Abstract
Emergent technologies in regenerative medicine may soon overcome the limitations of conventional diabetes therapies. Collaborative efforts across the subfields of stem cell technology, islet encapsulation, and biomaterial carriers seek to produce a bioengineered pancreas capable of restoring endocrine function in patients with insulin-dependent diabetes. These technologies rely on a robust understanding of the extracellular matrix (ECM), the supportive 3-dimensional network of proteins necessary for cellular attachment, proliferation, and differentiation. Although these functions can be partially approximated by biosynthetic carriers, novel decellularization protocols have allowed researchers to discover the advantages afforded by the native pancreatic ECM. The native ECM has proven to be an optimal platform for recellularization and whole-organ pancreas bioengineering, an exciting new field with the potential to resolve the dire shortage of transplantable organs. This review seeks to contextualize recent findings, discuss current research goals, and identify future challenges of regenerative medicine as it applies to diabetes management.
Keywords: diabetes mellitus, insulin, regenerative medicine, extracellular matrix, stem cells, bioartificial pancreas
The treatment of diabetes mellitus remains inadequate. Although exogenous insulin therapy is effective at preventing acute metabolic decompensation in type 1 diabetes, fewer than 40% of patients achieve and maintain therapeutic targets.1 As a result, hyperglycemia-related organ damage remains a significant cause of morbidity and mortality among the diabetic population. Intensive glycemic control achieved through dietary modification, physical activity, oral hypoglycemics, and exogenous insulin can significantly reduce, but not eliminate, the microvascular and macrovascular complications of diabetes mellitus.2 Current best-practice guidelines for the management of diabetes are centered on lifelong lifestyle and pharmaceutical intervention. While these measures reduce the incidence of diabetic emergency and complication, they do not offer the possibility of remission or cure. β-cell replacement, through whole pancreas or islet cell transplantation, is the sole treatment capable of establishing long-term, stable euglycemia in type 1 diabetic patients.
β-cell replacement through the transplantation of isolated pancreatic islets has been heavily scrutinized following the underperformance of the seminal “Edmonton Protocol.”3 Although 58% of patients achieved insulin-independence following transplantation, only 24% remained free of exogenous insulin requirements at the 2-year follow-up.4 Several physiological and biomechanical stressors have been proposed to explain the underperformance of therapeutic islet transplantation, including low isolation efficiency,4 the effects of immunosuppression on β-cell proliferation,5 inflammatory mediated host response,6 disruption of the basement membrane,7 recurrence of autoimmunity,8 and failed revascularization.9 Although subsequent studies have demonstrated improvements in diabetic complications and quality of life following islet transplantation,10-12 very few studies to date have reported improved outcomes over insulin therapy,13 though some results have been encouraging. Specifically, Thompson and Warnock et al reported that islet cell therapy is associated with less progression of retinopathic microvascular complications than intensive medical therapy.14 Nonetheless, islet transplantation requires 2 to 4 whole cadaveric pancreata per recipient,15 which would impoverish an already-limited organ pool. As a result of these shortcomings, in 2006 the American Diabetes Association recommended performing islet transplantation only in the context of controlled research studies.16 However, establishment of the Collaborative Islet Transplant Registry to monitor progress and safety of islet transplantation by using data from the United States, Canada, and several centers in Europe and Australia and supported by the Juvenile Diabetes Research Foundation, has allowed significant progress.17 As a consequence, the application for licensure of cadaveric islets as a biological product, allowing reimbursement from insurance companies is imminent in the United States, whereas in Canada, Switzerland, and other countries insurance companies have been covering islet as a clinical treatment for several years and it is not considered an experimental treatment any longer.
Comparatively, pancreas transplantation, first performed by Kelly et al,18 yields higher rates of insulin independence than islet transplantation.19 In addition, transplant recipients show greater improvements in secondary complications of diabetes20-24 and quality of life25,26 compared to patients treated with exogenous insulin. Despite these clear advantages, donor shortage, surgical morbidity, and the need for lifelong immunosuppression significantly limit clinical application. Furthermore, comparative analyses have shown that transplantation improves overall survival only when performed as part of a simultaneous pancreas and kidney transplant.19,27
Emerging technologies in the field of regenerative medicine (RM) seek to address the limitations of current diabetes treatment strategies. The regenerative technologies that are currently being implemented to bioengineer pancreatic tissues for transplant purposes can be broadly classified into the following categories: (1) islet encapsulation, (2) biomaterial carriers, and (3) whole-organ bioengineering. Collaborative efforts across these fields strive to produce a bioengineered pancreas capable of restoring endocrine function in patients with insulin-dependent diabetes. Although each of these topics will be discussed, the focus of this review is recent advancements in whole-organ bioengineering and their application to the endocrine pancreas.
Islet Encapsulation
RM promises to contribute to the advancement of islet transplantation through the development of microencapsulation technology and the exploitation of bioengineered microenvironments. Encapsulation is a means of immunoisolation which serves to “camouflage” the foreign antigens of the islet allo- or xeno-graft from host immune surveillance.28 Encapsulation protocols involve packaging islets within semipermeable, bioinert membranes that selectively allow the passage of oxygen, glucose, nutrients, waste products, and insulin while preventing penetration by immune cells.29,30 Theoretically, successful encapsulation eliminates the need for aggressive, lifelong immunosuppression, with consequent improvements in β-cell viability and host morbidity. Although promising results have been obtained in early animal studies, the clinical value of islet encapsulation has been limited by the following obstacles: (1) poor biocompatibility of capsule materials, (2) inadequate immunoisolation due to the penetration of small immune mediators, (3) hypoxia secondary to failed revascularization, and (4) mass transport, impeding the efficient entrance of nutrients into the capsule and exit of insulin to the bloodstream.29,31
Biomaterial Carriers
The immune-related obstacles facing islet encapsulation have prompted investigators to explore regenerative technologies using autologous cells and bioinert carriers. These emerging technologies employ recellularization protocols designed to seed pluripotent cells onto scaffolds capable of driving cellular proliferation and differentiation. The necessity of an extracellular scaffold is widely recognized in the bioengineering literature, as anchorage-dependent cells deprived of attachment sites quickly undergo apoptosis induced by loss of cellular adhesion, termed anoikis.32-38 Researchers have sought to artificially fulfill the anchorage requirement using biomaterial carriers that mimic the structure of native extracellular matrix (ECM). These carriers serve the functions of delivery platform, transitory structural support, and mechanical immune barrier, thus enabling islet transplantation into heterotopic sites.6 The properties of these carriers can also be precisely manipulated, allowing for the study of individual ECM components. Several biomaterial carriers have been investigated, including poly-lactic-co-glycolic acid,39 poly vinyl alcohol,40 poly(ethylene glycol),32,41 poly(N-isopropylacrylamide),6 and biopolymer films (see Borg and Bonifacio for a full review).42 These carriers vary in their structure, strength, stability, rigidity, biocompatibility, growth factor binding capacity, and amenability to manipulation.
The Importance of the Pancreatic Extracellular Matrix
Although these biomaterial carriers can satisfy the structural and anchorage requirements of the seeded cells, they cannot fulfill the full breadth of functions performed by the native ECM. Over the past decade, research has shown that the ECM plays a fundamental role in the welfare of cells, tissues, and organs. The role of the ECM extends far beyond mechanical support and architecture, influencing molecular composition, cell adhesion, and signaling and binding of growth factors. In addition, the mechanical stiffness and deformability of the ECM contribute significantly to the determination, differentiation, proliferation, survival, polarity, migration, and behavior of cells.43
The native pancreatic ECM is a 3-dimensional (3D), structural framework of proteins in a state of “dynamic reciprocity” with the cells of the endocrine pancreas.44 The ECM regulates essential aspects of islet biology including development, morphology, and differentiation,35,45-47 intracellular signaling,48 gene expression,49,50 adhesion and migration,51-53 proliferation,54,55 secretion,32,38,51,53,56 and survival.32,33,57 The ECM performs these functions via 3-D structure,6,32 signaling molecules, and the secretion and storage of growth factors and cytokines.58 As this functional breadth becomes increasingly recognized, the native ECM may become a valuable platform for pancreas bioengineering investigations.
The most well characterized components of the ECM are the laminins, a family of approximately 15 to 20 trimeric glycoproteins,59 which have been shown to independently enhance insulin release and survival.51 The ratio of laminin isoforms varies between embryonic development and adulthood, suggesting that individual isoforms may selectively promote different aspects of pancreatic maturation, proliferation and secretion.59,60 ECM-expressed laminins influence cellular processes through binding with integrins, a family of cell surface receptors responsible for cell-matrix adhesion61 and the transduction of external signals to the cytoskeleton.62 Integrins have been shown to initiate a number of intracellular signaling cascades in the endocrine pancreas, including focal adhesion kinase (FAK), paxillin, ERK1/2,48 phosphatidylinositol (PI), 3-kinase and mitogen-activated protein kinase (MAP kinase ERK), protein kinase B (PKB/Akt),57 and NF-KB.63 These laminin-activated signaling pathways have been shows to mediate the effects of ECM on islet spread, survival, and function.
Several features of the ECM appear to be essential for the function of the endocrine pancreas. The 3D structure of native ECM determines the topographical arrangement of pancreatic endocrine cells,64 which has been shown to influence islet secretory activity35 and survival.64 Furthermore, the constituent elements of the ECM, including collagens, glycoproteins, and glycosaminoglycans, have been shown to be independently capable of preventing β-cell apoptosis induced by loss of cellular adhesion, termed ankoisis.32-38 Specifically, Weber et al characterized the viability of pancreatic β-cells within several gel environments each containing an individual ECM protein and demonstrated that the presence of protein resulted in significantly decreased apoptosis compared to protein-free controls after 10 days with no significant differences amongst the proteins tested.28 Thus, an appropriate goal would be to recapture and improve on these beneficial effects in vivo by presenting islet cells with ECM elements in their native 3D infrastructural environments. ECM components have also been shown to enhance insulin secretion, even in the absence of glucose.64 The molecular basis of these prosurvival and prosecretory ECM signals has not been fully characterized, but the integrins have been implicated.57 Finally, the ECM is capable of binding, storing and regulating the activity of growth factors including TGF-β1,65,66 which plays a role in development,66 function, and regeneration65 of pancreatic islets. The dysregulation of these essential ECM-growth factor interactions have been shown to underlie a variety of pancreatic pathologies, including pancreatitis,67 fibrosis,68 and adenocarcinoma.69
Decellularization–Recellularization Technology
Emerging, cutting-edge technologies in RM have recently allowed researchers to exploit and appreciate the advantages of preserving innate ECM for pancreas bioengineering investigations.44,70-73 Indeed, the innate ECM represents a biochemically, geometrically and spatially ideal platform for regeneration and implantation because it is biocompatible,74 it has both basic components (proteins and polysaccharides) and matrix-bound growth factors and cytokines preserved at physiological levels,75 it retains an intact and patent vasculature capable of sustaining physiologic blood pressure when implanted in vivo (ie, contrast perfusion through the superior mesenteric and splenic arteries illuminates major vessels individually without extravasation),74 and it is able to drive differentiation of progenitor cells into an organ-specific phenotype.76,77 In other words, the native ECM represents the requisite environment for cell welfare because it contains all indispensable information for growth and function.43 RM is now investigating the use of intact, animal-derived ECM scaffolds as a platform for human organ bioengineering. Indeed, the decellularization and recellularization of the pancreata in our investigations is a core strategy reapplied to 1 specific organ. The principles readily generalize across the board, however.
ECM scaffolds from whole animal or human-cadaveric organs can be generated through detergent-based decellularization (Figure 1).70-72,78 Current decellularization techniques are capable of removing DNA, cellular material and cell surface antigens from the ECM scaffold while preserving attachment sites, structural integrity and vascular channels.79 Decellularization protocols involve the repeated irrigation of cadaveric tissues with detergents or acids through the innate vasculature, although organs with higher fat content, like the pancreas, often require the addition of lipid solvents, such as alcohol.80 Standard protocols employ a combination of ionic and nonionic detergents, enzymatic nucleases and antimicrobials, although the optimal composition and concentration of the detergent solution varies with the size, age, and density of the organ. Though previous studies reported that pancreatic decellularization may disrupt ECM protein ultrastructure,81 recent characterization studies of the decellularized porcine pancreas confirmed the sweeping presence of the essential structural proteins including different types of collagen, elastin, fibronectin, and laminin.82
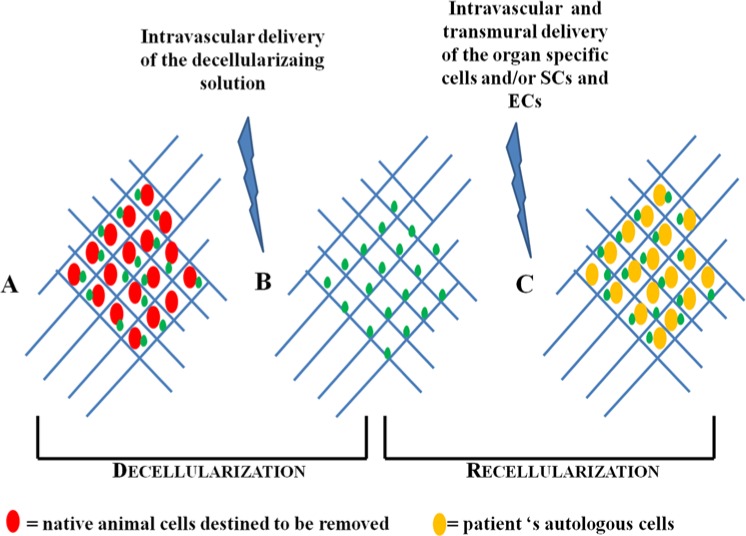
Figure 1.
Schematic representation of decellularization–recellularization technology. (A) Whole organ consisting of both the cellular compartment (red shapes) and the ECM (blue network), which contains also growth factors (green dots). (B) Acellular organ, after stripping of all cells (red shapes have been cleared off). Native ECM (blue network) supposedly remains intact and preserved in all its fundamental components, growth factors (green dots) included. (C) The new organ is reconstituted with autologous cells (yellow shapes). Adapted from Orlando et al,71 with permission.
Effective decellularization protocols are designed to achieve a series of key outcomes, including disruption of the cell membrane, cell lysis, removal of cytoplasmic contents, induction of endogenous nucleases, disruption of nuclear membranes, and degradation of nuclear material. It remains unclear whether detergent decellularization damages the essential components of the ECM, although irrigation through the existing vasculature is thought to limit potentially disruptive exposure. Complete decellularization is essential as residual cellular material may contain antigenic epitopes that trigger inflammatory responses83 and compromise subsequent recellularization.84 Following decellularization, gamma irradiation,85 ethylene oxide,86 or paracetic acid87 have been shown to effectively sterilize the ECM without denaturing the ECM proteins or growth factors, although the risk of viral contamination remains.44
The decellularized, sterilized ECM serves as the scaffold on which pluripotent cells are seeded with the intent to reconstitute the cellular compartment (recellularization). The successful recellularization of ECM scaffolds has been reported in several organ systems, including liver,88 respiratory tract,89 nerve,80 tendon,90 valve,91 bladder,92 and mammary gland.93 These results demonstrate the potential of RM to dramatically impact organ transplantation, with the possibility of upscaling to more complex, modular organs. Organs bioengineered from autologous cells may enable surgeons to successfully address the 2 major obstacles currently facing organ transplantation: (1) the need for a new, ideally inexhaustible source of organs and (2) the achievement of an immunosuppression-free state posttransplantation.
A critical advancement in the field of organ bioengineering has been the development of suitable bioreactors. These devices allow for the continuous support, monitoring, and manipulation of the biological, biochemical, and biophysical processes involved in organ bioengineering. Bioreactors facilitate the even distribution of seed populations on scaffolds while providing effective nutrient supply, waste removal, and hydrodynamic shear stress.94,95 Flow-dependent shear stress is a critical mediator of cellular development, as the mechanical stimulus activates signaling pathways that influence the cellular activity, differentiation, and function of the newly engrafted cells.96 Theoretically, bioreactors provide an environment where the reseeded scaffolds can mature under “in vivo–like” conditions in preparation for implantation.
An ideal bioreactor device must allow (1) precision control of environmental factors, (2) perfusion of innate channels with the seed population, (3) automated operation allowing for sterile conditions, (4) real-time monitoring, and (5) modifiable levels of shear stress. Perfusion bioreactors are essential for uniform cellular dispersion, as static culture conditions result in poor cellular migration, dispersion and adherence. As a result, static recellularization protocols are associated with a disproportionate deposition of cells around the organ exterior with a paucity of cells within the inner parenchyma.97 Poor seeding of the inner layers compromises organ integrity as it (1) fails to recapitulate the 3D structure of the native organ and (2) results in poor deposition of cells around the vascular channels. Close proximity to the arterial network is a necessity for the successful engraftment, proliferation and long-term viability of the seed population, due to the high metabolic requirements of differentiating cells. Perfusion bioreactors overcome this obstacle by distributing cells uniformly throughout the organ while providing shear stress via fluid flow.96
Whole-organ Pancreas Bioengineering
Pancreas bioengineering lags behind other organs in the field, as only a handful of studies report the successful repopulation of decellularized pancreatic ECM.98-100 De Carlo et al99 report the successful subcutaneous implantation of PVA/PEG tubular devices containing slices of rat pancreas and liver recellularized with differentiated murine islets. The pancreatic matrix significantly extended the duration of insulin function, suggesting that pancreas-specific matrix favors islet response to glucose over the long term. Upon transplantation, the islet devices effectively reduced hyperglycaemia, although normalization was not achieved. Conrad et al98 report abbreviated findings describing the successful recellularization of murine pancreatic matrix with human islet cells and supportive MSCs. The islets showed preserved glucose-stimulated insulin response, cell viability, subcellular anatomy, and attachments. However, complete findings have yet to be published.
Although these investigations serve as an important foundation for pancreas bioengineering, the scaffolds described in these studies do not have the physiological and structural capacity to support the critical mass of β-cells required to meet human insulin requirements.99 The application of decellularization–recellularization protocols to larger, complex organs can be challenging as perfusion decellularization relies in part on diffusion which can be impeded by their solid parenchymal compartments. Larger organs with greater parenchymal mass require higher perfusion pressures, stronger detergents and prolonged detergent exposure that may damage the native architecture and ECM proteins.74 To this end, we have recently reported the generation of intact, whole-organ ECM scaffolds from porcine pancreata.100 Successful decellularization was achieved by perfusing the innate vasculature with detergents administered via cannulas in the pancreatic duct and superior mesenteric vein. Although organs with a higher fat content, like the pancreas, are thought to require additional lipid solvents (Figure 2).80 (We were able to achieve optimal results with standard nonionic cellular disruption mediums.)101 Decellularization was confirmed through nuclear staining and electron microscopy. Imaging studies further demonstrated that our decellularization protocol preserved the vascular network intact, allowing for effective scaffold repopulation (Figure 3). Characterization studies of the acellular porcine pancreas showed widespread distribution of all essential structural proteins, including different types of collagen, elastin, fibronection, and laminin (Figure 4). The biocompatibility of the decellularized matrix was assessed by seeding the scaffold with human amniotic fluid stem cells, a potential stem cell source for the generation of β-cells.102,103 In our study, partial recellularization was histologically confirmed, demonstrating that porcine pancreatic ECM is capable of supporting the growth of human amniotic progenitor cells. These findings support the future possibility of “semi-xenotransplantation,” the transplantation of animal-derived matrices populated with human cells.67 We also demonstrated that the porcine pancreatic ECM promotes glucose-mediated insulin release when repopulated with differentiated islets in culture (Figure 5). Briefly, following overnight soaking in serum-free medium, scaffolds were sliced into 2-dimensional sections and subsequently seeded statically with progenitor cells in suspension. Our results demonstrate that the porcine pancreatic scaffolds serve as a formidable platform for insulin-producing bioengineered tissue because they (1) are easily explanted and decellularized, (2) retain native structural relationships and vascular channels, (3) are amenable to repopulation via immersion or perfusion, and (4) provide the cues necessary for cellular adhesion and proliferation.
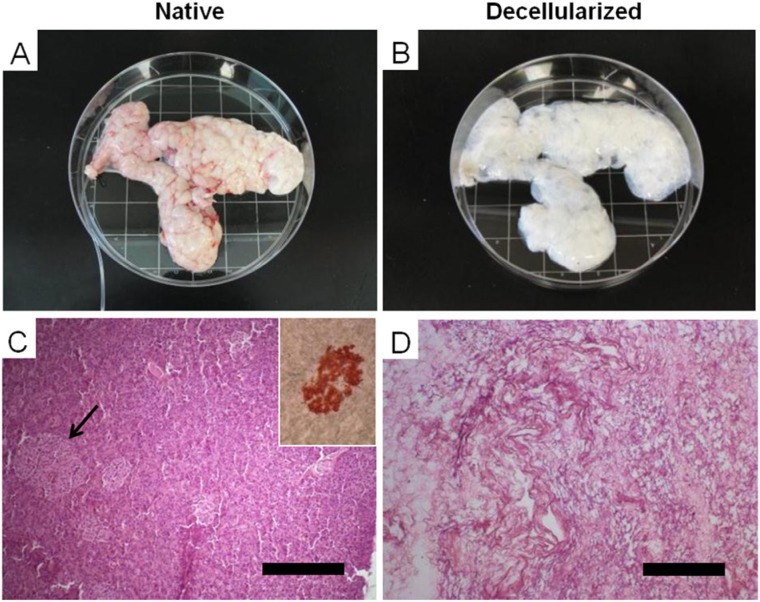
Figure 2.
Decellularization of whole porcine pancreas. (A) Porcine pancreas upon collection, with cannulated pancreatic duct. (B) The same pancreas after decellularization, showing a characteristic whitish/translucent appearance. (C, D) H&E staining of native and acellular porcine pancreas, respectively; the dense cellularity of the native pancreas is lost post detergent perfusion (arrow indicates an islet, which is extrapolated in the small cartoon). Adapted from Mirmalek-Sani et al,82 with permission.
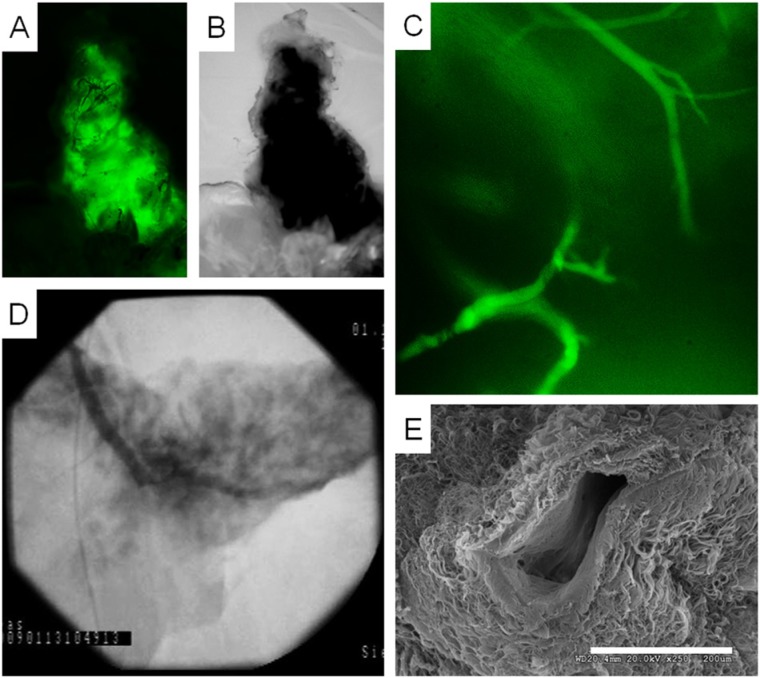
Figure 3.
Appearance of the innate, intrinsic vasculature in the decellularized porcine pancreas. (A, B) Perfusion of fluorescein isothiocyanate (FITC)–labeled dextran beads inside the decellularized pancreas, shown under fluorescent and bright light microscopy, respectively. (C) High magnification of panel A shows intact vessels inside the decellularized pancreas perfused with FITC-labeled dextran beads. (D) Fluoroangiograph of acellular porcine pancreas vasculature following perfusion of Conray® contrast agent. (F) Scanning electron micrograph of a preserved blood vessel with intact and smooth basal lamina layer. Scale bar = 200 µm. Adapted from Mirmalek-Sani et al,82 with permission.
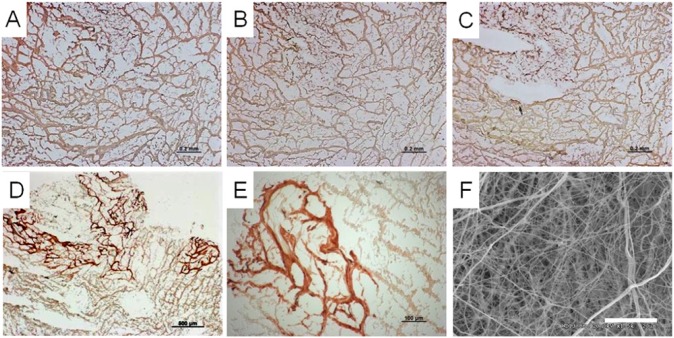
Figure 4.
Immunohistochemistry of acellular porcine pancreas ECM. IHC staining shows widespread expression of structural ECM proteins, namely collagen type I (A), collagen type III (B), collagen type IV (C), and laminin (D, E). (F) Scanning electron micrograph depicting dense and fibrous arrangement of matrix proteins (scale bars = 200 µm (A-C), 500 µm (D), 100 µm (E), and 20 µm (F)). Adapted from Mirmalek-Sani et al,82 with permission.
Figure 5.
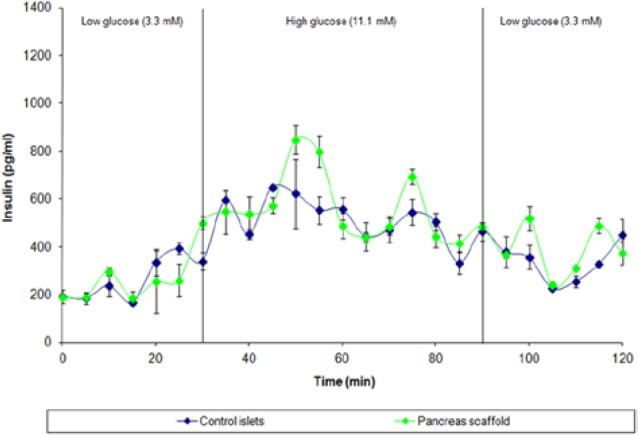
Functionality test of porcine islets seeded on porcine ECM. Time course data from perfusion of control (unseeded) porcine islets (blue) or islets seeded onto acellular pancreatic matrix (green). Groups were cultured for 3 days then subjected to a glucose challenge. Low glucose levels (3.3 mM) represented physiological euglycemia of 60 mg/dL, and the transition to high glucose (11.1 mM) represented the upper limit postprandial levels of 200 mg/dL. Both groups displayed increased insulin secretion during high glucose perfusion, with islets seeded onto scaffolds demonstrating higher peak insulin secretion values, observed specifically at 50, 55 and 75 minutes. Both groups showed a reduction of insulin secretion after a return to low (basal) glucose concentration. Adapted from Mirmalek-Sani et al,82 with permission.
Future Challenges
Several obstacles impede the clinical application of regenerative technologies in the treatment of insulin-dependent diabetes. On a molecular level, Otonkoski et al59 call for improved identification and understanding of ECM proteins and their islet receptors, as “specific laminin isoforms have not been tested in the context of human β-cell differentiation and proliferation in a physiological microenvironment.” Furthermore, the interaction of laminins and growth factors requires further investigation, as it remains unclear how growth factors augment proliferation and insulin response. With regard to islet transplantation, the major obstacles remain primary nonfunction of transplanted islets due to host inflammatory reactions, and the lack of a renewable cell source. Attempts to expand adults islets or committed pancreatic progenitors in culture have been unsuccessful, although Schulz et al104 have recently reported the scalable production of pancreatic progenitors from human embryonic stem cells.
In regard to whole-pancreas bioengineering, Goh et al recently made progress in regenerating 3D pancreata.105 Exocrine and endocrine cell suspensions were perfused through hepatic portal vein in successfully decellularized mouse pancreata. The resulting constructs showed upregulated insulin gene expression that the authors attributed to cell-ECM interactions. Nevertheless, progress is impeded by the unanswered questions that remain in the field. Although effective protocols have been established for whole-organ pancreatic decellularization in pigs and rats, similar studies involving nonhuman primates have not been attempted. Nonhuman primate studies will become increasingly important as the field advances, as porcine pancreata show marked differences in ECM architecture. The porcine pancreas has a sparse basement membrane with few cell-to-matrix adhesions compared to human or murine pancreata,106 which may facilitate decellularization. It remains unclear whether the decellularized pancreatic ECM can support the proliferation and differentiation of stem cells into the variety of cell types that compose the mature human pancreas. Even after the generation of a bioengineered pancreas has been achieved, further transplantation studies will be required to assess long-term viability, coagulation, revascularization, reinnervation, fibrosis, and potential for tumor growth.
Summary
We have reviewed recent advancements in RM as they apply to the pancreas engineering and the restoration of endocrine function. Emergent stem cell and biomaterial technologies have the potential to progress β-cell replacement through the advent of islet encapsulation and whole-organ bioengineering. These technologies depend on a robust understanding of the structural and functional roles of the pancreatic ECM. Although these roles may be roughly approximated with biosynthetic carriers, novel decellularization protocols have allowed researchers to exploit the advantages of the native ECM for effective recellularization and whole-organ bioengineering. Although the progress to date cannot be overstated, pancreatic bioengineering lags behind other organs in the field, as further research is required to maximize cell-matrix interactions in bioengineered pancreata, Despite these obstacles, RM technologies hold enormous potential to resolve the dire shortage of transplantable organs. Further collaborative efforts are required to drive the field forward toward the successful production of a bioengineered pancreas capable of restoring endocrine function in patients with end-stage disease.
Footnotes
Abbreviations: ECM, extracellular matrix; FAK, focal adhesion kinase; MAP, mitogen-activated protein kinase; PI, phosphatidylinositol; PKB, protein kinase B; RM, regenerative medicine; 3D, three dimensional.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Orlando G, Stratta RJ, Light J. Pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant. 2011;16:110-115. [DOI] [PubMed] [Google Scholar]
- 2. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289-1298. [PubMed] [Google Scholar]
- 3. Ruggenenti P, Remuzzi A, Remuzzi G. Decision time for pancreatic islet-cell transplantation. Lancet. 2008;371(9616):883-884. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318-1330. [DOI] [PubMed] [Google Scholar]
- 5. Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimizu H, et al. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials. 2009;30(30):5943-5949. [DOI] [PubMed] [Google Scholar]
- 7. Nagata NA, Inoue K, Tabata Y. Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polym Ed. 2002;13(5):579-590. [DOI] [PubMed] [Google Scholar]
- 8. Pugliese A, et al. Recurrence of autoimmunity in pancreas transplant patients: research update. Diabetes Manag (Lond). 2011;1(2):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45(6):749-763. [DOI] [PubMed] [Google Scholar]
- 10. Fiorina P, et al. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990-1997. [DOI] [PubMed] [Google Scholar]
- 11. Tharavanij T, et al. Improved long-term health-related quality of life after islet transplantation. Transplantation. 2008;86(9):1161-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warnock GL, et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation. 2008;86(12):1762-1766. [DOI] [PubMed] [Google Scholar]
- 13. Cravedi P, Remuzzi A, Remuzzi G. Comment on: Robertson (2010) Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes;59:1285-1291. Diabetes. 2010;59(9):e13; author reply e14. [DOI] [PubMed] [Google Scholar]
- 14. Thompson DM, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373-378. [DOI] [PubMed] [Google Scholar]
- 15. Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230-238. [DOI] [PubMed] [Google Scholar]
- 16. Robertson RP, et al. Pancreas and islet transplantation in type 1 diabetes. Diabetes Care. 2006;29(4):935. [DOI] [PubMed] [Google Scholar]
- 17. Barton FB, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35(7):1436-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly WD, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61(6):827-837. [PubMed] [Google Scholar]
- 19. Venstrom JM, et al. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA. 2003;290(21):2817-2823. [DOI] [PubMed] [Google Scholar]
- 20. Navarro X, et al. Influence of pancreas transplantation on cardiorespiratory reflexes, nerve conduction, and mortality in diabetes mellitus. Diabetes. 1990;39(7):802-806. [DOI] [PubMed] [Google Scholar]
- 21. Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997;42(5):727-736. [DOI] [PubMed] [Google Scholar]
- 22. Kennedy WR, et al. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med. 1990;322(15):1031-1037. [DOI] [PubMed] [Google Scholar]
- 23. Fioretto P, et al. Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet. 1993;342(8881):1193-1196. [DOI] [PubMed] [Google Scholar]
- 24. Bilous RW, et al. The effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med. 1989;321(2):80-85. [DOI] [PubMed] [Google Scholar]
- 25. Zehr PS, et al. Pancreas transplantation: assessing secondary complications and life quality. Diabetologia. 1991;34(suppl 1):S138-S140. [DOI] [PubMed] [Google Scholar]
- 26. Zehrer CL, Gross CR. Quality of life of pancreas transplant recipients. Diabetologia. 1991;34(suppl 1):S145-S149. [DOI] [PubMed] [Google Scholar]
- 27. Mohan P, et al. Improved patient survival in recipients of simultaneous pancreas-kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end-stage renal disease. Br J Surg. 2003;90(9):1137-1141. [DOI] [PubMed] [Google Scholar]
- 28. Orlando G. Immunosuppression-free transplantation reconsidered from a regenerative medicine perspective. Expert Rev Clin Immunol. 2012;8(2):179-187. [DOI] [PubMed] [Google Scholar]
- 29. Vaithilingam V, Tuch BE. Islet transplantation and encapsulation: an update on recent developments. Rev Diabet Stud. 2011;8(1):51-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Opara EC, et al. Design of a bioartificial pancreas. J Investig Med. 2010;58(7):831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weir GC. Islet encapsulation: advances and obstacles. Diabetologia. 2013;56(7):1458-1461. [DOI] [PubMed] [Google Scholar]
- 32. Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14(12):1959-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163(2):181-190. [DOI] [PubMed] [Google Scholar]
- 34. Rosenberg L, et al. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126(2):393-398. [PubMed] [Google Scholar]
- 35. Montesano R, et al. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol. 1983;97(3):935-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meda P, Hooghe-Peters EL, Orci L. Monolayer cultures of adult pancreatic islet cells on osmotically disrupted fibroblasts. Diabetes. 1980;29(6):497-500. [DOI] [PubMed] [Google Scholar]
- 37. Rabinovitch A, Russell T, Mintz DH. Factors from fibroblasts promote pancreatic islet B cell survival in tissue culture. Diabetes. 1979;28(12):1108-1113. [DOI] [PubMed] [Google Scholar]
- 38. Thivolet CH, et al. Morphological and functional effects of extracellular matrix on pancreatic islet cell cultures. Exp Cell Res. 1985;159(2):313-322. [DOI] [PubMed] [Google Scholar]
- 39. Dufour JM, et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9-10):1323-1331. [DOI] [PubMed] [Google Scholar]
- 40. Totani T, Teramura Y, Iwata H. Immobilization of urokinase on the islet surface by amphiphilic poly(vinyl alcohol) that carries alkyl side chains. Biomaterials. 2008;29(19):2878-2883. [DOI] [PubMed] [Google Scholar]
- 41. Kloxin AM, et al. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater. 2010;22(31):3484-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borg DJ, Bonifacio E. The use of biomaterials in islet transplantation. Curr Diab Rep. 2011;11(5):434-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17(8):424-432. [DOI] [PubMed] [Google Scholar]
- 45. Gittes GK, et al. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122(2):439-447. [DOI] [PubMed] [Google Scholar]
- 46. Hisaoka M, Haratake J, Hashimoto H. Pancreatic morphogenesis and extracellular matrix organization during rat development. Differentiation. 1993;53(3):163-172. [DOI] [PubMed] [Google Scholar]
- 47. Jiang FX, et al. Laminin-1 promotes differentiation of fetal mouse pancreatic beta-cells. Diabetes. 1999;48(4):722-730. [DOI] [PubMed] [Google Scholar]
- 48. Rondas D, et al. Novel mechanistic link between focal adhesion remodeling and glucose-stimulated insulin secretion. J Biol Chem. 2012;287(4):2423-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boretti MI, Gooch KJ. Effect of extracellular matrix and 3D morphogenesis on islet hormone gene expression by Ngn3-infected mouse pancreatic ductal epithelial cells. Tissue Eng Part A. 2008;14(12):1927-1937. [DOI] [PubMed] [Google Scholar]
- 50. Menke A, et al. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001;61(8):3508-3517. [PubMed] [Google Scholar]
- 51. Edamura K, et al. Effect of adhesion or collagen molecules on cell attachment, insulin secretion, and glucose responsiveness in the cultured adult porcine endocrine pancreas: a preliminary study. Cell Transplant. 2003;12(4):439-446. [DOI] [PubMed] [Google Scholar]
- 52. Ryschich E, et al. Promotion of tumor cell migration by extracellular matrix proteins in human pancreatic cancer. Pancreas. 2009;38(7):804-810. [DOI] [PubMed] [Google Scholar]
- 53. Kaido T, et al. Alphav-integrin utilization in human beta-cell adhesion, spreading, and motility. J Biol Chem. 2004;279(17):17731-17737. [DOI] [PubMed] [Google Scholar]
- 54. Hayek A, et al. Growth factor/matrix-induced proliferation of human adult beta-cells. Diabetes. 1995;44(12):1458-1460. [DOI] [PubMed] [Google Scholar]
- 55. Beattie GM, et al. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996;45(9):1223-1228. [DOI] [PubMed] [Google Scholar]
- 56. Kaiser N, et al. Monolayer culture of adult rat pancreatic islets on extracellular matrix: long term maintenance of differentiated B-cell function. Endocrinology. 1988;123(2):834-840. [DOI] [PubMed] [Google Scholar]
- 57. Hammar E, et al. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53(8):2034-2041. [DOI] [PubMed] [Google Scholar]
- 58. Beattie GM, et al. Ex vivo expansion of human pancreatic endocrine cells. J Clin Endocrinol Metab. 1997;82(6):1852-1856. [DOI] [PubMed] [Google Scholar]
- 59. Otonkoski T, et al. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10(suppl 4):119-127. [DOI] [PubMed] [Google Scholar]
- 60. Virtanen I, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51(7):1181-1191. [DOI] [PubMed] [Google Scholar]
- 61. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11-25. [DOI] [PubMed] [Google Scholar]
- 62. Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hammar EB, et al. Activation of NF-kappaB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J Biol Chem. 2005;280(34):30630-30637. [DOI] [PubMed] [Google Scholar]
- 64. Lucas-Clerc C, et al. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94(1):9-20. [DOI] [PubMed] [Google Scholar]
- 65. Han B, et al. TGF-beta i promotes islet beta-cell function and regeneration. J Immunol. 2011;186(10):5833-5844. [DOI] [PubMed] [Google Scholar]
- 66. Crisera CA, et al. Transforming growth factor-beta 1 in the developing mouse pancreas: a potential regulator of exocrine differentiation. Differentiation. 2000;65(5):255-259. [DOI] [PubMed] [Google Scholar]
- 67. Nakamura H, et al. Preferential increase of extracellular matrix expression relative to transforming growth factor beta1 in the pancreas during the early stage of acute hemorrhagic pancreatitis in rats. Pancreas. 2007;35(4):e23-e29. [DOI] [PubMed] [Google Scholar]
- 68. Blaine SA, et al. Epidermal growth factor receptor regulates pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G434-G441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grzesiak JJ, et al. The extracellular matrix differentially regulates the expression of PTHrP and the PTH/PTHrP receptor in FG pancreatic cancer cells. Pancreas. 2004;29(2):85-92. [DOI] [PubMed] [Google Scholar]
- 70. Orlando G, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transpl Int. 2011;24(3):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Orlando G, et al. Regenerative medicine and organ transplantation: past, present, and future. Transplantation. 2011;91(12):1310-1317. [DOI] [PubMed] [Google Scholar]
- 72. Orlando G, et al. Regenerative medicine as applied to general surgery. Ann Surg. 2012;255(5):867-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Badylak SF, et al. Engineered whole organs and complex tissues. Lancet. 2012;379(9819):943-952. [DOI] [PubMed] [Google Scholar]
- 74. Orlando G, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012;256(2):363-370. [DOI] [PubMed] [Google Scholar]
- 75. Wang Y, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53(1):293-305. [DOI] [PubMed] [Google Scholar]
- 76. Ross EA, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20(11):2338-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ng SL, et al. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32(30):7571-7580. [DOI] [PubMed] [Google Scholar]
- 78. Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675-3683. [DOI] [PubMed] [Google Scholar]
- 80. Crapo PM, et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33(13):3539-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tchen TT, et al. , et al. Decellularization of Pancreatic Extracellular Matrix for a Tissue-engineered Pancreas. Pittsburgh, PA: University of Pittsburgh; 2007. [Google Scholar]
- 82. Mirmalek-Sani SH, et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34(22):5488-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown BN, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sullivan DC, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33(31):7756-7764. [DOI] [PubMed] [Google Scholar]
- 86. Reing JE, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31(33):8626-8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brown B, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12(3):519-526. [DOI] [PubMed] [Google Scholar]
- 88. Baptista PM, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604-617. [DOI] [PubMed] [Google Scholar]
- 89. Song JJ, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92(3):998-1005; discussion 1005-1006. [DOI] [PubMed] [Google Scholar]
- 90. Martinello T, et al. ,. Successful recellularization of human tendon scaffolds using adipose-derived mesenchymal stem cells and collagen gel. J Tissue Eng Regen Med. 2012. [DOI] [PubMed]
- 91. Honge JL, et al. Recellularization of aortic valves in pigs. Eur J Cardiothorac Surg. 2011;39(6):829-834. [DOI] [PubMed] [Google Scholar]
- 92. Loai Y, et al. Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A. 2010;94(4):1205-1215. [DOI] [PubMed] [Google Scholar]
- 93. Wicha MS, et al. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982;79(10):3213-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Baiguera S, Birchall MA, Macchiarini P. Tissue-engineered tracheal transplantation. Transplantation. 2010;89(5):485-491. [DOI] [PubMed] [Google Scholar]
- 95. Rauh J, et al. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17(4):263-280. [DOI] [PubMed] [Google Scholar]
- 96. Chen HC, Hu YC. Bioreactors for tissue engineering. Biotechnol Lett. 2006;28(18):1415-1423. [DOI] [PubMed] [Google Scholar]
- 97. Martin I, et al. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Ann Biomed Eng. 1999;27(5):656-662. [DOI] [PubMed] [Google Scholar]
- 98. Conrad C, et al. Bio-engineered endocrine pancreas based on decellularized pancreatic matrix and mesenchymal stem cell/islet cell coculture. J Am Coll Surg. 2010;211(3):S62. [Google Scholar]
- 99. De Carlo E, et al. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and in vivo studies. Int J Mol Med. 2010;25(2):195-202. [PubMed] [Google Scholar]
- 100. Goh SK. Perfusion-decellularization of pancreatic matrix—a scaffold for bio-engineered pancreas. Paper presented at: AIChE; 2011; Minneapolis, MN. [Google Scholar]
- 101. Mirmalek-Sani SH, et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34:5488-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. De Coppi P, Bartsch G, Jr., Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic fluid stem cell lines with potential for therapy. Nat Biotechnol 2007;25(1):100-6. [DOI] [PubMed] [Google Scholar]
- 103. Li B, et al. Neuronal restrictive silencing factor silencing induces human amniotic fluid-derived stem cells differentiation into insulin-producing cells. Stem Cells Dev. 2011;20(7):1223-1231. [DOI] [PubMed] [Google Scholar]
- 104. Schulz TC, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLOS ONE. 2012;7(5):e37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goh SK, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34(28):6760-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van Deijnen JH, et al. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267(1):139-146. [DOI] [PubMed] [Google Scholar]