41 | 41 | |
| 10 µM | 34 | 43 | 39 | 39 | 39 | |
| NAC | 2 mM | 81 | 57 | 25 | 11 | 1 |
| 1.5 mM | 86 | 69 | 46 | 34 | 27 | |
| 1 mM | 81 | 63 | 42 | 31 | 23 | |
aEffectiveness is expressed as percentage value.
Table 1 shows that resveratrol was most effective at a concentration of 50 µM after 6, 12, 24, 36, and 48 h. The effectiveness of resveratrol declined progressively from 152% at 6 h to 85% at 48 h. Concentrations at 75 µM and 25 µM showed poorer results. Quercetin induced cytotoxicity at 20 µM (–7%) after 6 h of treatment, but this toxicity was gradually reverted and quercetin reached 22% of effectiveness after 48 h of incubation. At 15 µM, quercetin had 21% effectiveness compared with controls after 6 h of incubation. The effectiveness increased in a sustained manner and reached 41% after 48 h of incubation. NAC (1.5 mM) had an effectiveness of 86% after 6 h of incubation, which steadily decreased to 27% after 48 h. This was also observed with 2 mM (81%) and 1 mM (81%) following 6 h of incubation. After 48 h, 2 mM of NAC lost its effectiveness (1%).
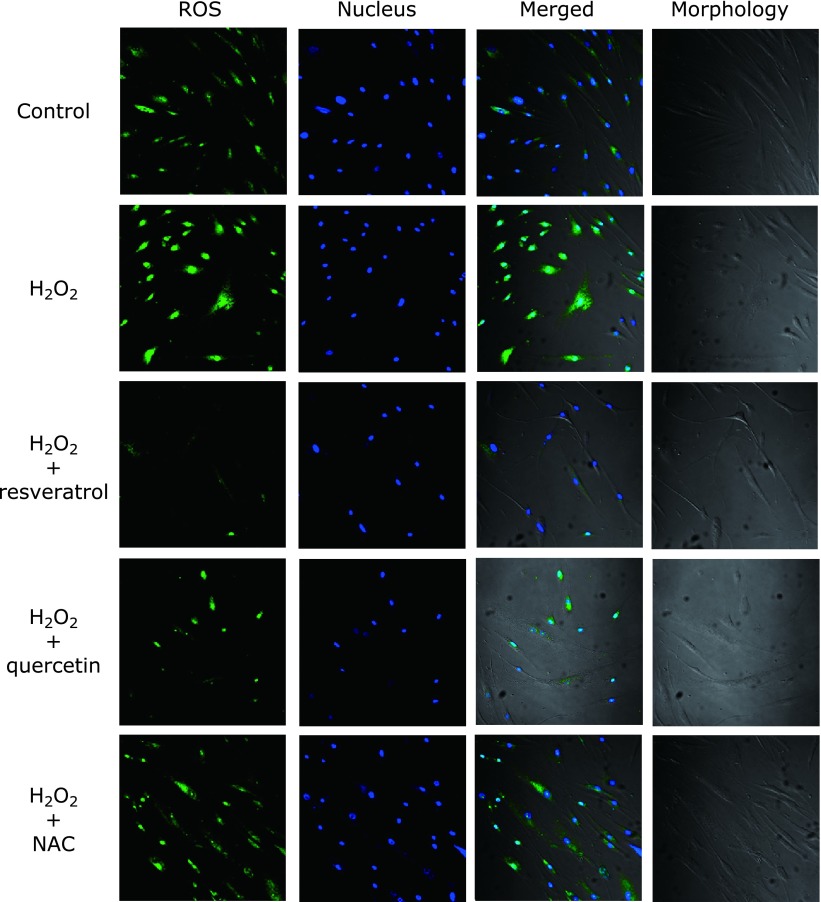
Resveratrol and quercetin reduce ROS production during oxidative stress
Fig. 2 shows cell images produced by transmitted light after H2O2 stimulation with or without antioxidants. The strength of the green intracellular fluorescence corresponds to intracellular production of ROS. In the H2O2 group, ROS production was remarkably increased compared with the control group. The group treated with resveratrol (50 µM) greatly diminished ROS production, followed by quercetin (15 µM). NAC (1.5 mM) showed similar fluorescence intensity to the untreated group.
Fig. 2.
Intracellular ROS scavenging activity of antioxidants in HGFs on oxidative stress. Representative double-stained, confocal laser microscopic images depicting ROS production induced by 0.23 mM H2O2 on HGFs (40×) with or without antioxidants after 30 min of incubation. Green fluorescence intensity corresponds to intracellular ROS production, and DAPI (blue) staining to viable cells. H2O2 affected the morphology of HGFs, which is typically large, flat, and elongated (spindle-shaped).
Resveratrol and quercetin promote upregulation of type 1 collagen gene transcription following oxidative stress
To investigate whether antioxidants alter gene expression levels of type I collagen following oxidative stress, we measured the amount of type I collagen mRNA expression in HGF-1 cells. Cells treated with resveratrol and quercetin showed significant upregulation of type I collagen, 1.70-fold (p<0.05) and 1.97-fold (p<0.01), respectively, following 3 h of ROS exposure. Collagen synthesis was downregulated in resveratrol-treated groups after 12 h, but was upregulated after 24 h (p<0.05). NAC did not stimulate the synthesis of type I collagen and rather decreased it in a constant manner from 0.30-fold (3 h) to 0.12-fold (72 h). The untreated group showed a sustained increase in gene expression, which reached its maximum (1.45-fold) at 24 h, and then progressively decreased in the following 48 (0.73-fold) and 72 (0.63-fold) h. The group exposed to 1 mM of H2O2 showed minimum gene expression, and its maximum value was 0.50-fold at 48 h of incubation. There was no significant difference found among the other groups (Fig. 3).
Fig. 3.
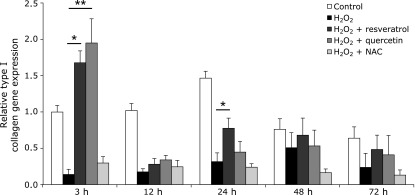
Effect of resveratrol, quercetin, and NAC on type I collagen gene expression in HGFs under oxidative stress induced by H2O2 after different incubation periods. Relative mRNA of Type I collagen was detected by qRT-PCR. Bars represent mRNA expression normalized to GAPDH and relative to the control group. Data are expressed as mean ± SEM based on four independent experiments (n = 9). *p<0.05, **p<0.01: significantly different from H2O2 group.
Resveratrol and quercetin enhance more mitochondrial respiration during oxidative stress
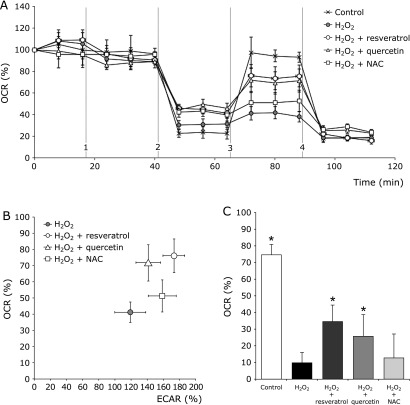
Because the mitochondria play a key role in controlling oxidative stress, mitochondrial respiratory profiles were examined following antioxidant treatment. The OCR was measured in HGFs following treatment with H2O2 and the mitochondrial respiratory capacity was observed in antioxidant-treated cells using the Seahorse XF24 flux analyzer. Compared with untreated cells, antioxidant-treated cells showed higher maximal respiratory capacity after 45 min (Fig. 4A). HGFs treated with resveratrol exhibited the highest maximal respiratory capacity, followed by quercetin and finally NAC.
Fig. 4.
Effect of antioxidants on mitochondrial respiratory profiles in HGFs under oxidative stress. (A) Changes in HGFs’ cellular respiration under oxidative stress. OCR was measured in HGFs cells after 30 min of treatment with resveratrol (50 µM), quercetin (15 µM), and NAC (1.5 mM). Seahorse OCR protocol (mitochondrial stress test) included (1) H2O2 (1 mM) and three mitochondrial ETC complex inhibitors: (2) oligomycin, (3) FCCP, and (4) antimycin A with rotenone. OCR measured before (1) represents basal respiration, between (3) and (4) represents maximal respiratory capacity. Plots shown are means ± SEM (n = 6). (B) Simultaneous comparison of OCR versus ECAR in HGFs stressed by H2O2 (1 mM) and treated with resveratrol (50 µM), quercetin (15 µM), and NAC (1.5 mM). A higher OCR value indicates predominance of mitochondrial respiration. Similarly, a high ECAR value indicates predominance of non-mitochondrial respiration. Plots indicated the SEM (n = 6). (C) Antioxidant treatment enhances OCR rate in oxidative stress conditions. The bars show the comparison of the total amount of oxygen consumed by mitochondrial respiration in HGFs stressed by H2O2 (1 mM) following treatment with resveratrol (50 µM), quercetin (15 µM), and NAC (1.5 mM). Data are expressed as mean ± SEM (n = 6). *p<0.05: significantly different from the H2O2 group.
After measuring OCR (mitochondrial respiration) and ECAR (non-mitochondrial respiration) simultaneously, and in real time, HGFs treated with resveratrol and quercetin stimulated more mitochondrial respiration than untreated cells. HGFs treated with NAC consumed less oxygen and enhanced more non-mitochondrial respiration (Fig. 4B). Finally, when we analyzed the spare respiratory capacity induced by each antioxidant, we found that HGFs treated with resveratrol or quercetin significantly increased the percentage of oxygen consumption to 28 and 20% more, respectively, compared with H2O2 treated group. There was no significant difference found between HGFs treated with NAC and H2O2 treated group (Fig. 4C).
Discussion
The effects of three different antioxidants were compared during oxidative stress induced by H2O2 in HGFs. Oxidative stress led to an increase in ROS production, which affected cellular viability, metabolism, and function. These findings demonstrate that antioxidants helped to control the detrimental effects produced by oxidative stress. In this study, the protective effects of resveratrol, quercetin, and NAC were examined. Resveratrol and quercetin enhanced cellular proliferation in oxidative stress conditions. The production of ROS was nearly inhibited by resveratrol, and greatly reduced by quercetin. Resveratrol and quercetin upregulated the synthesis of type I collagen gene after 3 h of incubation post-induction of oxidative stress, but only resveratrol kept it after 24 h. When the OCR was measured, resveratrol generated the highest maximal respiratory capacity, followed by quercetin and NAC, respectively. Further, the OCR and ECAR were evaluated and it was found that resveratrol and quercetin stimulated more mitochondrial respiration than cells without treatment. NAC treatment enhanced more non-mitochondrial respiration. Taken together, these results indicate that resveratrol was the most effective antioxidant to protect HGFs from damage under oxidative stress induced by H2O2.
Free radicals and other reactive small molecules have emerged as important regulators of many physiological and pathological processes.(32,33) In periodontitis, ROS serve as signaling messengers to induce immune responses.(5,33) Whether the effects of ROS are beneficial or harmful depends on the site, type, amount of ROS production, and the activity of the organism’s antioxidant defense system.(33,34) As with other diseases related to ROS overproduction, periodontitis shows impairment in gingival fibroblasts’ functioning owing to the toxic effects of ROS, resulting in reduced collagen synthesis, activation of metalloproteinases that break down type I collagen,(35) necrosis, and apoptosis of HGFs.(36)
In vitro and in vivo studies suggest that antioxidants can be used to supplement an organism’s defense system and delay or prevent the onset of periodontitis.(13,15,17,37) Until now, most of these in vitro studies have only described the final outcomes of antioxidant treatments, but the effects of antioxidants during all the treatment and the changes within cells have not been reported, yet. In the current study, two real-time systems were used. First, the xCELLigence system (ACEA Biosciences) that monitors and detects any cellular physiologic change by measuring the impedance variation. Changes such as ion concentration or whether the cells are attached or not to the electrodes are monitored in individual wells.(38–40) Second, the XF Extracellular Flux Analyzer (Seahorse Bioscience), which measures both OCR (mitochondrial respiration) and ECAR (non-mitochondrial respiration) in real time. It is possible to observe three important factors of mitochondrial performance: ATP turnover, proton leak, and maximal respiration.(41)
The antioxidants studied showed different effects on cellular viability and mitochondrial respiration. A possible explanation for this is that the antioxidants have different mechanisms of action. Previous studies also indicate that resveratrol is able to provide protection against oxidative stress induced by metals owing to its antioxidant capacity and can enhance the cellular viability of fibroblasts.(42) Studies also show that quercetin enhances wound healing potential in vitro yet an explanation for this result is not fully explained.(43)
Evidence suggests that resveratrol modulates the mitochondrial respiratory chain through complexes I and III, which are known to be the major source of ROS in normal respiring mitochondria.(12) Resveratrol modulation of complex I is sirtuin dependent, which augments the mitochondrial substrate supply pathways (i.e., the tricarboxylic acid cycle and fatty acid oxidation) raising the mitochondrial NAD+/NADH ratio.(44) Resveratrol decreases complex III activity by competing with coenzyme Q. This property is especially interesting as this complex is the site where ROS are generated. By decreasing the activity of complex III, resveratrol not only opposes the production of ROS but can also scavenge them.(45,46)
Quercetin, the most represented polyphenol in the human diet, is usually present as a glucoside-conjugate or as a methyl-glucoside-conjugate.(23) In addition to antioxidant activity, in vitro studies show that quercetin has a variety of actions including anti-mutagenic and anti-tumorigenic activities,(47) long-lasting anti-inflammatory activity,(48) protein kinase C inhibition, superoxide dismutase-like activity, and modulation of the cell cycle.(14) Despite all these benefits, quercetin has low solubility in water resulting in poor absorption and low bioavailability, limiting its potential clinical application.(49,50) Quercetin glucosides need to be converted to conjugate metabolites (aglycone) by deglycosylation followed by glucoronide/sulfate conjugation.(14) As a result, quercetin aglycone appears to be more accessible to cellular membranes because of its lipophilicity.(51) Unfortunately, the aglycone can function as a pro-oxidant, causing biological damage.(14) Moreover, it seems that quercetin might induce cytotoxicity at high concentrations (~40 µM).(48) Low solubility, conversion to another molecule, and the pro-oxidant activity of quercetin aglycone can explain the temporal drop of the CI value in the viability assay after 6 h of incubation (Fig. 1B). Shirai et al.(52) demonstrated that quercetin is more effective than its aglycone in preventing intracellular ROS production induced by H2O2 in mouse fibroblast-cultured cells. Additionally, it was not necessary that a large amount of quercetin reach the cytoplasm or cell nucleus compartment to observe this effect. The study concluded that quercetin metabolites play a role in the modulation of cellular redox regulation without invasion into the cytosol.
The antioxidant mechanism of NAC is associated with its ability to stimulate GSH synthesis. It is rapidly deacetylated to cysteine and increases intracellular GSH levels, providing a substrate for GSH synthesis and ROS scavenging.(53) In the current study, NAC had the lowest antioxidant activity. A possible explanation is that in addition to its activity as a GSH precursor, NAC is, per se, responsible for protective effects in the extracellular environment.(24) NAC directly scavenged ROS, and reduced the oxidative burst.(17) Sueishi et al.(54) proposed that the scavenging rates depends on the type of ROS being scavenged, yet it is a common practice to determine scavenging rates against a single ROS. Therefore, one should be cautious in judging the effectiveness of antioxidants.
In conclusion, our findings support the idea that antioxidants could enhance biological cellular functions during oxidative stress. Additionally, our results show that not all antioxidants have the same protective effects. We propose that this discrepancy can be explained by differing mechanisms of action and differing targets within cells. Given the fact that resveratrol enhanced cell proliferation, almost abolished ROS production, stimulated the synthesis of type I collagen gene, and stimulated mitochondrial respiration better than quercetin and NAC, we propose that resveratrol antioxidant is a beneficial supplement during the treatment of oxidative stress disorders. However, further studies using in vivo models are necessary to support the clinical use of antioxidants as a supplement to reduce oxidative stress and prevent periodontitis in humans.
Acknowledgments
We thank Dr. Masato Miyake from the Division of Molecular Biology, Institute for Genome Research, The University of Tokushima for his valuable support, instruction, and advice to improve this work; and Dr. Diego A. Vargas-Inchaustegui for assisting in the manuscript’s preparation. This work was partially supported by a Grant-in-Aid for Science Research (C) (No. 24593155) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- ECAR
extracellular acidification rate
- HGF
human gingival fibroblast
- NAC
N-acetylcysteine
- OCR
oxygen consumption rate
- ROS
reactive oxygen species
Conflict of Interest
Naofumi Tamaki was supported by a Yamada research grant in 2012. Resveratrol has been purchased from the Yamada Bee Farm (Okayama, Japan).
References
- 1.Murphy K, Travers P, Walport M, Janeway C.Janeway’s Immunobiology (7th ed) New York: Garland Science; 2008: 39–55. [Google Scholar]
- 2.Chapple IL, Matthews JB, Wright HJ, Scott AE, Griffiths HR, Grant MM. Ascorbate and α-tocopherol differentially modulate reactive oxygen species generation by neutrophils in response to FcγR and TLR agonists. Innate Immun. 2013;19:152–159. doi: 10.1177/1753425912455207. [DOI] [PubMed] [Google Scholar]
- 3.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Gulbins E, Zhang Y. Oxidative stress triggers Ca-dependent lysosome trafficking and activation of acid sphingomyelinase. Cell Physiol Biochem. 2012;30:815–826. doi: 10.1159/000341460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy MP, Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity. 2013;38:201–202. doi: 10.1016/j.immuni.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San Miguel SM, Opperman LA, Allen EP, Zielinski J, Svoboda KK. Bioactive polyphenol antioxidants protect oral fibroblasts from ROS-inducing agents. Arch Oral Biol. 2012;57:1657–1667. doi: 10.1016/j.archoralbio.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Sharma S. Reactive oxygen species and antioxidants in periodontics: a review. Int J Dent Clinics. 2011;3:44–47. [Google Scholar]
- 9.Petti S, Scully C. Polyphenols, oral health and disease: a review. J Dent. 2009;37:413–423. doi: 10.1016/j.jdent.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Bullon P, Cordero MD, Quiles JL, Morillo JM, del Carmen Ramirez-Tortosa M, Battino M. Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic Biol Med. 2011;50:1336–1343. doi: 10.1016/j.freeradbiomed.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Wanasundara PK, Shahidi F. Antioxidants: Science, Technology, and Applications. Bailey’s Industrial Oil and Fat Products. John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 12.Sassi N, Mattarei A, Azzolini M, et al. Cytotoxicity of mitochondria-targeted resveratrol derivatives: Interactions with respiratory chain complexes and ATP synthase. Biochim Biophys Acta. 2014;1837:1781–1789. doi: 10.1016/j.bbabio.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki N, Orihuela-Campos RC, Inagaki Y, Fukui M, Nagata T, Ito HO. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic Biol Med. 2014;75:222–229. doi: 10.1016/j.freeradbiomed.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 15.Napimoga MH, Clemente-Napimoga JT, Macedo CG, et al. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J Nat Prod. 2013;76:2316–2321. doi: 10.1021/np400691n. [DOI] [PubMed] [Google Scholar]
- 16.Kim do Y, Jun JH, Lee HL, et al. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch Pharm Res. 2007;30:1283–1292. doi: 10.1007/BF02980269. [DOI] [PubMed] [Google Scholar]
- 17.Toker H, Ozdemir H, Balcı H, Ozer H. N-acetylcysteine decreases alveolar bone loss on experimental periodontitis in streptozotocin-induced diabetic rats. J Periodontal Res. 2012;47:793–799. doi: 10.1111/j.1600-0765.2012.01497.x. [DOI] [PubMed] [Google Scholar]
- 18.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 19.Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol. 2012;3:141. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirola L, Fröjdö S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez Galdón B, Rodríguez Rodríguez EM, Díaz Romero C. Flavonoids in onion cultivars (Allium cepa L.) J Food Sci. 2008;73:C599–C605. doi: 10.1111/j.1750-3841.2008.00903.x. [DOI] [PubMed] [Google Scholar]
- 24.De Flora S, Izzotti A, D'Agostini F, Balansky RM. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]
- 25.Spagnuolo G, D'Antò V, Cosentino C, Schmalz G, Schweikl H, Rengo S. Effect of N-acetyl-l-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27:1803–1809. doi: 10.1016/j.biomaterials.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Tsutsumi K, Fujikawa H, Kajikawa T, Takedachi M, Yamamoto T, Murakami S. Effects of L-ascorbic acid 2-phosphate magnesium salt on the properties of human gingival fibroblasts. J Periodontal Res. 2012;47:263–271. doi: 10.1111/j.1600-0765.2011.01430.x. [DOI] [PubMed] [Google Scholar]
- 27.Colombo G, Dalle-Donne I, Orioli M, et al. Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic Biol Med. 2012;52:1584–1596. doi: 10.1016/j.freeradbiomed.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Kustermann S, Boess F, Buness A, et al. A label-free, impedance-based real time assay to identify drug-induced toxicities and differentiate cytostatic from cytotoxic effects. Toxicol In Vitro. 2013;27:1589–1595. doi: 10.1016/j.tiv.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Kang SW, Park HJ, Ban JY, Chung JH, Chun GS, Cho JO. Effects of nicotine on apoptosis in human gingival fibroblasts. Arch Oral Biol. 2011;56:1091–1097. doi: 10.1016/j.archoralbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Matsui S, Tsujimoto Y, Ozawa T, Matsushima K. Antioxidant effects of antioxidant biofactor on reactive oxygen species in human gingival fibroblasts. J Clin Biochem Nutr. 2011;48:209–213. doi: 10.3164/jcbn.10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Invernizzi F, D'Amato I, Jensen PB, Ravaglia S, Zeviani M, Tiranti V. Microscale oxygraphy reveals OXPHOS impairment in MRC mutant cells. Mitochondrion. 2012;12:328–335. doi: 10.1016/j.mito.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DD, Ridnour LA, Isenberg JS, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang HM, Chen CW, Lin TY, Kuo YH. N-phenethyl caffeamide and photodamage: protecting skin by inhibiting type I procollagen degradation and stimulating collagen synthesis. Food Chem Toxicol. 2014;72:154–161. doi: 10.1016/j.fct.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhou S, Sun W, Zhang Z, Zheng Y. The Role of Nrf2-Mediated Pathway in Cardiac Remodeling and Heart Failure. Oxid Med Cell Longev. 2014;2014:260429. doi: 10.1155/2014/260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fordham JB, Naqvi AR, Nares S. Leukocyte production of inflammatory mediators is inhibited by the antioxidants phloretin, silymarin, hesperetin, and resveratrol. Mediators Inflamm. 2014;2014:938712. doi: 10.1155/2014/938712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urcan E, Haertel U, Styllou M, Hickel R, Scherthan H, Reichl FX. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent Mater. 2010;26:51–58. doi: 10.1016/j.dental.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Öztürk F, Malkoc S, Ersöz M, Hakki SS, Bozkurt BS. Real-time cell analysis of the cytotoxicity of the components of orthodontic acrylic materials on gingival fibroblasts. Am J Orthod Dentofacial Orthop. 2011;140:e243–e249. doi: 10.1016/j.ajodo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Diemert S, Dolga AM, Tobaben S, et al. Impedance measurement for real time detection of neuronal cell death. J Neurosci Methods. 2012;203:69–77. doi: 10.1016/j.jneumeth.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier M, Billingham LK, Ramaswamy M, Siegel RM. Extracellular flux analysis to monitor glycolytic rates and mitochondrial oxygen consumption. Methods Enzymol. 2014;542:125–149. doi: 10.1016/B978-0-12-416618-9.00007-8. [DOI] [PubMed] [Google Scholar]
- 42.San Miguel SM, Opperman LA, Allen EP, Zielinski JE, Svoboda KK. Antioxidant combinations protect oral fibroblasts against metal-induced toxicity. Arch Oral Biol. 2013;58:299–310. doi: 10.1016/j.archoralbio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Muhammad AA, Pauzi NA, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res Int. 2013;2013:974580. doi: 10.1155/2013/974580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desquiret-Dumas V, Gueguen N, Leman G, et al. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J Biol Chem. 2013;288:36662–36675. doi: 10.1074/jbc.M113.466490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87–97. [PubMed] [Google Scholar]
- 46.Moldzio R, Radad K, Krewenka C, Kranner B, Duvigneau JC, Rausch WD. Protective effects of resveratrol on glutamate-induced damages in murine brain cultures. J Neural Transm. 2013;120:1271–1280. doi: 10.1007/s00702-013-1000-6. [DOI] [PubMed] [Google Scholar]
- 47.Schwingel TE, Klein CP, Nicoletti NF, et al. Effects of the compounds resveratrol, rutin, quercetin, and quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and neurotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:837–848. doi: 10.1007/s00210-014-0994-0. [DOI] [PubMed] [Google Scholar]
- 48.Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9:263–285. doi: 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- 49.Wang G, Song L, Wang H, Xing N. Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth and inducing apoptosis in human prostate cancer cells. Oncol Rep. 2013;30:357–363. doi: 10.3892/or.2013.2469. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Zhang J, Liu L, Sharma S, Dong Q. Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PLoS One. 2012;7:e51764. doi: 10.1371/journal.pone.0051764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajiya K, Ichiba M, Kuwabara M, Kumazawa S, Nakayama T. Role of lipophilicity and hydrogen peroxide formation in the cytotoxicity of flavonols. Biosci Biotechnol Biochem. 2001;65:1227–1229. doi: 10.1271/bbb.65.1227. [DOI] [PubMed] [Google Scholar]
- 52.Shirai M, Yamanishi R, Moon JH, Murota K, Terao J. Effect of quercetin and its conjugated metabolite on the hydrogen peroxide-induced intracellular production of reactive oxygen species in mouse fibroblasts. Biosci Biotechnol Biochem. 2002;66:1015–1021. doi: 10.1271/bbb.66.1015. [DOI] [PubMed] [Google Scholar]
- 53.Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;2:191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sueishi Y, Hori M, Ishikawa M, et al. Scavenging rate constants of hydrophilic antioxidants against multiple reactive oxygen species. J Clin Biochem Nutr. 2014;54:67–74. doi: 10.3164/jcbn.13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]